Assessment of Innovative Dry Powders for Inhalation of a Synergistic Combination Against Mycobacterium tuberculosis in Infected Macrophages and Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Dry Powders for Inhalation Based on VAN and THL
2.1.1. First Step of Production: Micronization of VAN
2.1.2. Second Step of Production: Preparation of Dry Powder for Inhalation
2.2. In Vitro Characterization of the SD-VAN and Dry Powders for Inhalation
2.2.1. Physicochemical Properties
- VAN and THL determination—HPLC-DAD method
- VAN and THL content
- Residual solvent, HCO content and yield
2.2.2. In Vitro Aerodynamic Properties
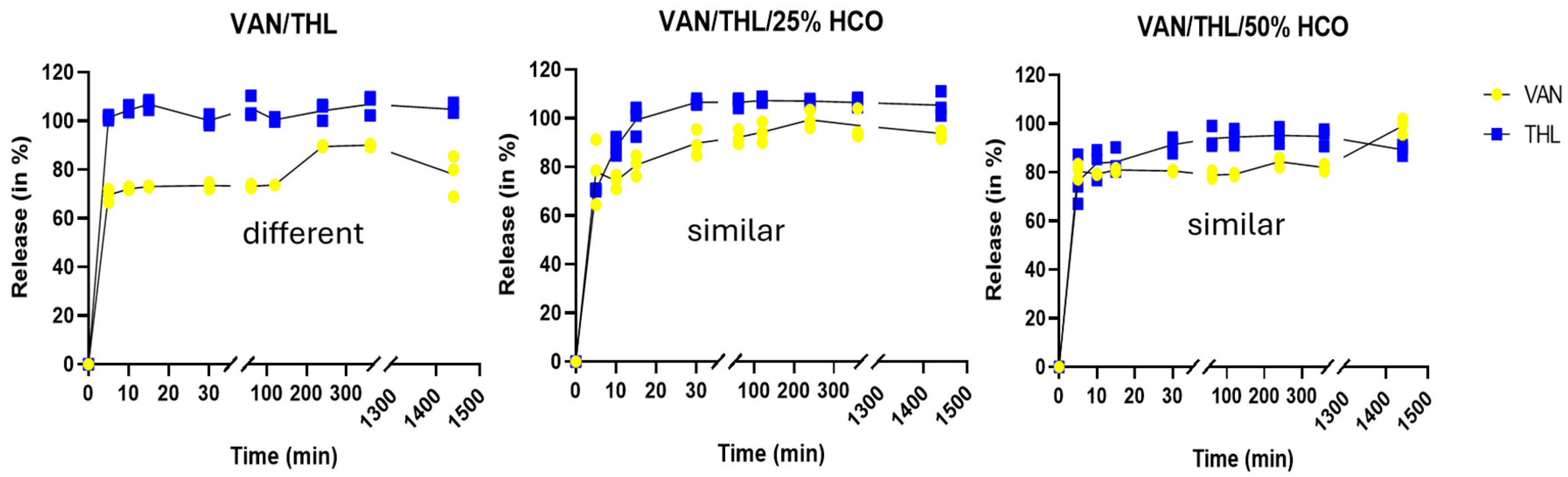
2.2.3. In Vitro Release Properties
2.3. In Vitro Efficacy Study on Infected Macrophages
2.3.1. Monocyte Differentiation into Macrophages
2.3.2. Bacterial Culture and Infection of Macrophages
2.3.3. Treatment and Analysis
2.4. In Vivo Efficacy Study in Mtb-Infected Mice
2.4.1. Animals
2.4.2. Infection
