Identifying Cellular Stress-Related mRNA Changes Induced by Novel Xanthone Derivatives in Ovarian Cancer Cells In Vitro
Abstract
1. Introduction

2. Materials and Methods
2.1. Chemicals for Biological Evaluations
2.2. Cell Culture and Treatment Conditions
2.3. In Silico Analysis
2.4. RNA Extraction and Real-Time RT PCR
3. Results and Discussion
3.1. In Silico Studies
3.1.1. Toxicity Prediction
| Novel Xanthone Derivatives | Control Drug | |||
|---|---|---|---|---|
| C8 | C7 | MAG | CIS | |
| Molecular weight | 385.88 | 420.33 | 410.46 | 259.21 |
| Octanol/water partition coefficient (logP) | 5.29 | 5.94 | 5.09 | −1.45 |
| Predicted LD50 [mg/kg]: | 450 | 723 | 1500 | 200 |
| Predicted Toxicity Class: | 4 | 4 | 4 | 3 |
| Hepatotoxicity | 0.77 | 0.77 | 0.70 | 0.72 |
| Carcinogenicity | 0.55 | 0.55 | 0.69 | 0.63 |
| Immunotoxicity | 0.64 | 0.57 | 0.84 | 0.99 |
| Mutagenicity | 0.58 | 0.58 | 0.53 | 0.56 |
| Cytotoxicity | 0.53 | 0.53 | 0.77 | 0.72 |
| Estrogen Receptor Alpha (ER) | 0.79 | 0.79 | 0.71 | 0.97 |
| Estrogen Receptor Ligand Binding Domain (ER-LBD) | 0.94 | 0.94 | 0.82 | 0.98 |
| Heat shock factor response element (HSE) | 0.92 | 0.92 | 0.81 | 0.81 |
| Mitochondrial Membrane Potential (MMP) | 0.88 | 0.88 | 0.67 | 0.94 |
| Phosphoprotein (Tumor Suppressor) p53 | 0.92 | 0.92 | 0.58 | 0.96 |
3.1.2. ADME Properties
| Novel Xanthone Derivatives | Control Drug | |||
|---|---|---|---|---|
| C8 | C7 | MAG | CIS | |
| Pharmacokinetics | ||||
| GI absorption | High | High | High | High |
| BBB permeant | Yes | Yes | No | No |
| P-gp substrate | Yes | Yes | No | No |
| CYP1A2 inhibitor | Yes | Yes | No | No |
| CYP2C19 inhibitor | Yes | Yes | No | No |
| CYP2C9 inhibitor | Yes | Yes | Yes | No |
| CYP2D6 inhibitor | Yes | Yes | No | No |
| CYP3A4 inhibitor | Yes | Yes | No | No |
| Druglikeness Criteria | ||||
| Lipinski | Yes | Yes | Yes | Yes |
| Ghose | Yes | Yes | Yes | No * |
| Veber | Yes | Yes | Yes | Yes |
| Egan | Yes | Yes | Yes | Yes |
