Advancements in Cyclodextrin Complexes with Bioactive Secondary Metabolites and Their Pharmaceutical Applications
Abstract
1. Introduction
- (a)
- Two decades ago (years 2005–2014), the number of articles on the topics of CDs and secondary metabolites was quite stable, with a predominance of studies on CD-PPs over CD-Alks, with the least interest in CD-TTs;
- (b)
- In the last decade (years 2015–2024), different trends were observed for various classes of secondary metabolites: (i) the number of articles on CD-PPs showed a progressively increasing interest, with a mean of around 40 articles/year over the last 7 years; (ii) the number of articles on CD-TTs remained relatively stagnant in the first half of the last decade, followed by a steady increase in the last 5 years, with a mean of around 10 articles/year in that period; and (iii) the number of articles on CD-Alks remained constant throughout the last decade, with a mean of around 10 articles/year over the last 10 years.
2. Structure, Properties, and Main Applications of Cyclodextrins
2.1. Structure of Cyclodextrins
2.2. Properties of Cyclodextrins
2.2.1. Hydrophilic/Hydrophobic Properties of Cyclodextrins
2.2.2. Solubility of Cyclodextrins
2.2.3. Toxicity of Cyclodextrins
2.2.4. Biocompatible and Biodegradable Properties of Cyclodextrins
2.3. Applications of Cyclodextrins
2.3.1. Application Fields of Cyclodextrins
2.3.2. Pharmaceutical Applications of Cyclodextrins
3. Inclusion Complexes of Cyclodextrins
3.1. Formation Mechanism of Inclusion Complexes of Cyclodextrins
3.2. Preparation Methods of Inclusion Complexes of Cyclodextrins
3.3. Confirmation and Analysis of Inclusion Complexes of Cyclodextrins
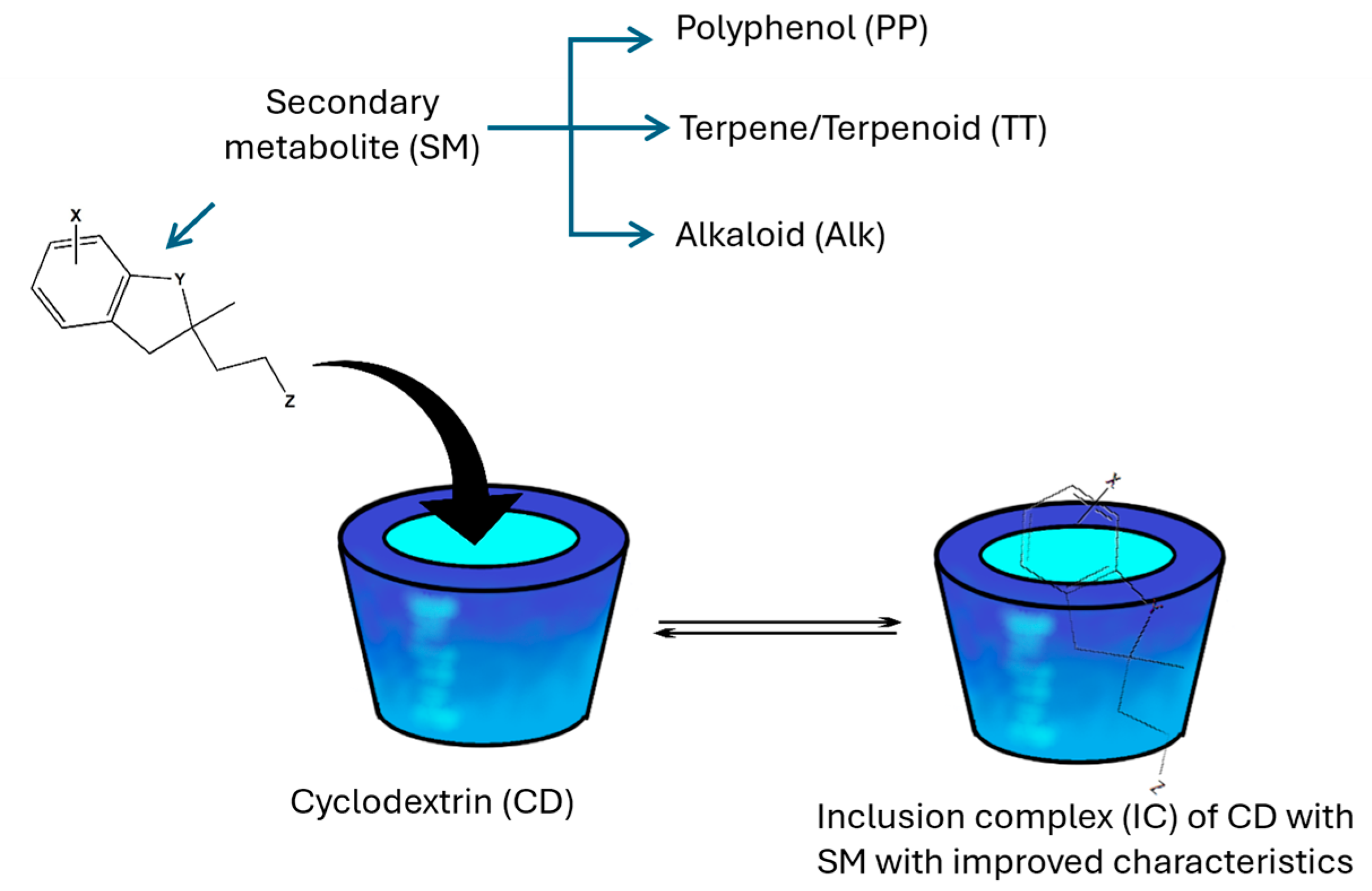
3.4. Guests of Cyclodextrins in This Review: The Secondary Metabolites
- (a)
- Phenolic compounds, most of them being polyphenols (PPs) [95];
- (b)
- Terpenes and their oxygenated derivatives, terpenoids (TTs); other isoprene-derived compounds, such as steroids, carotenoids, and gibberellic acid, are also included in this classification [96];
- (c)
- Nitrogen-containing compounds, such as alkaloids (Alks), cyanogenic glucosides, and non-proteinogenic amino acids [97].
4. Inclusion Complexes of Cyclodextrins with Secondary Metabolites
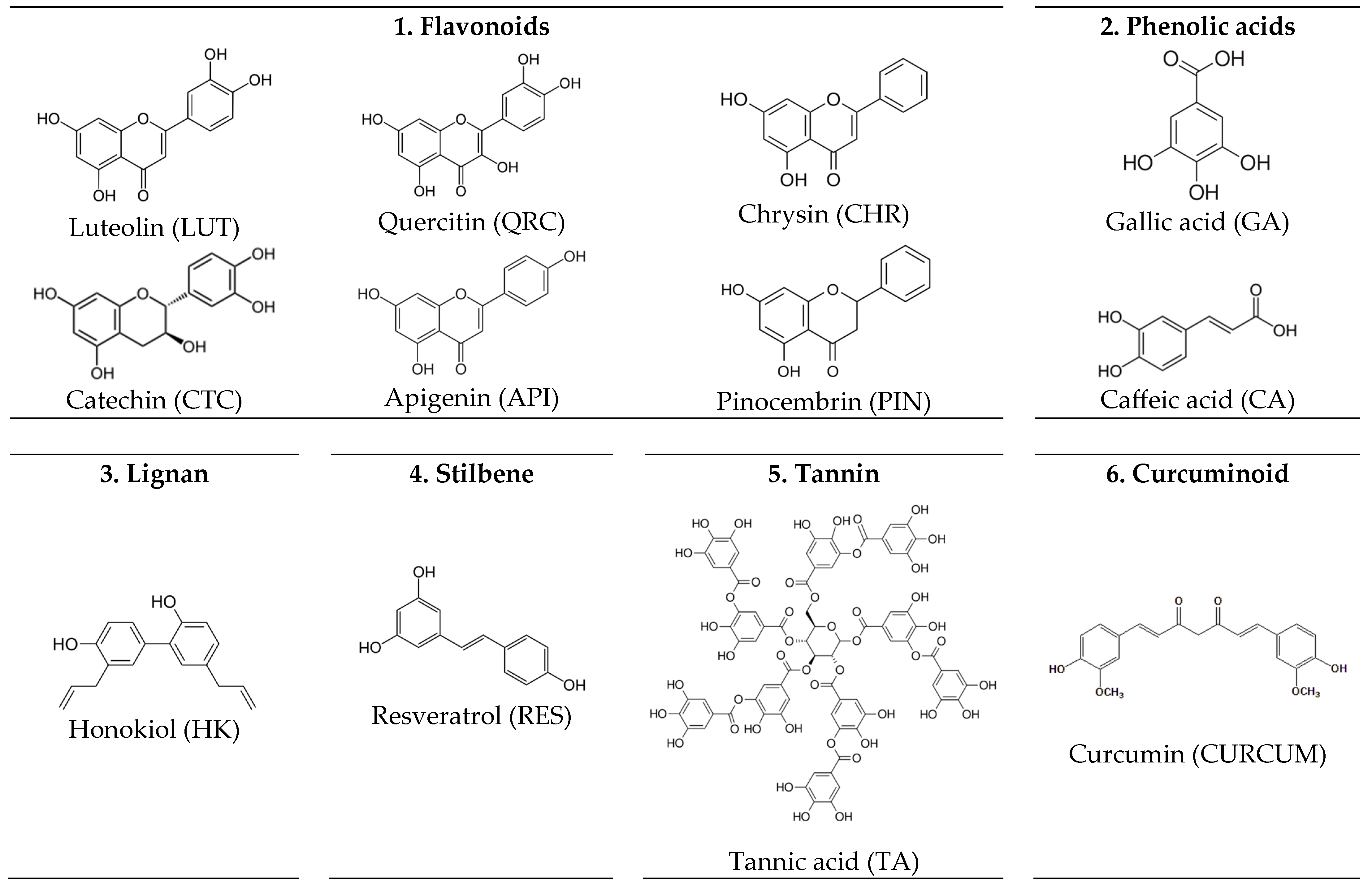
4.1. ICs of CDs-PPs with Pharmaceutical Applications
4.1.1. Molecular Structure and Biological Activity of Polyphenols
4.1.2. CD Interactions with Polyphenols from Plant-Based Sources
4.1.3. Brief Review of Studies on ICs of CDs-PPs
4.1.4. Inclusion Complexes of PPs with CD-Containing Composites
4.1.5. In Vitro and In Vivo Studies
4.1.6. Theoretical Studies
4.2. ICs of CDs-TTs with Pharmaceutical Applications
4.2.1. Molecular Structure and Biological Activity of Terpenes and Terpenoids
4.2.2. CDs Interactions with Pure TTs and TTs Rich Extracts from Plant-Based Sources
4.2.3. Inclusion Complexes of TTs with CD-Based Composites
4.2.4. In Vitro and In Vivo Studies
4.2.5. Theoretical Studies
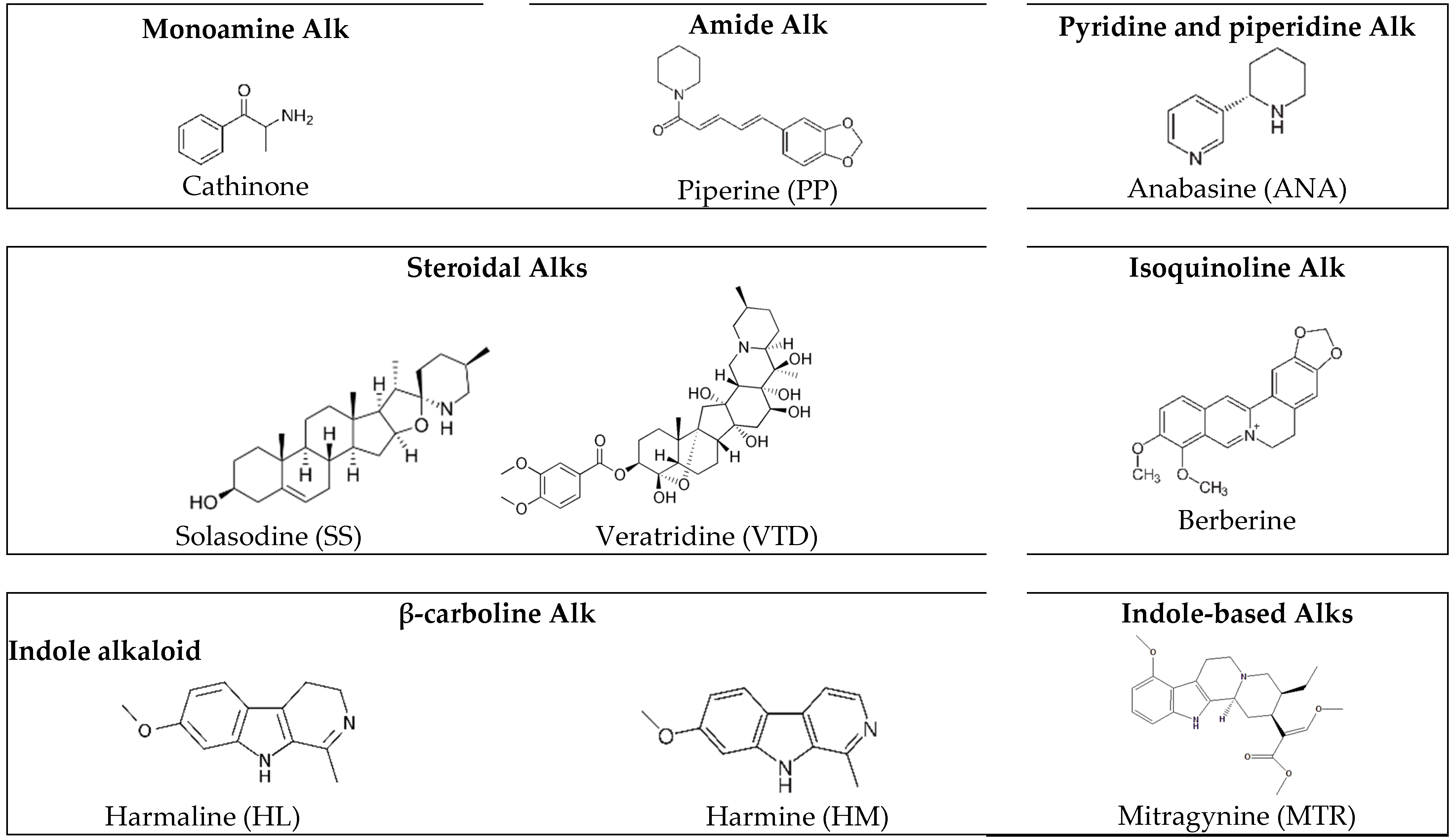
4.3. ICs of CDs-Alks with Pharmaceutical Applications
4.3.1. The Molecular Structure and Biological Activity of Alkaloids
4.3.2. CDs Interactions with Alks and Alk-Rich Extracts from Plant-Based Sources
- –
- Main properties of CD-Alk complexes indicate increased water solubility [156], stability, and bioavailability of CD-Alk complexes compared to free alkaloids [153]. Possible applications may be found in the case of mitragynine, an alkaloid with analgesic properties [156]. Lys-β-CD proved to be an effective complexation agent for berberine, yielding a potential sustained-release system, with applications in drug delivery and biomedical fields [160]. Other studies investigate the association strengths between SBE6.4-β-CD (SBE-β-CD with an average of 6.4 degrees of substitution) and various Alks [161] or between certain Alks (piperine, veratridine) and β-CD or its derivatives [155,156,157]. These studies indicated that SBE-β-CD forms more stable complexes with alkaloids than β-CD. A higher complex stability was also obtained when medium cavity-sized, negatively charged CDs were used for complexation [165,166].
- –
- Fluorescence spectroscopy allows for the analysis of fluorescent alkaloids. For example, harmaline (HL) and harmine (HM) exhibit superposed fluorescence spectra. Synchronous fluorescence measurements, following the inclusion of HL and HM in HP-β-CD, allowed for the separation of overlapping signals and the simultaneous determination of these Alks in various matrices, with high sensitivity and precision [158]. Other studies present the results of fluorescence spectroscopy measurements of berberine (BBR) and SBE10-β-CD complex [159,160]. Complexation with SBE10-β-CD increases the fluorescence intensity of BBR (~190-fold). In the presence of Cd2+ ions, the supramolecular complex SBE10-β-CD-BBR enables the detection of adenosine triphosphate (ATP), suggesting its potential use as a biosensor for ATP [159]. Other studies have investigated the potential of the same complex, SBE10-β-CD-BBR, to serve as a biosensor for the cancer biomarker spermine. Spermine is a metabolite whose concentration increases in urine and serum in the presence of malignant cells in the body [160].
- –
4.3.3. Inclusion Complexes of Alks with CD-Containing Composites
4.3.4. In Vitro and In Vivo Studies
4.3.5. Theoretical Studies
5. Pharmacokinetics and Pharmacodynamics of Cyclodextrin Inclusion Complexes with SMs
6. Risks and Limitations
7. Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDs | cyclodextrins |
| IC | inclusion complex |
| SMs | secondary metabolites |
| PPs | polyphenols |
| Alks | alkaloids |
| TTs | terpenes and terpenoids |
| FTIR | Fourier transform infrared spectroscopy |
| XRD | X-ray diffraction |
| UV-Vis | ultraviolet–visible spectroscopy |
| DSC | differential scanning calorimetry |
| NMR | nuclear magnetic resonance |
| ESI-MS/MS | electrospray ionization tandem mass spectrometry |
| CS-GA | gallic acid-grafted chitosan |
| HP-β-CD | 2-hydroxypropylated-β-CD |
| M-β-CD | methyl-β-CD |
| DM-β-CD | dimethyl-β-CD |
| SBE-β-CD | sulfobutyl ether-beta-cyclodextrin |
| QA-Ch | quaternary ammonium chitosan derivative |
References
- Sharma, K.; Thakur, I.; Kaushik, G. Occurrence and distribution of pharmaceutical compounds and their environmental impacts: A review. Bioresour. Technol. Rep. 2021, 16, 100841. [Google Scholar] [CrossRef]
- Ashiwaju, B.; Uzougbo, C.; Orikpete, O. Environmental Impact of Pharmaceuticals: A Comprehensive Review. Matrix Sci. Pharma 2024, 7, 85–94. [Google Scholar] [CrossRef]
- Kfoury, M.; Lichtfouse, E.; Fourmentin, S. The revival of cyclodextrins as active pharmaceutical ingredients. Environ. Chem. Lett. 2025, 23, 1–6. [Google Scholar] [CrossRef]
- Pandey, S.P.; Shukla, T.; Dhote, V.; Mishra, D.K.; Maheshwari, R.; Tekade, R.K. Chapter 4—Use of Polymers in Controlled Release of Active Agents. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: San Diego, CA, USA, 2019; pp. 113–172. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Nakahata, M. Supramolecular polymeric materials via cyclodextrin-guest interactions. Acc. Chem. Res. 2014, 47, 2128–2140. [Google Scholar] [CrossRef] [PubMed]
- Villiers, A. Sur la fermentation de la f’ecule par l’action du fermentbutyrique. Compt. Rend. Acad. Sci. 1891, 112, 536–538. [Google Scholar]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, E.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. History of Cyclodextrins. In The History of Cyclodextrins. Environmental Chemistry for a Sustainable World; Crini, G., Fourmentin, S., Lichtfouse, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; Volume 52, pp. 1–93. [Google Scholar] [CrossRef]
