Sonoporation with Echogenic Liposomes: The Evaluation of Glioblastoma Applicability Using In Vivo Xenograft Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Echogenic Liposomes and Encapsulation of Doxorubicin
2.2. Evaluation Population Characteristics of Formed Echogenic Liposomes
2.3. Confirmation of Drug Load Ability Through Electron Microscopy
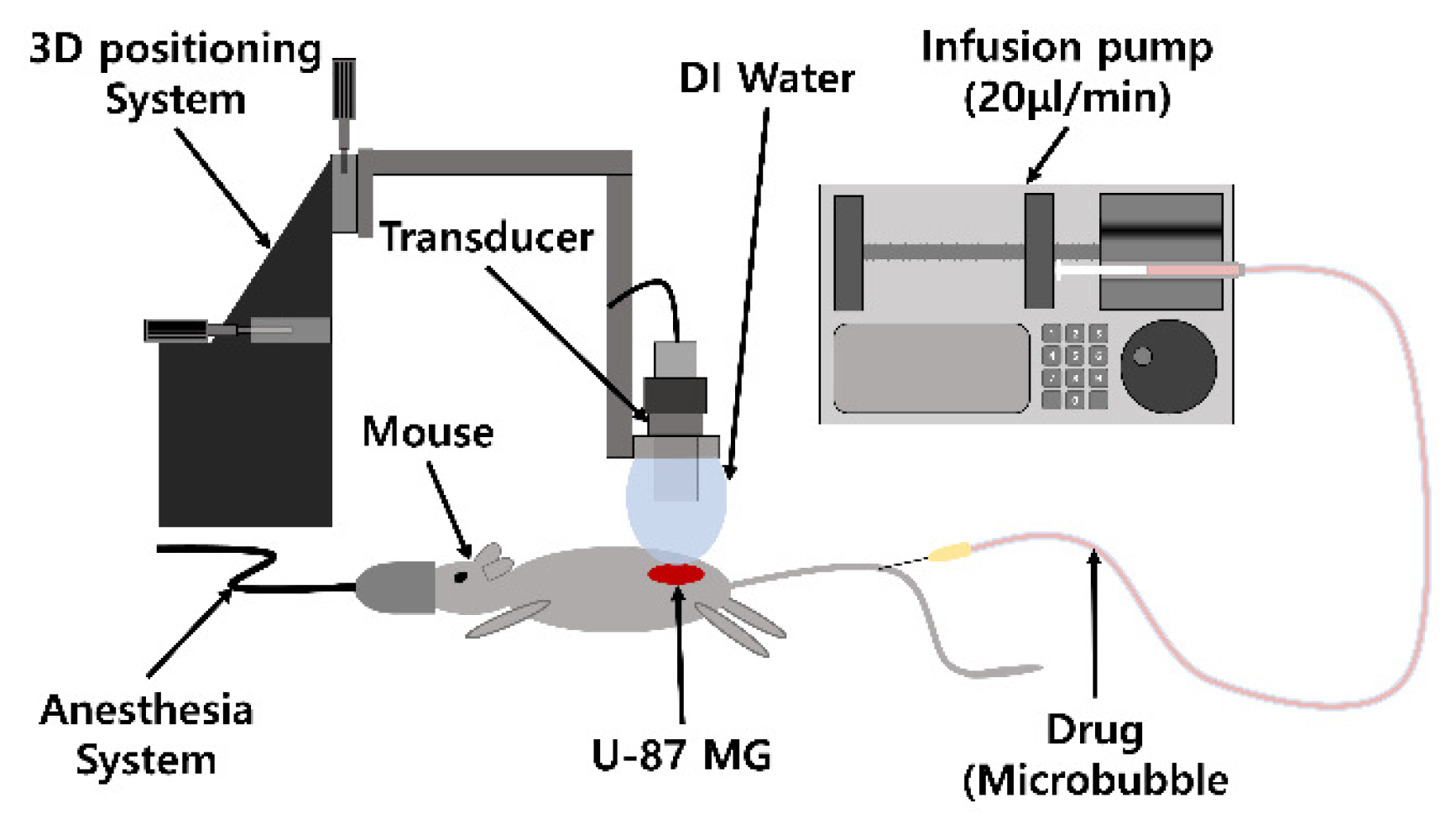
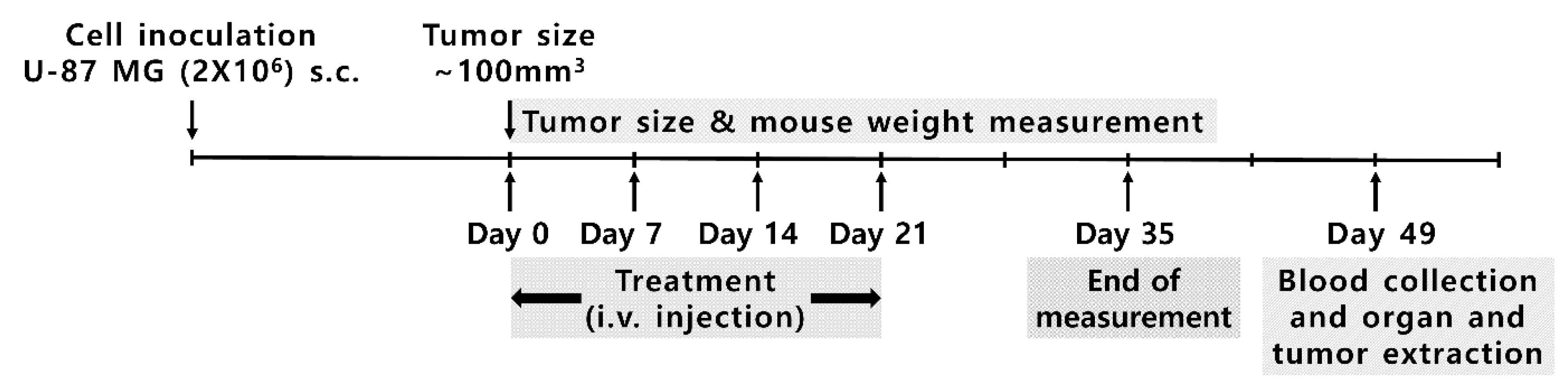
2.4. Doxorubicin Delivery to U-87 MG: In Vivo Experiment
3. Results
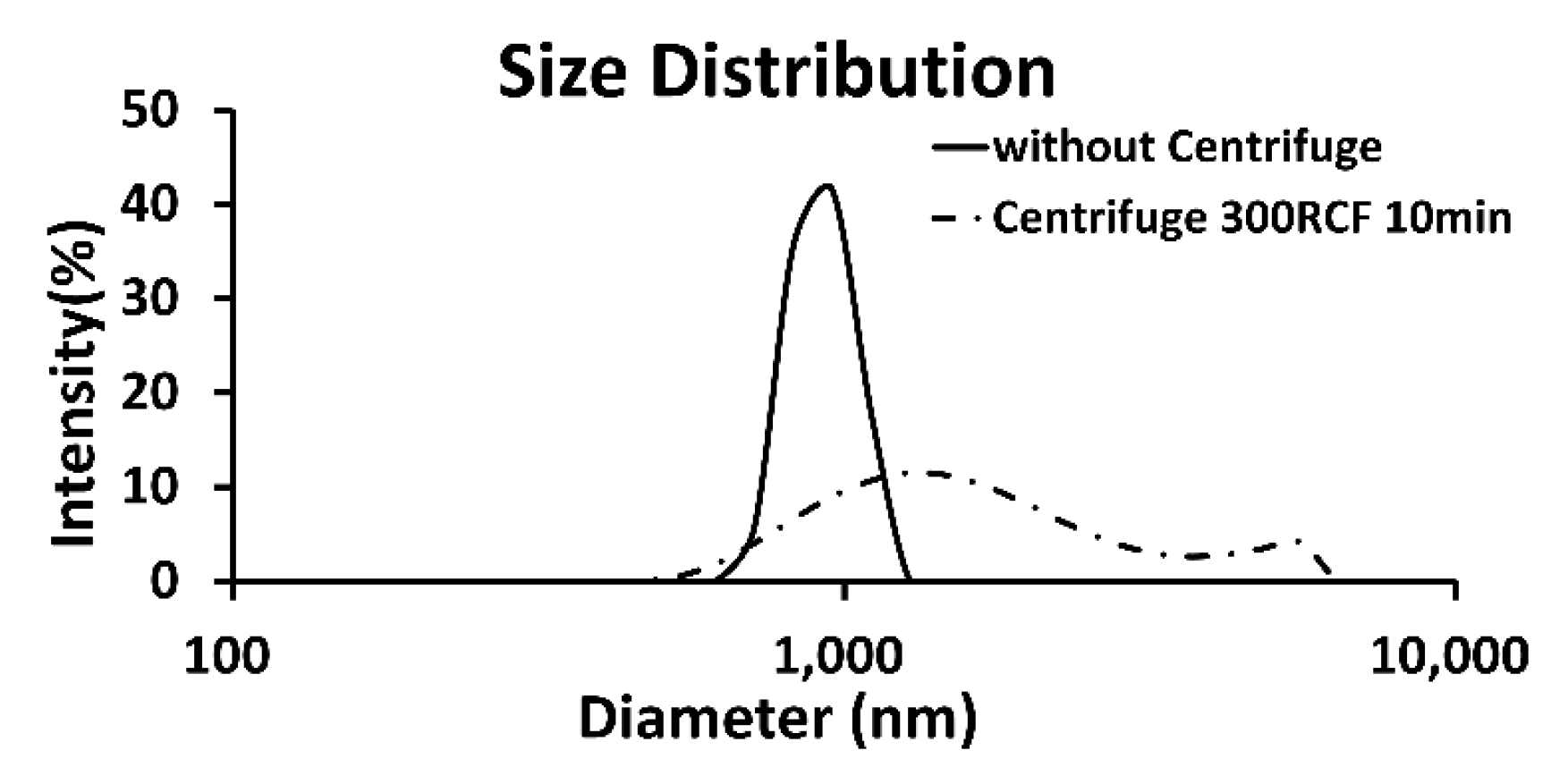
3.1. Population Characteristics of the Formed Echogenic Liposomes
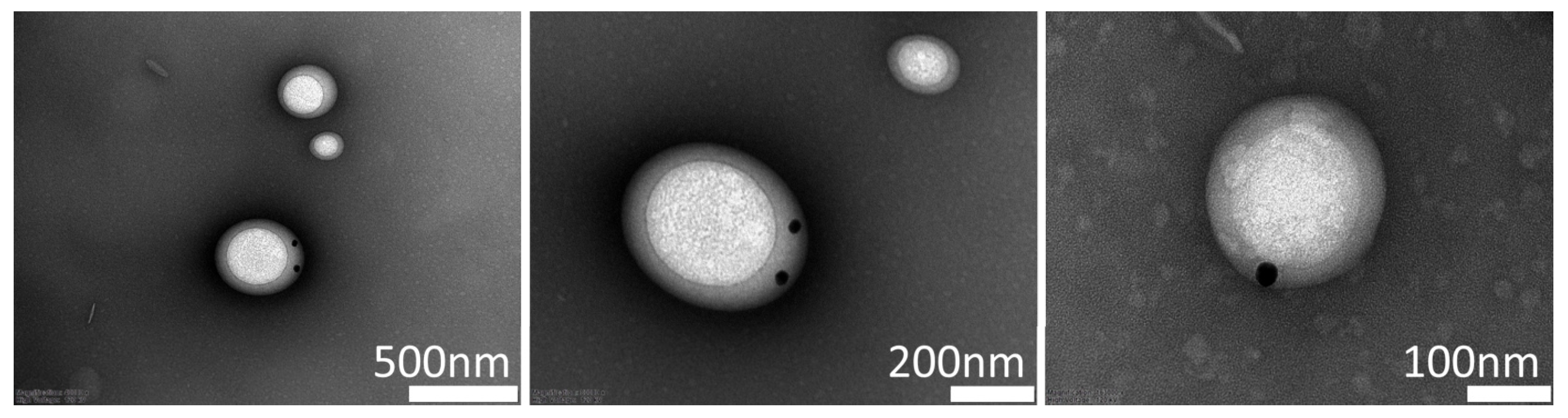
3.2. Visualization of Drug Load Ability Through Electron Microscopy
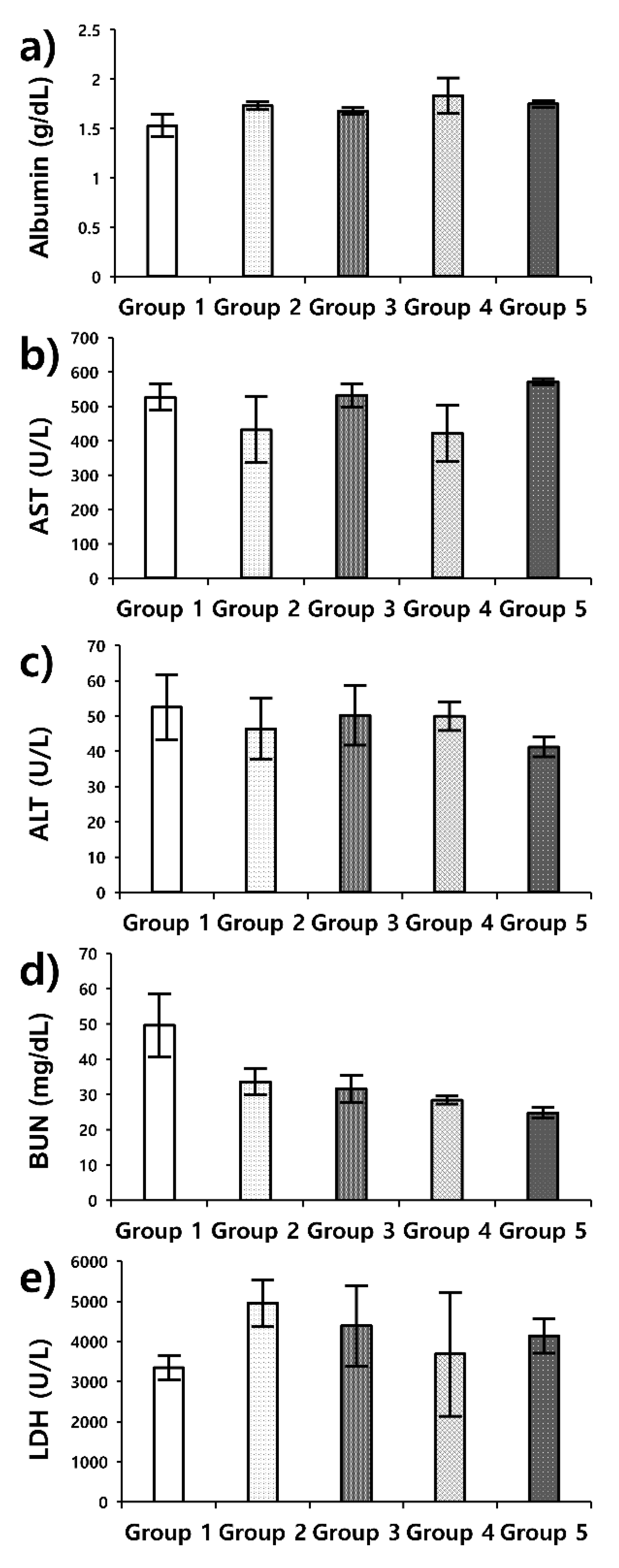
3.3. Doxorubicin Delivery to U-87 MG: In Vivo Experiment in a Xenograft Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neppiras, E.A. Acoustic Cavitation. Phys. Rep. 1980, 61, 159–251. [Google Scholar] [CrossRef]
- Stricker, L. Acoustic Cavitation and Sonochemistry; Universiteit Twente: Enschede, The Netherlands, 2013. [Google Scholar]
- Crum, L.A.; Reynolds, G.T. Sonoluminescence Produced by Stable Cavitation. J. Acoust. Soc. Am. 1985, 78, 137–139. [Google Scholar] [CrossRef]
- Gaitan, D.F.; Crum, L.A.; Church, C.C.; Roy, R.A. Sonoluminescence and Bubble Dynamics for a Single, Stable, Cavitation Bubble. J. Acoust. Soc. Am. 1992, 91, 3166–3183. [Google Scholar] [CrossRef]
