Abstract
The blood–brain barrier (BBB) is a highly selective and natural protective membrane that restricts the entry of therapeutic agents into the central nervous system (CNS). This restrictive nature poses a major challenge for pharmacological treatment of a wide range of CNS disorders, including neurodegenerative disorders, brain tumors, and psychiatric conditions. Many chemical drugs and biopharmaceuticals are unable to cross the BBB, and conventional drug delivery methods often fail to achieve sufficient brain concentrations, leading to reduced therapeutic efficacy and increased risk of systemic toxicity. In recent years, targeted drug delivery strategies have emerged as promising approaches to overcome the BBB and enhance the delivery of therapeutic agents to the brain. Among these, receptor-mediated transcytosis (RMT) and transporter-mediated transcytosis (TMT) are two of the most extensively studied mechanisms for transporting drugs across brain endothelial cells into the brain parenchyma. Advances in materials science and nanotechnology have facilitated the development of multifunctional carriers with optimized properties, improving drug targeting, stability, and release profiles within the brain. This review summarizes the physiological structure of the BBB and highlights recent innovations in RMT- and TMT-mediated brain drug delivery systems, emphasizing their potential not only to overcome current challenges in CNS drug development, but also to pave the way for next-generation therapies that enable more precise, effective, and personalized treatment of brain-related diseases.
1. Introduction
Brain disease, including brain cancers and central nervous system (CNS) disorders, are among the most prevalent, devastating and inadequately treated conditions. Developing safe and effective therapeutic strategies for CNS malignancies presents significant challenges, including high failure rates and extended timelines to reach the market compared to non-CNS therapies. Notably, the U.S Food and Drug Administration (FDA) has approved several drugs for utilization for the treatment of brain tumors, such as everolimus, bevacizumab, carmustine, dabrafenib mesylate, temozolomide, trametinib dimethyl sulfoxide, and vorasidenib citrate [1]. A critical obstacle in the development of CNS therapies is the blood–brain barrier (BBB), initially identified by Paul Ehrlich in the late 19th and early 20th centuries. Ehrlich’s observation that dyes injected into the bloodstream failed to stain brain tissue underscored the barrier’s selective permeability [2].
The BBB is a highly specialized and semipermeable structure that separates the CNS from the systemic circulation. It plays a crucial role in maintaining the brain microenvironment. The BBB acts as both a protective shield and a regulator, ensuring the CNS is isolated from toxins, pathogens, and potentially harmful fluctuations in blood composition, such as hormones or metabolites that could disrupt normal function [3]. By maintaining a tightly controlled environment, the BBB is essential for preserving neuronal activity and overall brain homeostasis.
Structurally, the BBB is composed of tightly bound endothelial cells (ECs) that line the brain’s capillaries. These ECs are reinforced by surrounding pericytes, astrocytes, and other supporting elements in the extracellular matrix (ECM) [4]. Pericytes and astrocytes not only provide structural support, but also contribute to the regulation of BBB permeability and respond to physiological or pathological changes in the brain. They are crucial for the development of accurate in vitro models of the BBB [5]. Other cellular and molecular components, such as enzymes and efflux transporters like P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), further enhance the barrier’s protective function by actively removing harmful substances [6]. Efflux transporters are proteins embedded in cell membranes that actively transport molecules out of the cells, essentially acting like “pumps” to remove unwanted substances. The BBB’s complex architecture and stringent selective permeability significantly hinder drug delivery to the CNS, excluding over 95% of drug candidates. The BBB allows the passive diffusion of only lipophilic molecules with a molecular weight under 400 Da and specific drugs with favorable physicochemical properties. Additionally, active transport systems facilitate the controlled entry of essential nutrients, such as glucose, amino acids, and ions required for neural metabolism [7,8]. However, large molecules, hydrophilic compounds, and many therapeutic agents, such as antibody–drug conjugate (ADC), are excluded, limiting the ability of systemic treatment to reach the brain. This exclusion becomes even more challenging in pathological conditions like CNS tumors. The progression of disease leads to the formation of a blood–tumor barrier (BTB), which imposes additional constraints on achieving effective drug concentrations within the tumor microenvironment [9].
In recent years, drug development for brain diseases has advanced substantially, with a particular focus on the utilization of polymeric and lipid-based nanoparticles (NPs) to enhance drug delivery to the brain. Nanocarriers can be modified to optimize brain targeting, enhance stability, and modify drug-release patterns. Despite these efforts, the success rate of CNS drug development remains among the lowest in the pharmaceutical industry, primarily due to the challenges posed by the BBB and BTB [7,8]. Effective and efficient drug delivery systems capable of traversing this protective barrier are indispensable for the development of therapies targeting CNS disorders. These systems must not only transport therapeutic agents across the BBB, but also ensure targeted delivery to specific brain regions, minimize systemic toxicity, and optimize pharmacokinetics and pharmacodynamics [10]. Lipid-based NPs, such as liposomes, lipid nanoparticles (LNPs), nanostructured lipid carriers (NLCs), solid lipid nanoparticles (SLNs), and emulsions, have emerged as promising carriers for drug delivery [11]. Their structural properties, including a lipid bilayer or lipid matrix, mimic biological membranes, facilitating the crossing of the BBB. Lipid-based NPs can encapsulate a wide range of drugs, including small molecules, peptides, and nucleic acids, protecting them from degradation and enabling sustained release, making them more attractive [12]. However, the potential immune reactions to NPs must be carefully considered and managed [13]. Polymeric NPs, including micelles, polyplexes, and polymeric hydrogels, offer additional advantages, such as a high drug-loading capacity, tunable release profiles, and structural versatility [14]. These NPs have been successfully used in preclinical and clinical research, delivering drugs for CNS diseases including medulloblastoma (MB), Alzheimer’s disease (AD), Parkinson’s disease, multiple sclerosis, and stroke [15]. One of the most notable advantages of NP-based systems is their ability to encapsulate drugs into nanocarriers, thereby facilitating sustained drug release. This capability not only reduces the dosing frequency, but also enhances patient compliance and optimizes therapeutic outcomes by ensuring that drug concentrations remain within the therapeutic window [16,17].
Surface modifications of NPs further enhance their functionality and ability to cross the BBB. Functionalization with targeting ligands, such as transferrin, lactoferrin, insulin, or low-density lipoprotein (LDL) receptors, facilitates receptor-mediated transcytosis (RMT) across the BBB [18]. Additionally, modifying NPs with cell-penetrating peptides or specific antibodies can improve their interaction with BBB ECs and promote selective delivery to the brain [19]. Transporter-mediated transcytosis (TMT), another promising strategy, exploits endogenous transport systems, such as glucose or amino acid transporters, to deliver drugs effectively into the brain [20]. TMT routes across the BBB include multiple mechanisms, each with unique challenges and opportunities for therapeutic delivery. For the BBB, the paracellular route allows water-soluble drugs to pass through tight junctions between ECs, though this pathway is highly restricted due to the barrier’s tightness [4]. The transcellular lipophilic pathway enables small, lipophilic molecules to diffuse across ECs, but limits the range of molecules that can be delivered [21]. Advanced strategies focus on exploiting active transport mechanisms such as RMT and TMT. RMT uses ligand–receptor interactions to transport macromolecules such as NPs, proteins, or peptides across the BBB, while TMT leverages transport proteins to ferry small molecules and nutrients [22]. Furthermore, adsorption-mediated transcytosis (AMT), driven by electrostatic interactions between positively charged NPs and negatively charged cell membranes, offers an alternative route for delivering therapeutics to the brain. However, AMT remains less specific than RMT or TMT, which limits its utility [23].
Ongoing research into the anatomical and pathological characteristics of the BBB is crucial for enhancing these strategies. Advancements in understanding how BBB integrity alters under pathological conditions provide insights into developing targeted therapies. Current research focuses on developing NPs with ligands or other modifications that can specifically bind to receptors on the BBB or/and the targeted cells to facilitate CNS drug delivery. While promising in preclinical studies, the translation of NP-based CNS drug delivery to clinical trials can be challenging due to safety concerns and complex formulation considerations. In this review, the structure and physiology of the BBB will be summarized, accompanied by an in-depth discussion of various RMT and TMT mechanisms for crossing the BBB. Particular attention will be given to the emerging roles of RMT and TMT as innovative strategies for overcoming the BBB, as well as recent advancements in drug delivery over the past five years in the treatment of CNS disorders. Through such focused research, novel approaches for crossing the BBB and achieving effective brain accumulation of therapeutics can be realized, ultimately addressing the unmet medical needs of CNS diseases.
2. BBB Structure and Physiology
The BBB is a complex and dynamic structure, primarily composed of ECs with tight junctions, supported by a basement membrane, astrocytes, pericytes, and extracellular matrix (ECM) components. Its major functional site is located in the capillaries (Figure 1) [4]. The BBB is considered the largest interface for blood–brain exchange, as it has a combined surface area of 12 and 18 m2 per average adult, based on an average microvessel surface area of 150 and 200 cm2 per gram of tissue [24]. Additionally, the blood–cerebrospinal fluid barrier (BCSFB) [25] and the arachnoid barrier represent the second and third barriers in the brain. However, the arachnoid barrier’s contribution to the exchange between the blood and the brain is insignificant, due to its avascular nature and relatively small surface area compared to the BBB and BCSFB [26].
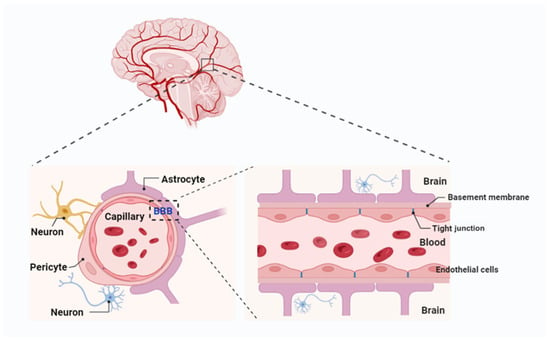
Figure 1.
Blood–brain barrier (BBB) components and composition. The BBB is composed of endothelial cells with tight junctions, supported by a basement membrane, astrocyte endfeet, pericytes, and extracellular matrix components, with its major functional site located in the capillaries. Created with Biorender.com.
The function of the BBB is contingent upon the coordinated contributions of multiple cell types within the neurovascular unit (NVU). This unit comprises astrocytes, pericytes, neurons, and microglia, all of which work together to maintain the integrity and selective permeability of the BBB [27]. Astrocytes, with their extensive “endfeet” covering 99% of the basal capillary membrane, play a vital role in the development and maintenance of the BBB by secreting factors such as GDNF, angiopoietin-1, and angiotensin II. These factors support EC integrity and regulate barrier function. Pericytes, which encircle ECs, provide structural support, regulate blood flow, and limit barrier permeability. Recent research highlights the location-dependent diversity of pericytes: those adjacent to arterioles regulate blood flow through contractile properties, while venous capillary pericytes maintain barrier integrity and modulate immune responses. These interactions between pericytes, astrocytes, and ECs are critical for preserving BBB integrity. Studies have demonstrated that pericyte deficiency increases BBB permeability, contributing to neurovascular dysfunction in diseases such as Alzheimer’s, stroke, and multiple sclerosis [28]. Consequently, these cell types form a highly coordinated system that safeguards the brain while facilitating the regulated exchange of nutrients and waste products.
The ECs that form the BBB are tightly joined by specialized junctions known as tight junctions (TJs), composed of transmembrane proteins such as claudins, occludins, and junctional adhesion molecules (JAMs) [29]. These proteins create a high-resistance paracellular barrier that limits the crossing of ions and molecules while ensuring selective permeability to essential substances such as oxygen and glucose. Claudins, particularly claudin-5, are crucial for maintaining BBB integrity; their disruption has been linked to various neurological disorders [30]. Additionally, ECs exhibit limited pinocytotic activity and express efflux transporters [31]. Efflux transporters in brain capillary ECs further enhance the BBB’s protective function by actively removing undesirable substances and metabolic byproducts from the brain into the systemic circulation. Key players include multidrug resistance transporters like P-glycoprotein, multidrug resistance proteins (MRPs), and BCRP [32]. While these systems safeguard the CNS, they also hinder the delivery of many therapeutic agents. Drugs designed to treat neurological disorders are often recognized as substrates and removed from the brain before achieving therapeutic levels. To address this, researchers are exploring strategies such as efflux transporter inhibitors, drug designs that evade transporter recognition, and advanced delivery systems. These approaches aim to balance the dual role of efflux transporters—protecting the brain while enabling effective drug delivery—without compromising the integrity or function of the BBB.
3. Transcytosis
Transcytosis is a fundamental transcellular transport mechanism that enables the internalization of molecules by endosomes on one side of a cell. These endosomes subsequently transport the molecules across the cytoplasm for release on the other side. Unlike paracellular transport, which occurs between cells, transcytosis facilitates the direct movement of molecules through a cell [33]. This process comprises three key steps: (1) internalization, where molecular uptake occurs via endocytosis, forming endosomes that encapsulate the cargo; (2) transport, where the endosomes traverse the cytoplasm, shuttling molecules across the cell membrane; and (3) release, where the endosomes fuse with the cell membrane on the opposite side, expelling their contents via exocytosis. At the BBB, transcytosis plays a pivotal role in transport across brain ECs. Given the BBB’s restrictive nature, this process is essential for delivering substances that cannot passively diffuse into the brain, ensuring the maintenance of brain homeostasis and neuronal function. Understanding transcytosis is vital for developing drug delivery strategies and therapeutics aimed at overcoming the BBB’s selective permeability. The regulation of endosomal trafficking plays a pivotal role in optimizing TMT and RMT efficiency at the BBB. This regulation influences the overall effectiveness of drug delivery strategies that target transcytosis pathways.
4. Mechanisms of BBB Crossing by Nanomaterials
Nanomaterials employ various mechanisms to cross the BBB, facilitating the targeted delivery of therapeutics to the brain. These mechanisms can be broadly categorized into passive and active transport pathways. Passive diffusion occurs when small, lipophilic nanomaterials cross the BBB without the involvement of specific transporters. In contrast, active transport mechanisms encompass TMT or carrier-mediated transport (CMT), RMT, and AMT. TMT exploits endogenous transporters expressed on brain ECs to actively shuttle molecules across the BBB, leveraging physiological pathways responsible for the transport of essential nutrients, peptides, and ions between the bloodstream and brain [3]. RMT relies on ligands, peptides, or antibodies conjugated to nanomaterials that engage specific receptors, such as transferrin receptors (TfRs) or low-density lipoprotein receptor-related proteins (LRP1 and LRP2), to facilitate endocytosis and transcytosis across the BBB [34]. AMT is mediated by electrostatic interactions between positively charged nanomaterials with negatively charged membrane components, promoting cellular uptake [12]. Beyond these traditional approaches, emerging strategies, such as cell-mediated transport via monocytes or exosomes, and stimuli-responsive nanomaterials that react to pH, temperature, or enzymatic activity, are being explored to enhance BBB permeability and improve therapeutic efficacy. These mechanisms can be further classified into invasive or non-invasive approaches. Invasive approaches involve the temporary disruption of the BBB through physical means, enabling nanomaterials to cross the BBB via the paracellular pathway. Techniques such as focused ultrasound-mediated BBB opening and local delivery strategies fall under this category, which is also referred to as the paracellular pathway [35,36]. Conversely, non-invasive strategies maintain BBB integrity during drug delivery and rely on the transcellular pathway to improve nanomaterial transport. TMT, RMT, and AMT, as well as emerging cell-mediated and stimuli-responsive nanomaterials, fall within this category, and can be collectively to referred to as transcellular mechanisms. These diverse transport mechanisms offer promising strategies for overcoming the restrictive nature of the BBB, thereby offering novel opportunities for the treatment of neurological disorders.
