Microfluidic Optimization of PEI-Lipid Hybrid Nanoparticles for Efficient DNA Delivery and Transgene Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmid DNA Purification
2.2. Formulation Preparation
- LNP formulations without PEI: For LNP-Luc (10) and LNP-Luc (40), we mixed plasmid DNA with the aqueous phase and dissolved the lipid mixture in ethanol to form the organic phase. The phases were combined via one-step microfluidic mixing (NanoAssemblr), producing LNPs encapsulating DNA.
- LNP formulations with PEI in the organic phase (one-step method): For LNP-Luc/PEI (10, Org) and LNP-Luc/PEI (40, Org), we added PEI to the organic phase with lipids and mixed it with the DNA-containing aqueous phase using one-step microfluidic mixing.
- LNP formulations with pre-complexed PEI-DNA in the aqueous phase (two-step method): For LNP-Luc/PEI 1:1, 1:2, and 1:3 (10) and (40), we pre-complexed PEI with DNA in the aqueous phase at ratios of 1:1, 1:2, or 1:3 by dropwise addition and 30-min incubation. The complexed solution was mixed with the organic lipid phase via two-step microfluidic mixing.
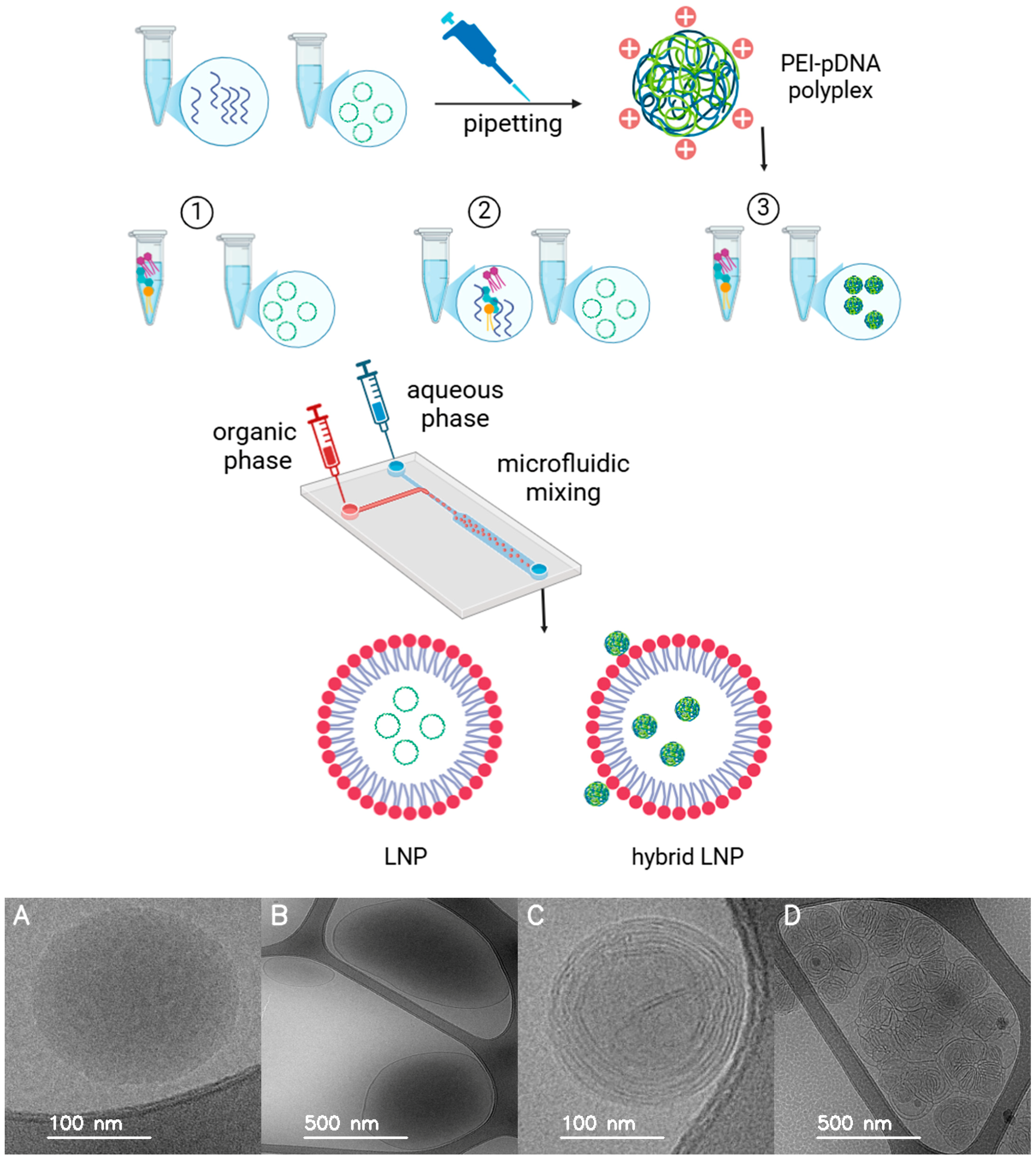
2.3. Characterization of Formulations
2.4. Cellular Uptake Study
2.5. Luciferase Expression Assay
2.6. Cell Viability Assay
2.7. GFP Expression Assay
2.8. Cryo-TEM Sample Preparation and Imaging
2.9. Statistical Analysis
3. Results and Discussion
3.1. Design of Lipid Nanoparticles for Luciferase DNA Delivery
3.2. Physico-Chemical Characterization of Luciferase DNA-Loaded LNPs
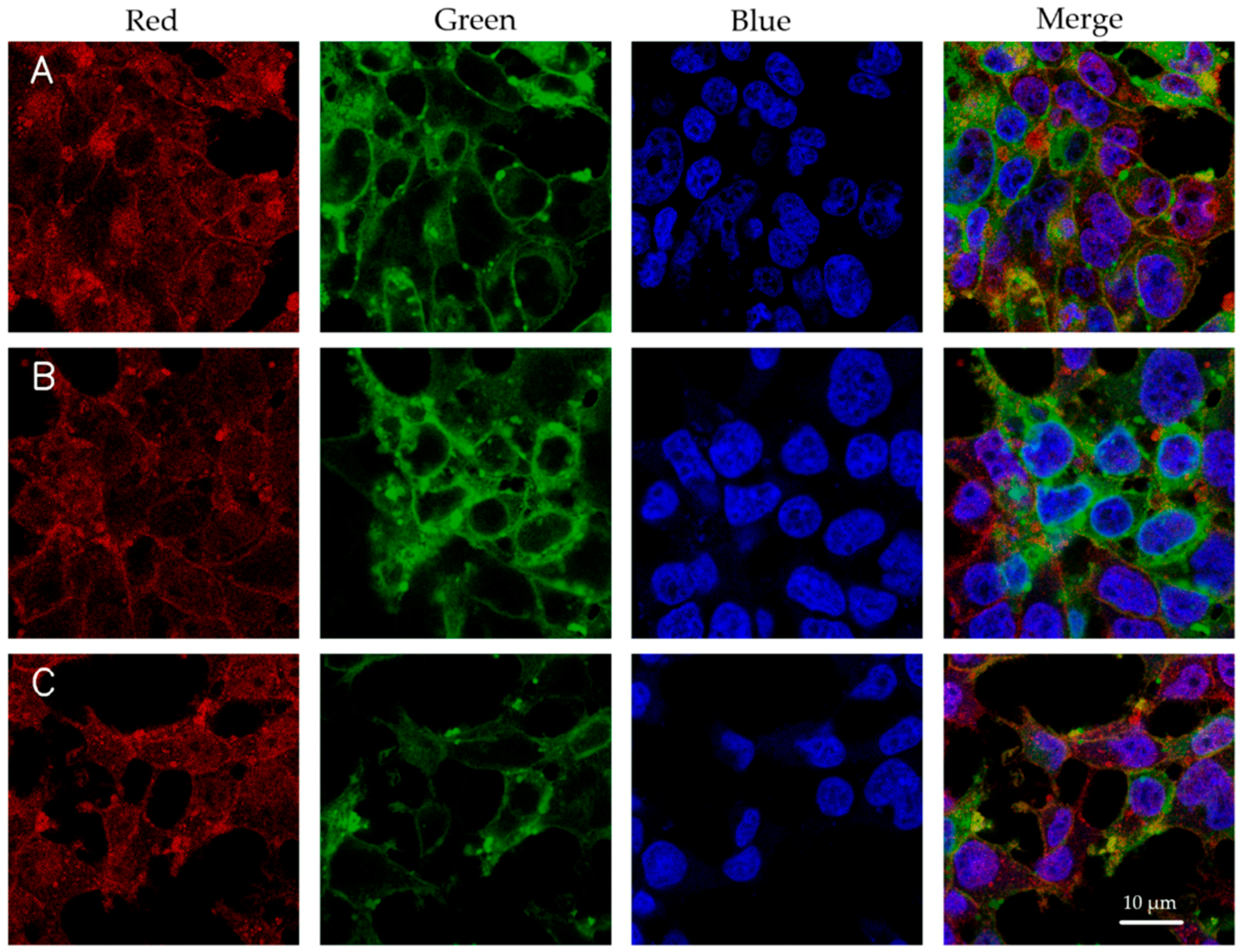
3.3. Cellular Uptake of Luciferase DNA-LNPs
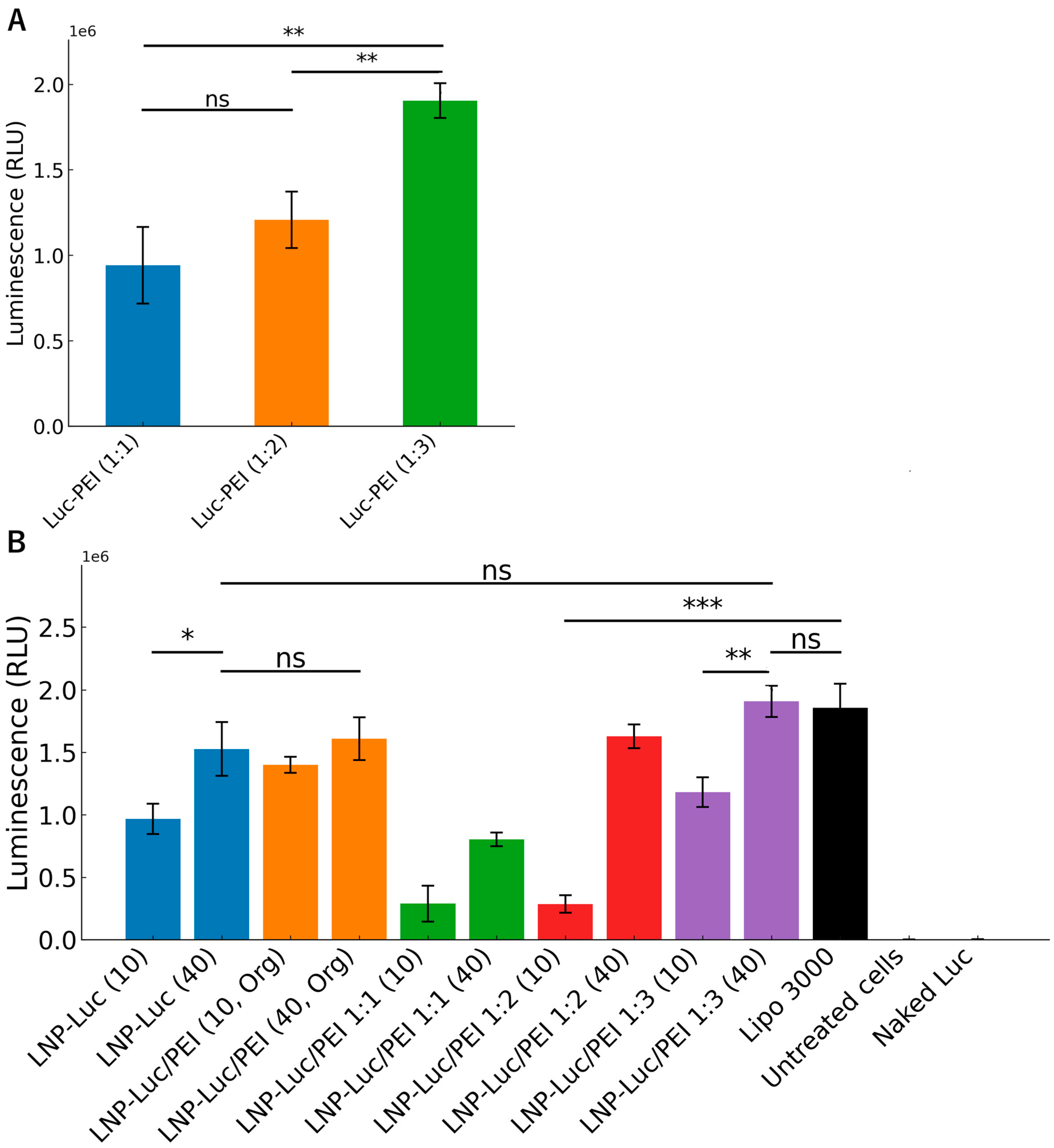
3.4. Cell Viability and Luciferase Expression In Vitro
3.5. Development of GFP-Based Formulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMG-PEG2000 | 1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 |
| DMSO | Dimethyl sulfoxide |
| DNA | Deoxyribonucleic acid |
| DOPE | Dioleoylphosphatidylethanolamine |
| DOTAP | 1,2-dioleoyl-3-trimethylammonium-propane |
| DSPC | 1,2-distearoyl-sn-glycero-3-phosphocholine |
