Novel Lipid-Based Formulation to Enhance Coenzyme Q10 Bioavailability: Preclinical Assessment and Phase 1 Pharmacokinetic Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. RP-HPLC-DAD Analysis
- Column temperature: 25 °C;
- Flow rate: 0.3 mL/min;
- Injection volume: 20 µL;
- Detection wavelength: 275 nm;
- Mobile phase: 55% acetonitrile (ACN), 40% tetrahydrofuran (THF), 5% water;
- Elution mode: isocratic;
- Run time: 12 min;
- Limit of detection (LOD): 0.05 µg/mL;
- Limit of quantification (LOQ): 0.25 µg/mL.
2.3. Preparation of CoQ10-Loaded LiBADDS Powder
2.4. Simulated Digestion of CoQ10-Loaded LiBADDS Sachets
2.5. Particle Size Determination of CoQ10 Dispersed Phase Using DLS
2.6. Calculation of CoQ10 Bioaccessible Fraction
2.7. Cell Culture Materials
2.8. In Vitro Model of Intestine
2.9. Caco-2 Cell Culture
2.10. Evaluation of the Formulations Impact on Enteric Cell Viability
2.11. Integrity of Barrier Function in the Model of Enteric Epithelium
2.12. Evaluation of CoQ10 Permeation Through the Enteric Epithelium
2.13. Cellular Uptake
2.14. Bioavailability Assessment
- Inclusion criteria
- Age between 20 and 60 years;
- No medications taken in the last 15 days;
- Informed consent obtained.
- Exclusion criteria
- Participation in competitive sports;
- BMI > 25;
- Ongoing chronic inflammatory disease (chronic autoimmune diseases such as rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, psoriasis, lupus, and connective tissue diseases);
- Ongoing or recently diagnosed cardiovascular disease (congestive heart failure, recent myocardial infarction, thrombophlebitis);
- Ongoing oncological disease and related anticancer treatment;
- Intake of coenzyme Q10 supplements in the last 30 days;
- Diet rich in meat (pork, beef, chicken), oily fish (herring, sardines, shellfish, mollusks) in the two weeks prior to the trial.
2.14.1. Dietary Recommendations
- ✓
- Avoid foods rich in coenzyme Q10 for the two weeks prior to and during the trial including:
- ○
- Meat (pork, beef, chicken);
- ○
- Oily fish (such as herring, sardines, mackerel);
- ○
- Shellfish and mollusks.
- ✓
- Limit intake of foods high in antioxidants like the following:
- ○
- Berries (blueberries, strawberries, raspberries);
- ○
- Leafy green vegetables (spinach, kale);
- ○
- Nuts and seeds (walnuts, almonds).
- ✓
- Maintain a consistent diet to avoid fluctuations in bioavailability that could affect the study outcomes. Avoid introducing new foods or significant dietary changes during the study period.
- ✓
- Avoid dietary supplements or multivitamins containing coenzyme Q10 or other antioxidants for at least 30 days prior to the trial and throughout the duration of the study.
- ✓
- Stay hydrated by drinking adequate amounts of water daily, as dehydration could influence absorption and blood sampling results.
- ✓
- Limit alcohol intake to avoid interference with the metabolism and absorption of CoQ10.
- ✓
- Balanced meals: follow a balanced and regular eating schedule, with no extreme fasting or overeating, to maintain stable metabolic conditions during the study.
- ✓
- After 2 h from intake of CoQ10 test product, volunteers were allowed a light breakfast without fatty foods (butter, oil, peanuts, milk to avoid any interference with CoQ10 bioaccessibility).
2.14.2. Scheduled Visits
- T0 (Day 0): screening visit + delivery of dietary recommendations, randomization visit.
- T1 (Day 15): first administration of CoQ10 product.
- Venous blood sampling at t = 0 min, t = 60 min, t = 120 min, t = 240 min, t = 360 min, t = 720 min.
- T2 (Day 45): second administration of CoQ10 product.
- Venous blood sampling at t = 0 min, t = 60 min, t = 120 min, t = 240 min, t = 360 min, t = 720 min.
2.14.3. Data Collected
- Demographic data (T0);
- Medical and pharmacological history (T0);
- Weight/height/BMI.
2.14.4. Pharmacokinetic Parameters
2.14.5. Bioanalytical Method
2.14.6. Sample Preparation
- Protein precipitation
- Step 1: 500 µL of “precipitation reagent 1” was added to the sample containing IS, mixed by vortexing for 30 s.
