Anti-Breast Cancer Properties and In Vivo Safety Profile of a Bis-Carbazole Derivative
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Cell Viability
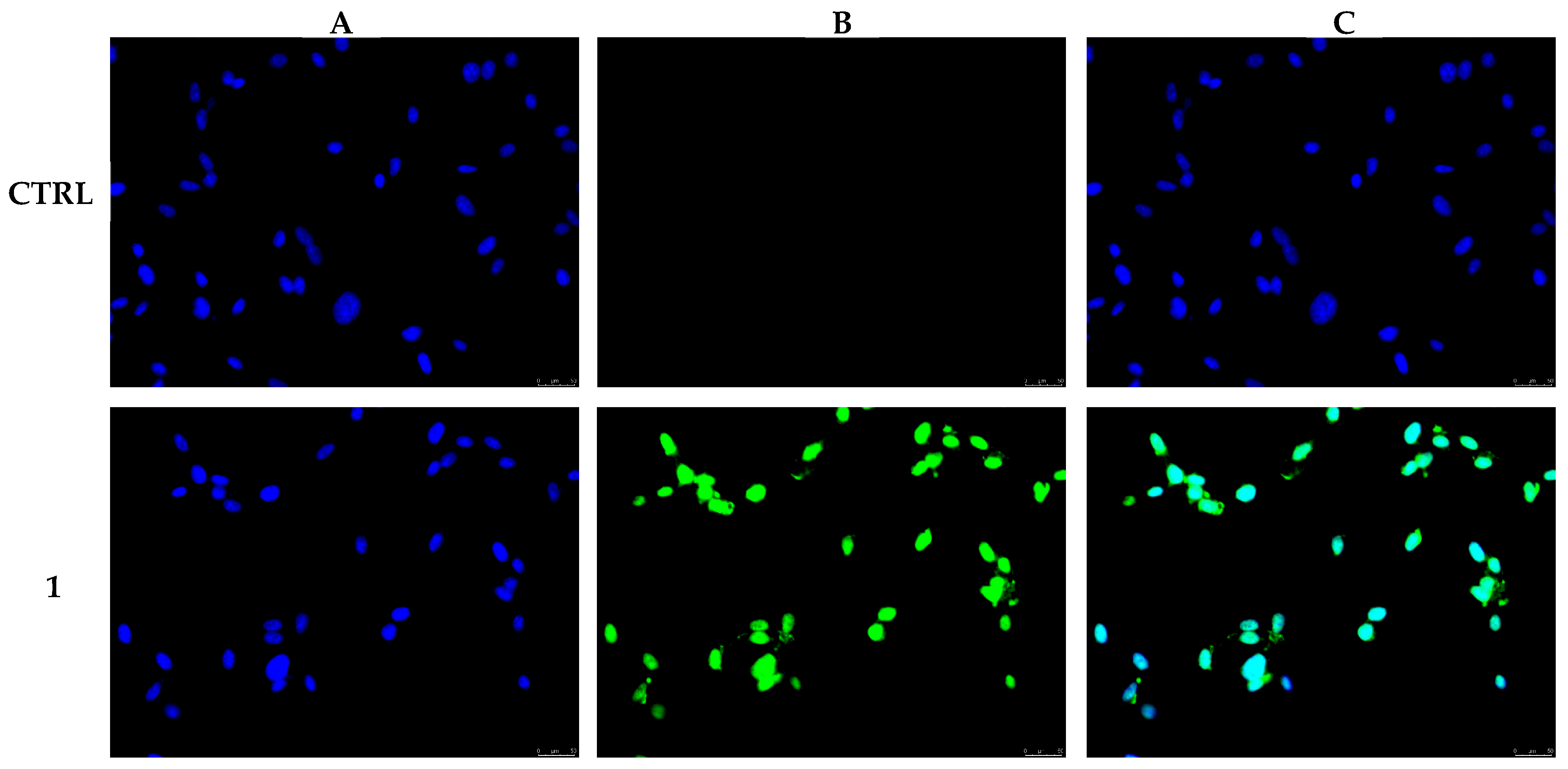
2.3. TUNEL Assay
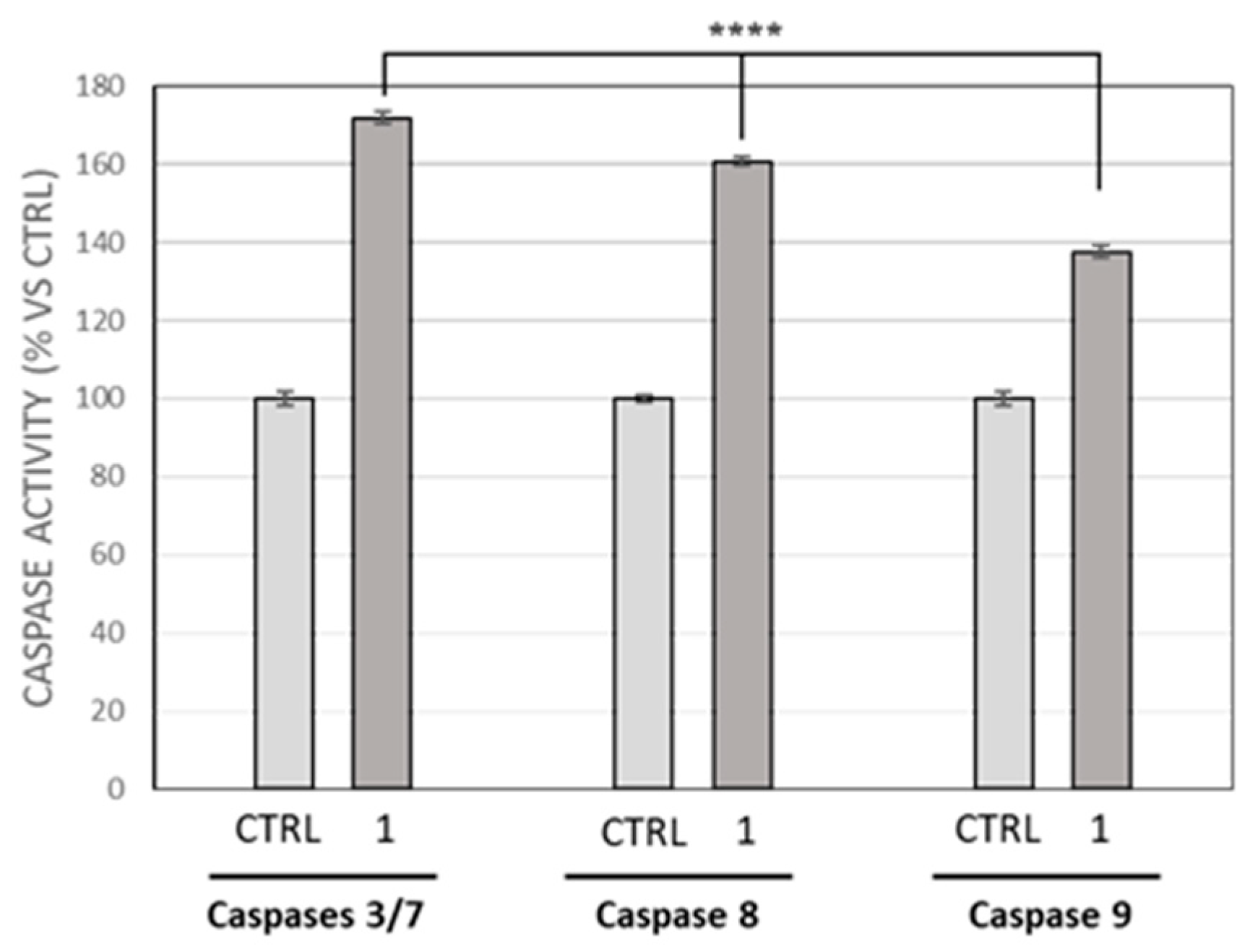
2.4. Caspases Assay
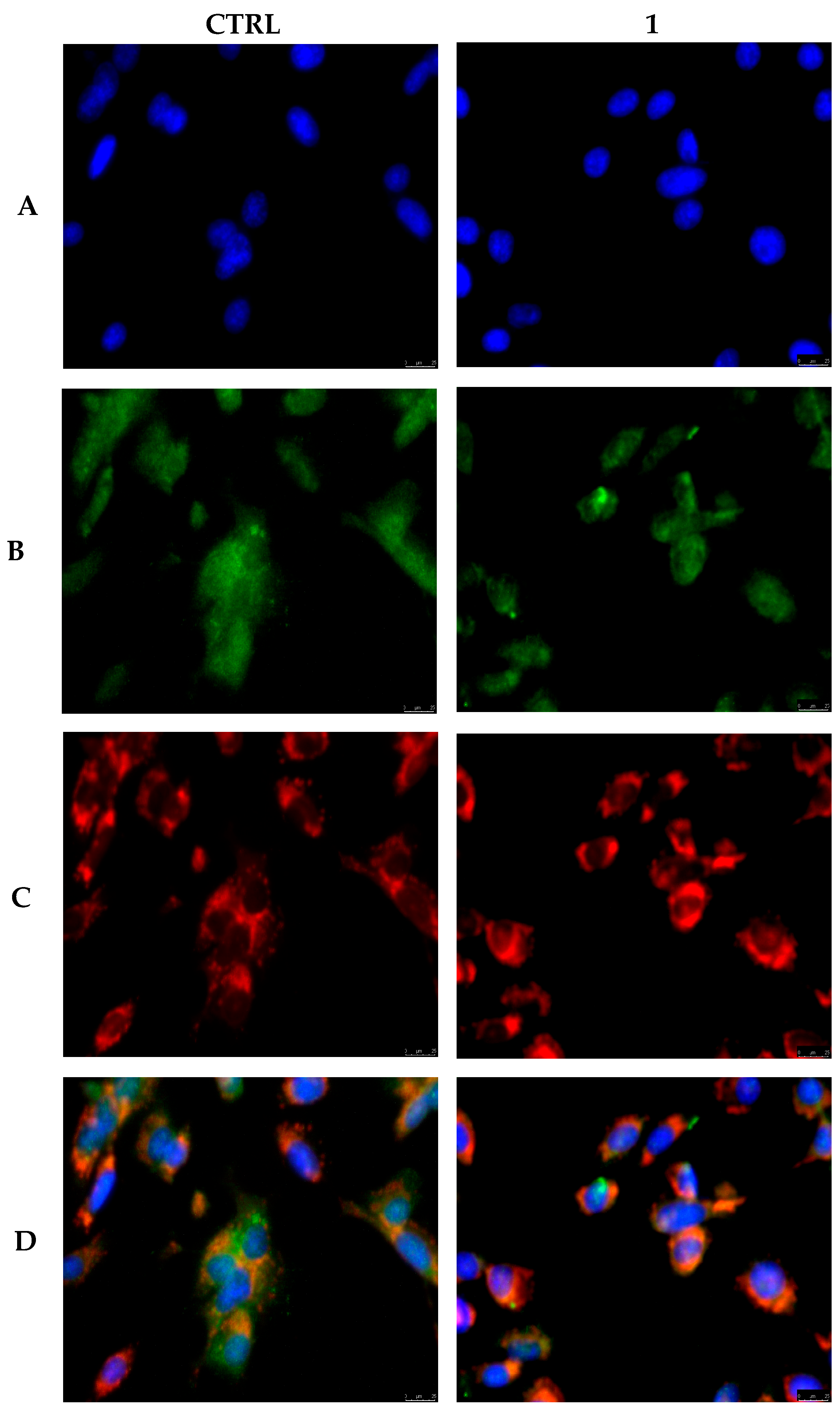
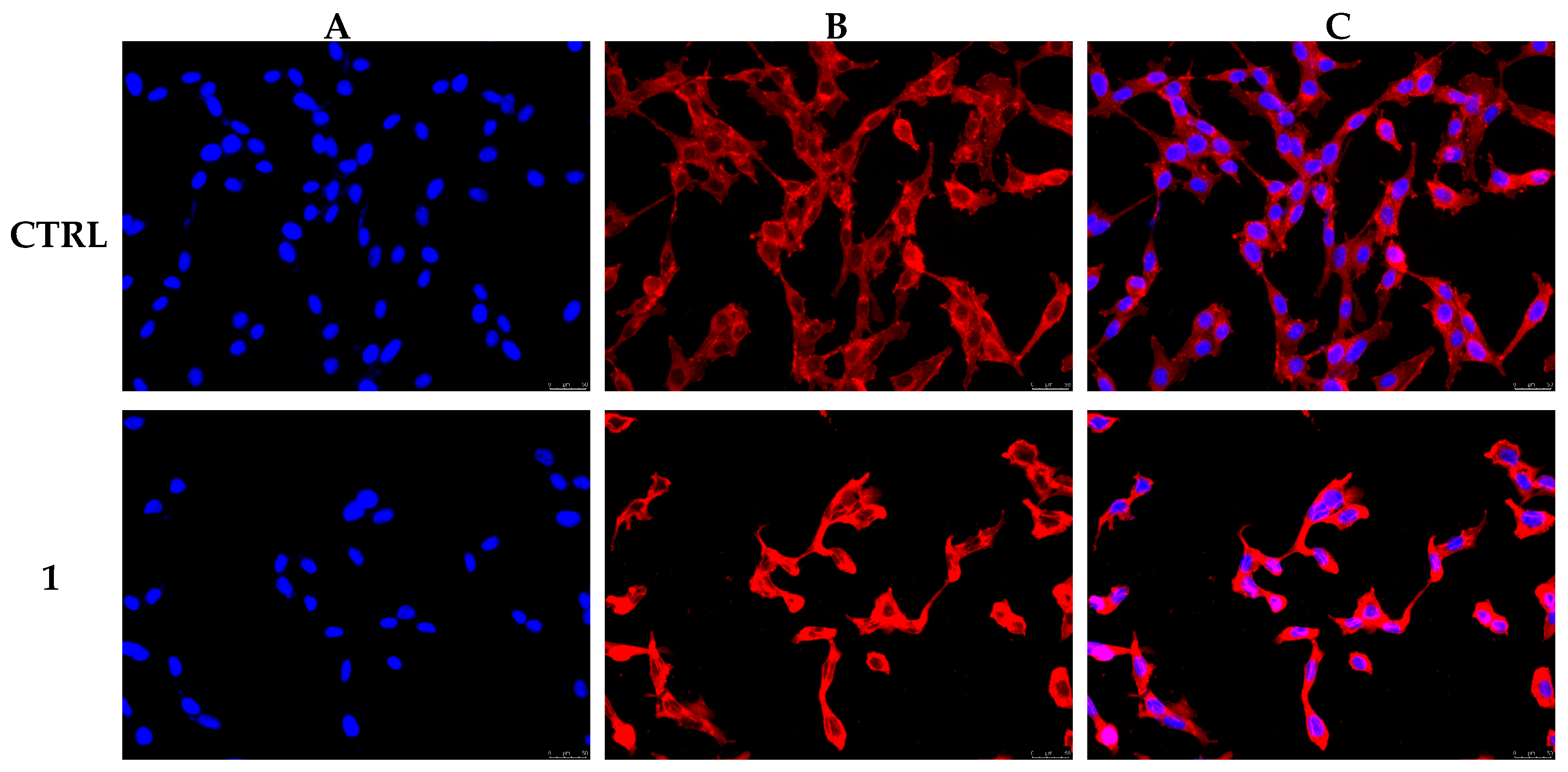
2.5. Immunofluorescence Studies and Mitochondria Staining
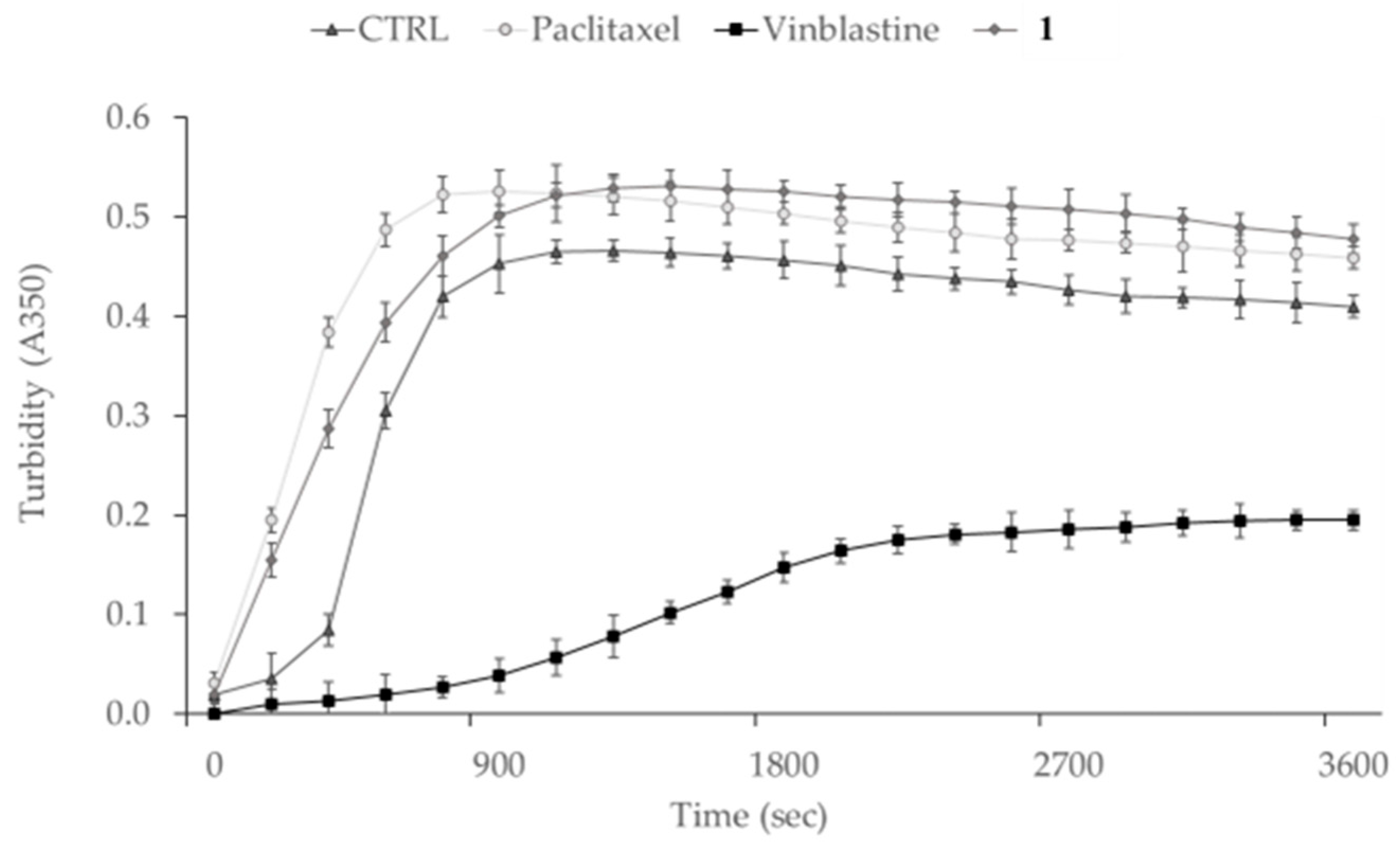
2.6. In Vitro Tubulin Polymerization Assay
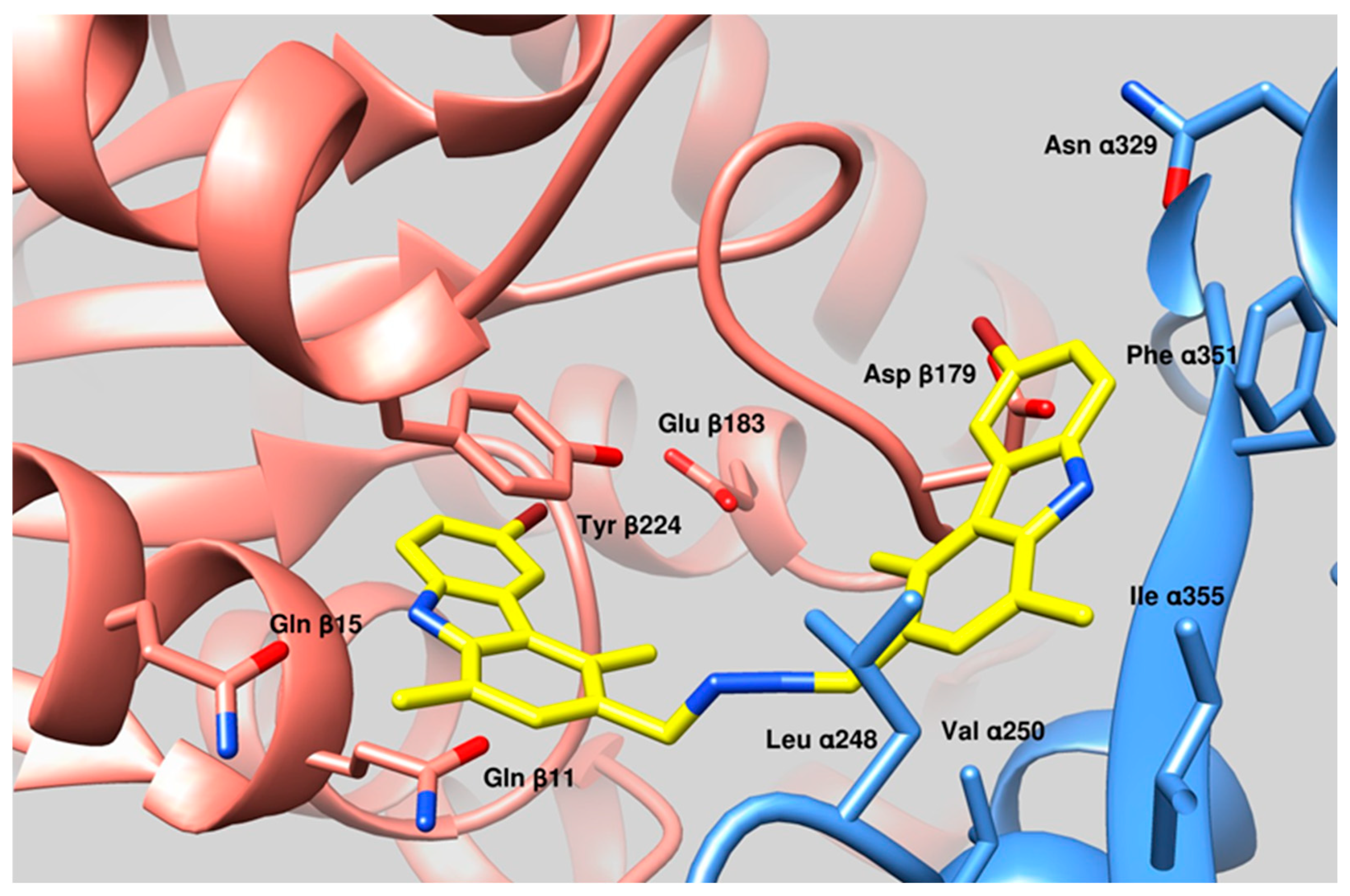
2.7. Molecular Docking
2.8. In Vivo Studies
2.9. Hydrolytic Stability Test
2.10. Statistical Analysis
3. Results and Discussion
3.1. Anticancer Activity and DNA Damage
3.2. Compound 1 Treatment Induces MDA-MB-231 Breast Cancer Cells Apoptosis
3.3. Docking Studies
3.4. Compound 1 Interferes with MDA-MB-231 Cells Cytoskeleton Dynamics
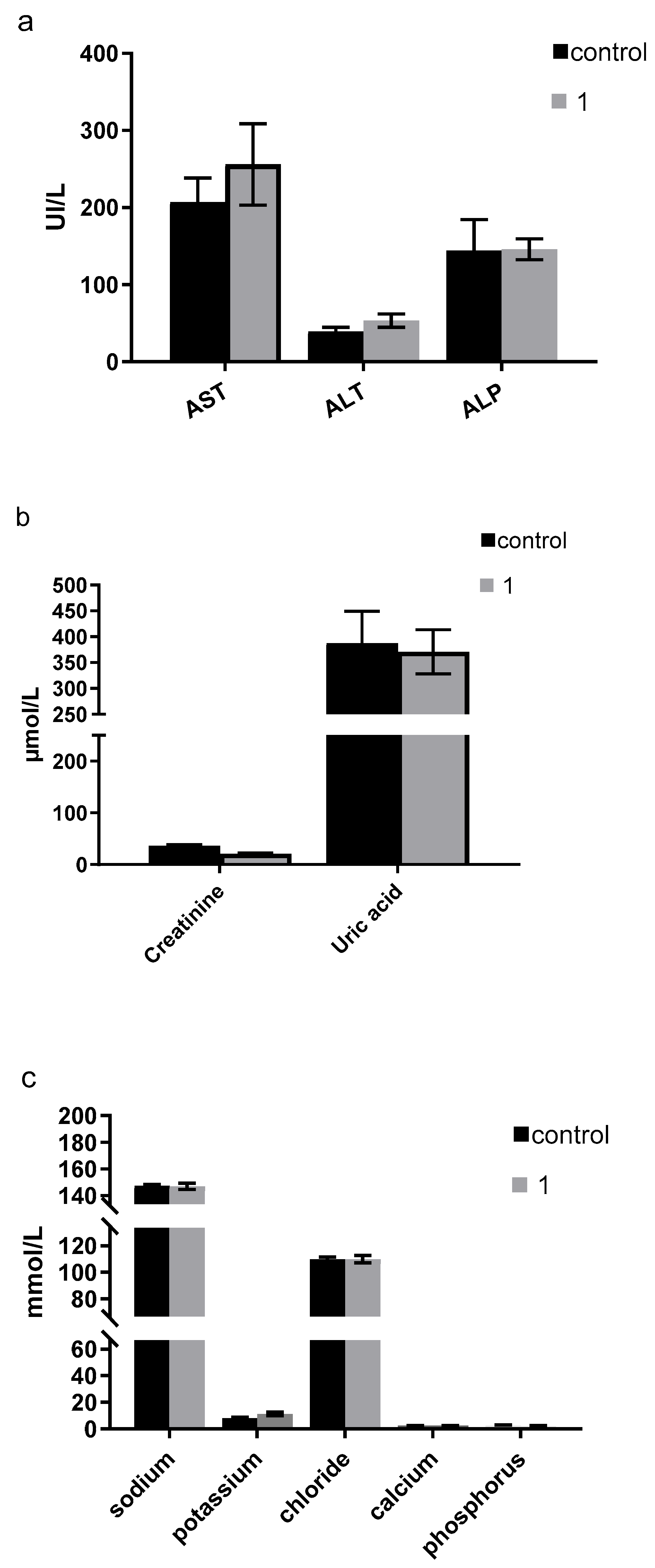
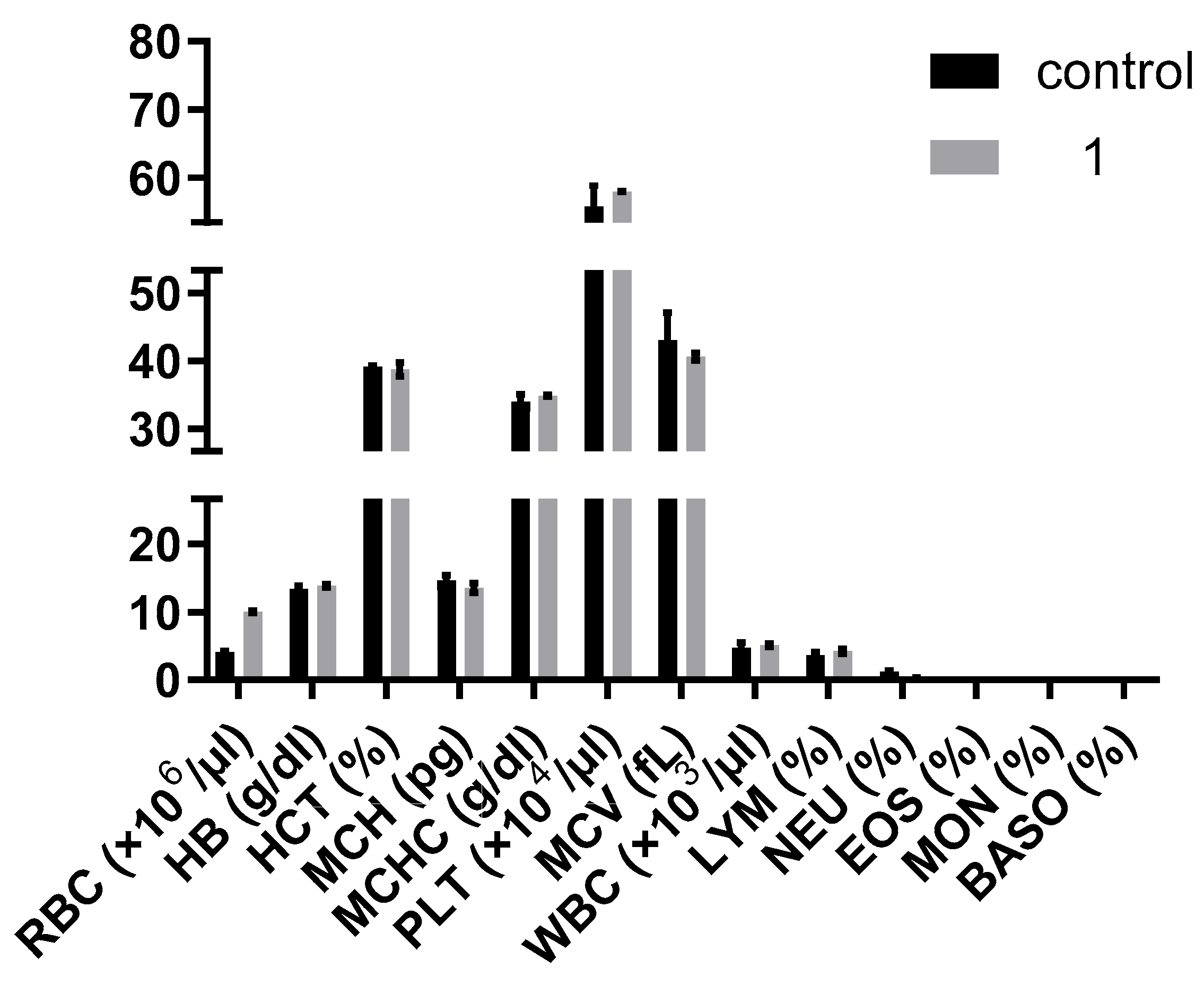
3.5. In Vivo Studies
3.6. Compound 1 Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MTAs | microtubule-targeting agents |
| MTT | 3-(4,5-dimethylthiazol-2-y1)-2,5-diphenyltetrazolium Bromide |
| DMSO | dimethyl sulfoxide |
| TUNEL | terminal deoxynucleotidyl transferase dUTP nick end labeling |
| DAPI | 4′,6-diamidino-2-phenylindole |
| SD | standard deviations |
| RMSD | root mean square deviation |
| AST | aspartate transaminase |
| ALT | alanine transaminase |
| ALP | alkaline phosphatase |
| RBC | red blood cell |
| HB | hemoglobin |
| HCT | hematocrit |
| MCH | mean corpuscular hemoglobin |
| WBC | white blood cell |
| MCV | mean corpuscular volume |
| MCHC | mean corpuscular hemoglobin concentration |
| PLT | platelet |
| WBC | white blood cells |
| LYM | lymphocytes |
| NEU | neutrophils |
| EOS | eosinophils |
| MAC | macrophages |
| BASO | basophile |
| OD | optical density |
| NC | negative control |
References
- Huang, L.; Feng, Z.-L.; Wang, Y.-T.; Lin, L.-G. Anticancer carbazole alkaloids and coumarins from Clausena plants: A review. Chin. J. Nat. Med. 2017, 15, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Issa, S.; Prandina, A.; Bedel, N.; Rongved, P.; Yous, S.; Le Borgne, M.; Bouaziz, Z. Carbazole scaffolds in cancer therapy: A review from 2012 to 2018. J. Enzym. Inhib. Med. Chem. 2019, 34, 1321–1346. [Google Scholar] [CrossRef]
