Solidago canadensis L. Herb Extract, Its Amino Acids Preparations and 3D-Printed Dosage Forms: Phytochemical, Technological, Molecular Docking and Pharmacological Research
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Assay of Main Phytochemicals by Spectrophotometry
2.3. Analysis of Phenolic Compounds by UPLC-MS/MS
2.4. Assay of Amino Acids by UPLC-MS/MS
2.5. Molecular Docking Analysis
2.6. Pharmacological Research
2.6.1. Acute Toxicity of the Extracts
2.6.2. Antimicrobial and Antifungal Activity of the Extracts
2.6.3. Anti-Inflammatory Activity of the Extracts
2.6.4. Hepatoprotective Activity of the Extracts
2.7. Three-Dimensional (3D) Printing of S. canadensis Extracts
2.8. Statistical Analysis
3. Results
3.1. Phytochemical Research
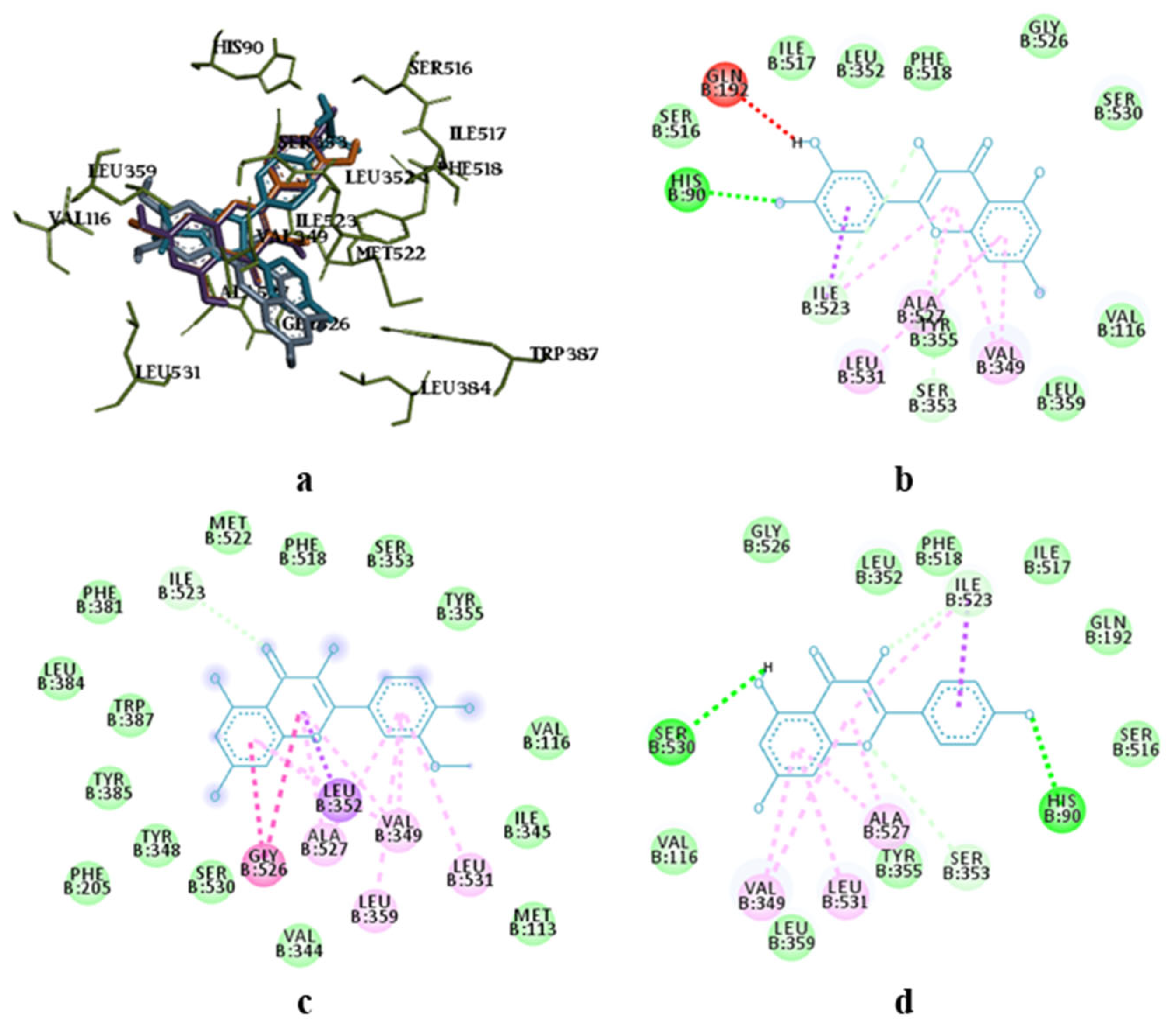
3.2. Molecular Docking Research
3.3. Acute Toxicity Study
3.4. Antimicrobial and Antifungal Activity
3.5. Anti-Inflammatory Activity
3.6. Hepatoprotective Activity
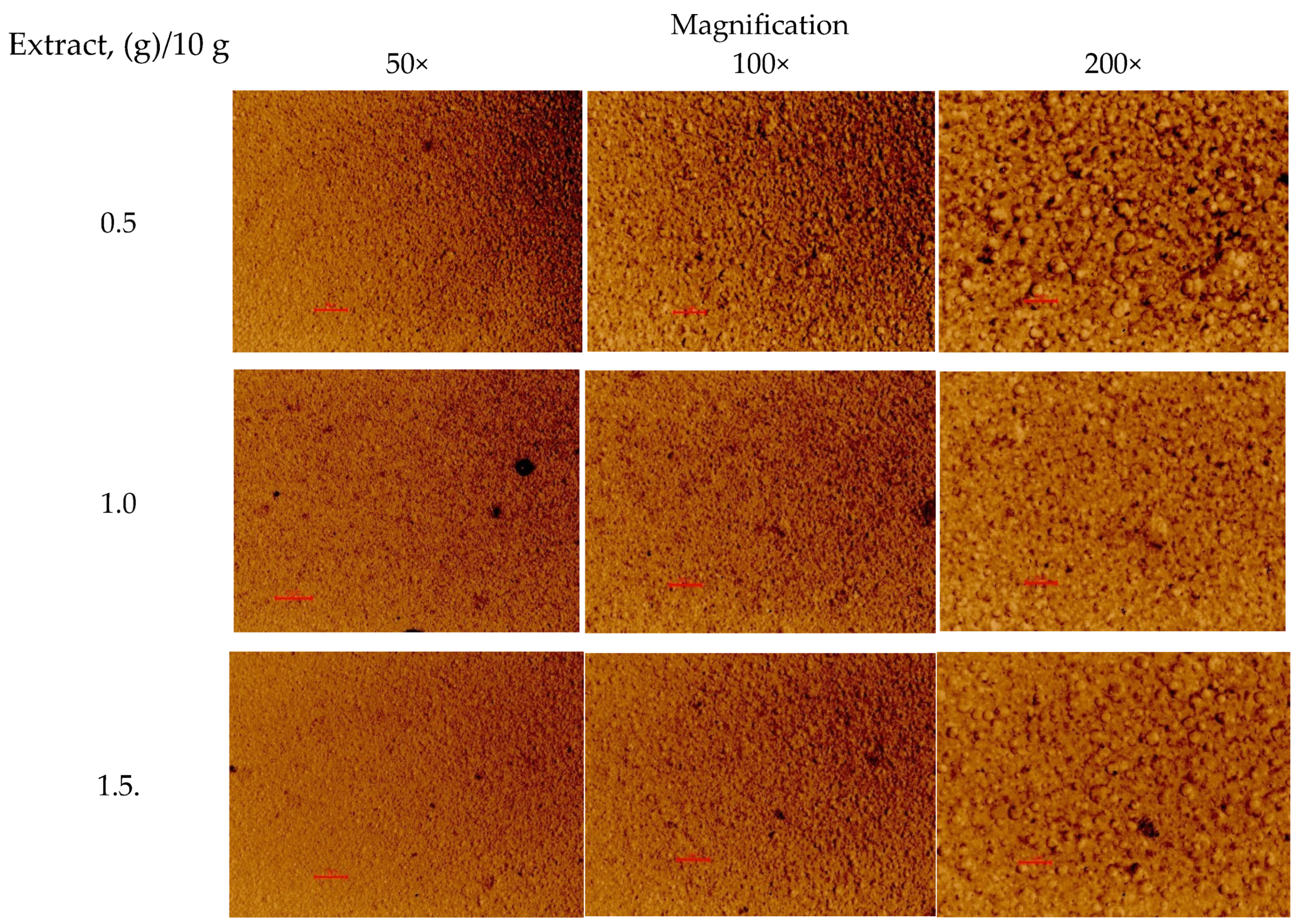
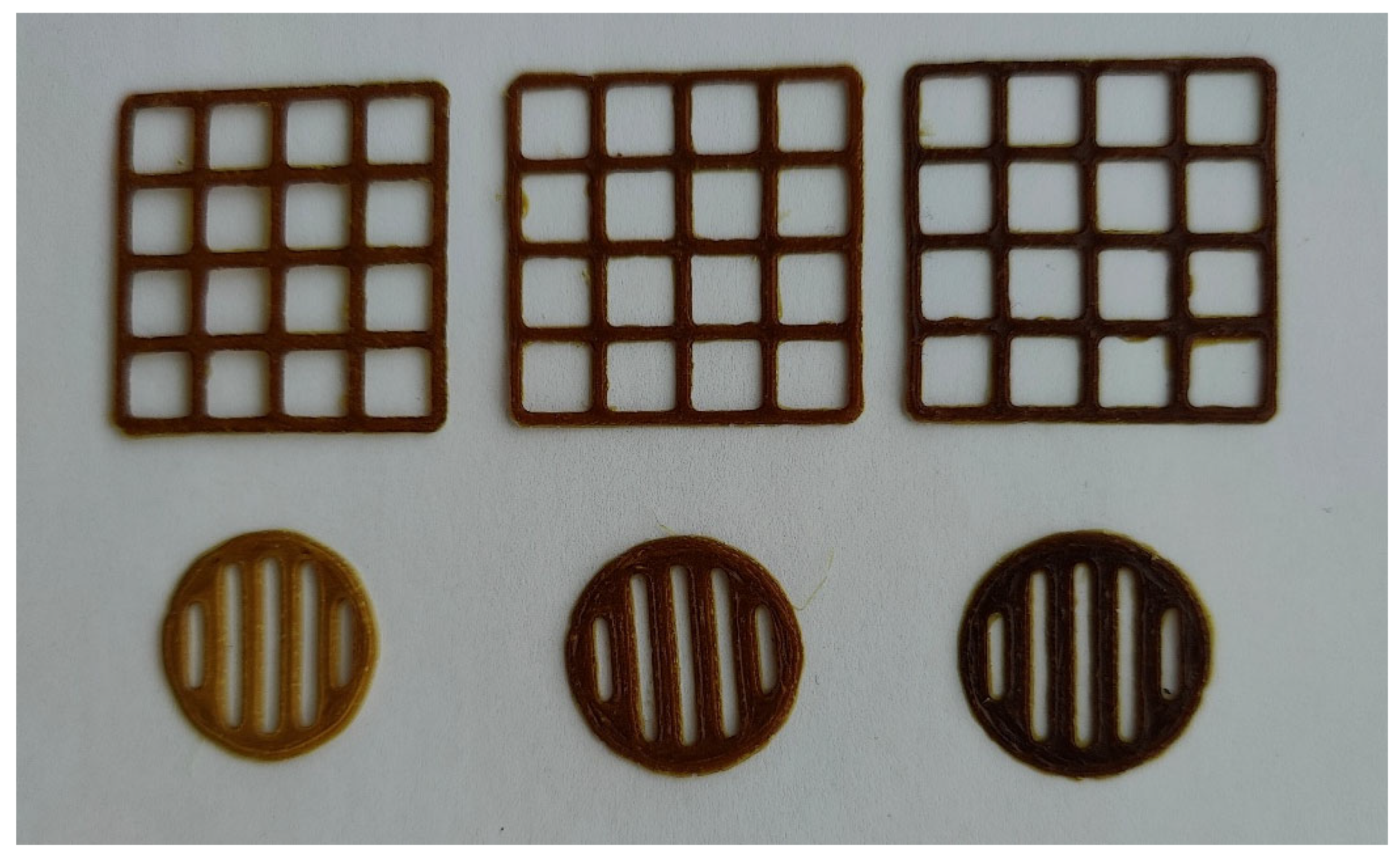
3.7. Novel 3D-Printed Oral Dosage Forms for S. canadensis Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pal, R.W.; Chen, S.; Nagy, D.U.; Callaway, R.M. Impacts of Solidago Gigantea on Other Species at Home and Away. Biol. Invasions 2015, 17, 3317–3325. [Google Scholar] [CrossRef]
- Poljuha, D.; Sladonja, B.; Uzelac Božac, M.; Šola, I.; Damijanić, D.; Weber, T. The Invasive Alien Plant Solidago canadensis: Phytochemical Composition, Ecosystem Service Potential, and Application in Bioeconomy. Plants 2024, 13, 1745. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Gruľová, D.; Baranová, B.; Caputo, L.; De Martino, L.; Sedlák, V.; Camele, I.; De Feo, V. Antimicrobial Activity and Chemical Composition of Essential Oil Extracted from Solidago canadensis L. Growing Wild in Slovakia. Molecules 2019, 24, 1206. [Google Scholar] [CrossRef] [PubMed]
- Shelepova, O.; Vinogradova, Y.; Vergun, O.; Grygorieva, O.; Brindza, J. Assessment of Flavonoids and Phenolic Compound Accumulation in Invasive Solidago canadensis L. in Slovakia. Potravin. Slovak J. Food Sci. 2020, 14, 587–594. [Google Scholar] [CrossRef]