- The samples were incubated at 4 °C in the dark for 10 min to facilitate protein precipitation, then centrifuged at 15,000× g for 5 min in a refrigerated centrifuge to obtain a solid pellet.
- Step 2: 100 µL of “precipitation reagent 2” was added to the same tubes, mixed by vortexing for 30 s, and centrifuged again at 15,000× g for 10 min in a refrigerated centrifuge.At that stage, a “clarified” supernatant free of plasma proteins was obtained.
- Solid-Phase Extraction (SPE)
- Columns, placed in glass collection tubes, were centrifuged at 700× g for 2 min to remove plasma contaminants and precipitation solvents (which were discarded), retaining ubiquinone within the solid-phase chromatographic matrix.
- This operation was repeated twice, using 500 µL of “washing buffer 1” and 160 µL of “washing buffer 2” to remove most matrix contaminants.
- Finally, columns were transferred to new collection tubes, and 250 µL of “elution solvent” was added to release ubiquinone from the chromatographic matrix, yielding the purified extract.
2.14.7. Chromatographic Separation and Data Processing
- The system was first decontaminated with isopropanol and water (both at 0.4 mL/min for 1 h).
- Then, the system was loaded with the mobile phase provided with the chromatographic kit for 30 min at 0.4 mL/min to remove traces of other solvents.
- The analytical column, equipped with a pre-column, was equilibrated with the mobile phase for 1 h at the working flow rate (2.5 mL/min) before starting the analysis.
- Flow rate: 2.5 mL/min, thermostat set at 30 °C.
- Run time: 14 min.
- Injection volume: 50 µL.
- Sampling needle wash solvent: isopropanol, as indicated in the kit.
- Ubiquinone chromatograms were acquired by monitoring UV absorbance at 275 nm, with IS and ubiquinone peaks observed at 7.5 and 12.5 min, respectively.Analytical results were processed using Empower 2 software (Waters, 102 Tide Mill Road, Hampton, NH, USA).
- Calibration was performed by repeatedly injecting the calibration standard until a consistent peak area was achieved, using a forced linear fitting through the origin of the axes. The quantification signal (“response”) was the ratio between the ubiquinone and IS peak areas.