| EE | Encapsulation efficiency |
| GFP | Green fluorescent protein |
| HD | Hydrodynamic diameter |
| LB | Lysogeny broth |
| LNPs | Lipid nanoparticles |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| PAMAM | Polyamidoamine |
| PBS | Phosphate-buffered saline |
| PDI | Polydispersity index |
| PEI | Polyethyleneimine |
| PFA | Paraformaldehyde |
| RLU | Relative light units |
| SEM | Standard error of the mean |
| siRNA | Small interfering RNA |
| TE | Tris-EDTA |
References
- Jensen, T.L.; Gøtzsche, C.R.; Woldbye, D.P.D. Current and Future Prospects for Gene Therapy for Rare Genetic Diseases Affecting the Brain and Spinal Cord. Front. Mol. Neurosci. 2021, 14, 695937. [Google Scholar] [CrossRef]
- Volodina, O.; Smirnikhina, S. The Future of Gene Therapy: A Review of In Vivo and Ex Vivo Delivery Methods for Genome Editing-Based Therapies. Mol. Biotechnol. 2024, 67, 425–437. [Google Scholar] [CrossRef]
- El-Zahaby, S.A.; Kaur, L.; Sharma, A.; Prasad, A.G.; Wani, A.K.; Singh, R.; Zakaria, M.Y. Lipoplexes’ Structure, Preparation, and Role in Managing Different Diseases. AAPS PharmSciTech 2024, 25, 131. [Google Scholar] [CrossRef]
- Dogbey, D.M.; Torres, V.E.S.; Fajemisin, E.; Mpondo, L.; Ngwenya, T.; Akinrinmade, O.A.; Perriman, A.W.; Barth, S. Technological advances in the use of viral and non-viral vectors for delivering genetic and non-genetic cargos for cancer therapy. Drug Deliv. Transl. Res. 2023, 13, 2719–2738. [Google Scholar] [CrossRef]
- Zu, H.; Gao, D. Non-viral Vectors in Gene Therapy: Recent Development, Challenges, and Prospects. AAPS J. 2021, 23, 78. [Google Scholar] [CrossRef]
- Selianitis, D.; Kafetzi, M.; Pippa, N.; Pispas, S.; Gazouli, M. Lipoplexes and Polyplexes for Targeted Gene Delivery. In Pharmaceutical Nanobiotechnology for Targeted Therapy; Barabadi, H., Mostafavi, E., Saravanan, M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 65–92. [Google Scholar] [CrossRef]
- Schlich, M.; Palomba, R.; Costabile, G.; Mizrahy, S.; Pannuzzo, M.; Peer, D.; Decuzzi, P. Cytosolic delivery of nucleic acids: The case of ionizable lipid nanoparticles. Bioeng. Transl. Med. 2021, 6, e10213. [Google Scholar] [CrossRef]