- Shelepova, O.; Vinogradova, Y.; Zaitchik, B.; Ruzhitsky, A.; Grygorieva, O.; Brindza, J. Constituents of the Essential Oil in Solidago canadensis L. from Eurasia. Potravin. Slovak J. Food Sci. 2018, 12, 20–25. [Google Scholar] [CrossRef]
- Valverde, S.S.; Santos, B.C.S.; De Oliveira, T.B.; Gonçalves, G.C.; De Sousa, O.V. Solidagenone from Solidago chilensis Meyen Inhibits Skin Inflammation in Experimental Models. Basic Clin. Pharmacol. Toxicol. 2021, 128, 91–102. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y.; Padilla-Zakour, O.; Yang, G. Polyphenols, Antioxidant and Antimicrobial Activities of Leaf and Bark Extracts of Solidago canadensis L. Ind. Crops Prod. 2015, 74, 803–809. [Google Scholar] [CrossRef]
- Apáti, P.; Szentmihályi, K.; Kristó, S.T.; Papp, I.; Vinkler, P.; Szoke, É.; Kéry, Á. Herbal Remedies of Solidago—Correlation of Phytochemical Characteristics and Antioxidative Properties. J. Pharm. Biomed. Anal. 2003, 32, 1045–1053. [Google Scholar] [CrossRef]
- Nkuimi Wandjou, J.G.; Quassinti, L.; Gudžinskas, Z.; Nagy, D.U.; Cianfaglione, K.; Bramucci, M.; Maggi, F. Chemical Composition and Antiproliferative Effect of Essential Oils of Four Solidago Species (S. canadensis, S. gigantea, S. virgaurea and S.×Niederederi). Chem. Biodivers. 2020, 17, e2000685. [Google Scholar] [CrossRef]
- Kraujalienė, V.; Pukalskas, A.; Venskutonis, P.R. Biorefining of Goldenrod (Solidago virgaurea L.) Leaves by Supercritical Fluid and Pressurized Liquid Extraction and Evaluation of Antioxidant Properties and Main Phytochemicals in the Fractions and Plant Material. J. Funct. Foods 2017, 37, 200–208. [Google Scholar] [CrossRef]
- Huang, B.; Lei, Y.; Qin, L.; Liu, J. Chemical Composition and Cytotoxic Activities of the Essential Oil from the Inflorescences of Solidago canadensis L., an Invasive Weed in Southeastern China. J. Essent. Oil Bear. Plants 2012, 15, 667–671. [Google Scholar] [CrossRef]