- Saenger, W.; Jacob, W.; Gessler, K.; Steiner, T.; Hoffmann, D.; Sanbe, H.; Koizumi, K.; Smith, S.M.; Takaha, T. Structures of the Common Cyclodextrins and Their Larger Analogues-Beyond the Doughnut. Chem. Rev. 1998, 98, 1787–1802. [Google Scholar] [CrossRef]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2022, 3, 1–31. [Google Scholar] [CrossRef]
- Wang, Q. Industrial Applications of Cyclodextrins. In Handbook of Macrocyclic Supramolecular Assembly; Liu, Y., Chen, Y., Zhang, H.Y., Eds.; Springer: Singapore, 2020; pp. 1665–1697. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, É.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 years of cyclodextrin discovery for health, food, agriculture, and the industry: A review. Environ. Chem. Lett. 2021, 19, 2581–2617. [Google Scholar] [CrossRef]
- Barba-Ostria, C.; Carrera-Pacheco, S.E.; Gonzalez-Pastor, R.; Heredia-Moya, J.; Mayorga-Ramos, A.; Rodríguez-Pólit, C.; Zúñiga-Miranda, J.; Arias-Almeida, B.; Guamán, L.P. Evaluation of Biological Activity of Natural Compounds: Current Trends and Methods. Molecules 2022, 27, 4490. [Google Scholar] [CrossRef]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, A.A., Jr.; Ikryannikova, L.N. Plant Secondary Metabolites in the Battle of Drugs and Drug-Resistant Bacteria: New Heroes or Worse Clones of Antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Compean, K. Antimicrobial Activity of Plant Secondary Metabolites: A Review. Res. J. Med. Plant. 2014, 8, 204–213. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef]
- Serra, A.C.S.; Souza, N.d.S.V.; Paes, G.d.S.; Migliolo, L.; Silva, L.M.G.E. Secondary metabolites with anti-tumor activity: A review. Res. Soc. Dev. 2022, 11, e49511326786. [Google Scholar] [CrossRef]
- Gordaliza, M. Natural products as leads to anticancer drugs. Clin. Transl. Oncol. 2007, 9, 767–776. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive Secondary Metabolites from Plant Sources: Types, Synthesis, and Their Therapeutic Uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Nisa, M.; Nadeem, M.; Babar, M.; Ahmed, M.; Gul, A. Pharmacological applications of bioactive secondary metabolites from plants. In Phytohormones and Stress Responsive Secondary Metabolites; Academic Press: San Diego, CA, USA, 2023; pp. 235–248. [Google Scholar] [CrossRef]
- McChesney, J.D.; Venkataraman, S.K.; Henri, J.T. Plant natural products: Back to the future or into extinction? Phytochemistry 2007, 68, 2015–2022. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Marris, E. Marine natural products: Drugs from the deep. Nature 2006, 443, 904–905. [Google Scholar] [CrossRef]
- Cardillo, A.B.; Perassolo, M.; Giulietti, A.M.; Talou, J.R. Cyclodextrins: A tool in plant cell and organ culture bioprocesses for the production of secondary metabolites. Plant Cell Tiss. Organ Cult. 2021, 146, 1–19. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Li, W.; Tang, Y.; Wang, S. Transcriptomics and Physiological Analyses Reveal Changes in Paclitaxel Production and Physiological Properties in Taxus cuspidata Suspension Cells in Response to Elicitors. Plants 2023, 12, 3817. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Gilardoni, G.; Lara, L.R.; Cumbicus, N.; Malagón, O. A New Leaf Essential Oil from Endemic Gynoxys laurifolia (Kunth) Cass. of Southern Ecuador: Chemical and Enantioselective Analyses. Plants 2023, 12, 2878. [Google Scholar] [CrossRef] [PubMed]
- Cumbicus, C.; Malagón, O.; Cumbicus, N.; Gilardoni, G. The Leaf Essential Oil of Gynoxys buxifolia (Kunth) Cass. (Asteraceae): A Good Source of Furanoeremophilane and Bakkenolide A. Plants 2023, 12, 1323. [Google Scholar] [CrossRef]
- Suvarna, V.; Bore, B.; Bhawar, C.; Mallya, R. Complexation of phytochemicals with cyclodextrins and their derivatives- an update. Biomed. Pharmacother. 2022, 149, 112862. [Google Scholar] [CrossRef]
- Christaki, S.; Spanidi, E.; Panagiotidou, E.; Athanasopoulou, S.; Kyriakoudi, A.; Mourtzinos, I.; Gardikis, K. Cyclodextrins for the Delivery of Bioactive Compounds from Natural Sources: Medicinal, Food and Cosmetics Applications. Pharmaceuticals 2023, 16, 1274. [Google Scholar] [CrossRef]
- Lima, P.; Lucchese, A.; Araújo-Filho, H.; Menezes, P.; Araújo, A.; Quintans-Júnior, L.; Quintans, J. Inclusion of Terpenes in Cyclodextrins: Preparation, Characterization and Pharmacological Approaches. Carbohydr. Polym. 2016, 151, 965–987. [Google Scholar] [CrossRef]
- Xu, Y.; Rashwan, A.K.; Osman, A.I.; Abd El-Monaem, E.M.; Elgarahy, A.M.; Eltaweil, A.S.; Omar, M.; Li, Y.; Mehanni, A.-H.E.; Chen, W.; et al. Synthesis and potential applications of cyclodextrin-based metal–organic frameworks: A review. Environ. Chem. Lett. 2023, 21, 447–477. [Google Scholar] [CrossRef]
- Periasamy, R. A systematic review on the significant roles of cyclodextrins in the construction of supramolecular systems and their potential usage in various fields. J. Carbohydr. Chem. 2020, 39, 189–216. [Google Scholar] [CrossRef]
- Sarabia-Vallejo, Á.; Caja, M.D.M.; Olives, A.I.; Martín, M.A.; Menéndez, J.C. Cyclodextrin Inclusion Complexes for Improved Drug Bioavailability and Activity: Synthetic and Analytical Aspects. Pharmaceutics 2023, 15, 2345. [Google Scholar] [CrossRef] [PubMed]
- Nicolaescu, O.E.; Belu, I.; Mocanu, A.G.; Manda, V.C.; Rău, G.; Pîrvu, A.S.; Ionescu, C.; Ciulu-Costinescu, F.; Popescu, M.; Ciocîlteu, M.V. Cyclodextrins: Enhancing Drug Delivery, Solubility and Bioavailability for Modern Therapeutics. Pharmaceutics 2025, 17, 288. [Google Scholar] [CrossRef]
- Esteso, M.A.; Romero, C.M. Cyclodextrins: Properties and Applications. Int. J. Mol. Sci. 2024, 25, 4547. [Google Scholar] [CrossRef]
- Morán-Serradilla, C.; Plano, D.; Sharma, A.K.; Sanmartín, C. Following the Trace of Cyclodextrins on the Selenium and Tellurium Odyssey. Int. J. Mol. Sci. 2024, 25, 7799. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.M.; Nguyen, T.V.A.; Bhandari, B.R. Amorphization of cyclodextrins by spray drying for producing encapsulated functional gas powders for agri-food applications. Dry Technol. 2022, 41, 843–858. [Google Scholar] [CrossRef]
- Yousaf, R.; Razzaq, F.; Asghar, S.; Irfan, M.; Ikram, U.K.; Syed, K. Cyclodextrins: An Overview of Fundamentals, Types, and Applications. In Cyclodextrins—Core Concepts and New Frontiers; Ali, R., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Szejtli, J. Utilization of cyclodextrins in industrial products and processes. J. Mater. Chem. 1997, 7, 575–587. [Google Scholar] [CrossRef]
- Hedges, A. Chapter 22—Cyclodextrins: Properties and Applications. In Starch, 3rd ed.; Food Science and Technology Series; BeMiller, J., Whistler, R., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 833–851. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Irie, T.; Uekama, K. Pharmaceutical applications of cyclodextrins. I.I.I. Toxicological issues and safety evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef]
- Ferreira, L.; Campos, J.; Veiga, F.; Cardoso, C.; Paiva-Santos, A.C. Cyclodextrin-based delivery systems in parenteral formulations: A critical update review. Eur. J. Pharm. Biopharm. 2022, 178, 35–52. [Google Scholar] [CrossRef]
- Cronin, S.; Lin, A.; Thompson, K.; Hoenerhoff, M.; Duncan, R. Hearing Loss and Otopathology Following Systemic and Intracerebroventricular Delivery of 2-Hydroxypropyl-Beta-Cyclodextrin. J. Assoc. Res. Otolaryngol. 2015, 16, 599–611. [Google Scholar] [CrossRef]
- Ding, D.; Manohar, S.; Jiang, H.; Salvi, R. Hydroxypropyl-β-cyclodextrin causes massive damage to the developing auditory and vestibular system. Hear. Res. 2020, 39, 108073. [Google Scholar] [CrossRef] [PubMed]