- Wang, B.; Shen, B.; Xiang, W.; Shen, H. Advances in the study of LNPs for mRNA delivery and clinical applications. Virus Genes 2024, 60, 577–591. [Google Scholar] [CrossRef]
- Cui, L.; Renzi, S.; Quagliarini, E.; Digiacomo, L.; Amenitsch, H.; Masuelli, L.; Bei, R.; Ferri, G.; Cardarelli, F.; Wang, J.; et al. Efficient Delivery of DNA Using Lipid Nanoparticles. Pharmaceutics 2022, 14, 1698. [Google Scholar] [CrossRef]
- Kong, W.; Wei, Y.; Dong, Z.; Liu, W.; Zhao, J.; Huang, Y.; Yang, J.; Wu, W.; He, H.; Qi, J. Role of size, surface charge, and PEGylated lipids of lipid nanoparticles (LNPs) on intramuscular delivery of mRNA. J. Nanobiotechnol. 2024, 22, 553. [Google Scholar] [CrossRef]
- Dinh, L.; Mahon, L.; Yan, B. Nano-Encapsulation and Conjugation Applied in the Development of Lipid Nanoparticles Delivering Nucleic Acid Materials to Enable Gene Therapies. Appl. Nano 2024, 5, 143–161. [Google Scholar] [CrossRef]
- Skandrani, N.; Barras, A.; Legrand, D.; Gharbi, T.; Boulahdour, H.; Boukherroub, R. Lipid nanocapsules functionalized with polyethyleneimine for plasmid DNA and drug co-delivery and cell imaging. Nanoscale 2014, 6, 7379–7390. [Google Scholar] [CrossRef] [PubMed]
- Gallops, C.; Ziebarth, J.; Wang, Y. A Polymer Physics Perspective on Why PEI Is an Effective Nonviral Gene Delivery Vector. In Polymers in Therapeutic Delivery; American Chemical Society: Washington, DC, USA, 2020; Volume 1350, pp. 1–12. [Google Scholar]
- Shen, Z.-l.; Xia, Y.-q.; Yang, Q.-s.; Tian, W.-d.; Chen, K.; Ma, Y.-q. Polymer–Nucleic Acid Interactions. Top. Curr. Chem. 2017, 375, 44. [Google Scholar] [CrossRef] [PubMed]
- Porello, I.; Bono, N.; Candiani, G.; Cellesi, F. Advancing nucleic acid delivery through cationic polymer design: Non-cationic building blocks from the toolbox. Polym. Chem. 2024, 15, 2800–2826. [Google Scholar] [CrossRef]
- Benjaminsen, R.V.; Mattebjerg, M.A.; Henriksen, J.R.; Moghimi, S.M.; Andresen, T.L. The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21, 149–157. [Google Scholar] [CrossRef]