- Mishra, D.; Joshi, S.; Bisht, G.; Pilkhwal, S. Chemical Composition and Antimicrobial Activity of Solidago canadensis Linn. Root Essential Oil. J. Basic Clin. Pharm. 2010, 1, 187–190. [Google Scholar]
- El-Sherei, M.; Khaleel, A.; Motaal, A.A.; Abd-Elbaki, P. Effect of Seasonal Variation on the Composition of the Essential Oil of Solidago canadensis Cultivated in Egypt. J. Essent. Oil Bear. Plants 2014, 17, 891–898. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2022. [Google Scholar]
- Woźniak, D.; Ślusarczyk, S.; Domaradzki, K.; Dryś, A.; Matkowski, A. Comparison of Polyphenol Profile and Antimutagenic and Antioxidant Activities in Two Species Used as Source of Solidaginis Herba-Goldenrod. Chem. Biodivers. 2018, 15, e1800023. [Google Scholar] [CrossRef]
- Dobjanschi, L.; Păltinean, R.; Vlase, L.; Babotă, M.; Fritea, L.; Tămaş, M. Comparative Phytochemical Research of Solidago Genus: S. graminifolia. Note I. Flavonoids. Acta Biol. Marisiensis 2018, 1, 18–26. [Google Scholar] [CrossRef]
- Dobjanschi, L.; Fritea, L.; Patay, E.B.; Tamas, M. Comparative Study of the Morphological and Phytochemical Characterization of Romanian Solidago Species. Pak. J. Pharm. Sci. 2019, 32, 1571–1579. [Google Scholar]
- Thiem, B.; Wesołowska, M.; Skrzypczak, L.; Budzianowski, J. Phenolic Compounds in Two Solidago L. Species from in Vitro Culture. Acta Pol. Pharm. 2001, 58, 277–281. [Google Scholar]
- Hrytsyk, Y.; Koshovyi, O.; Lepiku, M.; Jakštas, V.; Žvikas, V.; Matus, T.; Melnyk, M.; Grytsyk, L.; Raal, A. Phytochemical and Pharmacological Research in Galenic Remedies of Solidago canadensis L. Herb. Phyton 2024, 93, 2303–2315. [Google Scholar] [CrossRef]
- Fursenco, C.; Calalb, T.; Uncu, L.; Dinu, M.; Ancuceanu, R. Solidago virgaurea L.: A Review of Its Ethnomedicinal Uses, Phytochemistry, and Pharmacological Activities. Biomolecules 2020, 10, 1619. [Google Scholar] [CrossRef]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials–Process Perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef]