- Tiwari, A.; Mishra, B. Diverse pharmacological actions of potential carbazole derivatives by influencing various pathways of molecular signaling. Future J. Pharm. Sci. 2024, 10, 77. [Google Scholar] [CrossRef]
- Song, F.; Liu, D.; Huo, X.; Qiu, D. The anticancer activity of carbazole alkaloids. Arch. Pharm. 2021, 355, 2100277. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Iacopetta, D.; Caruso, A.; Mariconda, A.; Petrou, A.; Geronikaki, A.; Rosano, C.; Saturnino, C.; Catalano, A.; Longo, P.; et al. 5,8-Dimethyl-9H-carbazole Derivatives Blocking hTopo I Activity and Actin Dynamics. Pharmaceuticals 2023, 16, 353. [Google Scholar] [CrossRef]
- Miller, C.M.; O’Sullivan, E.C.; McCarthy, F.O. Novel 11-Substituted Ellipticines as Potent Anticancer Agents with Divergent Activity against Cancer Cells. Pharmaceuticals 2019, 12, 90. [Google Scholar] [CrossRef]
- Dziadziuszko, R.; Peters, S.; Ruf, T.; Cardona, A.; Guerini, E.; Kurtsikidze, N.; Smoljanovic, V.; Planchard, D. Clinical experience and management of adverse events in patients with advanced ALK-positive non-small-cell lung cancer receiving alectinib. ESMO Open 2022, 7, 100612. [Google Scholar] [CrossRef]
- Tzogani, K.; Yu, Y.; Meulendijks, D.; Herberts, C.; Hennik, P.; Verheijen, R.; Wangen, T.; Dahlseng Håkonsen, G.; Kaasboll, T.; Dalhus, M.; et al. European Medicines Agency review of midostaurin (Rydapt) for the treatment of adult patients with acute myeloid leukaemia and systemic mastocytosis. ESMO Open 2019, 4, e000606. [Google Scholar] [CrossRef]
- Wang, G.; Sun, S.; Guo, H. Current status of carbazole hybrids as anticancer agents. Eur. J. Med. Chem. 2022, 229, 113999. [Google Scholar] [CrossRef]
- D’Amato, A.; Iacopetta, D.; Ceramella, J.; Troiano, R.; Mariconda, A.; Catalano, A.; Marra, M.; Saturnino, C.; Rosano, C.; Sinicropi, M.S.; et al. Design, synthesis and biological evaluation of multitarget hybrid molecules containing NHC-Au(I) complexes and carbazole moieties. Eur. J. Med. Chem. 2024, 277, 116757. [Google Scholar] [CrossRef]
- Xu, X.; Xu, S.; Wan, J.; Wang, D.; Pang, X.; Gao, Y.; Ni, N.; Chen, D.; Sun, X. Disturbing cytoskeleton by engineered nanomaterials for enhanced cancer therapeutics. Bioact. Mater. 2023, 29, 50–71. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Song, Y.; Shen, M.; Xie, S.; Li, W.; Lu, Y.; Yang, Y.; Ou, G.; Zhou, J.; Wang, F.; et al. Microtubule-binding protein FOR20 promotes microtubule depolymerization and cell migration. Cell Discov. 2017, 3, 17032. [Google Scholar] [CrossRef] [PubMed]