5. Transporter-Mediated Transcytosis (TMT) for Crossing the BBB
Among the various strategies to overcome the BBB, TMT has gained significant attention due to its potential to facilitate efficient drug delivery to the CNS. TMT exploits specific endogenous transporters expressed on the ECs of the BBB to actively transport molecules across this tightly regulated barrier. By leveraging the natural mechanisms that transport essential nutrients, peptides, and ions between the bloodstream and brain, TMT enables efficient and targeted drug delivery to the brain (Figure 2) [37,38]. These TMT transporters are encoded by genes within the solute carrier (SLC) transporter gene family, which comprises over 300 genes that regulate the translocation of various substrates across the BBB [39]. Key transporters involved in TMT include glucose transporters (GLUTs), which mediate glucose and mannose uptake; neutral amino acid transporters (LAT1 and LAT2), responsible for transporting large neutral amino acids and certain drugs like L-dopa; and anionic/cationic amino acid transporters, which facilitate the transport of aspartate, glutamate, L-lysine, L-arginine, and L-ornithine [38]. Additionally, monocarboxylate transporters (MCTs) play a critical role in lactate transport, while lipid transporters, such as LRP1, facilitate the translocation of lipoproteins and other macromolecules. Dipeptide transporters such as PEPT2 also contribute to TMT by shuttling di- and tripeptides across the BBB [40,41,42].
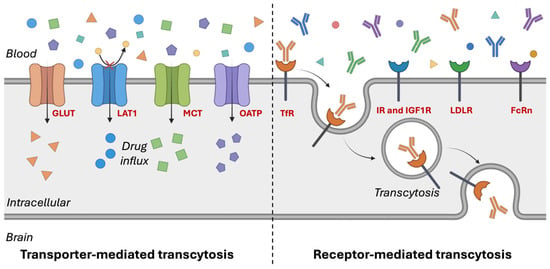
Figure 2.
Transporter- and receptor-mediated transcytosis in endothelial cells. Created with Biorender.com. Glucose transporters (GLUTs); L-type amino acid transporter 1 (LAT1); monocarboxylate transporters (MCTs); organic anion-transporting polypeptides (OATPs); transferrin receptors (TfRs); insulin receptors (IRs); insulin-like growth factor-1 receptor (IGF1R); low-density lipoprotein receptors (LDLRs); neonatal Fc receptor (FcRn).
The polarized distribution of transporters across the BBB contributes to its functional asymmetry. Certain transport proteins are exclusively localized to either the luminal or abluminal membrane, while others are expressed on both membranes of the ECs [43]. By harnessing these transport pathways, targeted drug delivery strategies can be developed to facilitate the efficient transport of therapeutic agents that would otherwise be excluded from the brain. Recent advancements in TMT-based drug delivery have focused on two primary approaches: (1) designing drug molecules that structurally mimic endogenous substrates of specific transporters, and (2) conjugating therapeutic compounds or nanocarriers with ligands that selectively bind to and engage transporter-mediated pathways [44]. TMT-based drug delivery systems have demonstrated significant potential in enhancing brain uptake of poorly permeable therapeutics, particularly through strategies such as LAT1-targeted prodrugs and GLUT1-based delivery platforms [45,46]. These innovative strategies improve the selective passage of therapeutics across the restrictive BBB, while preserving its structural integrity.
5.1. Glucose Transporters (GLUTs)
GLUTs, encoded by SLC2A1, play a crucial role in facilitating glucose transport across the BBB to meet the brain’s high energy demands [47,48]. Among the 14 identified human GLUT isoforms, GLUT1 to GLUT5 are the most extensively characterized, primarily transporting hexoses, notably D-glucose, while also facilitating the uptake of other hexoses, such as D-mannose and D-galactose, albeit with lower efficiency [48,49,50,51]. As a facilitative transporter, GLUT1 mediates passive glucose diffusion and is highly expressed on both the luminal (blood-facing) and abluminal (brain-facing) membranes of BBB ECs, ensuring a continuous glucose supply to the brain [52]. Unlike sodium-dependent glucose transporters (SGLTs), which require ion co-transport, GLUT1 functions independently of sodium gradients, allowing for energy-independent glucose uptake [53]. Beyond its fundamental physiological role, GLUT1 expression is tightly regulated in response to metabolic demands, and can be altered under pathological conditions [54]. In neurodegenerative disorders such as AD, decreased GLUT1 expression impairs glucose transport to the brain, contributing to cognitive decline [55]. Conversely, in glioblastoma and other malignancies, GLUT1 and GLUT3 cause rapid tumor proliferation by facilitating enhanced glucose uptake [55,56,57,58]. Furthermore, cerebrovascular conditions like ischemia can alter GLUT expression, thereby affecting glucose supply to the brain and further highlighting GLUTs as a potential therapeutic target [58,59].
Ligand-modified nanomaterials that mimic glucose structures or bind to GLUT1-binding domains have been developed to exploit GLUT1-mediated transport, enhancing CNS drug delivery. This understanding has led to an increasing interest in targeting GLUT1 for disease intervention and drug delivery. Recent studies have demonstrated that GLUT1-targeted NPs can improve the delivery of chemotherapeutic agents, neuroprotective peptides, and genetic material to the brain, leading to improved therapeutic efficacy [60]. By harnessing GLUT1-mediated transport, researchers seek to enhance drug bioavailability and optimize treatment strategies for brain-related diseases [61]. Given its critical role in BBB function and disease pathophysiology, GLUT1 remains a promising target for CNS drug delivery and therapeutic intervention [62].
One approach involved modifying liposomal NPs with mannose, a known GLUT1 ligand, to facilitate targeted delivery in AD treatment [60]. This strategy significantly enhanced brain-derived neurotrophic factor (BDNF) transport, achieving approximately 50% higher BBB permeability and a ~1.7-fold increase in BDNF levels in neuronal cells [60]. Similarly, Xie et al. developed glucose-modified liposomes incorporating phospholipids, glucose-derived cholesterols, and polyethylene glycol (PEG) linkers to optimize brain-targeting efficiency [63]. In one study, liposomes incorporating various glucose-conjugated cholesterols (GLU200-LIP, GLU1000-LIP, and GLU2000-LIP) demonstrated promising brain-targeting capabilities [63]. In addition, novel multivalent glucoside-based ligands with strong affinity for GLUT1 have been developed for brain-targeted liposomal drug delivery. Liposomes functionalized with these glucosides and encapsulated with docetaxel achieved significantly higher brain accumulation of docetaxel compared to both free docetaxel and non-targeted liposome formulations [64]. Xie et al. designed dual-sensitive nanomicelles functionalized with glucose molecules to enhance brain delivery of 3D6 antibody fragments (3D6-Fab) via GLUT1-mediated transport [65]. This strategy achieved a 41-fold increase in brain accumulation of 3D6-Fab, representing a promising platform for efficient delivery of therapeutic antibodies to the CNS for the treatment of neurological diseases. Further advancing GLUT1-mediated transcytosis, Hao et al. introduced sequential targeting in crosslinking (STICK) NPs, a polymer-based delivery system designed to enhance both BBB penetration and tumor targeting [66]. By incorporating maltobionic acid (a GLUT1-recognized glucose derivative) and 4-carboxyphenylboronic acid, this system improved drug stability, facilitated BBB transport, and significantly inhibited tumor growth in preclinical models of aggressive brain tumors [66,67]. Additionally, Zhou et al. developed glycosylated “triple-interaction” stabilized polymeric siRNA NPs (Gal-NP@siRNA) to target β-site amyloid precursor protein cleaving enzyme 1 (BACE1) in an amyloid precursor protein (APP)/PS1 transgenic AD mouse model [68]. These NPs demonstrated superior blood stability and efficiently penetrated the BBB via glycemia-controlled GLUT1-mediated transport. Once in the brain, the siRNAs effectively reduced BACE1 expression, modulating key pathological pathways in AD progression (Table 1) [68].

Table 1.
Literature review of glucose transporter-1 (GLUT-1)-targeted therapies for brain delivery.
These findings highlight the significant potential of GLUT1-targeted nanocarriers in CNS drug delivery. They demonstrate enhanced therapeutic efficacy through improved BBB transport, increased drug stability, and targeted disease modulation. However, challenges persist, particularly in studying human GLUTs in vivo, necessitating reliable in vitro models [61]. Future research is needed to optimize ligand specificity, enhance transcytosis efficiency, and minimize off-target effects. Additionally, inter-individual variability in GLUT1 expression poses a challenge for clinical translation. Addressing these issues will be key to advancing GLUT1-mediated drug delivery for neurological disorders, brain tumors, and cerebrovascular diseases, paving the way for more effective CNS therapeutics.
5.2. L-Type Amino Acid Transporters (LATs)
L-type amino acid transporter 1 (LAT1) is a high-affinity, sodium-independent transporter that facilitates the uptake of large neutral amino acids, including leucine, phenylalanine, tryptophan, and tyrosine [74]. Forming a heterodimeric complex with 4F2 heavy chain (SLC3A2), LAT1 is highly expressed in metabolically active tissues, such as the brain, placenta, testes, and various cancers, supporting protein synthesis, cell proliferation, and metabolic function [74]. At the BBB, LAT1 is primarily located on the luminal membrane of ECs, ensuring the continuous influx of essential amino acids for neuronal and glial function [75]. LAT1 is overexpressed in several cancers, including glioblastoma [76], brain metastases [77], and various solid tumors, as well as in neurological disorders, such as autism spectrum disorders [78]. Additionally, LAT1 facilitates the transport of CNS-active drugs, such as melphalan [79], levodopa (L-Dopa) [80], gabapentin [81], and pregabalin [82], making it integral for developing prodrugs with enhanced BBB permeability [83]. For example, LAT1 has been shown to exhibit a high maximal transport capacity (Vmax ≈ 40–60 nmol/min/g) and a substantial binding affinity (Km ≈ 10–200 µM), enabling the rapid exchange of high-affinity substrates across the BBB with half-times (half the time required to reach drug concentration equilibrium between the brain and blood) under 15 min [83].
Recent advancements in LAT1-targeted drug delivery have focused on designing prodrugs and NPs that exploit LAT1-mediated transport. Montaser et al. developed LAT1-targeted prodrugs to enhance the delivery of nonsteroidal anti-inflammatory drugs (NSAIDs) to the brain. Salicylic acid derivatives exhibited a five-fold increase in brain uptake, but challenges such as plasma protein binding and premature bioconversion remain [84]. Similarly, ferulic acid (FA)-based prodrugs have demonstrated LAT1-specific binding, enabling BBB penetration and cellular uptake. These amide-based prodrugs, incorporating an aromatic ring in the moiety, exhibited effective binding to LAT1, facilitating their cellular uptake in vitro and successfully crossing the BBB in mice [85]. Beyond small molecules, LAT1-targeted NPs have shown promise. L-DOPA-functionalized gold nanoflowers (L-DOPA-AuNFs) exhibited superior BBB permeability and uptake by brain macrophages without inducing inflammation [86]. In the context of glioma therapy, Zhang et al. developed LAT1-targeted liposomes co-loaded with temozolomide (TMZ) and sorafenib [87]. The high expression of LAT1 on BBB and glioma cells enabled these liposomes to cross the BBB more efficiently, enhancing drug delivery to the tumor site and improving therapeutic outcomes (Table 2) [87].
Given its broad substrate specificity, high transport efficacy, and key role in both CNS and tumor metabolism, LAT1 represents a powerful avenue for brain-targeted drug delivery [88]. Ongoing research into LAT1-mediated strategies, including optimized ligand conjugation, prodrug refinement, and NP engineering, holds promise for enhancing therapeutic outcomes in CNS diseases and brain tumors. By leveraging its strategic expression in key disease areas, LAT1 presents a critical opportunity for advancing more precise and effective treatments for complex neurological and oncological conditions. The following section delves into recent advancements based on LAT1-targeted strategies for overcoming the BBB.

Table 2.
Strategies to target L-type amino acid transporter 1 (LAT1) for brain-targeted drug delivery.
Table 2.
Strategies to target L-type amino acid transporter 1 (LAT1) for brain-targeted drug delivery.
| Drug | Malignancy | LAT1 Targeting Moiety | Delivery Vehicle | Inference | Reference |
|---|---|---|---|---|---|
| Salicylic acid, ibuprofen, naproxen, and flurbiprofen | Neurodegenerative diseases | L-Phenylalanine | Prodrug | Significant (5-fold) increase in brain uptake across BBB | [84] |
| Ferulic acid | Alzheimer’s disease | L-Phenylalanine | Prodrug | Amide-based prodrug with aromatic ring effectively binds to LAT1, facilitating cellular uptake in vitro and crossing BBB | [84] |
| Levodopa | Brain malignancies like Parkinson’s disease | L-Phenylalanine | L-DOPA-functionalized gold NPs | Significantly higher penetration across BBB, increased internalization in brain macrophages, and inflammatory responses as compared to non-targeted NPs | [86] |
| Temozolomide and sorafenib | Glioblastoma | Tyrosine | Lipid NPs (carboxylated polyethylene glycol stearate and PLGA-PEG) | Enhanced drug delivery across BBB, increased tumor accumulation, and improved anti-tumor efficacy | [87] |
| Morin hydrate | Alzheimer’s disease | Phenylalanine-phenylalanine dipeptide | Dipeptide-functionalized PLGA NPs | Significantly improved brain retention with liver and lung accumulation | [89] |
| Antisense oligonucleotide | Neurodegenerative diseases | Phenylalanine | Phenylalanine-functionalized PEG-PLL NPs | Sixty-four-fold higher brain accumulation when compared to non-targeted NPs | [90] |
| Saxagliptin | Alzheimer’s disease | L-valine-conjugated chitosan | Chitosan-L-valine NPs | Enhanced BBB permeability with ~50-fold higher brain uptake than free drug and 3.4-fold lower plasma concentration, suggesting stability in plasma and release in brain tissue | [91] |
| Levodopa and dopamine | Parkinson’s disease | Polyvinylpyrrolidone-functionalized selenium NPs | Facilitated BBB permeability and efficient internalization in brain endothelial cells | [92] | |
| Phenylalanine analogs | Glioblastoma multiforme | Phenylalanine | Free drug | Iodine substitution at second position improved LAT1 affinity, but reduced velocity; reducing one carbon reduced LAT1 affinity, and bicyclic and α-methyl phenylalanine showed similar velocity, with preferential tumor accumulation of bicyclic phenylalanine | [93] |
Poly(lactic-co-glycolic acid) (PLGA); polyethylene glycol (PEG); poly(ethylene glycol)-b-poly(l-lysine) (PEG-PLL).