2.4.3. Preparation and Characterization of the Powder Blends for Pulmonary Administration in Mice
2.4.4. Treatment
2.4.5. Sampling, Histopathology, and Determination of the Relative Light Unit (RLU)
2.5. In Vitro and In Vivo Efficacy Studies of VAN/THL Dry Powder for Inhalation Associated with First-Line Anti-TB Drug RIF
- In vitro efficacy study in Mtb
- In vivo efficacy study in Mtb-infected mice
2.6. Statistical Analysis
3. Results and Discussion
3.1. Production of Dry Powders for Inhalation and Physicochemical Properties
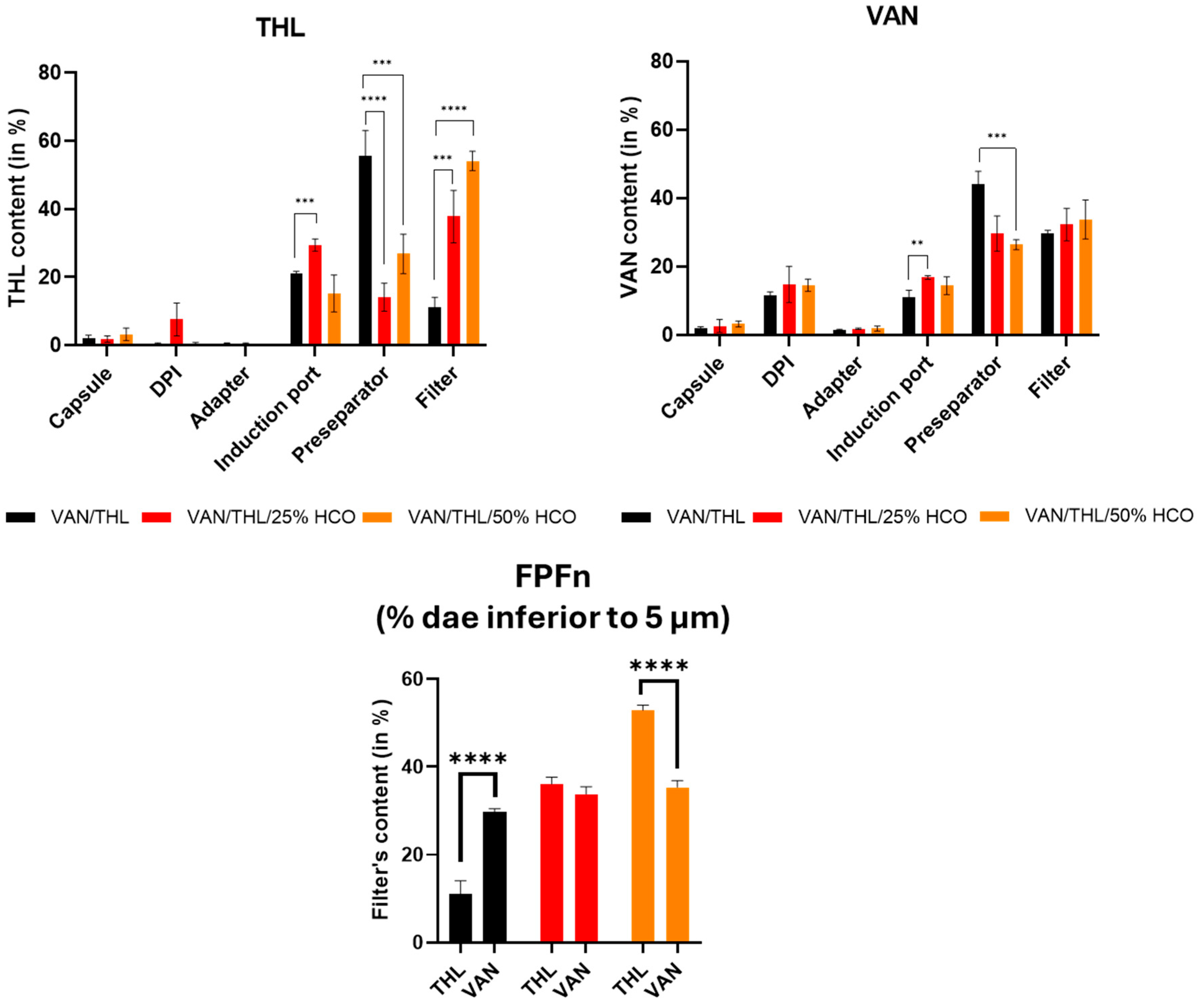
3.2. In Vitro Aerodynamic Performances of the Dry Powders for Inhalation
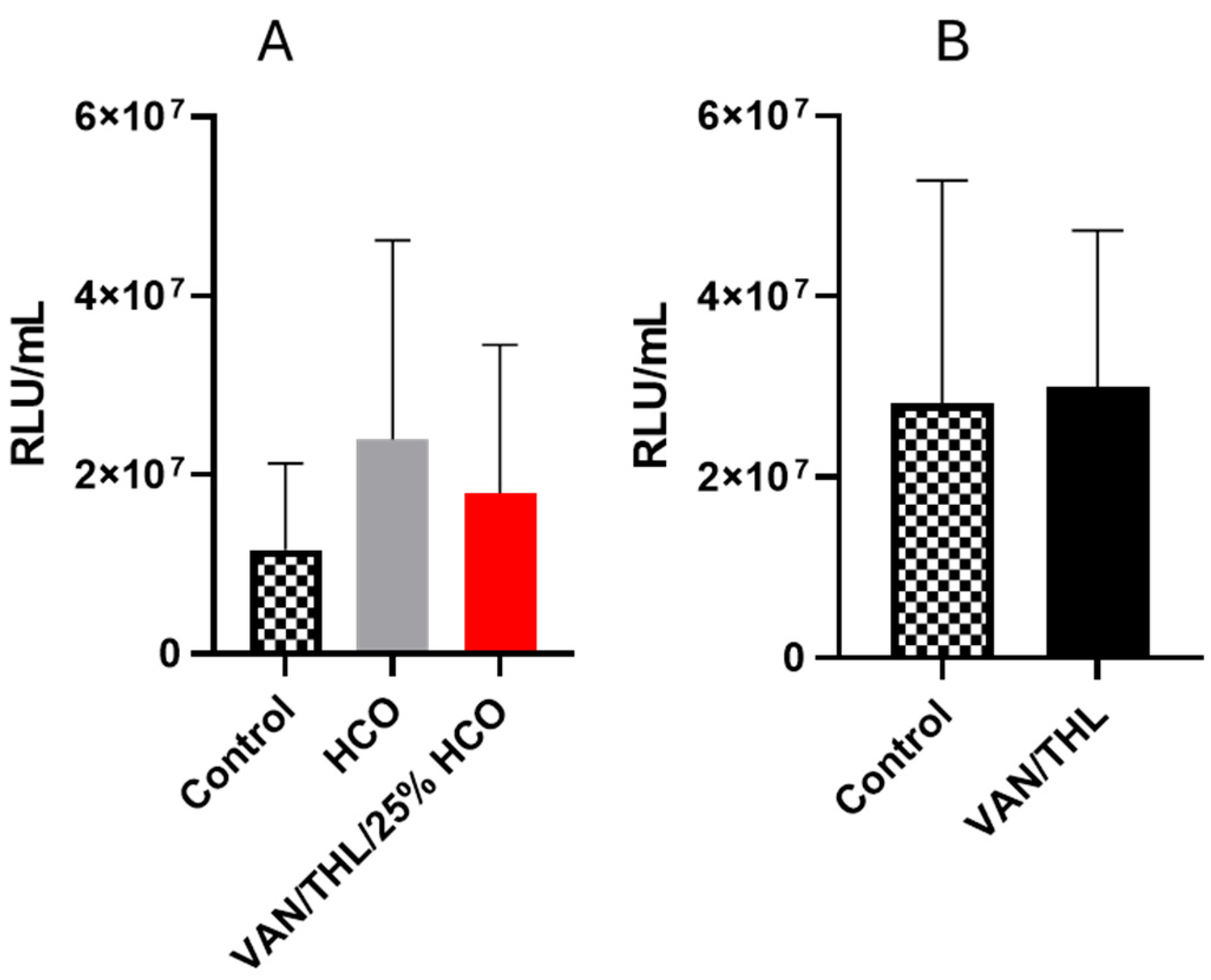
3.3. In Vitro Efficacy Study in M. bovis BCG::ESX-1Mmar-Infected Macrophages
3.4. In Vivo Efficacy Studies of the Dry Powder for Inhalation VAN/THL and VAN/THL/25% HCO in Mtb-Infected Mice
3.5. Evaluation of the Dry Powders for Inhalation in Combination with Rifampicin
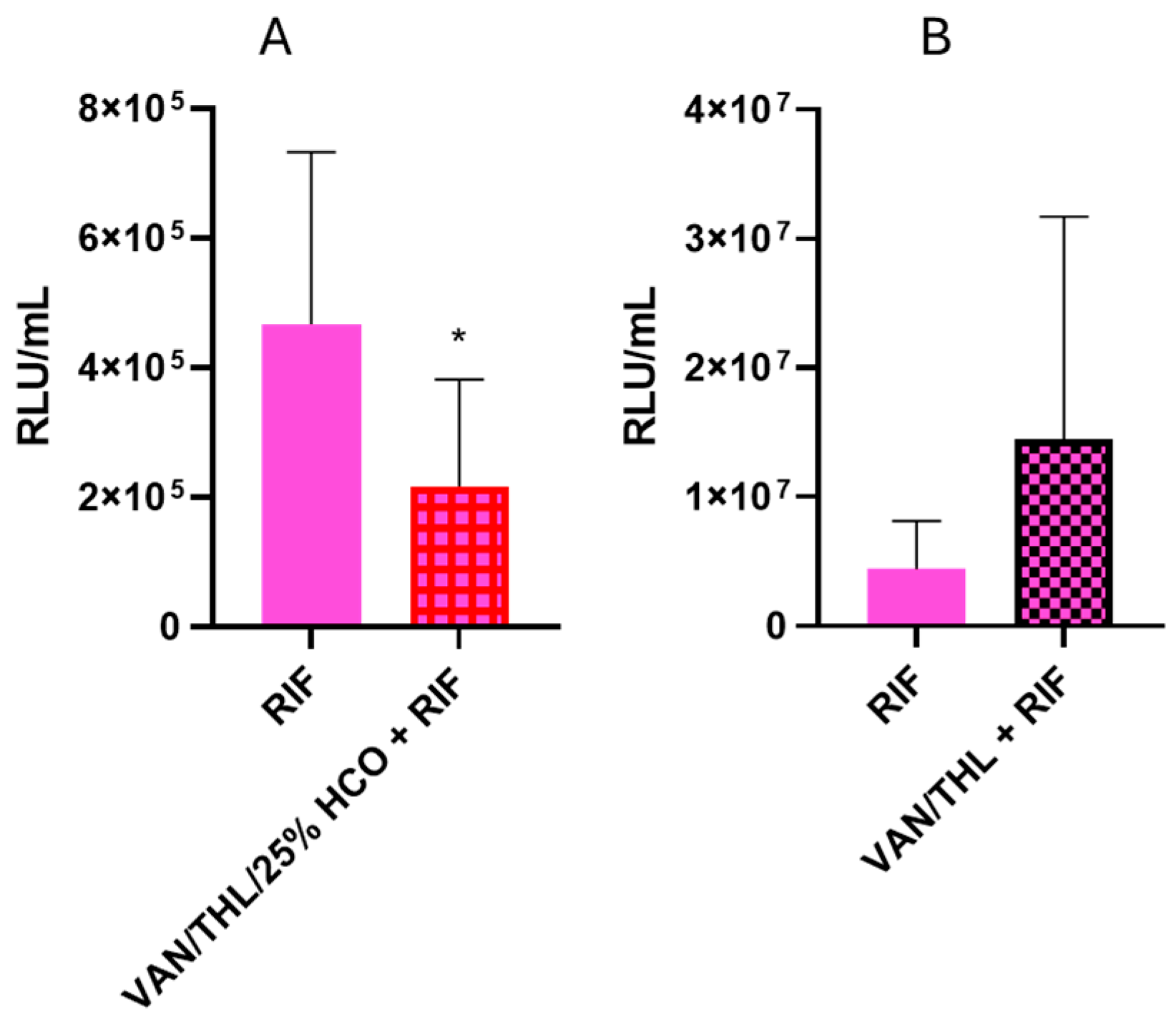
3.5.1. In Vitro Efficiency of RIF and THL Against Mtb