- El Aita, I.; Rahman, J.; Breitkreutz, J.; Quodbach, J. 3D-Printing with Precise Layer-Wise Dose Adjustments for Paediatric Use via Pressure-Assisted Microsyringe Printing. Eur. J. Pharm. Biopharm. 2020, 157, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, V.N. Compendium 2020. Medicines; MORION: Kyiv, Ukraine, 2020. [Google Scholar]
- Grodzinsky, A.M. Medicinal Plants: Encyclopedic Guide; Ukrainian Encyclopedia Named After M. P. Bazhana: Kyiv, Ukraine, 1990. [Google Scholar]
- Fedotova, V.V.; Chelombytko, V.A. Species of the Genus Solidago: Significance for Medical Practice, Study Prospects. Sci. Bull. 2012, 16, 136–145. [Google Scholar]
- Koshovyi, O.; Sepp, J.; Jakštas, V.; Žvikas, V.; Kireyev, I.; Karpun, Y.; Odyntsova, V.; Heinämäki, J.; Raal, A. German Chamomile (Matricaria chamomilla L.) Flower Extract, Its Amino Acid Preparations and 3D-Printed Dosage Forms: Phytochemical, Pharmacological, Technological, and Molecular Docking Study. Int. J. Mol. Sci. 2024, 25, 8292. [Google Scholar] [CrossRef]
- Zagayko, A.L.; Kolisnyk, T.Y.; Chumak, O.I.; Ruban, O.A.; Koshovyi, O.M. Evaluation of Anti-Obesity and Lipid-Lowering Properties of Vaccinium Myrtillus Leaves Powder Extract in a Hamster Model. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 697–703. [Google Scholar] [CrossRef]
- Kravchenko, G.; Krasilnikova, O.; Raal, A.; Mazen, M.; Chaika, N.; Kireyev, I.; Grytsyk, A.; Koshovyi, O. Arctostaphylos uva-ursi L. Leaves Extract and Its Modified Cysteine Preparation for the Management of Insulin Resistance: Chemical Analysis and Bioactivity. Nat. Prod. Bioprospect. 2022, 12, 30. [Google Scholar] [CrossRef]
- MacDougall, C. Pharmacokinetics of Valaciclovir. J. Antimicrob. Chemother. 2004, 53, 899–901. [Google Scholar] [CrossRef]
- Parfenov, V.A. Use of L-Lysine Aescinate in Central Nervous System Diseases. Neurol. Neuropsychiatry Psychosom. 2011, 3, 99. [Google Scholar] [CrossRef]
- Karpov, Y.A. Perindopril Arginine: A New ACE Inhibitor Salt Increases Therapeutic Potential. Cardiovasc. Ther. Prev. 2008, 7, 64–72. [Google Scholar]
- Koshovyi, O.; Raal, A.; Kireyev, I.; Tryshchuk, N.; Ilina, T.; Romanenko, Y.; Kovalenko, S.M.; Bunyatyan, N. Phytochemical and Psychotropic Research of Motherwort (Leonurus cardiaca L.) Modified Dry Extracts. Plants 2021, 10, 230. [Google Scholar] [CrossRef]
