From Mechanism to Therapy: The Role of MSC-EVs in Alleviating Radiation-Induced Injuries
Abstract
1. Introduction
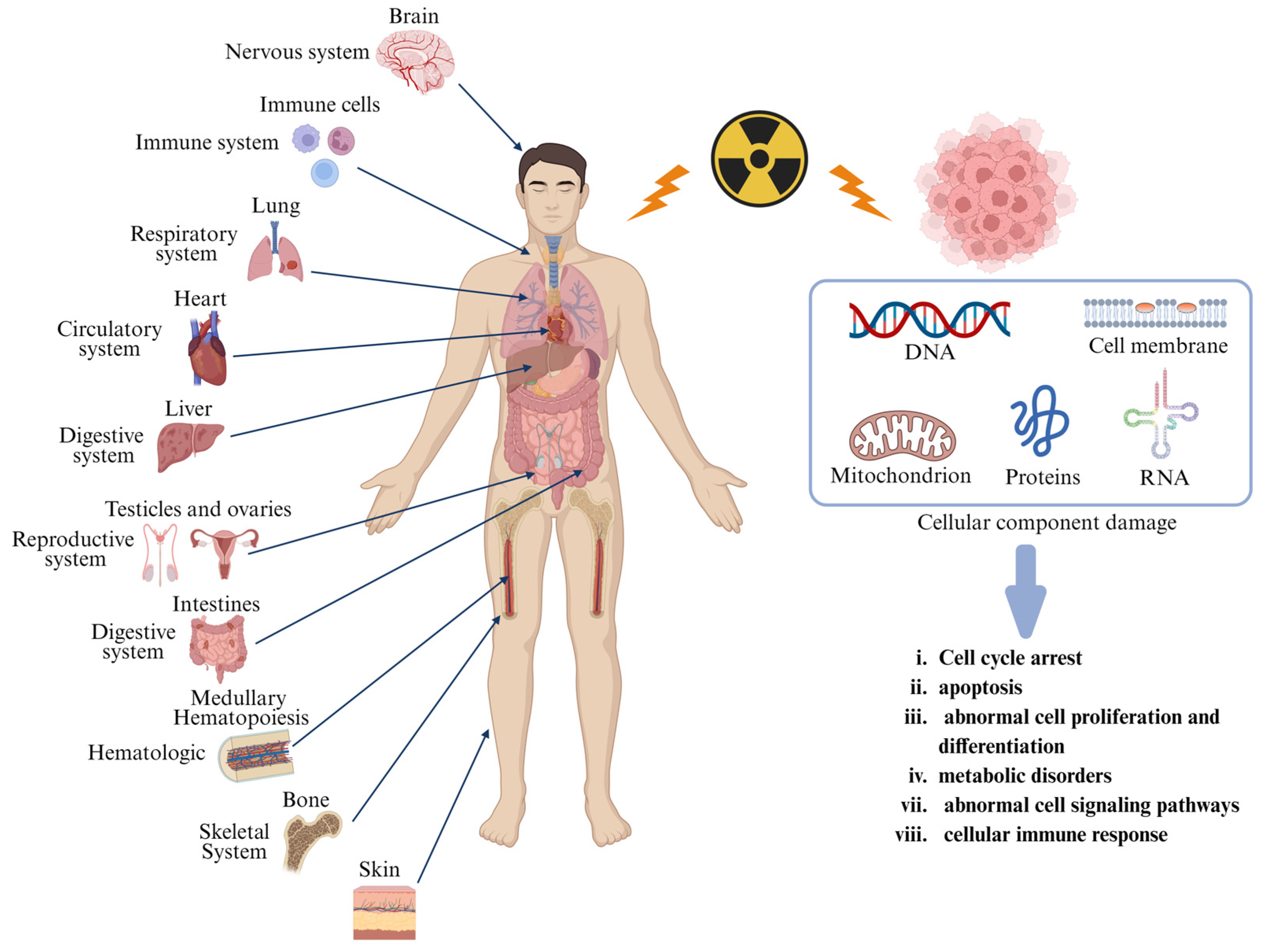
2. Pathophysiology and Current Therapeutic Landscape of Radiation Injury
2.1. Pathophysiology of Radiation Injury
2.2. Current Treatment of Radiation-Induced Diseases
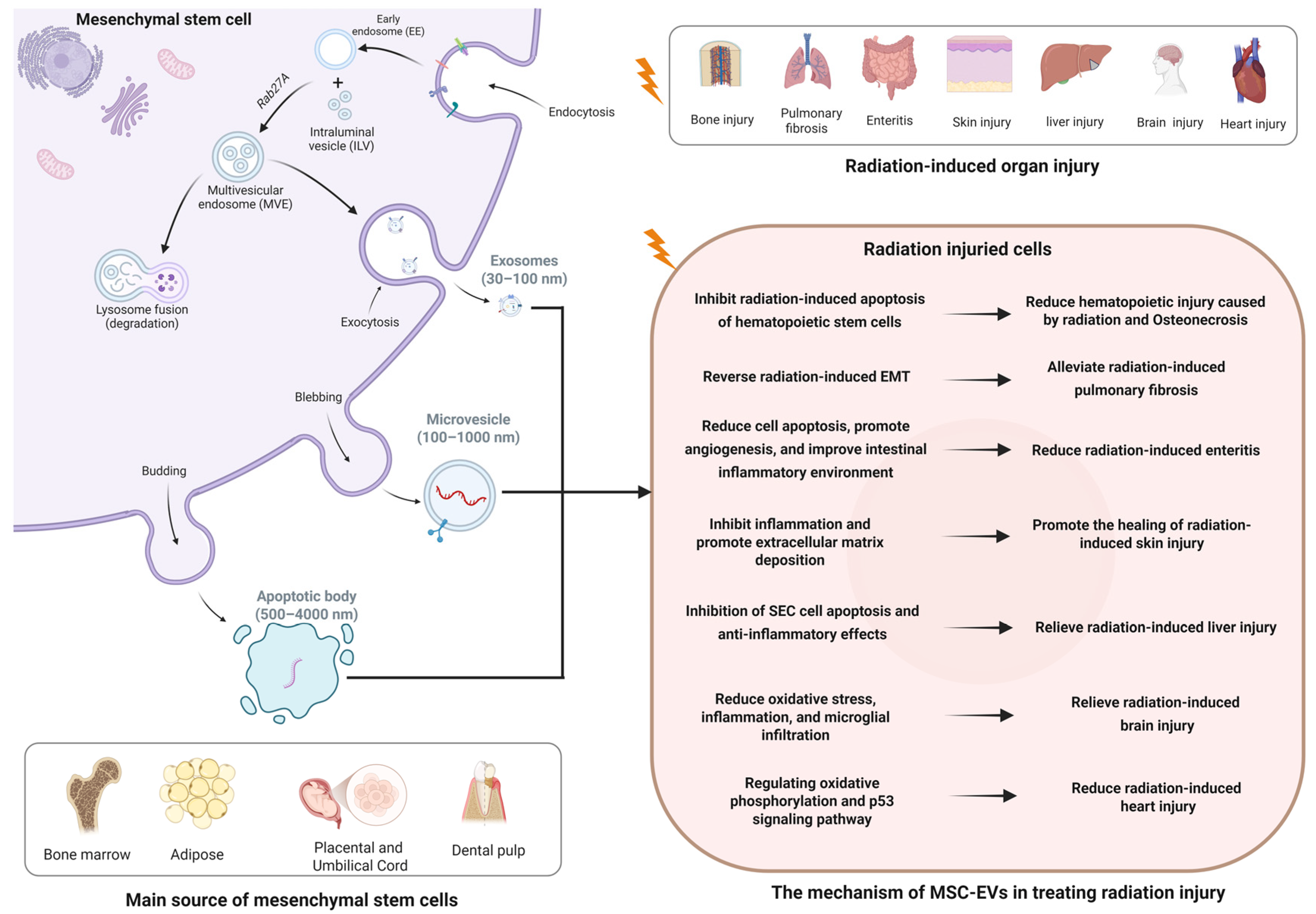
3. Biological Characteristics of MSC-EVs
4. Therapeutic Effects and Potential Mechanisms of MSC-EVs in Radiation Injury
4.1. Radiation-Induced Bone Injury
4.2. Radiation-Induced Lung Injury
4.3. Radiation-Induced Intestinal Injury
4.4. Radiation-Induced Skin Injury
4.5. Radiation-Induced Brain Injury
4.6. Radiation-Induced Cardiac Injury
4.7. Other Radiation-Induced Injuries
5. Clinical Development, Potential Limitations, and Challenges of EVs
5.1. Clinical Development of EVs
5.2. Potential Limitations and Challenges in the Clinical Translation of EVs
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, L.; Liang, Z.; Ma, S.; Li, L.; Liu, X. Radioprotective countermeasures for radiation injury (Review). Mol. Med. Rep. 2023, 27, 12953. [Google Scholar] [CrossRef] [PubMed]
- Ohba, T.; Tanigawa, K.; Liutsko, L. Evacuation after a nuclear accident: Critical reviews of past nuclear accidents and proposal for future planning. Environ. Int. 2021, 148, 106379. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T. Recent Trends in Medical Radiation Protection. Jpn. J. Radiol. Technol. 2022, 78, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Lebaron-Jacobs, L.; Herrera-Reyes, E. Basic concepts of radiation emergency medicine. J. Radiol. Prot. 2021, 41, S371–S390. [Google Scholar] [CrossRef]
- Jiao, Y.; Cao, F.; Liu, H. Radiation-induced Cell Death and Its Mechanisms. Health Phys. 2022, 123, 376–386. [Google Scholar] [CrossRef]
- Straub, J.M.; New, J.; Hamilton, C.D.; Lominska, C.; Shnayder, Y.; Thomas, S.M. Radiation-induced fibrosis: Mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1985–1994. [Google Scholar] [CrossRef]
- Oya, K.; Kakurai, M.; Ishii, Y.; Nomura, T. Atypical radiation recall dermatitis induced by radiotherapy targeting a different site from the previously irradiated site. J. Dermatol. 2024, 51, e11–e12. [Google Scholar] [CrossRef]
- Benveniste, M.F.; Gomez, D.; Carter, B.W.; Cuellar, S.L.B.; Shroff, G.S.; Benveniste, A.P.A.; Odisio, E.G.; Marom, E.M. Recognizing Radiation Therapy–related Complications in the Chest. RadioGraphics 2019, 39, 344–366. [Google Scholar] [CrossRef]
- Singh, V.K.; Seed, T.M. BIO 300: A promising radiation countermeasure under advanced development for acute radiation syndrome and the delayed effects of acute radiation exposure. Expert Opin. Investig. Drugs 2020, 29, 429–441. [Google Scholar] [CrossRef]
- Chua, H.L.; Plett, P.A.; Fisher, A.; Sampson, C.H.; Vemula, S.; Feng, H.; Sellamuthu, R.; Wu, T.; MacVittie, T.J.; Orschell, C.M. Lifelong Residual bone Marrow Damage in Murine Survivors of the Hematopoietic Acute Radiation Syndrome (H-ARS): A Compilation of Studies Comprising the Indiana University Experience. Health Phys. 2019, 116, 546–557. [Google Scholar] [CrossRef]