3.5.2. In Vivo Efficacy Studies of the Dry Powders for Inhalation in Combination with Oral RIF in Mtb-Infected Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VAN | vancomycin (hydrochloride) |
| RIF | rifampicin |
| THL | tetrahydrolipstatin |
| HCO | hydrogenated castor oil |
| Mtb | Mycobacterium tuberculosis |
| WHO | World Health Organization |
| TB | tuberculosis |
| DPI | dry powder inhaler |
| AM | alveolar macrophage |
| FPF | fine particle fraction |
| PDIM | phthiocerol dimycocerosate |
| MIC | minimum inhibitory concentration |
| SD | spray-drying |
| TPGS | PEGylated derivative of tocopherol succinate |
| FSI | fast-screening impactor |
| HPLC-DAD | High-Performance Liquid System connected to a diode array detector |
| FBS | fetal bovin serum |
| PBS | phosphate-buffer saline |
| PMA | phorbol 12-myristate 13-acetate |
| CFU | colony forming unit |
| ADC | Albumin-Dextrose-Catalase |
| OADC | Oleic-Albumin-Dextrose-Catalase |
| PADA | powder administration device for animals |
| FICI | fractional inhibitory concentration index |
| PSD | particle size distribution |
| TGA | thermogravimetric analysis |
| XRPD | X-ray powder diffraction |
| HE | haematoxylin and eosinophil coloration |
| BEV | bronchial epithelial vacuolation |
| PAS | periodic acid Schiff |
| Cong | congestion |
| ALM | alveolar luminal macrophages |
| IAF | intra-alveolar fibrin |
| IAH | intra-alveolar hemorrhage |
| PH | pneumocyte hyperplasia |
| AB | acute bronchopneumonia |
| LI | lobular infiltrate |
| PI | periportal infiltrate |
| FHN | focal hepatocyte necrosis |
| MM | mega-mitochondria |
| Stea | steatosis |
| IGI | intranuclear glycogen inclusion |
| GD | glycogen depletion |
| TN | tubular necrosis |
| IF | intestinal fibrosis |
| TA | tubular atrophy |
| ITN | inflammation or tubulointerstitial nephritis |
References
- World Health Organization. Global Tuberculosis Report 2024. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2024 (accessed on 7 March 2025).
