Dissolving Microneedles Containing Lactoferrin Nanosuspension for Enhancement of Antimicrobial and Anti-Inflammatory Effects in the Treatment of Dry Eye Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. High-Performance Liquid Chromatography Method of Lactoferrin (BLF)
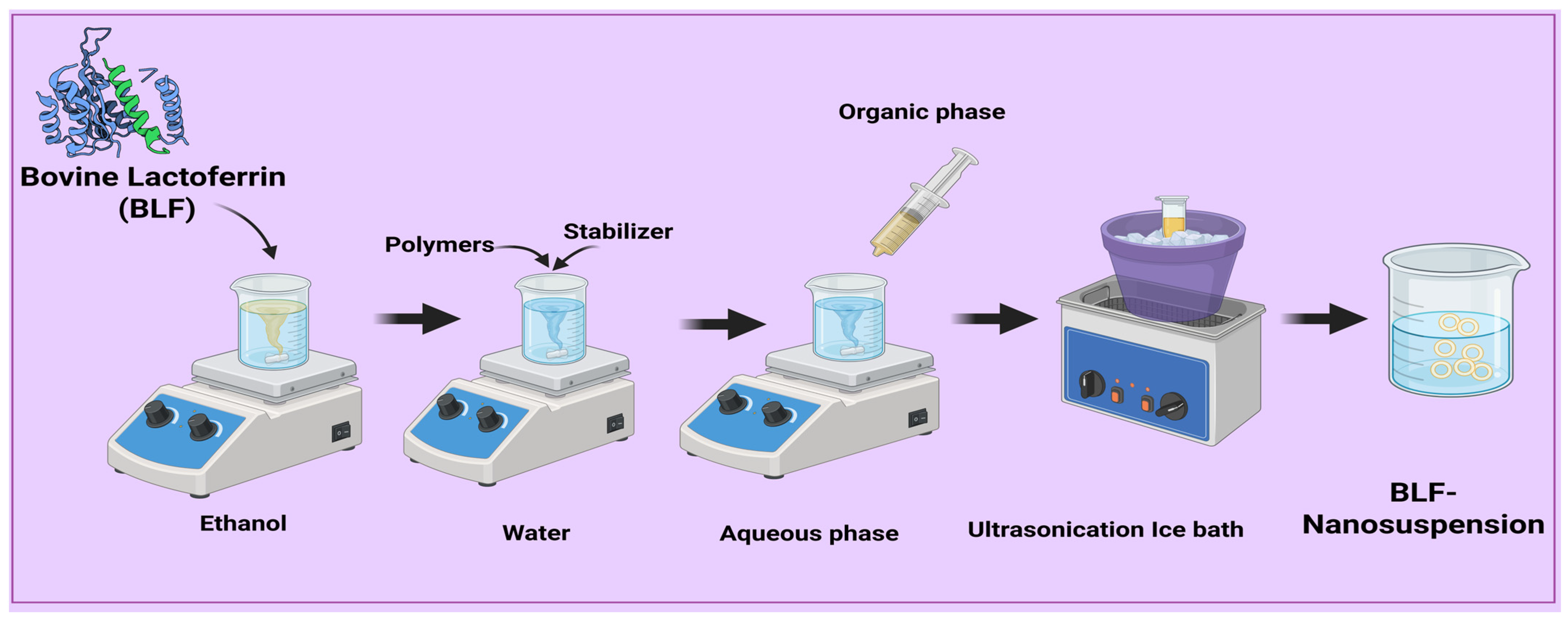
2.3. Preparation of Lactoferrin Nanosuspensions
2.4. Optimization of Formulation
2.5. Investigation of Lactoferrin-Loaded Nanosuspension
2.5.1. Measurement of Particle Size, Polydispersity Index, and Zeta Potential
2.5.2. Drug Content Analysis of Lactoferrin Nanosuspension
2.6. Characterization of Lactoferrin Nanosuspension
2.6.1. Transmission Electron Microscopy (TEM)
2.6.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.6.3. Differential Scanning Calorimetry (DSC)
2.6.4. Drug Release Studies
In Vitro Drug Release Q24 of Lactoferrin from BLF-NS
Ex Vivo Permeation Study
2.6.5. Stability Study
2.7. Fabrication of PVP/HPMC Microneedles Loading Freeze-Dried BLF-NS
2.8. Microneedle Morphological Characterizations
2.8.1. Physical Characterization of MN
2.8.2. Mechanical Strength/Breaking Strength
2.8.3. Drug Content Studies
2.8.4. Physicochemical Characterization for the Optimized MN
Scanning Electron Microscopy (SEM)
Fourier Transform Infrared Spectroscopy (FTIR)
Differential Scanning Calorimetry (DSC)
Drug Release Studies for Microneedles Formulation
2.9. Antimicrobial Study
2.9.1. Diffusion Agar Method
2.9.2. Quantitative Determination of the Minimum Inhibitory Concentration (MIC)
2.10. In Vivo Study
2.10.1. Animals
2.10.2. Dry Eye Models
2.10.3. Eye Irritancy Test (Draize Test)
2.10.4. Schirmer Tear Test (Measurement of Tear Secretion)
2.10.5. mRNA Expression in Cornea and Conjunctiva Detected by qRT-PCR
2.10.6. Inflammatory Cytokines in Conjunctival Tissue Detected by ELISA
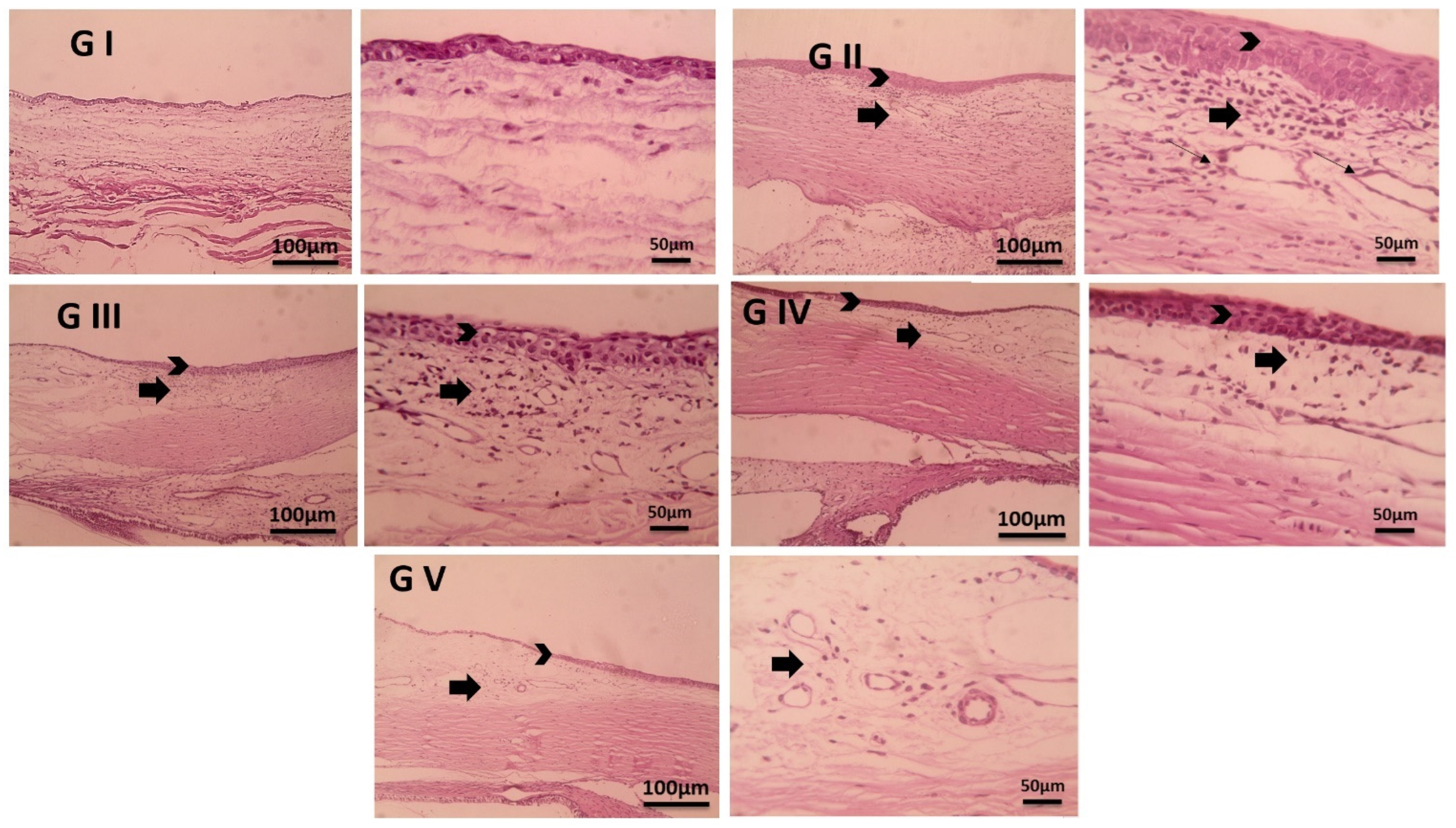
