Development and Characterization of a Primary Ciliated Porcine Airway Model for the Evaluation of In Vitro Mucociliary Clearance and Mucosal Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Cell Culture
2.2. Histology
2.2.1. Tissue Procession
2.2.2. Alcian Blue Staining
2.2.3. Immunofluorescence (IF) Staining
2.2.4. Quantification of Ciliation, Cilia Length and Epithelial Thickness
2.2.5. Electron Microscopy
2.3. Gene Expression Analysis Using RT-qPCR
2.4. Transepithelial Electrical Resistance (TEER) Measurement
2.5. Determination of Permeability
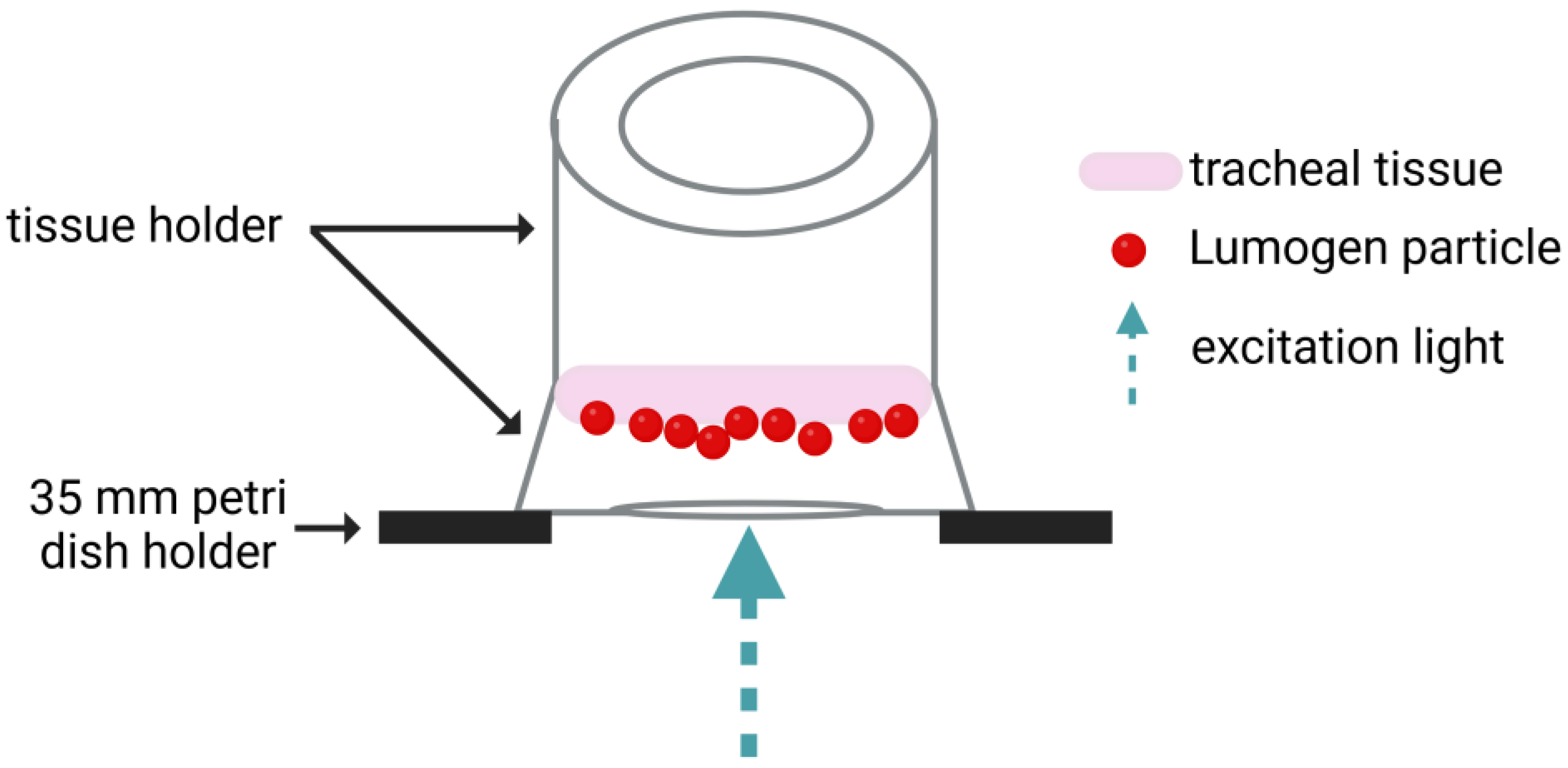
2.6. Mucociliary Clearance (MCC) Assay
2.7. Statistical Analysis
3. Results and Discussion
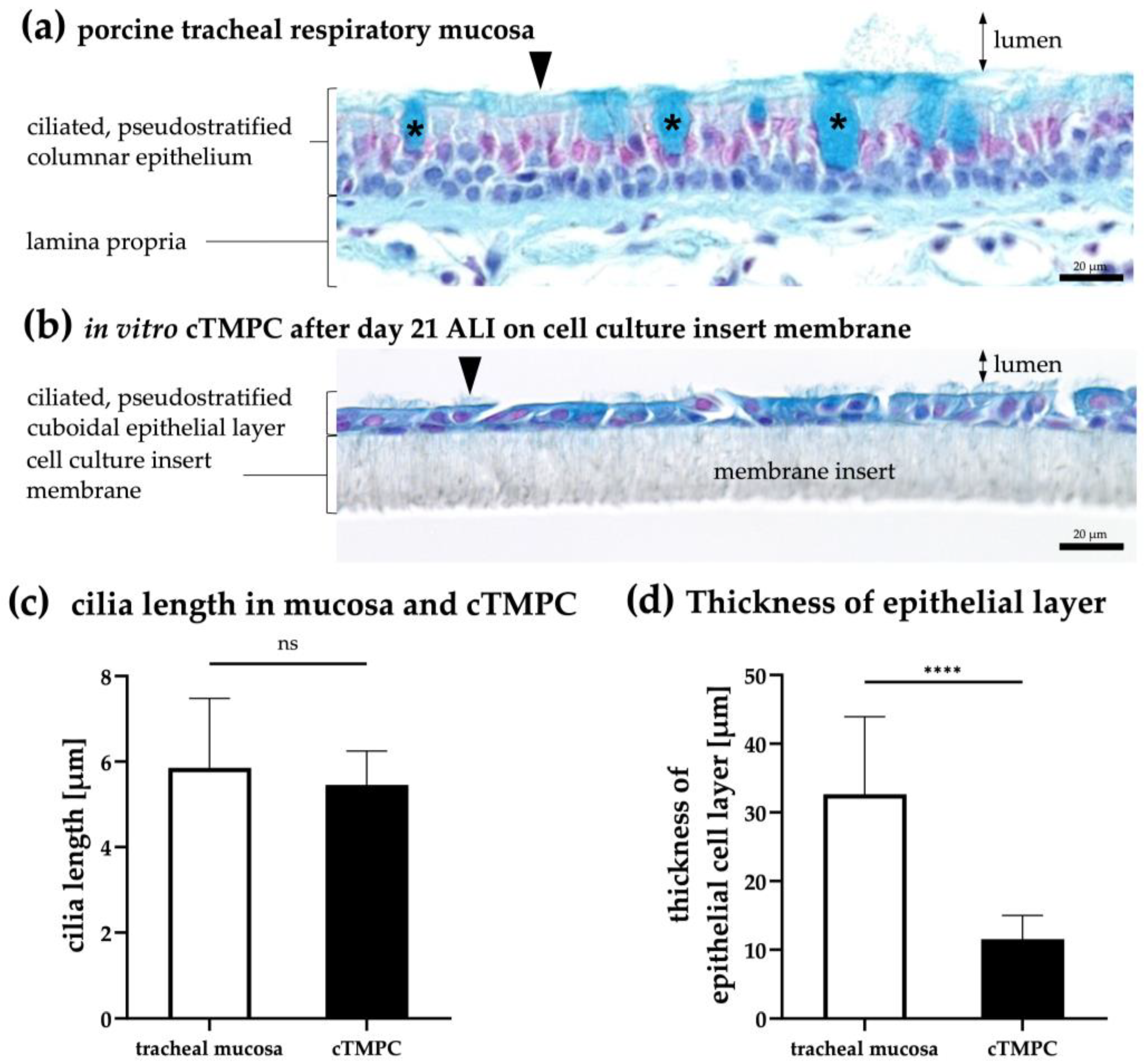
3.1. Determination of Epithelial Thickness and Cilial Length in the cTMPC Model
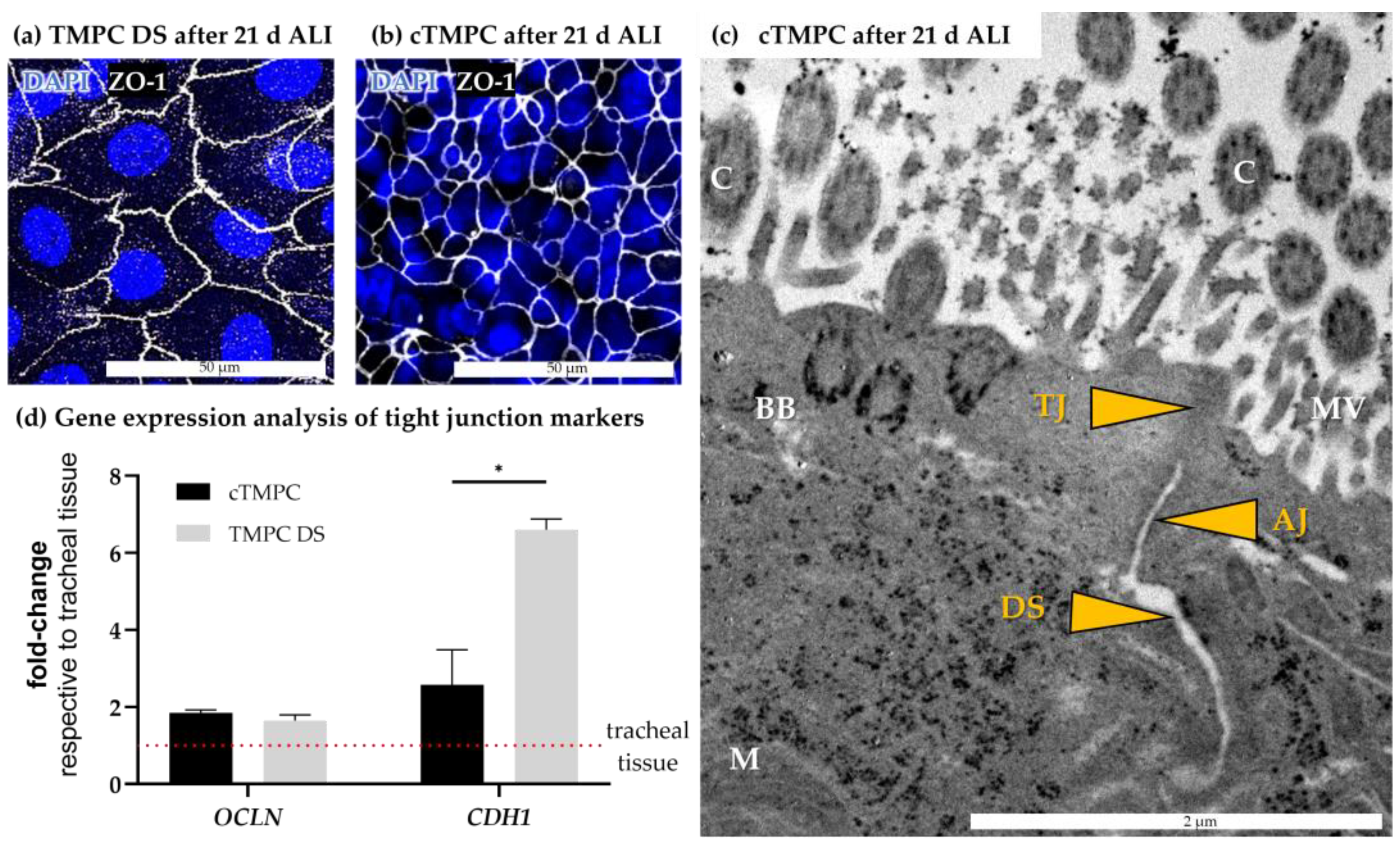
3.2. Morphological Characterization of Barrier Integrity
3.3. Basal Cell Characterization and 3D Structure of the Cell Modell
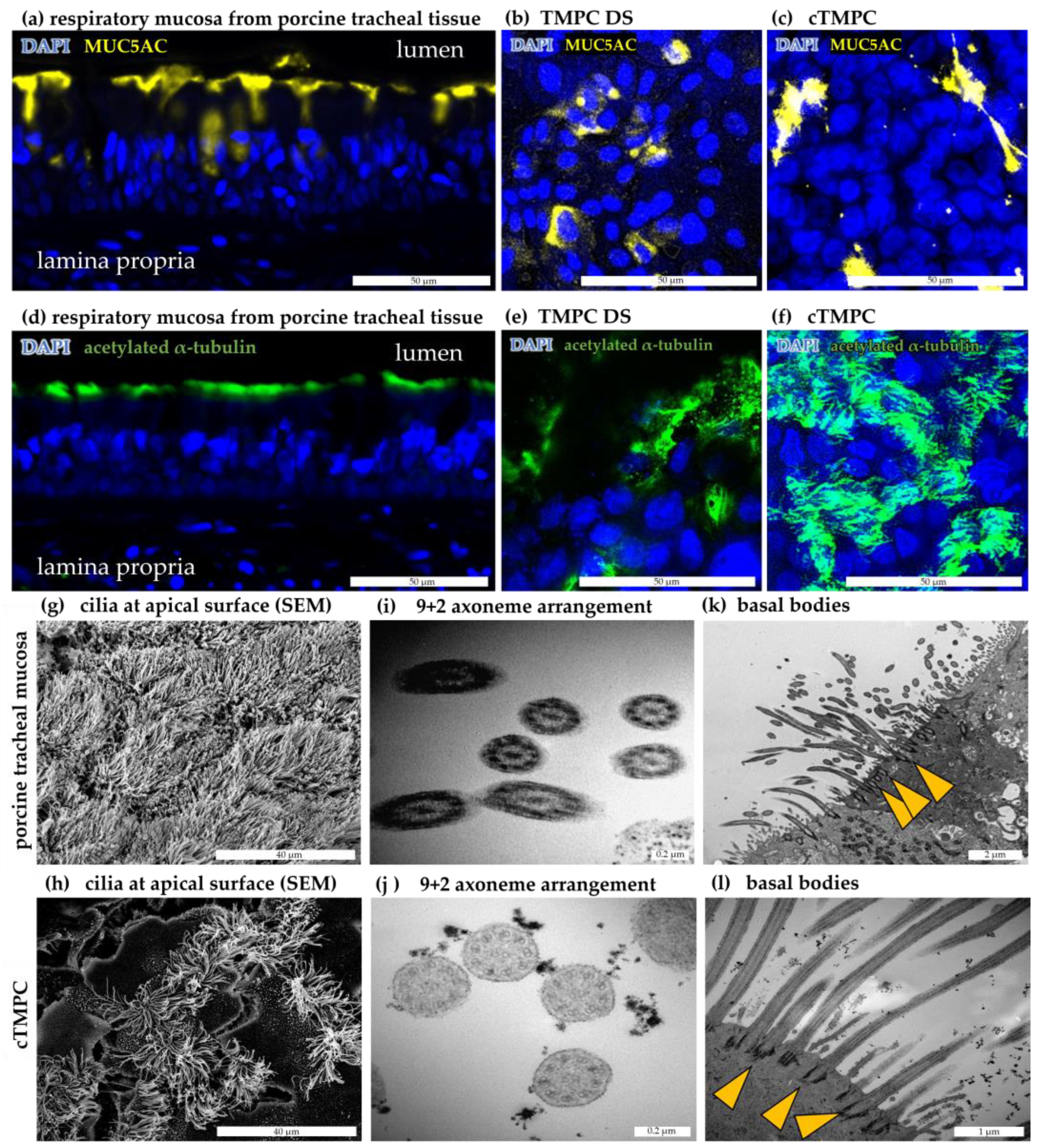
3.4. Mucus Production and Secretion
3.5. Formation and Ultrastructure of Cilia in cTMPC
3.6. Assesment of Ciliary Clearance in cTMPC Compared to Specimens from Airway Mucosa
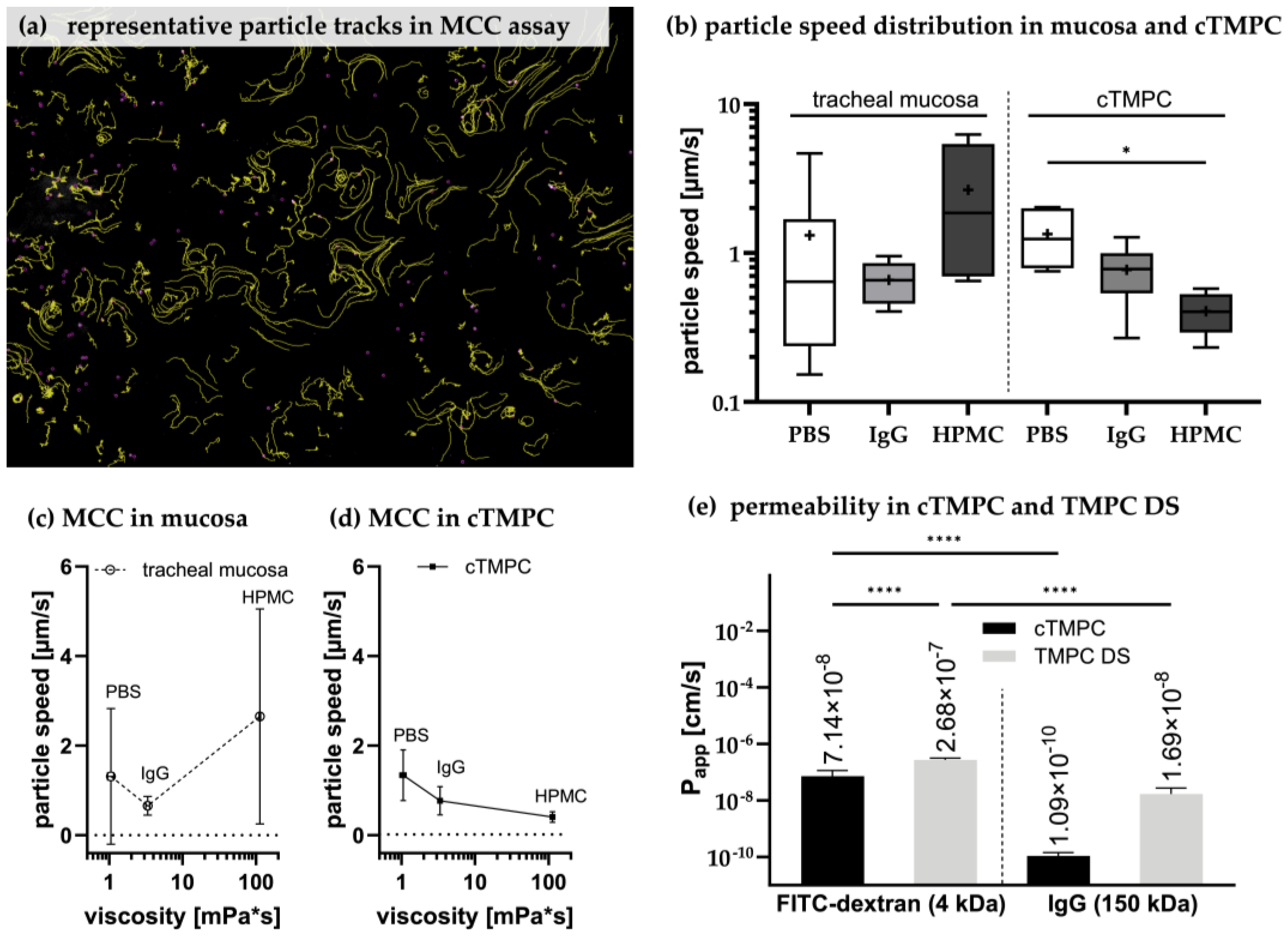
3.7. Permeability of cTMPC Compared to TMPC DS to Evaluate Drug Transport
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, M.M.; Zhao, M.; Islam, M.S.; Dong, K.; Saha, S.C. Aerosol Particle Transport and Deposition in Upper and Lower Airways of Infant, Child and Adult Human Lungs. Atmosphere 2021, 12, 1402. [Google Scholar] [CrossRef]
- Yeh, H.C.; Phalen, R.F.; Raabe, O.G. Factors influencing the deposition of inhaled particles. Environ. Health Perspect. 1976, 15, 147–156. [Google Scholar] [CrossRef]
- Wanner, A.; Salathé, M.; O’Riordan, T.G. Mucociliary clearance in the airways. Am. J. Respir. Crit. Care Med. 1996, 154, 1868–1902. [Google Scholar] [CrossRef]
- Downey, R.P.; Samra, N.S. Anatomy, Thorax, Tracheobronchial Tree; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Maiti, S. Nanometric Biopolymer Devices for Oral Delivery of Macromolecules with Clinical Significance; Elsevier: Amsterdam, The Netherlands, 2017; pp. 109–138. [Google Scholar] [CrossRef]
- Ladel, S.; Schlossbauer, P.; Flamm, J.; Luksch, H.; Mizaikoff, B.; Schindowski, K. Improved In Vitro Model for Intranasal Mucosal Drug Delivery: Primary Olfactory and Respiratory Epithelial Cells Compared with the Permanent Nasal Cell Line RPMI 2650. Pharmaceutics 2019, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Junker, F.; Gordon, J.; Qureshi, O. Fc Gamma Receptors and Their Role in Antigen Uptake, Presentation, and T Cell Activation. Front. Immunol. 2020, 11, 1393. [Google Scholar] [CrossRef]
- Lisi, S.; Sisto, M.; Lofrumento, D.D.; D’Amore, S.; D’Amore, M. Advances in the understanding of the Fc gamma receptors-mediated autoantibodies uptake. Clin. Exp. Med. 2011, 11, 1–10. [Google Scholar] [CrossRef]
- Abuqayyas, L.; Balthasar, J.P. Investigation of the role of FcγR and FcRn in mAb distribution to the brain. Mol. Pharm. 2013, 10, 1505–1513. [Google Scholar] [CrossRef]
- Ishikawa, T.; Takizawa, T.; Iwaki, J.; Mishima, T.; Ui-Tei, K.; Takeshita, T.; Matsubara, S.; Takizawa, T. Fc gamma receptor IIb participates in maternal IgG trafficking of human placental endothelial cells. Int. J. Mol. Med. 2015, 35, 1273–1289. [Google Scholar] [CrossRef]
- Ladel, S.; Maigler, F.; Flamm, J.; Schlossbauer, P.; Handl, A.; Hermann, R.; Herzog, H.; Hummel, T.; Mizaikoff, B.; Schindowski, K. Impact of Glycosylation and Species Origin on the Uptake and Permeation of IgGs through the Nasal Airway Mucosa. Pharmaceutics 2020, 12, 1014. [Google Scholar] [CrossRef]
- Smith, K.G.C.; Clatworthy, M.R. FcgammaRIIB in autoimmunity and infection: Evolutionary and therapeutic implications. Nat. Rev. Immunol. 2010, 10, 328–343. [Google Scholar] [CrossRef]
- Abelson, D.; Frater, C.; Cottee, A.; Pearson, M.; Morgan, L. Mucociliary clearance (MCC) in the trachea in mild-moderate COPD occurs in spirals. Eur. Respir. J. 2015, 46, PA2285. [Google Scholar]
- Houtmeyers, E.; Gosselink, R.; Gayan-Ramirez, G.; Decramer, M. Regulation of mucociliary clearance in health and disease. Eur. Respir. J. 1999, 13, 1177–1188. [Google Scholar] [CrossRef]