- Ji, L.; Cui, P.; Zhou, S.; Qiu, L.; Huang, H.; Wang, C.; Wang, J. Advances of Amifostine in Radiation Protection: Administration and Delivery. Mol. Pharm. 2023, 20, 5383–5395. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Seed, T.M. The efficacy and safety of amifostine for the acute radiation syndrome. Expert Opin. Drug Saf. 2019, 18, 1077–1090. [Google Scholar] [CrossRef]
- Singh, V.K.; Seed, T.M.; Olabisi, A.O. Drug discovery strategies for acute radiation syndrome. Expert Opin. Drug Discov. 2019, 14, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Guillamat-Prats, R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells 2021, 10, 1729. [Google Scholar] [CrossRef] [PubMed]
- Koniusz, S.; Andrzejewska, A.; Muraca, M.; Srivastava, A.K.; Janowski, M.; Lukomska, B. Extracellular Vesicles in Physiology, Pathology, and Therapy of the Immune and Central Nervous System, with Focus on Extracellular Vesicles Derived from Mesenchymal Stem Cells as Therapeutic Tools. Front. Cell Neurosci. 2016, 10, 109. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef]
- You, B.; Zhou, C.; Yang, Y. MSC-EVs alleviate osteoarthritis by regulating microenvironmental cells in the articular cavity and maintaining cartilage matrix homeostasis. Ageing Res. Rev. 2023, 85, 101864. [Google Scholar] [CrossRef]
- Christensen, D.M.; Iddins, C.J.; Sugarman, S.L. Ionizing Radiation Injuries and Illnesses. Emerg. Med. Clin. N. Am. 2014, 32, 245–265. [Google Scholar] [CrossRef]
- Ning, J.; Chen, L.; Zeng, Y.; Xiao, G.; Tian, W.; Wu, Q.; Tang, J.; He, S.; Tanzhu, G.; Zhou, R. The scheme, and regulative mechanism of pyroptosis, ferroptosis, and necroptosis in radiation injury. Int. J. Biol. Sci. 2024, 20, 1871–1883. [Google Scholar] [CrossRef]
- Albanese, J.; Dainiak, N. Modulation of intercellular communication mediated at the cell surface and on extracellular, plasma membrane-derived vesicles by ionizing radiation. Exp. Hematol. 2003, 31, 455–464. [Google Scholar] [CrossRef]
- Averbeck, D.; Rodriguez-Lafrasse, C. Role of Mitochondria in Radiation Responses: Epigenetic, Metabolic, and Signaling Impacts. Int. J. Mol. Sci. 2021, 22, 11047. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhu, J.; Liu, Y.; Zhou, P.; Gu, Y. Mechanisms of radiation-induced tissue damage and response. MedComm 2024, 5, e725. [Google Scholar] [CrossRef] [PubMed]
- Molinar-Inglis, O.; DiCarlo, A.L.; Lapinskas, P.J.; Rios, C.I.; Satyamitra, M.M.; Silverman, T.A.; Winters, T.A.; Cassatt, D.R. Radiation-induced multi-organ injury. Int. J. Radiat. Biol. 2024, 100, 486–504. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Hay, K.D.; Macdonald, H.; Rich, A.M. Retrospective audit of the use of the Marx Protocol for prophylactic hyperbaric oxygen therapy in managing patients requiring dental extractions following radiotherapy to the head and neck. N. Z. Dent. J. 2009, 105, 47–50. [Google Scholar]
- Nolen, D.; Cannady, S.B.; Wax, M.K.; Scharpf, J.; Puscas, L.; Esclamado, R.M.; Fritz, M.; Freiberger, J.; Lee, W.T. Comparison of complications in free flap reconstruction for osteoradionecrosis in patients with or without hyperbaric oxygen therapy. Head Neck 2014, 36, 1701–1704. [Google Scholar] [CrossRef]