- Gierke, S.; Kumar, P.; Wittmann, T. Analysis of Microtubule Polymerization Dynamics in Live Cells. In Microtubules: In Vivo, Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 15–33. [Google Scholar]
- Lafanechère, L. The microtubule cytoskeleton: An old validated target for novel therapeutic drugs. Front. Pharmacol. 2022, 13, 969183. [Google Scholar] [CrossRef]
- Wordeman, L.; Vicente, J.J. Microtubule Targeting Agents in Disease: Classic Drugs, Novel Roles. Cancers 2021, 13, 5650. [Google Scholar] [CrossRef]
- Čermák, V.; Dostál, V.; Jelínek, M.; Libusová, L.; Kovář, J.; Rösel, D.; Brábek, J. Microtubule-targeting agents and their impact on cancer treatment. Eur. J. Cell Biol. 2020, 99, 1515075. [Google Scholar] [CrossRef]
- Vlaar, C.P.; Castillo-Pichardo, L.; Medina, J.I.; Marrero-Serra, C.M.; Vélez, E.; Ramos, Z.; Hernández, E. Design, synthesis and biological evaluation of new carbazole derivatives as anti-cancer and anti-migratory agents. Bioorganic Med. Chem. 2018, 26, 884–890. [Google Scholar] [CrossRef]
- Butler-Fernández, K.M.; Ramos, Z.; Francis-Malavé, A.M.; Bloom, J.; Dharmawardhane, S.; Hernández, E. Synthesis, Anti-Cancer and Anti-Migratory Evaluation of 3,6-Dibromocarbazole and 5-Bromoindole Derivatives. Molecules 2019, 24, 2686. [Google Scholar] [CrossRef]
- Sinicropi, M.; Lappano, R.; Caruso, A.; Santolla, M.; Pisano, A.; Rosano, C.; Capasso, A.; Panno, A.; Lancelot, J.; Rault, S.; et al. (6-Bromo-1,4-dimethyl-9H-carbazol-3-yl-methylene)-hydrazine (Carbhydraz) Acts as a GPER Agonist in Breast Cancer Cells. Curr. Top. Med. Chem. 2015, 15, 1035–1042. [Google Scholar] [CrossRef]
- Yin, X.-M. Signal transduction mediated by Bid, a pro-death Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways. Cell Res. 2000, 10, 161–167. [Google Scholar] [CrossRef]
- Kantari, C.; Walczak, H. Caspase-8 and Bid: Caught in the act between death receptors and mitochondria. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2011, 1813, 558–563. [Google Scholar] [CrossRef]
- Fielden, M.R.; Kolaja, K.L. The role of earlyin vivotoxicity testing in drug discovery toxicology. Expert Opin. Drug Saf. 2008, 7, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Iacopetta, D.; Catalano, A.; Ceramella, J.; Barbarossa, A.; Carocci, A.; Fazio, A.; La Torre, C.; Caruso, A.; Ponassi, M.; Rosano, C.; et al. Synthesis, anticancer and antioxidant properties of new indole and pyranoindole derivatives. Bioorg. Chem. 2020, 105, 104440. [Google Scholar] [CrossRef]