- Fulcher, M.L.; Gabriel, S.; Burns, K.A.; Yankaskas, J.R.; Randell, S.H. Well-differentiated human airway epithelial cell cultures. Methods Mol. Med. 2005, 107, 183–206. [Google Scholar] [CrossRef]
- You, Y.; Richer, E.J.; Huang, T.; Brody, S.L. Growth and differentiation of mouse tracheal epithelial cells: Selection of a proliferative population. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L1315–L1321. [Google Scholar] [CrossRef] [PubMed]
- Lodes, N.; Seidensticker, K.; Perniss, A.; Nietzer, S.; Oberwinkler, H.; May, T.; Walles, T.; Hebestreit, H.; Hackenberg, S.; Steinke, M. Investigation on Ciliary Functionality of Different Airway Epithelial Cell Lines in Three-Dimensional Cell Culture. Tissue Eng. Part A 2020, 26, 432–440. [Google Scholar] [CrossRef]
- Pickles, R.J. Human airway epithelial cell cultures for modeling respiratory syncytial virus infection. Curr. Top. Microbiol. Immunol. 2013, 372, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Benam, K.H.; Novak, R.; Nawroth, J.; Hirano-Kobayashi, M.; Ferrante, T.C.; Choe, Y.; Prantil-Baun, R.; Weaver, J.C.; Bahinski, A.; Parker, K.K.; et al. Matched-Comparative Modeling of Normal and Diseased Human Airway Responses Using a Microengineered Breathing Lung Chip. Cell Syst. 2016, 3, 456–466.e4. [Google Scholar] [CrossRef]
- Benam, K.H.; Villenave, R.; Lucchesi, C.; Varone, A.; Hubeau, C.; Lee, H.-H.; Alves, S.E.; Salmon, M.; Ferrante, T.C.; Weaver, J.C.; et al. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat. Methods 2016, 13, 151–157. [Google Scholar] [CrossRef]
- Sakagami, M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv. Drug Deliv. Rev. 2006, 58, 1030–1060. [Google Scholar] [CrossRef]
- Inoue, D.; Tanaka, A.; Kimura, S.; Kiriyama, A.; Katsumi, H.; Yamamoto, A.; Ogawara, K.-I.; Kimura, T.; Higaki, K.; Yutani, R.; et al. The relationship between in vivo nasal drug clearance and in vitro nasal mucociliary clearance: Application to the prediction of nasal drug absorption. Eur. J. Pharm. Sci. 2018, 117, 21–26. [Google Scholar] [CrossRef]
- Henning, A.; Schneider, M.; Bur, M.; Blank, F.; Gehr, P.; Lehr, C.-M. Embryonic chicken trachea as a new in vitro model for the investigation of mucociliary particle clearance in the airways. AAPS PharmSciTech 2008, 9, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Iwasaki, A. Method for Measuring Mucociliary Clearance and Cilia-generated Flow in Mice by Ex Vivo Imaging. Bio Protoc. 2020, 10, e3554. [Google Scholar] [CrossRef]
- Smith, D.J.; Gaffney, E.A.; Blake, J.R. Modelling mucociliary clearance. Respir. Physiol. Neurobiol. 2008, 163, 178–188. [Google Scholar] [CrossRef]
- Fliegauf, M.; Sonnen, A.F.-P.; Kremer, B.; Henneke, P. Mucociliary clearance defects in a murine in vitro model of pneumococcal airway infection. PLoS ONE 2013, 8, e59925. [Google Scholar] [CrossRef]
- Becker, M.E.; Martin-Sancho, L.; Simons, L.M.; McRaven, M.D.; Chanda, S.K.; Hultquist, J.F.; Hope, T.J. Live imaging of airway epithelium reveals that mucociliary clearance modulates SARS-CoV-2 spread. Nat. Commun. 2024, 15, 9480. [Google Scholar] [CrossRef]
- Bustamante-Marin, X.M.; Ostrowski, L.E. Cilia and Mucociliary Clearance. Cold Spring Harb. Perspect. Biol. 2017, 9, a028241. [Google Scholar] [CrossRef]
- Tilley, A.E.; Walters, M.S.; Shaykhiev, R.; Crystal, R.G. Cilia dysfunction in lung disease. Annu. Rev. Physiol. 2015, 77, 379–406. [Google Scholar] [CrossRef]
- Sears, P.R.; Yin, W.-N.; Ostrowski, L.E. Continuous mucociliary transport by primary human airway epithelial cells in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L99–L108. [Google Scholar] [CrossRef]
- Raju, S.V.; Lin, V.Y.; Liu, L.; McNicholas, C.M.; Karki, S.; Sloane, P.A.; Tang, L.; Jackson, P.L.; Wang, W.; Wilson, L.; et al. The Cystic Fibrosis Transmembrane Conductance Regulator Potentiator Ivacaftor Augments Mucociliary Clearance Abrogating Cystic Fibrosis Transmembrane Conductance Regulator Inhibition by Cigarette Smoke. Am. J. Respir. Cell Mol. Biol. 2017, 56, 99–108. [Google Scholar] [CrossRef]