| Muegge | Yes | Yes | No ** | No *** |
| Bioavailability Score | 0.55 | 0.55 | 0.55 | 0.55 |
3.2. qPCR of Cellular Stress-Related Proteins
3.2.1. Antioxidant and Pro-Oxidant Enzymes
3.2.2. Xenobiotic Metabolism—p450
3.2.3. Other Xenobiotic Metabolism Genes
3.2.4. Molecular Chaperones
3.2.5. Heat Shock Proteins
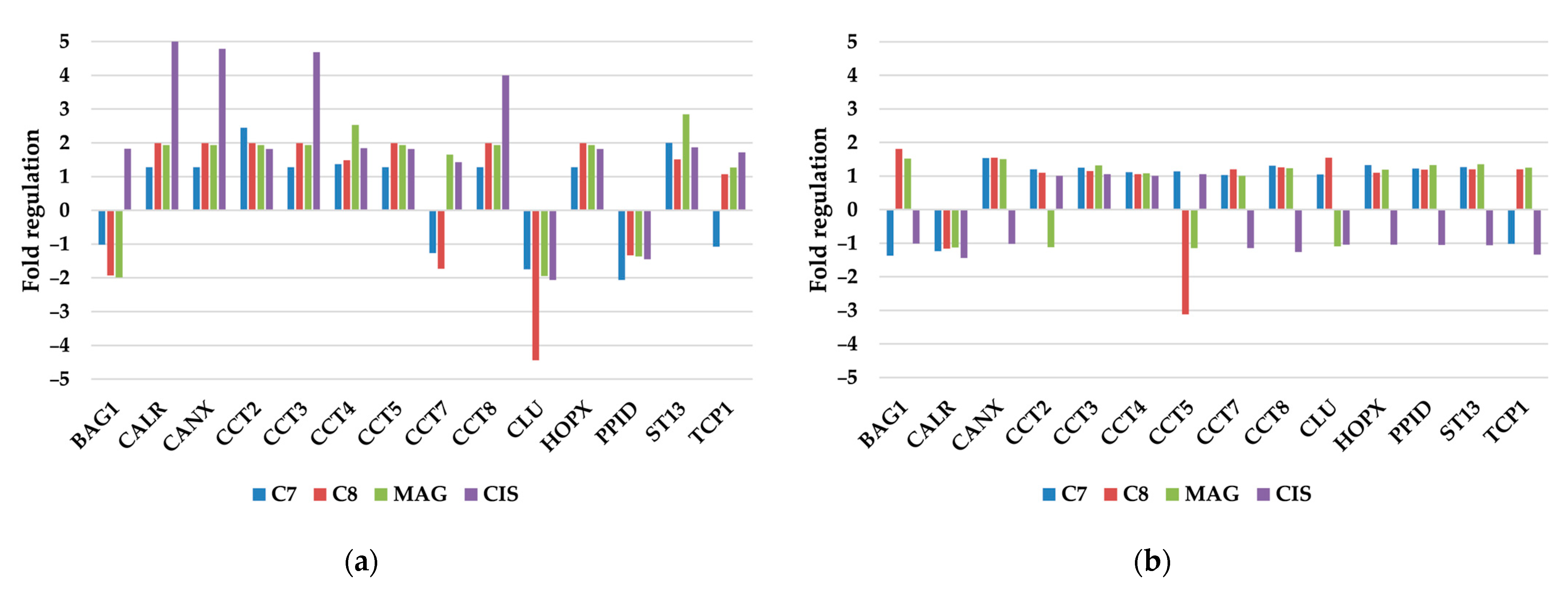
3.2.6. Other Molecular Chaperones
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMET | Absorption, distribution, metabolism, elimination, toxicity |
| ADME | Absorption, distribution, metabolism, elimination |
| AhR | Hydrocarbon receptor |
| BBB | Blood–brain barrier |
| CIS | Cisplatin |
| CYP | Cytochrome p450 isoenzyme |
| GI | Gastrointestinal |
| MAG | α-Mangostin |
| P-gp | Permeability glycoprotein |
| ROS | Reactive oxygen species |
References
- Brett, M.R.; Jennifer, B.P.; Thomas, A.S.; Brett, M.R.; Jennifer, B.P.; Thomas, A.S. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef] [PubMed]
- Rech, J.; Wilińska, J.; Borecka, A.; Turek, A. Application of fibrin in drug technology: Achievements and perspectives. Postep. Hig. Med. Dosw. 2020, 74, 322–330. [Google Scholar] [CrossRef]
- Olakowska, E.; Właszczuk, A.; Turek, A.; Borecka, A.; Liśkiewicz, A.; Wawro, D.; Kasperczyk, J.; Jędrzejowska-Szypułka, H. Effects of 17-β-estradiol released from shape-memory terpolymer rods on sciatic nerve regeneration after injury and repair with chitosan nerve conduit in female rats. J. Appl. Biomed. 2022, 20, 87–97. [Google Scholar] [CrossRef]
- Turek, A.; Cwalina, B.; Kobielarz, M. Radioisotopic investigation of crosslinking density in bovine pericardium used as a biomaterial. Nukleonika 2013, 58, 511–517. [Google Scholar]
- Wilińska, J.; Turek, A.; Rech, J.; Janeczek, H.; Pastusiak, M.; Kordyka, A.; Borecka, A.; Kobielarz, M.; Kasperczyk, J. Hot Melt Extrusion as a Formulation Method of Terpolymer Rods with Aripiprazole: A Preliminary Study. Appl. Sci. 2023, 13, 9521. [Google Scholar] [CrossRef]
