Novel Delivery of Cyclic-Diguanylate Monophosphate Utilizing Amyloid Depots
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Development of Receptor-Interacting Protein Peptide and Cyclic-di-GMP Amyloid Formulation
2.4. Development of Lysozyme and Cyclic-di-GMP Amyloid Formulation
2.5. Size, Surface Charge, and Morphology
2.6. Thioflavin T (ThT) Fluorescence
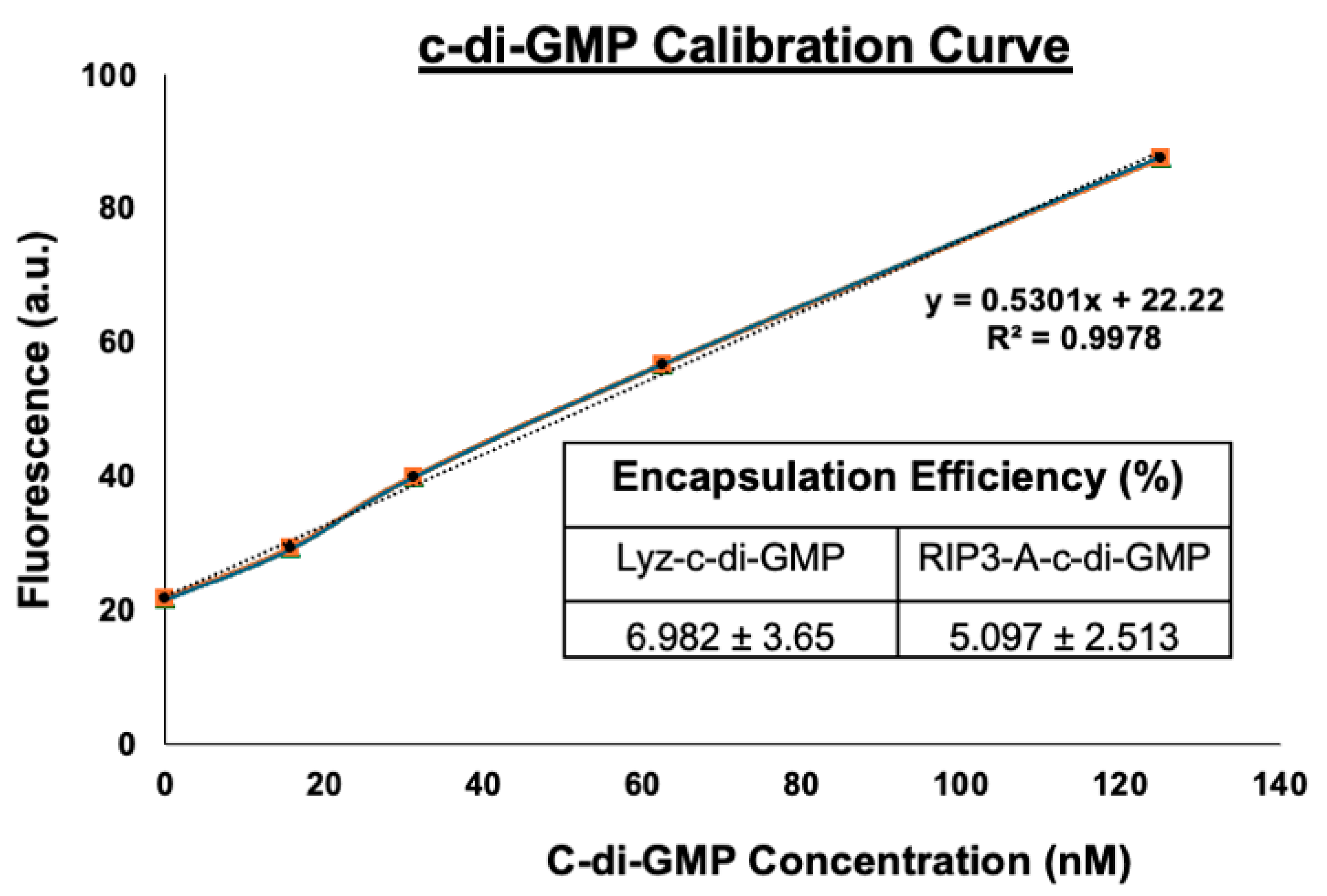
2.7. Cyclic-di-GMP Calibration and Encapsulation
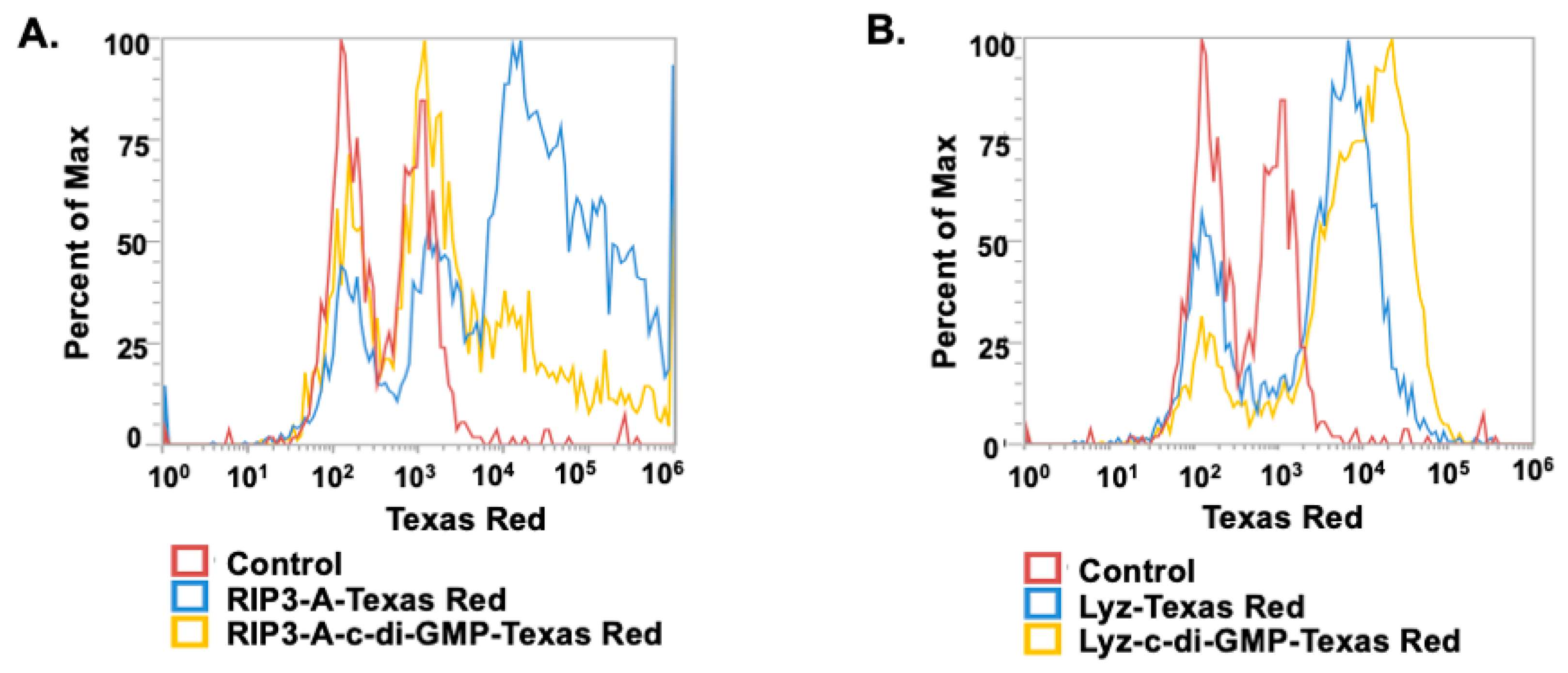
2.8. Cellular Uptake
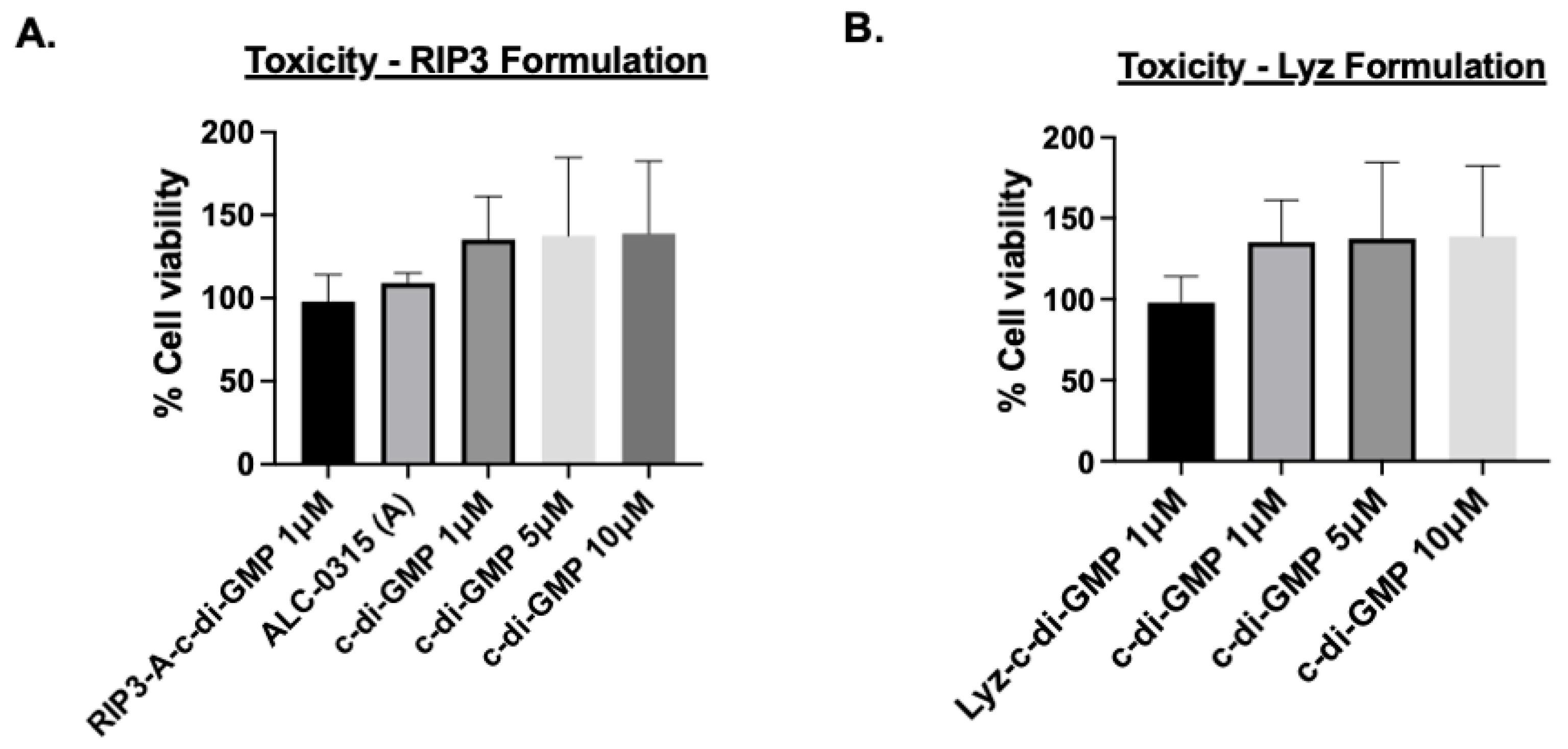
2.9. Cellular Toxicity
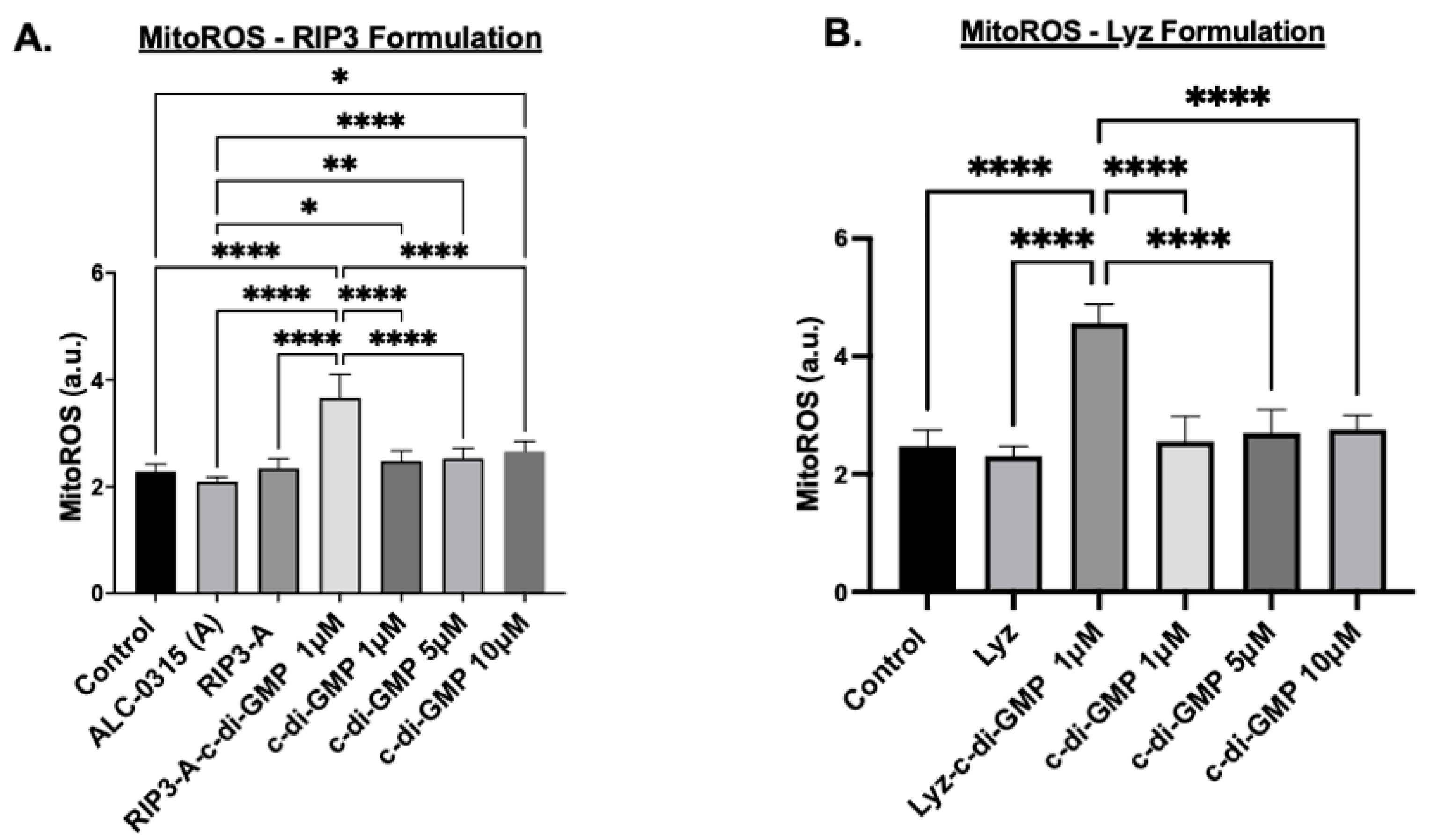
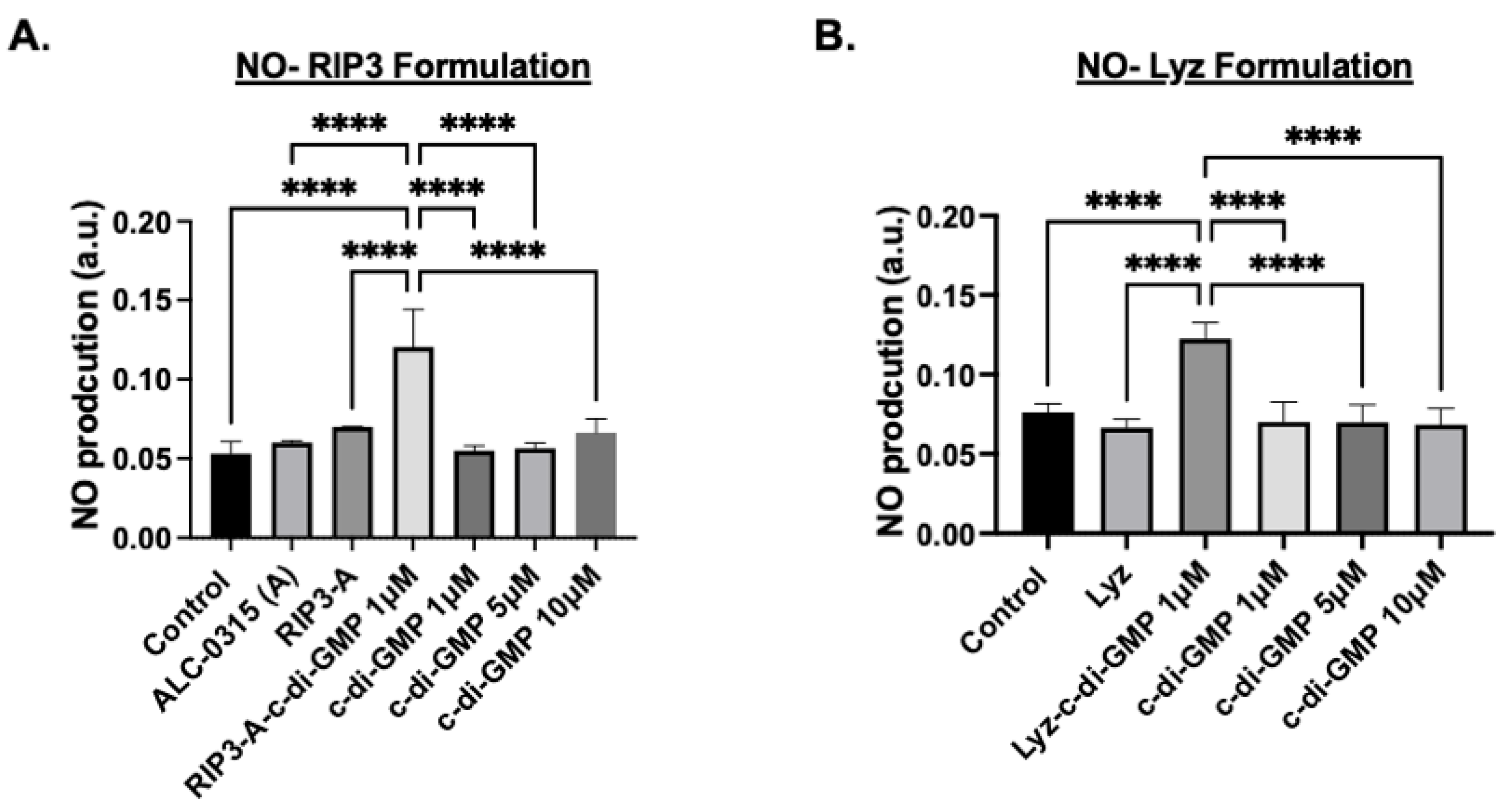
2.10. Nitric Oxide (NO) Assay and Mitochondrial ROS (MitoROS)
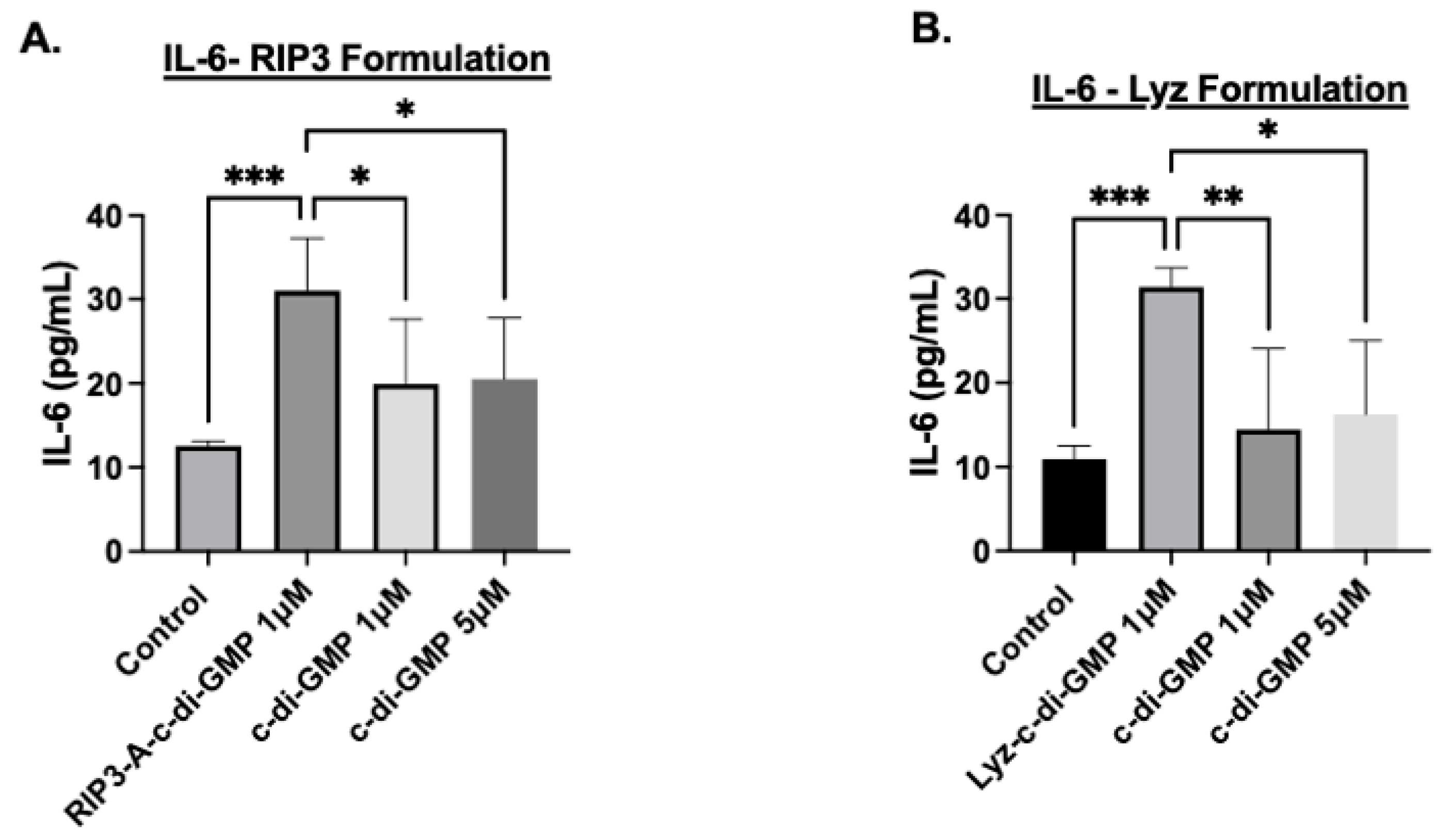
2.11. IL-6 ELISA Assay
2.12. Statistical Analysis
3. Results
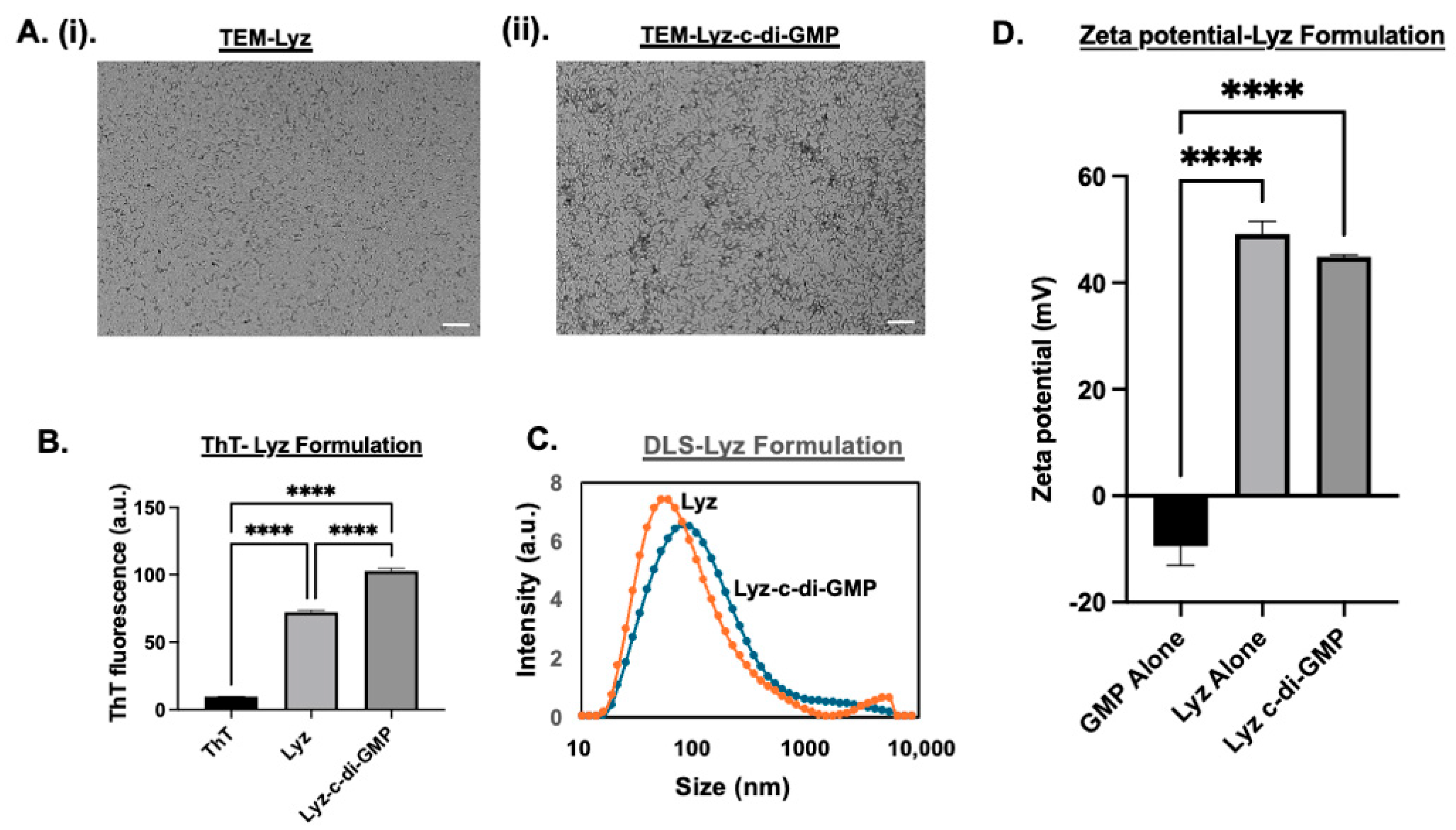
3.1. Characterization and Efficacy Studies
3.1.1. Physicochemical Characterization
3.1.2. Cellular Efficacy Studies
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romling, U.; Amikam, D. Cyclic di-GMP as a second messenger. Curr. Opin. Microbiol. 2006, 9, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Miyabe, H.; Hyodo, M.; Sato, Y.; Hayakawa, Y.; Harashima, H. Liposomes loaded with a STING pathway ligand, cyclic di-GMP, enhance cancer immunotherapy against metastatic melanoma. J. Control Release 2015, 216, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Karaolis, D.K.; Means, T.K.; Yang, D.; Takahashi, M.; Yoshimura, T.; Muraille, E.; Philpott, D.; Schroeder, J.T.; Hyodo, M.; Hayakawa, Y.; et al. Bacterial c-di-GMP is an immunostimulatory molecule. J. Immunol. 2007, 178, 2171–2181. [Google Scholar] [CrossRef]