- Saturnino, C.; Caruso, A.; Iacopetta, D.; Rosano, C.; Ceramella, J.; Muià, N.; Mariconda, A.; Bonomo, M.G.; Ponassi, M.; Rosace, G.; et al. Inhibition of Human Topoisomerase II by N,N,N-Trimethylethanammonium Iodide Alkylcarbazole Derivatives. ChemMedChem 2018, 13, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Mariconda, A.; Sirignano, M.; Iacopetta, D.; Rosano, C.; Catalano, A.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Novel Au Carbene Complexes as Promising Multi-Target Agents in Breast Cancer Treatment. Pharmaceuticals 2022, 15, 507. [Google Scholar] [CrossRef]
- Iacopetta, D.; Mariconda, A.; Saturnino, C.; Caruso, A.; Palma, G.; Ceramella, J.; Muià, N.; Perri, M.; Sinicropi, M.S.; Caroleo, M.C.; et al. Novel Gold and Silver Carbene Complexes Exert Antitumor Effects Triggering the Reactive Oxygen Species Dependent Intrinsic Apoptotic Pathway. ChemMedChem 2017, 12, 2054–2065. [Google Scholar] [CrossRef]
- Ceramella, J.; La Torre, C.; De Luca, M.; Iacopetta, D.; Fazio, A.; Catalano, A.; Ragno, G.; Longo, P.; Sinicropi, M.S.; Rosano, C. Exploring the anticancer and antioxidant properties of Vicia faba L. pods extracts, a promising source of nutraceuticals. PeerJ 2022, 10, e13683. [Google Scholar] [CrossRef]
- Iacopetta, D.; Rosano, C.; Sirignano, M.; Mariconda, A.; Ceramella, J.; Ponassi, M.; Saturnino, C.; Sinicropi, M.S.; Longo, P. Is the Way to Fight Cancer Paved with Gold? Metal-Based Carbene Complexes with Multiple and Fascinating Biological Features. Pharmaceuticals 2020, 13, 91. [Google Scholar] [CrossRef]
- Ceramella, J.; Mariconda, A.; Rosano, C.; Iacopetta, D.; Caruso, A.; Longo, P.; Sinicropi, M.S.; Saturnino, C. α–ω Alkenyl-bis-S-Guanidine Thiourea Dihydrobromide Affects HeLa Cell Growth Hampering Tubulin Polymerization. ChemMedChem 2020, 15, 2306–2316. [Google Scholar] [CrossRef]
- Landskron, L.; Bak, J.; Adamopoulos, A.; Kaplani, K.; Moraiti, M.; van den Hengel, L.G.; Song, J.-Y.; Bleijerveld, O.B.; Nieuwenhuis, J.; Heidebrecht, T.; et al. Posttranslational modification of microtubules by the MATCAP detyrosinase. Science 2022, 376, eabn6020. [Google Scholar] [CrossRef]