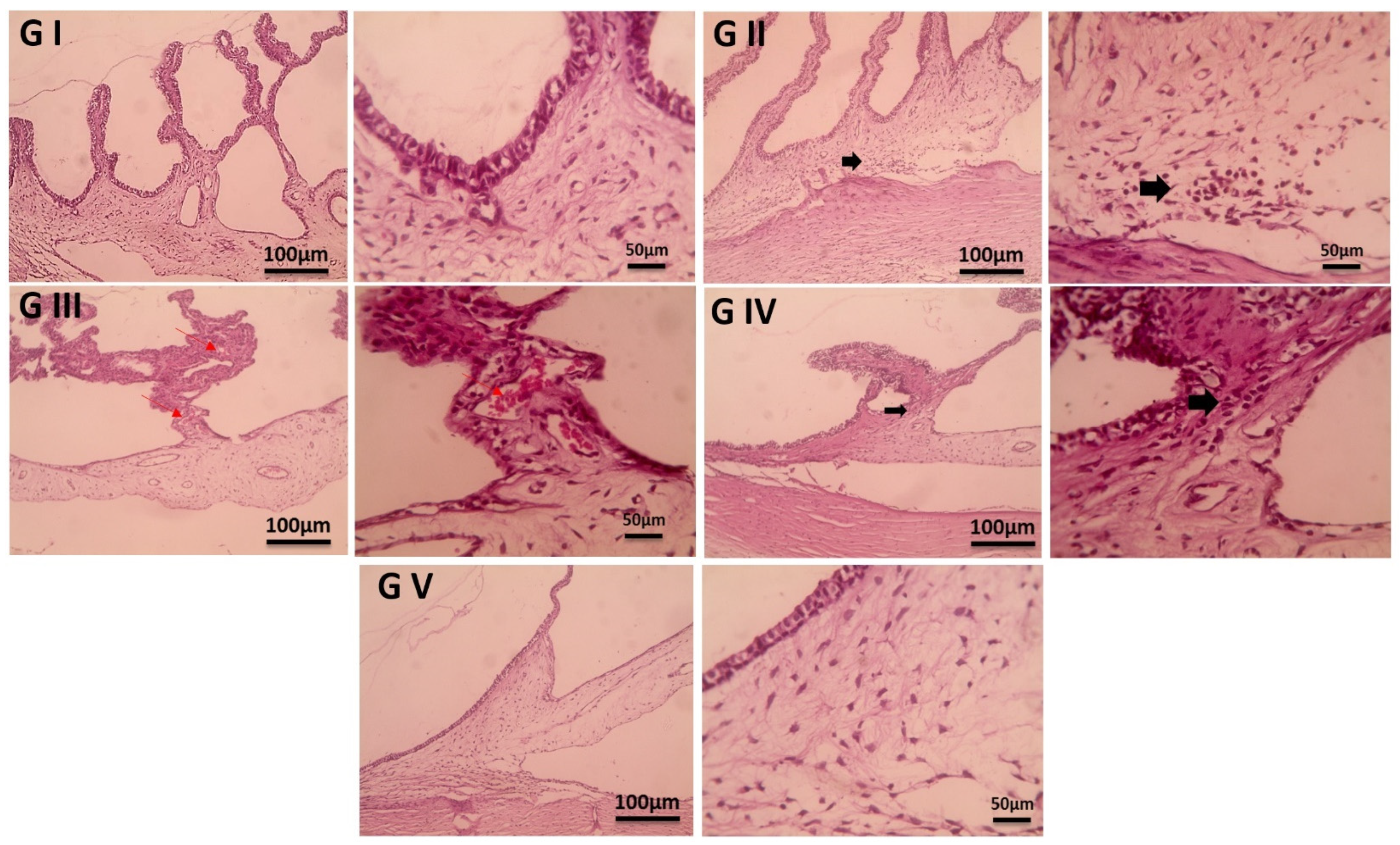
2.11. Histopathological Examination
2.12. Statistical Analysis
3. Results and Discussion
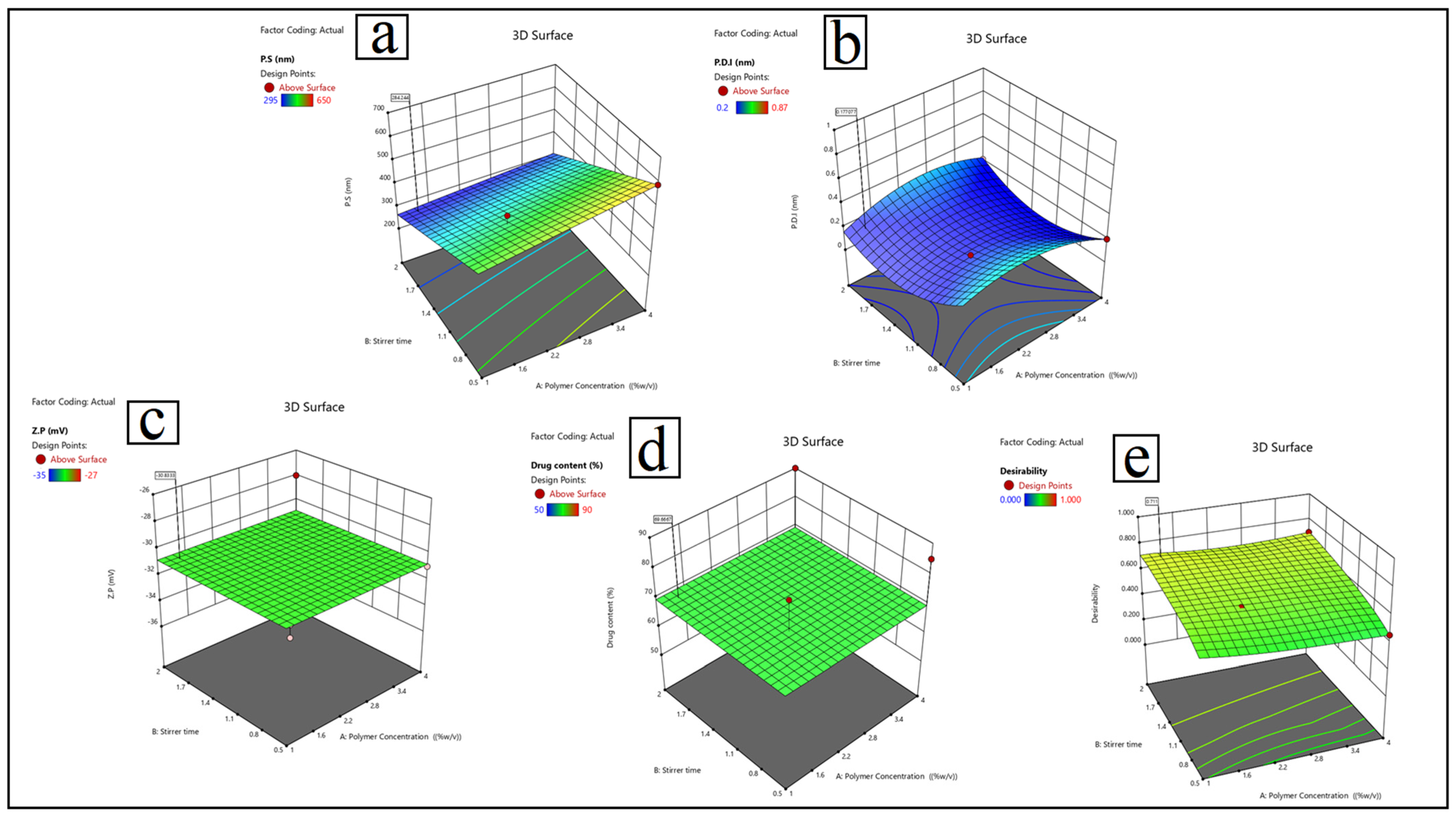
3.1. Formulation Optimization of Lactoferrin Nanosuspension: Entrapment Efficiency (EE%), Particle Size (P.S), Polydispersity Index (P.D.I), and Zeta Potential (Z.P)
- Particle size (Y1): Y1 = 420 + 25A − 30B − 15C + 10D − 12AB + 8AC − 5BC + 18A2.
- Polydispersity index (Y2) is calculated as: 0.25 + 0.08A − 0.05B − 0.12C + 0.03D + 0.02AB − 0.04AC + 0.01BC − 0.06A2 + 0.04B2.
- Zeta potential (Y3) is calculated as: Y3 = −28.5 + 1.2A − 2.8B + 3.5C − 1.8D + 0.9AB − 1.2AC + 0.5BC + 0.7A2 − 1.1C2
- Drug content (Y4) = 75 + 5A + 3B − 8C + 12D − 4AB + 7AD.
3.2. Physicochemical Characterization of Lactoferrin Nanosuspension
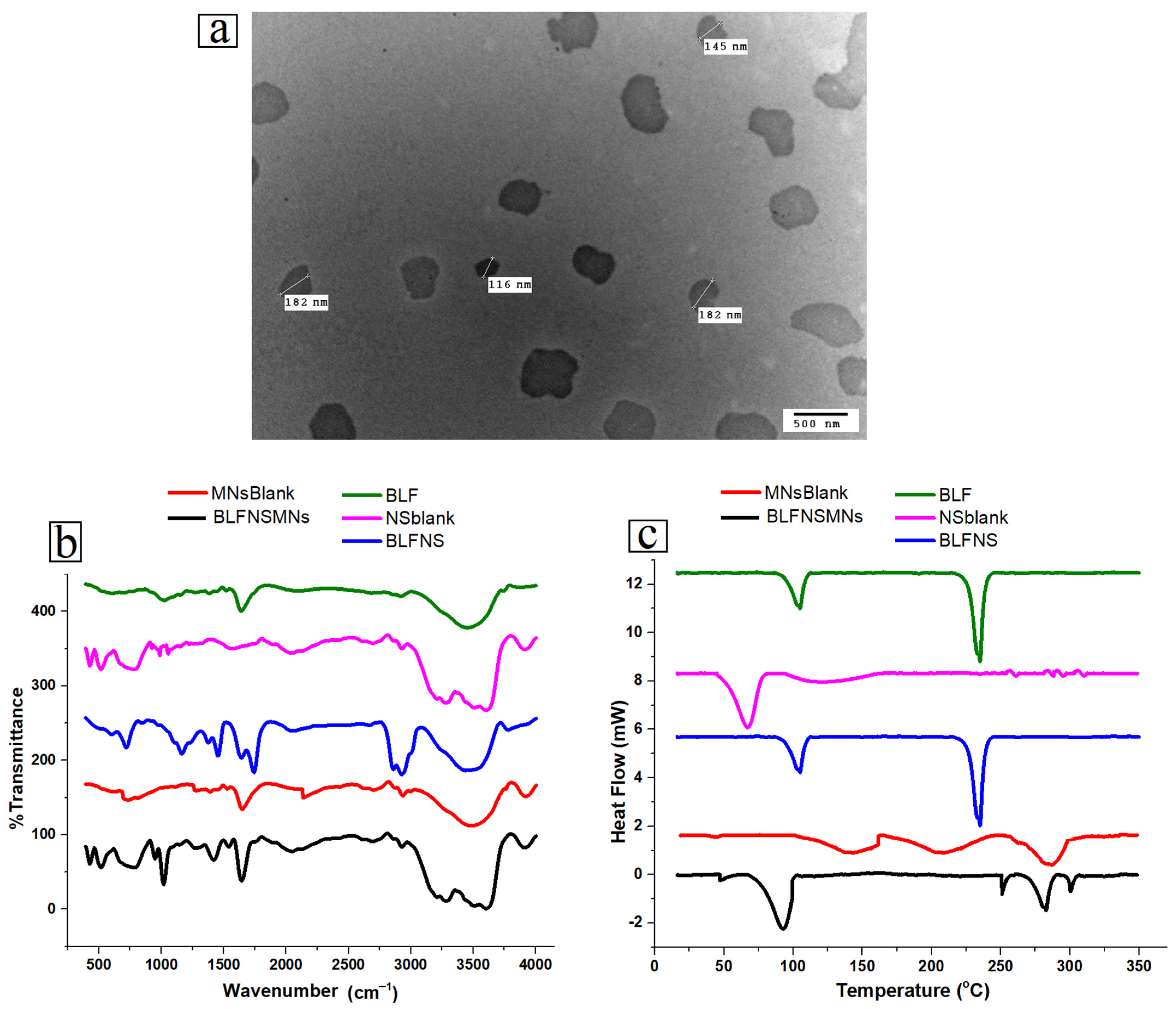
3.2.1. Transmission Electron Microscopy (TEM)
3.2.2. Fourier Transform Infrared Spectroscopy (FT-IR)
3.2.3. Differential Scanning Calorimetry (DSC)
3.2.4. Drug Release Studies
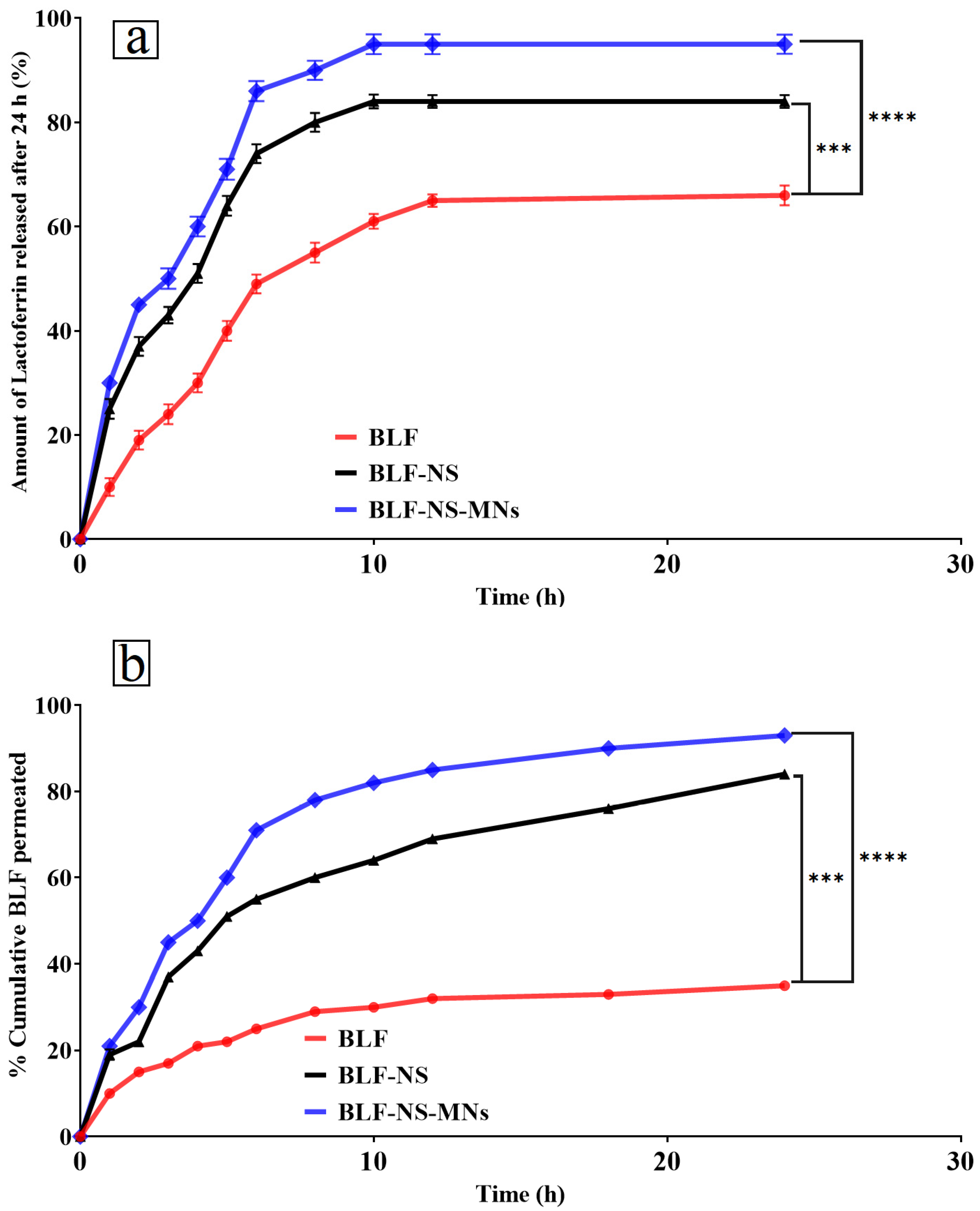
In Vitro Drug Release Q24 of Lactoferrin from BLF-NS
Ex Vivo Permeation Study
3.3. Stability Study
3.4. Fabrication of PVP/HPMC Microneedles Loading Freeze-Dried BLF-NS
3.4.1. Microneedle Characterizations
Physical Characterization of Microneedles—Mechanical Strength/Breaking Strength
Drug Content Studies
3.4.2. Characterization of the Optimized MN
Scanning Electron Microscopy (SEM)
Fourier Transmission Infrared Spectroscopy (FTIR)
Differential Scanning Calorimetry (DSC)
In Vitro Drug Release Q24 of Lactoferrin from BLF-NS-MNs
Ex Vivo Permeation Study
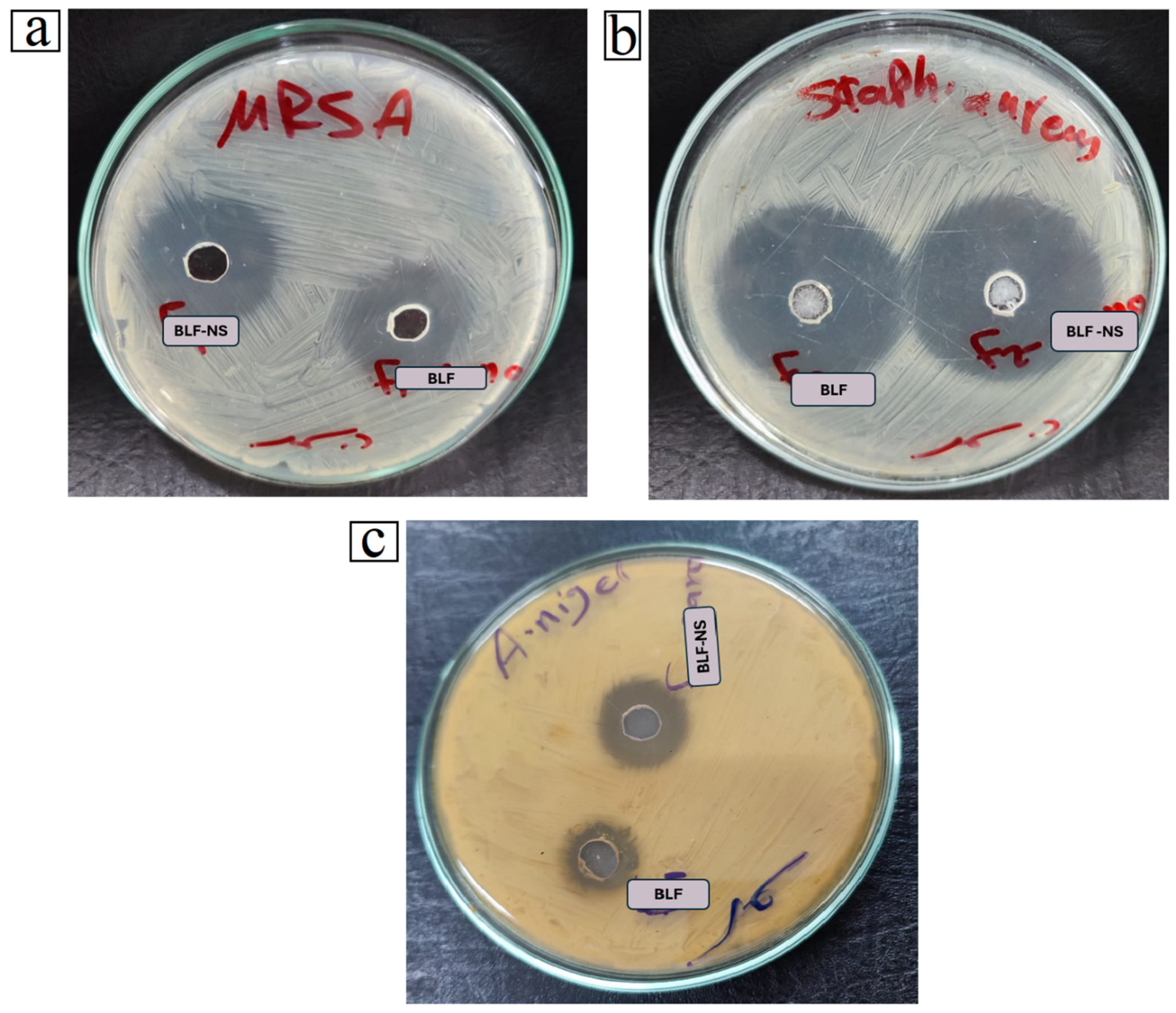
3.5. Antimicrobial Study
3.6. In Vivo Study
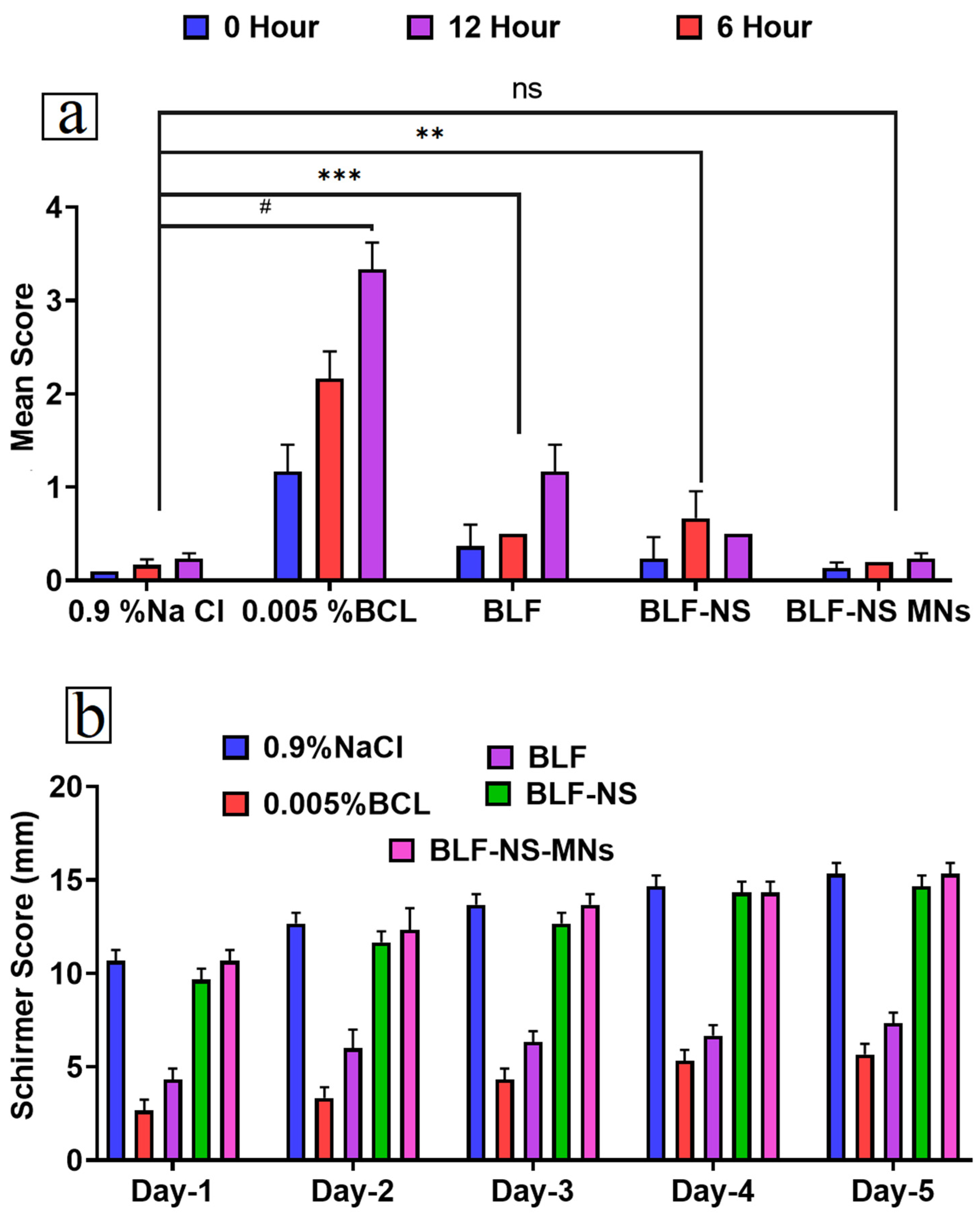
3.6.1. Eye Irritancy Test (Draize Test)
3.6.2. Schirmer Tear Test (Measurement of Tear Secretion)
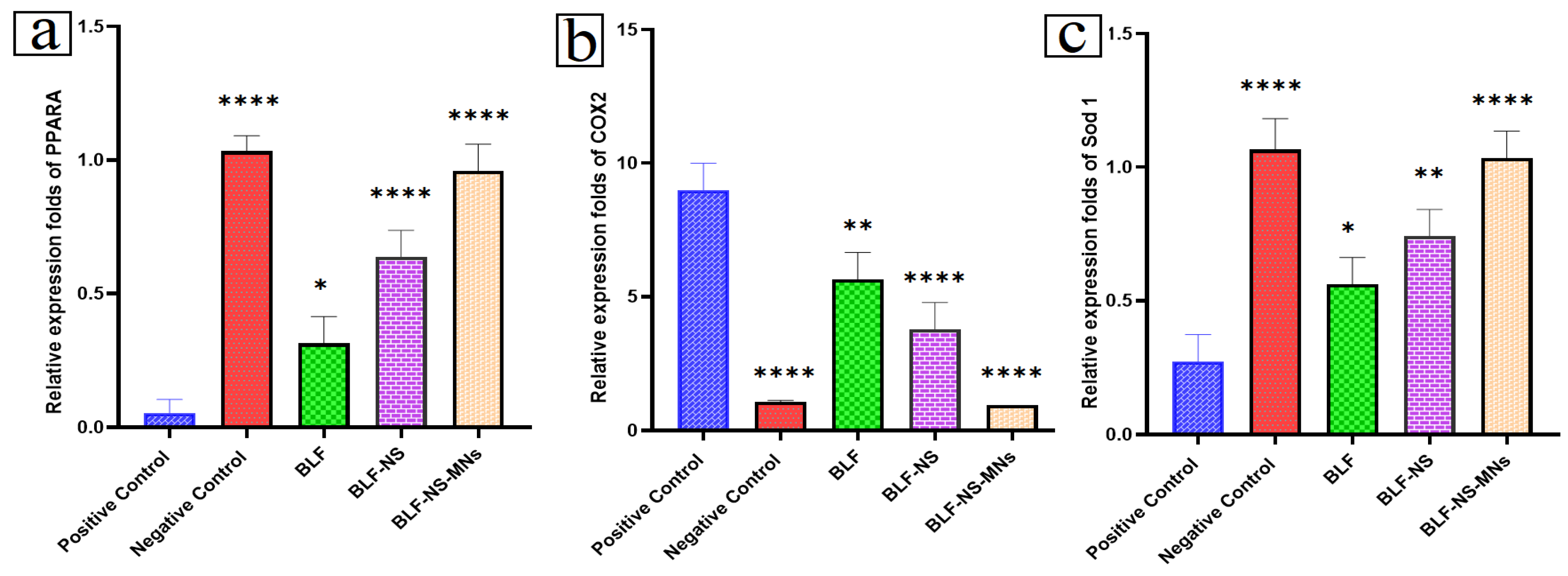