- Woo, S.R.; Fuertes, M.B.; Corrales, L.; Spranger, S.; Furdyna, M.J.; Leung, M.Y.; Duggan, R.; Wang, Y.; Barber, G.N.; Fitzgerald, K.A.; et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 2014, 41, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Burdette, D.L.; Monroe, K.M.; Sotelo-Troha, K.; Iwig, J.S.; Eckert, B.; Hyodo, M.; Hayakawa, Y.; Vance, R.E. STING is a direct innate immune sensor of cyclic di-GMP. Nature 2011, 478, 515–518. [Google Scholar] [CrossRef]
- Shu, C.; Yi, G.; Watts, T.; Kao, C.C.; Li, P. Structure of STING bound to cyclic di-GMP reveals the mechanism of cyclic dinucleotide recognition by the immune system. Nat. Struct. Mol. Biol. 2012, 19, 722–724. [Google Scholar] [CrossRef]
- Wang, H.H.; Tsourkas, A. Cytosolic delivery of inhibitory antibodies with cationic lipids. Proc. Natl. Acad. Sci. USA 2019, 116, 22132–22139. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Xiao, M.; Zhang, Q.; Liu, Z.; Liu, M.; Zhang, P.; Zeng, Y. Peptide nanotube loaded with a STING agonist, c-di-GMP, enhance cancer immunotherapy against melanoma. Nano Res. 2023, 16, 5206–5215. [Google Scholar] [CrossRef]
