1. Introduction
Loxoprofen sodium (LXP) is a prodrug type of pharmaceutical. It is a non-steroidal anti-inflammatory drug (NSAID) of the 2-phenylpropionate class and is sold in at least 28 countries [
1]. In Japan, LXP is the most frequently prescribed NSAID, being prescribed to 32.5% of patients [
2]. It is widely used for the treatment of osteoarthritis, adhesive capsulitis, rheumatoid arthritis, arthritis, dental pain, and postoperative pain [
3]. In addition to oral formulations such as tablets and granules, various dosage forms including gels, patches, and ointments are also available. In Japan, LXP was switched to being sold as an over-the-counter drug in 2011 and to being one of the first-choice analgesics.
Furthermore, research into LXP formulations is ongoing [
4]. Antipyretic and analgesic drugs other than LXP, such as ibuprofen (IB) and acetaminophen (APAP), have been used for a long time in Japan. However, LXP differs significantly from IB and APAP. For example, both IB and APAP exist in the free form and show low solubility in water [
5]. In contrast, LXP is a salt, has high solubility in water, and exhibits viscosity upon dissolution, resulting in poor disintegration characteristics. Furthermore, IB and APAP are needle-shaped crystals, which are associated with poor compressibility and various tableting issues such as capping [
6,
7]. Additionally, IB has a low melting point, which has been reported to cause adhesion problems [
8]. On the other hand, there are few reports on the compressibility of LXP.
LXP is a dihydrate, and its hydration level significantly influences the manufacturing process. For instance, it can easily dehydrate under low-humidity conditions or when heated [
9]. The dehydration of hydrates alters their physical and chemical properties, which can have a considerable impact on their manufacture [
10,
11]. Therefore, it is essential to establish appropriate temperature and humidity conditions during the manufacturing process [
12]. Furthermore, hydrates generally tend to form hydrogen bonds with other molecules, which is known to impact the tablet hardness [
13]. To ensure consistent product quality and efficacy in pharmaceuticals, optimizing the formulation process is essential [
14]. Understanding how manufacturing conditions affect the physical properties of tablets is crucial for this optimization. Various methods for tablet manufacturing are employed, with the wet granulation method being one of the most commonly used. Wet granulation involves adding a solvent to a powder and includes a drying process. Granules obtained by wet granulation are characteristically close to being spherical and exhibit a wide particle size distribution [
15]. Thus, wet granulation is chosen for cases where ensuring content uniformity proves challenging due to segregation, when flowability is poor, or when the compression property is low. On the other hand, use of a solvent and drying of the wet granules are essential in the wet granulation process. Therefore, establishing drying conditions based on the stability of the drug and the solvent used, as well as the melting point of the drug, is critical [
15,
16,
17]. Therefore, there are concerns that the physical properties of LXP may vary depending on the manufacturing conditions. Thus, elucidating the mechanisms of LXP’s compressibility is crucial for advancing its formulation. In this study, we investigated the impact of the solvent types and drying temperature on the properties of tablets produced from wet granulation of LXP. Our findings indicate that the tablet hardness varies with different solvents and temperature conditions. Furthermore, we analyzed the formulations produced, identified the physicochemical characteristics related to changes in the tablet properties due to variations in the solvents and drying temperatures, and elucidated the mechanisms involved in tablet hardness. This research is expected to facilitate the optimization of manufacturing processes and ensure production of high-quality pharmaceuticals for patients.
2. Materials and Methods
2.1. Materials
We used “Loxoprofen Sodium Dihydrate powder grade” manufactured by Daiwa Pharmaceutical Co., Ltd. (Toyama-shi, Toyama, Japan). as LXP. We used “Purified Water Yoshida” conforming to the Japanese Pharmacopoeia sourced from Yoshida Pharmaceutical Co., Ltd. (Chuo-ku, Tokyo, Japan). and “Ethanol 95” conforming to the Japanese Pharmacopoeia procured from Nippon Alcohol Sales Co., Ltd. (Chuo-ku, Tokyo, Japan). We also used “Magnesium stearate Light grade” conforming to the Japanese Pharmacopoeia from Taihei Chemical Industry Co., Ltd. (Osaka-shi, Osaka, Japan).