- At each analytical session, the two quality controls provided with the kit were injected at the beginning and end of the session to verify analytical performance within EMA guideline acceptance ranges (maximum 15% deviation from nominal value for accuracy, and maximum 15% relative standard deviation for precision). These criteria were met in all sessions. All samples were analyzed in two independent analytical sessions, alternating samples from the first and second evaluations (blinded to the treatment received by the patients) to minimize the effects of minor intra-session analytical variations.
2.15. Statistical Analyses and Calculations
- A complete descriptive analysis of all parameters studied: mean, geometric mean, standard deviation, coefficient of variation, 95% confidence interval, normality test (Shapiro–Wilk);
- A power of 80% for n = 5 was considered sufficient according to the following equation:
3. Results and Discussion
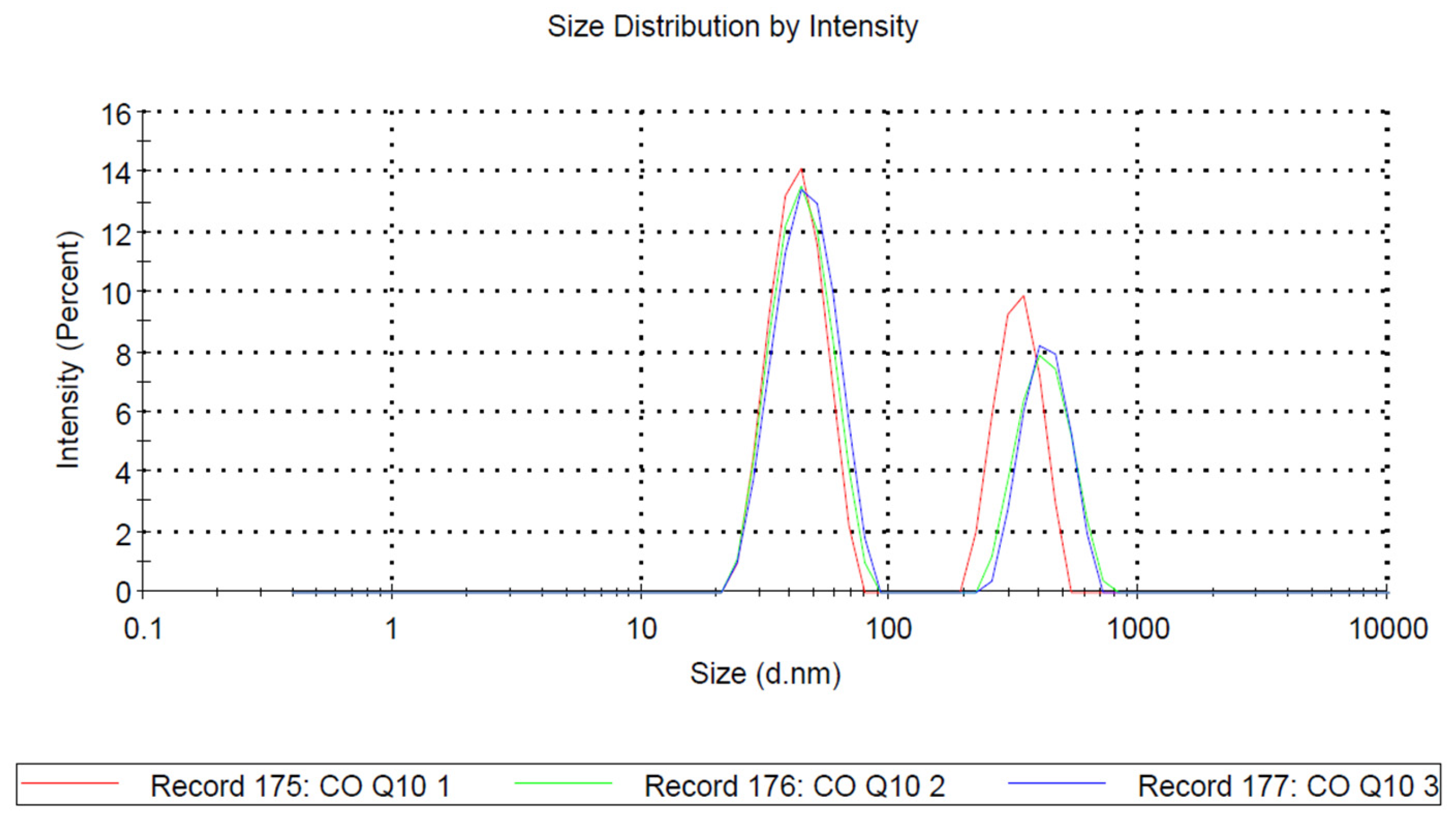
3.1. Dispersed Phase Size in FaSSIF by Dynamic Light Scattering (DLS)
3.2. Bioaccessibility of CoQ10 LiBADDS Dispersion in FaSSIF
3.3. Integrity of Barrier Function in the Model of Enteric Epithelium
3.4. CoQ10 Permeation
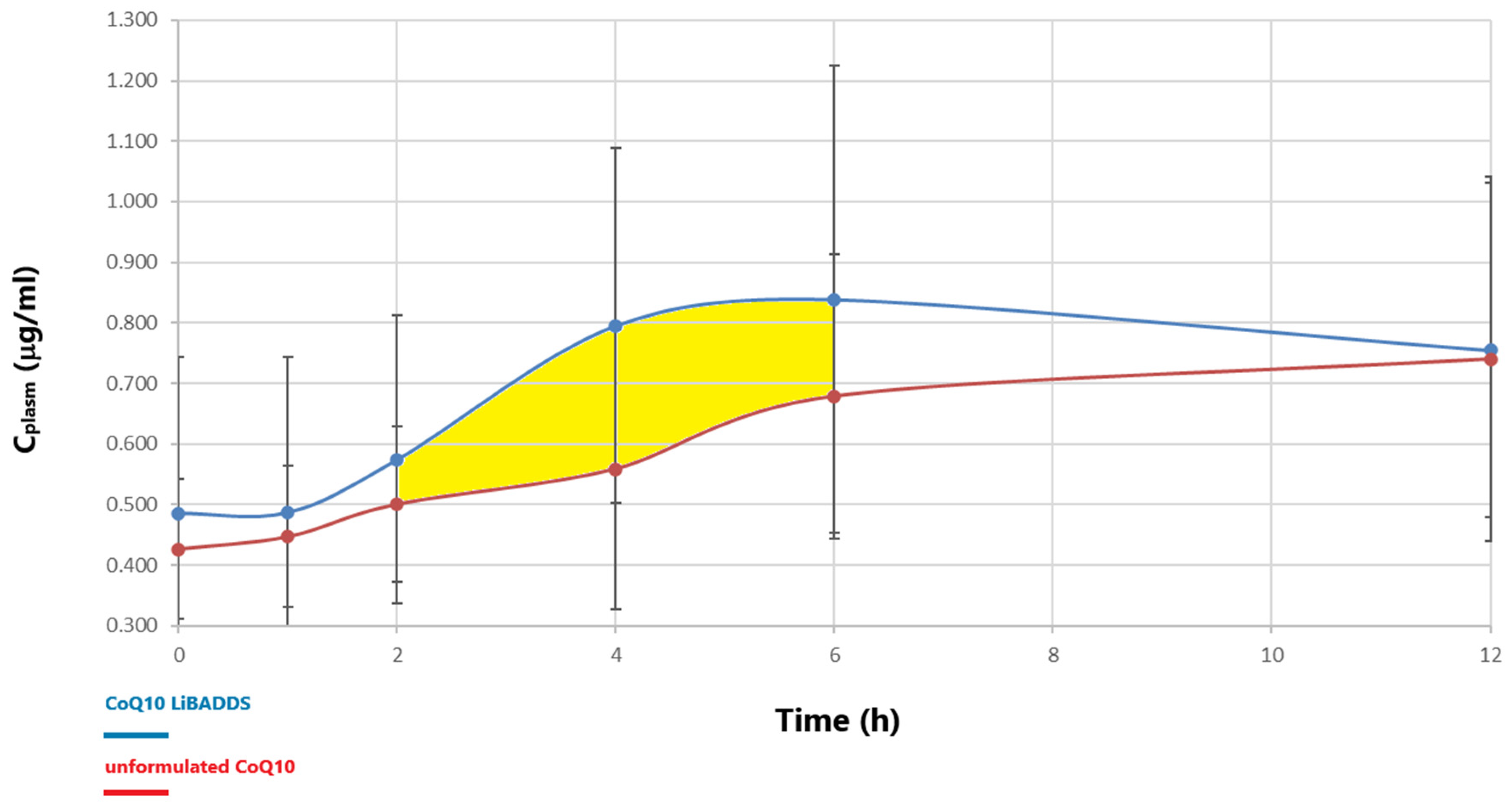
3.5. Bioavailability Assessment
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martelli, A.; Testai, L.; Colletti, A.; Cicero, A.F.G. Coenzyme Q10: Clinical Applications in Cardiovascular Diseases. Antioxidants 2020, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Onur, S.; Niklowitz, P.; Menke, T.; Laudes, M.; Rimbach, G.; Döring, F. Coenzyme Q10 Status as a Determinant of Muscular Strength in Two Independent Cohorts. PLoS ONE 2016, 11, e0167124. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.H.; Chang, P.S.; Chang, Y.H.; Lin, P.T. Identification of Coenzyme Q10 and Skeletal Muscle Protein Biomarkers as Potential Factors to Assist in the Diagnosis of Sarcopenia. Antioxidants 2022, 11, 725. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.G.; Lee, E.H.; Sail, R.; Denham, L.; Nagaraj, G.; Hsueh, C.T. Anthracycline-induced cardiotoxicity: A case report and review of literature. World J. Cardiol. 2021, 13, 28–37. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef]
- Kamphuis, J.A.M.; Linschoten, M.; Cramer, M.J.; Doevendans, P.A.; Asselbergs, F.W.; Teske, A.J. Early- and late anthracycline-induced cardiac dysfunction: Echocardiographic characterization and response to heart failure therapy. Cardiooncology 2020, 6, 23. [Google Scholar] [CrossRef]
- Osoro, I.; Sharma, A.; Amir, M.; Vohra, M.; Kumar, R.; Kumar, H.; Zargar, A.; Bangar, H. Prevention and management of anthracycline induced cardiotoxicity: A review. Health Sci. Rev. 2022, 5, 100070. [Google Scholar] [CrossRef]