3.6.3. mRNA Expression in Cornea and Conjunctiva Detected by qRT-PCR
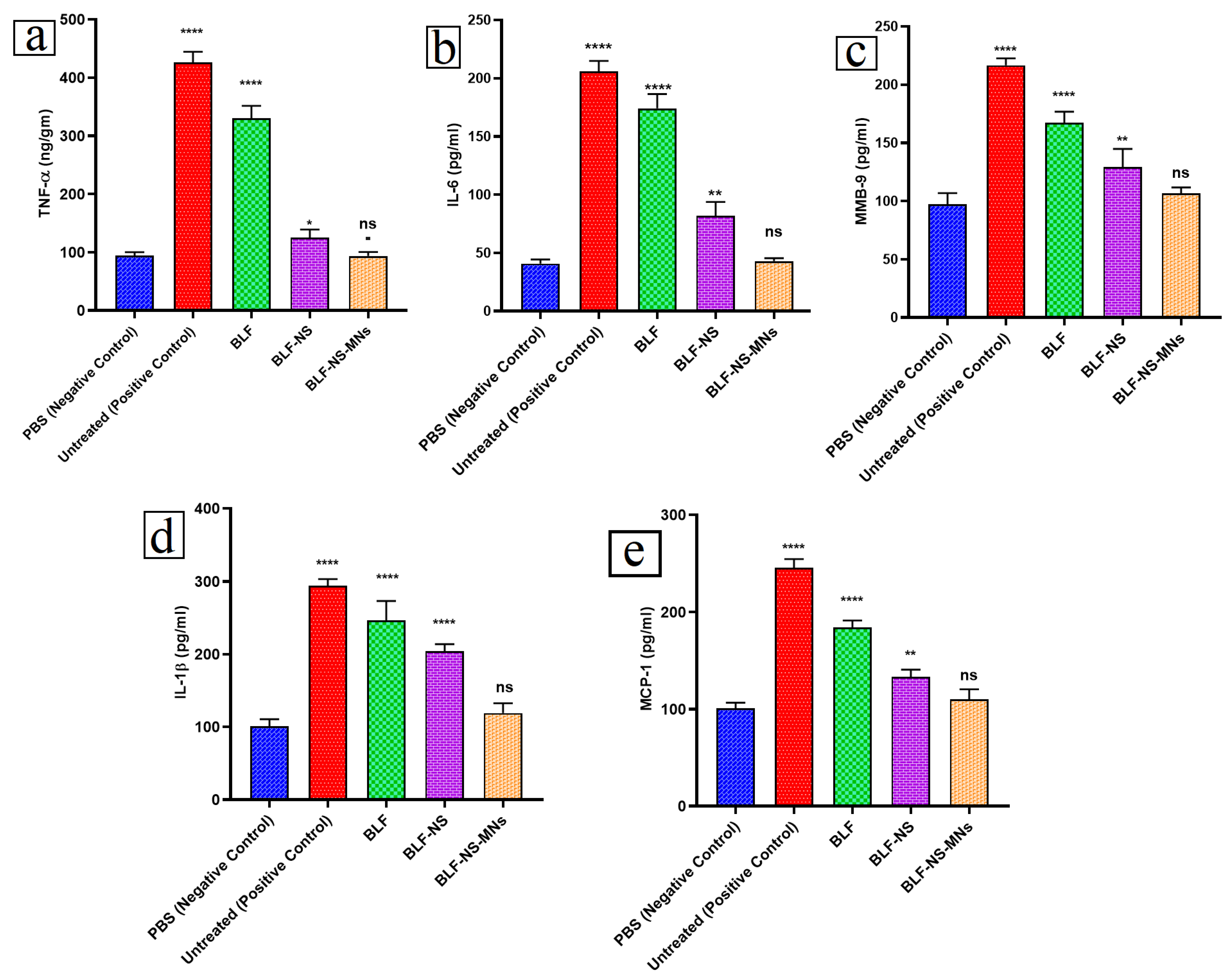
3.6.4. Inflammatory Cytokines in Conjunctival Tissue Detected by ELISA
3.7. Histopathological Examination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BLF | Bovine Lactoferrin |
| DED | Dry Eye Disease |
| BCL | Benzalkonium Chloride |
| BLF-NS | BLF-Nanosuspension |
| BLF-NS-MNs | BLF Nanosuspension Microneedles |
| qRT-PCR | Quantitative Real-Time Polymerase Chain Reaction |
| PPARA | Peroxisome Proliferator-Activated Receptor Alpha |
| COX-2 | Cyclooxygenase-2 |
| SOD 1 | Superoxide Dismutase 1 |
| TNF-α | Tumor Necrosis Factor Alpha |
| IL-6 | Interleukin-6 |
| MMP | Matrix Metalloproteinase-9 |
| IL-1β | Interleukin-1 Beta |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| H&E | Hematoxylin and Eosin |
| PVP | Polyvinylpyrrolidone |
| HPMC | Hydroxypropyl Methylcellulose |
| P.D.I | Polydispersity Index |
| Z.P | Zeta Potential |
| MIC | Minimum Inhibitory Concentration |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| SEM | Scanning Electron Microscopy |
| FTIR | Fourier Transform Infrared Spectroscopy |
| DSC | Differential Scanning Calorimetry |
| HPLC | High-Performance Liquid Chromatography |
| ANOVA | Analysis of Variance |
| BBD | Box–Behnken Design |
References
- Makrynioti, D.; Zagoriti, Z.; Koutsojannis, C.; Morgan, P.B.; Lagoumintzis, G. Ocular Conditions and Dry Eye Due to Traditional and New Forms of Smoking: A Review. Contact Lens Anterior Eye 2020, 43, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Pucker, A.D.; Yim, T.W.; Rueff, E.; Ngo, W.; Tichenor, A.A.; Conto, J.E. LipiFlow for the Treatment of Dry Eye Disease. Cochrane Database Syst. Rev. 2024, 2024, CD015448. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, Y.; Han, Y.; Wu, Y.; Wang, D.; Zhang, B. Recommendations for Nutritional Supplements for Dry Eye Disease: Current Advances. Front. Pharmacol. 