5.3. Monocarboxylate Transporters (MCTs)
MCTs constitute a family of transmembrane proteins responsible for the transport of monocarboxylates, including lactate, pyruvate, ketone bodies (β-hydroxybutyrate and acetoacetate), and short-chain fatty acids, across biological membranes. These proton-coupled symporters facilitate energy-independent transport by co-transporting protons (H+) along substrate concentration gradients [40]. Among the MCT family, MCT1 (SLC16A1), MCT2 (SLC16A7), and MCT4 (SLC16A3) hold particular significance in brain metabolism and BBB function.
At the BBB, MCT1 is predominantly expressed on the luminal membrane of endothelial cells, facilitating the uptake of ketone bodies during periods of low glucose availability, such as fasting, exercise, or ketogenic diets [44,94,95]. Additionally, MCT1 plays a crucial role in transporting lactate from the bloodstream into the brain, where it serves as an energy substrate for neurons and glial cells [96]. MCT2, primarily expressed in neurons, supports lactate uptake for metabolic processes, whereas MCT4, found in astrocytes, facilitates lactate export to sustain neuronal energy demands [97]. Given their essential role in brain metabolism, MCTs have emerged as promising targets for drug delivery, particularly in conditions associated with metabolic dysfunction and neurodegeneration.
Taking advantage of the natural substrate affinity of MCT1, researchers have developed MCT1-mediated drug delivery systems, including NPs, prodrugs, and inhibitors, to enhance drug penetration into the brain. For instance, Venishetty et al. designed β-hydroxybutyric acid (HBA)-grafted docetaxel-loaded solid lipid NPs (HD-SLNs) to exploit MCT1-mediated transport, achieving significantly higher brain concentrations of docetaxel than Taxotere® [98]. This system also demonstrated enhanced cellular uptake and controlled drug release, highlighting its potential for CNS drug delivery [98]. Similarly, Güliz Ak et al. developed HBA-conjugated solid lipid NPs (SLNs) for glioblastoma therapy, facilitating the transport of carmustine (BCNU) and TMZ across the BBB [99]. These dual drug-loaded SLNs exhibited controlled release, increased cytotoxicity against glioblastoma cells, and reduced toxicity to healthy cells, making them a promising targeted therapy for glioblastoma multiforme (GBM) [99]. Beyond drug-loaded NPs, inhibitors targeting MCT1 have shown potential in modulating tumor metabolism. Pluronic P85 (P85) NPs were found to inhibit MCT1-mediated lactate transport across brain microvascular ECs without significantly affecting GLUT1 function. This demonstrated a concentration-dependent impact on brain monocarboxylate metabolism, while maintaining safety [100]. In another approach, Huang et al. developed ultra-pH-sensitive NPs encapsulating AZD3965, a selective MCT1 inhibitor [101]. This formulation effectively blocked MCT1 activity in tumors, reversing lactic acid-induced immunosuppression and enhancing the effectiveness of cancer immunotherapy, while reducing systemic toxicity [101]. AZD3965 exhibited rapid oral absorption, high bioavailability, and target engagement with minimal systemic toxicity. However, its potential for brain cancer therapy remains underexplored [102]. The study also revealed nonlinear pharmacokinetics, with dose-dependent increases in exposure and changes in clearance, indicating potential target-mediated drug disposition (TMDD) for AZD3965 [102]. Given its ability to inhibit MCT1 in tumors expressing high MCT1 levels, AZD3965 could enhance drug penetration across the BBB and modulate the tumor microenvironment, warranting further investigation in glioblastoma models (Table 3).

Table 3.
Literature review of monocarboxylate Transporter 1 (MCT1)-targeted therapies for brain delivery.
In conclusion, MCT1-targeted drug delivery has demonstrated significant potential for improving brain drug penetration, particularly in glioblastoma and other CNS disorders. The development of HBA-grafted NPs and MCT1 inhibitors such as AZD3965 offers promising avenues for overcoming the BBB limitations and enhancing drug efficacy. However, further research is necessary to optimize these formulations, assess their long-term safety, and expand their clinical applicability. Additionally, more specific studies focusing on MCT1’s role in brain tumors are crucial for refining these strategies and improving therapeutic outcomes for patients with CNS malignancies.
5.4. Organic Anion-Transporting Polypeptides (OATPs)
OATPs, a subfamily of the solute carrier (SLC) superfamily, play a critical role in the uptake and distribution of amphipathic molecules, including steroid hormones, bile acids, statins, antihypertensives, antibiotics, antifungals, and chemotherapeutic agents [104]. Their broad tissue distribution and capacity to transport structurally diverse compounds make them key regulators of drug disposition in major organs, including the liver, kidney, intestine, and brain [104,105]. OATP isoforms such as OATP1B1, OATP1B3, OATP1A2, and OATP2B1 are particularly relevant to BBB transport, influencing CNS drug permeability and offering potential targets for enhancing drug delivery to the brain [104,105]. Given their role in modulating drug transport across the BBB, OATPs also represent promising targets for enhancing CNS drug delivery, particularly in neurological disorders, where drug penetration remains a significant challenge.
The expression and function of OATPs are influenced by genetic polymorphisms, age, gender, and dietary factors, contributing to inter-individual variability in drug absorption, distribution, efficacy, and toxicity. Genetic polymorphisms in OATP transporters also impact their functional capacity, leading to variations in pharmacokinetics and pharmacodynamics. Notably, OATP1A4 and OATP1A5 exhibit age- and gender-specific expression patterns, affecting brain drug-transport dynamics [106,107,108]. A deeper understanding of these variations is crucial for optimizing CNS drug delivery strategies, particularly in neurological disorders. Advancements in nanotechnology have enabled the development of OATP-targeted drug delivery systems to enhance CNS drug uptake, offering novel insights into transporter-mediated brain drug delivery, improving BBB penetration and therapeutic efficacy [109,110]. For example, Yang et al. developed OATP2B1-targeted dendrigraft poly-l-lysine-polyethylene glycol NPs for siRNA delivery, demonstrating enhanced targeting precision and gene-silencing efficiency in the mouse brain [109]. Similarly, Reichel et al. designed the HMC-FMX nanoprobe, which facilitated OATP-mediated BBB transport and glioblastoma accumulation, highlighting its potential for brain tumor therapy [110]. Additionally, Thompson et al. found that hypoxia/reoxygenation stress upregulates OATP1A4 expression at the BBB, enhancing atorvastatin transport into the brain, suggesting its potential as a drug delivery target (Table 4) [111].

Table 4.
Literature review of organic anion-transporting polypeptide (OATP)-targeted therapies for brain delivery.
6. Receptor-Mediated Transcytosis (RMT) for Crossing the BBB
RMT is another crucial mechanism that facilitates the transport of macromolecules across the BBB, offering a promising strategy for the delivery of CNS drugs. This process relies on receptor–ligand interactions to selectively transport therapeutics across ECs while maintaining BBB integrity. In this section, we discuss the biological mechanisms of RMT, recent advancements in therapeutic applications, and future directions for optimizing BBB drug delivery. RMT involves three key steps: (1) therapeutic molecules or ligands designed to mimic endogenous substrates bind to specific receptors expressed on the luminal (blood-facing) surface of the ECs; (2) the receptor–ligand complex undergoes vesicular endocytosis, protecting the cargo from degradation and facilitating intracellular transport; and (3) vesicles translocate across ECs and release their cargo into the brain’s interstitial fluid, where the therapeutic agent exerts its effects [115]. Several receptors facilitate RMT across the BBB, including the TfR, insulin receptor (IR), insulin-like growth factor-1 receptor (IGF1R), low-density lipoprotein receptor-related proteins (LRP1 and LRP2), neonatal Fc receptor (FcRn), and angiotensin-converting enzyme receptor (ACE). By targeting receptors involved in the physiological uptake of essential nutrients and signaling molecules, RMT provides an endogenous and efficient pathway for CNS drug delivery. RMT-based approaches have shown significant potential for improving CNS drug bioavailability and enhancing the efficacy of biologics, NPs, and small molecules. However, several challenges must be addressed for clinical translation, including receptor saturation, immunogenicity, and off-target effects. Additionally, optimizing ligand specificity and transport efficiently remains critical for maximizing therapeutic outcomes while minimizing systemic exposure.
6.1. Transferrin Receptor (TfR)
The TfR is a transmembrane glycoprotein primarily responsible for iron uptake through the binding and endocytosis of transferrin. Two main isoforms exist: TfR1 (CD71), which is widely expressed at low levels in various tissues; and TfR2, predominantly found in hepatocytes [116,117]. The TfR is highly expressed on the luminal surface of BBB ECs, making it a key target for RMT-based CNS drug/gene delivery [118].
By conjugating therapeutic agents, such as NPs or biologics, to transferrin or TfR-targeting ligands, drug transport across the BBB can be enhanced. However, challenges such as competition with endogenous ligands, variations in receptor–ligand binding affinity, efflux transporter activity, and the endothelial glycocalyx barriers limit the efficiency of TfR-targeted delivery [116]. Additionally, the basement membrane limits the movement of drugs into the brain parenchyma. Addressing these barriers is essential to improving drug delivery to the brain, with TfR-targeted strategies emerging as a promising approach for overcoming these challenges [116].
Friden et al. showed that TfR-targeting antibodies facilitate drug transport across the BBB via RMT [119]. More recently, Lengerich et al. engineered an antibody transport vehicle (ATV) incorporating a monovalent TfR-binding site to enhance TREM2 activation in an AD mouse model, improving brain distribution, microglial function, and glucose metabolism [120]. Researchers at Denali Therapeutics developed an oligonucleotide (ASO) transport vehicle (OTV) by engineering a TfR-binding molecule for ASO delivery [121]. Studies in human TfR knockin (TfRmu/hu KI) mice and nonhuman primates (NHPs) confirmed the broad distribution of therapeutic ASOs across brain regions, highlighting the OTV’s potential for treating AD [121]. This delivery method enhanced therapeutic potential by targeting the brain broadly, including the cortex and the hippocampus. Since transferrin receptors are an entry point for ASOs, this technology has broader implications, and is not limited to CNS disorders. It also encompasses peripheral health conditions influenced by the brain, such as obesity and aging. However, the delivery system’s lack of specificity to the brain, or specific cell types within the brain, poses a limitation.
Monoclonal antibodies such as anti-transferrin receptor IgG2a (OX26), which specifically binds to TfRs, have been widely used for drug delivery across the BBB [122]. By targeting the extracellular domain of TfRs, OX26 facilitates RMT, enabling the transport of drug-loaded NPs or liposomes into the brain without interfering with the natural transferrin-binding process [123]. Studies have shown that OX26 has significantly greater uptake by brain capillary ECs than non-specific IgG2a, making it an effective vector for BBB-targeted drug delivery [124]. Pardridge et al. initially introduced this strategy in the 1990s for peptide delivery across the BBB. The approach was subsequently refined by conjugating OX26 to liposomes, enhancing drug transport efficiency into the brain [125,126,127]. Additionally, focused ultrasound (FUS) combined with microbubbles has further improved TfR-targeted liposomal delivery, increasing drug accumulation in brain ECs [128]. In Parkinson’s disease, Sela et al. designed transferrin-coated liposomes to deliver SynO4 monoclonal antibodies, effectively reducing alpha-synuclein aggregation and improving neuronal viability [129]. Similarly, Gabold et al. developed transferrin-functionalized chitosan NPs for nasal drug delivery, facilitating rapid epithelial transport and improved brain targeting, offering a promising approach for targeted drug delivery to the brain [130].
TfR-targeted nanocarriers have also shown promise in glioma therapy. Marrocco et al. engineered a ferritin-based stimuli-sensitive nanocarrier (The-0504) for glioma treatment, successfully delivering a topoisomerase 1 inhibitor (Genz-644282) and reducing tumor growth in glioma-bearing mice [131]. Sonkar et al. developed transferrin-coated gold-based theranostic liposomes co-loaded with docetaxel and glutathione-reduced gold NPs, demonstrating enhanced BBB penetration, improved drug delivery compared to Docel™, and sustained drug release, making them a promising platform for brain-targeted drug delivery and brain imaging (Table 5) [132].
The pivotal role of TfR in BBB transport has driven significant research over the past 30–35 years, significantly advancing TfR-mediated drug delivery to the CNS. Numerous preclinical studies have demonstrated the potential of TfR-targeted strategies, as highlighted in a recent comprehensive review on BBB transport of TfR-targeted NPs [133]. However, clinical translation remains challenging due to variability in receptor–ligand interactions, limited transport efficiency, and additional biological barriers within the brain microenvironment. Addressing these challenges necessitates a deeper understanding of the mechanistic aspects of TfR-mediated transport, in order to refine existing strategies and enhance the effectiveness of TfR-targeted drug delivery for neurological disorders [116].

Table 5.
Literature review of transferrin receptor (TfR)-mediated therapies for brain delivery.
Table 5.