- Klein-Júnior, L.C.; Campos, A.; Niero, R.; Corrêa, R.; Vander Heyden, Y.; Filho, V.C. Xanthones and Cancer: From Natural Sources to Mechanisms of Action. Chem. Biodivers. 2020, 17, e1900499. [Google Scholar] [CrossRef]
- Szkaradek, N.; Sypniewski, D.; Waszkielewicz, A.M.; Gunia-Krzyżak, A.; Galilejczyk, A.; Gałka, S.; Marona, H.; Bednarek, I. Synthesis and in vitro Evaluation of the Anticancer Potential of New Aminoalkanol Derivatives of Xanthone. Anticancer Agents Med. Chem. 2016, 16, 1587–1604. [Google Scholar] [CrossRef]
- Mazur, G.; Pańczyk-Straszak, K.; Waszkielewicz, A.M. Xanthone Derivatives in the Fight against Glioblastoma and Other Cancers. Appl. Sci. 2023, 13, 2897. [Google Scholar] [CrossRef]
- Do, H.T.T.; Cho, J. Mangosteen Pericarp and Its Bioactive Xanthones: Potential Therapeutic Value in Alzheimer’s Disease, Parkinson’s Disease, and Depression with Pharmacokinetic and Safety Profiles. Int. J. Mol. Sci. 2020, 21, 6211. [Google Scholar] [CrossRef]
- Staszkiewicz, R.; Sobański, D.; Pulka, W.; Gładysz, D.; Gadzieliński, M.; Strojny, D.; Grabarek, B.O. Variances in the Expression Profile of Circadian Clock-Related Genes in Astrocytic Brain Tumors. Cancers 2024, 16, 2335. [Google Scholar] [CrossRef]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, W.P.; Da Conceição Braga, L.; Gonçales, N.G.; Silva, L.M.; Da Silva Filho, A.L. HSPA1A, HSPA1L and TRAP1 heat shock genes may be associated with prognosis in ovarian epithelial cancer. Oncol. Lett. 2020, 19, 359–367. [Google Scholar] [CrossRef]
- Rech, J.; Żelaszczyk, D.; Marona, H.; Gunia-Krzyżak, A.; Żmudzki, P.; Bednarek, I.A. Hyperthermia Intensifies α-Mangostin and Synthetic Xanthones’ Antimalignancy Properties. Int. J. Mol. Sci. 2024, 25, 8874. [Google Scholar] [CrossRef]
- Rech, J.; Rogóż, W.; Borecka, A.; Turek, A. Application of fibrin in tissue engineering. Achievements and perspectives. Postep. Hig. Med. Dosw. 2021, 75, 749–761. [Google Scholar] [CrossRef]