- Chen, Y.P.; Xu, L.; Tang, T.W.; Chen, C.H.; Zheng, Q.H.; Liu, T.P.; Mou, C.Y.; Wu, C.H.; Wu, S.H. STING Activator c-di-GMP-Loaded Mesoporous Silica Nanoparticles Enhance Immunotherapy Against Breast Cancer. ACS Appl. Mater. Interfaces 2020, 12, 56741–56752. [Google Scholar] [CrossRef]
- Abdelrahman, S.; Alghrably, M.; Lachowicz, J.I.; Emwas, A.H.; Hauser, C.A.E.; Jaremko, M. “What Doesn’t Kill You Makes You Stronger”: Future Applications of Amyloid Aggregates in Biomedicine. Molecules 2020, 25, 5245. [Google Scholar] [CrossRef]
- Li, J.; McQuade, T.; Siemer, A.B.; Napetschnig, J.; Moriwaki, K.; Hsiao, Y.S.; Damko, E.; Moquin, D.; Walz, T.; McDermott, A.; et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 2012, 150, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, Y.; Xie, J.; Qu, X.; Liu, C. Lysozyme Amyloid Fibril-Integrated PEG Injectable Hydrogel Adhesive with Improved Antiswelling and Antibacterial Capabilities. Biomacromolecules 2022, 23, 1376–1391. [Google Scholar] [CrossRef] [PubMed]
- Iconomidou, V.A.; Hamodrakas, S.J. Natural protective amyloids. Curr. Protein Pept. Sci. 2008, 9, 291–309. [Google Scholar] [CrossRef] [PubMed]
- Waku, T.; Tanaka, N. Recent advances in nanofibrous assemblies based on β-sheet-forming peptides for biomedical applications. Polym. Int. 2017, 66, 277–288. [Google Scholar] [CrossRef]
- Lai, Y.R.; Wang, S.S.; Hsu, T.L.; Chou, S.H.; How, S.C.; Lin, T.H. Application of Amyloid-Based Hybrid Membranes in Drug Delivery. Polymers 2023, 15, 1444. [Google Scholar] [CrossRef]