- Bus, T.; Traeger, A.; Schubert, U.S. The great escape: How cationic polyplexes overcome the endosomal barrier. J. Mater. Chem. B 2018, 6, 6904–6918. [Google Scholar] [CrossRef]
- Xue, L.; Yan, Y.; Kos, P.; Chen, X.; Siegwart, D.J. PEI fluorination reduces toxicity and promotes liver-targeted siRNA delivery. Drug Deliv. Transl. Res. 2021, 11, 255–260. [Google Scholar] [CrossRef]
- Pinnapireddy, S.R.; Duse, L.; Strehlow, B.; Schäfer, J.; Bakowsky, U. Composite liposome-PEI/nucleic acid lipopolyplexes for safe and efficient gene delivery and gene knockdown. Colloids Surf. B Biointerfaces 2017, 158, 93–101. [Google Scholar] [CrossRef]
- Yang, S.; Wang, D.; Sun, Y.; Zheng, B. Delivery of antisense oligonucleotide using polyethylenimine-based lipid nanoparticle modified with cell penetrating peptide. Drug Deliv. 2019, 26, 965–974. [Google Scholar] [CrossRef]
- Ewe, A.; Aigner, A. Cationic Lipid-Coated Polyplexes (Lipopolyplexes) for DNA and Small RNA Delivery. Methods Mol. Biol. 2016, 1445, 187–200. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Lee, R.J.; Qi, Y.; Wang, K.; Hao, F.; Zhang, Y.; Lu, J.; Meng, Q.; Li, S.; et al. Synthesis of Polymer-Lipid Nanoparticles by Microfluidic Focusing for siRNA Delivery. Molecules 2016, 21, 1314. [Google Scholar] [CrossRef]
- Rajendran, A.P.; Ogundana, O.; Morales, L.C.; Meenakshi Sundaram, D.N.; Kucharski, C.; Kc, R.; Uludağ, H. Transfection Efficacy and Cellular Uptake of Lipid-Modified Polyethyleneimine Derivatives for Anionic Nanoparticles as Gene Delivery Vectors. ACS Appl. Bio Mater. 2023, 6, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Xun, M.-M.; Huang, Z.; Xiao, Y.-P.; Liu, Y.-H.; Zhang, J.; Zhang, J.-H.; Yu, X.-Q. Synthesis and Properties of Low-Molecular-Weight PEI-Based Lipopolymers for Delivery of DNA. Polymers 2018, 10, 1060. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Essex, S.; Sawant, R.R.; Biswas, S.; Nagesha, D.; Sridhar, S.; de Ilarduya, C.T.; Torchilin, V.P. Phospholipid-modified polyethylenimine-based nanopreparations for siRNA–mediated gene silencing: Implications for transfection and the role of lipid components. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Jerzykiewicz, J.; Czogalla, A. Polyethyleneimine-Based Lipopolyplexes as Carriers in Anticancer Gene Therapies. Materials 2021, 15, 179. [Google Scholar] [CrossRef]
- Chang, P.K.C.; Prestidge, C.A.; Bremmell, K.E. PAMAM versus PEI complexation for siRNA delivery: Interaction with model lipid membranes and cellular uptake. Pharm. Res. 2022, 39, 1151–1163. [Google Scholar] [CrossRef]
- Santhanes, D.; Wilkins, A.; Zhang, H.; John Aitken, R.; Liang, M. Microfluidic formulation of lipid/polymer hybrid nanoparticles for plasmid DNA (pDNA) delivery. Int. J. Pharm. 2022, 627, 122223. [Google Scholar] [CrossRef]
- González-García, D.; Tapia, O.; Évora, C.; García-García, P.; Delgado, A. Conventional and microfluidic methods: Design and optimization of lipid-polymeric hybrid nanoparticles for gene therapy. Drug Deliv. Transl. Res. 2024, 15, 908–924. [Google Scholar] [CrossRef]
- Kambar, N.; Leal, C. Microfluidic synthesis of multilayered lipid–polymer hybrid nanoparticles for the formulation of low solubility drugs. Soft Matter 2023, 19, 1596–1605. [Google Scholar] [CrossRef]
- Vogelaar, A.; Marcotte, S.; Cheng, J.; Oluoch, B.; Zaro, J. Use of Microfluidics to Prepare Lipid-Based Nanocarriers. Pharmaceutics 2023, 15, 1053. [Google Scholar] [CrossRef]
- Maeki, M.; Uno, S.; Sugiura, K.; Sato, Y.; Fujioka, Y.; Ishida, A.; Ohba, Y.; Harashima, H.; Tokeshi, M. Development of Polymer–Lipid Hybrid Nanoparticles for Large-Sized Plasmid DNA Transfection. ACS Appl. Mater. Interfaces 2024, 16, 2110–2119. [Google Scholar] [CrossRef]
- Kuan, C.-Y.; Yang, I.-H.; Chang, C.-T.; Chen, Z.-Y.; Lin, J.-N.; Kuo, W.-T.; Lin, Y.-Y.; Yueh, A.; Lin, F.-H. Enhanced non-viral gene delivery via calcium phosphate/DNA co-precipitates with low-voltage pulse electroporation in NK-92 cells for immunocellular therapy. APL Bioeng. 2024, 8, 036107. [Google Scholar] [CrossRef]