- Wang, F.; Ma, H.; Liu, Z.; Huang, W.; Xu, X.; Zhang, X. α-Mangostin inhibits DMBA/TPA-induced skin cancer through inhibiting inflammation and promoting autophagy and apoptosis by regulating PI3K/Akt/mTOR signaling pathway in mice. Biomed. Pharmacother. 2017, 92, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Akao, Y.; Kobayashi, E.; Ohguchi, K.; Ito, T.; Tanaka, T.; Iinuma, M.; Nozawa, Y. Induction of Apoptosis by Xanthones from Mangosteen in Human Leukemia Cell Lines. J. Nat. Prod. 2003, 66, 1124–1127. [Google Scholar] [CrossRef]
- Joshi, P.; Vishwakarma, R.A.; Bharate, S.B. Natural alkaloids as P-gp inhibitors for multidrug resistance reversal in cancer. Eur. J. Med. Chem. 2017, 138, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Sypniewski, D.; Szkaradek, N.; Loch, T.; Waszkielewicz, A.M.; Gunia-Krzyżak, A.; Matczyńska, D.; Sołtysik, D.; Marona, H.; Bednarek, I. Contribution of reactive oxygen species to the anticancer activity of aminoalkanol derivatives of xanthone. Investig. New Drugs 2018, 36, 355–369. [Google Scholar] [CrossRef]
- Fujii, J.; Homma, T.; Osaki, T. Superoxide Radicals in the Execution of Cell Death. Antioxidants 2022, 11, 501. [Google Scholar] [CrossRef]
- Cidade, H.; Rocha, V.; Palmeira, A.; Marques, C.; Tiritan, M.E.; Ferreira, H.; Lobo, J.S.; Almeida, I.F.; Sousa, M.E.; Pinto, M. In silico and in vitro antioxidant and cytotoxicity evaluation of oxygenated xanthone derivatives. Arab. J. Chem. 2020, 13, 17–26. [Google Scholar] [CrossRef]
- Gallorini, M.; Carradori, S.; Resende, D.I.S.P.; Saso, L.; Ricci, A.; Palmeira, A.; Cataldi, A.; Pinto, M.; Sousa, E. Natural and Synthetic Xanthone Derivatives Counteract Oxidative Stress via Nrf2 Modulation in Inflamed Human Macrophages. Int. J. Mol. Sci. 2022, 23, 13319. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Q.; Peng, M.; Xu, J.; Guo, Y. Design, Synthesis, Biological Evaluation, and Preliminary Mechanistic Study of a Novel Mitochondrial-Targeted Xanthone. Molecules 2023, 28, 1016. [Google Scholar] [CrossRef] [PubMed]