2024, 15, 1388787. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, P.; Tang, H.; Zhang, J. Rapid Detection of Tear Lactoferrin for Diagnosis of Dry Eyes by Using Fluorescence Polarization-Based Aptasensor. Sci. Rep. 2023, 13, 15179. [Google Scholar] [CrossRef]
- Elhabal, S.F.; El-Nabarawi, M.; Elrefai, M.F.M.; Teaima, M.H.; Shoela, M.S.; Khamis, G.M.; Faheem, A.M.; kholeif, N.A.; Sanad, M.T. Nano-Spanlastics-Loaded Dissolving Microneedle Patches for Ketotifen Fumarate: Advanced Strategies for Allergic Conjunctivitis Treatment and Molecular Insights. Drug Deliv. Transl. Res. 2025, 1–24. [Google Scholar] [CrossRef]
- Maity, M.; Galor, A.; Basu, S.; Singh, S. Tear Film Dynamics in Visual Display Terminal Users: A Review of Impact on Goblet Cells, Lacrimal and Meibomian Gland Function. Semin. Ophthalmol. 2024, 40, 306–319. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Chen, X.; Chen, D.; Hua, X.; Bian, F.; Deng, R.; Lu, F.; Li, Z.; Pflugfelder, S.C.; et al. Pollen/TLR4 Innate Immunity Signaling Initiates IL-33/ST2/Th2 Pathways in Allergic Inflammation. Sci. Rep. 2016, 6, 36150. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, P.; Ghate, V.M.; Nampoothiri, M.; Lewis, S.A. Cyclosporine a Eluting Nano Drug Reservoir Film for the Management of Dry Eye Disease. AAPS PharmSciTech 2025, 26, 109. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Y.; Sullivan, A.G.; Zheng, T.; Ke, M. The Impact of Eyeliner Usage on Dry Eye Symptoms. Sci. Rep. 2025, 15, 14039. [Google Scholar] [CrossRef]
- Flanagan, J.L.; Willcox, M.D.P. Role of Lactoferrin in the Tear Film. Biochimie 2009, 91, 35–43. [Google Scholar] [CrossRef]
- LACTOFERRIN DRY EYE—Search Results—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=LACTOFERRIN+DRY+EYE&sort=date (accessed on 24 April 2025).