- Scopulovic, L.; Francis, D.; Pandzic, E.; Francis, R. Quantifying cilia beat frequency using high-speed video microscopy: Assessing frame rate requirements when imaging different ciliated tissues. Physiol. Rep. 2022, 10, e15349. [Google Scholar] [CrossRef]
- Glorieux, S.; van den Broeck, W.; van der Meulen, K.M.; van Reeth, K.; Favoreel, H.W.; Nauwynck, H.J. In vitro culture of porcine respiratory nasal mucosa explants for studying the interaction of porcine viruses with the respiratory tract. J. Virol. Methods 2007, 142, 105–112. [Google Scholar] [PubMed]
- Lobasso, S.; Lopalco, P.; Angelini, R.; Baronio, M.; Fanizzi, F.P.; Babudri, F.; Corcelli, A. Lipidomic analysis of porcine olfactory epithelial membranes and cilia. Lipids 2010, 45, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Ober, R.J.; Radu, C.G.; Ghetie, V.; Ward, E.S. Differences in promiscuity for antibody-FcRn interactions across species: Implications for therapeutic antibodies. Int. Immunol. 2001, 13, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Meurens, F.; Summerfield, A.; Nauwynck, H.; Saif, L.; Gerdts, V. The pig: A model for human infectious diseases. Trends Microbiol. 2012, 20, 50–57. [Google Scholar] [CrossRef]
- Fulcher, M.L.; Randell, S.H. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol. Biol. 2013, 945, 109–121. [Google Scholar] [CrossRef]
- Martin, J.; Rittersberger, R.; Treitler, S.; Kopp, P.; Ibraimi, A.; Koslowski, G.; Sickinger, M.; Dabbars, A.; Schindowski, K. Characterization of a primary cellular airway model for inhalative drug delivery in comparison with the established permanent cell lines CaLu3 and RPMI 2650. Vitr. Models 2024, 3, 183–203. [Google Scholar] [CrossRef]
- Scott, J.E.; Quintarelli, G.; Dellovo, M.C. The chemical and histochemical properties of Alcian Blue. I. The mechanism of Alcian Blue staining. Histochemie 1964, 4, 73–85. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Schütz, D.; Rode, S.; Read, C.; Müller, J.A.; Glocker, B.; Sparrer, K.M.J.; Fackler, O.T.; Walther, P.; Münch, J. Viral Transduction Enhancing Effect of EF-C Peptide Nanofibrils Is Mediated by Cellular Protrusions. Adv. Funct. Mater. 2021, 31, 2104814. [Google Scholar] [CrossRef]
- Olari, L.-R.; Bauer, R.; Gil Miró, M.; Vogel, V.; Cortez Rayas, L.; Groß, R.; Gilg, A.; Klevesath, R.; Rodríguez Alfonso, A.A.; Kaygisiz, K.; et al. The C-terminal 32-mer fragment of hemoglobin alpha is an amyloidogenic peptide with antimicrobial properties. Cell. Mol. Life Sci. 2023, 80, 151. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Abraham, G.; Zizzadoro, C.; Kacza, J.; Ellenberger, C.; Abs, V.; Franke, J.; Schoon, H.-A.; Seeger, J.; Tesfaigzi, Y.; Ungemach, F.R. Growth and differentiation of primary and passaged equine bronchial epithelial cells under conventional and air-liquid-interface culture conditions. BMC Vet. Res. 2011, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Spindler, L.M.; Feuerhake, A.; Ladel, S.; Günday, C.; Flamm, J.; Günday-Türeli, N.; Türeli, E.; Tovar, G.E.M.; Schindowski, K.; Gruber-Traub, C. Nano-in-Micro-Particles Consisting of PLGA Nanoparticles Embedded in Chitosan Microparticles via Spray-Drying Enhances Their Uptake in the Olfactory Mucosa. Front. Pharmacol. 2021, 12, 732954. [Google Scholar] [CrossRef]
- Tinevez, J.-Y.; Perry, N.; Schindelin, J.; Hoopes, G.M.; Reynolds, G.D.; Laplantine, E.; Bednarek, S.Y.; Shorte, S.L.; Eliceiri, K.W. TrackMate: An open and extensible platform for single-particle tracking. Methods 2017, 115, 80–90. [Google Scholar] [CrossRef]
- Shadi, I.; Chowdhry, B.; Snowden, M.; Withnall, R. Semi-quantitative trace analysis of nuclear fast red by surface enhanced resonance Raman scattering. Anal. Chim. Acta 2001, 450, 115–122. [Google Scholar] [CrossRef]
- Norden, C. Pseudostratified epithelia-cell biology, diversity and roles in organ formation at a glance. J. Cell Sci. 2017, 130, 1859–1863. [Google Scholar] [CrossRef]
- Aoki, F.G.; Varma, R.; Marin-Araujo, A.E.; Lee, H.; Soleas, J.P.; Li, A.H.; Soon, K.; Romero, D.; Moriya, H.T.; Haykal, S.; et al. De-epithelialization of porcine tracheal allografts as an approach for tracheal tissue engineering. Sci. Rep. 2019, 9, 12034. [Google Scholar] [CrossRef]