- Koshovyi, O.; Granica, S.; Piwowarski, J.P.; Stremoukhov, O.; Kostenko, Y.; Kravchenko, G.; Krasilnikova, O.; Zagayko, A. Highbush Blueberry (Vaccinium corymbosum L.) Leaves Extract and Its Modified Arginine Preparation for the Management of Metabolic Syndrome—Chemical Analysis and Bioactivity in Rat Model. Nutrients 2021, 13, 2870. [Google Scholar] [CrossRef]
- Koshovyi, O.; Vlasova, I.; Laur, H.; Kravchenko, G.; Krasilnikova, O.; Granica, S.; Piwowarski, J.P.; Heinämäki, J.; Raal, A. Chemical Composition and Insulin-Resistance Activity of Arginine-Loaded American Cranberry (Vaccinium Macrocarpon Aiton, Ericaceae) Leaf Extracts. Pharmaceutics 2023, 15, 2528. [Google Scholar] [CrossRef] [PubMed]
- Hrytsyk, Y.; Koshovyi, O.; Hrytsyk, R.; Raal, A. Extracts of the Canadian Goldenrod (Solidago canadensis L.)—Promising Agents with Antimicrobial, Anti-Inflammatory and Hepatoprotective Activity. Sci. Pharm. Sci. 2024, 4, 78–87. [Google Scholar] [CrossRef]
- Dobrochaeva, D.N.; Kotov, M.I.; Prokudin, Y.N.; Barbarich, A.I. Key to Higher Plants of Ukraine; Naukova Dumka: Kyiv, Ukraine, 1999. [Google Scholar]
- Vlasova, I.; Gontova, T.; Grytsyk, L.; Zhumashova, G.; Sayakova, G.; Boshkayeva, A.; Shanaida, M.; Koshovyi, O. Determination of Standardization Parameters of Oxycoccus macrocarpus (Ait.) Pursh and Oxycoccus Palustris Pers. Leaves. Sci. Pharm. Sci. 2022, 3, 48–57. [Google Scholar] [CrossRef]
- Kovaleva, A.M.; Georgievskyi, G.V.; Kovalev, V.M.; Komissarenko, A.M.; Timchenko, M.M. Development of the Method of Standardization of the New Medicinal Product Piflamin. Pharmacom 2002, 2, 92–97. [Google Scholar]
- Krivoruchko, E.; Markin, A.; Samoilova, V.A.; Ilina, T.; Koshovyi, O. Research in the Chemical Composition of the Bark of Sorbus Aucuparia. Ceska A Slov. Farm. 2018, 67, 113–115. [Google Scholar]
- Vilkickyte, G.; Raudone, L.; Petrikaite, V. Phenolic Fractions from Vaccinium vitis-idaea L. and Their Antioxidant and Anticancer Activities Assessment. Antioxidants 2020, 9, 1261. [Google Scholar] [CrossRef]