- Sanner, M.F.; Duncan, B.S.; J. Carrillo, C.; Olson, A.J. Integrating Computation and Visualization for Biomolecular Analysis: An Example Using Python and Avs. In Proceedings of the Biocomputing ’99, 1998, Big Island, HI, USA, 4–9 January 1999; pp. 401–412. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Christner, S.M.; Clausen, D.M.; Beumer, J.H.; Parise, R.A.; Guo, J.; Huang, Y.; Dömling, A.S.; Eiseman, J.L. In vitro cytotoxicity and in vivo efficacy, pharmacokinetics, and metabolism of pyrazole-based small molecule inhibitors of Mdm2/4–p53 interaction. Cancer Chemother. Pharmacol. 2015, 76, 287–299. [Google Scholar] [CrossRef] [PubMed]
- van Meer, P.; Raber, J. Mouse behavioural analysis in systems biology. Biochem. J. 2005, 389, 593–610. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Robles-Planells, C.; Liu, D.; Graves, S.A.; Vasquez-Martinez, G.; Mayoral-Andrade, G.; Lee, D.; Rastogi, P.; Marks, B.M.; Sagastume, E.A.; et al. Pre-clinical evaluation of biomarkers for the early detection of nephrotoxicity following alpha-particle radioligand therapy. Eur. J. Nucl. Med. Mol. Imaging 2023, 51, 1395–1408. [Google Scholar] [CrossRef]
- Abbasnezhad, A.; Salami, F.; Mohebbati, R. A review: Systematic research approach on toxicity model of liver and kidney in laboratory animals. Anim. Models Exp. Med. 2022, 5, 436–444. [Google Scholar] [CrossRef]
- Krawczyk, P.; Kula, S.; Seklecka, K.; Łączkowski, K.Z. Synthesis, electrochemical, optical and biological properties of new carbazole derivatives. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2022, 267, 120497. [Google Scholar] [CrossRef]
- Kuo, P.-L.; Hsu, Y.-L.; Chang, C.-H.; Lin, C.-C. The mechanism of ellipticine-induced apoptosis and cell cycle arrest in human breast MCF-7 cancer cells. Cancer Lett. 2005, 223, 293–301. [Google Scholar] [CrossRef]
- Kim, R.; Kin, T.; Beck, W.T. Impact of Complex Apoptotic Signaling Pathways on Cancer Cell Sensitivity to Therapy. Cancers 2024, 16, 984. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial Membrane Permeabilization in Cell Death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef]
- Debatin, K.-M.; Krammer, P.H. Death receptors in chemotherapy and cancer. Oncogene 2004, 23, 2950–2966. [Google Scholar] [CrossRef]
- Yuan, L.; Cai, Y.; Zhang, L.; Liu, S.; Li, P.; Li, X. Promoting Apoptosis, a Promising Way to Treat Breast Cancer With Natural Products: A Comprehensive Review. Front. Pharmacol. 2022, 12, 801662. [Google Scholar] [CrossRef] [PubMed]