- Puddu, P.; Valenti, P.; Gessani, S. Immunomodulatory Effects of Lactoferrin on Antigen Presenting Cells. Biochimie 2009, 91, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zong, W.; Jiang, L.; Chen, J.; Wu, D.; Sun, Z. Protective Effects and Mechanisms of Lactoferrin and HIF-1α on Dry Eye Syndrome in Mice. Exp. Eye Res. 2025, 255, 110339. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Comis, S.; Jannuzzi, V.; Camposampiero, D.; Ponzin, D.; Cambria, S.; Santocono, M.; Lavorante, N.P.; Del Noce, C.; Scorcia, V.; et al. Effect of Liposomal-Lactoferrin-Based Eye Drops on the Conjunctival Microflora of Patients Undergoing Cataract Surgery. Ophthalmol. Ther. 2023, 12, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.-N.; Xu, Q.-F.; Yang, M.; Wang, R.-H.; Shu, G.-W.; Li, G.-L. Pretreatment-Free Aptasensing of Lactoferrin in Complex Biological Samples by Portable Electrophoretic Mobility Shift Assay. Int. J. Biol. Macromol. 2025, 286, 138265. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Hong, C.; Hsu, M.Y.; Lai, T.T.; Huang, C.W.; Lu, C.Y.; Chen, W.L.; Cheng, C.M. Fluorescence-Based Reagent and Spectrum-Based Optical Reader for Lactoferrin Detection in Tears: Differentiating Sjögren’s Syndrome from Non-Sjögren’s Dry Eye Syndrome. Sci. Rep. 2024, 14, 14505. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Cheng, S.J. Brain Targeted Delivery of Carmustine Using Solid Lipid Nanoparticles Modified with Tamoxifen and Lactoferrin for Antitumor Proliferation. Int. J. Pharm. 2016, 499, 10–19. [Google Scholar] [CrossRef]
- Pattamatta, U.; Willcox, M.; Stapleton, F.; Garrett, Q. Bovine Lactoferrin Promotes Corneal Wound Healing and Suppresses IL-1 Expression in Alkali Wounded Mouse Cornea. Curr. Eye Res. 2013, 38, 1110–1117. [Google Scholar] [CrossRef]
- Kiratli, H.; Irkeç, M.; Orhan, M. Tear Lactoferrin Levels in Chronic Meibomitis Associated with Acne Rosacea. Eur. J. Ophthalmol. 2000, 10, 11–14. [Google Scholar]
- Vagge, A.; Senni, C.; Bernabei, F.; Pellegrini, M.; Scorcia, V.; Traverso, C.E.; Giannaccare, G. Therapeutic Effects of Lactoferrin in Ocular Diseases: From Dry Eye Disease to Infections. Int. J. Mol. Sci. 2020, 21, 6668. [Google Scholar] [CrossRef]
- Jin, H.; Chen, X.; Ji, F.; Liu, Y.; Sheng, Y.; Wang, G.; Han, L.; Wang, X.; Ding, H.; Liu, J.; et al. Changes in Tear Cytokine and Lactoferrin Levels in Postmenopausal Women with Primary Acquired Nasolacrimal Duct Obstruction Complicated with Obstructed Meibomian Gland Dysfunction. BMC Ophthalmol 2025, 25, 29. [Google Scholar] [CrossRef]
- Dogru, M.; Matsumoto, Y.; Yamamoto, Y.; Goto, E.; Saiki, M.; Shimazaki, J.; Takebayashi, T.; Tsubota, K. Lactoferrin in Sjögren’s Syndrome. Ophthalmology 2007, 114, 2366–2367.e4. [Google Scholar] [CrossRef]
- Gao, S.; Yi, X.; Gao, X.; Long, Z.; Guo, J.; Xia, G.; Shen, X. Stabilization of β-Carotene Liposomes with Chitosan–Lactoferrin Coating System: Vesicle Properties and Anti-Inflammatory In Vitro Studies. Foods 2025, 14, 968. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Luo, H.; Zhu, J.; Ma, H.; Zhang, Y.; Xing, J.; Liu, Y.; Cai, Y.; Sun, W.; Luo, P. In Vitro and in Vivo Assessment of Diosmetin-Loaded Lactoferrin-Modified Liposomes for Brain Delivery in Intracerebral Hemorrhage Therapy. Drug Deliv. Transl. Res. 2025. [Google Scholar] [CrossRef] [PubMed]
- Dieyi, L.; Guner, G.; Chattoraj, S.; Morrison, C. Impact of Air Entrainment on Wet Bead Media Milling of Drug Nanosuspensions and Approaches for Monitoring Entrained Air. J. Pharm. Sci. 2025, 103798. [Google Scholar] [CrossRef]
- Elhabal, S.F.; El-Nabarawi, M.A.; Abdelaal, N.; Mohamed Elrefai, M.F.; Ghaffar, S.A.; Khalifa, M.M.; Mohie, P.M.; Waggas, D.S.; Hamdan, A.M.; Alshawwa, S.Z.; et al. Development of Canagliflozin Nanocrystals Sublingual Tablets in the Presence of Sodium Caprate Permeability Enhancer: Formulation Optimization, Characterization, In-Vitro, In Silico, and In-Vivo Study. Drug Deliv. 2023, 30, 2241665. [Google Scholar] [CrossRef] [PubMed]
- Aldeeb, M.M.E.; Wilar, G.; Suhandi, C.; Mohammed, A.F.A.; El-Rayyes, A.; Elamin, K.M.; Wathoni, N. Formulation and Characterization of Sonchus Arvensis L. Nanosuspension for Enhanced Antioxidant and Lipid-Lowering Activities. Int. J. Nanomed. 2025, 20, 5457–5473. [Google Scholar] [CrossRef]
- Zulbeari, N.; Lund, N.E.; Holm, R. Small-Scale Aqueous Suspension Preparation Using Dual Centrifugation: The Effect of Process Parameters on the Sizes of Drug Particles. Eur. J. Pharm. Sci. 2025, 205, 106980. [Google Scholar] [CrossRef]
- Nagra, U.; Barkat, K.; Ashraf, M.U.; Shabbir, M. Feasibility of Enhancing Skin Permeability of Acyclovir through Sterile Topical Lyophilized Wafer on Self-Dissolving Microneedle-Treated Skin. Dose Response 2022, 20, 15593258221097594. [Google Scholar] [CrossRef]
- Chanabodeechalermrung, B.; Chaiwarit, T.; Chaichit, S.; Udomsom, S.; Baipaywad, P.; Worajittiphon, P.; Jantrawut, P. HPMC/PVP K90 Dissolving Microneedles Fabricated from 3D-Printed Master Molds: Impact on Microneedle Morphology, Mechanical Strength, and Topical Dissolving Property. Polymers 2024, 16, 452. [Google Scholar] [CrossRef]
- Chanabodeechalermrung, B.; Chaiwarit, T.; Udomsom, S.; Rachtanapun, P.; Piboon, P.; Jantrawut, P. Determination of Vat-Photopolymerization Parameters for Microneedles Fabrication and Characterization of HPMC/PVP K90 Dissolving Microneedles Utilizing 3D-Printed Mold. Sci. Rep. 2024, 14, 16174. [Google Scholar] [CrossRef]
- Shabbir, M.; Barkat, K.; Ashraf, M.U.; Nagra, U. Development of a Novel Self-Dissolving Microneedle-Assisted Percutaneous Delivery System of Diacerein through Solid Dispersion Gel: Solubility Enhancement, Proof of Anti-Inflammatory Activity and Safety. Curr. Drug Deliv. 2022, 20, 1351–1367. [Google Scholar] [CrossRef] [PubMed]
- Chaiwarit, T.; Chanabodeechalermrung, B.; Jantrawut, P.; Ruksiriwanich, W.; Sainakham, M. Fabrication and Characterization of Dissolving Microneedles Containing Oryza sativa L. Extract Complex for Enhancement of Transfollicular Delivery. Polymers 2024, 16, 2377. [Google Scholar] [CrossRef]
- Frueh, J.L.; Shu, P.; Vennard, T.R.; Gray, M.A.; Phillips, S.C. Determination of Bovine Lactoferrin in Powdered Infant Formula and Adult Nutritionals by Heparin Affinity Extraction and Reverse-Phase High-Performance Liquid Chromatography/Ultraviolet Detection (HPLC/UV): Single-Laboratory Validation, First Action 2021.10. J. AOAC Int. 2024, 107, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Metzger, K.; Gimsa, U.; Otten, W. Technical Note: Optimizing and Validating an RP-HPLC Method to Determine Lactoferrin in Porcine Colostrum and Milk. J. Anim. Sci. 2025, 103, skaf068. [Google Scholar] [CrossRef]
- Girard, V.; Marchal-Heussler, L.; Chapuis, H.; Brosse, N.; Canilho, N.; Ziegler-Devin, I. Modeling the Production Process of Lignin Nanoparticles Through Anti-Solvent Precipitation for Properties Prediction. Nanomaterials 2024, 14, 1786. [Google Scholar] [CrossRef]
- Hamed, R.; Alhadidi, H.F.I. Minoxidil Nanosuspension-Loaded Dissolved Microneedles for Hair Regrowth. AAPS PharmSciTech 2024, 25, 75. [Google Scholar] [CrossRef]
- Al-Shoubki, A.A.; Teaima, M.H.M.; Abdelmonem, R.A.A.B.; El-Nabarawi, M.A.; Elhabal, S.F. Potential Application of Sucrose Acetate Isobutyrate, and Glyceryl Monooleate for Nanonization and Bioavailability Enhancement of Rivaroxaban Tablets. Pharm. Sci. Adv. 2024, 2, 100015. [Google Scholar] [CrossRef]
- El-Nawawy, T.M.; Adel, Y.A.; Teaima, M.H.M.; Nassar, N.N.; Nemr, A.A.; Al-Samadi, I.; El-Nabarawi, M.A.; Elhabal, S.F. Intranasal Bilosomes in Thermosensitive Hydrogel: Advancing Desvenlafaxine Succinate Delivery for Depression Management. Pharm. Dev. Technol. 2024, 29, 663–674. [Google Scholar] [CrossRef]