- Strelkova Petersen, D.M.; Chaudhary, N.; Arral, M.L.; Weiss, R.M.; Whitehead, K.A. The mixing method used to formulate lipid nanoparticles affects mRNA delivery efficacy and organ tropism. Eur. J. Pharm. Biopharm. 2023, 192, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Bezelya, A.; Küçüktürkmen, B.; Bozkır, A. Microfluidic Devices for Precision Nanoparticle Production. Micro 2023, 3, 822–866. [Google Scholar] [CrossRef]
- Tan, E.; Chin, C.S.H.; Lim, Z.F.S.; Ng, S.K. HEK293 Cell Line as a Platform to Produce Recombinant Proteins and Viral Vectors. Front. Bioeng. Biotechnol. 2021, 9, 796991. [Google Scholar] [CrossRef] [PubMed]
- Sciolino, N.; Reverdatto, S.; Premo, A.; Breindel, L.; Yu, J.; Theophall, G.; Burz, D.S.; Liu, A.; Sulchek, T.; Schmidt, A.M.; et al. Messenger RNA in lipid nanoparticles rescues HEK 293 cells from lipid-induced mitochondrial dysfunction as studied by real time pulse chase NMR, RTPC-NMR, spectroscopy. Sci. Rep. 2022, 12, 22293. [Google Scholar] [CrossRef]
- Jiang, P.; Ren, L.; Zhi, L.; Hu, X.; Xiao, R.P. Protocol for cell preparation and gene delivery in HEK293T and C2C12 cells. STAR Protoc. 2021, 2, 100497. [Google Scholar] [CrossRef]
- Thanki, K.; Zeng, X.; Foged, C. Preparation, Characterization, and In Vitro Evaluation of Lipidoid-Polymer Hybrid Nanoparticles for siRNA Delivery to the Cytosol. Methods Mol. Biol. 2019, 1943, 141–152. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Darjuan, M.M.; Mercer, J.E.; Chen, S.; van der Meel, R.; Thewalt, J.L.; Tam, Y.Y.C.; Cullis, P.R. On the Formation and Morphology of Lipid Nanoparticles Containing Ionizable Cationic Lipids and siRNA. ACS Nano 2018, 12, 4787–4795. [Google Scholar] [CrossRef]
- Navarro, G.; Sawant, R.R.; Essex, S.; Tros de Ilarduya, C.; Torchilin, V.P. Phospholipid-polyethylenimine conjugate-based micelle-like nanoparticles for siRNA delivery. Drug Deliv. Transl. Res. 2011, 1, 25–33. [Google Scholar] [CrossRef]
- Song, H.; Wang, G.; He, B.; Li, L.; Li, C.; Lai, Y.; Xu, X.; Gu, Z. Cationic lipid-coated PEI/DNA polyplexes with improved efficiency and reduced cytotoxicity for gene delivery into mesenchymal stem cells. Int. J. Nanomed. 2012, 7, 4637–4648. [Google Scholar] [CrossRef]