- Omoteso, O.A.; Fadaka, A.O.; Walker, R.B.; Khamanga, S.M. Innovative Strategies for Combating Multidrug-Resistant Tuberculosis: Advances in Drug Delivery Systems and Treatment. Microorganisms 2025, 13, 722. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, L.; Sethuraman, V.; Kazantseva, M.; Hickey, A. Efficacy of Combined Rifampicin Formulations Delivered by the Pulmonary Route to Treat Tuberculosis in the Guinea Pig Model. Pharmaceutics 2021, 13, 1309. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.K.; Roy, T.; Verma, K.; Yadav, C.; Verma, S.; Deivreddy, V.S.R.; Sofi, H.S.; Bharti, R.; Sharma, R.; Bansode, H.; et al. Dry powder Inhalation of lytic mycobacteriophages for adjunct therapy in a mouse model of infection with Mycobacterium tuberculosis. Tuberculosis 2025, 152, 102631. [Google Scholar] [CrossRef] [PubMed]
- Laohapojanart, N.; Ratanajamit, C.; Kawkitinarong, K.; Srichana, T. Efficacy and safety of combined isoniazid-rifampicin-pyrazinamide-levofloxacin dry powder inhaler in treatment of pulmonary tuberculosis: A randomized controlled trial. Pulm. Pharmacol. Ther. 2021, 70, 102056. [Google Scholar] [CrossRef]
- Greenwood, J.; Schwarz, C.; Sommerwerck, U.; Nash, E.F.; Tamm, M.; Cao, W.; Mastoridis, P.; Debonnett, L.; Hamed, K. Ease of use of tobramycin inhalation powder compared with nebulized tobramycin and colistimethate sodium: A crossover study in cystic fibrosis patients with pulmonary Pseudomonas aeruginosa infection. Ther. Adv. Respir. Dis. 2017, 11, 249–260. [Google Scholar] [CrossRef]
- Krishnan, V.; Nath, S.; Nair, P.; Das, B. Mycobacterium tuberculosis and its clever approaches to escape the deadly macrophage. World J. Microbiol. Biotechnol. 2023, 39, 1–21. [Google Scholar] [CrossRef]
- Dartois, V. The path of anti-tuberculosis drugs: From blood to lesions to mycobacterial cells. Nat. Rev. Microbiol. 2014, 12, 159–167. [Google Scholar] [CrossRef]
- Chaurasiya, B.; Zhao, Y.Y. Dry powder for pulmonary delivery: A comprehensive review. Pharmaceutics 2021, 13, 31. Available online: https://pubmed.ncbi.nlm.nih.gov/33379136/ (accessed on 7 March 2025). [CrossRef]
- Wauthoz, N.; Amighi, K. Formulation Strategies for Pulmonary Drug Delivery of Poorly Soluble Drugs; Martin, G., Nokhodchi, A., Eds.; Advances and Challenges in Pulmonary Drug Delivery 2015; John Wiley & Sons, Limited: Hoboken, NJ, USA, 2015; 352p. [Google Scholar] [CrossRef]
- Liu, H.; Lv, H.; Duan, X.; Du, Y.; Tang, Y.; Xu, W. Advancements in Macrophage-Targeted Drug Delivery for Effective Disease Management. Int. J. Nanomed. 2023, 18, 6915–6940. [Google Scholar] [CrossRef]
- Pandey, R.; Khuller, G.K. Solid lipid particle-based inhalable sustained drug delivery system against experimental tuberculosis. Tuberculosis 2005, 85, 227–234. [Google Scholar] [CrossRef]
- Sharma, P.R.; Dravid, A.A.; Kalapala, Y.C.; Gupta, V.K.; Jeyasankar, S.; Goswami, A.; Agarwal, R. Cationic inhalable particles for enhanced drug delivery to M. tuberculosis infected macrophages. Biomater. Adv. 2022, 133, 112612. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Hasegawa, T.; Hinata, H.; Ito, F.; Inagawa, H.; Kochi, C.; Soma, G.-I.; Makino, K.; Terada, H. Optimum conditions for efficient phagocytosis of rifampicin-loaded PLGA microspheres by alveolar macrophages. J. Control Release 2007, 119, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Soetaert, K.; Rens, C.; Wang, X.M.; De Bruyn, J.; Lanéelle, M.A.; Laval, F.; Lemassu, A.; Daffé, M.; Bifani, P.; Fontaine, V.; et al. Increased vancomycin susceptibility in mycobacteria: A new approach to identify synergistic activity against multidrug-resistant mycobacteria. Antimicrob. Agents Chemother. 2015, 59, 5057–5060. Available online: https://pubmed.ncbi.nlm.nih.gov/26033733/ (accessed on 7 March 2025). [CrossRef]