- Uminska, K.; Gudžinskas, Z.; Ivanauskas, L.; Georgiyants, V.; Kozurak, A.; Skibytska, M.; Mykhailenko, O. Amino Acid Profiling in Wild Chamaenerion Angustifolium Populations Applying Chemometric Analysis. J. Appl. Pharm. Sci. 2023, 13, 171–180. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.H.; Buijsman, M.N.C.P.; Van Amelsvoort, J.M.M.; Katan, M.B. Chlorogenic Acid, Quercetin-3-Rutinoside and Black Tea Phenols Are Extensively Metabolized in Humans. J. Nutr. 2003, 133, 1806–1814. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.-P.; Wang, Y.-Q.; Shi, L.; Zhang, Z.-K.; Dong, F.; Li, H.-R.; Zhang, J.-Y.; Man, Y.-Q. Comparative Metabolism Study on Chlorogenic Acid, Cryptochlorogenic Acid and Neochlorogenic Acid Using UHPLC-Q-TOF MS Coupled with Network Pharmacology. Chin. J. Nat. Med. 2021, 19, 212–224. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic Acids: Chemistry, Biosynthesis, Occurrence, Analytical Challenges, and Bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, Y.; Liu, Z.; Wang, Z.; Liu, Y. The Metabolite Profiling of 3,4-dicaffeoylquinic Acid in Sprague–Dawley Rats Using Ultra-high Performance Liquid Chromatography Equipped with Linear Ion trap-Orbitrap MS. Biomed. Chromatogr. 2022, 36, e5276. [Google Scholar] [CrossRef]
- Rimon, G.; Sidhu, R.S.; Lauver, D.A.; Lee, J.Y.; Sharma, N.P.; Yuan, C.; Frieler, R.A.; Trievel, R.C.; Lucchesi, B.R.; Smith, W.L. Coxibs Interfere with the Action of Aspirin by Binding Tightly to One Monomer of Cyclooxygenase-1. Proc. Natl. Acad. Sci. USA 2010, 107, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Yuan, C.; Orlando, B.J.; Malkowski, M.G.; Smith, W.L. Fatty Acid Binding to the Allosteric Subunit of Cyclooxygenase-2 Relieves a Tonic Inhibition of the Catalytic Subunit. J. Biol. Chem. 2016, 291, 25641–25655. [Google Scholar] [CrossRef]
- Stefanov, O.V. Preclinical Studies of Drugs; Avicenna: Kyiv, Ukraine, 2001. [Google Scholar]