- Crumling, M.A.; King, K.A.; Duncan, R.A. Cyclodextrins and Iatrogenic Hearing Loss: New Drugs with Significant Risk. Front. Cell. Neurosci. 2017, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ding, D.; Chen, G.-D.; Li, L.; Jiang, H.; Salvi, R. 2-Hydroxypropyl-β-cyclodextrin Ototoxicity in Adult Rats: Rapid Onset and Massive Destruction of Both Inner and Outer Hair Cells Above a Critical Dose. Neurotox. Res. 2020, 38, 808–823. [Google Scholar] [CrossRef] [PubMed]
- Munnangi, S.R.; Youssef, A.A.A.; Narala, N.; Lakkala, P.; Narala, S.; Vemula, S.K.; Repka, M. Drug complexes: Perspective from Academic Research and Pharmaceutical Market. Pharm. Res. 2023, 40, 1519–1540. [Google Scholar] [CrossRef]
- Mu, K.; Jiang, K.; Wang, Y.; Zhao, Z.; Cang, S.; Bi, K.; Li, Q.; Liu, R. The Biological Fate of Pharmaceutical Excipient β-Cyclodextrin: Pharmacokinetics, Tissue Distribution, Excretion, and Metabolism of β-Cyclodextrin in Rats. Molecules 2022, 27, 1138. [Google Scholar] [CrossRef]
- Shan, M.; Martin del Valle, E. Cyclodextrins as worlds smallest beauty cases. Int. J. Bioassays 2013, 2, 1174–1179. [Google Scholar] [CrossRef]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J. Applications of cyclodextrins in food science: A review. Trends Food Sci. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Crini, G.; Aleya, L. Cyclodextrin applications in pharmacy, biology, medicine, and environment. Environ. Sci. Pollut. Res. 2022, 29, 167–170. [Google Scholar] [CrossRef]
- Quaratesi, I.; Bruno, I.; Pauciulo, A.; Bartiromo, A.; Badea, E.; Carsote, C.; Neri, P.; Talotta, C.; Gliubizzi, R.; Di Tullio, V.; et al. Side-chain Poly[2]pseudorotaxanes containing β-cyclodextrin for more sustainable tanning process. Polym. Test. 2023, 129, 108268. [Google Scholar] [CrossRef]
- Quaratesi, I.; Călinescu, I.; Chipurici, P.; Dumbravă, E.-G.; Cucos, A.; Zaki, M.Y.; La Manna, P.; Bercea, A.; Stan, M.S.; Michalik, S.; et al. Ultrasound-assisted synthesis of β-cyclodextrin/hydroxyapatite composites as a green and safe additive for enhancing leather properties. J. Mol. Struct. 2025, 1358, 141299. [Google Scholar] [CrossRef]
- Braga, S.S. Cyclodextrin Superstructures for Drug Delivery. J. Drug Deliv. Sci. Technol. 2022, 75, 103650. [Google Scholar] [CrossRef]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Musuc, A.M. Cyclodextrins: Advances in Chemistry, Toxicology, and Multifaceted Applications. Molecules 2024, 29, 5319. [Google Scholar] [CrossRef] [PubMed]
- Aiassa, V.; Garnero, C.; Zoppi, A.; Longhi, M.R. Cyclodextrins and Their Derivatives as Drug Stability Modifiers. Pharmaceuticals 2023, 16, 1074. [Google Scholar] [CrossRef]
- Cho, E.; Yun, D.; Jeong, D.; Jeong, D.; Im, J.; Kim, H.; Dindulkar, S.D.; Choi, Y.; Jung, S. Regioselective self-acylating cyclodextrins in organic solvent. Sci. Rep. 2016, 6, 23740. [Google Scholar] [CrossRef]
- Rajamohan, R.; Ashokkumar, S.; Murugavel, K.; Lee, Y.R. Preparation and Characterization of a Nano-Inclusion Complex of Quercetin with β-Cyclodextrin and Its Potential Activity on Cancer Cells. Micromachines 2023, 14, 1352. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, B.; Gong, Z.; Huang, H.; Gong, Y.; Xiao, W. Metagenomics Approach to the Intestinal Microbiome Structure and Abundance in High-Fat-Diet-Induced Hyperlipidemic Rat Fed with (−)-Epigallocatechin-3-Gallate Nanoparticles. Molecules 2022, 27, 4894. [Google Scholar] [CrossRef]
- Fu, L.; Gu, Q.; Zhang, S.; Wang, J.; Cai, Z.; Fu, Y. Simultaneous extraction and encapsulation of polyphenols from Cajanus cajan leaves and the evaluation of their biological activity. Microchem. J. 2023, 193, 109187. [Google Scholar] [CrossRef]
- Costa, R.H.S.d.; Pessoa, R.T.; Silva, E.d.S.; Araujo, I.M.; Gonçalves, S.A.; Rocha, J.E.; Pereira, F.N., Jr.; Oliveira, N.C.; Oliveira, V.M.d.; Rocha, M.N.d.; et al. Antibacterial and Inhibitory Activity of Nora and Mepa Efflux Pumps of Estragole Complexed to β-Cyclodextrin (ES/β-CD) In Vitro Against Staphylococcus aureus Bacteria, Molecular Docking and MPO-Based Pharmacokinetics Prediction. Pharmaceutics 2024, 16, 1469. [Google Scholar] [CrossRef]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.; Pan, P.; Huang, Y.; Chen, T.; Yuan, T.; Ma, Y.; Han, G.; Li, J.; Jin, Y.; et al. Pulmonary delivery of resveratrol-β-cyclodextrin inclusion complexes for the prevention of zinc chloride smoke-induced acute lung injury. Drug Deliv. 2022, 29, 1122–1131. [Google Scholar] [CrossRef]
- Lavania, K.; Garg, A. Inclusion Complex of Chrysin with Hydroxypropyl-β-cyclodextrin (HP-β-CD) Preparation, Characterization, and Dissolution Study. BioNanoSci. 2023, 13, 616–624. [Google Scholar] [CrossRef]
- Song, M.; Wang, C.; Zhang, S.; Song, J.; Hu, Z.; Zhou, S.; Li, Y.; Wang, L.; Cao, F. Screening and inclusion of luteolin for β-cyclodextrin: Molecular simulations and experiments. Green Mater. 2024, in press. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, H.Y.; Jiang, S.; Dai, S.; Wang, Y.; Yang, J.; Chen, Y.; Chen, J.; Park, H. Inclusion Complexation of Flavonoids with Cyclodextrin: Molecular Docking and Experimental Study. ChemistrySelect 2024, 9, e202403606. [Google Scholar] [CrossRef]
- Krstic, L.; Jarho, P.; Ruponen, M.; Urtti, A.; Gonzalez-Garcia, M.; Diebold, Y. Improved Ocular Delivery of Quercetin and Resveratrol: A Comparative Study Between Binary and Ternary Cyclodextrin Complexes. Int. J. Pharm. 2022, 624, 122028. [Google Scholar] [CrossRef]
- Ntuli, S.; Leuschner, M.; Bester, M.J.; Serem, J.C. Stability, Morphology, and Effects of In Vitro Digestion on the Antioxidant Properties of Polyphenol Inclusion Complexes with β-Cyclodextrin. Molecules 2022, 27, 3808. [Google Scholar] [CrossRef]
- Song, W.; Chen, X.; Dai, C.; Lin, D.; Pang, X.; Zhang, D.; Liu, G.; Jin, Y.; Lin, J. Comparative Study of Preparation, Evaluation, and Pharmacokinetics in Beagle Dogs of Curcumin β-Cyclodextrin Inclusion Complex, Curcumin Solid Dispersion, and Curcumin Phospholipid Complex. Molecules 2022, 27, 2998. [Google Scholar] [CrossRef]
- Yu, C.; Naeem, A.; Liu, Y.; Guan, Y. Ellagic Acid Inclusion Complex-Loaded Hydrogels as an Efficient Controlled Release System: Design, Fabrication and In Vitro Evaluation. J. Funct. Biomater. 2023, 14, 278. [Google Scholar] [CrossRef]
- Stergiou, A.; Binou, P.; Igoumenidis, P.E.; Chiou, A.; Yannakopoulou, K.; Karathanos, V.Τ. Host-guest inclusion complexes of hydroxytyrosol with cyclodextrins: Development of a potential functional ingredient for food application. J. Food Sci. 2022, 87, 2678–2691. [Google Scholar] [CrossRef]
- Catarino, M.D.; Costa, B.S.B.; Circuncisão, A.R.; Silva, A.M.S.; Cardoso, S.M.; Braga, S.S. γ-Cyclodextrin Inclusion of Phloroglucinol: Solid State Studies and Antioxidant Activity throughout the Digestive Tract. Appl. Sci. 2022, 12, 2340. [Google Scholar] [CrossRef]
- Zhan, S.; Zhao, Z.; Ai, P.; Fang, X.; Sun, Q.; Li, H. Facile synthesis and crystalline stability of cubic nano-γ-cyclodextrin metal-organic frameworks for hydrophobic polyphenols carrier. Food Biosci. 2025, 63, 105655. [Google Scholar] [CrossRef]
- Noreldin, A.E.; Elzoghby, A.O.; Khattab, S.N. Green self-assembled lactoferrin carboxymethyl cellulose nanogels for synergistic chemo/herbal breast cancer therapy. Colloids Surf. B Biointerfaces 2022, 217, 112657. [Google Scholar] [CrossRef]
- Cai, D.; Wang, X.; Wang, Q.; Tong, P.; Niu, W.; Guo, X.; Yu, J.; Chen, X.; Liu, X.; Zhou, D.; et al. Controlled release characteristics of alkyl gallates and gallic acid from β-cyclodextrin inclusion complexes of alkyl gallates. Food Chem. 2024, 460, 140726. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Wang, X.; Wang, Q.; Tong, P.; Niu, W.; Guo, X.; Yu, J.; Chen, X.; Liu, X.; Zhou, D.; et al. β-cyclodextrin inclusion complexes with short-chain phenolipids: An effective formulation for the dual sustained-release of phenolic compounds. Food Res. Int. 2024, 187, 114423. [Google Scholar] [CrossRef]