2.2. Preparation of Granules
LXP (100%,
w/
w) was thoroughly mixed in a mortar until there were no agglomerates. Various solvents (10%,
v/
v) were added to the mixed LXP and the mixture was kneaded for 5 min. The resulting wet granules were dried under various temperature conditions for 24 h to remove the solvent and obtain dry granules. The obtained dry granules were then sieved, and granules classified between 106 μm and 180 μm were used for examination (
Table 1).
2.3. Particle Size Distributions
Approximately 5 g of each granule was analyzed using a particle size distribution measurement device (Robot Shifter RPS-01, Seishin Enterprise Co., Ltd., Chiyoda-ku, Tokyo, Japan). Sieve mesh sizes of 710, 500, 355, 250, 180, 150, 106, and 75 μm were employed, and the samples were vibrated for 3 min with Level 4 vibration and a pulse interval of 1 s (
n = 1). The relative width of the particle size distribution (
Rw) was calculated using the following equation:
where
D10,
D50, and
D90 are 10, 50, and 90%, respectively, of the accumulated particle size on a screen.
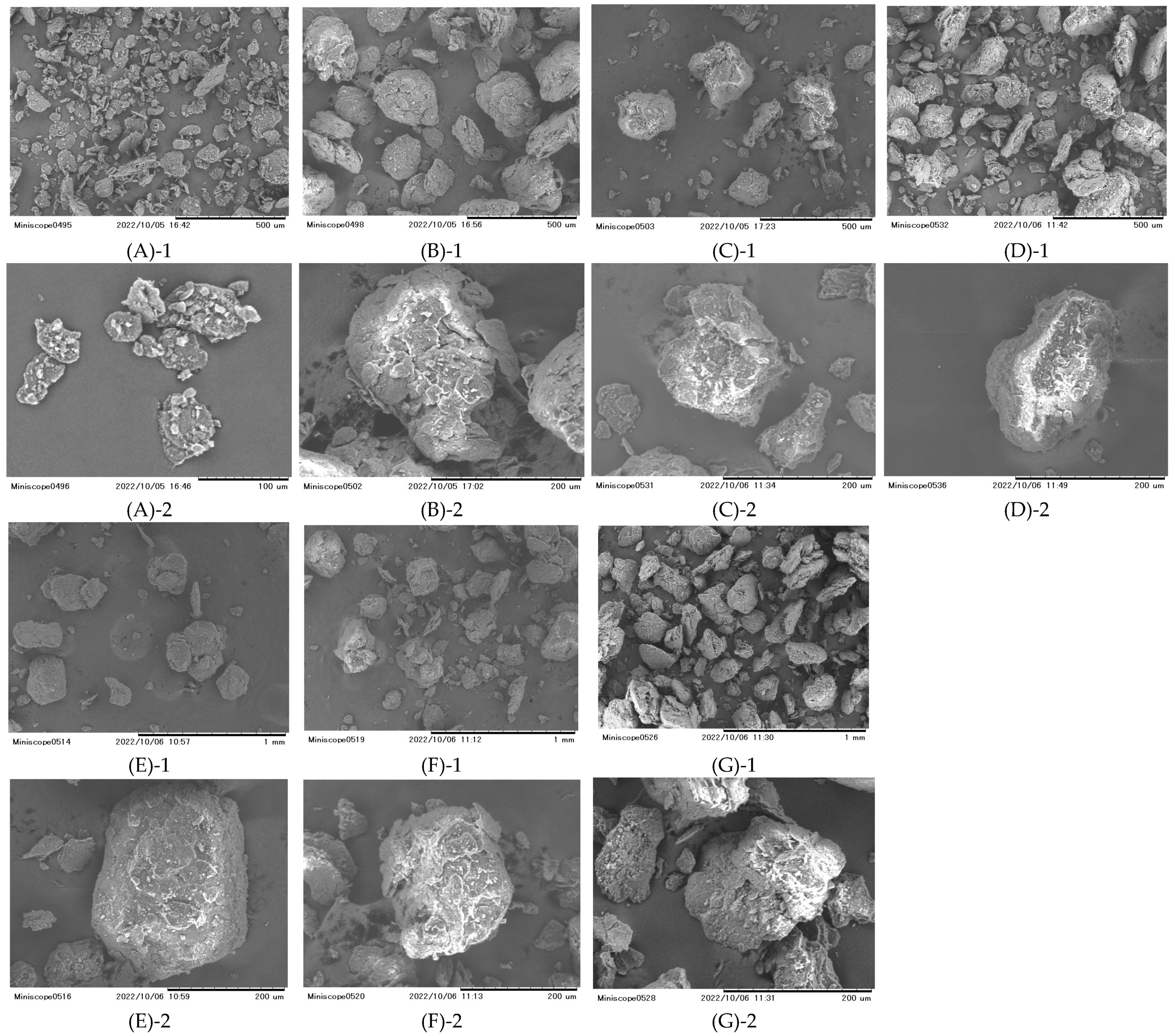
2.4. Scanning Electron Microscopy (SEM)
Particle surfaces were examined using a scanning electron microscope (SEM) (TM-1000 Tabletop Scanning Electron Microscope, Hitachi High Technologies, Minato-ku, Tokyo, Japan) which could be operated without the need for metal deposition. Samples were placed on a plate without being coated with Au before imaging. An accelerating voltage was set to 5 kV.