- Ferrera, A.; Fiorentini, V.; Reale, S.; Solfanelli, G.; Tini, G.; Barbato, E.; Volpe, M.; Battistoni, A. Anthracyclines-induced cardiac dysfunction: What every clinician should know. Rev. Cardiovasc. Med. 2023, 24, 148. [Google Scholar] [CrossRef]
- Childs, A.C.; Phaneuf, S.L.; Dirks, A.J.; Phillips, T.; Leeuwenburgh, C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis as well as increased mitochondrial efficiency superoxide dismutase activity and Bcl-2 Bax ratio. Cancer Res. 2002, 62, 4592–4598. [Google Scholar]
- Wang, G.W.; Klein, J.B.; Kang, Y.J. Metallothionein inhibits doxorubicin-induced mitochondrial cytochrome c release and caspase-3 activation in cardiomyocytes. J. Pharmacol. Exp. Ther. 2001, 298, 461–468. [Google Scholar] [CrossRef]
- Wallace, K.B.; Sardão, V.A.; Oliveira, P.J. Mitochondrial determinants of doxorubicin-induced cardiomyopathy. Circ. Res. 2020, 126, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Vincent, D.T.; Ibrahim, Y.F.; Espey, M.G.; Suzuki, Y.J. The role of antioxidants in the era of cardio-oncology. Cancer Chemother. Pharmacol. 2013, 72, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Guo, M.; Chai, H.; Wang, W.T.; Gao, Z.Y.; Shi, D.Z. Effects of coenzyme Q10 on statin-induced myopathy: An updated meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2018, 7, e009835. [Google Scholar] [CrossRef]
- Kennedy, C.; Köller, Y.; Surkova, E. Effect of Coenzyme Q10 on statin-associated myalgia and adherence to statin therapy: A systematic review and meta-analysis. Atherosclerosis 2020, 299, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Serban, C.; Sahebkar, A.; Ursoniu, S.; Rysz, J.; Muntner, P.; Toth, P.P.; Jones, S.R.; Rizzo, M.; Glasser, S.P.; et al. Lipid and Blood Pressure Meta-analysis Collaboration Group. Effects of coenzyme Q10 on statin-induced myopathy: A meta-analysis of randomized controlled trials. Mayo Clin. Proc. 2015, 90, 24–34. [Google Scholar] [CrossRef]
- Wei, H.; Xin, X.; Zhang, J.; Xie, Q.; Naveed, M.; Kaiyan, C.; Xiao, P. Effects of coenzyme Q10 supplementation on statin-induced myopathy: A meta-analysis of randomized controlled trials. Ir. J. Med. Sci. 2022, 191, 719–725. [Google Scholar] [CrossRef]
- Banach, M.; Serban, C.; Ursoniu, S.; Rysz, J.; Muntner, P.; Toth, P.P.; Jones, S.R.; Rizzo, M.; Glasser, S.P.; Watts, G.F.; et al. Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group. Statin therapy and plasma coenzyme Q10 concentrations—A systematic review and meta-analysis of placebo-controlled trials. Pharmacol. Res. 2015, 99, 329–336. [Google Scholar] [CrossRef]