- Rens, C.; Laval, F.; Daffé, M.; Denis, O.; Frita, R.; Baulard, A.; Wattiez, R.; Lefèvre, P.; Fontaine, V. Effects of lipid-lowering drugs on vancomycin susceptibility of mycobacteria. Antimicrob. Agents Chemother. 2016, 60, 6193–6199. Available online: https://pubmed.ncbi.nlm.nih.gov/27503643/ (accessed on 7 March 2025). [CrossRef]
- Ravon, F.; Menchi, E.; Lambot, C.; Al Kattar, S.; Chraibi, S.; Remmelink, M.; Fontaine, V.; Wauthoz, N. In vitro and in vivo local tolerability of a synergistic anti-tuberculosis drug combination intended for pulmonary delivery. J. Appl. Toxicol. 2023, 43, 298–311. [Google Scholar] [CrossRef]
- Jouhikainen, T. Inhaled Vancomycin Tolerability, Safety and Pharmacokinetics. ClinicalTrials.gov ID: NCT01537666. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/results/NCT01537666?term=vancomycin+for+inhalation&draw=2&rank=3 (accessed on 7 March 2025).
- Waterer, G.; Lord, J.; Hofmann, T.; Jouhikainen, T. Phase I, dose-escalating study of the safety and pharmacokinetics of inhaled dry-powder vancomycin (AeroVAnc) in volunteers and patients with cystic fibrosis: A New Approach to Therapy for Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2020, 64, e01776-19. Available online: https://pubmed.ncbi.nlm.nih.gov/31964790/ (accessed on 7 March 2025). [CrossRef] [PubMed]
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef]
- Watson, W.C.; Gordon, R.S. Studies on the digestion, absorption and metabolism of castor oil. Biochem. Pharmacol. 1962, 11, 229–236. [Google Scholar] [CrossRef]
- Inhatarget Therapeutics I. A Phase I/IIa First-in-Human Single-Arm Open-Label Multicentre Clinical Trial to Investigate the Safety, Tolerability, Pharmacokinetics and Anti-Tumour Activity of Ascending Doses of a Cisplatin-Based Formulation Administered as Dry Powder for Inhalation 2022-501183-17-00. Available online: https://www.inhatarget.com/clinical-trial (accessed on 7 March 2025).
- Levet, V.; Merlos, R.; Rosière, R.; Amighi, K.; Wauthoz, N. Platinum pharmacokinetics in mice following inhalation of cisplatin dry powders with different release and lung retention properties. Int. J. Pharm. 2017, 517, 359–372. [Google Scholar] [CrossRef]
- Chraibi, S.; Rosière, R.; Larbanoix, L.; Gérard, P.; Hennia, I.; Laurent, S.; Vermeersch, M.; Amighi, K.; Wauthoz, N. The combination of an innovative dry powder for inhalation and a standard cisplatin-based chemotherapy in view of therapeutic intensification against lung tumours. Eur. J. Pharm. Biopharm. 2021, 164, 93–104. [Google Scholar] [CrossRef]
- Levet, V.; Rosière, R.; Merlos, R.; Fusaro, L.; Berger, G.; Amighi, K.; Wauthoz, N. Development of controlled-release cisplatin dry powders for inhalation against lung cancers. Int. J. Pharm. 2016, 515, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.H.; Bauer, M.; Boussac, N.; Khan-Malek, R.; Munden, P.; Sardaro, M. An evaluation of fit factors and dissolution efficiency for the comparison of in vitro dissolution profiles. J. Pharm. Biomed. Anal. 1998, 17, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Gröschel, M.I.; Sayes, F.; Shin, S.J.; Frigui, W.; Pawlik, A.; Orgeur, M.; Canetti, R.; Honoré, N.; Simeone, R.; van der Werf, T.S.; et al. Recombinant BCG Expressing ESX-1 of Mycobacterium marinum Combines Low Virulence with Cytosolic Immune Signaling and Improved TB Protection. Cell Rep. 2017, 18, 2752–2765. [Google Scholar] [CrossRef]