- The Law of Ukraine “On the Protection of Animals from Cruel Treatment” Dated 12/15/2009. Available online: https://zakon.rada.gov.ua/laws/show/3447-15#Text (accessed on 8 September 2021).
- On Approval of the Procedure for Preclinical Study of Medicinal Products and Examination of Materials of Preclinical Study of Medicinal Products; EMA: Amsterdam, The Netherlands, 2009.
- Council of the European Union. European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes; Council of the European Union: Brussels, Belgium, 1999. [Google Scholar]
- Bergey, D.H. Bergey’s Manual of Determinative Bacteriology; Baltimore, Williams & Wilkins Co.: Baltimore, MD, USA, 1957. [Google Scholar]
- Kutsyk, R.V. Screening Study of the Antimicrobial Activity of Medicinal Plants of the Carpathian Region against Polyantibiotic-Resistant Clinical Strains of Staphylococci. Message 1. Galician Med. Bull. 2004, 11, 44–48. [Google Scholar]
- Hrytsyk, R.A.; Kutsyk, R.V.; Yurchyshyn, O.I.; Struk, O.A.; Kireev, I.V.; Grytsyk, A.R. The Investigation of Antimicrobial and Antifungal Activity of Some Artemisia L. Species. Pharmacia 2021, 68, 93–100. [Google Scholar] [CrossRef]
- Shanaida, M.; Oleschuk, O.; Lykhatskyi, P.; Kernychna, I. Study of the Hepatoprotective Activity of the Liquid Extract of the Garden Thyme Herb in Tetrachloromethane Hepatitis. Pharm. J. 2017, 42, 92–97. [Google Scholar]
- Korobeinikova, E.N. Modification of Determination of Lipid Peroxidation Products in the Reaction with Thiobarbituric Acid. Lab. Work 1989, 7, 8–10. [Google Scholar]
- Viidik, L.; Seera, D.; Antikainen, O.; Kogermann, K.; Heinämäki, J.; Laidmäe, I. 3D-Printability of Aqueous Poly(Ethylene Oxide) Gels. Eur. Polym. J. 2019, 120, 109206. [Google Scholar] [CrossRef]
- Koshovyi, O.; Heinämäki, J.; Raal, A.; Laidmäe, I.; Topelius, N.S.; Komisarenko, M.; Komissarenko, A. Pharmaceutical 3D-Printing of Nanoemulsified Eucalypt Extracts and Their Antimicrobial Activity. Eur. J. Pharm. Sci. 2023, 187, 106487. [Google Scholar] [CrossRef]
- Riegel, J.; Mayer, W.; Havre, Y.V. FreeCAD (Version 0.19.24291). 2001. Available online: http://www.freecad.org (accessed on 6 February 2024).