Literature review of transferrin receptor (TfR)-mediated therapies for brain delivery.
| Therapeutic | Malignancy | TfR Targeting Moiety | Delivery Vehicle | Inference | Reference |
|---|---|---|---|---|---|
| Cisplatin (focused ultrasound) | Brain malignancies | OX26 | Liposomes (DSPC, Cholesterol, DSPE-PEG, DSPE-PEG-maleimide, and DiD) | Significant increase in brain uptake and enhanced accumulation in hemisphere (40%) | [128] |
| SynO4 | Parkinson’s disease | Human holo-Transferrin (T4132) | Liposomes (DPPC, cholesterol, DSPE-PEG1000-NH2, and DSPE-PEG1000-NH2) | Significant increase in brain accumulation (7-fold higher), enhanced motor function, and minimal adverse effects | [129] |
| Labeled β-galactosidase (for enzymatic activity) | Glioblastoma | Transferrin | Azide-functionalized chitosan NPs | Rapid target specific accumulation, and transferrin concentration was directly proportional to cellular uptake | [130] |
| Genz-644282 | Glioblastoma | Ferritin | Nanocarrier formed with The-05 (ferritin linked with peptide that is cleavable by metalloproteases 2 and 9, and polypeptide comprising proline, alanine, serine, and glutamic acid) | Significant deposition of nanocarriers in glioma, reduced tumor burden, and enhanced survival rate | [131] |
| Docetaxel | Brain malignancies | Transferrin | Glutathione reduced gold NPs, incorporated in liposomes (TPGS, egg lecithin, and cholesterol) | Sustained drug release in 72 h with 2.7–4-fold higher drug concentrations in brain in comparison with marketed formulation | [132] |
| Lurasidone hydrochloride | Schizophrenia | Transferrin | Chitosan-modified NPs | Increased brain concentration of drug, thereby enhancing neuroprotective action | [134] |
| Caffeine | Strengthening physical performance | Transferrin | Liposomes (SPC, cholesterol, DSPE-PEG-MAL, and DSPE-PEG-2000) | Higher drug concentration in brain, increased dopamine release, thereby improving physical performance with targeted formulation | [135] |
| Curcumin | Neurodegenerative conditions | Transferrin | SLNs and nanostructured lipid carriers (cetyl palmitate, tween 60, miglyol-812, and PEG) | Controlled drug release, with 1.5-fold higher permeability with transferrin conjugation leading to higher brain uptake, and non-significant cytotoxicity. However, transferrin conjugation interfered with curcumin entrapment efficiency over time | [136] |
| Anti-miRNA103/107 | Ischemic brain damage | Transferrin | LNPs (DSPC, cholesterol, DODAP, PEG2000-Cer16, and DSPE-PEG-Mal) | Enhanced BBB penetration of formulation over free miRNA led to significant improvement in ischemic damage repair | [137] |
| Docetaxel and gadolinium | Brain cancer | Transferrin | Micelles (TPGS) | Sustained drug release up to 72 h, higher brain accumulation, and enhanced therapeutic efficacy of formulation | [138] |
| Rivastigmine and resveratrol | Alzheimer’s disease | Transferrin | NLC (Geleol, gelucire 50/13, transcutol HP, and tween 80) | Sustained drug release, non-toxic formulation with 1.7-fold higher uptake by brain cells | [139] |
1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC); 2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG-2000); 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt (DiD); dipalmitoyl phosphatidylcholine (DPPC); 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)] (ammonium salt) (DSPE-PEG); tocopheryl polyethylene glycol (TPGS); soy phosphatidylcholine (SPC); 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-n-[poly(ethylene glycol)]-maleimide (DSPE-PEG-MAL); N-palmitoyl-sphingosine-1-succinyl[methoxy(polyethylene glycol)2000] (PEG2000-Cer16); 1,2-dioleoyl-3-dimethylammonium-propane (DODAP).
6.2. Insulin Receptor (IR) and Insulin-like Growth Factor-1 Receptor (IGF1R)
The insulin receptor (IR), located on the luminal surface of brain microvascular ECs, facilitates the transport of insulin and other molecules into the brain. Proteomic analyses have confirmed the presence of IR expression in brain microvessels isolated from humans, monkeys, and mice [140]. At the BBB, the IR serves two primary functions: (1) mediating the transport of circulating insulin into the brain, a mechanism with potential for targeted drug delivery; and (2) regulating BBB function, implicating insulin resistance in the pathophysiology of CNS disorders and type 2 diabetes [141]. Although the role of IR in insulin transport across the BBB is limited, IR-mediated targeting has been explored for drug delivery, offering the potential to enhance drug bioavailability while minimizing peripheral side effects by ensuring precise delivery to brain tissue. Furthermore, while the TfR remains a major target for BBB drug delivery, continued research into NP design and IR-targeting strategies is essential for developing innovative therapies for CNS disorders (Table 6). Notably, anti-receptor antibodies targeting the IR have been investigated as vectors for transporting therapeutics across the BBB.
In addition to the IR, the insulin-like growth factor-1 receptor (IGF1R) has emerged as a promising receptor for RMT delivery. This receptor facilitates the transport of IGF-1 across the BBB, and exhibits elevated expression in brain ECs compared to peripheral tissue. Alata et al. developed camelid single-domain antibodies (sdAbs, VHHs) against IGF1R, and demonstrated their ability to facilitate drug delivery via the RMT pathway [142]. Furthermore, they characterized IGF1R5, an sdAb that specifically binds to IGF1Rs at the BBB, serving as a ligand to trigger RMT, effectively delivering therapeutic cargo across the barrier [143]. Recently, insulin-fusion proteins, termed hippocampal neuron-targeting (Ht) proteins, have been engineered for protein-targeted drug delivery [144]. In vitro studies have demonstrated that insulin and Ht proteins are internalized by hippocampal neurons via insulin receptor-mediated macropinocytosis. Notably, the presence of cysteine residues is critical for efficient Ht protein delivery, with an insulin B chain mutant showing superior efficacy in transporting cargo proteins, highlighting the potential of IR- and IGF1R-mediated transport systems for CNS drug delivery (Table 6) [144].

Table 6.
Literature examples of insulin receptor (IR) and insulin-like growth factor-1 receptor (IGF1R) used as brain targeting ligands.
Table 6.
Literature examples of insulin receptor (IR) and insulin-like growth factor-1 receptor (IGF1R) used as brain targeting ligands.
| Therapeutic | Malignancy | IR or IGF1R Targeting Moiety | Delivery Vehicle | Inference | Reference |
|---|---|---|---|---|---|
| Loperamide | Opioid withdrawal syndrome and nociception | Anti-insulin receptor monoclonal antibody, 29B4 (IR) | Human serum albumin NP | Therapeutic activity of drug was 3.7 times higher when conjugated with insulin and 4.4 times higher when conjugated with 29B4 | [145] |
| Glial cell line-derived neurotrophic factor (GDNF) | Parkinson’s disease | Fusion protein (IR) | Human insulin receptor monoclonal antibody | Enabled CNS uptake of GDNF; however, was not efficacious in Parkinson’s disease model with insufficient neurons | [146] |
| Iduronate 2-sulfatase (enzyme) | Mucopolysaccharidosis Type II | Fusion protein (IR) | Human insulin receptor monoclonal antibody | Enabled CNS uptake of iduronate 2-sulfatase | [147] |
| BMSC and insulin-like growth factor-1 | Cerebral ischemia | Insulin-like growth factor-1 (IGF1R) | BMSC treated with insulin-like growth factor-1 | Improved survival migratory function of BMSC, with significant improvement in cerebral blood flow and behavioral outcomes | [148] |
| IGF1R3, IGF1R4, and IGF1R5 | Brain malignancies | Anti-IGF1R antibodies (IGF1R3, IGF1R4, and IGF1R5; IGF1R) | Free single-domain anti-IGF1R antibodies | Significant, saturable accumulation of non-permeable antibodies in brain via IGF1 receptor | [142] |
| Caffeic acid | Diabetic neuropathy | Caffeic acid (IGF1R) | Free drug | Reduced inflammation and oxidative stress and acted as potential target for IGF1R | [149] |
| LR3-IGF-1 | Alzheimer’s disease | LR3-IGF-1 (IGF1R) | Free peptide | Enhanced actin remodeling, improved restored body composition, and reduced filamentous plaques; however, no improvement in cognitive functions | [150] |
| Acrylic acid | Neural disease (IGF1R) | Insulin-like growth factor-1 | Polyethylene-glycol diacrylate microparticles conjugated with acrylic acid | Stable formulation with sustained release of IGF1R, acts as dual delivery system, and has potential to restore health of injured neurons | [151] |
Bone marrow mesenchymal stem cell (BMSC); insulin-like growth factor-1 (IGF1); ling arginine 3-insulin-like growth factor-1 (LR3-IGF-1).
6.3. Lipoprotein Receptor-Related Proteins
The low-density lipoprotein receptor (LDLR) family of cell membrane glycoproteins plays a crucial role in lipid homeostasis, energy metabolism, and synthesis of the cell membrane and hormones. This family comprises LDLRs, very-low-density lipoprotein receptors (VLDLRs), and low-density lipoprotein-related protein receptors (LRP), including, LRP1, LRP2 (megalin), LRP5, LRP6, and LRP8 (apoER2) [152]. The LDLR primarily interacts with a cholesteryl ester containing a lipoprotein particle, low-density lipoprotein (LDL), forming an LDL-LDLR complex at neutral pH. LDLRs are detected on the ECs of brain capillaries; however, other members of the LDLR family, including the LDLR, LRP1, and LRP2, are also expressed on astrocytes facilitating RMT [153,154]. In the systemic circulation, other circulating lipoprotein particles, including apolipoprotein E (apoE) and very-low-, intermediate-, and high-density lipoproteins, also exhibit affinity towards LDLRs [155]. The substrate–receptor complex is internalized by endosomes via the clathrin-dependent pathway, followed by lysosomal hydrolysis, where the lipid is dissociated from the complex due to a low pH, and the receptor is recycled back to the cell surface [156,157]. The transcription of LDLRs in the nucleus regulates cholesterol [157]. The function of LDLRs is well understood in cardiovascular diseases, cholesterol homeostasis, and atherosclerosis. However, despite the critical role of lipids in myelin synthesis, the metabolism of lipoproteins in the central nervous system remains vague [158]. While most of the lipids required for the normal functioning of the brain are endogenously synthesized via de novo synthesis, some brain uptake of lipoproteins, fatty acids, and cholesterol from the systemic circulation suggests the presence of lipid transporters. Dysregulated lipoprotein metabolism in brain endothelia is linked with various chronic neurological disorders. For example, the endocytosis of apolipoprotein E (apoE), which binds to LDLRs, influences the accumulation and the clearance of amyloid β peptide, a key factor in the progression of Alzheimer’s disease [153,159]. Additionally, LRP5 and LRP6 are involved in the Wnt/β catenin signaling pathway, elucidating its importance in gliomas and medulloblastomas [160].
LRP1 and LRP2, integral members of the LDL receptor family, are highly expressed in the BBB, making them attractive targets for drug delivery systems designed to facilitate brain penetration. Given their elevated expression in glioma cells, LRP ligands are also employed for brain tumor therapy. LRP1 mediates ligand internalization, contributes to BBB disruption following ischemic events, regulates tight-junction proteins, and facilitates the clearance of extracellular matrix (ECM)-degrading proteinases [161]. Leveraging LRP1 and LRP2 for drug delivery involves the conjugation of therapeutic agents or NPs with ligands, peptides (such as Angiopep-2), or antibodies that specifically bind to these receptors, thereby promoting transcytosis into the brain parenchyma. This strategy is particularly beneficial for treating neurodegenerative diseases, brain tumors, and lysosomal storage disorders, as it enhances drug delivery efficiency while minimizing peripheral distribution and potential toxicity [162]. For example, Guo et al. developed statin-loaded Angiopep-2-anchored NPs (S@A-NPs), which selectively upregulate LRP1 expression in both brain microvascular ECs and brain metastatic tumor cells [163]. These NPs efficiently and self-promotingly cross the BBB and target brain metastases via Angiopep-2-mediated endocytosis, presenting a promising approach for the clinical management of brain metastasis [163]. Additionally, functionalized LNPs incorporating peptides that target receptors overexpressed on brain ECs and neurons—such as RVG29, T7, AP2, and mApoE—have been demonstrated to enhance mRNA transfection in the mouse brain, while reducing hepatic accumulation following systemic administration [164].
Functionalizing lipidic, polymeric, and metallic NPs with LDLR substrates—such as apoE, apolipoprotein B (apoB), Angiopep-2, polysorbate-80, and lecithin—further enhances brain uptake via RMT [165]. Studies have shown that functionalization of liposomes with apoE or its mimetics enhances the likelihood of NPs undergoing endocytosis by LDL receptors on the brain endothelium [165,166]. Seo et al. synthesized apolipoproteinB29-conjugated (apoB29) gold NPs (ApoB@AuNP), which exhibited a 20-fold increase in astrocyte uptake compared to non-targeted NPs, enhancing reactive oxygen species production and thereby improving therapeutic efficacy [167]. Another emerging approach involves modifying exosomes for targeted brain delivery. While unmodified exosomes tend to accumulate in the spleen and liver, apoE conjugation redirects their uptake to the brain and glioblastoma cells, improving treatment outcomes [168]. These studies lay the groundwork for the development of new treatment strategies for brain disorders.
Clinical trials (NCT01967810, NCT01480583, NCT02048059, and NCT01497665), such as those investigating ANG1005 and GRN1005, have explored LRP1-targeting therapeutics, where paclitaxel molecules are covalently linked to Angiopep-2 to enhance BBB penetration and tumor uptake while circumventing P-gp-mediated drug efflux (Table 7) [169,170]. Given that LDLRs are overexpressed in rapidly proliferating tumor cells, they represent promising targets for brain cancer therapy. Many LDLR substrates are biocompatible and biodegradable, facilitating efficient drug transport across the BBB. However, as LDLRs are known to be expressed in normal tissues, off-target effects and systemic toxicity remain concerns, potentially reducing the drug concentration at the intended site. Despite these challenges, LDLR-targeted strategies hold significant promise for treating neurological disorders and brain malignancies [165].

Table 7.
Strategies to target low-density lipoprotein receptors (LDLR) for brain-targeted drug delivery.
6.4. Neonatal Fc Receptor (FcRn)
The FcRn, also known as the Brambell receptor, is responsible for binding to the Fc region of immunoglobulin G (IgG) and to albumin, two of the most abundant proteins in the systemic circulation. It is predominantly expressed in the brain microvascular endothelium and the choroid plexus epithelium, where it plays a crucial role in extending the half-life of IgG and albumin by recycling them, rather than degrading them. The FcRn mediates both efflux (from the brain to the bloodstream) and potentially influx (from the bloodstream to the brain) transcytosis of IgG, which may have significant implications for the delivery of drugs to the CNS [178]. Studies have demonstrated that the FcRn facilitates the rapid efflux of IgG from the brain parenchyma back into the bloodstream, a process known as “reverse transcytosis”, which may limit the therapeutic efficacy of antibodies targeting the brain [179]. Consequently, understanding the FcRn’s role in IgG transport across the BBB has led to efforts to modulate antibody–FcRn interactions to either enhance or reduce brain delivery of therapeutic antibodies [178]. The FcRn binds to the Fc portion of IgG in a pH-dependent manner, preventing IgG from degradation and facilitating its transcellular transport. This interaction is characterized by high affinity at acidic pH (~6.0–6.5), which allows the FcRn to bind IgG. However, as the environment becomes more neutral (~7.4), the receptor’s affinity decreases significantly, leading to the release of IgG back into the circulation [180]. In addition to IgG, the FcRn also binds to albumin under mildly acidic conditions, which plays a crucial role in extending albumin’s half-life in the bloodstream to approximately 21–28 days [181]. This interaction helps to maintain albumin’s high plasma concentration (35–55 g/L) by recycling it through endosomal pathways and protecting it from degradation [182].