- Remígio, M.S.d.N.; Greco, T.; Silva Júnior, J.O.C.; Converti, A.; Ribeiro-Costa, R.M.; Rossi, A.; Barbosa, W.L.R. Spray-Drying Microencapsulation of Bauhinia ungulata L. var. obtusifolia Aqueous Extract Containing Phenolic Compounds: A Comparative Study Using Different Wall Materials. Pharmaceutics 2024, 16, 488. [Google Scholar] [CrossRef]
- Radan, M.; Živković, J.; Nedeljković, S.; Janković, T.; Drinić, Z.; Bigovic, D.; Šavikin, K. Influence of hydroxypropyl-β-cyclodextrin complexation on the extraction efficiency of rutin, quercetin and total polyphenols from Fagopyrum esculentum Moench. Sustain. Chem. Pharm. 2023, 35, 101220. [Google Scholar] [CrossRef]
- Blagojević, B.; Agić, D.; Četojević-Simin, D.; Lazzara, G.; Vranješ, M.; Popovic, B. β-Cyclodextrin as a green booster for the extraction of polyphenols from blackthorn fruits: Bioactivity determination and molecular docking analysis. Food Bioprod. Process. 2023, 140, 84–98. [Google Scholar] [CrossRef]
- Zambito, Y.; Piras, A.M.; Fabiano, A. Bergamot Essential Oil: A Method for Introducing It in Solid Dosage Forms. Foods 2022, 11, 3860. [Google Scholar] [CrossRef]
- Sip, S.; Gościniak, A.; Szulc, P.; Walkowiak, J.; Cielecka-Piontek, J. Assisted Extraction with Cyclodextrins as a Way of Improving the Antidiabetic Activity of Actinidia Leaves. Pharmaceutics 2022, 14, 2473. [Google Scholar] [CrossRef]
- Kossel, A. Ueber die chemische Zusammensetzung der Zelle. Arch. Anat. Physiol. Physiol. Abteilung 1891, 278, 181–186. [Google Scholar]
- Czapek, F. Biochemie d. Pflanzen, 3rd ed.; Jena, Fischer-Verlag: Jena, Germany, 1922–1925; p. 852. [Google Scholar]
- Talapatra, S.; Talapatra, B.; Nicolaou, K.C. Chemistry of Plant Natural Products: Stereochemistry, Conformation, Synthesis, Biology, and Medicin; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Croteau, R.; Kutchan, T.M.; Lewis, N.G. Natural Products (Secondary Metabolites). In Biochemistry & Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; American Society of Plant Biologists: Rockville, MD, USA, 2000; Volume 24, pp. 1250–1319. [Google Scholar]
- Kabera, J. Plant Secondary Metabolites: Biosynthesis, Classification, Function and Pharmacological Classification, Function and Pharmacological Properties. J. Pharm. Pharmacol. 2014, 2, 377–392. [Google Scholar]
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S.B. From Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and In Vitro Approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef] [PubMed]
- Teoh, E.S. Secondary Metabolites of Plants. Med. Orchid. Asia 2015, 5, 59–73. [Google Scholar] [CrossRef]
- Nawrot-Chorabik, K.; Sułkowska, M.; Gumulak, N. Secondary Metabolites Produced by Trees and Fungi: Achievements So Far and Challenges Remaining. Forests 2022, 13, 1338. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Roy, A. A review on the alkaloids an important therapeutic compound from plants. IJPB 2017, 3, 1–9. [Google Scholar]
- Lobiuc, A.; Pavăl, N.-E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.-C.; Amăriucăi-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Meng, X.; Li, S.; Gan, R.Y.; Li, Y.; Li, H.B. Bioactivity, Health Benefits, and Related Molecular Mechanisms of Curcumin: Current Progress, Challenges, and Perspectives. Nutrients 2018, 10, 1553. [Google Scholar] [CrossRef]
- Christou, A.; Parisis, N.; Tzakos, A.; Gerothanassis, I.; Goulas, V. Optimization of β-cyclodextrin based ultrasound-assisted extraction: A green strategy to enhance the extraction of bioactive compounds from taro leaf byproduct. Sustain. Chem. Pharm. 2024, 41, 101728. [Google Scholar] [CrossRef]
- Tsitlakidou, P.; Kamplioni, D.; Kyriakoudi, A.; Irakli, M.; Biliaderis, C.G.; Mourtzinos, I. Antioxidant-Enhanced Alginate Beads for Stabilizing Rapeseed Oil: Utilizing Extracts from Post-Distillation Waste Residues of Rosemary. Foods 2024, 13, 2142. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gutiérrez, E. Study of Influence of Extraction Method on the Recovery Bioactive Compounds from Peel Avocado. Molecules 2023, 28, 2557. [Google Scholar] [CrossRef]
- To, N.; Ha, T.; Nguyien, V.M.; Tran, T. Production of instant pomelo peel powder by spray drying: Optimization of wall material composition to microencapsulate phenolic compounds. Food Sci. Technol. 2022, 42, e102621. [Google Scholar] [CrossRef]
- Jovanović, M.S.; Krgović, N.; Šavikin, K.; Živković, J. Ultrasound-Assisted Water Extraction of Gentiopicroside, Isogentisin, and Polyphenols from Willow Gentian “Dust” Supported by Hydroxypropyl-β-Cyclodextrin as Cage Molecules. Molecules 2022, 27, 7606. [Google Scholar] [CrossRef] [PubMed]
- Diwani, N.; Chelly, M.; Athmouni, K.; Chelly, S.; Gammoudi, S.; Turki, M.; Boudawara, T.; Ayadi, H.; Bouaziz-Ketata, H. β-cyclodextrin microencapsulation enhanced antioxidant and antihyperlipidemic properties of Tunisian Periploca angustifolia roots condensed tannins in rats. Environ. Sci. Pollut. Res. 2022, 29, 61049–61064. [Google Scholar] [CrossRef]
- He, Y.; Xiang, J.; Chen, J.; Fang, S.; Guo, Z.; Liang, X. Improving Bioaccessibility and Bioavailability of Isoflavone Aglycones from Chickpeas by Germination and Forming β-Cyclodextrin Inclusion Complexes. Pharmaceutics 2023, 15, 2684. [Google Scholar] [CrossRef]
- Moreira, R.; Novais, J.; Silva, R.; Nunes, R.; Abreu, L.; Dias, E.; Castro, H.; do Carmo, F.; Rangel, C.; Sousa, V.; et al. Preparation and evaluation of red propolis and nystatin cyclodextrin inclusion complexes against oral microbiome opportunistic microorganisms. Food Sci. Technol. Campinas 2022, 42, e118022. [Google Scholar] [CrossRef]
- Frangopoulos, T.A. Green Efficient Approach on Extraction of Polyphenols from Fenugreek Seeds (Trigonella foenum-graecum): DES and β-Cyclodextrin Assisted Extraction. Waste Biomass. Valor. 2022, 13, 4403–4415. [Google Scholar] [CrossRef]
- Cegledi, E.; Garofulić, I.E.; Zorić, Z.; Roje, M.; Dragović-Uzelac, V. Effect of Spray Drying Encapsulation on Nettle Leaf Extract Powder Properties, Polyphenols and Their Bioavailability. Foods 2022, 11, 2852. [Google Scholar] [CrossRef] [PubMed]
- Vhangani, L.N.; Favre, L.C.; Rolandelli, G.; Van Wyk, J.; del Pilar Buera, M. Optimising the Polyphenolic Content and Antioxidant Activity of Green Rooibos (Aspalathus linearis) Using Beta-Cyclodextrin Assisted Extraction. Molecules 2022, 27, 3556. [Google Scholar] [CrossRef]
- Messias, M.A.; Ferreira, S.M.; Tavares, L.; Santos, L. A Comparative Study between Onion Peel Extracts, Free and Complexed with β-Cyclodextrin, as a Natural UV Filter to Cosmetic Formulations. Int. J. Mol. Sci. 2023, 24, 15854. [Google Scholar] [CrossRef]
- Li, N.; Feng, B.; Bi, Y.; Kong, F.; Wang, Z.; Tan, S. Sulfobutyl ether cyclodextrin inclusion complexes containing tea polyphenols: Preparation, characterization, antioxidant activity, α-glucosidase inhibition, and in vitro release property. J. Mol. Struct. 2023, 1295, 136686. [Google Scholar] [CrossRef]
- Dobroslavić, E.; Elez Garofulić, I.; Zorić, Z.; Pedisić, S.; Roje, M.; Dragović-Uzelac, V. Physicochemical Properties, Antioxidant Capacity, and Bioavailability of Laurus nobilis L. Leaf Polyphenolic Extracts Microencapsulated by Spray Drying. Foods 2023, 12, 1923. [Google Scholar] [CrossRef]
- Sruthi, P.; Madhava Naidu, M.; Rao, P.J. Valorization of cashew nut testa phenolics through nano-complexes stabilized with whey protein isolate and β-cyclodextrin: Characterization, anti-oxidant activity, stability and in vitro release. Food Res. Int. 2024, 181, 114110. [Google Scholar] [CrossRef]