- Qu, H.; Meng, Y.Y.; Chai, H.; Liang, F.; Zhang, J.Y.; Gao, Z.Y.; Shi, D.Z. The effect of statin treatment on circulating coenzyme Q10 concentrations: An updated meta-analysis of randomized controlled trials. Eur. J. Med. Res. 2018, 23, 57. [Google Scholar] [CrossRef]
- Bhagavan, H.N.; Chopra, R.K. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic. Res. 2006, 40, 445–453. [Google Scholar] [CrossRef]
- Zaki, N.M. Strategies for oral delivery and mitochondrial targeting of CoQ10. Drug Deliv. 2016, 23, 1868–1881. [Google Scholar] [CrossRef]
- Bhagavan, H.N.; Chopra, R.K. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion 2007, 7, S78–S88. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Aurich, M.K.; Jonsson, J.J.; Thiele, I. Membrane transporters in a human genome-scale metabolic knowledgebase and their implications for disease. Front. Physiol. 2014, 5, 91. [Google Scholar]
- Takekawa, Y.; Sato, Y.; Yamaki, Y.; Imai, M.; Noto, K.; Sumi, M.; Takekuma, Y.; Iseki, K.; Sugawara, M. An approach to improve intestinal absorption of poorly absorbed water-insoluble components via Niemann–Pick C1-Like 1. Biol. Pharm. Bull. 2016, 39, 301–307. [Google Scholar] [PubMed]
- Bhagavan, H.N.; Chopra, R.K.; Craft, N.E.; Chitchumroonchokchai, C.; Failla, M.L. Assessment of coenzyme Q10 absorption using an in vitro digestion-Caco-2 cell model. Int. J. Pharm. 2007, 333, 112–117. [Google Scholar] [CrossRef]
- Liu, Z.X.; Artmann, C. Relative bioavailability comparison of different coenzyme Q10 formulations with a novel delivery system. Altern. Ther. Health Med. 2009, 15, 42–46. [Google Scholar] [PubMed]
- López-Lluch, G.; Del Pozo-Cruz, J.; Sánchez-Cuesta, A.; Cortés-Rodríguez, A.B.; Navas, P. Bioavailability of coenzyme Q10 supplements depends on carrier lipids and solubilization. Nutrition 2019, 57, 133–140. [Google Scholar] [CrossRef]
- Pravst, I.; Rodríguez Aguilera, J.C.; Cortes Rodriguez, A.B.; Jazbar, J.; Locatelli, I.; Hristov, H.; Žmitek, K. Comparative bioavailability of different coenzyme Q10 formulations in healthy elderly individuals. Nutrients 2020, 12, 784. [Google Scholar] [CrossRef]
- Beg, S.; Javed, S.; Kohli, K. Bioavailability enhancement of coenzyme Q10: An extensive review of patents. Recent Pat. Drug Deliv. Formul. 2010, 4, 245–255. [Google Scholar] [CrossRef]