FcRn-targeted drug delivery platforms involve the engineering of therapeutic antibodies or fusion constructs with optimized Fc regions that enhance their interaction with FcRns and promote transcytosis into the brain. Engineered antibodies with modified Fc domains showed improved transport across the BBB in a mouse model [178]. Recent studies have further explored this pathway to enhance the brain delivery of various therapeutics. Additionally, Haqqani et al. reviewed RMT mechanisms for brain delivery of therapeutics, emphasizing the significance of receptors like the FcRn in facilitating this transport [115]. Holst et al. investigated the subcellular trafficking and transcytosis efficacy of various receptor types, including the FcRn, for therapeutic antibody delivery at the BBB, providing insights into optimizing such delivery systems [182]. Simonneau et al. utilized BBB organoid arrays to study receptor-mediated antibody transcytosis, highlighting the FcRn’s potential for mediating drug delivery across the BBB [183]. Furthermore, Mhaske explored receptor-assisted nanotherapeutics for overcoming the BBB, underscoring the role of the FcRn in enhancing drug delivery to the brain [184]. Albumin, another FcRn ligand, has been extensively explored as a drug delivery carrier, due to its favorable properties. Albumin-based NPs are particularly attractive, due to their excellent biocompatibility, low immunogenicity, and prolonged systemic circulation. Their extended half-life is primarily attributed to FcRn-mediated recycling, which protects albumin from lysosomal degradation via a pH-dependent retrieval mechanism [185]. In glioma models, albumin NPs functionalized with the cell-penetrating peptide have demonstrated enhanced brain penetration and tumor-targeting capabilities [186]. Additionally, the free thiol group of cysteine residue (Cys34) on albumin’s domain I has been employed in drug conjugation strategies, such as the Drug Affinity Complex (DAC) approach. A notable example is Aldoxorubicin, an acid-sensitive doxorubicin prodrug that binds covalently to albumin via Cys34 following intravenous administration [187]. This conjugate has shown promise in clinical trials for the treatment of sarcoma and glioblastoma [187] (Table 8).
These findings highlight FcRn’s emerging role in CNS drug delivery, and the potential for optimizing Fc engineering and NP formulations to enhance transcytosis efficiency. Additionally, a comprehensive review of FcRn-IgG immunobiology, including its functional and pathological roles, along with an overview of FcRn-targeted therapy development, provides valuable insights into the advancing field [188].

Table 8.
Strategies to target the neonatal Fc receptor (FcRn) for brain-targeted drug delivery.
Table 8.
Strategies to target the neonatal Fc receptor (FcRn) for brain-targeted drug delivery.
| Therapeutic | Malignancy | FcRn Targeting Moiety | Delivery Vehicle | Inference | Reference |
|---|---|---|---|---|---|
| IgG1 N434A and IgG1 H435A | Brain malignancies | IgG1 N434A (high-affinity) and IgG1 H435A (low-affinity) | Free monoclonal antibodies | N434A and H435A have affinity towards efflux transporter FcRn, making them potential targeting moieties for brain delivery | [189,190] |
| Anti-PD-L1 IgG | Brain glioblastoma | FcRn high-affinity 89Zr-DFO-C4 with FSU | C4 radioligands | Leveraging FcRn confers improved kinetic properties to 89Zr-DFO-C4 | [191] |
| Doxorubicin | Glioma | Albumin | Acid-sensitive prodrug | Showed promise in clinical trials for treatment of sarcoma and glioblastoma | [187] |
Immunoglobulin-G (IgG1), focused ultrasound (FSU).
7. Conclusions
TMT and RMT represent a promising and potential transformative approach to overcoming the challenges of drug delivery across the BBB. Significant progress has been made in understanding the biology of RMT/TMT and its applications in treating CNS disorders. However, challenges such as receptor or transporter saturation, immunogenicity, and cargo limitations must be addressed to achieve widespread clinical adoption. Continued advancements in ligand design, nanotechnology, and personalized medicine will be instrumental in realizing the full therapeutic potential of utilizing RMT/TMT and improving outcomes for patients with neurological diseases. With ongoing advancements in drug design, NP engineering, and transporter and receptor biology, TMT/RMT are poised to play a central role in the development of next-generation therapies for the treatment of CNS disorders. Continued research and innovation will be crucial to unlocking their full potential and bringing TMT/RMT-based therapies closer to clinical reality.
Author Contributions
L.D. conceived the idea, developed the figures, and designed and wrote the original manuscript. P.K. helped with the writing of the original manuscript. P.A. helped with the writing of the original manuscript and the preparation of Figure 2. D.J.M. reviewed and edited the manuscript. Supervision was conducted by D.J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The figures in this review were created with BioRender.com.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- National Cancer Institute. Drugs Approved for Brain Tumors; National Cancer Institute: Bethesda, MD, USA, 2024. [Google Scholar]
- Saunders, N.R.; Dreifuss, J.-J.; Dziegielewska, K.M.; Johansson, P.A.; Habgood, M.D.; Møllgård, K.; Bauer, H.-C. The rights and wrongs of blood-brain barrier permeability studies: A walk through 100 years of history. Front. Neurosci. 2014, 8, 404. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yao, Y.; Tsirka, S.E.; Cao, Y. Cell-culture models of the blood–brain barrier. Stroke 2014, 45, 2514–2526. [Google Scholar] [CrossRef]
- Miller, D.S.; Bauer, B.; Hartz, A.M. Modulation of P-glycoprotein at the blood-brain barrier: Opportunities to improve central nervous system pharmacotherapy. Pharmacol. Rev. 2008, 60, 196–209. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation, drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Dong, X. Current strategies for brain drug delivery. Theranostics 2018, 8, 1481. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Drug delivery systems, CNS protection, and the blood brain barrier. BioMed Res. Int. 2014, 2014, 869269. [Google Scholar] [CrossRef]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-based nanoparticles for drug/gene delivery: An overview of the production techniques and difficulties encountered in their industrial development. ACS Mater. Au 2023, 3, 600–619. [Google Scholar] [CrossRef]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the blood-brain barrier: Advances in nanoparticle technology for drug delivery in neuro-oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jeong, M.; Park, J.; Jung, H.; Lee, H. Immunogenicity of lipid nanoparticles and its impact on the efficacy of mRNA vaccines and therapeutics. Exp. Mol. Med. 2023, 55, 2085–2096. [Google Scholar] [CrossRef]
- Zhang, W.; Mehta, A.; Tong, Z.; Esser, L.; Voelcker, N.H. Development of polymeric nanoparticles for blood–brain barrier transfer—Strategies and challenges. Adv. Sci. 2021, 8, 2003937. [Google Scholar] [CrossRef]
- Ekhator, C.; Qureshi, M.Q.; Zuberi, A.W.; Hussain, M.; Sangroula, N.; Yerra, S.; Devi, M.; Naseem, M.A.; Bellegarde, S.B.; Pendyala, P.R. Advances and opportunities in nanoparticle drug delivery for central nervous system disorders: A review of current advances. Cureus 2023, 15, e44302. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Jagaran, K.; Singh, M. Lipid nanoparticles: Promising treatment approach for Parkinson’s disease. Int. J. Mol. Sci. 2022, 23, 9361. [Google Scholar] [CrossRef]
- Jones, A.R.; Shusta, E.V. Blood–brain barrier transport of therapeutics via receptor-mediation. Pharm. Res. 2007, 24, 1759–1771. [Google Scholar] [CrossRef]
- Teixeira, M.I.; Lopes, C.M.; Amaral, M.H.; Costa, P.C. Surface-modified lipid nanocarriers for crossing the blood-brain barrier (BBB): A current overview of active targeting in brain diseases. Colloids Surf. B Biointerfaces 2023, 221, 112999. [Google Scholar] [CrossRef]
- Song, J.; Lu, C.; Leszek, J.; Zhang, J. Design and development of nanomaterial-based drug carriers to overcome the blood–brain barrier by using different transport mechanisms. Int. J. Mol. Sci. 2021, 22, 10118. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, L.; Cao, F.; Wang, H.; Gong, P.; Ma, C.; Ren, L.; Lin, Y.; Lin, X. The barrier and interface mechanisms of the brain barrier, and brain drug delivery. Brain Res. Bull. 2022, 190, 69–83. [Google Scholar] [CrossRef]
- Pardridge, W.M. Advanced blood–brain barrier drug delivery. Pharmaceutics 2022, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Hervé, F.; Ghinea, N.; Scherrmann, J.-M. CNS delivery via adsorptive transcytosis. AAPS J. 2008, 10, 455–472. [Google Scholar] [CrossRef] [PubMed]
- Nag, S. Blood Brain Barrier, Exchange of Metabolites and Gases, Pathology and Genetics: Cerebrovascular Diseases; ISN Neuropath Press: Basel, Switzerland, 2005; pp. 22–29. [Google Scholar]
- Brown, P.; Davies, S.; Speake, T.; Millar, I. Molecular mechanisms of cerebrospinal fluid production. Neuroscience 2004, 129, 955–968. [Google Scholar] [CrossRef]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M.; Siegelbaum, S.; Hudspeth, A.J.; Mack, S. Principles of Neural Science; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and dysfunction of the blood-brain barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef]
- Li, P.; Fan, H. Pericyte Loss in Diseases. Cells 2023, 12, 1931. [Google Scholar] [CrossRef]
- Kaya, M.; Ahishali, B. Basic physiology of the blood-brain barrier in health and disease: A brief overview. Tissue Barriers 2021, 9, 1840913. [Google Scholar] [CrossRef]
- Maridaki, Z.; Syrros, G.; Delichatsiou, S.G.; Warsh, J.; Konstantinou, G.N. Claudin-5 and occludin levels in patients with psychiatric disorders—A systematic review. Brain Behav. Immun. 2024, 123, 865–875. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2005, 2, 86–98. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog. Neurobiol. 2005, 76, 22–76. [Google Scholar] [CrossRef]
- Fung, K.Y.; Fairn, G.D.; Lee, W.L. Transcellular vesicular transport in epithelial and endothelial cells: Challenges and opportunities. Traffic 2018, 19, 5–18. [Google Scholar] [CrossRef]
- Lajoie, J.M.; Shusta, E.V. Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Stamp, M.E.M.; Halwes, M.; Nisbet, D.; Collins, D.J. Breaking barriers: Exploring mechanisms behind opening the blood-brain barrier. Fluids Barriers CNS 2023, 20, 87. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Panja, S.; Zaman, L.A.; Yeapuri, P.; Bhattarai, S.; Gorantla, S.; Chang, L.; Heredia, A.; Walczak, P.; Hanson, B. CCR5-ligand decorated rilpivirine lipid-based nanoparticles for sustained antiretroviral responses. Nat. Commun. 2025, 16, 513. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Transendothelial transport and its role in therapeutics. Int. Sch. Res. Not. 2014, 2014, 309404. [Google Scholar] [CrossRef]
- Barar, J.; Rafi, M.A.; Pourseif, M.M.; Omidi, Y. Blood-brain barrier transport machineries and targeted therapy of brain diseases. BioImpacts BI 2016, 6, 225. [Google Scholar] [CrossRef]
- Lin, L.; Yee, S.W.; Kim, R.B.; Giacomini, K.M. SLC transporters as therapeutic targets: Emerging opportunities. Nat. Rev. Drug Discov. 2015, 14, 543–560. [Google Scholar] [CrossRef]
- Vijay, N.; Morris, M.E. Role of monocarboxylate transporters in drug delivery to the brain. Curr. Pharm. Des. 2014, 20, 1487–1498. [Google Scholar] [CrossRef]
- Storck, S.E.; Meister, S.; Nahrath, J.; Meißner, J.N.; Schubert, N.; Di Spiezio, A.; Baches, S.; Vandenbroucke, R.E.; Bouter, Y.; Prikulis, I. Endothelial LRP1 transports amyloid-β 1–42 across the blood-brain barrier. J. Clin. Investig. 2016, 126, 123–136. [Google Scholar] [CrossRef]
- Wang, C.; Chu, C.; Ji, X.; Luo, G.; Xu, C.; He, H.; Yao, J.; Wu, J.; Hu, J.; Jin, Y. Biology of peptide transporter 2 in mammals: New insights into its function, structure and regulation. Cells 2022, 11, 2874. [Google Scholar] [CrossRef]
- Begley, D.J.; Brightman, M.W. Structural and functional aspects of the blood-brain barrier. In Peptide Transport and Delivery into the Central Nervous System; Springer: Berlin/Heidelberg, Germany, 2003; pp. 39–78. [Google Scholar]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Puris, E.; Gynther, M.; Auriola, S.; Huttunen, K.M. L-Type amino acid transporter 1 as a target for drug delivery. Pharm. Res. 2020, 37, 88. [Google Scholar] [CrossRef] [PubMed]