- Taleyarkhan, R.P.; West, C.D.; Cho, J.S.; Lahey, R.T.; Nigmatulin, R.I.; Block, R.C. Evidence for nuclear emissions during Acoustic cavitation. Science 2002, 295, 1868–1873. [Google Scholar] [CrossRef]
- Wrenn, S.P.; Dicker, S.M.; Small, E.F.; Dan, N.R.; Mleczko, M.; Schmitz, G.; Lewin, P.A. Bursting bubbles and bilayers. Theranostics 2012, 2, 1140–1159. [Google Scholar] [CrossRef]
- Church, C.C.; Carstensen, E.L. “Stable” inertial cavitation. Ultrasound Med. Biol. 2001, 27, 1435–1437. [Google Scholar] [CrossRef]
- Miller, D.L. Overview of experimental studies of biological effects of medical ultrasound caused by gas body activation and inertial cavitation. Prog. Biophys. Mol. Biol. 2007, 93, 314–330. [Google Scholar] [CrossRef]
- Fabiilli, M.; Haworth, K.; Fakhri, N.; Kripfgans, O.; Carson, P.; Fowlkes, J. The Role of Inertial Cavitation in Acoustic Droplet Vaporization. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 1006–1017. [Google Scholar] [CrossRef]
- Zhong, W.; Sit, W.H.; Wan, J.M.; Yu, A.C. Sonoporation induces apoptosis and cell cycle arrest in human promyelocytic leukemia cells. Ultrasound Med. Biol. 2011, 37, 2149–2159. [Google Scholar] [CrossRef]
- Miller, D.L.; Quddus, J. Sonoporation of monolayer cells by diagnostic ultrasound activation of contrast-agent gas bodies. Ultrasound Med. Biol. 2000, 26, 661–667. [Google Scholar] [CrossRef]