- Petrangolini, G.; Ronchi, M.; Frattini, E.; De Combarieu, E.; Allegrini, P.; Riva, A. A new food-grade coenzyme Q10 formulation improves bioavailability: Single and repeated pharmacokinetic studies in healthy volunteers. Curr. Drug Deliv. 2019, 16, 759–767. [Google Scholar] [CrossRef]
- Niu, Z.; Acevedo-Fani, A.; McDowell, A.; Barnett, A.; Loveday, S.M.; Singh, H. Nanoemulsion structure and food matrix determine the gastrointestinal fate and in vivo bioavailability of coenzyme Q10. J. Control. Release 2020, 327, 444–455. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Lee, B.J.; Oh, D.H.; Kim, J.O.; Lee, Y.I.; Kim, D.D.; Jee, J.P.; Lee, Y.B.; Woo, J.S.; Yong, C.S.; et al. Enhanced oral bioavailability of coenzyme Q10 by self-emulsifying drug delivery systems. Int. J. Pharm. 2009, 374, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Xiao, C.; Morgantini, C.; Lewis, G.F. New insights into the regulation of chylomicron production. Annu. Rev. Nutr. 2015, 35, 265–294. [Google Scholar] [CrossRef] [PubMed]
- Geboers, S.; Stappaerts, J.; Tack, J.; Annaert, P.; Augustijns, P. In vitro and in vivo investigation of the gastrointestinal behavior of simvastatin. Int. J. Pharm. 2016, 510, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Rakusa, Z.T.; Kristl, A.; Roskar, R. Quantification of reduced and oxidized coenzyme Q10 in supplements and medicines by HPLC-UVAnal. Methods 2020, 12, 2580. [Google Scholar]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef]
- Tavelin, S.; Gråsjö, J.; Taipalensuu, J.; Ocklind, G.; Artursson, P. Applications of epithelial cell culture in studies of drug transport. Methods Mol. Biol. 2002, 188, 233–272. [Google Scholar] [CrossRef]
- Safaya, M.; Rotliwala, Y.C. Nanoemulsions: A review on low energy formulation methods, characterization, applications and optimization technique. Mater. Today Proc. 2020, 27, 454–459. [Google Scholar] [CrossRef]
- Peng, X.; Sun, Y.; Qi, W.; Su, R.; He, Z. Study of the interaction between coenzyme Q10 and human serum albumin: Spectroscopic approach. J. Solut. Chem. 2014, 43, 585–607. [Google Scholar] [CrossRef]
- Matsushita, N.; Oshima, T.; Takahashi, H.; Baba, Y. Enhanced water dispersibility of coenzyme Q10 by complexation with albumin hydrolysate. J. Agric. Food Chem. 2013, 61, 5972–5978. [Google Scholar] [CrossRef]
- Guideline on the Investigation of Bioequivalence. 2010. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf (accessed on 8 February 2025).