- Anraku, Y.; Kuwahara, H.; Fukusato, Y.; Mizoguchi, A.; Ishii, T.; Nitta, K.; Matsumoto, Y.; Toh, K.; Miyata, K.; Uchida, S. Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nat. Commun. 2017, 8, 1001. [Google Scholar] [CrossRef] [PubMed]
- Jafri, S.; Shah, S.I.A.; Jaffari, S. Molecular Structure, Biochemical Functions, Genetics, and Emerging Clinical Relevance of Glucose Transporters. Glob. J. Med. Pharm. Biomed. Update 2023, 18, 23. [Google Scholar] [CrossRef]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Asp. Med. 2013, 34, 121–138. [Google Scholar] [CrossRef]
- Thorens, B.; Mueckler, M. Glucose transporters in the 21st Century. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E141–E145. [Google Scholar] [CrossRef]
- Schmidl, S.; Ursu, O.; Iancu, C.V.; Oreb, M.; Oprea, T.I.; Choe, J.-Y. Identification of new GLUT2-selective inhibitors through in silico ligand screening and validation in eukaryotic expression systems. Sci. Rep. 2021, 11, 13751. [Google Scholar] [CrossRef]
- Dawson, P.A.; Mychaleckyj, J.C.; Fossey, S.C.; Mihic, S.J.; Craddock, A.L.; Bowden, D.W. Sequence and Functional Analysis of GLUT10: A Glucose Transporter in the Type 2 Diabetes-Linked Region of Chromosome 20q12–13.1. Mol. Genet. Metab. 2001, 74, 186–199. [Google Scholar] [CrossRef]
- Devraj, K.; Klinger, M.E.; Myers, R.L.; Mokashi, A.; Hawkins, R.A.; Simpson, I.A. GLUT-1 glucose transporters in the blood-brain barrier: Differential phosphorylation. J. Neurosci. Res. 2011, 89, 1913–1925. [Google Scholar] [CrossRef]
- Albaik, M.; Saleh, D.S.; Kauther, D.; Mohammed, H.; Alfarra, S.; Alghamdi, A.; Ghaboura, N.; Sindi, I.A. Bridging the gap: Glucose transporters, Alzheimer’s, and future therapeutic prospects. Front. Cell Dev. Biol. 2024, 12, 1344039. [Google Scholar] [CrossRef]
- Xiuli, G.; Meiyu, G.; Guanhua, D.; Transporter, G. Distribution in the Brain and in Neural Disorders: Its Relationship With Transport of Neuroactive Drugs Through the Blood-Brain Barrier. Biochem. Genet. 2005, 43, 175–187. [Google Scholar] [CrossRef]
- Chen, Y.; Joo, J.; Chu, J.M.-T.; Chang, R.C.-C.; Wong, G.T.-C. Downregulation of the glucose transporter GLUT 1 in the cerebral microvasculature contributes to postoperative neurocognitive disorders in aged mice. J. Neuroinflammation 2023, 20, 237. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, L. Brain Glucose Transporters: Role in Pathogenesis and Potential Targets for the Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 8142. [Google Scholar] [CrossRef] [PubMed]
- Pucci, G.; Minafra, L.; Bravatà, V.; Calvaruso, M.; Turturici, G.; Cammarata, F.P.; Savoca, G.; Abbate, B.; Russo, G.; Cavalieri, V.; et al. Glut-3 Gene Knockdown as a Potential Strategy to Overcome Glioblastoma Radioresistance. Int. J. Mol. Sci. 2024, 25, 2079. [Google Scholar] [CrossRef]
- Zhang, S.; Zuo, W.; Guo, X.-F.; He, W.-B.; Chen, N.-H. Cerebral glucose transporter: The possible therapeutic target for ischemic stroke. Neurochem. Int. 2014, 70, 22–29. [Google Scholar] [CrossRef]
- Vannucci, S.J.; Seaman, L.B.; Vannucci, R.C. Effects of Hypoxia-Ischemia on GLUT1 and GLUT3 Glucose Transporters in Immature Rat Brain. J. Cereb. Blood Flow Metab. 1996, 16, 77–81. [Google Scholar] [CrossRef]
- Arora, S.; Sharma, D.; Singh, J. GLUT-1: An Effective Target To Deliver Brain-Derived Neurotrophic Factor Gene Across the Blood Brain Barrier. ACS Chem. Neurosci. 2020, 11, 1620–1633. [Google Scholar] [CrossRef]
- Patching, S.G. Glucose Transporters at the Blood-Brain Barrier: Function, Regulation and Gateways for Drug Delivery. Mol. Neurobiol. 2017, 54, 1046–1077. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Z.; Guan, J.; Lu, W.; Zhan, C. Unraveling GLUT-mediated transcytosis pathway of glycosylated nanodisks. Asian J. Pharm. Sci. 2021, 16, 120–128. [Google Scholar] [CrossRef]
- Xie, F.; Yao, N.; Qin, Y.; Zhang, Q.; Chen, H.; Yuan, M.; Tang, J.; Li, X.; Fan, W.; Zhang, Q.; et al. Investigation of glucose-modified liposomes using polyethylene glycols with different chain lengths as the linkers for brain targeting. Int. J. Nanomed. 2012, 7, 163–175. [Google Scholar] [CrossRef]
- Qu, B.; Li, X.; Guan, M.; Li, X.; Hai, L.; Wu, Y. Design, synthesis and biological evaluation of multivalent glucosides with high affinity as ligands for brain targeting liposomes. Eur. J. Med. Chem. 2014, 72, 110–118. [Google Scholar] [CrossRef]
- Xie, J.; Gonzalez-Carter, D.; Tockary, T.A.; Nakamura, N.; Xue, Y.; Nakakido, M.; Akiba, H.; Dirisala, A.; Liu, X.; Toh, K.; et al. Dual-Sensitive Nanomicelles Enhancing Systemic Delivery of Therapeutically Active Antibodies Specifically into the Brain. ACS Nano 2020, 14, 6729–6742. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lu, H.; Xiao, W.; Yang, J.; Du, H.; Shen, Y.; Qu, H.; Jia, B.; Manna, S.K.; Ramachandran, M.; et al. Sequential Targeting in Crosslinking Nanotheranostics for Tackling the Multibarriers of Brain Tumors. Adv. Mater. 2020, 32, e1903759. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Yu, Z.; Xu, T.; Wang, L.; Meng, N.; Jin, H.; Xu, B. Novel Nano-Drug Delivery System for Brain Tumor Treatment. Cells 2022, 11, 3761. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, F.; Liu, Y.; Zheng, M.; Wang, Y.; Zhang, D.; Anraku, Y.; Zou, Y.; Li, J.; Wu, H.; et al. Blood-brain barrier-penetrating siRNA nanomedicine for Alzheimer’s disease therapy. Sci. Adv. 2020, 6, 3761. [Google Scholar] [CrossRef]
- Caro, C.; Paez-Muñoz, J.M.; Pernía Leal, M.; Carayol, M.; Feijoo-Cuaresma, M.; García-Martín, M.L. Metabolically-Driven Active Targeting of Magnetic Nanoparticles Functionalized with Glucuronic Acid to Glioblastoma: Application to MRI-Tracked Magnetic Hyperthermia Therapy. Adv. Healthc. Mater. 2025, 14, 2404391. [Google Scholar] [CrossRef]
- Liu, J.; Sun, M.; Li, Z.; Xiang, H.; Wang, Q.; Xin, X.; Shen, Y. Catalytic nanoreactors promote GLUT1-mediated BBB permeation by generating nitric oxide for potentiating glioblastoma ferroptosis. Chem. Eng. J. 2024, 483, 149233. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, X.; Chen, Y.; Yang, H.; Liu, J.; Yang, Z.; Lei, J.; Lu, F.; Hao, D.; Feng, L. Nanoparticles modified with glucose analogs to enhance the permeability of the blood-brain barrier and its accumulation in epileptic brain. J. Mater. Chem. B 2025, 13, 2810–2819. [Google Scholar] [CrossRef]
- Mahanta, A.K.; Chaulagain, B.; Trivedi, R.; Singh, J. Mannose-Functionalized Chitosan-Coated PLGA Nanoparticles for Brain-Targeted Codelivery of CBD and BDNF for the Treatment of Alzheimer’s Disease. ACS Chem. Neurosci. 2024, 15, 4021–4032. [Google Scholar] [CrossRef]
- Temizyürek, A.; Yılmaz, C.U.; Emik, S.; Akcan, U.; Atış, M.; Orhan, N.; Arıcan, N.; Ahishali, B.; Tüzün, E.; Küçük, M.; et al. Blood-brain barrier targeted delivery of lacosamide-conjugated gold nanoparticles: Improving outcomes in absence seizures. Epilepsy Res. 2022, 184, 106939. [Google Scholar] [CrossRef]
- Wang, Q.; Holst, J. L-type amino acid transport and cancer: Targeting the mTORC1 pathway to inhibit neoplasia. Am. J. Cancer Res. 2015, 5, 1281–1294. [Google Scholar]
- Delescluse, J.; Simonnet, M.M.; Ziegler, A.B.; Piffaretti, K.; Alves, G.; Grosjean, Y.; Manière, G. A LAT1-Like Amino Acid Transporter Regulates Neuronal Activity in the Drosophila Mushroom Bodies. Cells 2024, 13, 1340. [Google Scholar] [CrossRef] [PubMed]
- Cappoli, N.; Jenkinson, M.D.; Russo, C.D.; Dickens, D. LAT1, a novel pharmacological target for the treatment of glioblastoma. Biochem. Pharmacol. 2022, 201, 115103. [Google Scholar] [CrossRef] [PubMed]
- Faried, A.; Bolly, H.M.B.; Hermanto, Y.; Achmad, A.; Halim, D.; Tjahjono, F.P.; Agustina, H.; Kartamihardja, A.H.S.; Arifin, M.Z. Prognostic significance of L-type amino acid transporter-1 (LAT-1) expression in human astrocytic gliomas. Interdiscip. Neurosurg. 2021, 23, 100939. [Google Scholar] [CrossRef]
- Tărlungeanu, D.C.; Deliu, E.; Dotter, C.P.; Kara, M.; Janiesch, P.C.; Scalise, M.; Galluccio, M.; Tesulov, M.; Morelli, E.; Sonmez, F.M.; et al. Impaired Amino Acid Transport at the Blood Brain Barrier Is a Cause of Autism Spectrum Disorder. Cell 2016, 167, 1481–1494.e18. [Google Scholar] [CrossRef]
- Cornford, E.M.; Young, D.; Paxton, J.W.; Finlay, G.J.; Wilson, W.R.; Pardridge, W.M. Melphalan penetration of the blood-brain barrier via the neutral amino acid transporter in tumor-bearing brain. Cancer Res. 1992, 52, 138–143. [Google Scholar]
- Lerner, R.P.; Francardo, V.; Fujita, K.; Bimpisidis, Z.; Jourdain, V.A.; Tang, C.C.; Dewey, S.L.; Chaly, T.; Cenci, M.A.; Eidelberg, D. Levodopa-induced abnormal involuntary movements correlate with altered permeability of the blood-brain-barrier in the basal ganglia. Sci. Rep. 2017, 7, 16005. [Google Scholar] [CrossRef]
- Dickens, D.; Webb, S.D.; Antonyuk, S.; Giannoudis, A.; Owen, A.; Rädisch, S.; Hasnain, S.S.; Pirmohamed, M. Transport of gabapentin by LAT1 (SLC7A5). Biochem. Pharmacol. 2013, 85, 1672–1683. [Google Scholar] [CrossRef]
- Takahashi, Y.; Nishimura, T.; Higuchi, K.; Noguchi, S.; Tega, Y.; Kurosawa, T.; Deguchi, Y.; Tomi, M. Transport of Pregabalin Via L-Type Amino Acid Transporter 1 (SLC7A5) in Human Brain Capillary Endothelial Cell Line. Pharm. Res. 2018, 35, 246. [Google Scholar] [CrossRef]
- Rautio, J.; Gynther, M.; Laine, K. LAT1-mediated prodrug uptake: A way to breach the blood–brain barrier? Ther. Deliv. 2013, 4, 281–284. [Google Scholar] [CrossRef]
- Montaser, A.B.; Järvinen, J.; Löffler, S.; Huttunen, J.; Auriola, S.; Lehtonen, M.; Jalkanen, A.; Huttunen, K.M. L-Type Amino Acid Transporter 1 Enables the Efficient Brain Delivery of Small-Sized Prodrug across the Blood–Brain Barrier and into Human and Mouse Brain Parenchymal Cells. ACS Chem. Neurosci. 2020, 11, 4301–4315. [Google Scholar] [CrossRef]
- Puris, E.; Gynther, M.; Huttunen, J.; Auriola, S.; Huttunen, K.M. L-type amino acid transporter 1 utilizing prodrugs of ferulic acid revealed structural features supporting the design of prodrugs for brain delivery. Eur. J. Pharm. Sci. 2019, 129, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Carter, D.A.; Ong, Z.Y.; McGilvery, C.M.; Dunlop, I.E.; Dexter, D.T.; Porter, A.E. L-DOPA functionalized, multi-branched gold nanoparticles as brain-targeted nano-vehicles. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, Q.; Xue, Y.; Yao, K.; Syeda, M.Z.; Xu, J.; Wu, J.; Wang, Z.; Tang, L.; Mu, Q. LAT1 targeted brain delivery of temozolomide and sorafenib for effective glioma therapy. Nano Res. 2023, 16, 9743–9751. [Google Scholar] [CrossRef]
- Hayashi, K.; Anzai, N. L-type amino acid transporter 1 as a target for inflammatory disease and cancer immunotherapy. J. Pharmacol. Sci. 2022, 148, 31–40. [Google Scholar] [CrossRef]
- Alonso, M.; Barcia, E.; González, J.-F.; Montejo, C.; García-García, L.; Villa-Hermosilla, M.-C.; Negro, S.; Fraguas-Sánchez, A.-I.; Fernández-Carballido, A. Functionalization of morin-loaded PLGA nanoparticles with phenylalanine dipeptide targeting the brain. Pharmaceutics 2022, 14, 2348. [Google Scholar] [CrossRef]
- Lim, Y.N.; Ryu, I.S.; Jung, Y.-J.; Helmlinger, G.; Kim, I.; Park, H.W.; Kang, H.; Lee, J.; Lee, H.J.; Lee, K.S. l-Type amino acid transporter 1-targeting nanoparticles for antisense oligonucleotide delivery to the CNS. Mol. Ther. Nucleic Acids 2024, 35, 102340. [Google Scholar] [CrossRef]
- Fernandes, J.; Ghate, M.V.; Mallik, S.B.; Lewis, S.A. Amino acid conjugated chitosan nanoparticles for the brain targeting of a model dipeptidyl peptidase-4 inhibitor. Int. J. Pharm. 2018, 547, 563–571. [Google Scholar] [CrossRef]
- Kalčec, N.; Peranić, N.; Mamić, I.; Beus, M.; Hall, C.R.; Smith, T.A.; Sani, M.A.; Turčić, P.; Separovic, F.; Vrček, I.V. Selenium nanoparticles as potential drug-delivery systems for the treatment of Parkinson’s disease. ACS Appl. Nano Mater. 2023, 6, 17581–17592. [Google Scholar] [CrossRef]
- Chen, S.; Jin, C.; Ohgaki, R.; Xu, M.; Okanishi, H.; Kanai, Y. Structure-activity characteristics of phenylalanine analogs selectively transported by L-type amino acid transporter 1 (LAT1). Sci. Rep. 2024, 14, 4651. [Google Scholar] [CrossRef]
- Pérez-Escuredo, J.; Van Hée, V.F.; Sboarina, M.; Falces, J.; Payen, V.L.; Pellerin, L.; Sonveaux, P. Monocarboxylate transporters in the brain and in cancer. Biochim. Biophys. Acta 2016, 1863, 2481–2497. [Google Scholar] [CrossRef]
- Ueno, M.; Chiba, Y.; Murakami, R.; Miyai, Y.; Matsumoto, K.; Wakamatsu, K.; Takebayashi, G.; Uemura, N.; Yanase, K. Distribution of Monocarboxylate Transporters in Brain and Choroid Plexus Epithelium. Pharmaceutics 2023, 15, 2062. [Google Scholar] [CrossRef] [PubMed]