- Aljohani, S.; Fliefel, R.; Brunner, T.F.; Chronopoulos, A.; Binmadi, N.; Otto, S. Fluorescence-guided surgery for osteoradionecrosis of the jaw: A retrospective study. J. Int. Med. Res. 2022, 50, 3000605221104186. [Google Scholar] [CrossRef]
- Xu, J.J.; Zhou, J.; Wang, S.L. Osteoradionecrosis and bisphosphonate-related osteonecrosis of the jaw: A review of the pathogenesis and treatment. Zhonghua Kou Qiang Yi Xue Za Zhi 2021, 56, 404–409. [Google Scholar]
- Arqueros-Lemus, M.; Mariño-Recabarren, D.; Niklander, S.; Martínez-Flores, R.; Moraga, V. Pentoxifylline and tocopherol for the treatment of osteoradionecrosis of the jaws. A systematic review. Med. Oral Patol. Oral Cir. Bucal 2023, 28, e293–e300. [Google Scholar] [CrossRef]
- King, M.; Joseph, S.; Albert, A.; Thomas, T.V.; Nittala, M.R.; Woods, W.C.; Vijayakumar, S.; Packianathan, S. Use of Amifostine for Cytoprotection during Radiation Therapy: A Review. Oncology 2020, 98, 61–80. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, H.; Yang, Z.; Chen, Z.; Zhang, S.; Zhu, Y.; Yang, M.; Zhong, H.; Zhou, F.; Xie, Y.; et al. Treatment of Radiation Bone Injury with Transplanted hUCB-MSCs via Wnt/β-Catenin. Stem Cells Int. 2021, 2021, 5660927. [Google Scholar] [CrossRef]
- Loge, L.; Florescu, C.; Alves, A.; Menahem, B. Radiation enteritis: Diagnostic and therapeutic issues. J. Visc. Surg. 2020, 157, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wang, Y.; Chen, Y.; Zhang, Y.; Deng, H.; Yao, D. External treatment of traditional Chinese medicine for radiation enteritis: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e26014. [Google Scholar] [CrossRef] [PubMed]
- Hale, M.F. Radiation enteritis: From diagnosis to management. Curr. Opin. Gastroenterol. 2020, 36, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Hanania, A.N.; Mainwaring, W.; Ghebre, Y.T.; Hanania, N.A.; Ludwig, M. Radiation-Induced Lung Injury: Assessment and Management. Chest 2019, 156, 150–162. [Google Scholar] [CrossRef]
- Jin, H.; Yoo, Y.; Kim, Y.; Kim, Y.; Cho, J.; Lee, Y.-S. Radiation-Induced Lung Fibrosis: Preclinical Animal Models and Therapeutic Strategies. Cancers 2020, 12, 1561. [Google Scholar] [CrossRef]
- Qin, W.; Liu, B.; Yi, M.; Li, L.; Tang, Y.; Wu, B.; Yuan, X. Antifibrotic Agent Pirfenidone Protects against Development of Radiation-Induced Pulmonary Fibrosis in a Murine Model. Radiat. Res. 2018, 190, 396–403. [Google Scholar] [CrossRef]
- Soriano, J.L.; Calpena, A.C.; Souto, E.B.; Clares, B. Therapy for prevention and treatment of skin ionizing radiation damage: A review. Int. J. Radiat. Biol. 2019, 95, 537–553. [Google Scholar] [CrossRef]
- Liu, Z.; Rao, Z.; Sheng, X.; Long, Y.; Zhou, X. Effect of adipose-derived stem cells on radiation-induced acute skin injury in rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2019, 44, 150–157. [Google Scholar]
- Turnquist, C.; Harris, B.T.; Harris, C.C. Radiation-induced brain injury: Current concepts and therapeutic strategies targeting neuroinflammation. Neuro-Oncol. Adv. 2020, 2, vdaa057. [Google Scholar] [CrossRef]