- Perak Junaković, E.; Šandor, K.; Terzić, S.; Vujnović, A.; Andrišić, M.; Benić, M.; Fajdić, D.; Sinković, S.; Pehnec, M.; Žarković, I. Influence of Encapsulation of Propolis Extract with 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD) on Polyphenolic Contents during In Vitro Simulation of Digestion. Appl. Sci. 2023, 13, 9357. [Google Scholar] [CrossRef]
- Jakupović, L.; Bačić, I.; Jablan, J.; Marguí, E.; Marijan, M.; Inić, S.; Nižić Nodilo, L.; Hafner, A.; Zovko Končić, M. Hydroxypropyl-β-Cyclodextrin-Based Helichrysum italicum Extracts: Antioxidant and Cosmeceutical Activity and Biocompatibility. Antioxidants 2023, 12, 855. [Google Scholar] [CrossRef]
- Marijan, M.; Jakupović, L.; Končić, M.Z. Hydroxypropyl-β-Cyclodextrin-Glycerol-Assisted Extraction of Phenolics from Satureja montana L.: Optimization, Anti-Elastase and Anti-Hyaluronidase Properties of the Extracts. Processes 2023, 11, 1117. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Miklaszewski, A.; Michniak-Kohn, B.; Cielecka-Piontek, J. The Antioxidant Potential of Resveratrol from Red Vine Leaves Delivered in an Electrospun Nanofiber System. Antioxidants 2023, 12, 1777. [Google Scholar] [CrossRef]
- Athanasopoulou, S.; Spanidi, E.; Panagiotidou, E.; Cavagnino, A.; Bobier, A.; Gardikis, K. An Advanced Combinatorial System from Vitis vinifera Leaves and Propolis Enhances Antioxidants’ Skin Delivery and Fibroblasts Functionality. Pharmaceuticals 2024, 17, 1610. [Google Scholar] [CrossRef] [PubMed]
- Kazlauskaite, J.A.; Ivanauskas, L.; Marksa, M.; Bernatoniene, J. The Effect of Traditional and Cyclodextrin-Assisted Extraction Methods on Trifolium pratense L. (Red Clover) Extracts Antioxidant Potential. Antioxidants 2022, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Aree, T. How cyclodextrin encapsulation improves molecular stability of apple polyphenols phloretin, phlorizin, and ferulic acid: Atomistic insights through structural chemistry. Food Chem. 2023, 409, 135326. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, H.; Meng, H.; Li, S. Preparation and Physicochemical Characterization of Inclusion Complex of Pterostilbene-Hydroxypropyl-Β-Cyclodextrin for Enhancing the Application of Pterostilbene in Cosmetics. J. Photochem. Photobiol. 2024, 447, 115221. [Google Scholar] [CrossRef]
- Yang, X.; Shen, J.; Liu, J.; Yang, Y.; Hu, A.; Ren, N.; Cheng, Z.; Liu, W. Spray-Drying of Hydroxypropyl β-Cyclodextrin Microcapsules for Co-Encapsulation of Resveratrol and Piperine with Enhanced Solubility. Crystals 2022, 12, 596. [Google Scholar] [CrossRef]
- Li, H.; Song, J.; Liu, C.; Wang, X.; Liu, Y.; Han, M.; Liang, J.; Gao, Z. Corn starch/β-Cyclodextrin composite nanoparticles for encapsulation of tea polyphenol and development of oral targeted delivery systems with pH-responsive properties. Food Hydrocoll. 2024, 151, 109823. [Google Scholar] [CrossRef]
- Ma, C.; Xie, Y.; Huang, X.; Zhang, L.; Julian McClements, D.; Zou, L.; Liu, W. Encapsulation of (-)-epigallocatechin gallate (EGCG) within phospholipid-based nanovesicles using W/O emulsion-transfer methods: Masking bitterness and delaying release of EGCG. Food Chem. 2024, 437, 137913. [Google Scholar] [CrossRef]
- Radan, M.; Ćujić Nikolić, N.; Kuzmanović Nedeljković, S.; Mutavski, Z.; Krgović, N.; Stević, T.; Marković, S.; Jovanović, A.; Živković, J.; Šavikin, K. Multifunctional Pomegranate Peel Microparticles with Health-Promoting Effects for the Sustainable Development of Novel Nutraceuticals and Pharmaceuticals. Plants 2024, 13, 281. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, F.; Fan, Z.; He, Z.; Tai, L.; Peng, Q.; Zhang, Y.; Chao, Z.; Jiang, W.; Jia, L.; et al. An Oral Polyphenol Host-Guest Nanoparticle for Targeted Therapy of Inflammatory Bowel Disease. Acta Biomater. 2023, 169, 422–433. [Google Scholar] [CrossRef]
- Meurisse, N.; Wylin, T.; Heedfeld, V.; Fieuws, S.; Ceulemans, L.; Jochmans, I.; Pirenne, J.; Monbaliu, D. Effects of Cyclodextrin Curcumin Formulation on Ischemia-Reperfusion Injury in Porcine DCD Liver Transplantation. Transplantation 2024, 108, 2366–2373. [Google Scholar] [CrossRef]
- Gao, C.H.; Pan, L.X.; Tan, Z.J.; Sun, H.Z.; Sun, M.X.; Wang, J.J.; Shen, X.; Su, F.; Yu, R.L. Double-network polyphenol chitosan hydrogels with instant aldehyde-β-cyclodextrin-based structure as potential for treating bacterially infected wounds. Int. J. Biol. Macromol. 2024, 278, 134819. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, E.B.; Kurus, M.; Turkcuoglu, I.; Melekoglu, R.; Balcioglu, S.; Yigitcan, B.; Ates, B.; Koytepe, S. Synthesis of Cyclodextrin-Based Multifunctional Biocompatible Hydrogels and Their Use in the Prevention of Intrauterine Adhesions (Asherman’s Syndrome) after Surgical Injury. ACS Omega 2024, 9, 31957–31973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.Z.; Tan, M.; Zeng, J.; Qi, X.W.; Zhang, Y.T.; Che, Y.T.; Zhang, S.; Li, B.J. A Supramolecular Assembly of EGCG for Long-Term Treatment of Allergic Rhinitis. ACS Biomater. Sci. Eng. 2024, 10, 2282–2298. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, R.; Kawamura, A.; Matsumoto, Y.; Iida, Y.; Kanayama, M.; Kurokawa, M.; Aida, Y. Epigallocatechin Gallate Stabilized by Cyclodextrin Inactivates Influenza Virus and Human Coronavirus 229E. Microorganisms 2022, 10, 1796. [Google Scholar] [CrossRef]
- Guzmán-Oyarzo, D.; Hernández-Montelongo, J.; Rosas, C.; Leal, P.; Weber, H.; Alvear, M.; Salazar, L.A. Controlled Release of Caffeic Acid and Pinocembrin by Use of nPSi-βCD Composites Improves Their Antiangiogenic Activity. Pharmaceutics 2022, 14, 484. [Google Scholar] [CrossRef]
- Kumar, P.; Bhardwaj, V.K.; Purohit, R. Unveiling the Intricate Supramolecular Chemistry of γ-Cyclodextrin-Epigallocatechin Gallate Inclusion Complexes. Ind. Eng. Chem. Res. 2024, 63, 1021. [Google Scholar] [CrossRef]
- Jackson, M.B. Nature’s chemicals. The natural products that shaped our world. Ann. Bot. 2010, 106, vi–vii. [Google Scholar] [CrossRef]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of Terpenes and Recent Advances in Plant Protection. Int. J. Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef]
- Alves, Â.V.F. Ent-kaurenoic acid-enriched Mikania glomerata leaves-complexed β-cyclodextrin: Pharmaceutical development and in vivo antitumor activity in a sarcoma 180 mouse model. Int. J. Pharm. 2023, 631, 122497. [Google Scholar] [CrossRef]
- Ez-zoubi, A.; Ez-zoubi, Y.; Ramzi, A.; Fadil, M.; El Ouali Lalami, A.; Farah, A. Ethanol and glycerol green emulsifying solvent for the formation of a Lavandula stoechas essential oil/β-cyclodextrin inclusion complex: Mixture design and adulticidal activity against Culex pipiens. Heliyon 2022, 8, e10204. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.; Tavares, Y.; Novaes, G.; Santos, V.; Oliveira, A.; Leal, C.; Alencar Filho, E.; Picot, L.; Almeida, J.R.; de Oliveira, R., Jr. Anti-Melanoma Potential of Inclusion Complexes Containing Phyllacanthone in β-Cyclodextrin and Sulfobutyl-Ether-β-Cyclodextrin. J. Drug. Deliv. Technol. 2023, 89, 105020. [Google Scholar] [CrossRef]
- Heimfarth, L.; Rezende, M.M.; Pereira, E.W.; Passos, F.R.; Monteiro, B.S.; Santos, T.K.; Lima, N.T.; Souza, I.C.L.; Cavalcanti de Albuquerque, R.L., Jr.; de Souza Siqueira Lima, P.; et al. Pharmacological effects of a complex α-bisabolol/β-cyclodextrin in a mice arthritis model with involvement of IL-1β, IL-6 and MAPK. Biomed. Pharmacother. 2022, 151, 113142. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.G.R.; da Silva, L.Y.S.; Pessoa, R.T.; Neto, L.J.d.L.; da Costa, R.H.S.; Martins, A.O.P.B.B.; de Oliveira, M.R.C.; da Silva, C.P.; Coutinho, H.D.M.; Quintans, L.J.; et al. Screening of the action mechanisms involved in the antinociceptive effect of isopulegol and its complex in cyclodextrin using acute nociception models in mice. Carbohydr. Polym. Technol. Appl. 2023, 6, 100383. [Google Scholar] [CrossRef]