- Diaz-Caballero, M.; Fernandez, M.R.; Navarro, S.; Ventura, S. Prion-based nanomaterials and their emerging applications. Prion 2018, 12, 266–272. [Google Scholar] [CrossRef]
- Pena-Diaz, S.; Olsen, W.P.; Wang, H.; Otzen, D.E. Functional Amyloids: The Biomaterials of Tomorrow? Adv. Mater. 2024, 36, e2312823. [Google Scholar] [CrossRef]
- Torok, M. Amyloids in synthetic applications. Sci. Rep. 2022, 12, 21095. [Google Scholar] [CrossRef]
- Yang, L.; Li, H.; Yao, L.; Yu, Y.; Ma, G. Amyloid-Based Injectable Hydrogel Derived from Hydrolyzed Hen Egg White Lysozyme. ACS Omega 2019, 4, 8071–8080. [Google Scholar] [CrossRef]
- Swaminathan, R.; Ravi, V.K.; Kumar, S.; Kumar, M.V.; Chandra, N. Lysozyme: A model protein for amyloid research. Adv. Protein Chem. Struct. Biol. 2011, 84, 63–111. [Google Scholar] [CrossRef]
- Nawaz, N.; Wen, S.; Wang, F.; Nawaz, S.; Raza, J.; Iftikhar, M.; Usman, M. Lysozyme and Its Application as Antibacterial Agent in Food Industry. Molecules 2022, 27, 6305. [Google Scholar] [CrossRef]
- Derde, M.; Lechevalier, V.; Guerin-Dubiard, C.; Cochet, M.F.; Jan, S.; Baron, F.; Gautier, M.; Vie, V.; Nau, F. Hen egg white lysozyme permeabilizes Escherichia coli outer and inner membranes. J. Agric. Food Chem. 2013, 61, 9922–9929. [Google Scholar] [CrossRef]
- Morimoto, R.; Horita, M.; Yamaguchi, D.; Nakai, H.; Nakano, S.I. Evaluation of weak interactions of proteins and organic cations with DNA duplex structures. Biophys. J. 2022, 121, 2873–2881. [Google Scholar] [CrossRef]