2.5. Flow Properties
The flow properties of the granulated particles were measured using an FT4 powder rheometer (Freeman Technology, Tewkesbury, UK) [
18,
19,
20]. To achieve highly reproducible results, FT4 conditioned the powder before measurement. The conditioning was performed using a blade that moved at a specified helix angle and tip speed, resulting in a uniform and highly reproducible state. Approximately 70 g of each batch of granules were filled into an 85 mL measuring container (
n = 1). A 48 mm blade was used to determine the total energy required for the downward test on the conditioned powder, as well as the conditioned bulk density (CBD) and basic flow energy (BFE).
2.6. Shear Force
The shear stress was measured using a shear force kit of the FT4 powder rheometer (Freeman Technology, Tewkesbury, UK). To achieve highly reproducible results, FT4 conditioned the powder before measurement. The conditioning was performed using a blade that moved at a specified helix angle and tip speed, resulting in a uniform and highly reproducible state. The constant load shear testing method involved shearing the powder layer while applying a constant vertical stress on the upper surface of the powder layer, allowing for the relationship between vertical stress and shear stress to be obtained (n = 1). This method was used to calculate the angle of internal friction (AIF), flow function (FF), and cohesion.
2.7. Surface Free Energy
The surface tension of each sample (20 ± 0.5 °C) was measured using the capillary method with a tensiometer (Tensiio, KRUSS, KRÜSS GmbH, Hamburg, Germany). A fixed amount of the sample packed in a glass tube was immersed in solutions with known surface free energy (SFE) values—hexane, tetrachloromethane, 1-chlorobutane, and 1-nitropropane—at room temperature (20 ± 0.5 °C). The point of liquid contact with the powder was designated as zero for measuring the contact angle. Each measurement was repeated five times and the average value obtained (
n = 5). The contact angles were analyzed using the Owens–Wendt–Rabel–Kaelble equation to calculate the polar component of the solid’s SFE, γ(p), and γ(d) [
21,
22].
In the above equation, θ signifies the contact angle, while and represent the SFE of the liquid and solid, respectively. The variables p and d indicate the polar and dispersive components of the SFE, respectively.
2.8. Moisture Content
The moisture content of each sample was measured using a heated drying moisture analyzer (MX-50, A&D). Approximately 5 g of each sample was placed in a stainless steel weighing pan and heated at 105 °C for 30 min to determine the moisture content (Loss on Drying, LOD). Each measurement was repeated three times and the average values obtained (n = 1).
2.9. Thermogravimetric Analysis
Differential scanning calorimetry (DSC) was performed using a differential scanning calorimeter (Thermo plus EVO2 DSCvesta, Rigaku, Shibuya-ku, Tokyo, Japan). Approximately 5 mg of each sample was accurately weighed in an aluminum pan. The sample pan was heated from 30 °C to 250 °C at a rate of 5 °C per minute, using an empty aluminum pan as reference. Closed pans were utilized for the DSC measurements. We used each LXP sample excluding Mg-st for the measurements. Thermogravimetric analysis was carried out using a Thermogravimetric Analyzer (Thermo plus EVO2 TGDTA8122, Rigaku, Shibuya-ku, Tokyo, Japan). The sample pan was heated from 30 °C to 250 °C at a rate of 5 °C per minute, using an empty aluminum pan as reference.