- Zmitek, J.; Smidovnik, A.; Fir, M.; Prosek, M.; Zmitek, K.; Walczak, J.; Pravst, I. Relative bioavailability of two forms of a novel water-soluble coenzyme Q10. Ann. Nutr. Metab. 2008, 52, 281–287. [Google Scholar] [CrossRef]
- Maciejewska-Stupska, K.; Czarnecka, K.; Szymański, P. Bioavailability enhancement of coenzyme Q10: An update of novel approaches. Arch. Pharm. 2024, 357, e2300676. [Google Scholar] [CrossRef]

| CoQ10 LiBADDS 028 1 Sachet | CoQ10 LiBADDS 027 1 Sachet | |
|---|---|---|
| CoQ10 (g) | 0.0200 | 0.020 |
| Labrafac lipophile WL 1349 (MCT) (g) | 0.0400 | 0.040 |
| Cleargum CO 001 (SOS) | 0.0500 | 0.0500 |
| Veremul T 80 (PS 80) g | 0.1200 | 0.1200 |
| Glucidex 19 D (g) | Up to 5.0 g | Up to 5.0 g |
| Emul AC (g) | 0.5000 | 0 |
| Tonalin A 80 (CLA) (g) | 0.0020 | 0.0020 |
| Tocopherol acetate 66% (g) | 0.0025 | 0.0025 |
| Amorphous silica (g) | 0.3500 | 0.3500 |
| Total (g) | 5.0000 | 5.0000 |
| Size (d.nm) | % Intensity | St Dev (d.nm) | ζ Potential | ||
|---|---|---|---|---|---|
| Z-Average (d.nm): 128.4 | Peak 1: | 43.24 | 62.7 | 10.15 | −18.24 Mv+/−3.67 |
| PDI: 0.373 | Peak 2: | 329.8 | 37.3 | 65.35 | |
| Intercept: 0.964 | Peak 3: | 0.000 | 0.0 | 0.000 |
| CoQ10 Unform. | CoQ10 LiBADDS 021 (Sachet) | CoQ10 LiBADDS 022 (Sachet) | CoQ10 LiBADDS 023 (Sachet) | CoQ10 LiBADDS 024 (Sachet) | CoQ10 LiBADDS 025 (Sachet) | CoQ10 LiBADDS 026 (Sachet) | CoQ10 LiBADDS 027 (Sachet) | CoQ10 LiBADDS 028 (Sachet) | |
|---|---|---|---|---|---|---|---|---|---|
| CoQ10 (theoretical) (g) | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 |
| CoQ10 (measured) (g) | 0.019 +/− 0.0022 | 0.019 +/− 0.0026 | 0.019 +/− 0.0026 | 0.019 +/− 0.0036 | 0.019 +/− 0.0034 | 0.019 +/− 0.0020 | 0.019 +/− 0.0029 | 0.019 +/− 0.0034 | 0.019 +/− 0.0030 |
| oxCoQ10 | 0.0078 +/− 0.0005 | 0.0076 +/− 0.0006 | 0.0077 +/− 0.0006 | 0.0078 +/− 0.0004 | 0.0078 +/− 0.0005 | 0.0079 +/− 0.0004 | 0.0078 +/− 0.0004 | 0.0080 +/− 0.0006 | 0.0074 +/− 0.0003 |
| rCoQ10 | 0.011 +/− 0.0003 | 0.011 +/− 0.0004 | 0.012 +/− 0.0005 | 0.010 +/− 0.0004 | 0.012 +/− 0.0003 | 0.011 +/− 0.0004 | 0.012 +/− 0.0002 | 0.012 +/− 0.0003 | 0.011 +/− 0.0006 |
| BF (%) | 13.0 +/− 1.10 | 17.6 +/− 1.42 | 19.8 +/− 2.41 | 20.4 +/− 3.41 | 73.8 +/− 8.68 | 96.5 +/− 8.48 | 96.4 +/− 10.31 | 92.8 +/− 9.11 | 76.8 +/− 8.88 |
| Ratio PS80/CoQ10 | - | 6 | 8 | 10 | 8 | 4 | 2 | 1 | 0 |
| NaC | - | 0.100 | 0.100 | 0.100 | - | - | - | - | - |
| Labrafac Lipophile WL 1379 (MCT) g | - | - | - | - | 0.020 | 0.040 | 0.040 | 0.040 | 0.040 |
| Cleargum CR 01 (SOS) | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | |
| Tonalin A 80 (CLA) g | - | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 |
| Industrial machinability |  |  |  |  |  |  |  |  |  |
CoQ10 LiBADDS 027 (Ap  Bl) Bl)C = 0.030 mM | CoQ10 LiBADDS 027 (Bl  Ap) Ap)C = 0.030 mM |