- Yang, X.; Ren, H.; Fu, J. Treatment of Radiation-Induced Brain Necrosis. Oxid. Med. Cell Longev. 2021, 2021, 4793517. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Jung, Y.; Seo, J.; Bae, Y.; Kim, H.-S.; Jeong, W. Roles of extracellular vesicles from mesenchymal stem cells in regeneration. Mol. Cells 2024, 47, 100151. [Google Scholar] [CrossRef] [PubMed]
- Shekari, F.; Nazari, A.; Kashani, S.A.; Hajizadeh-Saffar, E.; Lim, R.; Baharvand, H. Pre-clinical investigation of mesenchymal stromal cell-derived extracellular vesicles: A systematic review. Cytotherapy 2021, 23, 277–284. [Google Scholar] [CrossRef]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef]
- Hazawa, M.; Tomiyama, K.; Saotome-Nakamura, A.; Obara, C.; Yasuda, T.; Gotoh, T.; Tanaka, I.; Yakumaru, H.; Ishihara, H.; Tajima, K. Radiation increases the cellular uptake of exosomes through CD29/CD81 complex formation. Biochem. Biophys. Res. Commun. 2014, 446, 1165–1171. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, K.; Zhang, N.; Zhao, Y.; Ma, Q.; Shen, J.; Lin, Y.; Xiang, P.; Tang, Y.; Hu, X.; et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1659–1670. [Google Scholar] [CrossRef]
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Lo Sicco, C.; Reverberi, D.; Balbi, C.; Ulivi, V.; Principi, E.; Pascucci, L.; Becherini, P.; Bosco, M.C.; Varesio, L.; Franzin, C.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl. Med. 2017, 6, 1018–1028. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; de Voogt, W.S.; Tognoli, M.L.; van der Werff, L.; Gitz-Francois, J.J.; Seinen, C.W.; Schiffelers, R.M.; de Jong, O.G.; Vader, P. Integrin beta 1 and fibronectin mediate extracellular vesicle uptake and functional RNA delivery. J. Biol. Chem. 2025, 301, 108305. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Wang, X.; Chen, J.; Du, C.; Wang, J.; Liao, W. Insights into ionizing radiation-induced bone marrow hematopoietic stem cell injury. Stem Cell Res. Ther. 2024, 15, 222. [Google Scholar] [CrossRef] [PubMed]
- Fliedner, T.; Cronkite, E.; Bond, V. Pathogenesis and regeneration of radiation induced bone marrow injury, and therapeutic implications. Strahlentherapie 1962, 51, 263–278. [Google Scholar]
- Zhang, J.; Qiu, X.; Xi, K.; Hu, W.; Pei, H.; Nie, J.; Wang, Z.; Ding, J.; Shang, P.; Li, B.; et al. Therapeutic ionizing radiation induced bone loss: A review of in vivo and in vitro findings. Connect. Tissue Res. 2018, 59, 509–522. [Google Scholar] [CrossRef]
- Wen, S.; Dooner, M.; Cheng, Y.; Papa, E.; Del Tatto, M.; Pereira, M.; Deng, Y.; Goldberg, L.; Aliotta, J.; Chatterjee, D.; et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia 2016, 30, 2221–2231. [Google Scholar] [CrossRef]
- Kink, J.A.; Bellio, M.A.; Forsberg, M.H.; Lobo, A.; Thickens, A.S.; Lewis, B.M.; Ong, I.M.; Khan, A.; Capitini, C.M.; Hematti, P. Large-scale bioreactor production of extracellular vesicles from mesenchymal stromal cells for treatment of acute radiation syndrome. Stem Cell Res. Ther. 2024, 15, 72. [Google Scholar] [CrossRef]
- Kink, J.A.; Forsberg, M.H.; Reshetylo, S.; Besharat, S.; Childs, C.J.; Pederson, J.D.; Gendron-Fitzpatrick, A.; Graham, M.; Bates, P.D.; Schmuck, E.G.; et al. Macrophages Educated with Exosomes from Primed Mesenchymal Stem Cells Treat Acute Radiation Syndrome by Promoting Hematopoietic Recovery. Biol. Blood Marrow Transplant. 2019, 25, 2124–2133. [Google Scholar] [CrossRef]