2.10. Powder X-Ray Diffraction Measurement
Each granulated sample was analyzed using a powder X-ray diffractometer (SmartLab, Rigaku, Shibuya-ku, Tokyo, Japan). Measurements were conducted with Cu Kα radiation at 40 kV and 30 mA, over a scanning range of 0° to 50°, with a scan rate of 0.5° per minute.
2.11. Preparation of Tablets
Tablets were obtained through compression molding using a single-punch tablet press (HANDTAB, Ichihashiseiki Co., Ltd., Kyoto-shi, Kyoto, Japan). The granules were mixed to achieve a composition of 99.0% (w/w) LXP and 1.0% (w/w) Mg-st, resulting in a homogeneous mixture.
The reason for adding 1% Mg-st is that when tablets were pressed using only LXP, adhesion occurred, making it impossible to form tablets. Therefore, we decided to add a minimum of 1% Mg-st to enable tablet formation. The mixture of granulated particles and Mg-st were passed through a sieve with a mesh opening of 710 μm, then placed in a plastic bag and mixed for 3 min. This mixture was then compressed into tablets with a target mass of approximately 250 mg using an 8 mm flat-faced punch at compression pressures of 5 kN and 10 kN.
2.12. Evaluation of Tablets
The tablet hardness was measured using a tablet hardness tester (Schleuniger 8M tester, Pharmatron Dr. Schleuniger, Thun, Switzerland) by applying vertical compression at a rate of 0.2 mm/sec to determine the breaking strength, which is the force required to fracture the tablet (
n = 3). The tensile strength (TS) was then calculated using the following formulation based on the obtained results. The tensile strength is considered as an indicator of the tablet hardness. Measurements were performed in triplicate for each sample, and the average value was determined. Results are expressed as the mean ± standard deviation of three independent experiments.
F: Tablet hardness [N]; D: Tablet diameter [mm]; t: Tablet thickness [mm].
2.13. Statistical Analysis
Two-way ANOVA was performed on the data of TS. The data analysis was con-ducted using JMP (version 18, SAS Institute Inc., Cary, NC, USA). Differences in solution (X1) and dry temperature (X2) were used as explanatory variables for this analysis, and p-values of <0.05, 0.01, or 0.001 were considered statistically significant. Furthermore, Tukey’s test was conducted to compare the differences in TS between each sample, and p-values of <0.05, 0.01, or 0.001 were considered statistically significant.