- Pereira, E.W.M.; Heimfarth, L.; Santos, T.K.; Passos, F.R.; Siqueira-Lima, P.; Scotti, L.; Scotti, M.T.; Almeida, J.R.G.d.S.; Campos, A.R.; Coutinho, H.D.; et al. Limonene, a citrus monoterpene, non-complexed and complexed with hydroxypropyl-β-cyclodextrin attenuates acute and chronic orofacial nociception in rodents: Evidence for involvement of the PKA and PKC pathway. Phytomedicine 2022, 96, 153893. [Google Scholar] [CrossRef]
- Stasiłowicz-Krzemień, A.; Szymanowska, D.; Szulc, P.; Cielecka-Piontek, J. Antimicrobial, Probiotic, and Immunomodulatory Potential of Cannabis sativa Extract and Delivery Systems. Antibiotics 2024, 13, 369. [Google Scholar] [CrossRef]
- Rocha, G.N.d.S.A.O.; Silva, J.Y.R.; Santos, D.K.D.D.N.; Pereira, A.C.M.V.; Rocha, J.V.R.; Alves, C.d.S.C.; Almeida, J.R.G.d.S.; Gomes, A.S.L.; Bakuzis, A.F.; Junior, S.A. Design of a magnetic nanocarrier containing phyllacanthone as delivery of anticancer phytochemical: Characterization and theranostic in vitro applications. J. Alloys Compd. 2025, 1010, 177860. [Google Scholar] [CrossRef]
- Ibrahim, B.M.M.; Darwish, A.B.; Taleb, S.A.; Mourad, R.M.; Yassen, N.N.; Hessin, A.F.; Gad, S.A.; Mohammed, M.A. Appraisal terpenoids rich Boswellia carterri ethyl acetate extract in binary cyclodextrin oligomer nano complex for improving respiratory distress. Sci. Rep. 2024, 14, 16779. [Google Scholar] [CrossRef]
- Lacerda, E.; Teixeira, G.; Anconi, C. GFN2-xTB study of the inclusion of thymol and carvacrol in β-cyclodextrin. J. Incl. Phenom. Macro. 2024, 104, 487–499. [Google Scholar] [CrossRef]
- Adamski, Z.; Blythe, L.L.; Milella, L.; Bufo, S.A. Biological Activities of Alkaloids: From Toxicology to Pharmacology. Toxins 2020, 12, 210. [Google Scholar] [CrossRef]
- Debnath, B.; Singh, S.; Das, M.; Goswami, S.; Singh, M.; Maiti, D.; Manna, K. Role of plant alkaloids on human health: A review of biological activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Azzeme, A.; Kamarul Zaman, M. Plant toxins: Alkaloids and their toxicities. GSC Biol. Pharm. Sci. 2019, 6, 21–29. [Google Scholar] [CrossRef]
- Chang, C.-H.; Chen, M.-C.; Chiu, T.-H.; Li, Y.-H.; Yu, W.-C.; Liao, W.-L.; Oner, M.; Yu, C.-T.R.; Wu, C.-C.; Yang, T.-Y.; et al. Arecoline Promotes Migration of A549 Lung Cancer Cells through Activating the EGFR/Src/FAK Pathway. Toxins 2019, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Muldakhmetov, Z.; Fazylov, S.; Nurkenov, O.; Gazaliev, A.; Sarsenbekova, A.; Pustolaikina, I.; Nurmaganbetov, Z.; Seilkhanov, O.; Alsfouk, A.A.; Elkaeed, E.B.; et al. Combined Computational and Experimental Studies of Anabasine Encapsulation by Beta-Cyclodextrin. Plants 2022, 11, 2283. [Google Scholar] [CrossRef]
- Christoforides, E.; Andreou, A.; Papaioannou, A.; Bethanis, K. Structural Studies of Piperine Inclusion Complexes in Native and Derivative β-Cyclodextrins. Biomolecules 2022, 12, 1762. [Google Scholar] [CrossRef]
- Ali, S.; Saokaew, P.; Aman, A.; Todsaporn, D.; Sanachai, K.; Krusong, K.; Hannongbua, S.; Wolschann, P.; Mahalapbutr, P.; Rungrotmongkol, T. Enhancing solubility and stability of piperine using β-cyclodextrin derivatives: Computational and experimental investigations. J. Biomol. Struct. Dyn. 2024, 43, 2596–2609. [Google Scholar] [CrossRef]
- Várnai, B.; Zsila, F.; Szakács, Z.; Garádi, Z.; Malanga, M.; Béni, S. Sulfobutylation of Beta-Cyclodextrin Enhances the Complex Formation with Mitragynine: An NMR and Chiroptical Study. Int. J. Mol. Sci. 2022, 23, 3844. [Google Scholar] [CrossRef]
- Uribe, L.A.; Leonardo, S.; Nielsen, T.T.; Steinmann, C.; Campàs, M.; Fragoso, A. Supramolecular Complexes of Plant Neurotoxin Veratridine with Cyclodextrins and Their Antidote-like Effect on Neuro-2a Cell Viability. Pharmaceutics 2022, 14, 598. [Google Scholar] [CrossRef]
- Abdelazim, A.H.; Algarni, M.A.; Almalki, A.H. Innovative spectrofluorometric method for determination of harmaline and harmine in different matrices. Sci. Rep. 2023, 13, 19951. [Google Scholar] [CrossRef]
- Chakraborty, G.; Chittela, R.K.; Nilaya, P.; Pal, H. Supramolecular Modulation in Photophysical Features of Berberine and Its Application towards ATP Sensing. J. Mol. Liq. 2022, 359, 119316. [Google Scholar] [CrossRef]
- Hasan, F.; Sarkar, S.; Chakraborty, G. Berberine and cyclodextrin based supramolecular assembly for the detection of a cancer biomarker in complex bio-matrices via indicator displacement assay. Microchem. J. 2023, 194, 109366. [Google Scholar] [CrossRef]
- Miskolczy, Z.; Megyesi, M.; Biczók, L. Entropy-Driven Inclusion of Natural Protoberberine Alkaloids in Sulfobutylether-β-Cyclodextrin. Molecules 2022, 27, 7514. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, H.Y.; Ren, L.; Yang, J.; Hao, P.; Tong, J.; Chen, Y.; Chen, J.; Park, H. Optimization, Characterization, Molecular Docking, and In Vitro Release of BBH/Lysine-β-cyclodextrin Inclusion Complex. ChemistrySelect 2023, 8, e202303620. [Google Scholar] [CrossRef]
- Kalydi, E.; Sebák, F.; Fiser, B.; Minofar, B.; Moussong, É.; Malanga, M.; Bodor, A.; Kardos, J.; Béni, S. Exceptional stability of the sugammadex-solasodine complex: Insights from experimental and theoretical studies. Carbohydr. Polym. 2024, 348, 122819. [Google Scholar] [CrossRef] [PubMed]
- Dohárszky, A.; Vági, E.M.; Könczöl, Á.; Simon, A.; Várnagy, E.; Muratov, M.; Steiger, K.I.; Várnai, B.; Béni, S.; Riethmüller, E.; et al. Kratom Alkaloids: A Blood–Brain Barrier Specific Membrane Permeability Assay-Guided Isolation and Cyclodextrin Complexation Study. Molecules 2024, 29, 5302. [Google Scholar] [CrossRef]
- Dohárszky, A.; Kalydi, E.; Völgyi, G.; Béni, S.; Fejős, I. Cyclodextrin-Enabled Enantioselective Complexation Study of Cathinone Analogs. Molecules 2024, 29, 876. [Google Scholar] [CrossRef]
- Casado Hidalgo, G.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Magnetic material based on mesostructured silica functionalized with β-cyclodextrin to extract opium alkaloids in poppy seed infusions. Adv. Sample Prep. 2023, 6, 100056. [Google Scholar] [CrossRef]
- Sangar, F.H.; Farahpour, M.R.; Tabatabaei, Z.G. Facile synthesis of 2-hydroxy-β-cyclodextrin/polyacrylamide/carbazole hydrogel and its application for the treatment of infected wounds in a murine model. Int. J. Biol. Macromol. 2024, 267, 131252. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Mahdy, N.K.; Al Mulla, H.; ElMeshad, A.N.; Issa, M.Y.; Azzazy, H.M.E.-S. PLGA/PEG Nanoparticles Loaded with Cyclodextrin-Peganum harmala Alkaloid Complex and Ascorbic Acid with Promising Antimicrobial Activities. Pharmaceutics 2022, 14, 142. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B. Cyclodextrin complexes: Perspective from drug delivery and formulation. Drug Dev. Res. 2018, 79, 201–217. [Google Scholar] [CrossRef]
- Yao, Y.; Xie, Y.; Hong, C.; Li, G.; Shen, H.; Ji, G. Development of a myricetin/hydroxypropyl-β-cyclodextrin inclusion complex: Preparation, characterization, and evaluation. Carbohydr. Polym. 2014, 110, 329–337. [Google Scholar] [CrossRef]
- Loftsson, T.; Moya-Ortega, M.D.; Alvarez-Lorenzo, C.; Concheiro, A. Pharmacokinetics of cyclodextrins and drugs after oral and parenteral administration of drug/cyclodextrin complexes. J. Pharm. Pharmacol. 2016, 68, 544–555. [Google Scholar] [CrossRef]