- Bershteyn, A.; Chaparro, J.; Yau, R.; Kim, M.; Reinherz, E.; Ferreira-Moita, L.; Irvine, D.J. Polymer-supported lipid shells, onions, and flowers. Soft Matter 2008, 4, 1787–1791. [Google Scholar] [CrossRef] [PubMed]
- Golan-Paz, S.; Frizzell, H.; Woodrow, K.A. Cross-Platform Comparison of Therapeutic Delivery from Multilamellar Lipid-Coated Polymer Nanoparticles. Macromol. Biosci. 2019, 19, e1800362. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Pippa, N.; Forys, A.; Trzebicka, B.; Pispas, S. Structure-Based Evaluation of Hybrid Lipid-Polymer Nanoparticles: The Role of the Polymeric Guest. Polymers 2024, 16, 290. [Google Scholar] [CrossRef] [PubMed]




| Formulation | DNA/Lipid (w/w) | DNA/PEI (w/w) | Size (nm, Mean ± SD) | PDI (Mean ± SD) | Zeta Potential (mV, Mean ± SD) | Preparation Method |
|---|---|---|---|---|---|---|
| LNP-Luc (10) | 1:10 | - | 188.8 ± 3.88 | 0.213 ± 0.018 | 0.068 ± 0.091 | One-step (NanoAssemblr direct) |
| LNP-Luc (40) | 1:40 | - | 136.27 ± 1.69 | 0.034 ± 0.009 | 6.003 ± 0.357 | One-step (NanoAssemblr direct) |
| LNP-Luc/PEI (10, Org) | 1:10 | 1:1 | 200.4 ± 3.78 | 0.083 ± 0.036 | 0.155 ± 0.067 | One-step (PEI in organic phase, NanoAssemblr) |
| LNP-Luc/PEI (40, Org) | 1:40 | 1:1 | 109.9 ± 2.02 | 0.144 ± 0.021 | 0.225 ± 0.058 | One-step (PEI in organic phase, NanoAssemblr) |
| LNP-Luc/PEI 1:1 (10) | 1:10 | 1:1 | 177.4 ± 11.6 | 0.325 ± 0.029 | 0.313 ± 0.162 | Two-step (PEI/DNA complex, NanoAssemblr) |
| LNP-Luc/PEI 1:1 (40) | 1:40 | 1:1 | 111.07 ± 4.64 | 0.195 ± 0.008 | 0.622 ± 0.244 | Two-step (PEI/DNA complex, NanoAssemblr) |
| LNP-Luc/PEI 1:2 (10) | 1:10 | 1:2 | 250.2 ± 22.6 | 0.300 ± 0.018 | 0.016 ± 0.009 | Two-step (PEI/DNA complex, NanoAssemblr) |
| LNP-Luc/PEI 1:2 (40) | 1:40 | 1:2 | 201.4 ± 14.9 | 0.289 ± 0.023 | 0.184 ± 0.023 | Two-step (PEI/DNA complex, NanoAssemblr) |
| LNP-Luc/PEI 1:3 (10) | 1:10 | 1:3 | 182.1 ± 18.4 | 0.379 ± 0.049 | 0.336 ± 0.086 | Two-step (PEI/DNA complex, NanoAssemblr) |