- Lv, M.; Wang, M.; Lu, K.; Peng, L.; Zhao, Y. DNA/Lysozyme-binding affinity study of novel peptides from TAT (47–57) and BRCA1 (782–786) in vitro by spectroscopic analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 209, 109–117. [Google Scholar] [CrossRef]
- Steinrauf, L.K.; Shiuan, D.; Yang, W.J.; Chiang, M.Y. Lysozyme association with nucleic acids. Biochem. Biophys. Res. Commun. 1999, 266, 366–370. [Google Scholar] [CrossRef]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Ismail, M.; Kanapathipillai, M. Novel Ultrasound-Responsive Amyloid Formulation. Pharmaceuticals 2024, 17, 777. [Google Scholar] [CrossRef]
- Wu, X.L.; Hu, H.; Dong, X.Q.; Zhang, J.; Wang, J.; Schwieters, C.D.; Liu, J.; Wu, G.X.; Li, B.; Lin, J.Y.; et al. The amyloid structure of mouse RIPK3 (receptor interacting protein kinase 3) in cell necroptosis. Nat. Commun. 2021, 12, 1627. [Google Scholar] [CrossRef]
- Hu, H.; Wu, X.; Wu, G.; Nan, N.; Zhang, J.; Zhu, X.; Zhang, Y.; Shu, Z.; Liu, J.; Liu, X.; et al. RIP3-mediated necroptosis is regulated by inter-filament assembly of RIP homotypic interaction motif. Cell Death Differ. 2021, 28, 251–266. [Google Scholar] [CrossRef]
- Wu, X.; Ma, Y.; Zhao, K.; Zhang, J.; Sun, Y.; Li, Y.; Dong, X.; Hu, H.; Liu, J.; Wang, J.; et al. The structure of a minimum amyloid fibril core formed by necroptosis-mediating RHIM of human RIPK3. Proc. Natl. Acad. Sci. USA 2021, 118, e2022933118. [Google Scholar] [CrossRef]