- Genna, V.G.; Adamo, D.; Galaverni, G.; Lepore, F.; Boraldi, F.; Quaglino, D.; Lococo, F.; Pellegrini, G. Validation of airway porcine epithelial cells as an alternative to human in vitro preclinical studies. Sci. Rep. 2023, 13, 16290. [Google Scholar] [CrossRef]
- Jeffery, P.K.; Reid, L. New observations of rat airway epithelium: A quantitative and electron microscopic study. J. Anat. 1975, 120, 295–320. [Google Scholar]
- Roperto, F.; Rossacco, P.; Tartaro, A.; Galati, P. Abnormal length of respiratory cilia in a pig. An ultrastructural study. J. Submicrosc. Cytol. Pathol. 1994, 26, 75–78. [Google Scholar] [PubMed]
- Tam, A.; Wadsworth, S.; Dorscheid, D.; Man, S.F.P.; Sin, D.D. The airway epithelium: More than just a structural barrier. Ther. Adv. Respir. Dis. 2011, 5, 255–273. [Google Scholar] [CrossRef]
- Gizurarson, S. The effect of cilia and the mucociliary clearance on successful drug delivery. Biol. Pharm. Bull. 2015, 38, 497–506. [Google Scholar] [CrossRef]
- Chung, S.W.; Hil-lal, T.A.; Byun, Y. Strategies for non-invasive delivery of biologics. J. Drug Target. 2012, 20, 481–501. [Google Scholar] [CrossRef] [PubMed]
- Vuković, L.D.; Jevtić, P.; Edens, L.J.; Levy, D.L. New Insights into Mechanisms and Functions of Nuclear Size Regulation. Int. Rev. Cell Mol. Biol. 2016, 322, 1–59. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P.; Veiga, A.B.G.d.t. Biologia Molecular da Célula, 4th ed.; Artmed: Porto Alegre, RS, USA, 2006; ISBN 0-8153-3218-1. [Google Scholar]
- Pezzulo, A.A.; Starner, T.D.; Scheetz, T.E.; Traver, G.L.; Tilley, A.E.; Harvey, B.-G.; Crystal, R.G.; McCray, P.B.; Zabner, J. The air-liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 300, L25–L31. [Google Scholar] [CrossRef]
- Lin, H.; Li, H.; Cho, H.-J.; Bian, S.; Roh, H.-J.; Lee, M.-K.; Kim, J.S.; Chung, S.-J.; Shim, C.-K.; Kim, D.-D. Air-liquid interface (ALI) culture of human bronchial epithelial cell monolayers as an in vitro model for airway drug transport studies. J. Pharm. Sci. 2007, 96, 341–350. [Google Scholar] [CrossRef]
- Wang, H.; He, L.; Liu, B.; Feng, Y.; Zhou, H.; Zhang, Z.; Wu, Y.; Wang, J.; Gan, Y.; Yuan, T.; et al. Establishment and comparison of air-liquid interface culture systems for primary and immortalized swine tracheal epithelial cells. BMC Cell Biol. 2018, 19, 10. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Crystal, R.G.; Randell, S.H.; Engelhardt, J.F.; Voynow, J.; Sunday, M.E. Airway epithelial cells: Current concepts and challenges. Proc. Am. Thorac. Soc. 2008, 5, 772–777. [Google Scholar] [CrossRef]
- Ruysseveldt, E.; Martens, K.; Steelant, B. Airway Basal Cells, Protectors of Epithelial Walls in Health and Respiratory Diseases. Front. Allergy 2021, 2, 787128. [Google Scholar] [CrossRef]
- Inayama, Y.; Hook, G.E.; Brody, A.R.; Jetten, A.M.; Gray, T.; Mahler, J.; Nettesheim, P. In vitro and in vivo growth and differentiation of clones of tracheal basal cells. Am. J. Pathol. 1989, 134, 539–549. [Google Scholar]
- Arason, A.J.; Jonsdottir, H.R.; Halldorsson, S.; Benediktsdottir, B.E.; Bergthorsson, J.T.; Ingthorsson, S.; Baldursson, O.; Sinha, S.; Gudjonsson, T.; Magnusson, M.K. deltaNp63 has a role in maintaining epithelial integrity in airway epithelium. PLoS ONE 2014, 9, e88683. [Google Scholar] [CrossRef]
- Cozens, D.; Sutherland, E.; Marchesi, F.; Taylor, G.; Berry, C.C.; Davies, R.L. Temporal differentiation of bovine airway epithelial cells grown at an air-liquid interface. Sci. Rep. 2018, 8, 14893. [Google Scholar] [CrossRef]
- Daniely, Y.; Liao, G.; Dixon, D.; Linnoila, R.I.; Lori, A.; Randell, S.H.; Oren, M.; Jetten, A.M. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am. J. Physiol. Cell Physiol. 2004, 287, C171–C181. [Google Scholar] [CrossRef]
- O’Boyle, N.; Sutherland, E.; Berry, C.C.; Davies, R.L. Temporal dynamics of ovine airway epithelial cell differentiation at an air-liquid interface. PLoS ONE 2017, 12, e0181583. [Google Scholar] [CrossRef]
- Rock, J.R.; Randell, S.H.; Hogan, B.L.M. Airway basal stem cells: A perspective on their roles in epithelial homeostasis and remodeling. Dis. Model. Mech. 2010, 3, 545–556. [Google Scholar] [CrossRef]