- Chasseigneaux, S.; Cochois-Guégan, V.; Lecorgne, L.; Lochus, M.; Nicolic, S.; Blugeon, C.; Jourdren, L.; Gomez-Zepeda, D.; Tenzer, S.; Sanquer, S.; et al. Fasting upregulates the monocarboxylate transporter MCT1 at the rat blood-brain barrier through PPAR δ activation. Fluids Barriers CNS 2024, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Lee, D.; Xiong, W.C.; Metabolism, L. Signaling, and Function in Brain Development, Synaptic Plasticity, Angiogenesis, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 13398. [Google Scholar] [CrossRef]
- Venishetty, V.K.; Samala, R.; Komuravelli, R.; Kuncha, M.; Sistla, R.; Diwan, P.V. β-Hydroxybutyric acid grafted solid lipid nanoparticles: A novel strategy to improve drug delivery to brain, Nanomedicine: Nanotechnology. Biol. Med. 2013, 9, 388–397. [Google Scholar] [CrossRef]
- Ak, G.; Ünal, A.; Karakayalı, T.; Özel, B.; Selvi Günel, N.; Hamarat Şanlıer, Ş. Brain-targeted, drug-loaded solid lipid nanoparticles against glioblastoma cells in culture. Colloids Surf. B. Biointerfaces 2021, 206, 111946. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Zhang, Y.; Li, Y.; Li, S.; Vinogradov, S.V.; Persidsky, Y.; Alakhov, V.Y.; Miller, D.W.; Kabanov, A.V. Effects of pluronic P85 on GLUT1 and MCT1 transporters in the blood-brain barrier. Pharm. Res. 2004, 21, 1993–2000. [Google Scholar] [CrossRef]
- Huang, T.; Feng, Q.; Wang, Z.; Li, W.; Sun, Z.; Wilhelm, J.; Huang, G.; Vo, T.; Sumer, B.D.; Gao, J. Tumor-Targeted Inhibition of Monocarboxylate Transporter 1 Improves T-Cell Immunotherapy of Solid Tumors. Adv. Healthc. Mater. 2021, 10, e2000549. [Google Scholar] [CrossRef]
- Guan, X.; Morris, M.E. Pharmacokinetics of the Monocarboxylate Transporter 1 Inhibitor AZD3965 in Mice: Potential Enterohepatic Circulation and Target-Mediated Disposition. Pharm. Res. 2019, 37, 5. [Google Scholar] [CrossRef]
- Halford, S.; Veal, G.J.; Wedge, S.R.; Payne, G.S.; Bacon, C.M.; Sloan, P.; Dragoni, I.; Heinzmann, K.; Potter, S.; Salisbury, B.M.; et al. A Phase I Dose-escalation Study of AZD3965, an Oral Monocarboxylate Transporter 1 Inhibitor, in Patients with Advanced Cancer. Clin. Cancer Res. 2023, 29, 1429–1439. [Google Scholar] [CrossRef]
- Schulte, R.R.; Ho, R.H. Organic Anion Transporting Polypeptides: Emerging Roles in Cancer Pharmacology. Mol. Pharmacol. 2019, 95, 490–506. [Google Scholar] [CrossRef]
- Westholm, D.E.; Rumbley, J.N.; Salo, D.R.; Rich, T.P.; Anderson, G.W. Organic anion-transporting polypeptides at the blood-brain and blood-cerebrospinal fluid barriers. Curr. Top. Dev. Biol. 2008, 80, 135–170. [Google Scholar] [PubMed]
- Gong, I.Y.; Kim, R.B. Impact of genetic variation in OATP transporters to drug disposition and response. Drug Metab. Pharmacokinet 2013, 28, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.Y.; Xu, S.F.; Zhu, Q.N.; Lu, Y.F.; Cheng, X.G.; Liu, J. Age- and sex-related differences of organic anion-transporting polypeptide gene expression in livers of rats. Toxicol. Appl. Pharmacol. 2014, 280, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Wang, J.; Zhang, Q.; Qin, L.; Wang, Y.; He, Y. The role of organic anion transport protein 1a4 in drug delivery and diseases: A review. Food Sci. Technol. 2023, 43, e114122. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Li, L.; Wang, L.; Xu, M.-C.; An, S.; Jiang, C.; Gu, J.-K.; Wang, Z.-J.J.; Yu, L.-S.; Zeng, S. siRNA capsulated brain-targeted nanoparticles specifically knock down OATP2B1 in mice: A mechanism for acute morphine tolerance suppression. Sci. Rep. 2016, 6, 33338. [Google Scholar] [CrossRef]
- Reichel, D.; Sagong, B.; Teh, J.; Zhang, Y.; Wagner, S.; Wang, H.; Chung, L.W.K.; Butte, P.; Black, K.L.; Yu, J.S.; et al. Near Infrared Fluorescent Nanoplatform for Targeted Intraoperative Resection and Chemotherapeutic Treatment of Glioblastoma. ACS Nano 2020, 14, 8392–8408. [Google Scholar] [CrossRef]
- Thompson, B.J.; Sanchez-Covarrubias, L.; Slosky, L.M.; Zhang, Y.; Laracuente, M.-L.; Ronaldson, P.T. Hypoxia/Reoxygenation Stress Signals an Increase in Organic Anion Transporting polypeptide 1a4 (Oatp1a4) at the Blood–Brain Barrier: Relevance to CNS Drug Delivery. J. Cereb. Blood Flow Metab. 2014, 34, 699–707. [Google Scholar] [CrossRef]
- Tonduru, A.K.; Maljaei, S.H.; Adla, S.K.; Anamea, L.; Tampio, J.; Králová, A.; Jalkanen, A.J.; Espada, C.; Santos, I.F.; Montaser, A.B.; et al. Targeting Glial Cells by Organic Anion-Transporting Polypeptide 1C1 (OATP1C1)-Utilizing l-Thyroxine-Derived Prodrugs. J. Med. Chem. 2023, 66, 15094–15114. [Google Scholar] [CrossRef]
- Klein, I.; Isensee, J.; Wiesen, M.H.; Imhof, T.; Wassermann, M.K.; Müller, C.; Hucho, T.; Koch, M.; Lehmann, H.C. Glycyrrhizic acid prevents paclitaxel-induced neuropathy via inhibition of OATP-mediated neuronal uptake. Cells 2023, 12, 1249. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.J.; Zheng, X.R.; Zhou, Z.J.; Duan, J.Q.; Liu, H.Y.; Shao, Y.Y.; Hou, R.G. (+)-Borneol enhances the protective effect of edaravone against cerebral ischemia/reperfusion injury by targeting OAT3/P-gp transporters for drug delivery into the brain. Phytomedicine 2025, 139, 156521. [Google Scholar] [CrossRef]
- Haqqani, A.S.; Bélanger, K.; Stanimirovic, D.B. Receptor-mediated transcytosis for brain delivery of therapeutics: Receptor classes and criteria. Front. Drug Deliv. 2024, 4, 1360302. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Burkhart, A.; Thomsen, L.B.; Andresen, T.L.; Moos, T. Targeting the transferrin receptor for brain drug delivery. Prog. Neurobiol. 2019, 181, 101665. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.R.; Bernabeu, E.; Rodríguez, J.A.; Patel, S.; Kozman, M.; Chiappetta, D.A.; Holler, E.; Ljubimova, J.Y.; Helguera, G.; Penichet, M.L. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim. Biophys. Acta 2012, 1820, 291–317. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Loureiro, J.A.; Coelho, M.A.N.; Pereira, M.C. Transferrin Receptor-Targeted Nanocarriers: Overcoming Barriers to Treat Glioblastoma. Pharmaceutics 2022, 14, 279. [Google Scholar] [CrossRef]
- Friden, P.M.; Walus, L.R.; Musso, G.F.; Taylor, M.A.; Malfroy, B.; Starzyk, R.M. Anti-transferrin receptor antibody and antibody-drug conjugates cross the blood-brain barrier. Proc. Natl. Acad. Sci. USA 1991, 88, 4771–4775. [Google Scholar] [CrossRef]
- van Lengerich, B.; Zhan, L.; Xia, D.; Chan, D.; Joy, D.; Park, J.I.; Tatarakis, D.; Calvert, M.; Hummel, S.; Lianoglou, S.; et al. A TREM2-activating antibody with a blood-brain barrier transport vehicle enhances microglial metabolism in Alzheimer’s disease models. Nat. Neurosci. 2023, 26, 416–429. [Google Scholar] [CrossRef]
- Barker, S.J.; Thayer, M.B.; Kim, C.; Tatarakis, D.; Simon, M.J.; Dial, R.; Nilewski, L.; Wells, R.C.; Zhou, Y.; Afetian, M. Targeting the transferrin receptor to transport antisense oligonucleotides across the mammalian blood-brain barrier. Sci. Transl. Med. 2024, 16, eadi2245. [Google Scholar] [CrossRef]
- Yue, P.-J.; He, L.; Qiu, S.-W.; Li, Y.; Liao, Y.-J.; Li, X.-P.; Xie, D.; Peng, Y. OX26/CTX-conjugated PEGylated liposome as a dual-targeting gene delivery system for brain glioma. Mol. Cancer 2014, 13, 191. [Google Scholar] [CrossRef]
- Haqqani, A.S.; Thom, G.; Burrell, M.; Delaney, C.E.; Brunette, E.; Baumann, E.; Sodja, C.; Jezierski, A.; Webster, C.; Stanimirovic, D.B. Intracellular sorting and transcytosis of the rat transferrin receptor antibody OX26 across the blood-brain barrier in vitro is dependent on its binding affinity. J. Neurochem. 2018, 146, 735–752. [Google Scholar] [CrossRef]
- Gosk, S.; Vermehren, C.; Storm, G.; Moos, T. Targeting anti-transferrin receptor antibody (OX26) and OX26-conjugated liposomes to brain capillary endothelial cells using in situ perfusion. J. Cereb. Blood Flow Metab. 2004, 24, 1193–1204. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Pardridge, W.M. Biotin delivery to brain with a covalent conjugate of avidin and a monoclonal antibody to the transferrin receptor. J. Pharmacol. Exp. Ther. 1992, 263, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Bickel, U.; Yoshikawa, T.; Landaw, E.M.; Faull, K.F.; Pardridge, W.M. Pharmacologic effects in vivo in brain by vector-mediated peptide drug delivery. Proc. Natl. Acad. Sci. USA 1993, 90, 2618–2622. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef]
- Olsman, M.; Sereti, V.; Mühlenpfordt, M.; Johnsen, K.B.; Andresen, T.L.; Urquhart, A.J.; Davies, C.D.L. Focused Ultrasound and Microbubble Treatment Increases Delivery of Transferrin Receptor-Targeting Liposomes to the Brain. Ultrasound Med. Biol. 2021, 47, 1343–1355. [Google Scholar] [CrossRef]
- Sela, M.; Poley, M.; Mora-Raimundo, P.; Kagan, S.; Avital, A.; Kaduri, M.; Chen, G.; Adir, O.; Rozencweig, A.; Weiss, Y.; et al. Brain-Targeted Liposomes Loaded with Monoclonal Antibodies Reduce Alpha-Synuclein Aggregation and Improve Behavioral Symptoms in Parkinson’s Disease. Adv. Mater. 2023, 35, e2304654. [Google Scholar] [CrossRef]
- Gabold, B.; Adams, F.; Brameyer, S.; Jung, K.; Ried, C.L.; Merdan, T.; Merkel, O.M. Transferrin-modified chitosan nanoparticles for targeted nose-to-brain delivery of proteins. Drug Deliv. Transl. Res. 2023, 13, 822–838. [Google Scholar] [CrossRef]
- Marrocco, F.; Falvo, E.; Mosca, L.; Tisci, G.; Arcovito, A.; Reccagni, A.; Limatola, C.; Bernardini, R.; Ceci, P.; D’Alessandro, G.; et al. Nose-to-brain selective drug delivery to glioma via ferritin-based nanovectors reduces tumor growth and improves survival rate. Cell Death Dis. 2024, 15, 262. [Google Scholar] [CrossRef] [PubMed]
- Sonkar, R.; Sonali; Jha, A.; Viswanadh, M.K.; Burande, A.S.; Narendra; Pawde, D.M.; Patel, K.K.; Singh, M.; Koch, B.; et al. Gold liposomes for brain-targeted drug delivery: Formulation and brain distribution kinetics. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111652. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Johnsen, K.B.; Kucharz, K.; Lauritzen, M.; Moos, T. Blood-Brain Barrier Transport of Transferrin Receptor-Targeted Nanoparticles. Pharmaceutics 2022, 14, 2237. [Google Scholar] [CrossRef]
- Annu; Sartaj, A.; Qamar, Z.; Aldosari, E.; Baboota, S.; Ali, J. Intranasal delivery of transferrin conjugated chitosan nanoparticles to the brain: A comparative analysis of conjugated and un-conjugated nanoparticles in vivo. J. Drug Deliv. Sci. Technol. 2025, 104, 106550. [Google Scholar] [CrossRef]
- Yun, H.; Su, W.; You, T.; Wang, J.; Ying, Y.; Wang, C.; Ren, Y.; Lu, B.; Li, Y.; Liu, C. Boosting physical performance in SD rats through brain-targeted delivery of caffeine-loaded transferrin liposomes. Heliyon 2024, 10, e34617. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; van der Putten, L.; Queiroz, J.F.; Pinheiro, M.; Reis, S. Transferrin-functionalized lipid nanoparticles for curcumin brain delivery. J. Biotechnol. 2021, 331, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Cepparulo, P.; Cuomo, O.; Campani, V.; Vinciguerra, A.; Sisalli, M.J.; Nele, V.; Anzilotti, S.; Valsecchi, V.; Casamassa, A.; Brancaccio, P.; et al. Anti-miRNA103/107 encapsulated in transferrin-conjugated lipid nanoparticles crosses blood-brain barrier and reduces brain ischemic damage. Mol. Ther.-Nucleic Acids 2024, 35, 102131. [Google Scholar] [CrossRef]
- Chauhan, M.; Singh, R.P.; Sonali; Yadav, B.; Shekhar, S.; Kumar, L.; Mehata, A.K.; Jhawat, V.; Dutt, R.; Garg, V.; et al. Dual-targeted transferrin and AS1411 aptamer conjugated micelles for improved therapeutic efficacy and imaging of brain cancer. Colloids Surf. B Biointerfaces 2023, 231, 113544. [Google Scholar] [CrossRef]
- Jain, D.; Hasan, N.; Zafar, S.; Thakur, J.; Haider, K.; Parvez, S.; Ahmad, F.J. Transferrin functionalized nanostructured lipid carriers for targeting Rivastigmine and Resveratrol to Alzheimer’s disease: Synthesis, in vitro characterization and brain uptake analysis. J. Drug Deliv. Sci. Technol. 2023, 86, 104555. [Google Scholar] [CrossRef]
- Hançer, N.J.; Qiu, W.; Cherella, C.; Li, Y.; Copps, K.D.; White, M.F. Insulin and metabolic stress stimulate multisite serine/threonine phosphorylation of insulin receptor substrate 1 and inhibit tyrosine phosphorylation. J. Biol. Chem. 2014, 289, 12467–12484. [Google Scholar] [CrossRef]
- Ohtsuki, S. Insulin receptor at the blood-brain barrier: Transport and signaling. Vitam Horm. 2024, 126, 113–124. [Google Scholar]
- Alata, W.; Yogi, A.; Brunette, E.; Delaney, C.E.; van Faassen, H.; Hussack, G.; Iqbal, U.; Kemmerich, K.; Haqqani, A.S.; Moreno, M.J. Targeting insulin-like growth factor-1 receptor (IGF1R) for brain delivery of biologics. FASEB J. 2022, 36, e22208. [Google Scholar] [CrossRef]