- Gallyas, F.; Wu, Y.; Zhao, D.; Zhuang, J.; Zhang, F.; Xu, C. Caspase-8 and Caspase-9 Functioned Differently at Different Stages of the Cyclic Stretch-Induced Apoptosis in Human Periodontal Ligament Cells. PLoS ONE 2016, 11, e0168268. [Google Scholar] [CrossRef]
- Çapan, İ.; Hawash, M.; Jaradat, N.; Sert, Y.; Servi, R.; Koca, İ. Design, synthesis, molecular docking and biological evaluation of new carbazole derivatives as anticancer, and antioxidant agents. BMC Chem. 2023, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Patil, S.A.; Ble-González, E.A.; Isbel, S.R.; Hampton, S.M.; Bugarin, A. Carbazole Derivatives as Potential Antimicrobial Agents. Molecules 2022, 27, 6575. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Tavani, C.; Rosano, C.; Ceramella, J.; Iacopetta, D.; Barbarossa, A.; Bianchi, L.; Benzi, A.; Maccagno, M.; Ponassi, M.; et al. A Nitrocarbazole as a New Microtubule-Targeting Agent in Breast Cancer Treatment. Appl. Sci. 2021, 11, 9139. [Google Scholar] [CrossRef]
- Aseervatham, J. Cytoskeletal Remodeling in Cancer. Biology 2020, 9, 385. [Google Scholar] [CrossRef]
- Zhang, D.; Kanakkanthara, A. Beyond the Paclitaxel and Vinca Alkaloids: Next Generation of Plant-Derived Microtubule-Targeting Agents with Potential Anticancer Activity. Cancers 2020, 12, 1721. [Google Scholar] [CrossRef]
- Izdebska, M.; Zielińska, W.; Hałas-Wiśniewska, M.; Grzanka, A. Involvement of Actin and Actin-Binding Proteins in Carcinogenesis. Cells 2020, 9, 2245. [Google Scholar] [CrossRef]
- Pimm, M.L.; Henty-Ridilla, J.L.; Bement, W. New twists in actin–microtubule interactions. Mol. Biol. Cell 2021, 32, 211–217. [Google Scholar] [CrossRef]
- Dogterom, M.; Koenderink, G.H. Actin–microtubule crosstalk in cell biology. Nat. Rev. Mol. Cell Biol. 2018, 20, 38–54. [Google Scholar] [CrossRef]
- Coles, C.H.; Bradke, F. Coordinating Neuronal Actin–Microtubule Dynamics. Curr. Biol. 2015, 25, R677–R691. [Google Scholar] [CrossRef] [PubMed]
- Tokuraku, K.; Kuragano, M.; Uyeda, T.Q.P. Long-Range and Directional Allostery of Actin Filaments Plays Important Roles in Various Cellular Activities. Int. J. Mol. Sci. 2020, 21, 3209. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Manneville, S. Actin and Microtubules in Cell Motility: Which One is in Control? Traffic 2004, 5, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.; Xu, S.; Zhang, H.; Gui, W.; Zhao, Y.; Duan, L.; Hu, W. In vitro and in vivo efficacies of carbazole aminoalcohols in the treatment of alveolar echinococcosis. Acta Trop. 2018, 185, 138–143. [Google Scholar] [CrossRef]