- Alamgir, A.; Ghosal, S.; DeLisa, M.P.; Alabi, C.A. Bioreversible Anionic Cloaking Enables Intracellular Protein Delivery with Ionizable Lipid Nanoparticles. ACS Cent. Sci. 2024, 10, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Zuris, J.A.; Thompson, D.B.; Shu, Y.; Guilinger, J.P.; Bessen, J.L.; Hu, J.H.; Maeder, M.L.; Joung, J.K.; Chen, Z.Y.; Liu, D.R. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol. 2015, 33, 73–80. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Witzigmann, D.; Chen, S.; Cullis, P.R.; van der Meel, R. Lipid Nanoparticle Technology for Clinical Translation of siRNA Therapeutics. Acc. Chem. Res. 2019, 52, 2435–2444. [Google Scholar] [CrossRef]
- Frare, E.; Mossuto, M.F.; de Laureto, P.P.; Tolin, S.; Menzer, L.; Dumoulin, M.; Dobson, C.M.; Fontana, A. Characterization of oligomeric species on the aggregation pathway of human lysozyme. J. Mol. Biol. 2009, 387, 17–27. [Google Scholar] [CrossRef]
- Sooreshjani, M.A.; Gursoy, U.K.; Aryal, U.K.; Sintim, H.O. Proteomic analysis of RAW macrophages treated with cGAMP or c-di-GMP reveals differentially activated cellular pathways. RSC Adv. 2018, 8, 36840–36851. [Google Scholar] [CrossRef]
- Yildiz, S.; Alpdundar, E.; Gungor, B.; Kahraman, T.; Bayyurt, B.; Gursel, I.; Gursel, M. Enhanced immunostimulatory activity of cyclic dinucleotides on mouse cells when complexed with a cell-penetrating peptide or combined with CpG. Eur. J. Immunol. 2015, 45, 1170–1179. [Google Scholar] [CrossRef]
- Gauley, J.; Pisetsky, D.S. The release of microparticles by RAW 264.7 macrophage cells stimulated with TLR ligands. J. Leukoc. Biol. 2010, 87, 1115–1123. [Google Scholar] [CrossRef]