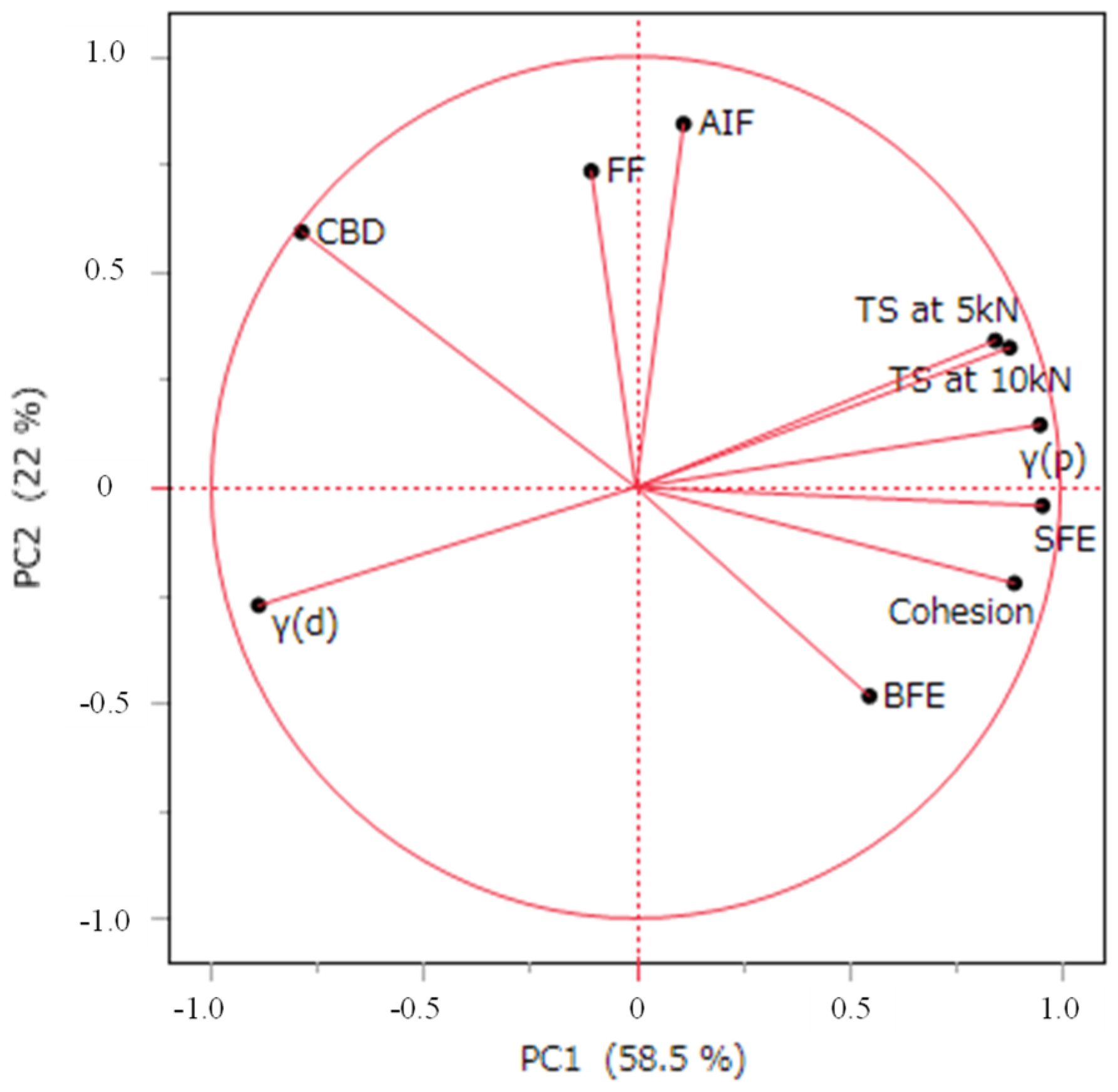
Principal component analysis (PCA) was conducted using JMP (version 18, SAS Institute Inc., Cary, NC, USA) on the data for BFE, CBD, Cohesion, FF, AIF, γ(p), γ(d), SFE, TS at 5 kN and TS at 10 kN. The data used for the PCA were standardized [
23]. Additionally, correlation analysis of the average values of the data was performed using the JMP software.
5. Conclusions
LXP granules were prepared by a wet granulation process under various manufacturing conditions to evaluate the effects on the tablet properties. The hardness of the tablets increased with the granulation, with granules produced using ethanol as a solvent exhibiting greater hardness than those produced with water. Additionally, the tablet hardness varied with the drying temperature. Thus, the granulation conditions were confirmed to exert an impact on the hardness of tablets containing LXP. PCA showed a positive correlation between tablet hardness and SFE, γ(p), and adhesion strength.
In this study, we identified parameters affecting the tablet hardness of LXP-containing tablets. A detailed investigation of the mechanisms revealed that the increase in γ(p) value associated with the use of ethanol as the granulation solvent can be attributed to differences in the particle surface state caused by variations in the solubility of LXP in water and ethanol. We also confirmed that the γ(p) value was influenced by the hydration state of the granules, which was affected by the drying temperature. According to these results, we suggest that controlling the hydration state allows control of the tab-let hardness, serving as valuable information for optimizing the formulation design. For example, in the formulation of active ingredients prone to hardness reduction, evaluating the surface free energy composition facilitates the selection of suitable excipients to enhance the hardness. Future research must focus on identifying effective excipients based on surface free energy considerations. Moreover, our findings indicate that the surface free energy of LXP varies with manufacturing conditions, including choice of the solvent and drying temperature, underscoring the relevance of surface free energy in selecting optimal manufacturing conditions. Thus, this research pro-vides insights for provides insights for both optimization of the formulation design and the manufacturing parameters for LXP tablets.