- You, X.; Zhu, D.; Lu, W.; Sun, Y.; Qiao, S.; Luo, B.; Du, Y.; Pi, R.; Hu, Y.; Huang, P.; et al. Design, synthesis and biological evaluation ofN-arylsulfonyl carbazoles as novel anticancer agents. RSC Adv. 2018, 8, 17183–17190. [Google Scholar] [CrossRef]
- Sellers, R.S.; Mortan, D.; Michael, B.; Roome, N.; Johnson, J.K.; Yano, B.L.; Perry, R.; Schafer, K. Society of Toxicologic Pathology Position Paper: Organ Weight Recommendations for Toxicology Studies. Toxicol. Pathol. 2007, 35, 751–755. [Google Scholar] [CrossRef]
- Andersen, H.; Larsen, S.; Spliid, H.; Christensen, N.D. Multivariate statistical analysis of organ weights in toxicity studies. Toxicology 1999, 136, 67–77. [Google Scholar] [CrossRef]
- Rejeski, K.; Subklewe, M.; Aljurf, M.; Bachy, E.; Balduzzi, A.; Barba, P.; Bruno, B.; Benjamin, R.; Carrabba, M.G.; Chabannon, C.; et al. Immune effector cell–associated hematotoxicity: EHA/EBMT consensus grading and best practice recommendations. Blood 2023, 142, 865–877. [Google Scholar] [CrossRef]
- Mansoori, S.; Noei, A.; Maali, A.; Seyed-Motahari, S.S.; Sharifzadeh, Z. Recent updates on allogeneic CAR-T cells in hematological malignancies. Cancer Cell Int. 2024, 24, 304. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Li, A.-Y. Gastrodin—A potential drug used for the treatment of Tourette Syndrome. J. Pharmacol. Sci. 2021, 145, 289–295. [Google Scholar] [CrossRef]
- Mačianskienė, R.; Zigmantaitė, V.; Andriulė, I.; Pangonytė, D.; Sadauskienė, I.; Arandarčikaitė, O.; Stankevičius, A.; Grigas, J.; Pautienius, A.; Treinys, R.; et al. Acute and Sub-Chronic Intraperitoneal Toxicity Studies of the Elsholtzia ciliata Herbal Extract in Balb/c Mice. Pharmaceutics 2023, 15, 2417. [Google Scholar] [CrossRef] [PubMed]
- Bossardi Ramos, R.; Adam, A.P. Molecular Mechanisms of Vascular Damage During Lung Injury. In Lung Inflammation in Health and Disease, Volume II, Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2021; pp. 95–107. [Google Scholar]
- Nguyen, T.M.C.; Hoang, L.D.C.; Nguyen, T.K.G.; Nguyen, T.N.; Nguyen, Q.C.; Nguyen, T.B.; Dang, H.H.Q.; Bui, V.C.; Pham, T.M.; Nguyen, T.T. Safety assessment, radioiodination and preclinical evaluation of antinuclear antibody as novel medication for prostate cancer in mouse xenograft model. Sci. Rep. 2023, 13, 18753. [Google Scholar] [CrossRef]