- Yu, A.S.; Hanner, F.; Peti-Peterdi, J. Intercellular Junctions, 347–368. In Seldin and Giebisch’s The Kidney, 5th ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 347–368. [Google Scholar] [CrossRef]
- Wu, P.; Gong, H.; Richman, R.; Freddo, T.F. Localization of occludin, ZO-1, and pan-cadherin in rabbit ciliary epithelium and iris vascular endothelium. Histochem. Cell Biol. 2000, 114, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; van Winkle, L.S.; Fanucchi, M.V.; Plopper, C.G.; Evans, M.J.; van Winkle, L.S.; Fanucchi, M.V.; Plopper, C.G. Cellular and molecular characteristics of basal cells in airway epithelium. Exp. Lung Res. 2001, 27, 401–415. [Google Scholar] [CrossRef]
- Prytherch, Z.; Job, C.; Marshall, H.; Oreffo, V.; Foster, M.; BéruBé, K. Tissue-Specific stem cell differentiation in an in vitro airway model. Macromol. Biosci. 2011, 11, 1467–1477. [Google Scholar] [CrossRef]
- Swami, D.; Aich, J.; Bisht, B.; Paul, M.K. Reconstructing the lung stem cell niche in vitro. In Advances in Stem Cells and their Niches; Elsevier: Amsterdam, The Netherlands, 2022; Volume 6, pp. 97–143. [Google Scholar] [CrossRef]
- Ganesan, S.; Comstock, A.T.; Sajjan, U.S. Barrier function of airway tract epithelium. Tissue Barriers 2013, 1, e24997. [Google Scholar] [CrossRef]
- Groneberg, D.A.; Eynott, P.R.; Oates, T.; Lim, S.; Wu, R.; Carlstedt, I.; Nicholson, A.G.; Chung, K.F. Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung. Respir. Med. 2002, 96, 81–86. [Google Scholar] [CrossRef]
- Okuda, K.; Chen, G.; Subramani, D.B.; Wolf, M.; Gilmore, R.C.; Kato, T.; Radicioni, G.; Kesimer, M.; Chua, M.; Dang, H.; et al. Localization of Secretory Mucins MUC5AC and MUC5B in Normal/Healthy Human Airways. Am. J. Respir. Crit. Care Med. 2019, 199, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Prince, O.A.; Krunkosky, T.M.; Krause, D.C. In vitro spatial and temporal analysis of Mycoplasma pneumoniae colonization of human airway epithelium. Infect. Immun. 2014, 82, 579–586. [Google Scholar] [CrossRef]
- Delgado-Ortega, M.; Olivier, M.; Sizaret, P.-Y.; Simon, G.; Meurens, F. Newborn pig trachea cell line cultured in air-liquid interface conditions allows a partial in vitro representation of the porcine upper airway tissue. BMC Cell Biol. 2014, 15, 14. [Google Scholar] [CrossRef]
- Liu, X.; Krawczyk, E.; Suprynowicz, F.A.; Palechor-Ceron, N.; Yuan, H.; Dakic, A.; Simic, V.; Zheng, Y.-L.; Sripadhan, P.; Chen, C.; et al. Conditional reprogramming and long-term expansion of normal and tumor cells from human biospecimens. Nat. Protoc. 2017, 12, 439–451. [Google Scholar] [CrossRef]
- Davis, A.S.; Chertow, D.S.; Moyer, J.E.; Suzich, J.; Sandouk, A.; Dorward, D.W.; Logun, C.; Shelhamer, J.H.; Taubenberger, J.K. Validation of normal human bronchial epithelial cells as a model for influenza A infections in human distal trachea. J. Histochem. Cytochem. 2015, 63, 312–328. [Google Scholar] [CrossRef]
- Schagen, J.; Sly, P.D.; Fantino, E. Characterizing well-differentiated culture of primary human nasal epithelial cells for use in wound healing assays. Lab. Investig. 2018, 98, 1478–1486. [Google Scholar] [CrossRef]
- Bukowy-Bieryłło, Z.; Daca-Roszak, P.; Jurczak, J.; Przystałowska-Macioła, H.; Jaksik, R.; Witt, M.; Ziętkiewicz, E. In vitro differentiation of ciliated cells in ALI-cultured human airway epithelium-The framework for functional studies on airway differentiation in ciliopathies. Eur. J. Cell Biol. 2022, 101, 151189. [Google Scholar] [CrossRef]
- de Jong, P.M.; van Sterkenburg, M.A.; Hesseling, S.C.; Kempenaar, J.A.; Mulder, A.A.; Mommaas, A.M.; Dijkman, J.H.; Ponec, M. Ciliogenesis in human bronchial epithelial cells cultured at the air-liquid interface. Am. J. Respir. Cell Mol. Biol. 1994, 10, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, K.; Dabbagh, K.; Lee, H.M.; Agustí, C.; Lausier, J.A.; Ueki, I.F.; Grattan, K.M.; Nadel, J.A. Epidermal growth factor system regulates mucin production in airways. Proc. Natl. Acad. Sci. USA 1999, 96, 3081–3086. [Google Scholar] [CrossRef]
- Gray, T.E.; Guzman, K.; Davis, C.W.; Abdullah, L.H.; Nettesheim, P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 1996, 14, 104–112. [Google Scholar] [CrossRef]