- Sharif Neaz, W.; Alam, M.; Imran, A.B. Advancements in cyclodextrin-based controlled drug delivery: Insights into pharmacokinetic and pharmacodynamic profiles. Heliyon 2024, 10, e39917. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, S.; Wei, S.; Liu, J.; Bingren Tian, B. Pharmacokinetics and pharmacodynamics of cyclodextrin-based oral drug delivery formulations for disease therapy. Carbohydr. Polym. 2024, 329, 121763. [Google Scholar] [CrossRef] [PubMed]
- Khajir, S.; Karimzadeh, Z.; Khoubnasabjafari, M.; Jouyban-Gharamaleki, V.; Rahimpour, E.; Jouyban, A. A Rayleigh light scattering technique based on β- cyclodextrin modified gold nanoparticles for phenytoin determination in exhaled breath condensate. J. Pharm. Biomed. Anal. 2023, 223, 115141. [Google Scholar] [CrossRef]
- Zeng, Y.; Lv, Y.; Hu, M.; Guo, F.; Zhang, C. Curcumin-loaded hydroxypropyl-β-cyclodextrin inclusion complex with enhanced dissolution and oral bioavailability for epilepsy treatment. Xenobiotica 2022, 52, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Pereira de Lima, E.; Laurindo, L.F.; Catharin, V.C.S.; Direito, R.; Tanaka, M.; Santos German, I.J.; Barbalho Lamas, C.; Landgraf Guiguer, E.; Cressoni Araújo, A.; Ragassi Fiorini, A.M.; et al. Polyphenols, Alkaloids, and Terpenoids Against Neurodegeneration: Evaluating the Neuroprotective Effects of Phytocompounds Through a Comprehensive Review of the Current Evidence. Metabolites 2025, 15, 124. [Google Scholar] [CrossRef]
- Gul, A.; Bakht, J.; Mehmood, F. Huperzine-A response to cognitive impairment and task switching deficits in patients with Alzheimer’s disease. J. Chin. Med. Assoc. 2019, 82, 40–43. [Google Scholar] [CrossRef]
- Szmeja, S.; Gubica, T.; Ostrowski, A.; Zalewska, A.; Szeleszczuk, Ł.; Zawada, K.; Zielińska-Pisklak, M.; Skowronek, K.; Wiweger, M. Caffeine-Cyclodextrin Complexes as Solids: Synthesis, Biological and Physicochemical Characterization. Int. J. Mol. Sci. 2021, 22, 4191. [Google Scholar] [CrossRef]
- Terekhova, I.V.; Kumeev, R.S.; Al’per, G.A. The interaction of caffeine with substituted cyclodextrins in water. Russ. J. Phys. Chem. 2007, 81, 1071–1075. [Google Scholar] [CrossRef]
- Rescifina, A.; Ugo Chiacchio, U.; Iannazzo, D.; Anna Piperno, A.; Romeo, G. β-Cyclodextrin and Caffeine Complexes with Natural Polyphenols from Olive and Olive Oils: NMR, Thermodynamic, and Molecular Modeling Studies. J. Agric. Food Chem. 2010, 58, 11876–11882. [Google Scholar] [CrossRef] [PubMed]



| Plant-Based Sources | Green Cosolvent | Extraction Method | Ref. |
|---|---|---|---|
| Colocasia esculenta L. leaves | β-CD | ultrasonication of leaves with an ethanolic solution of β-CD | Christou et al., 2024 [102] |
| post-distillation rosemary solid residues | solid–liquid extraction with an aqueous solution of β-CD | Tsitlakidou et al., 2024 [103] | |
| avocado peel | maceration plus β-CD | Martínez-Gutiérrez 2023 [104] | |
| pomelo peel (Citrus maxima) | microencapsulation by spray drying | To et al., 2022 [105] | |
| Gentiana asclepiadea L.; willow gentian underground parts, an unexploited herbal tea by-product | HP-β-CD | ultrasound-assisted extraction | Jovanović et al., 2022 [106] |
| Plant-Based Sources of PPs | CDs | Main Findings/Benefits of Complexation | Reference |
|---|---|---|---|
| Tannins from Periploca angustifolia roots | β-CD | Encapsulation efficacy of 70% in β-CD was obtained; tannin release with enhanced antioxidant and antihyperlipidemic activities of ICs; pure and encapsulated tannins demonstrated effective inhibition of pancreatic lipase activity. | Diwani et al., 2022 [107] |
| Chickpea sprouts isoflavones | The content of isoflavones in chickpeas increased through sprouting; efficient extraction of components with antioxidant activity in the presence of β-CD; increased bioavailability of extracted flavonoids through encapsulation. | He et al., 2023 [108] | |
| Red propolis (Prop) | Antimicrobial activity against microorganisms from the oral cavity. | Moreira et al., 2022 [109] | |
| Blackthorn fruits | Antioxidant capacity increased by over 60%; β-CD enables simultaneous extraction and encapsulation. | Blagojević et al., 2023 [84] | |
| Fenugreek Seeds (Trigonella foenum-graecum) | β-CD-assisted extraction allowed for a higher yield of components with antioxidant, anti-inflammatory, and hypoglycemic effects. | Frangopoulos, 2022 [110] | |
| Nettle leaves (Urtica dioica L.) | Extracts obtained using spray encapsulation in the presence of β-CD contained the highest phenolic content and antioxidant activity; encapsulation increased the bioavailability of polyphenols. | Cegledi et al., 2022 [111] | |
| Green rooibos (Aspalathus linearis) | An increase in β-CD concentration generated an increase in the extraction yield of polyphenols, which correlated with an increase in antioxidant activity. | Vhangani et al., 2022 [112] | |
| Onion peel (OP) extract rich in quercetin and resveratrol | Encapsulation efficiency: 91.8%; microencapsulation protected OP extract, prolonging its shelf life; OP extract, whether in its natural form or encapsulated, can be used as a natural sunscreen, allowing for the replacement of synthetic sunscreens. | Messias et al., 2023 [113] | |
| Red Clover aerial parts (Trifoliu pratense L.) | β-CD; γ-CD | Significant increase in extracted total phenolic content (TPC): 20.29% increase in aqueous samples, using β-CD, and 22.26% increase in ethanolic samples, using γ-CD; a direct correlation was observed between TPC and antioxidant activity. | Kazlauskaite et al., 2022 [122] |
| Tea polyphenols (TP) | SBE-β-CD | Improved antioxidant activity, α-glucosidase scavenging ability, and thermal stability; designed as a nutraceutical with antioxidant and hypoglycemic properties. | Li et al., 2023 [114] |
| Aqueous extract of Bauhinia ungulata var. obtusifolia leaves | MD-CMC-β-CD - | Antioxidant and antidiabetic properties of identified phenolic compounds with thermal stability, such as p-coumaric acid, chlorogenic acid, rutin, and isoquercitrin. | Remígio et al., 2024 [82] |
| Laurel (Laurus nobilis L.) leaves | β-CD; MD-β-CD; GA-gum Arabic-β-CD | β-CD was less efficient than β-CD + MD/GA in preserving the flavonols during digestion; β-CD + MD induced optimal solubility, hygroscopicity, and antioxidant capacity. | Dobroslavić et al., 2023 [115] |
| Cashew nut testa (CNT), an underutilized cashew by-product rich in polyphenols | WPI-β-CD- | Higher solubility, stability, antioxidant activity, and increased controlled release of PPs in the encapsulated form. | Sruthi et al., 2024 [116] |
| Propolis | HP-β-CD | Complexation enables a good solubility of polyphenols; stable IC with good bio-accessibility of main PPs at the small intestine level. | Perak et al., 2023 [117] |
| Cajanus cajan (C. cajan) leaves | Inhibitory effects on Gram-positive bacteria and strong antioxidant activity; might be applied for pharmaceutical preparations due to their high load capacity, high solubility and increased biological activity. | Fu et al., 2023 [65] | |
| Fagopyrum esculentum Moench (common buckwheat) | Positive impact on the extraction efficiency of rutin, quercetin, and total polyphenols and on their stability when exposed to stress conditions. | Radan et al., 2023 [83] | |
| Two Helichrysum italicum extracts: OPT-1 (rich in phenolic acids) and OPT-2 (rich in total phenols and flavonoids) | In most of the assays, the antioxidant and cosmeceutical activities of tested compounds were better than those of positive controls. | Jakupović et al., 2023 [118] | |
| Satureja montana L. | HP-β-CD | The extracts displayed good anti-elastase and excellent anti-hyaluronidase activity, making them suitable components of natural cosmetic products. | Marijan et al., 2023 [119] |