- Kong, F.; Wu, C.-T.; Geng, P.; Liu, C.; Xiao, F.; Wang, L.-S.; Wang, H. Dental Pulp Stem Cell-Derived Extracellular Vesicles Mitigate Haematopoietic Damage after Radiation. Stem Cell Rev. Rep. 2021, 17, 318–331. [Google Scholar] [CrossRef]
- Zuo, R.; Liu, M.; Wang, Y.; Li, J.; Wang, W.; Wu, J.; Sun, C.; Li, B.; Wang, Z.; Lan, W.; et al. BM-MSC-derived exosomes alleviate radiation-induced bone loss by restoring the function of recipient BM-MSCs and activating Wnt/β-catenin signaling. Stem. Cell Res. Ther. 2019, 10, 30. [Google Scholar] [CrossRef]
- Zhai, R.; Tai, F.; Ding, K.; Tan, X.; Li, H.; Cao, Z.; Ge, C.; Zheng, X.; Fu, H. Comparative Analysis of the Therapeutic Effects of MSCs From Umbilical Cord, Bone Marrow, and Adipose Tissue and Investigating the Impact of Oxidized RNA on Radiation-Induced Lung Injury. Stem Cells Int. 2024, 2024, 7419270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, J.; Verma, V.; Liu, X.; Wu, M.; Yu, J.; Chen, D. Crossed Pathways for Radiation-Induced and Immunotherapy-Related Lung Injury. Front. Immunol. 2021, 12, 774807. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Cha, H.J.; Lee, E.-M.; Lee, S.-J.; Seo, S.-K.; Jin, H.-O.; Park, I.-C.; Jin, Y.-W.; An, S. Alteration of miRNA profiles by ionizing radiation in A549 human non-small cell lung cancer cells. Int. J. Oncol. 2009, 35, 81–86. [Google Scholar] [PubMed]
- Zhang, W.-Y.; Wen, L.; Du, L.; Liu, T.T.; Sun, Y.; Chen, Y.-Z.; Lu, Y.-X.; Cheng, X.-C.; Sun, H.-Y.; Xiao, F.-J.; et al. S-RBD-modified and miR-486-5p-engineered exosomes derived from mesenchymal stem cells suppress ferroptosis and alleviate radiation-induced lung injury and long-term pulmonary fibrosis. J. Nanobiotechnol. 2024, 22, 662. [Google Scholar] [CrossRef]
- Lei, X.; He, N.; Zhu, L.; Zhou, M.; Zhang, K.; Wang, C.; Huang, H.; Chen, S.; Li, Y.; Liu, Q.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Radiation-Induced Lung Injury via miRNA-214-3p. Antioxid. Redox Signal. 2021, 35, 849–862. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Z.; Jiang, X.; Wang, Y.; Yang, Z.; Mao, Y.; Wu, Z.; Li, G.; Chen, H. Mouse mesenchymal stem cell-derived exosomal miR-466f-3p reverses EMT process through inhibiting AKT/GSK3β pathway via c-MET in radiation-induced lung injury. J. Exp. Clin. Cancer Res. 2022, 41, 128. [Google Scholar] [CrossRef]
- Li, W.; Lin, Y.; Luo, Y.; Wang, Y.; Lu, Y.; Li, Y.; Guo, H. Vitamin D Receptor Protects against Radiation-Induced Intestinal Injury in Mice via Inhibition of Intestinal Crypt Stem/Progenitor Cell Apoptosis. Nutrients 2021, 13, 2910. [Google Scholar] [CrossRef]
- Yang, L.; Fang, C.; Song, C.; Zhang, Y.; Zhang, R.; Zhou, S. Mesenchymal Stem Cell-Derived Exosomes are Effective for Radiation Enteritis and Essential for the Proliferation and Differentiation of Lgr5+ Intestinal Epithelial Stem Cells by Regulating Mir-195/Akt/β-Catenin Pathway. Tissue Eng. Regen. Med. 2023, 20, 739–751. [Google Scholar] [CrossRef]
- He, N.; Dong, M.; Sun, Y.; Yang, M.; Wang, Y.; Du, L.; Ji, K.; Wang, J.; Zhang, M.; Gu, Y.; et al. Mesenchymal stem cell-derived extracellular vesicles targeting irradiated intestine exert therapeutic effects. Theranostics 2024, 14, 5492–5511. [Google Scholar] [CrossRef]