- Yogi, A.; Hussack, G.; van Faassen, H.; Haqqani, A.S.; Delaney, C.E.; Brunette, E.; Sandhu, J.K.; Hewitt, M.; Sulea, T.; Kemmerich, K.; et al. Brain Delivery of IGF1R5, a Single-Domain Antibody Targeting Insulin-like Growth Factor-1 Receptor. Pharmaceutics 2022, 14, 1452. [Google Scholar] [CrossRef]
- Kamei, N.; Ikeda, K.; Ohmoto, Y.; Fujisaki, S.; Shirata, R.; Maki, M.; Miyata, M.; Miyauchi, Y.; Nishiyama, N.; Yamada, M.; et al. Insulin-inspired hippocampal neuron-targeting technology for protein drug delivery. Proc. Natl. Acad. Sci. USA 2024, 121, e2407936121. [Google Scholar] [CrossRef]
- Ulbrich, K.; Knobloch, T.; Kreuter, J. Targeting the insulin receptor: Nanoparticles for drug delivery across the blood–brain barrier (BBB). J. Drug Target. 2011, 19, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Treatment of Parkinson’s disease with biologics that penetrate the blood-brain barrier via receptor-mediated transport. Front. Aging Neurosci. 2023, 15, 1276376. [Google Scholar] [CrossRef] [PubMed]
- Boado, R.J.; Ka-Wai Hui, E.; Zhiqiang Lu, J.; Pardridge, W.M. Insulin receptor antibody-iduronate 2-sulfatase fusion protein: Pharmacokinetics, anti-drug antibody, and safety pharmacology in Rhesus monkeys. Biotechnol. Bioeng. 2014, 111, 2317–2325. [Google Scholar] [CrossRef]
- Shen, H.; Gu, X.; Wei, Z.Z.; Wu, A.; Liu, X.; Wei, L. Combinatorial intranasal delivery of bone marrow mesenchymal stem cells and insulin-like growth factor-1 improves neurovascularization and functional outcomes following focal cerebral ischemia in mice. Exp. Neurol. 2021, 337, 113542. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Ma, J.; Feng, X.; Chen, J.; Dong, B.H.; Xiao, L.; Zhang, X.; Guo, B. Caffeic acid and diabetic neuropathy: Investigating protective effects and insulin-like growth factor 1 (IGF-1)-related antioxidative and anti-inflammatory mechanisms in mice. Heliyon 2024, 10, e32623. [Google Scholar] [CrossRef]
- Engel, M.G.; Narayan, S.; Cui, M.-H.; Branch, C.A.; Zhang, X.; Gandy, S.E.; Ehrlich, M.; Huffman, D.M. Intranasal long R3 insulin-like growth factor-1 treatment promotes amyloid plaque remodeling in cerebral cortex but fails to preserve cognitive function in male 5XFAD mice. J. Alzheimer’s Dis. 2025, 103, 113–126. [Google Scholar] [CrossRef]
- Ferrer, P.R.; Sakiyama-Elbert, S. Acrylic Acid Modified Poly-ethylene Glycol Microparticles for Affinity-Based release of Insulin-Like Growth Factor-1 in Neural Applications. bioRxiv 2024. [Google Scholar] [CrossRef]
- Go, G.W.; Mani, A. Low-density lipoprotein receptor (LDLR) family orchestrates cholesterol homeostasis. Yale J. Biol. Med. 2012, 85, 19–28. [Google Scholar]
- Demeule, M.; Currie, J.-C.; Bertrand, Y.; Ché, C.; Nguyen, T.; Régina, A.; Gabathuler, R.; Castaigne, J.-P.; Béliveau, R. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector Angiopep-2. J. Neurochem. 2008, 106, 1534–1544. [Google Scholar] [CrossRef]
- Méresse, S.; Delbart, C.; Fruchart, J.-C.; Cecchelli, R. Low-Density Lipoprotein Receptor on Endothelium of Brain Capillaries. J. Neurochem. 1989, 53, 340–345. [Google Scholar] [CrossRef]
- Jeon, H.; Blacklow, S.C. Structure and physiologic function of the low-density lipoprotein receptor. Annu. Rev. Biochem. 2005, 74, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Defesche, J.C. Low-Density Lipoprotein Receptor-Its Structure, Function, and Mutations, Seminars in Vascular Medicine; Thieme Medical Publishers, Inc.: New York, NY, USA, 2004; pp. 5–11. [Google Scholar]
- Gent, J.; Braakman, I. Low-density lipoprotein receptor structure and folding. Cell. Mol. Life Sci. CMLS 2004, 61, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Iijima, H.; Goto, K.; Sakai, J.; Ishii, H.; Kim, H.-J.; Suzuki, H.; Kondo, H.; Saeki, S.; Yamamoto, T. Human Apolipoprotein E Receptor 2: A novel lipoprotein receptor of the low density lipoprotein receptor family predominantly expressed in brain. J. Biol. Chem. 1996, 271, 8373–8380. [Google Scholar] [CrossRef]
- Herz, J. Apolipoprotein E receptors in the nervous system. Curr. Opin. Lipidol. 2009, 20, 190–196. [Google Scholar] [CrossRef]
- Mastrantuono, E.; Ghibaudi, M.; Matias, D.; Battaglia, G. The multifaceted therapeutical role of low-density lipoprotein receptor family in high-grade glioma. Mol. Oncol. 2024, 18, 2966–2976. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, D.; Zhao, J.; Song, J.; Zhao, Y. The role of the low-density lipoprotein receptor-related protein 1 (LRP-1) in regulating blood-brain barrier integrity. Rev. Neurosci. 2016, 27, 623–634. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, X.; Gong, M.; Zhang, J. Delivery of a peptide-drug conjugate targeting the blood brain barrier improved the efficacy of paclitaxel against glioma. Oncotarget 2016, 7, 79401–79407. [Google Scholar] [CrossRef]
- Guo, Q.; Zhu, Q.; Miao, T.; Tao, J.; Ju, X.; Sun, Z.; Li, H.; Xu, G.; Chen, H.; Han, L. LRP1-upregulated nanoparticles for efficiently conquering the blood-brain barrier and targetedly suppressing multifocal and infiltrative brain metastases. J. Control Release 2019, 303, 117–129. [Google Scholar] [CrossRef]
- Han, E.L.; Tang, S.; Kim, D.; Murray, A.M.; Swingle, K.L.; Hamilton, A.G.; Mrksich, K.; Padilla, M.S.; Palanki, R.; Li, J.J. Peptide-Functionalized Lipid Nanoparticles for Targeted Systemic mRNA Delivery to the Brain. Nano Lett. 2024, 25, 800–810. [Google Scholar] [CrossRef]
- Pawar, S.; Koneru, T.; McCord, E.; Tatiparti, K.; Sau, S.; Iyer, A.K. LDL receptors and their role in targeted therapy for glioma: A review. Drug Discov. Today 2021, 26, 1212–1225. [Google Scholar] [CrossRef]
- Di, L.; Maiseyeu, A. Low-density lipoprotein nanomedicines: Mechanisms of targeting, biology, theranostic potential. Drug Deliv. 2021, 28, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Kim, E.H.; Chang, W.-S.; Lee, W.-S.; Kim, K.-H.; Kim, J.-K. Enhanced proton treatment with a LDLR-ligand peptide-conjugated gold nanoparticles targeting the tumor microenvironment in an infiltrative brain tumor model. Am. J. Cancer Res. 2022, 12, 198. [Google Scholar] [PubMed]
- Li, B.; Chen, X.; Qiu, W.; Zhao, R.; Duan, J.; Zhang, S.; Pan, Z.; Zhao, S.; Guo, Q.; Qi, Y.; et al. Synchronous Disintegration of Ferroptosis Defense Axis via Engineered Exosome-Conjugated Magnetic Nanoparticles for Glioblastoma Therapy. Adv. Sci. 2022, 9, 2105451. [Google Scholar] [CrossRef]
- Dmello, C.; Brenner, A.; Piccioni, D.; Wen, P.Y.; Drappatz, J.; Mrugala, M.; Lewis, L.D.; Schiff, D.; Fadul, C.E.; Chamberlain, M.; et al. Phase II trial of blood-brain barrier permeable peptide-paclitaxel conjugate ANG1005 in patients with recurrent high-grade glioma. Neurooncol. Adv. 2024, 6, vdae186. [Google Scholar] [CrossRef]
- Kurzrock, R.; Gabrail, N.; Chandhasin, C.; Moulder, S.; Smith, C.; Brenner, A.; Sankhala, K.; Mita, A.; Elian, K.; Bouchard, D.; et al. Safety, Pharmacokinetics, and Activity of GRN1005, a Novel Conjugate of Angiopep-2, a Peptide Facilitating Brain Penetration, and Paclitaxel, in Patients with Advanced Solid Tumors. Mol. Cancer Ther. 2012, 11, 308–316. [Google Scholar] [CrossRef]
- Wünsch, A.; Mulac, D.; Langer, K. Lipoprotein imitating nanoparticles: Lecithin coating binds ApoE and mediates non-lysosomal uptake leading to transcytosis over the blood-brain barrier. Int. J. Pharm. 2020, 589, 119821. [Google Scholar] [CrossRef]
- Sankari, S.S.; Urade, R.; Chiu, C.-C.; Wang, L.-F. Neuropeptide-functionalized gold nanorod enhanced cellular uptake and improved in vitro photothermal killing in LRP1-positive glioma cells. Pharmaceutics 2022, 14, 1939. [Google Scholar] [CrossRef]
- Liang, J.; Li, L.; Tian, H.; Wang, Z.; Liu, G.; Duan, X.; Guo, M.; Liu, J.; Zhang, W.; Nice, E.C.; et al. Drug Repurposing-Based Brain-Targeting Self-Assembly Nanoplatform Using Enhanced Ferroptosis against Glioblastoma. Small 2023, 19, 2303073. [Google Scholar] [CrossRef]
- Thomas, F.C.; Taskar, K.; Rudraraju, V.; Goda, S.; Thorsheim, H.R.; Gaasch, J.A.; Mittapalli, R.K.; Palmieri, D.; Steeg, P.S.; Lockman, P.R.; et al. Uptake of ANG1005, A Novel Paclitaxel Derivative, Through the Blood-Brain Barrier into Brain and Experimental Brain Metastases of Breast Cancer. Pharm. Res. 2009, 26, 2486–2494. [Google Scholar] [CrossRef]
- Fritzen, L.; Wienken, K.; Wagner, L.; Kurtyka, M.; Vogel, K.; Körbelin, J.; Weggen, S.; Fricker, G.; Pietrzik, C.U. Truncated mini LRP1 transports cargo from luminal to basolateral side across the blood brain barrier. Fluids Barriers CNS 2024, 21, 74. [Google Scholar] [CrossRef]
- Awad, S.; Araújo, M.; Faria, P.; Sarmento, B.; Martins, C. Chemical engineering of zein with polyethylene glycol and Angiopep-2 to manufacture a brain-targeted docetaxel nanomedicine for glioblastoma treatment. Drug Deliv. Transl. Res. 2024, 14, 3585–3598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.A.; Xin, X.; Liu, C.; Liu, Y.H.; Duan, H.X.; Qi, L.L.; Zhang, Y.Y.; Zhao, H.M.; Chen, L.Q.; Jin, M.J.; et al. Novel brain-targeted nanomicelles for anti-glioma therapy mediated by the ApoE-enriched protein corona in vivo. J. Nanobiotechnol. 2021, 19, 453. [Google Scholar] [CrossRef] [PubMed]
- Tien, J.; Leonoudakis, D.; Petrova, R.; Trinh, V.; Taura, T.; Sengupta, D.; Jo, L.; Sho, A.; Yun, Y.; Doan, E.; et al. Modifying antibody-FcRn interactions to increase the transport of antibodies through the blood-brain barrier. mAbs 2023, 15, 2229098. [Google Scholar] [CrossRef] [PubMed]
- Caram-Salas, N.; Boileau, E.; Farrington, G.K.; Garber, E.; Brunette, E.; Abulrob, A.; Stanimirovic, D. In vitro and in vivo methods for assessing FcRn-mediated reverse transcytosis across the blood-brain barrier. Methods Mol. Biol. 2011, 763, 383–401. [Google Scholar]
- Jensen, P.F.; Schoch, A.; Larraillet, V.; Hilger, M.; Schlothauer, T.; Emrich, T.; Rand, K.D. A two-pronged binding mechanism of IgG to the neonatal Fc receptor controls complex stability and IgG serum half-life. Mol. Cell. Proteom. 2017, 16, 451–456. [Google Scholar] [CrossRef]
- Sand, K.M.; Bern, M.; Nilsen, J.; Noordzij, H.T.; Sandlie, I.; Andersen, J.T. Unraveling the Interaction between FcRn and Albumin: Opportunities for Design of Albumin-Based Therapeutics. Front. Immunol. 2014, 5, 682. [Google Scholar] [CrossRef]
- Holst, M.R.; de Wit, N.M.; Ozgür, B.; Brachner, A.; Hyldig, K.; Appelt-Menzel, A.; Sleven, H.; Cader, Z.; de Vries, H.E.; Neuhaus, W. Subcellular trafficking and transcytosis efficacy of different receptor types for therapeutic antibody delivery at the blood–brain barrier. Fluids Barriers CNS 2023, 20, 82. [Google Scholar] [CrossRef]
- Simonneau, C.; Duschmalé, M.; Gavrilov, A.; Brandenberg, N.; Hoehnel, S.; Ceroni, C.; Lassalle, E.; Kassianidou, E.; Knoetgen, H.; Niewoehner, J.; et al. Investigating receptor-mediated antibody transcytosis using blood-brain barrier organoid arrays. Fluids Barriers CNS 2021, 18, 43. [Google Scholar] [CrossRef]
- Mhaske, A.; Shukla, S.; Ahirwar, K.; Singh, K.K.; Shukla, R. Receptor-Assisted Nanotherapeutics for Overcoming the Blood-Brain Barrier. Mol. Neurobiol. 2024, 61, 8702–8738. [Google Scholar] [CrossRef]
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing albumin as a carrier for cancer therapies. Adv. Drug Deliv. Rev. 2018, 130, 73–89. [Google Scholar] [CrossRef]
- Lin, T.; Zhao, P.; Jiang, Y.; Tang, Y.; Jin, H.; Pan, Z.; He, H.; Yang, V.C.; Huang, Y. Blood-Brain-Barrier-Penetrating Albumin Nanoparticles for Biomimetic Drug Delivery via Albumin-Binding Protein Pathways for Antiglioma Therapy. ACS Nano 2016, 10, 9999–10012. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Yan, J.; Forscher, C.; Hendifar, A. Aldoxorubicin: A tumor-targeted doxorubicin conjugate for relapsed or refractory soft tissue sarcomas. Drug Des. Dev. Ther. 2018, 12, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Pyzik, M.; Kozicky, L.K.; Gandhi, A.K.; Blumberg, R.S. The therapeutic age of the neonatal Fc receptor. Nat. Rev. Immunol. 2023, 23, 415–432. [Google Scholar] [CrossRef]
- Cooper, P.R.; Ciambrone, G.J.; Kliwinski, C.M.; Maze, E.; Johnson, L.; Li, Q.; Feng, Y.; Hornby, P.J. Efflux of monoclonal antibodies from rat brain by neonatal Fc receptor, FcRn. Brain Res. 2013, 1534, 13–21. [Google Scholar] [CrossRef]
- Schellhammer, L.; Beffinger, M.; Salazar, U.; Laman, J.D.; Buch, T.; vom Berg, J. Exit pathways of therapeutic antibodies from the brain and retention strategies. iScience 2023, 26, 108132. [Google Scholar] [CrossRef]
- Chevaleyre, C.; Novell, A.; Tournier, N.; Dauba, A.; Dubois, S.; Kereselidze, D.; Selingue, E.; Jego, B.; Maillère, B.; Larrat, B.; et al. Efficient PD-L1 imaging of murine glioblastoma with FUS-aided immunoPET by leveraging FcRn-antibody interaction. Theranostics 2023, 13, 5584–5596. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).