- Snewin, V.A.; Gares, M.P.; Gaora, P.O.; Hasan, Z.; Brown, I.N.; Young, D.B. Assessment of immunity to mycobacterial infection with luciferase reporter constructs. Infect Immun. 1999, 67, 4586–4593. [Google Scholar] [CrossRef]
- Pilcer, G.; Wauthoz, N.; Amighi, K. Lactose characteristics and the generation of the aerosol. Adv. Drug Deliv. Rev. 2012, 64, 233–256. [Google Scholar] [CrossRef]
- NCCLS. Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes; Approved Standard. NCCLS Document M24-A; NCCLS: Wayne, PA, USA, 2003; Volume 23, pp. 1–71. ISBN 1-56238-500-3. [Google Scholar]
- Pillai, S.K.; Moellering, R.C.; Eliopoulos, G.M. Antimicrobial Combinations. In Antibiotics in Laboratory Medicine, 5th ed.; Lorian, V., Ed.; The Lippincott Williams & Wilkins Co.: Philadelphia, PA, USA, 2005; pp. 365–440. [Google Scholar]
- Santos, N.C.S.; Scodro, R.B.L.; de Almeida, A.L.; Baldin, V.P.; Nakamura de Vasconcelos, S.S.; Siqueira, V.L.D.; Caleffi-Ferracioli, K.R.; Campanerut-Sá, P.A.Z.; Cardoso, R.F. Combinatory activity of linezolid and levofloxacin with antituberculosis drugs in Mycobacterium tuberculosis. Tuberculosis 2018, 111, 41–44. [Google Scholar] [CrossRef]
- Young, P.M.; Sung, A.; Traini, D.; Kwok, P.; Chiou, H.; Chan, H.K. Influence of humidity on the electrostatic charge and aerosol performance of dry powder inhaler carrier based systems. Pharm. Res. 2007, 24, 963–970. [Google Scholar] [CrossRef]
- Peng, T.; Lin, S.; Niu, B.; Wang, X.; Huang, Y.; Zhang, X.; Li, G.; Pan, X.; Wu, C. Influence of physical properties of carrier on the performance of dry powder inhalers. Acta Pharm. Sin. B. 2016, 6, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.P.; El-Gendy, N.; Kuehl, C.; Berkland, C. Pulmonary Delivery of Vancomycin Dry Powder Aerosol to Intubated Rabbits. Mol. Pharm. 2015, 12, 2665–2674. [Google Scholar] [CrossRef]
- USP. Residual Solvents. Clinical Tests [Internet]. 2017, pp. 587–592. Available online: http://www.edimark.fr/Front/frontpost/ge-files/18116.pdf%0Ahttp://doi.wiley.com/10.2903/j.efsa.2017.4720%0Ahttps://link.springer.com/content/pdf/10.1007%2F978-94-015-8277-3.pdf%0Ahttps://link.springer.com/content/pdf/10.1007%2F978-94-011-4681-4.pdf%0Ahttp (accessed on 7 March 2025).
- Kim, D.H.; Kim, J.Y.; Kim, R.M.; Maharjan, P.; Ji, Y.G.; Jang, D.J.; Min, K.A.; Koo, T.S.; Cho, K.H. Orlistat-loaded solid SNEDDS for the enhanced solubility, dissolution, and in vivo performance. Int. J. Nanomed. 2018, 13, 7095–7106. [Google Scholar] [CrossRef]
- BASF. Kolliwax® HCO [Internet]. 2023. Available online: https://pharma.basf.com/technicalinformation/30674274/kolliwax-hco (accessed on 7 March 2025).
- Gröschel, M.I.; Sayes, F.; Simeone, R.; Majlessi, L.; Brosch, R. ESX secretion systems: Mycobacterial evolution to counter host immunity. Nat. Rev. Microbiol. 2016, 14, 677–691. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopeia 11, 01/2017:20906_Uniformity of Content of Single-Dose Preparations. In The European Pharmacopoeia. Available online: https://pheur.edqm.eu/internal/7f0cd8bb3da54a65aa964a15800ae890/11-8/11-8/page/20906E.pdf (accessed on 7 March 2025).