- Sachs, L.A.; Finkbeiner, W.E.; Widdicombe, J.H. Effects of media on differentiation of cultured human tracheal epithelium. Vitr. Cell. Dev. Biol. Anim. 2003, 39, 56–62. Available online: https://www.jstor.org/stable/4295421 (accessed on 12 February 2025).
- Yoon, J.H.; Gray, T.; Guzman, K.; Koo, J.S.; Nettesheim, P. Regulation of the secretory phenotype of human airway epithelium by retinoic acid, triiodothyronine, and extracellular matrix. Am. J. Respir. Cell Mol. Biol. 1997, 16, 724–731. [Google Scholar] [CrossRef]
- O’Boyle, N.; Sutherland, E.; Berry, C.C.; Davies, R.L. Optimisation of growth conditions for ovine airway epithelial cell differentiation at an air-liquid interface. PLoS ONE 2018, 13, e0193998. [Google Scholar] [CrossRef]
- Moon, S.K.; Yoo, J.H.; Kim, H.N.; Lim, D.J.; Chung, M.H. Effects of retinoic acid, triiodothyronine and hydrocortisone on mucin and lysozyme expression in cultured human middle ear epithelial cells. Acta Otolaryngol. 2000, 120, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Zhou, X.; Kolosov, V.P.; Perelman, J.M. Triiodothyronine represses MUC5AC expression by antagonizing Sp1 binding to its promoter in human bronchial epithelial HBE16 cells. J. Biomed. Biotechnol. 2012, 2012, 648170. [Google Scholar] [CrossRef]
- Atherton, H.C.; Jones, G.; Danahay, H. IL-13-induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3-kinase regulation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L730–L739. [Google Scholar] [CrossRef]
- Gerovac, B.J.; Fregien, N.L. IL-13 Inhibits Multicilin Expression and Ciliogenesis via Janus Kinase/Signal Transducer and Activator of Transcription Independently of Notch Cleavage. Am. J. Respir. Cell Mol. Biol. 2016, 54, 554–561. [Google Scholar] [CrossRef]
- You, Y.; Brody, S.L. Culture and differentiation of mouse tracheal epithelial cells. Methods Mol. Biol. 2013, 945, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Mercer, R.R.; Russell, M.L.; Roggli, V.L.; Crapo, J.D. American Journal of Respiratory Cell and Molecular Biology//Cell Number and Distribution in Human and Rat Airways, Electronic version; American Thoracic Society: New York, NY, USA, 1994. [Google Scholar]
- Boers, J.E.; Ambergen, A.W.; Thunnissen, F.B. Number and proliferation of clara cells in normal human airway epithelium. Am. J. Respir. Crit. Care Med. 1999, 159, 1585–1591. [Google Scholar] [CrossRef]
- Davis, J.D.; Wypych, T.P. Cellular and functional heterogeneity of the airway epithelium. Mucosal Immunol. 2021, 14, 978–990. [Google Scholar] [CrossRef]
- Cumplido-Laso, G.; Benitez, D.A.; Mulero-Navarro, S.; Carvajal-Gonzalez, J.M. Transcriptional Regulation of Airway Epithelial Cell Differentiation: Insights into the Notch Pathway and Beyond. Int. J. Mol. Sci. 2023, 24, 14789. [Google Scholar] [CrossRef]
- Rock, J.R.; Gao, X.; Xue, Y.; Randell, S.H.; Kong, Y.-Y.; Hogan, B.L.M. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 2011, 8, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Nishinakamura, R.; Saga, Y.; Kopan, R. Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development 2012, 139, 4365–4373. [Google Scholar] [CrossRef] [PubMed]
- Kopan, R. Notch signaling. Cold Spring Harb. Perspect. Biol. 2012, 4, a011213. [Google Scholar] [CrossRef]
- Byrnes, L.E.; Deleon, R.; Reiter, J.F.; Choksi, S.P. Opposing transcription factors MYCL and HEY1 mediate the Notch-dependent airway stem cell fate decision. bioRxiv 2022, 2022-10. [Google Scholar] [CrossRef]
- Hua, K.; Ferland, R.J. Fixation methods can differentially affect ciliary protein immunolabeling. Cilia 2017, 6, 5. [Google Scholar] [CrossRef]
- Wallace, P.; Kennedy, J.R.; Mendicino, J. Transdifferentiation of outgrowth cells and cultured epithelial cells from swine trachea. Vitr. Cell. Dev. Biol. Anim. 1994, 30A, 168–180. [Google Scholar] [CrossRef]
- Wang, Z. Regulation of Cell Cycle Progression by Growth Factor-Induced Cell Signaling. Cells 2021, 10, 3327. [Google Scholar] [CrossRef]
- Kent, K.D.; Bomser, J.A. Bovine pituitary extract provides remarkable protection against oxidative stress in human prostate epithelial cells. Vitr. Cell. Dev. Biol. Anim. 2003, 39, 388. Available online: https://www.jstor.org/stable/4295490 (accessed on 12 February 2025).