- Lemmon, J.C.; McFarland, R.J.; Rybicka, J.M.; Balce, D.R.; McKeown, K.R.; Krohn, R.M.; Matsunaga, T.O.; Yates, R.M. In vitro and in vivo transfection of primary phagocytes via microbubble-mediated intraphagosomal sonoporation. J. Immunol. Methods 2011, 371, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, M.Y.; Lee, S.D.; Lee, W.; Chiu, A.; Yoo, S.S. Suppression of EEG visual-evoked potentials in rats through neu-romodulatory focused ultrasound. Neuroreport 2015, 26, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Pitt, W.G.; Husseini, G.A.; Staples, B.J. Ultrasonic drug delivery—A general review. Expert Opin. Drug Deliv. 2004, 1, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Coussios, C.C.; Roy, R.A. Applications of acoustics and cavitation to noninvasive therapy and drug delivery. Annu. Rev. Fluid. Mech. 2008, 40, 395–420. [Google Scholar] [CrossRef]
- Klibanov, A.L. Ultrasound contrast agents: Development of the field and current status. Top Curr. Chem. 2002, 222, 73–106. [Google Scholar]
- Zhao, Y.Z.; Lu, C.T.; Fu, H.X.; Li, X.K.; Zhou, Z.C.; Zhao, G.T.; Tian, J.L.; Gao, H.S.; Jiang, Y.N.; Hu, S.P.; et al. Phospho-lipid-based ultrasonic microbubbles for loading protein and ultrasound-triggered release. Drug Dev. Ind. Pharm. 2009, 35, 1121–1127. [Google Scholar] [CrossRef]
- Goldberg, B.B.; Raichlen, J.S.; Forsberg, F. Ultrasound Contrast Agents: Basic Principles and Clinical Applications, 2nd ed.; Martin Dunitz: London, UK, 2001. [Google Scholar]
- Stride, E.; Saffari, N. Microbubble ultrasound contrast agents: A review. Proc. Inst. Mech. Eng. Part. H J. Eng. Med. 2003, 217, 429–447. [Google Scholar] [CrossRef]