- Yan, W.; Yan, Y.; Luo, X.; Dong, Y.; Liang, G.; Miao, H.; Huang, Z.; Jiang, H. Lipopolysaccharide (LPS)-induced inflammation in RAW264.7 cells is inhibited by microRNA-494-3p via targeting lipoprotein-associated phospholipase A2. Eur. J. Trauma. Emerg. Surg. 2024, 50, 3289–3298. [Google Scholar] [CrossRef]
- Sawaya, M.R.; Hughes, M.P.; Rodriguez, J.A.; Riek, R.; Eisenberg, D.S. The expanding amyloid family: Structure, stability, function, and pathogenesis. Cell 2021, 184, 4857–4873. [Google Scholar] [CrossRef]
- Westermark, P.; Benson, M.D.; Buxbaum, J.N.; Cohen, A.S.; Frangione, B.; Ikeda, S.; Masters, C.L.; Merlini, G.; Saraiva, M.J.; Sipe, J.D. A primer of amyloid nomenclature. Amyloid 2007, 14, 179–183. [Google Scholar] [CrossRef]
- Yakupova, E.I.; Bobyleva, L.G.; Shumeyko, S.A.; Vikhlyantsev, I.M.; Bobylev, A.G. Amyloids: The History of Toxicity and Functionality. Biology 2021, 10, 394. [Google Scholar] [CrossRef] [PubMed]
- Kushnirov, V.V.; Vishnevskaya, A.B.; Alexandrov, I.M.; Ter-Avanesyan, M.D. Prion and nonprion amyloids: A comparison inspired by the yeast Sup35 protein. Prion 2007, 1, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Si, K.; Lindquist, S.; Kandel, E.R. A neuronal isoform of the aplysia CPEB has prion-like properties. Cell 2003, 115, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Guyonnet, B.; Egge, N.; Cornwall, G.A. Functional amyloids in the mouse sperm acrosome. Mol. Cell Biol. 2014, 34, 2624–2634. [Google Scholar] [CrossRef]