| Red vine leaf | nanofibers containing HP-β-CD | In the nanofiber combination, resveratrol had increased solubility and better buccal penetration. | Paczkowska-Walendowska et al., 2023 [120]; |
| Vine leaves PPs and propolis PPs | HP-β-CD liposome | PPs/HP-β-CD/liposome system delivered anti-aging compounds for human skin. | Athanasopoulou et al., 2024 [121] |
| Bergamot essential oil (BEO) (rich in polyphenols and limonene) | M-β-CD/QA-Ch | A conjugate, BEO/QA-CH/M-CD was obtained, allowing for the stabilization of volatile compounds of BEO and the elimination of its unpleasant taste; conversion of the oil into a solid dosage form (powders, granules, tablets); better protection of PPs and limonene. | Zambito et al., 2022 [85] |
| Actinidia leaves (Geneva, Jumbo, Ken’s Red, Kijivska Hibridna, and Sentyabraskaya) | α-CD β-CD γ-CD | Actinida leaves main constituents: quercetin, rutin, epicatechin, chlorogenic acid, and kaempferol; the highest biological activity (antioxidant and enzyme inhibition assays) was found in Ken’s Red variety; extraction in the presence of CDs increases the biological activity of Ken’s Red leaves. | Sip et al., 2022 [86] |
| Polyphenol | CD or CD-Derivative | Preparation Methods | Confirmation Techniques | Reference |
|---|---|---|---|---|
| Luteolin (LUT) | β-CD | Freeze-drying method | FTIR, XRD, DSC, UV-Vis | Song et al., 2024 [70] |
| Catechin (CAT) | Kneading Freeze-drying method | SEM, ESI-MS/MS | Ntuli et al., 2022 [73] | |
| Epigallocatechin-3-gallate (EGCG) | ||||
| Gallic acid (GA) | ||||
| Alkyl gallates (dodecyl gallate, butyl gallate, octyl gallate, and ethyl gallate) | Freeze-drying method | HPLC-UV analysis | Cai et al., 2024 [80] | |
| Alkyl gallates (butyl, propyl, ethyl, and methyl gallates) | Freeze-drying method | NMR, SEM, XRD, FTIR | Cai et al., 2024 [81] | |
| Phloretin (PRT) Phlorizin (PRZ) Ferulic acid (FEA) | Solvent evaporation of concentrated solutions containing β-CD and PPs | XRD | Aree 2023 [123] | |
| Curcumin (CURCUM) | Coprecipitation | SEM, XRD, FTIR | Song et al., 2022 [74] | |
| Quercetin (QRC) | Nanoprecipitation, lyophilization | FTIR, NMR, SAED *, XRD | Rajamohan et al., 2023 [63] | |
| Hydroxytyrosol (HT) | α-CD, β-CD | Freeze-drying method | UV-Vis, NMR, DSC, FTIR | Stergiou et al., 2022 [76] |
| Phloroglucinol (PGL) | γ-CD | Co-dissolution and freeze drying | FTIR, TG, DTA, XRD | Catarino et al., 2022 [77] |
| Chrysin (CHR) | HP-β-CD | Kneading and coprecipitation | FTIR, TG, SEM, NMR | Lavania et al., 2023 [69] |
| Pterostilbene (PTS) | HP-β-CD | Freeze-drying method | XRD, SEM, NMR, TG, DSC | Yang et al., 2024 [124] |
| Resveratrol (RES) | HP-β-CD | Spray-drying technique | HPLC, FTIR, DSC, XRD | Yang et al., 2022 [125] |
| TTs | CDs | Main Findings/Benefits of Complexation | Reference |
|---|---|---|---|
| Estragole (ES) (Terpenoid) | β-CD | Encapsulation of ES in β-CD was calculated to be 25.45%. In vitro: β-CD-ES potentiates the antibiotic effect of some compounds but does not exhibit antibiotic activity when administered alone. | Costa et al., 2024 [66] |
| Mikania glomerata leaves extract rich in Ent-kaurenoic acid (ERKA) (Terpenoid) | ERKA inclusion in β-CD using the malaxation method (65.37%) was superior to inclusion using the co-evaporation method (13.64%). In vivo: antitumor activity in mice and low systemic toxicity. | Alves et al., 2023 [139] | |
| Lavandula stoechas essential oil (terpenes and terpenoids) | Encapsulation efficiency increased when ethanol and glycerol were used as green emulsifiers during the encapsulation process. In vivo: encapsulation increased the thermal stability of L. stoechas essential oil and its insecticidal effect on adult mosquitoes. | Ez-zoubi et al., 2022 [140] | |
| Phyllacanthone (PHY) (terpene) isolated from stem barks of Cnidoscolus quercifolius | β-CD SBE-β-CD | ICs of PHY with β-CD and SBE-β-CD have been obtained; complexation improves the water solubility of PHY. In vitro studies: free and complexed FHY mitigate the growth of melanoma cells. | Alves et al., 2023 [141] |
| Alks | CDs | Main Findings/Benefits of Complexation | Reference |
|---|---|---|---|
| Anabasine (ANA) | β-CD | Increased stability and bioavailability of ANA. | Muldakhmetov et al., 2022 [153] |
| Harmaline (HL) and harmine (HM) | HP-β-CD | Fluorescence spectroscopy indicates that complexation allows the simultaneous determination of HL and HM from various matrices. | Abdelazim et al., 2023 [158] |
| Berberine (BER) | SBE10-β-CD | Fluorescence spectroscopy indicated that SBE10-β-CD-BBR-Cd2+ may serve as a biosensor for the bio-analyte ATP. | Chakraborty et al., 2022 [159] |
| SBE10-β-CD | Fluorescence spectroscopy indicated that SBE10-β-CD-BBR may serve as a biosensor for the cancer biomarker spermine. | Hasan et al., 2023 [160] | |
| Lysine-modified β-cyclodextrin (Lys-β-CD) | Lys-β-CD-BER complex may act as a potential sustained-release system, with applications in drug delivery and biomedical fields. | Liu et al., 2023 [162] | |
| Berberine, coptisine, palmatine, epiberberine, dehydrocorydaline | SBE6.4-β-CD | The association constant decreases eightfold in the series berberine ≈ coptisine >> palmatine > epiberberine > dehydrocorydaline. | Miskolczy et al., 2022 [161] |
| Piperine (PIP) | β-CD methylated derivatives of β-CD HP-β-CD | Complexation efficiency was more elevated using randomly methylated-β-CD and HP-β-CD than using β-CD. | Christoforides et al., 2022 [154] |
| β-CD SBE-β-CD HP-β-CD DM-β-CD | PP formed the most stable complexes with SBE-β-CD, followed by HP-β-CD. | Ali et al., 2024 [155] | |
| Mitragynine (MTR) | β-CD SBE-β-CD | Increased water solubility and bioavailability of MTR; MTR forms more stable complexes with SBE-β-CD than with β-CD. | Várnai et al., 2022 [156] |
| Veratridine (VTD) | β-CD δ-CD SBE-β-CD | VTD formed more stable complexes with γ-CD and SBE-β-CD than with β-CD. Possible use as antidotes for VTD-induced toxicity. | Uribe et al., 2022 [157] |
| Solasodine (SS) | Sugammadex (SGM) (a modified γ-cyclodextrin) | Possible use as an antidote for SS-induced toxicity. | Kalydi et al., 2024 [163] |
| Kratom alkaloids | 40 CD derivatives (native and synthetic) | Affinity capillary electrophoresis indicated that the highest stability of complexes was achieved for the medium cavity-sized, negatively charged CDs; potential in creating antidotes for kratom and cathinone analogs. | Dohárszky et al., 2024 [164,165] |
| Cathionine and four of its derivatives |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolaescu, O.E.; Ionescu, C.; Samide, A.; Tigae, C.; Spînu, C.I.; Oprea, B. Advancements in Cyclodextrin Complexes with Bioactive Secondary Metabolites and Their Pharmaceutical Applications. Pharmaceutics 2025, 17, 506. https://doi.org/10.3390/pharmaceutics17040506
Nicolaescu OE, Ionescu C, Samide A, Tigae C, Spînu CI, Oprea B. Advancements in Cyclodextrin Complexes with Bioactive Secondary Metabolites and Their Pharmaceutical Applications. Pharmaceutics. 2025; 17(4):506. https://doi.org/10.3390/pharmaceutics17040506
Chicago/Turabian StyleNicolaescu, Oana Elena, Cătălina Ionescu, Adriana Samide, Cristian Tigae, Cezar Ionuţ Spînu, and Bogdan Oprea. 2025. "Advancements in Cyclodextrin Complexes with Bioactive Secondary Metabolites and Their Pharmaceutical Applications" Pharmaceutics 17, no. 4: 506. https://doi.org/10.3390/pharmaceutics17040506
APA StyleNicolaescu, O. E., Ionescu, C., Samide, A., Tigae, C., Spînu, C. I., & Oprea, B. (2025). Advancements in Cyclodextrin Complexes with Bioactive Secondary Metabolites and Their Pharmaceutical Applications. Pharmaceutics, 17(4), 506. https://doi.org/10.3390/pharmaceutics17040506