- Rübe, C.E.; Freyter, B.M.; Tewary, G.; Roemer, K.; Hecht, M.; Rübe, C. Radiation Dermatitis: Radiation-Induced Effects on the Structural and Immunological Barrier Function of the Epidermis. Int. J. Mol. Sci. 2024, 25, 3320. [Google Scholar] [CrossRef]
- Spałek, M. Chronic radiation-induced dermatitis: Challenges and solutions. Clin. Cosmet. Investig. Dermatol. 2016, 9, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Shibuya, Y.; Imai, Y.; Oshima, J.; Sasaki, M.; Sasaki, K.; Aihara, Y.; Khanh, V.C.; Sekido, M. Therapeutic Potential of Adipose-Derived Stem Cell-Conditioned Medium and Extracellular Vesicles in an In Vitro Radiation-Induced Skin Injury Model. Int. J. Mol. Sci. 2023, 24, 17214. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Li, W.; Xu, J.; Pang, L.; Tang, L.; Yu, S.; Li, A.; Ge, H.; Huang, R.; Cheng, H. Advances in the study of the molecular biological mechanisms of radiation-induced brain injury. Am. J. Cancer Res. 2023, 13, 3275–3299. [Google Scholar] [PubMed]
- Ratushnyak, M.G.; Semochkina, Y.P.; Yastremsky, E.V.; Kamyshinsky, R.A. Stem Cell Exosomes Improve Survival of Neural Stem Cells After Radiation Exposure. Cell Technol. Biol. Med. 2022, 173, 544–552. [Google Scholar] [CrossRef]
- Liu, M.; Yang, Y.; Zhao, B.; Yang, Y.; Wang, J.; Shen, K.; Yang, X.; Hu, D.; Zheng, G.; Han, J. Exosomes Derived From Adipose-Derived Mesenchymal Stem Cells Ameliorate Radiation-Induced Brain Injury by Activating the SIRT1 Pathway. Front. Cell Dev. Biol. 2021, 9, 693782. [Google Scholar] [CrossRef]
- Wang, H.; Wei, J.; Zheng, Q.; Meng, L.; Xin, Y.; Yin, X.; Jiang, X. Radiation-induced heart disease: A review of classification, mechanism and prevention. Int. J. Biol. Sci. 2019, 15, 2128–2138. [Google Scholar] [CrossRef]
- Cao, H.; Yue, L.; Shao, J.; Kong, F.; Liu, S.; Huai, H.; He, Z.; Mao, Z.; Yang, Y.; Tan, Y.; et al. Small extracellular vesicles derived from umbilical cord mesenchymal stem cells alleviate radiation-induced cardiac organoid injury. Stem Cell Res. Ther. 2024, 15, 493. [Google Scholar] [CrossRef]
- Luo, L.; Yan, C.; Fuchi, N.; Kodama, Y.; Zhang, X.; Shinji, G.; Miura, K.; Sasaki, H.; Li, T.-S. Mesenchymal stem cell-derived extracellular vesicles as probable triggers of radiation-induced heart disease. Stem Cell Res. Ther. 2021, 12, 422. [Google Scholar] [CrossRef]
- Benderitter, M.; Caviggioli, F.; Chapel, A.; Coppes, R.P.; Guha, C.; Klinger, M.; Malard, O.; Stewart, F.; Tamarat, R.; van Luijk, P.; et al. Stem cell therapies for the treatment of radiation-induced normal tissue side effects. Antioxid. Redox Signal. 2014, 21, 338–355. [Google Scholar] [CrossRef]
- Wen, S.; Dooner, M.; Papa, E.; Del Tatto, M.; Pereira, M.; Borgovan, T.; Cheng, Y.; Goldberg, L.; Liang, O.; Camussi, G.; et al. Biodistribution of Mesenchymal Stem Cell-Derived Extracellular Vesicles in a Radiation Injury Bone Marrow Murine Model. Int. J. Mol. Sci. 2019, 20, 5468. [Google Scholar] [CrossRef]