- McFadyen, M.C.E.; Cruickshank, M.E.; Miller, I.D.; McLeod, H.L.; Melvin, W.T.; Haites, N.E.; Parkin, D.; Murray, G.I. Cytochrome P450 CYP1B1 over-expression in primary and metastatic ovarian cancer. Br. J. Cancer 2001, 85, 242–246. [Google Scholar] [CrossRef]
- Therachiyil, L.; Krishnankutty, R.; Ahmad, F.; Mateo, J.M.; Uddin, S.; Korashy, H.M. Aryl Hydrocarbon Receptor Promotes Cell Growth, Stemness Like Characteristics, and Metastasis in Human Ovarian Cancer via Activation of PI3K/Akt, β-Catenin, and Epithelial to Mesenchymal Transition Pathways. Int. J. Mol. Sci. 2022, 23, 6395. [Google Scholar] [CrossRef]
- Trousil, S.; Lee, P.; Edwards, R.J.; Maslen, L.; Lozan-Kuehne, J.P.; Ramaswami, R.; Aboagye, E.O.; Clarke, S.; Liddle, C.; Sharma, R. Altered cytochrome 2E1 and 3A P450-dependent drug metabolism in advanced ovarian cancer correlates to tumour-associated inflammation. Br. J. Pharmacol. 2019, 176, 3712–3722. [Google Scholar] [CrossRef]
- Režen, T.; Rozman, D.; Kovács, T.; Kovács, P.; Sipos, A.; Bai, P.; Mikó, E. The role of bile acids in carcinogenesis. Cell. Mol. Life Sci. 2022, 79, 243. [Google Scholar] [CrossRef]
- Nawa, A.; Tanino, T.; Luo, C.; Iwaki, M.; Kajiyama, H.; Shibata, K.; Yamamoto, E.; Ino, K.; Nishiyama, Y.; Kikkawa, F. Gene Directed Enzyme Prodrug Therapy for Ovarian Cancer: Could GDEPT Become a Promising Treatment Against Ovarian Cancer? Anticancer Agents Med. Chem. 2008, 8, 232–239. [Google Scholar] [CrossRef]
- Liu, J.; Xu, X.; Xie, P.; Yang, X.; Ye, Y.; Zhao, Y. A novel activatable fluorescent probe for revealing the dynamic of esterase and viscosity during ferroptosis and DILI. Sens. Actuators B Chem. 2023, 396, 134594. [Google Scholar] [CrossRef]
- Cicchillitti, L.; Penci, R.; Di Michele, M.; Filippetti, F.; Rotilio, D.; Donati, M.B.; Scambia, G.; Ferlini, C. Proteomic characterization of cytoskeletal and mitochondrial class III β-tubulin. Mol. Cancer Ther. 2008, 7, 2070–2079. [Google Scholar] [CrossRef]