| LNP-Luc/PEI 1:3 (40) | 1:40 | 1:3 | 154.6 ± 12.6 | 0.254 ± 0.013 | 7.46 ± 0.833 | Two-step (PEI/DNA complex, NanoAssemblr) |
| Formulation | DNA/Lipid (w/w) | DNA/PEI (w/w) | Preparation Method | Size (nm, Mean ± SD) | PDI (Mean ± SD) | Zeta Potential (mV, Mean ± SD) |
|---|---|---|---|---|---|---|
| LNP-GFP (40) | 1:40 | - | One-step (NanoAssemblr direct) | 69.17 ± 1.30 | 0.23 ± 0.04 | 0.12 ± 1.61 |
| LNP-GFP/PEI 1:1 (40) | 1:40 | 1:1 | Two-step (PEI/DNA complex, NanoAssemblr) | 119.53 ± 7.75 | 0.20 ± 0.01 | 3.43 ± 2.00 |
| LNP-GFP/PEI 1:3 (40) | 1:40 | 1:3 | Two-step (PEI/DNA complex, NanoAssemblr) | 183.03 ± 17.42 | 0.23 ± 0.01 | 12.67 ± 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosseini-Kharat, M.; Wignall, A.; Mekonnen, Z.A.; Ung, B.S.-Y.; Chereda, B.; Bremmell, K.E.; Grubor-Bauk, B.; Prestidge, C.A. Microfluidic Optimization of PEI-Lipid Hybrid Nanoparticles for Efficient DNA Delivery and Transgene Expression. Pharmaceutics 2025, 17, 454. https://doi.org/10.3390/pharmaceutics17040454
Hosseini-Kharat M, Wignall A, Mekonnen ZA, Ung BS-Y, Chereda B, Bremmell KE, Grubor-Bauk B, Prestidge CA. Microfluidic Optimization of PEI-Lipid Hybrid Nanoparticles for Efficient DNA Delivery and Transgene Expression. Pharmaceutics. 2025; 17(4):454. https://doi.org/10.3390/pharmaceutics17040454
Chicago/Turabian StyleHosseini-Kharat, Mahboubeh, Anthony Wignall, Zelalem A. Mekonnen, Ben S.-Y. Ung, Bradley Chereda, Kristen E. Bremmell, Branka Grubor-Bauk, and Clive A. Prestidge. 2025. "Microfluidic Optimization of PEI-Lipid Hybrid Nanoparticles for Efficient DNA Delivery and Transgene Expression" Pharmaceutics 17, no. 4: 454. https://doi.org/10.3390/pharmaceutics17040454
APA StyleHosseini-Kharat, M., Wignall, A., Mekonnen, Z. A., Ung, B. S.-Y., Chereda, B., Bremmell, K. E., Grubor-Bauk, B., & Prestidge, C. A. (2025). Microfluidic Optimization of PEI-Lipid Hybrid Nanoparticles for Efficient DNA Delivery and Transgene Expression. Pharmaceutics, 17(4), 454. https://doi.org/10.3390/pharmaceutics17040454