- Al-Shoubki, A.A.; Teaima, M.H.M.; Abdelmonem, R.A.A.B.; El-Nabarawi, M.A.; Elhabal, S.F. Sucrose Acetate Isobutyrate (SAIB) and Glyceryl Monooleate (GMO) Hybrid Nanoparticles for Bioavailability Enhancement of Rivaroxaban: An Optimization Study. Pharm. Dev. Technol. 2023, 28, 928–938. [Google Scholar] [CrossRef]
- Zarif Attalla, K.; Hassan, D.H.; Teaima, M.H.; Yousry, C.; El-Nabarawi, M.A.; Said, M.A.; Elhabal, S.F. Enhanced Intranasal Delivery of Atorvastatin via Superparamagnetic Iron-Oxide-Loaded Nanocarriers: Cytotoxicity and Inflammation Evaluation and In Vivo, In Silico, and Network Pharmacology Study for Targeting Glioblastoma Management. Pharmaceuticals 2025, 18, 421. [Google Scholar] [CrossRef]
- Elhabal, S.F.; Abdelmonem, R.A.A.B.; El Nashar, R.M.; Mohamed Elrefai, M.F.; Hamdan, A.M.; Safwat, N.A.; Shoela, M.S.; Hassan, F.E.S.; Rizk, A.; Kabil, S.L.; et al. Enhanced Antibacterial Activity of Clindamycin Using Molecularly Imprinted Polymer Nanoparticles Loaded with Polyurethane Nanofibrous Scaffolds for the Treatment of Acne Vulgaris. Pharmaceutics 2024, 16, 947. [Google Scholar] [CrossRef] [PubMed]
- Elhabal, S.F.; Al-Zuhairy, S.A.; El-Nabarawi, M.A.; Mohamed Elrefai, M.F.; Shoela, M.S.; Hababeh, S.; Nelson, J.; Khalek, M.A.; Fady, M.; Elzohairy, N.A.; et al. Enhancing Photothermal Therapy for Antibiofilm Wound Healing: Insights from Graphene Oxide-Cranberry Nanosheet Loaded Hydrogel in Vitro, in Silico, and in Vivo Evaluation. Int. J. Nanomed. 2024, 19, 12999–13027. [Google Scholar] [CrossRef] [PubMed]
- Al-Zuhairy, S.A.K.S.; Elhabal, S.F.; Mohamed Elrefai, M.F.; Hababeh, S.; Nelson, J.; Fady, M.; Elzohairy, N.A.; Ewedah, T.M.; Mousa, I.S.; Hamdan, A.M.E. Polylactic-Co-Glycolic Acid/Alginate/Neem Oil-Reduced Graphene Oxide as a PH-Sensitive Nanocarrier for Hesperidin Drug Delivery: Antimicrobial and Acute Otitis Media Assessments. Pharmaceuticals 2025, 18, 381. [Google Scholar] [CrossRef] [PubMed]
- ELhabal, S.F.; El-Nabarawi, M.A.; Hassanin, S.O.; Hassan, F.E.; Abbas, S.S.; Gebril, S.M.; Albash, R. Transdermal Fluocinolone Acetonide Loaded Decorated Hyalurosomes Cellulose Acetate/Polycaprolactone Nanofibers Mitigated Freund’s Adjuvant-Induced Rheumatoid Arthritis in Rats. J. Pharm. Investig. 2025, 55, 113–132. [Google Scholar] [CrossRef]
- Mohammed, M.H.H.; El-Sayed Hamed, A.N.; Elhabal, S.F.; Mokhtar, F.A.; Abdelmohsen, U.R.; Fouad, M.A.; Kamel, S.M. Chemical Composition and Anti-Proliferative Activities of Hyophorbe lagenicaulis Aerial Parts and Their Biogenic Nanoparticles Supported by Network Pharmacology Study. S. Afr. J. Bot. 2023, 156, 398–410. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Mangukiya, M.A.; Patel, P.A.; Vaidya, R.J.; Koli, A.R.; Ranch, K.M.; Shah, D.O. Extended Release of Ketotifen from Silica Shell Nanoparticle-Laden Hydrogel Contact Lenses: In Vitro and in Vivo Evaluation. J. Mater. Sci. Mater. Med. 2016, 27, 113. [Google Scholar] [CrossRef]
- Elhabal, S.F.; Faheem, A.M.; Hababeh, S.; Nelson, J.; Elzohairy, N.A.; Ibrahim, Y.F.; Ewedah, T.M.; Mousa, I.S.; Allam, K.M.; Hamdan, A.M.E. Augmented Marshmallow Extract Lipid Nanoparticles with Clove Oil Embedded in Collagen Sponge for Ultimate Antimicrobial Healing of Diabetic Mouth Ulcer. Pharmaceutics 2025, 17, 611. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, T.; Tan, D.; Liu, J.; Gao, Y.; Wang, H.; Gao, F.; Yang, Z. Preparation, Characterization, and Ex Vivo Evaluation of Isoxanthohumol Nanosuspension. Int. J. Pharm. 2024, 667, 124909. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Zhang, L.; Wang, Q.; Zhang, D. Stability of Nanosuspensions in Drug Delivery. J. Control. Release 2013, 172, 1126–1141. [Google Scholar] [CrossRef]
- Pyrzynska, K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yue, J.; Guo, S.; Rahmatulla, A.; Li, S.; Liu, Y.; Chen, Y. Dissolving Microneedles: A Transdermal Drug Delivery System for the Treatment of Rheumatoid Arthritis. Int. J. Pharm. 2025, 671, 125206. [Google Scholar] [CrossRef] [PubMed]
- Asaf, M.B.; Khairiyah; Kurniawan, I.; Achmad, N.A.; Tuna, R.W.; Himawan, A.; Rahman, L.; Aliyah; Agustina, R.; Dominguez-Robles, J.; et al. Amphotericin B Nanocrystals Integrated with Bilayer Dissolving Microneedles: A New Strategy for Transmucosal Delivery of Amphotericin B to Improve the Effectiveness of Oral Candidiasis Therapy. J. Pharm. Investig. 2025, 1–19. [Google Scholar] [CrossRef]
- Alkhiro, A.R.; Ghareeb, M.M. Formulation and Evaluation of Iornoxicam as Dissolving Microneedle Patch. Iraqi J. Pharm. Sci. 2020, 29, 184–194. [Google Scholar] [CrossRef]
- Ewedah, T.M.; Abdalla, A.M.; Hagag, R.S.; Elhabal, S.F.; Teaima, M.H.M.; El-Nabarawi, M.A.; Schlatter, G.; Shoueir, K.R. Enhancing Cellular Affinity for Skin Disorders: Electrospun Polyurethane/Collagen Nanofiber Mats Coated with Phytoceramides. Int. J. Pharm. 2024, 663, 124541. [Google Scholar] [CrossRef]
- Nagaraja, K.; Mallika, B.; Arunpandian, M.; Ravindran, E.; Tae Hwan, O.H. Green Synthesis of Gold-Decorated BaTiO3-ZnO Nanocomposites Using Arabic Gum Polymer for Efficient Photocatalytic Degradation of Emerging Textile Dyes, Antimicrobial, and Toxicological Evaluation. Int. J. Biol. Macromol. 2025, 311, 143396. [Google Scholar] [CrossRef]
- Di Giulio, M.; Zappacosta, R.; Di Lodovico, S.; Di Campli, E.; Siani, G.; Fontana, A.; Cellini, L. Antimicrobial and Antibiofilm Efficacy of Graphene Oxide against Chronic Wound Microorganisms. Antimicrob. Agents Chemother. 2018, 62, e00547-18. [Google Scholar] [CrossRef] [PubMed]
- Sachin, K.S.; Shetty, K.S.; Jeyalakshmi, K.B.; Harishma, S.; Harshini, S. Assessing the Antimicrobial Properties of Bioceramic Sealers Enhanced with Herbal Extracts against E. faecalis. Folia Med. 2025, 67, e142966. [Google Scholar] [CrossRef]
- Mishra, A.; Halder, J.; Saha, I.; Rai, V.K.; Mahanty, R.; Pradhan, D.; Dash, P.; Das, C.; Rajwar, T.K.; Satpathy, B.; et al. Biogenic Amino Acid Cross-Linked Hyaluronic Acid Nanoparticles Containing Dexamethasone for the Treatment of Dry Eye Syndrome. AAPS PharmSciTech 2025, 26, 97. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, B.; Lin, J.; Wang, Q.; Zhang, L.; Li, Y.; Liu, X.; Liu, Y.; Li, C.; Zhao, G. Piperine Inhibits Fungal Growth and Protects against Pyroptosis in Aspergillus fumigatus Keratitis by Regulating the MTOR/HIF-1α Pathway. Int. Immunopharmacol. 2025, 150, 114286. [Google Scholar] [CrossRef]
- Gu, L.; Chi, M.; Wang, Z.; Fu, Y.; Li, N.; Niu, Y.; Yu, B.; Lin, J.; Li, C.; Zhao, G. Hydroxytyrosol Downregulates Inflammatory Responses via Nrf2/HO-1 Axis during Fungal Keratitis and Exerts Antifungal Effects. Int. Immunopharmacol. 2025, 149, 114202. [Google Scholar] [CrossRef]
- van Vollenhoven, W.M.A.; Hensen, E.F.; de Boer, N.P.; Koot, R.W.; Jansen, J.C.; Malessy, M.J.A. Tear Production after Vestibular Schwannoma Surgery and Intermediate Nerve Function. J. Neurosurg. 2025, 1–7. [Google Scholar] [CrossRef]
- Agnifili, L.; Ruggeri, M.L.; Figus, M.; Corboli, L.V.; Fornaro, M.; Covello, G.; Mastropasqua, R.; Di Nicola, M.; Marotta, A.; Mastropasqua, L. Unraveling the Effects of Serial Intravitreal Aflibercept Injections on the Ocular Surface of Patients with Glaucoma and Retinal Comorbidity. Sci. Rep. 2025, 15, 12980. [Google Scholar] [CrossRef] [PubMed]
- Doğan, C.S.; Akkoc, O.; Özağır, M.; Avci, S.; Biçer Baikoglu, S.; Kurudirek, M.İ.; Ulucan, K. Comparison of Athletic Performance of Turkish Ice Hockey Players with ACE I/D (Rs1799752), ACTN3 (Rs1815739), PPARA (Rs4253778) and HIF1A (Rs11549465) Polymorphisms. Cell. Mol. Biol. 2024, 70, 142–153. [Google Scholar] [CrossRef]