- Kanakkanthara, A.; Miller, J.H. βIII-tubulin overexpression in cancer: Causes, consequences, and potential therapies. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188607. [Google Scholar] [CrossRef]
- Singh, R.R.; Reindl, K.M. Glutathione S-Transferases in Cancer. Antioxidants 2021, 10, 701. [Google Scholar] [CrossRef]
- Hoter, A.; Naim, H.Y. Heat shock proteins and ovarian cancer: Important roles and therapeutic opportunities. Cancers 2019, 11, 1389. [Google Scholar] [CrossRef] [PubMed]
- Sterrenberg, J.N.; Blatch, G.L.; Edkins, A.L. Human DNAJ in cancer and stem cells. Cancer Lett. 2011, 312, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Cyran, A.M.; Zhitkovich, A. Heat Shock Proteins and HSF1 in Cancer. Front. Oncol. 2022, 12, 860320. [Google Scholar] [CrossRef]
- Di Michele, M.; Marcone, S.; Cicchillitti, L.; Della Corte, A.; Ferlini, C.; Scambia, G.; Donati, M.B.; Rotilio, D. Glycoproteomics of paclitaxel resistance in human epithelial ovarian cancer cell lines: Towards the identification of putative biomarkers. J. Proteom. 2010, 73, 879–898. [Google Scholar] [CrossRef]
- Zhong, B.-X.; Shen, F.-M.; Chen, J.-K. The role of HSP40 in cancer: Recent advances. Histol. Histopathol. 2024, 39, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Ni, M.; Zhou, J.; Zhu, Z.; Xu, Q.; Yin, Z.; Wang, Y.; Zheng, Z.; Zhao, H. Shikonin and cisplatin synergistically overcome cisplatin resistance of ovarian cancer by inducing ferroptosis via upregulation of HMOX1 to promote Fe2+ accumulation. Phytomedicine 2023, 112, 154701. [Google Scholar] [CrossRef]
- Grabosch, S.; Bulatovic, M.; Zeng, F.; Ma, T.; Zhang, L.; Ross, M.; Brozick, J.; Fang, Y.S.; Tseng, G.; Kim, E.; et al. Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene 2019, 38, 2380–2393. [Google Scholar] [CrossRef]
- Kielbik, M.; Szulc-kielbik, I.; Klink, M. Calreticulin—Multifunctional chaperone in immunogenic cell death: Potential significance as a prognostic biomarker in ovarian cancer patients. Cells 2021, 10, 130. [Google Scholar] [CrossRef]