| Papp Not detectable C CoQ10 intracellular 24.3 +/− 2.6 nmoles/mg proteins %internalization (IR) (A-B) (3 h) 39.2 +/− 5.3 BF (%) 93.8 +/− 10.2 Mass balance 83.7 +/− 10.5 | Papp Not detectable C CoQ10 intracellular 36.6 +/− 3.1 nmoles/mg proteins %internalization (IR) (B-A) (3 h) 51.2 +/− 8.6 Mass balance 78.3 +/− 12.9 |
Unformulated CoQ10 (Ap  Bl) Bl) C = 0.030 mM | Unformulated CoQ10 (Bl  Ap) Ap) C = 0.030 mM |
| Papp Not detectable C CoQ10 intracellular 14.4 +/− 2.8 nmoles/mg proteins %internalization (IR) (A-B) (3 h) 28.3 +/− 4.4 BF (%) 13.0 +/− 1.10 Mass balance 80.4 +/− 14.2 | Papp Not detectable C CoQ10 intracellular 20.9 +/− 3.9 nmoles/mg proteins %internalization (IR) (B-A) (3 h) 44.6 +/− 3.6 Mass balance 82.6 +/− 11.1 |
| Parameter (Mean) +/− SD | Cmax (mg) CI 95% | DCmax (mg) CI 95% | AUC0−12h (mg/L/h) Cl 95% | DAUC0−12h (mg/L/h) CI 95% | AUC2−6h (mg/L/h) Cl 95% | DAUC2−6h (mg/L/h) Cl95% | Tmax (h) Cl95% |
|---|---|---|---|---|---|---|---|
| CoQ10 LiBADDS Unform. CoQ10 | 1.08 +/− 0.15 | 0.33 +/− 0.18 | 10.2 +/− 4.2 | 4.38 +/− 0.16 | 4.12 +/− 1.55 | 1.15 +/− 0.17 | 6.8 +/− 3.03 |
| (0.93–1.23) | (0.15–0.51) | (6.08–14.3) | (4.22–4.54) | (2.60–5.64) | (0.98–1.32) | (3.83–9.77) | |
| 0.85 +/− 0.09 | 0.29 +/− 0.06 | 7.9 +/− 2.1 | 3.34 +/− 0.55 | 2.86 +/− 0.62 | 0.56 +/− 0.35 | 7.6 +/− 4.34 | |
| (0.76–0.94) | (0.23–0.35) | (5.84–9.96) | (2.80–3.88) | (2.25–3.45) | (0.22–0.90) | (3.35–11.85) | |
| Ratio for mean % (CoQ10 LiBADDS)/(Unform.CoQ10) | 114 | 131 | 205 | ||||
| Ratio for mean % Log geometric mean CI 90% 80–125% (FDA/EMEA guidelines) (CoQ10 LiBADDS)/(Unform. CoQ10) | 126 | 126 | 141 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fratter, A.; Colletti, A.; Cravotto, G.; Pellizzato, M.; Papetti, A.; Pellicorio, V.; Bolego, C.; Simiele, M.; D’Avolio, A.; Cignarella, A. Novel Lipid-Based Formulation to Enhance Coenzyme Q10 Bioavailability: Preclinical Assessment and Phase 1 Pharmacokinetic Trial. Pharmaceutics 2025, 17, 414. https://doi.org/10.3390/pharmaceutics17040414
Fratter A, Colletti A, Cravotto G, Pellizzato M, Papetti A, Pellicorio V, Bolego C, Simiele M, D’Avolio A, Cignarella A. Novel Lipid-Based Formulation to Enhance Coenzyme Q10 Bioavailability: Preclinical Assessment and Phase 1 Pharmacokinetic Trial. Pharmaceutics. 2025; 17(4):414. https://doi.org/10.3390/pharmaceutics17040414
Chicago/Turabian StyleFratter, Andrea, Alessandro Colletti, Giancarlo Cravotto, Marzia Pellizzato, Adele Papetti, Vanessa Pellicorio, Chiara Bolego, Marco Simiele, Antonio D’Avolio, and Andrea Cignarella. 2025. "Novel Lipid-Based Formulation to Enhance Coenzyme Q10 Bioavailability: Preclinical Assessment and Phase 1 Pharmacokinetic Trial" Pharmaceutics 17, no. 4: 414. https://doi.org/10.3390/pharmaceutics17040414
APA StyleFratter, A., Colletti, A., Cravotto, G., Pellizzato, M., Papetti, A., Pellicorio, V., Bolego, C., Simiele, M., D’Avolio, A., & Cignarella, A. (2025). Novel Lipid-Based Formulation to Enhance Coenzyme Q10 Bioavailability: Preclinical Assessment and Phase 1 Pharmacokinetic Trial. Pharmaceutics, 17(4), 414. https://doi.org/10.3390/pharmaceutics17040414