- Willand, N.; Dirié, B.; Carette, X.; Bifani, P.; Singhal, A.; Desroses, M.; Leroux, F.; Willery, E.; Mathys, V.; Déprez-Poulain, R.; et al. Synthetic EthR inhibitors boost antituberculous activity of ethionamide. Nat. Med. 2009, 15, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Larenas-Muñoz, F.; Ruedas-Torres, I.; Hunter, L.; Bird, A.; Agulló-Ros, I.; Winsbury, R.; Clark, S.; Rayner, E.; Salguero, F.J. Characterisation and Development of Histopathological Lesions in a Guinea Pig Model of Mycobacterium Tuberculosis Infection. Front. Vet. Sci. 2023, 10, 1264200. [Google Scholar] [CrossRef]
- Gumbo, T.; Lenaerts, A.J.; Hanna, D.; Romero, K.; Nuermberger, E. Nonclinical Models for Antituberculosis Drug Development: A Landscape Analysis. J. Infect. Dis. 2015, 211, S83–S95. [Google Scholar] [CrossRef] [PubMed]
- Via, L.E.; Lin, P.L.; Ray, S.M.; Carrillo, J.; Allen, S.S.; Eum, S.Y.; Taylor, K.; Klein, E.; Manjunatha, U.; Gonzales, J.; et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008, 76, 2333–2340. [Google Scholar] [CrossRef]
- ICH. ICH Q2 (R2) Guideline on Validation of Analytical Procedures [Internet]. European Agency for the Evaluation of Medicinal Products, International Commission on Harmonisation, (CPMP/ICH/281/95). 2023. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q2r2-guidelinevalidation-analytical-procedures-step-5-revision-1_en.pdf (accessed on 7 March 2025).
- Biswas, P.; Sen, D.; Bouwman, W. Structural characterization of spray-dried microgranules by spin-echo small angle neutron scattering. Powder Technol. 2021, 378 Pt A, 680–684. [Google Scholar] [CrossRef]
- Boyd, A.; Cain, O.; Chauhan, A.; Webb, G.J. Medical liver biopsy: Background, indications, procedure and histopathology. Frontline Gastroenterol. 2020, 11, 40–47. [Google Scholar] [CrossRef] [PubMed]

| Formulations | VAN Content (%) | THL Content (%) | Residual Solvent (%) | HCO Content (%) | Yield (%) |
|---|---|---|---|---|---|
| SD-VAN | 97 ± 1 | NA | 8.13 ± 0.02 | NA | 85 |
| VAN/THL | 86 ± 6 (7.8) | 11 ± 2 (1) | 7.95 ± 0.02 | NA | 76 |
| VAN/THL/HCO 25% | 65 ± 2 (10.1) | 6.4 ± 0.4 (1) | 5.39 ± 0.04 | 23.1 | 70 |
| VAN/THL/HCO 50% | 47 ± 2 (10.4) | 4.8 ± 0.4 (1) | 4.71 ± 0.02 | 43.5 | 69 |
| Drugs | MIC99 (µg/mL) | FICI |
|---|---|---|
| RIF | 0.25–1 | 0.185–0.3 |
| RIF (+THL 1 µg/mL) | <0.06–1.25 | |
| THL | 5–10 | |
| THL (+RIF 0.01 µg/mL) | 0.3–1.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravon, F.; Berns, E.; Lambert, I.; Rens, C.; Adnet, P.-Y.; Kiass, M.; Megalizzi, V.; Delporte, C.; Baulard, A.; Mathys, V.; et al. Assessment of Innovative Dry Powders for Inhalation of a Synergistic Combination Against Mycobacterium tuberculosis in Infected Macrophages and Mice. Pharmaceutics 2025, 17, 705. https://doi.org/10.3390/pharmaceutics17060705
Ravon F, Berns E, Lambert I, Rens C, Adnet P-Y, Kiass M, Megalizzi V, Delporte C, Baulard A, Mathys V, et al. Assessment of Innovative Dry Powders for Inhalation of a Synergistic Combination Against Mycobacterium tuberculosis in Infected Macrophages and Mice. Pharmaceutics. 2025; 17(6):705. https://doi.org/10.3390/pharmaceutics17060705
Chicago/Turabian StyleRavon, Faustine, Emilie Berns, Isaline Lambert, Céline Rens, Pierre-Yves Adnet, Mehdi Kiass, Véronique Megalizzi, Cédric Delporte, Alain Baulard, Vanessa Mathys, and et al. 2025. "Assessment of Innovative Dry Powders for Inhalation of a Synergistic Combination Against Mycobacterium tuberculosis in Infected Macrophages and Mice" Pharmaceutics 17, no. 6: 705. https://doi.org/10.3390/pharmaceutics17060705
APA StyleRavon, F., Berns, E., Lambert, I., Rens, C., Adnet, P.-Y., Kiass, M., Megalizzi, V., Delporte, C., Baulard, A., Mathys, V., Boarbi, S., Wauthoz, N., & Fontaine, V. (2025). Assessment of Innovative Dry Powders for Inhalation of a Synergistic Combination Against Mycobacterium tuberculosis in Infected Macrophages and Mice. Pharmaceutics, 17(6), 705. https://doi.org/10.3390/pharmaceutics17060705