- Cosgrove, D. Ultrasound contrast agents: An overview. Eur. J. Radiol. 2006, 60, 324–330. [Google Scholar] [CrossRef]
- Doinikov, A.A.; Bouakaz, A. Review of shell models for contrast agent microbubbles. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 981–993. [Google Scholar] [CrossRef]
- AlkanOnyuksel, H.; Demos, S.M.; Lanza, G.M.; Vonesh, M.J.; Klegerman, M.E.; Kane, B.J.; Kuszak, J.; McPherson, D.D. Development of Inherently Echogenic Liposomes as an Ultrasonic Contrast Agent. J. Pharm. Sci. 1996, 85, 486–490. [Google Scholar] [CrossRef]
- Kheirolomoom, A.; Dayton, P.A.; Lum, A.F.; Little, E.; Paoli, E.E.; Zheng, H.; Ferrara, K.W. Acoustically-active microbubbles conjugated to liposomes: Characterization of a proposed drug delivery vehicle. J. Control. Release 2007, 118, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Tiukinhoy-Laing, S.D.; Huang, S.; Klegerman, M.; Holland, C.K.; McPherson, D.D. Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. Thromb. Res. 2007, 119, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-L. Liposomes in ultrasonic drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1167–1176. [Google Scholar] [CrossRef]
- Suzuki, R.; Oda, Y.; Utoguchi, N.; Maruyama, K. Progress in the development of ultrasound-mediated gene delivery systems utilizing nano- and microbubbles. J. Control. Release 2011, 149, 36–41. [Google Scholar] [CrossRef]