- van Scott, M.R.; Lee, N.P.; Yankaskas, J.R.; Boucher, R.C. Effect of hormones on growth and function of cultured canine tracheal epithelial cells. Am. J. Physiol. 1988, 255, C237–C245. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Iwano, T.; Takeda, S. Estrogen and EGFR Pathways Regulate Notch Signaling in Opposing Directions for Multi-Ciliogenesis in the Fallopian Tube. Cells 2019, 8, 933. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.C.; Karasin, A.I.; Olsen, C.W. Differentiated swine airway epithelial cell cultures for the investigation of influenza A virus infection and replication. Influenza Other Respir. Viruses 2013, 7, 139–150. [Google Scholar] [CrossRef]
- Mao, H.; Wang, Y.; Yuan, W.; Wong, L.B. Ciliogenesis in cryopreserved mammalian tracheal epithelial cells cultured at the air-liquid interface. Cryobiology 2009, 59, 250–257. [Google Scholar] [CrossRef]
- Gerovac, B.J.; Valencia, M.; Baumlin, N.; Salathe, M.; Conner, G.E.; Fregien, N.L. Submersion and hypoxia inhibit ciliated cell differentiation in a notch-dependent manner. Am. J. Respir. Cell Mol. Biol. 2014, 51, 516–525. [Google Scholar] [CrossRef]
- Gan, Y.; Xie, X.; Zhang, L.; Xiong, Q.; Shao, G.; Feng, Z. Establishment of a model of Mycoplasma hyopneumoniae infection using Bama miniature pigs. Food Prod. Process Nutr. 2020, 2, 19. [Google Scholar] [CrossRef]
- Dvornikov, D.; Halavatyi, A.; Khan, M.M.; Zimmermann, N.; Cross, A.; Poeckel, D.; Melnikov, E.; Tischer, C.; Leyrer, J.; Schneider, M.A.; et al. Quantitative imaging reveals PI3Kδ inhibition reduces rhinovirus-induced damage of small airway epithelia in ex vivo cultured human precision cut lung slices from COPD patients. bioRxiv 2022, 2022-03. [Google Scholar] [CrossRef]
- Roth, D.; Şahin, A.T.; Ling, F.; Senger, C.N.; Quiroz, E.J.; Calvert, B.A.; van der Does, A.M.; Güney, T.G.; Tepho, N.; Glasl, S.; et al. Structure-function relationships of mucociliary clearance in the human airways. Nat. Commun. 2025, 16, 2446. [Google Scholar] [CrossRef]
- Reynolds, M.J.; Phetruen, T.; Fisher, R.L.; Chen, K.; Pentecost, B.T.; Gomez, G.; Ounjai, P.; Sui, H. The Developmental Process of the Growing Motile Ciliary Tip Region. Sci. Rep. 2018, 8, 7977. [Google Scholar] [CrossRef] [PubMed]
- Viswanadha, R.; Sale, W.S.; Porter, M.E. Ciliary Motility: Regulation of Axonemal Dynein Motors. Cold Spring Harb. Perspect. Biol. 2017, 9, a018325. [Google Scholar] [CrossRef]
- Mukherjee, I.; Roy, S.; Chakrabarti, S. Identification of Important Effector Proteins in the FOXJ1 Transcriptional Network Associated with Ciliogenesis and Ciliary Function. Front. Genet. 2019, 10, 23. [Google Scholar] [CrossRef]
- Saint-Criq, V.; Delpiano, L.; Casement, J.; Onuora, J.C.; Lin, J.; Gray, M.A. Choice of Differentiation Media Significantly Impacts Cell Lineage and Response to CFTR Modulators in Fully Differentiated Primary Cultures of Cystic Fibrosis Human Airway Epithelial Cells. Cells 2020, 9, 2137. [Google Scholar] [CrossRef]
- M. Ways, T.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, D.; Tong, S.; Liu, F.; Wei, W.; Liu, Z. Experimental study on shear viscosity and rheopexy of Escherichia coli suspensions. Rheol. Acta 2022, 61, 271–280. [Google Scholar] [CrossRef]
- Mersich, C.; Ahrer, K.; Buchacher, A.; Ernegger, T.; Kohla, G.; Kannicht, C.; Pock, K.; Römisch, J. Biochemical characterization and stability of immune globulin intravenous 10% liquid (Panzyga®). Biologicals 2017, 45, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Gheber, L.; Korngreen, A.; Priel, Z. Effect of viscosity on metachrony in mucus propelling cilia. Cell Motil. Cytoskelet. 1998, 39, 9–20. Available online: https://onlinelibrary.wiley.com/doi/10.1002/(SICI)1097-0169(1998)39:1%3C9::AID-CM2%3E3.0.CO;2-3 (accessed on 12 February 2025). [CrossRef]
- Lee, W.L.; Jayathilake, P.G.; Tan, Z.; Le, D.V.; Lee, H.P.; Khoo, B.C. Muco-ciliary transport: Effect of mucus viscosity, cilia beat frequency and cilia density. Comput. Fluids 2011, 49, 214–221. [Google Scholar] [CrossRef]
- Robinot, R.; Hubert, M.; de Melo, G.D.; Lazarini, F.; Bruel, T.; Smith, N.; Levallois, S.; Larrous, F.; Fernandes, J.; Gellenoncourt, S.; et al. SARS-CoV-2 infection induces the dedifferentiation of multiciliated cells and impairs mucociliary clearance. Nat. Commun. 2021, 12, 4354. [Google Scholar] [CrossRef]
- Hofmann, W.; Asgharian, B. The effect of lung structure on mucociliary clearance and particle retention in human and rat lungs. Toxicol. Sci. 2003, 73, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Henning, A.; Schneider, M.; Nafee, N.; Muijs, L.; Rytting, E.; Wang, X.; Kissel, T.; Grafahrend, D.; Klee, D.; Lehr, C.-M. Influence of particle size and material properties on mucociliary clearance from the airways. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 233–241. [Google Scholar] [CrossRef]
- Ershov, D.; Phan, M.-S.; Pylvänäinen, J.W.; Rigaud, S.U.; Le Blanc, L.; Charles-Orszag, A.; Conway, J.R.W.; Laine, R.F.; Roy, N.H.; Bonazzi, D.; et al. TrackMate 7: Integrating state-of-the-art segmentation algorithms into tracking pipelines. Nat. Methods 2022, 19, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Kürti, L.; Veszelka, S.; Bocsik, A.; Ozsvári, B.; Puskás, L.G.; Kittel, A.; Szabó-Révész, P.; Deli, M.A. Retinoic acid and hydrocortisone strengthen the barrier function of human RPMI 2650 cells, a model for nasal epithelial permeability. Cytotechnology 2013, 65, 395–406. [Google Scholar] [CrossRef]
- Arbach, O.; Taumberger, A.B.; Wietek, S.; Cervinek, L.; Salama, A. Efficacy and safety of a new intravenous immunoglobulin (Panzyga® ) in chronic immune thrombocytopenia. Transfus. Med. 2019, 29, 48–54. [Google Scholar] [CrossRef]
- Pasman, T.; Baptista, D.; van Riet, S.; Truckenmüller, R.K.; Hiemstra, P.S.; Rottier, R.J.; Hamelmann, N.M.; Paulusse, J.M.J.; Stamatialis, D.; Poot, A.A. Development of an In Vitro Airway Epithelial-Endothelial Cell Culture Model on a Flexible Porous Poly(Trimethylene Carbonate) Membrane Based on Calu-3 Airway Epithelial Cells and Lung Microvascular Endothelial Cells. Membranes 2021, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Sibinovska, N.; Žakelj, S.; Roškar, R.; Kristan, K. Suitability and functional characterization of two Calu-3 cell models for prediction of drug permeability across the airway epithelial barrier. Int. J. Pharm. 2020, 585, 119484. [Google Scholar] [CrossRef]
- Selo, M.A.; Sake, J.A.; Kim, K.-J.; Ehrhardt, C. In vitro and ex vivo models in inhalation biopharmaceutical research-advances, challenges and future perspectives. Adv. Drug Deliv. Rev. 2021, 177, 113862. [Google Scholar] [CrossRef]
- Inoue, D.; Furubayashi, T.; Tanaka, A.; Sakane, T.; Sugano, K. Quantitative estimation of drug permeation through nasal mucosa using in vitro membrane permeability across Calu-3 cell layers for predicting in vivo bioavailability after intranasal administration to rats. Eur. J. Pharm. Biopharm. 2020, 149, 145–153. [Google Scholar] [CrossRef]