- Helissey, C.; Guitard, N.; Théry, H.; Goulinet, S.; Mauduit, P.; Girleanu, M.; Favier, A.-L.; Drouet, M.; Parnot, C.; Chargari, C.; et al. Two New Potential Therapeutic Approaches in Radiation Cystitis Derived from Mesenchymal Stem Cells: Extracellular Vesicles and Conditioned Medium. Biology 2022, 11, 980. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, J.; Yin, Y.; Yang, L.; Gao, M.; Wu, Z.; Lu, B.; Luo, S.; Wang, W.; Li, R. MSC-EXs inhibits uranium nephrotoxicity by competitively binding key proteins and inhibiting ROS production. Ecotoxicol. Environ. Saf. 2025, 289, 117654. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Zhang, L.; Zhang, P.; Xu, Y.; Wang, J.; Zhao, X.; Dai, Z.; Zhou, H.; Zhao, S.; Fan, A. Human UC-MSC-derived exosomes facilitate ovarian renovation in rats with chemotherapy-induced premature ovarian insufficiency. Front. Endocrinol. 2023, 14, 1205901. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Sakai, K.; Koma, Y.; Watanabe, J.; Liu, K.; Maruyama, H.; Sakaguchi, K.; Hibi, H. Dental pulp stem cell-derived small extracellular vesicle in irradiation-induced senescence. Biochem. Biophys. Res. Commun. 2021, 575, 28–35. [Google Scholar] [CrossRef]
- Guo, X.; Huang, Z.; Wu, F.; Jiang, W.; Li, Y.; Wang, T.; Tran, S.D.; Lin, Z.; Su, X. Exosomes of human adipose stem cells mitigate irradiation injury to salivary glands by inhibiting epithelial-mesenchymal transition through miR-199a-3p targeting Twist1 and regulating TGFβ1/Smad3 pathway. Theranostics 2025, 15, 1622–1641. [Google Scholar] [CrossRef]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Exosomes as a new frontier of cancer liquid biopsy. Mol. Cancer 2022, 21, 56. [Google Scholar] [CrossRef]
- Samiei, H.; Ajam, F.; Gharavi, A.; Abdolmaleki, S.; Kokhaei, P.; Mohammadi, S.; Memarian, A. Simultaneous disruption of circulating miR-21 and cytotoxic T lymphocytes (CTLs): Prospective diagnostic and prognostic markers for esophageal squamous cell carcinoma (ESCC). J. Clin. Lab. Anal. 2022, 36, e24125. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef]
- Shi, M.M.; Yang, Q.Y.; Monsel, A.; Yan, J.Y.; Dai, C.X.; Zhao, J.Y.; Shi, G.C.; Zhou, M.; Zhu, X.M.; Li, S.K.; et al. Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12134. [Google Scholar] [CrossRef]
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Kimiz-Gebologlu, I.; Oncel, S.S. Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Control. Release 2022, 347, 533–543. [Google Scholar] [CrossRef]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, C.; Chen, X.; Fu, E.; Zhang, K.; Tao, H.; Han, Z.; Han, Z.-C.; Li, Z. Phosphatidylserine-mediated uptake of extracellular vesicles by hepatocytes ameliorates liver ischemia-reperfusion injury. Apoptosis 2025, 30, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Jiang, Z.; Chen, Y.; Shang, D.; Miao, P.; Gao, J. MiR-455-5p upregulation in umbilical cord mesenchymal stem cells attenuates endometrial injury and promotes repair of damaged endometrium via Janus kinase/signal transducer and activator of transcription 3 signaling. Bioengineered 2021, 12, 12891–12904. [Google Scholar] [CrossRef]
- Ma, N.; Liu, Y.; Chen, D.; Wu, C.; Meng, Z. In Vivo Imaging of Exosomes Labeled with NIR-II Polymer Dots in Liver-Injured Mice. Biomacromolecules 2022, 23, 4825–4833. [Google Scholar] [CrossRef]