- Ibsen, S.; Benchimol, M.; Simberg, D.; Schutt, C.; Steiner, J.; Esener, S. A novel nested liposome drug delivery vehicle capable of ultrasound triggered release of its payload. J. Control. Release 2011, 155, 358–366. [Google Scholar] [CrossRef]
- Park, J. Echogenic liposome as a carrier of siRNA for sonoporation: An alternative microbubble for sonoporation. In Proceedings of the 2015 IEEE International Ultrasonics Symposium (IUS), Taipei, Taiwan, 21–24 October 2015. [Google Scholar]
- Wallace, N.; Wrenn, S. Ultrasound triggered drug delivery with liposomal nested microbubbles. Ultrasonics 2015, 63, 31–38. [Google Scholar] [CrossRef]
- Shekhar, H.; Bader, K.B.; Huang, S.; Peng, T.; Huang, S.; McPherson, D.D.; Holland, C.K. In vitro thrombolytic efficacy of echogenic liposomes loaded with tissue plasminogen activator and octafluoropropane gas. Phys. Med. Biol. 2017, 62, 517–538. [Google Scholar] [CrossRef]
- Park, J.; Park, D.; Shin, U.; Moon, S.; Kim, C.; Kim, H.S.; Park, H.; Choi, K.; Jung, B.; Oh, J.; et al. Synthesis of Laboratory Ultrasound Contrast Agents. Molecules 2013, 18, 13078–13095. [Google Scholar] [CrossRef]
- Park, D.; Jung, H.C.; Park, J.; Bae, S.; Shin, U.; Kim, S.W.; Kim, C.W.; Lee, Y.H.; Seo, J. Synthesis of echogenic liposomes for sonoporation. Micro Nano Lett. 2022, 17, 276–285. [Google Scholar] [CrossRef]
- Sheikov, N.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.; Hynynen, K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med. Biol. 2004, 30, 979–989. [Google Scholar] [CrossRef]