- Silva, C.M.P.C.; Massoni, V.V.; Araujo, L.D.C.; Lima, R.B.; de Freitas Miranda-Filho, A.E.; Pucinelli, C.M.; Lucisano, M.P.; Nelson-Filho, P.; Consolaro, A.; da Silva, R.A.B.; et al. Correlation between REDOX Enzymes and NET Markers Expression during the Development of Periapical Lesions in Mice. Clin. Oral Investig. 2025, 29, 166. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, S.T.; Mirzaei, S.A.; Mohamadhashem, F.; Naghizadeh, M.M.; Razavi, A.N.; Mansoori, Y.; Daraei, A.; Mohamadhashem, F. Pathological Expression of Mitochondrial Genome-Derived CircRNA SCAR/Mc-COX2 and Its CeRNA Network in Colorectal Cancer: Implications for Clinical Significance. BMC Cancer 2025, 25, 466. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fu, C.; Li, F. Acetate Affects the Process of Lipid Metabolism in Rabbit Liver, Skeletal Muscle and Adipose Tissue. Animals 2019, 9, 799. [Google Scholar] [CrossRef]
- Schnupf, P.; Sansonetti, P.J. Quantitative RT-PCR Profiling of the Rabbit Immune Response: Assessment of Acute Shigella flexneri Infection. PLoS ONE 2012, 7, e36446. [Google Scholar] [CrossRef]
- Abou-Hashim, F.; Khalifa, W.H.; Shalaby, M.B.; Kassem, S.M.; Khalil, W.K.B. Evaluation of Fasting and Probiotics in Reducing Postweaning Stress in Rabbits: Study of Their Effects on Biochemical and Gene Expression Patterns. Appl. Biochem. Biotechnol. 2024, 196, 558–572. [Google Scholar] [CrossRef]
- Villani, E.; Campagna, G.; Gentili, V.; Postorino, E.I.; Genovese, P.; Palino, P.; Maini, G.; Carbucicchio, A.; Ferioli, E.; Nucci, P.; et al. Hydroxypropyl-Methylcellulose and GlicoPro® Eyedrops in the Treatment of Dry Eye Disease: In Vitro and Clinical Study. Ophthalmol. Ther. 2025, 14, 787–803. [Google Scholar] [CrossRef]
- Yu, M.; Xia, M.L.; Yu, M.; Jiang, W.J.; Zhou, Z.; Si, J.Q.; Li, L. Effect of TNF-α on Cisplatin-Induced Permeability Change of Blood Labyrinth Barrier in Cochlea of C57BL/6J Mice. Chin. J. Otorhinolaryngol. Head Neck Surg. 2025, 60, 447–456. [Google Scholar] [CrossRef]
- Chelnis, J.G.; Chelnis, A. Dynamic Muscle Stimulation of the Periorbital Area for Improvement of Blinking in Dry Eye Patients. Clin. Ophthalmol. 2025, 19, 1057–1071. [Google Scholar] [CrossRef]
- Thomas, R.G.; Kim, J.H.; Kim, J.H.; Yoon, J.; Choi, K.H.; Jeong, Y.Y. Treatment of Ischemic Stroke by Atorvastatin-Loaded PEGylated Liposome. Transl. Stroke Res. 2024, 15, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, L.; Wang, J.; Gong, M.; Yuan, R.; Lu, J.; Xiao, X.; Liu, X. Effects of RAmb a 1-Loaded PLGA-PEG Nanoparticles in a Murine Model of Allergic Conjunctivitis. Molecules 2022, 27, 598. [Google Scholar] [CrossRef]
- Cheng, Y.; Farasati Far, B.; Jahanbakhshi, M.; Bahrami, S.; Tamimi, P.; Sedaghat, M.; Ghazizadeha, E. Exploring the Potential of a Polyvinyl Alcohol/Chitosan-Based Nanofibrous Matrix for Erythromycin Delivery: Fabrication, In Vitro and In Vivo Evaluation. RSC Adv. 2023, 13, 18450–18460. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, S.; Zhao, X.; Lian, J.; Gao, Y. Preparation, Characterization, and in Vivo Evaluation of Levonorgestrel-Loaded Thermostable Microneedles. Drug Deliv. Transl. Res. 2022, 12, 944–956. [Google Scholar] [CrossRef]
- Ananda, P.W.R.; Elim, D.; Zaman, H.S.; Muslimin, W.; Tunggeng, M.G.R.; Permana, A.D. Combination of Transdermal Patches and Solid Microneedles for Improved Transdermal Delivery of Primaquine. Int. J. Pharm. 2021, 609, 121204. [Google Scholar] [CrossRef]
- Guo, X.; Qiao, X.; Li, X.; Zhou, W.; Liu, C.; Yu, F.; Chen, Q.; Pan, M.; Niu, X.; Wang, X.; et al. Lactoferrin-Modified Organic-Inorganic Hybrid Mesoporous Silica for Co-Delivery of Levodopa and Curcumin in the Synergistic Treatment of Parkinson’s Disease. Phytomedicine 2025, 140, 156547. [Google Scholar] [CrossRef] [PubMed]
- Kral, Ö.; Ilbasmis-Tamer, S.; Han, S.; Tirnaksiz, F. Development of Dermal Lidocaine Nanosuspension Formulation by the Wet Milling Method Using Experimental Design: In Vitro/In Vivo Evaluation. ACS Omega 2024, 9, 50992–51008. [Google Scholar] [CrossRef] [PubMed]
- Schaffazick, S.R.; Guterres, S.S.; De Lucca Freitas, L.; Pohlmann, A.R. Physicochemical Characterization and Stability of the Polymeric Nanoparticle Systems for Drug Administration. Quim. Nova 2003, 26, 726–737. [Google Scholar] [CrossRef]
- Jeong, S.J.; Lee, J.S.; Lee, H.G. Nanoencapsulation of Synergistic Antioxidant Fruit and Vegetable Concentrates and Their Stability during in Vitro Digestion. J. Sci. Food Agric. 2020, 100, 1056–1063. [Google Scholar] [CrossRef]
- Gade, S.; Glover, K.; Mishra, D.; Sharma, S.; Guy, O.; Donnelly, R.F.; Vora, L.K.; Thakur, R.R.S. Hollow Microneedles for Ocular Drug Delivery. J. Control. Release 2024, 371, 43–66. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.; Roy, G.; Garg, P.; Venuganti, V.V.K. Ocular Delivery of Cyclosporine A Using Dissolvable Microneedle Contact Lens. J. Drug Deliv. Sci. Technol. 2022, 70, 103211. [Google Scholar] [CrossRef]
- Jin, M.; Jeon, W.J.; Lee, H.; Jung, M.; Kim, H.E.; Yoo, H.; Won, J.H.; Kim, J.C.; Park, J.H.; Yang, M.J.; et al. Preparation and Evaluation of Rapid Disintegrating Formulation from Coated Microneedle. Drug Deliv. Transl. Res. 2022, 12, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, X.; Zheng, X.; Yang, J.; Hui, J.; Fan, D. Rapid Dissolution Microneedle Based on Polyvinyl Alcohol/Chitosan for Local Oral Anesthesia. Int. J. Biol. Macromol. 2024, 257, 128629. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, F.; Jin, Q. Polymer-Mediated Delivery of Amphotericin B for Fungal Infections. Macromol. Rapid Commun. 2025, 2500013. [Google Scholar] [CrossRef]
- Men, Z.; Su, T.; Tang, Z.; Liang, J.; Shen, T. Tacrolimus Nanocrystals Microneedle Patch for Plaque Psoriasis. Int. J. Pharm. 2022, 627, 122207. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, D.; Chen, S.; Wang, X.; Zheng, Z.; Gui, S.; He, N. Minimally Invasive Treatment of Fungal Keratitis with Voriconazole Microneedle Corneal Patch. Eur. J. Pharm. Biopharm. 2025, 211, 114717. [Google Scholar] [CrossRef]
- Sabbagh, F.; Kim, B.S. Ex Vivo Transdermal Delivery of Nicotinamide Mononucleotide Using Polyvinyl Alcohol Microneedles. Polymers 2023, 15, 2031. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Pandya, A.K.; Demartis, S.; Domínguez-Robles, J.; Moreno-Castellanos, N.; Li, H.; Gavini, E.; Patravale, V.B.; Donnelly, R.F. Liposome-Loaded Polymeric Microneedles for Enhanced Skin Deposition of Rifampicin. Int. J. Pharm. 2023, 646, 123446. [Google Scholar] [CrossRef]
- Rad, Z.F.; Nordon, R.E.; Anthony, C.J.; Bilston, L.; Prewett, P.D.; Arns, J.Y.; Arns, C.H.; Zhang, L.; Davies, G.J. High-Fidelity Replication of Thermoplastic Microneedles with Open Microfluidic Channels. Microsyst. Nanoeng. 2017, 3, 17034. [Google Scholar] [CrossRef]
- Fouad, S.A.; Malaak, F.A.; Teaima, M.H.M.; Omar, S.M.; Kutkat, O.M.; Elhabal, S.F.; El-Nabarawi, M.A. Novel Inhalable Nano-Based/Microparticles for Enhanced Sustained Pulmonary Delivery of Remdesivir—A Patient Malleable Treatment for Coronaviruses Infection: In Vitro Aerosolization, Cytotoxicity Assays and Antiviral Activity Studies. J. Drug Deliv. Sci. Technol. 2024, 101, 106196. [Google Scholar] [CrossRef]
- Mohanty, D.L.; Divya, N.; Zafar, A.; Warsi, M.H.; Parida, G.R.; Padhi, P.; Khalid, M.; Yasir, M.; Mujtaba, M.A. Development of Etoricoxib-Loaded Mesoporous Silica Nanoparticles Laden Gel as Vehicle for Transdermal Delivery: Optimization, Ex Vivo Permeation, Histopathology, and in Vivo Anti-Inflammatory Study. Drug Dev. Ind. Pharm. 2025, 51, 506–521. [Google Scholar] [CrossRef] [PubMed]
- Raab, C.; Brugger, S.; Lechner, J.-S.; Barbalho, G.N.; Gratieri, T.; Agarwal, P.; Rupenthal, I.D.; Keck, C.M. Utilizing an Ex Vivo Skin Penetration Analysis Model for Predicting Ocular Drug Penetration: A Feasibility Study with Curcumin Formulations. Pharmaceutics 2024, 16, 1302. [Google Scholar] [CrossRef]
- Ong, R.; Cornish, J.; Wen, J. Nanoparticular and Other Carriers to Deliver Lactoferrin for Antimicrobial, Antibiofilm and Bone-Regenerating Effects: A Review. Biometals 2022, 36, 709–727. [Google Scholar] [CrossRef]