- Wang, J.; Ottosson, J.; Tentarelli, S. Development of hydrolytic stability screening methods for early drug discovery with high differentiation and predictive power. J. Pharm. Biomed. Anal. 2023, 233, 115478. [Google Scholar] [CrossRef]



| IC50 (µM) | SI | ||||
|---|---|---|---|---|---|
| MCF-7 | MDA-MB-231 | MCF-10A | MCF-7 | MDA-MB-231 | |
| Compound 1 | 0.6 ± 0.3 | 0.3 ± 0.2 | >100 | >166.7 | >333.3 |
| Ellipticine | 1.3 ± 0.2 | 1.9 ± 0.1 | 1.2 ± 0.3 | 0.9 | 0.8 |
| Vinblastine | (36.8 ± 0.7) × 10−3 | (130.2 ± 1.1) × 10−3 | (17.2 ± 0.6) × 10−3 | 0.13 | 0.47 |
| Paclitaxel | (1.3 ± 0.7) × 10−2 | (1.9 ± 0.5) × 10−2 | (6.4 ± 0.8) × 10−1 | 33.7 | 49.2 |
| Organ Coefficient (mg/10 g) = 10 × Body Weight/Weight of the Mouse | ||||||
|---|---|---|---|---|---|---|
| Spleen | Liver | Lung | Brain | Heart | Kidney | |
| NC | 0.056 ± 0.019 | 0.48 ± 0.047 | 0.08 ± 0.018 | 0.133 ± 0.025 | 0.057 ± 0.007 | 0.069 ± 0.006 |
| 1 | 0.07 ± 0.02 | 0.54 ± 0.03 | 0.11 ± 0.01 | 0.13 ± 0.03 | 0.06 ± 0.01 | 0.07 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceramella, J.; Rosano, C.; Iacopetta, D.; Ben Toumia, I.; Chekir-Ghedira, L.; Maatouk, M.; Mariconda, A.; Longo, P.; Dallemagne, P.; Rochais, C.; et al. Anti-Breast Cancer Properties and In Vivo Safety Profile of a Bis-Carbazole Derivative. Pharmaceutics 2025, 17, 415. https://doi.org/10.3390/pharmaceutics17040415
Ceramella J, Rosano C, Iacopetta D, Ben Toumia I, Chekir-Ghedira L, Maatouk M, Mariconda A, Longo P, Dallemagne P, Rochais C, et al. Anti-Breast Cancer Properties and In Vivo Safety Profile of a Bis-Carbazole Derivative. Pharmaceutics. 2025; 17(4):415. https://doi.org/10.3390/pharmaceutics17040415
Chicago/Turabian StyleCeramella, Jessica, Camillo Rosano, Domenico Iacopetta, Iméne Ben Toumia, Leila Chekir-Ghedira, Mouna Maatouk, Annaluisa Mariconda, Pasquale Longo, Patrick Dallemagne, Christophe Rochais, and et al. 2025. "Anti-Breast Cancer Properties and In Vivo Safety Profile of a Bis-Carbazole Derivative" Pharmaceutics 17, no. 4: 415. https://doi.org/10.3390/pharmaceutics17040415
APA StyleCeramella, J., Rosano, C., Iacopetta, D., Ben Toumia, I., Chekir-Ghedira, L., Maatouk, M., Mariconda, A., Longo, P., Dallemagne, P., Rochais, C., & Sinicropi, M. S. (2025). Anti-Breast Cancer Properties and In Vivo Safety Profile of a Bis-Carbazole Derivative. Pharmaceutics, 17(4), 415. https://doi.org/10.3390/pharmaceutics17040415