- Konofagou, E.E.; Tung, Y.S.; Choi, J.; Deffieux, T.; Baseri, B.; Vlachos, F. Ultrasound-Induced Blood-Brain Barrier Opening. Curr. Pharm. Biotechnol. 2012, 13, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-H.; Hsiao, M.-Y.; Kung, Y.; Huang, A.P.-H.; Chen, W.-S. Investigation of the Therapeutic Effect of Doxorubicin Combined With Focused Shockwave on Glioblastoma. Front. Oncol. 2021, 11, 711088. [Google Scholar] [CrossRef]
- Lah, T.T.; Novak, M.; Breznik, B. Brain malignancies: Glioblastoma and brain metastases. Semin. Cancer Biol. 2020, 60, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, X.; Xu, H.; Teschendorff, A.E.; Xu, L.; Li, J.; Fu, M.; Liu, J.; Zhou, H.; Wang, Y.; et al. Integrative analysis of genomic and epigenomic regulation reveals miRNA mediated tumor heterogeneity and immune evasion in lower grade glioma. Commun. Biol. 2024, 7, 824. [Google Scholar] [CrossRef] [PubMed]
- Tomayko, M.M.; Reynolds, C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 1989, 24, 148–154. [Google Scholar] [CrossRef]
- Klaunberg, B.A.; O’Malley, J.; Clark, T.; Davis, J.A. Euthanasia of mouse fetuses and neonates. Contemp. Top Lab. Anim. Sci. 2004, 43, 29–34. [Google Scholar]
- Quimby, F.W. The Clinical Chemistry of Laboratory Animals, 2nd ed.; Taylor & Francis: Philadelphia, PA, USA, 1999. [Google Scholar]