- Tong, J.; Liu, Z.; Zhou, K.; Wang, K.; Guo, S.; Zhang, H. Thermosensitive Bovine Lactoferricin-Loaded Chitosan Hydrogels for Sustained Antibacterial Release: An Alternative to Antibiotics for Treating Bovine Mastitis. Int. J. Biol. Macromol. 2025, 303, 140673. [Google Scholar] [CrossRef]
- Ramamourthy, G.; Vogel, H.J. Antibiofilm Activities of Lactoferricin-Related Trp- and Arg-Rich Antimicrobial Hexapeptides against Pathogenic Staphylococcus aureus and Pseudomonas aeruginosa Strains. Biochem. Cell Biol. 2025, 103, 1–18. [Google Scholar] [CrossRef]
- Carney, F.P.; Morris, C.A.; Milthorpe, B.; Flanagan, J.L.; Willcox, M.D.P. In Vitro Adsorption of Tear Proteins to Hydroxyethyl Methacrylate-Based Contact Lens Materials. Eye Contact Lens 2009, 35, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Muller, S.J.; Radke, C.J. Wettability of Silicone-Hydrogel Contact Lenses in the Presence of Tear-Film Components. Curr. Eye Res. 2004, 28, 93–108. [Google Scholar] [CrossRef]
- Song, J.H.; Park, S.Y. Safety and Efficacy of Combined Acupuncture (Body and Intradermal Acupuncture) for Dry Eye Disease: Study Protocol for a Pilot, Single-Centre, Assessor-Blinded, Randomised, Artificial Tear Drop-Controlled Trial at Naju Dongshin University Korean Medicine Hospital. BMJ Open 2024, 14, e077913. [Google Scholar] [CrossRef]
- Mishra, A.; Halder, J.; Saha, I.; Rai, V.K.; Mahanty, R.; Pradhan, D.; Dash, P.; Das, C.; Rajwar, T.K.; Satpathy, B.; et al. Quercetin Loaded Biogenic Squalene Nano-Lipid Carriers for the Treatment of Dry Eye Syndrome. Int. J. Pharm. 2025, 674, 125457. [Google Scholar] [CrossRef]
- Chu, D.; Zhao, M.; Rong, S.; Jhe, W.; Cai, X.; Xiao, Y.; Zhang, W.; Geng, X.; Li, Z.; Zhang, X.; et al. Dual-Atom Nanozyme Eye Drops Attenuate Inflammation and Break the Vicious Cycle in Dry Eye Disease. Nanomicro Lett. 2024, 16, 120. [Google Scholar] [CrossRef] [PubMed]


| Factors (Independent Variables) | Levels | ||
| A: Polymer Conc (w/v%) | 1 | 2 | 4 |
| B: Stirrer time (h) | 0.5 | 1 | 2 |
| C: Polymer Type | P188 | TPGS | Soy lecithin |
| D: Plasticizer Type | PG | PEG | Glycerol |
| Responses (Dependent Variables) | Constraints | ||
| Y1: P.S (nm) | Minimize | ||
| Y2: P.D.I | Minimize | ||
| Y3: Drug content | Maximize | ||
| Y4: Z.P | Maximize (absolute value) | ||
| Formulation | PVP | HPMC |
|---|---|---|
| MNs1 | 15 | 10 |
| MNs2 | 10 | 15 |
| MNs3 | 5 | 25 |
| Gene | Accession Number | Primer Direction | Primer Sequence (5′-3′) | Product Size (bp) | Reference |
|---|---|---|---|---|---|
| PPARA | XM_002723354.3 | Forward | AGGCCCTCTTCAGAACCTGT | 112 | [67] |
| Reverse | GTGGCTTTCTGTTCCCAGAG | ||||
| COX-2 | NM_001082388.1 | Forward | CGGATTCTACGGTGAAAACTGC | 124 | [68] |
| Reverse | GACGATGTTCCAGACTCCCTTG | ||||
| SOD 1 | NM_001082627 | Forward | ACCTGGGTAATGTGACTGCA | 132 | [69] |
| Reverse | CAATGACACCACAGGCCAAA | ||||
| β-actin | NM_001101683 | Forward | CGCAGAAACGAGACGAGATT | 168 | [69] |
| Reverse | GCAGAACTTTGGGGACTTTG |
| Factors | Responses | |||||||
|---|---|---|---|---|---|---|---|---|
| Run | A: Polymer Concentration (w/v%) | B: Stirrer Time (h) | C: Polymer Type | D: Plasticizer Type | P.S nm | P.D.I Nm | Z.P mV | Drug Content % |
| 1 | 1 | 0.5 | P188 | PG | 480 ± 0.01 | 0.54 ± 0.03 | −32 ± 0.71 | 75 ± 0.67 |
| 2 | 2 | 2 | P188 | PG | 650 ± 0.43 | 0.30 ± 0.09 | −28 ± 0.45 | 70 ± 0.87 |
| 3 | 2 | 1 | P188 | PEG | 420 ± 0.45 | 0.25 ± 0.01 | −34 ± 0.91 | 80 ± 0.61 |
| 4 | 4 | 0.5 | P188 | PEG | 590 ± 0.97 | 0.20 ± 0.06 | −31 ± 0.58 | 85 ± 0.58 |
| 5 | 2 | 1 | TPGS | PEG | 510 ± 0.82 | 0.22 ± 0.08 | −29 ± 0.43 | 82 ± 0.48 |
| 6 | 4 | 2 | TPGS | PEG | 330 ± 0.78 | 0.65 ± 0.03 | −35 ± 0.91 | 60 ± 0.81 |
| 7 | 1 | 2 | TPGS | PG | 310 ± 0.67 | 0.20 ± 0.01 | −30 ± 0.37 | 68 ± 0.75 |
| 8 | 1 | 0.5 | Soy Lecithin | PEG | 540 ± 0.54 | 0.31 ± 0.03 | −27 ± 0.65 | 55 ± 0.05 |
| 9 | 1 | 2 | Soy Lecithin | PEG | 310 ± 0.92 | 0.23 ± 0.05 | −33 ± 0.91 | 65 ± 0.58 |
| 10 | 4 | 1 | Soy Lecithin | PEG | 390 ± 0.89 | 0.20 ± 0.06 | −32 ± 0.56 | 57 ± 0.91 |
| 11 | 4 | 2 | P188 | Glycerol | 530 ± 0.82 | 0.28 ± 0.09 | −30 ± 0.46 | 50 ± 0.78 |
| 12 | 1 | 0.5 | TPGS | Glycerol | 420 ± 0.86 | 0.25 ± 0.02 | −31 ± 0.38 | 85 ± 0.82 |
| 13 | 1 | 2 | P188 | Glycerol | 500 ± 0.96 | 0.22 ± 0.03 | −32 ± 0.71 | 80 ± 0.56 |
| 14 | 2 | 1 | P188 | PEG | 480 ± 0.56 | 0.20 ± 0.06 | −35 ± 0.51 | 58 ± 0.81 |
| 15 | 2 | 1 | Soy Lecithin | Glycerol | 490 ± 0.38 | 0.20 ± 0.01 | −33 ± 0.71 | 65 ± 0.75 |
| 16 | 4 | 0.5 | Soy Lecithin | Glycerol | 410 ± 0.72 | 0.23 ± 0.01 | −32 ± 0.65 | 83 ± 0.67 |
| 17 | 4 | 1 | TPGS | Glycerol | 530 ± 0.67 | 0.28 ± 0.04 | −28 ± 0.91 | 78 ± 0.84 |
| 18 | 4 | 2 | P188 | PEG | 215 ± 0.45 | 0.21 ± 0.06 | −28 ± 0.34 | 90 ± 0.66 |
| 19 | 2 | 1 | Soy Lecithin | PG | 605 ± 0.93 | 0.54 ± 0.02 | −27 ± 0.05 | 77 ± 0.65 |
| 20 | 2 | 0.5 | P188 | Glycerol | 450 ± 0.71 | 0.45 ± 0.12 | −29 ± 0.43 | 69 ± 0.35 |
| 21 | 2 | 2 | TPGS | Glycerol | 390 ± 0.75 | 0.65 ± 0.05 | −33 ± 0.06 | 65 ± 0.54 |
| 22 | 4 | 0.5 | TPGS | PG | 490 ± 0.47 | 0.87 ± 0.09 | −29 ± 0.34 | 55 ± 0.72 |
| 23 | 2 | 0.5 | TPGS | PG | 450 ± 0.96 | 0.65 ± 0.05 | −30 ± 0.62 | 61 ± 0.57 |
| 24 | 4 | 2 | Soy Lecithin | PG | 554 ± 0.81 | 0.76 ± 0.03 | −32 ± 0.41 | 59 ± 0.98 |
| Parameters | BLF-GLY Freshly Prepared | BLF-GLY After 3 Months of Storage at 4 °C | BLF-GLY After 3 Months of Storage at 25 °C |
|---|---|---|---|
| P.S (nm) | 215 ± 0.45 | 211 ± 0.05 | 209 ± 0.81 |
| P.D.I | 0.21 ± 0.06 | 0.21 ± 0.02 | 0.22 ± 0.01 |
| Z.P (mV) | −28 ± 0.34 | −27 ± 0.01 | −29 ± 0.46 |
| EE (%) | 99 ± 0.66 | 98 ± 0.98 | 97 ± 0.46 |
| Strain | Zone Inhibition (mm) | MIC (µg/mL) | ||||
|---|---|---|---|---|---|---|
| Control | BLF | BLF-NS | Control | BLF | BLF-NS | |
| (MRSA) | 15 ± 0.37 | 18 ± 0.81 | 20 ± 0.23 | 400 ± 12.71 | 31.25 ± 0.13 | 15.63 ± 0.56 |
| Staphylococcus aureus | 24 ± 0.23 | 31 ± 0.43 | 35 ± 0.23 | 10 ± 0.12 | 10 ± 0.04 | 10 ± 0.62 |
| Aspergillus niger | 15 ± 0.78 | 13 ± 0.94 | 17 ± 0.13 | 500 ± 0.05 | 500 ± 0.13 | 62.50.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhabal, S.F.; Faheem, A.M.; Hababeh, S.; Nelson, J.; Elzohairy, N.A.; AbdelGhany Morsy, S.A.; Ewedah, T.M.; Mousa, I.S.; Fouad, M.A.; Hamdan, A.M.E. Dissolving Microneedles Containing Lactoferrin Nanosuspension for Enhancement of Antimicrobial and Anti-Inflammatory Effects in the Treatment of Dry Eye Disease. Pharmaceutics 2025, 17, 653. https://doi.org/10.3390/pharmaceutics17050653
Elhabal SF, Faheem AM, Hababeh S, Nelson J, Elzohairy NA, AbdelGhany Morsy SA, Ewedah TM, Mousa IS, Fouad MA, Hamdan AME. Dissolving Microneedles Containing Lactoferrin Nanosuspension for Enhancement of Antimicrobial and Anti-Inflammatory Effects in the Treatment of Dry Eye Disease. Pharmaceutics. 2025; 17(5):653. https://doi.org/10.3390/pharmaceutics17050653
Chicago/Turabian StyleElhabal, Sammar Fathy, Ahmed Mohsen Faheem, Sandra Hababeh, Jakline Nelson, Nahla A. Elzohairy, Suzan Awad AbdelGhany Morsy, Tassneim M. Ewedah, Ibrahim S. Mousa, Marwa A. Fouad, and Ahmed Mohsen Elsaid Hamdan. 2025. "Dissolving Microneedles Containing Lactoferrin Nanosuspension for Enhancement of Antimicrobial and Anti-Inflammatory Effects in the Treatment of Dry Eye Disease" Pharmaceutics 17, no. 5: 653. https://doi.org/10.3390/pharmaceutics17050653
APA StyleElhabal, S. F., Faheem, A. M., Hababeh, S., Nelson, J., Elzohairy, N. A., AbdelGhany Morsy, S. A., Ewedah, T. M., Mousa, I. S., Fouad, M. A., & Hamdan, A. M. E. (2025). Dissolving Microneedles Containing Lactoferrin Nanosuspension for Enhancement of Antimicrobial and Anti-Inflammatory Effects in the Treatment of Dry Eye Disease. Pharmaceutics, 17(5), 653. https://doi.org/10.3390/pharmaceutics17050653