- Liu, W.; Lu, Y.; Yan, X.; Lu, Q.; Sun, Y.; Wan, X.; Li, Y.; Zhao, J.; Li, Y.; Jiang, G. Current understanding on the role of CCT3 in cancer research. Front. Oncol. 2022, 12, 961733. [Google Scholar] [CrossRef] [PubMed]
- Temiz, E.; Koyuncu, İ.; Sahin, E. CCT3 suppression prompts apoptotic machinery through oxidative stress and energy deprivation in breast and prostate cancers. Free Radic. Biol. Med. 2021, 165, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Xue, T.; Wang, J.; Chen, B.; Lei, Y.; Huang, Y.; Wang, H.; Xin, X. Roles of clusterin in progression, chemoresistance and metastasis of human ovarian cancer. Int. J. Cancer 2009, 125, 791–806. [Google Scholar] [CrossRef]
- Praharaj, P.P.; Patra, S.; Panigrahi, D.P.; Patra, S.K.; Bhutia, S.K. Clusterin as modulator of carcinogenesis: A potential avenue for targeted cancer therapy. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188500. [Google Scholar] [CrossRef] [PubMed]
- Kapper, C.; Oppelt, P.; Arbeithuber, B.; Gyunesh, A.A.; Vilusic, I.; Stelzl, P.; Rezk-Füreder, M. Targeting ferroptosis in ovarian cancer: Novel strategies to overcome chemotherapy resistance. Life Sci. 2024, 349, 122720. [Google Scholar] [CrossRef]
- Kalinina, E.V.; Hasan, A.A.S.; Tatarskiy, V.V.; Volodina, Y.L.; Petrova, A.S.; Novichkova, M.D.; Zhdanov, D.D.; Nurmuradov, N.K.; Chernov, N.N.; Shtil, A.A. Suppression of PI3K/Akt/mTOR Signaling Pathway and Antioxidant System and Reversal of Cancer Cells Resistance to Cisplatin under the Effect of Curcumin. Bull. Exp. Biol. Med. 2022, 173, 371–375. [Google Scholar] [CrossRef]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luís, A.; McCarthy, N.; Montibeller, L.; More, S.; et al. Endoplasmic reticulum stress signalling—From basic mechanisms to clinical applications. FEBS J. 2019, 286, 241–278. [Google Scholar] [CrossRef]
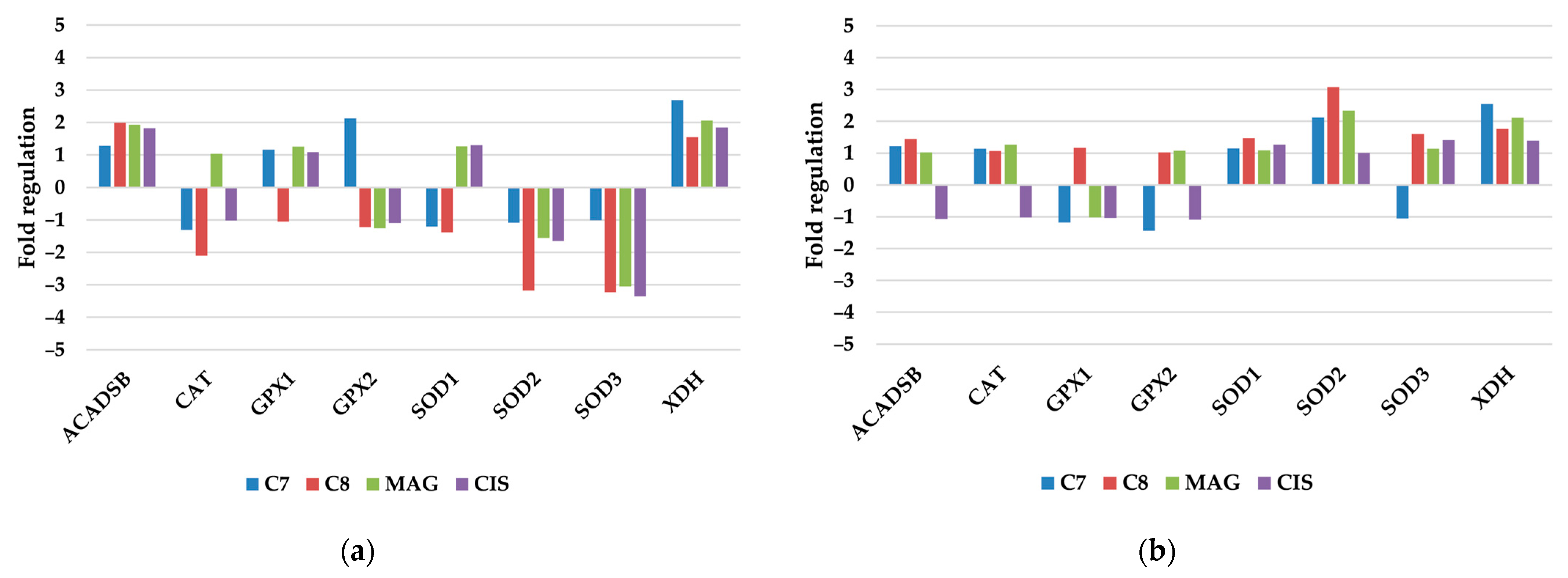
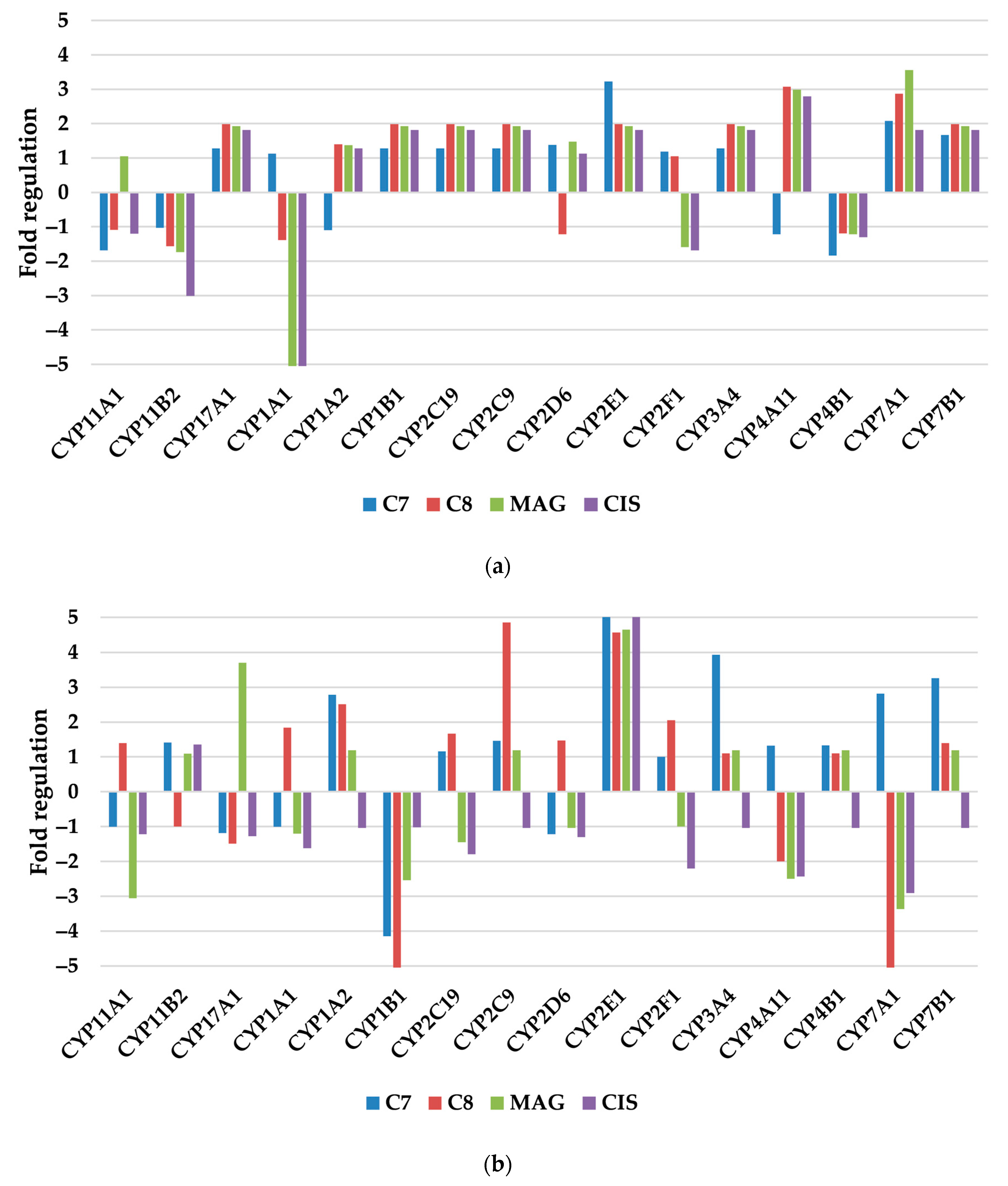
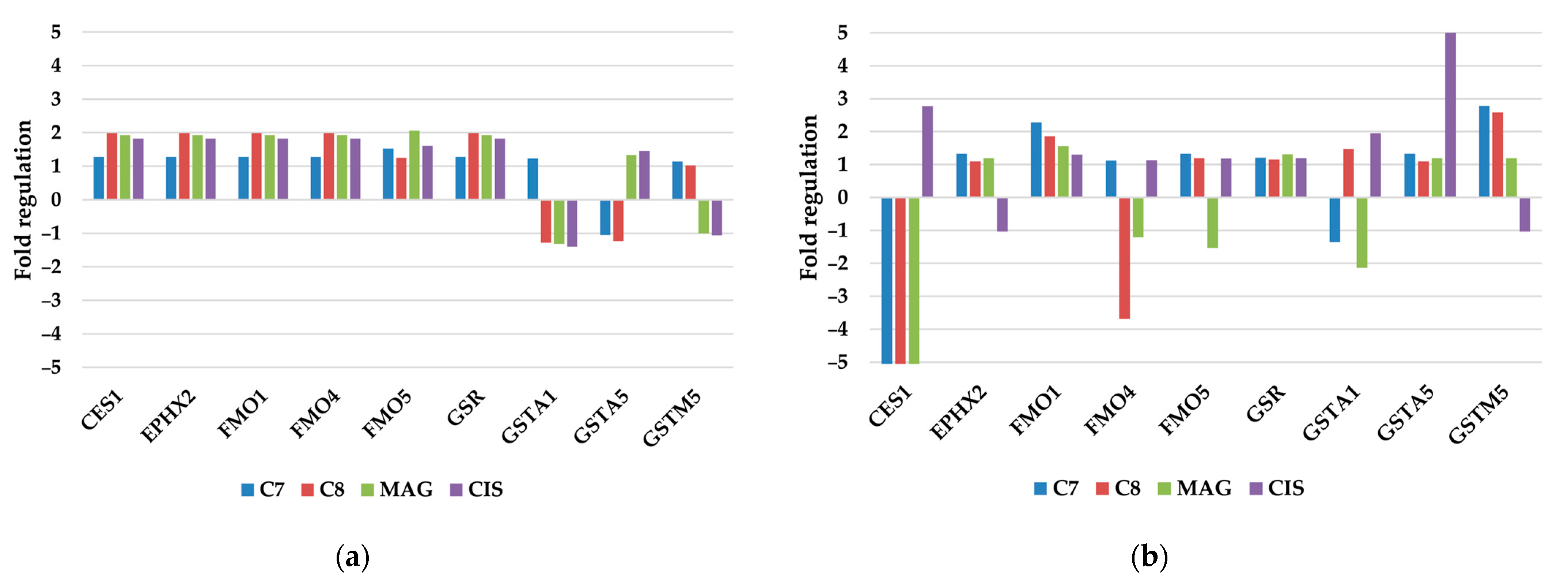
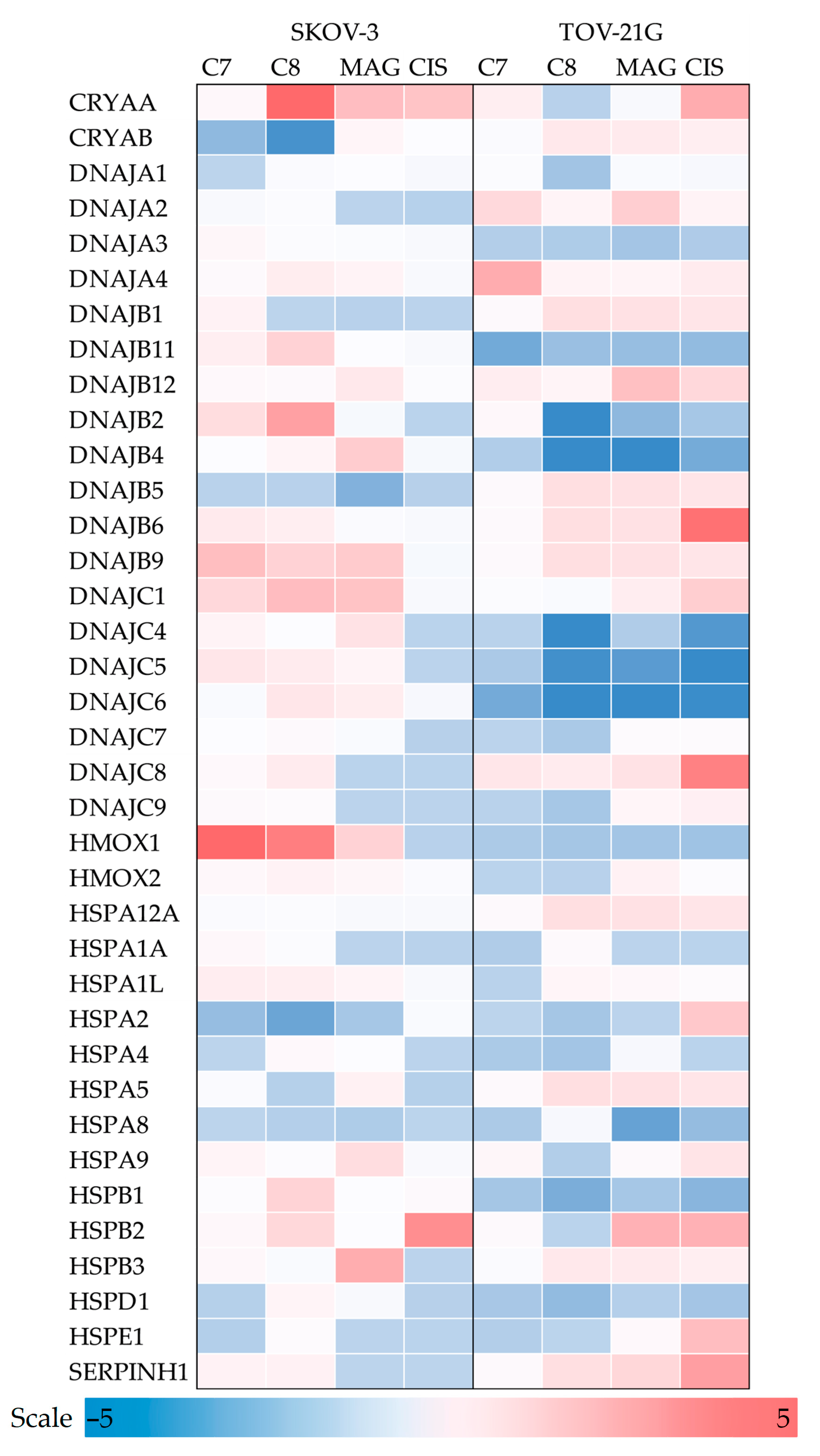