- Furubayashi, T.; Inoue, D.; Nishiyama, N.; Tanaka, A.; Yutani, R.; Kimura, S.; Katsumi, H.; Yamamoto, A.; Sakane, T. Comparison of Various Cell Lines and Three-Dimensional Mucociliary Tissue Model Systems to Estimate Drug Permeability Using an In Vitro Transport Study to Predict Nasal Drug Absorption in Rats. Pharmaceutics 2020, 12, 79. [Google Scholar] [CrossRef]
- Hashida, R.; Anamizu, C.; Yagyu-Mizuno, Y.; Ohkuma, S.; Takano, T. Transcellular transport of fluorescein dextran through an arterial endothelial cell monolayer. Cell Struct. Funct. 1986, 11, 343–349. [Google Scholar] [CrossRef]
- Grainger, C.I.; Greenwell, L.L.; Lockley, D.J.; Martin, G.P.; Forbes, B. Culture of Calu-3 cells at the air interface provides a representative model of the airway epithelial barrier. Pharm. Res. 2006, 23, 1482–1490. [Google Scholar] [CrossRef]
- Lorenz, T.; Kirschke, M.; Ledwig, V.; Reichl, S.; Dietzel, A. Microfluidic System for In Vivo-Like Drug Permeation Studies with Dynamic Dilution Profiles. Bioengineering 2021, 8, 58. [Google Scholar] [CrossRef]
- Spiekermann, G.M.; Finn, P.W.; Ward, E.S.; Dumont, J.; Dickinson, B.L.; Blumberg, R.S.; Lencer, W.I. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: Functional expression of FcRn in the mammalian lung. J. Exp. Med. 2002, 196, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lee, J.H.; Lee, J.S.; Kim, D.C.; Yang, J.W.; An, H.J.; Na, J.M.; Shin, M.C.; Song, D.H. Fc Receptor Expression as a Prognostic Factor in Patients with Non-small-cell Lung Cancer. Vivo 2022, 36, 2708–2713. [Google Scholar] [CrossRef]
- Wengst, A.; Reichl, S. RPMI 2650 epithelial model and three-dimensional reconstructed human nasal mucosa as in vitro models for nasal permeation studies. Eur. J. Pharm. Biopharm. 2010, 74, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Ladel, S.; Flamm, J.; Zadeh, A.S.; Filzwieser, D.; Walter, J.-C.; Schlossbauer, P.; Kinscherf, R.; Lischka, K.; Luksch, H.; Schindowski, K. Allogenic Fc Domain-Facilitated Uptake of IgG in Nasal Lamina Propria: Friend or Foe for Intranasal CNS Delivery? Pharmaceutics 2018, 10, 107. [Google Scholar] [CrossRef]
- Hoffmeyer, F.; Witte, K.; Schmidt, R.E. The high-affinity Fc gamma RI on PMN: Regulation of expression and signal transduction. Immunology 1997, 92, 544–552. [Google Scholar] [CrossRef]
- Boruchov, A.M.; Heller, G.; Veri, M.-C.; Bonvini, E.; Ravetch, J.V.; Young, J.W. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J. Clin. Investig. 2005, 115, 2914–2923. [Google Scholar] [CrossRef]
- Hayes, J.M.; Wormald, M.R.; Rudd, P.M.; Davey, G.P. Fc gamma receptors: Glycobiology and therapeutic prospects. J. Inflamm. Res. 2016, 9, 209–219. [Google Scholar] [CrossRef]
- Rothen-Rutishauser, B.; Blank, F.; Mühlfeld, C.; Gehr, P. In vitro models of the human epithelial airway barrier to study the toxic potential of particulate matter. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Herzog, H.; Glöckler, S.; Flamm, J.; Ladel, S.; Maigler, F.; Pitzer, C.; Schindowski, K. Intranasal Nose-to-Brain Drug Delivery via the Olfactory Region in Mice: Two In-Depth Protocols for Region-Specific Intranasal Application of Antibodies and for Expression Analysis of Fc Receptors via In Situ Hybridization in the Nasal Mucosa. Methods Mol. Biol. 2024, 2754, 387–410. [Google Scholar] [CrossRef] [PubMed]
- Pyzik, M.; Rath, T.; Lencer, W.I.; Baker, K.; Blumberg, R.S. FcRn: The Architect Behind the Immune and Nonimmune Functions of IgG and Albumin. J. Immunol. 2015, 194, 4595–4603. [Google Scholar] [CrossRef] [PubMed]
- Rayner, R.E.; Makena, P.; Prasad, G.L.; Cormet-Boyaka, E. Optimization of Normal Human Bronchial Epithelial (NHBE) Cell 3D Cultures for in vitro Lung Model Studies. Sci. Rep. 2019, 9, 500. [Google Scholar] [CrossRef]
- Bur, M.; Huwer, H.; Lehr, C.-M.; Hagen, N.; Guldbrandt, M.; Kim, K.-J.; Ehrhardt, C. Assessment of transport rates of proteins and peptides across primary human alveolar epithelial cell monolayers. Eur. J. Pharm. Sci. 2006, 28, 196–203. [Google Scholar] [CrossRef]


| Target | Gene | Sequence (5′-3′) | Product Length (bp) | |
|---|---|---|---|---|
| Genes involved in ciliogenesis | DEUP1 | F | GCAGCGCAGTGCAGGTTAAA | 75 |
| R | TGGGCTTGGTTCTCCATGTC | |||
| MCIDAS | F | CTGGACAGGAAGTTCGCTCC | 170 | |
| R | CCGAGTAACGAGGAGCAGTC | |||
| TP73 | F | TCAGGCGGCACCAGAGTG | 134 | |
| R | AAAATAGGTGCTGTCCGGCT | |||
| Ciliated cells | DNALI1 | F | CCAAGCTCCCCTCAACTTCC | 96 |
| R | TGTCCTCCACCCATTCCCTTG | |||
| DNAH5 | F | TGCTCTGACTGGGGTTTGTG | 91 | |
| R | GTTGAAGCTCACCTCCCGAT | |||
| RSPH4A | F | CCAGGGAAATTTAGAAGGAGCTG | 120 | |
| R | TCCGTGCCCAAACCAACTCCAG | |||
| CCDC40 | F | TGGAGAAAAAGCGCATCCTGCA | 125 | |
| R | GTCCATGGACTTGGCTTGATGC | |||
| R | GCATAGTCCGAAAGGGGAGG | |||
| Characteristic | cTMPC | Human In Vitro Airway Model |
|---|---|---|
| cilia length [µm] | 5–6 | 4–6; [149] * |
| epithelial thickness [µm] | 11.5 ± 3.5 | 35–40; [149] * |
| TEER [Ω×cm2] | 822.19 ± 206.91 | 700–1200; [59] * |
| tight-junctions | yes | yes; [149] * |
| mucus production | yes | yes; [149] * |
| beating cilia | yes | Yes; [149] * |
| particle speed MCC [µm/s] | 0.41–1.34 | ~ 40–180; [30] * |
| CBF [beats/s] | not measured | ~ 6–18; [30,149] * |
| Papp [cm/s]–4 kDa | 7.14 × 10−8 ± 4.29 × 10−8 | 2.20 × 10−7 ± 0.30 × 10−7; [150] **/# |
| Papp [cm/s]–150 kDa | 1.09 × 10−10 ± 0.342 × 10−10 | 0.36 × 10−7 ± 0.22 × 10−7; [150] ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, J.; Neubauer, V.; Rittersberger, R.; Treitler, S.; Kopp, P.; Günday, C.; Shrimo, I.; Dabbars, A.; Rosenau, F.; Türeli, A.E.; et al. Development and Characterization of a Primary Ciliated Porcine Airway Model for the Evaluation of In Vitro Mucociliary Clearance and Mucosal Drug Delivery. Pharmaceutics 2025, 17, 462. https://doi.org/10.3390/pharmaceutics17040462
Martin J, Neubauer V, Rittersberger R, Treitler S, Kopp P, Günday C, Shrimo I, Dabbars A, Rosenau F, Türeli AE, et al. Development and Characterization of a Primary Ciliated Porcine Airway Model for the Evaluation of In Vitro Mucociliary Clearance and Mucosal Drug Delivery. Pharmaceutics. 2025; 17(4):462. https://doi.org/10.3390/pharmaceutics17040462
Chicago/Turabian StyleMartin, Janik, Veronika Neubauer, Rebecca Rittersberger, Simon Treitler, Patrick Kopp, Cemre Günday, Iman Shrimo, Annabelle Dabbars, Frank Rosenau, Akif Emre Türeli, and et al. 2025. "Development and Characterization of a Primary Ciliated Porcine Airway Model for the Evaluation of In Vitro Mucociliary Clearance and Mucosal Drug Delivery" Pharmaceutics 17, no. 4: 462. https://doi.org/10.3390/pharmaceutics17040462
APA StyleMartin, J., Neubauer, V., Rittersberger, R., Treitler, S., Kopp, P., Günday, C., Shrimo, I., Dabbars, A., Rosenau, F., Türeli, A. E., Günday-Türeli, N., Haedicke-Peters, O., & Schindowski, K. (2025). Development and Characterization of a Primary Ciliated Porcine Airway Model for the Evaluation of In Vitro Mucociliary Clearance and Mucosal Drug Delivery. Pharmaceutics, 17(4), 462. https://doi.org/10.3390/pharmaceutics17040462