- Ryan, P.C.; Jakubczak, J.L.; Stewart, D.A.; Hawkins, L.K.; Cheng, C.; Clarke, L.M.; Ganesh, S.; Hay, C.; Huang, Y.; Kaloss, M.; et al. Antitumor efficacy and tumor-selective replication with a single intravenous injection of OAS403, an oncolytic adenovirus dependent on two prevalent alterations in human cancer. Cancer Gene Ther. 2004, 11, 555–569. [Google Scholar] [CrossRef]
- Deng, Z.H.; Gao, S.S.; Xiao, X.; Yin, N.; Ma, S.Y.; Li, W.P.; Li, Y.S. The effect of earthworm extract on mice S180 tumor growth and apoptosis. Biomed. Pharmacother. 2019, 115, 108979. [Google Scholar] [CrossRef]
- Liu, H.; Guo, C.; Shang, Y.; Zeng, L.; Jia, H.; Wang, Z. A Supramolecular Nanoparticle of Pemetrexed Improves the Anti-Tumor Effect by Inhibiting Mitochondrial Energy Metabolism. Front. Bioeng. Biotechnol. 2021, 9, 804747. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Park, D.H.; Jung, B.K.; Lee, Y.S.; Jang, J.Y.; Kim, M.K.; Lee, J.K.; Park, H.; Seo, J.; Kim, C.W. Evaluation of in vivo antitumor effects of ANT2 shRNA delivered using PEI and ultrasound with microbubbles. Gene Ther. 2015, 22, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, G.L.; Zhang, J.C. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xie, Y.T.; Song, J.Y.; Liu, R.R.; Chen, J.Y.; Weitz, D.A.; Sheng, J.P.; Liang, T.B.; Chen, D. Self-Assembly of Biocompatible Core-Shell Nanocapsules with Tunable Surface Functionality by Microfluidics for Enhanced Drug Delivery. Adv. Funct. Mater. 2024, 34, 2407112. [Google Scholar] [CrossRef]
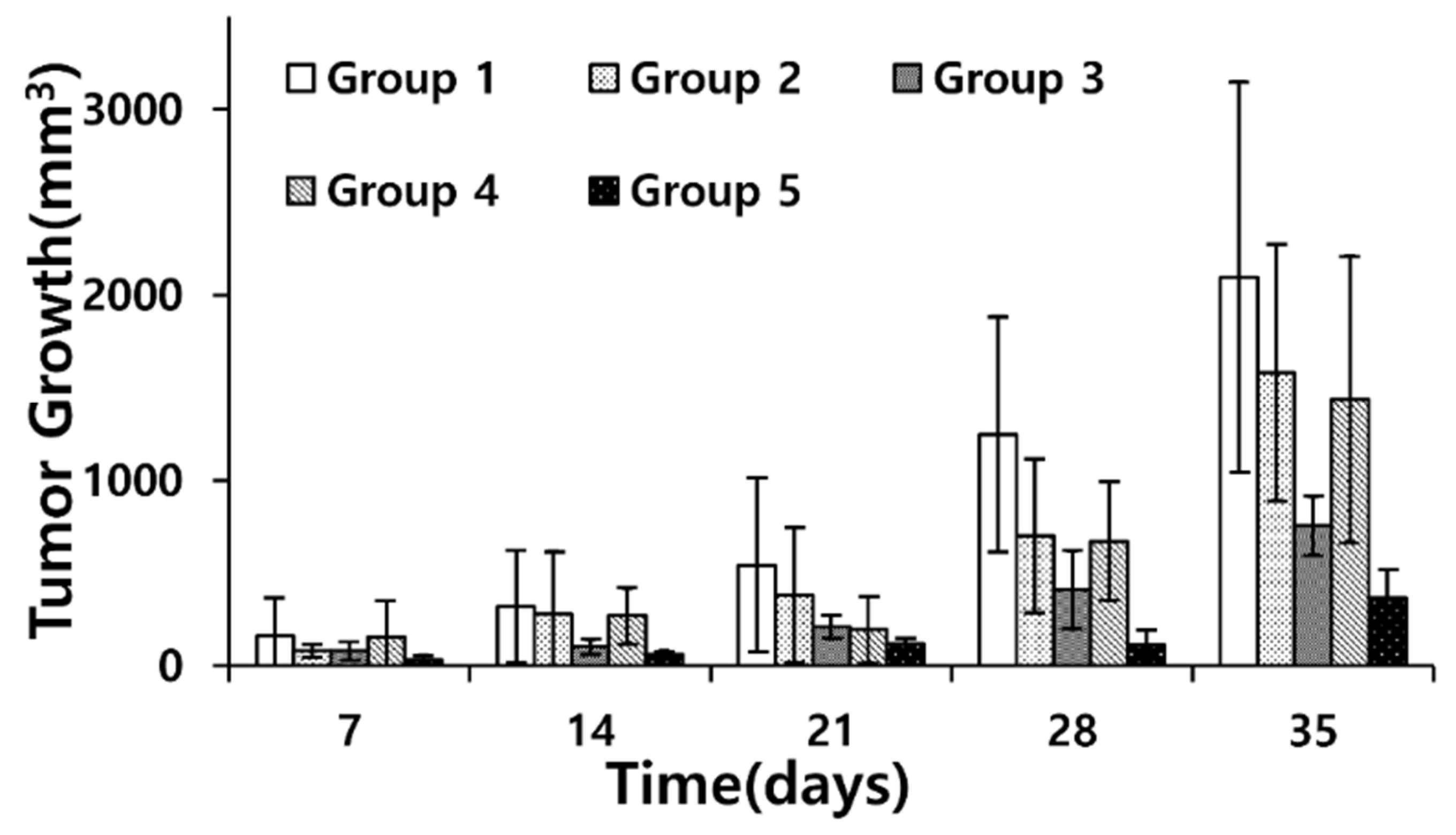