- Salunkhe, S.; Dheeraj; Basak, M.; Chitkara, D.; Mittal, A. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: Strategies and significance. J. Control. Release 2020, 326, 599–614. [Google Scholar] [CrossRef]
- Tian, S.; Zhou, X.; Zhang, M.; Cui, L.; Li, B.; Liu, Y.; Su, R.; Sun, K.; Hu, Y.; Yang, F.; et al. Mesenchymal stem cell-derived exosomes protect against liver fibrosis via delivering miR-148a to target KLF6/STAT3 pathway in macrophages. Stem. Cell Res. Ther. 2022, 13, 330. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, L.; Yu, H. Potential Druggability of Mesenchymal Stem/Stromal Cell-derived Exosomes. Curr. Stem Cell Res. Ther. 2024, 19, 1195–1209. [Google Scholar] [CrossRef]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Tian, J.; Han, Z.; Song, D.; Peng, Y.; Xiong, M.; Chen, Z.; Duan, S.; Zhang, L. Engineered Exosome for Drug Delivery: Recent Development and Clinical Applications. Int. J. Nanomed. 2023, 18, 7923–7940. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, M. Therapeutic Potential of Mesenchymal Stromal Cells and Extracellular Vesicles in the Treatment of Radiation Lesions—A Review. Cells 2021, 10, 427. [Google Scholar] [CrossRef] [PubMed]
| Radiation Injuries | Source of EVs | Ingredients | References |
|---|---|---|---|
| Radiation-induced hematopoietic injury | BMSC-EVs DPSC-EVs | Let-7 miR-143 | [56,57,58,59] |
| Radiation-induced bone loss | BMSC-EVs | -- | [60] |
| Radiation-induced lung injury | UCMSC-EVs PL-MSC-EVs BMSC-EVs | miR-486-5p miR-214-3p MiR-466f-3p | [64,65,66] |
| Radiation-induced intestinal injury | PL-MSC-EVs BMSC-EVs | miR-195 miR-455-3p | [68,69] |
| Radiation-induced skin injury | ADSC-EVs | -- | [72] |
| Radiation-induced brain injury | ADSC-EVs | -- | [75] |
| Radiation-induced heart disease | PL-MSC-EVs UCMSC-EVs | miR-23a-5p miR-29a-3p miR-146a-5p | [77] |
| Radiation-induced cystitis | BMSC-EVs | -- | [81] |
| Radiation-induced kidney injury | UC-MSC-EVs | -- | [82] |
| Radiation-induced premature ovarian injury | UC-MSC-EVs | -- | [83] |
| Radiation-induced salivary gland injury | ADSC-EVs | miR-199a-3p | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Li, H.; Zhang, Z.; Mou, T.; Wang, D.; Li, C.; Tian, L.; Zong, C. From Mechanism to Therapy: The Role of MSC-EVs in Alleviating Radiation-Induced Injuries. Pharmaceutics 2025, 17, 652. https://doi.org/10.3390/pharmaceutics17050652
Huang C, Li H, Zhang Z, Mou T, Wang D, Li C, Tian L, Zong C. From Mechanism to Therapy: The Role of MSC-EVs in Alleviating Radiation-Induced Injuries. Pharmaceutics. 2025; 17(5):652. https://doi.org/10.3390/pharmaceutics17050652
Chicago/Turabian StyleHuang, Chong, Heng Li, Zhiyue Zhang, Ting Mou, Dandan Wang, Chenlu Li, Lei Tian, and Chunlin Zong. 2025. "From Mechanism to Therapy: The Role of MSC-EVs in Alleviating Radiation-Induced Injuries" Pharmaceutics 17, no. 5: 652. https://doi.org/10.3390/pharmaceutics17050652
APA StyleHuang, C., Li, H., Zhang, Z., Mou, T., Wang, D., Li, C., Tian, L., & Zong, C. (2025). From Mechanism to Therapy: The Role of MSC-EVs in Alleviating Radiation-Induced Injuries. Pharmaceutics, 17(5), 652. https://doi.org/10.3390/pharmaceutics17050652