- Fowler, D.M.; Koulov, A.V.; Alory-Jost, C.; Marks, M.S.; Balch, W.E.; Kelly, J.W. Functional amyloid formation within mammalian tissue. PLoS Biol. 2006, 4, e6. [Google Scholar] [CrossRef]
- Cao, C.; Tian, L.; Li, J.; Raveendran, R.; Stenzel, M.H. Mix and Shake: A Mild Way to Drug-Loaded Lysozyme Nanoparticles. ACS Appl. Mater. Interfaces 2024, 16, 27177–27186. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, M.; Beluzo, B.; Chuikov, S.; Keshamouni, V.G.; Kanapathipillai, M. Novel Delivery of Cyclic-Diguanylate Monophosphate Utilizing Amyloid Depots. Pharmaceutics 2025, 17, 668. https://doi.org/10.3390/pharmaceutics17050668
Ismail M, Beluzo B, Chuikov S, Keshamouni VG, Kanapathipillai M. Novel Delivery of Cyclic-Diguanylate Monophosphate Utilizing Amyloid Depots. Pharmaceutics. 2025; 17(5):668. https://doi.org/10.3390/pharmaceutics17050668
Chicago/Turabian StyleIsmail, Maytham, Benjamin Beluzo, Sergei Chuikov, Venkateshwar G. Keshamouni, and Mathumai Kanapathipillai. 2025. "Novel Delivery of Cyclic-Diguanylate Monophosphate Utilizing Amyloid Depots" Pharmaceutics 17, no. 5: 668. https://doi.org/10.3390/pharmaceutics17050668
APA StyleIsmail, M., Beluzo, B., Chuikov, S., Keshamouni, V. G., & Kanapathipillai, M. (2025). Novel Delivery of Cyclic-Diguanylate Monophosphate Utilizing Amyloid Depots. Pharmaceutics, 17(5), 668. https://doi.org/10.3390/pharmaceutics17050668