| Group Pairs | p-Value |
|---|---|
| 2–3 | 0.18 |
| 2–4 | 0.98 × 10−3 |
| 2–5 | 0.31 × 10−1 |
| 3–4 | 0.31 |
| 3–5 | 0.73 |
| 4–5 | 0.61 × 10−1 |
| Marker | Normal Range |
|---|---|
| Albumin | 2.7–4.9 g/dL |
| AST | 46–221 U/L |
| ALT | 22–133 U/L |
| BUN | 2–71 mg/dL |
| LDH | 140–280 U/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-H.; Lee, Y.-K.; Lee, H.; Choi, D.-H.; Rhee, K.-J.; Kim, H.S.; Seo, J.-B. Sonoporation with Echogenic Liposomes: The Evaluation of Glioblastoma Applicability Using In Vivo Xenograft Models. Pharmaceutics 2025, 17, 509. https://doi.org/10.3390/pharmaceutics17040509
Park J-H, Lee Y-K, Lee H, Choi D-H, Rhee K-J, Kim HS, Seo J-B. Sonoporation with Echogenic Liposomes: The Evaluation of Glioblastoma Applicability Using In Vivo Xenograft Models. Pharmaceutics. 2025; 17(4):509. https://doi.org/10.3390/pharmaceutics17040509
Chicago/Turabian StylePark, Ju-Hyun, Yoo-Kyung Lee, Hana Lee, Dong-Hyun Choi, Ki-Jong Rhee, Han Sung Kim, and Jong-Bum Seo. 2025. "Sonoporation with Echogenic Liposomes: The Evaluation of Glioblastoma Applicability Using In Vivo Xenograft Models" Pharmaceutics 17, no. 4: 509. https://doi.org/10.3390/pharmaceutics17040509
APA StylePark, J.-H., Lee, Y.-K., Lee, H., Choi, D.-H., Rhee, K.-J., Kim, H. S., & Seo, J.-B. (2025). Sonoporation with Echogenic Liposomes: The Evaluation of Glioblastoma Applicability Using In Vivo Xenograft Models. Pharmaceutics, 17(4), 509. https://doi.org/10.3390/pharmaceutics17040509