| Antioxidant and Pro-Oxidant Enzymes |
|---|
| ACADSB, CAT, GPX1, GPX2, SOD1, SOD2, SOD3, XDH |
| Molecular Chaperones |
| Heat Shock Proteins |
| CRYAA, CRYAB, DNAJA1, DNAJA2, DNAJA3, DNAJA4, DNAJB1, DNAJB11, DNAJB12, DNAJB2, DNAJB4, DNAJB5, DNAJB6, DNAJB9, DNAJC1, DNAJC4, DNAJC5, DNAJC6, DNAJC7, DNAJC8, DNAJC9, HMOX1, HMOX2, HSPA12A, HSPA1A (HSP70-1A), HSPA1L, HSPA2, HSPA4(HSP70), HSPA5 (GRP78), HSPA8, HSPA9, HSPB1 (HSP27), HSPB2, HSPB3, HSPD1, HSPE1, SERPINH1 (HSP47) |
| Other Molecular Chaperones BAG1, CALR, CANX, CCT2, CCT3, CCT4, CCT5, CCT7, CCT8, CLU, HOPX, PPID, ST13, TCP1 |
| Xenobiotic Metabolism |
| Cytochrome P450s |
| CYP11A1, CYP11B2, CYP17A1, CYP1A1, CYP1A2, CYP1B1, CYP2C19, CYP2C9, CYP2D6, CYP2E1, CYP2F1, CYP3A4, CYP4A11, CYP4B1, CYP7A1, CYP7B1 |
| Other Xenobiotic Metabolism Genes |
| CES1, EPHX2, FMO1, FMO4, FMO5, GSR, GSTA1, GSTA5 (YC2), GSTM5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rech, J.; Żelaszczyk, D.; Marona, H.; Bednarek, I.A. Identifying Cellular Stress-Related mRNA Changes Induced by Novel Xanthone Derivatives in Ovarian Cancer Cells In Vitro. Pharmaceutics 2025, 17, 816. https://doi.org/10.3390/pharmaceutics17070816
Rech J, Żelaszczyk D, Marona H, Bednarek IA. Identifying Cellular Stress-Related mRNA Changes Induced by Novel Xanthone Derivatives in Ovarian Cancer Cells In Vitro. Pharmaceutics. 2025; 17(7):816. https://doi.org/10.3390/pharmaceutics17070816
Chicago/Turabian StyleRech, Jakub, Dorota Żelaszczyk, Henryk Marona, and Ilona Anna Bednarek. 2025. "Identifying Cellular Stress-Related mRNA Changes Induced by Novel Xanthone Derivatives in Ovarian Cancer Cells In Vitro" Pharmaceutics 17, no. 7: 816. https://doi.org/10.3390/pharmaceutics17070816
APA StyleRech, J., Żelaszczyk, D., Marona, H., & Bednarek, I. A. (2025). Identifying Cellular Stress-Related mRNA Changes Induced by Novel Xanthone Derivatives in Ovarian Cancer Cells In Vitro. Pharmaceutics, 17(7), 816. https://doi.org/10.3390/pharmaceutics17070816