- Ukrainian Scientific Pharmacopoeial Center of Drugs Quality. State Pharmacopoeia of Ukraine, 2nd ed.; Ukrainian Scientific Pharmacopoeial Center of Drugs Quality: Kharkiv, Ukraine, 2015. [Google Scholar]
- Lapach, S.N.; Chubenko, A.V.; Babich, P.N. Statistical Methods in Biomedical Research Using Excel; MORION: Kyiv, Ukraine, 2000. [Google Scholar]
- Zhu, Q.; Han, Y.; He, Y.; Fu, Y.; Yang, H.; Chen, Y.; Shi, Y. Kaempferol Improves Breast Cancer-Related Depression through the COX-2/PGE2 Pathway. Front. Biosci. 2023, 28, 311. [Google Scholar] [CrossRef]
- Khoswanto, C. Molecular Docking Analysis of Quercetin and Diclofenac as Cox-2 Potential Inhibitors. J. Int. Dent. Med. Res. 2022, 15, 552–555. [Google Scholar]
- Bare, Y.; Krisnamurti, G.C.; Elizabeth, A.; Rachmad, Y.T.; Sari, D.R.T.; Wahyusari, M.R.; Lorenza, G. The Potential Role of Caffeic Acid in Coffee as Cyclooxygenase-2 (COX-2) Inhibitor: In Silico Study. Biointerface Res. Appl. Chem. 2019, 9, 4424–4427. [Google Scholar] [CrossRef]
- Mohalkar, R.; Poul, B.; Patil, S.S.; Hetkar, M.A.; Chavan, S.D. A Review on Immediate Release Drug Delivery Systems. PharmaTutor 2014, 2, 95–109. [Google Scholar]
- Kute, V.G.; Patil, R.S.; Kute, V.G.; Kaluse, P.D. Immediate-release dosage form; focus on disintegrants use as a promising excipient. J. Drug Deliv. Ther. 2023, 13, 170–180. [Google Scholar] [CrossRef]


| Compound | Content in the Dry Extract, mg/g | ||||||
|---|---|---|---|---|---|---|---|
| S [19] | S-Phe | S-Arg | S-Gly | S-β-Ala | S-Lys | S-Val | |
| Neochlorogenic acid | 0.86 ± 0.08 | 0.78 ± 0.01 | 0.78 ± 0.02 | 0.94 ± 0.02 | 0.87 ± 0.01 | 0.85 ± 0.03 | 0.84 ± 0.02 |
| Kaempferol-3-O-rutinoside | 0.21 ± 0.08 | 0.20 ± 0.01 | 0,21 ± 0.02 | 0.20 ± 0.02 | 0.20 ± 0.01 | 0.20 ± 0.03 | 0.22 ± 0.03 |
| Isoquercitrin | 8.07 ± 0.24 | 0.40 ± 0.03 | 0,42 ± 0.02 | 0.45 ± 0.01 | 0.46 ± 0.02 | 0.46 ± 0.02 | 0.43 ± 0,034 |
| Chlorogenic acid | 11.87 ± 0.42 | 14.34 ± 0.44 | 14.53 ± 0.17 | 16.54 ± 0.34 | 15.57 ± 1.42 | 15.62 ± 0.23 | 15.40 ± 0.41 |
| Quercetin | 8.43 ± 0.19 | 5.964 ± 0.29 | 6.83 ± 0.19 | 6.87 ± 0.08 | 6.50 ± 0.27 | 8.21 ± 0.22 | 6.85 ± 0.10 |
| Isorhamnetin-3-O-rutinoside | 2.98 ± 0.21 | 0.31 ± 0.03 | 0.32 ± 0.00 | 0.36 ± 0.01 | 0.31 ± 0.02 | 0.35 ± 0.01 | 0.36 ± 0.03 |
| p-Coumaric acid | 0.05 ± 0.01 | 0.04 ± 0,01 | 0.04 ± 0.00 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 |
| Ferulic acid | 0.05 ± 0.01 | 0.05 ± 0.01 | 0,04 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.01 | 0.04 ± 0.01 |
| Vanilic acid | 0.21 ± 0.03 | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.17 ± 0.01 | 0.17 ± 0.01 | 0.15 ± 0.01 | 0.15 ± 0.02 |
| Caffeic acid | 0.19 ± 0.01 | 0.22 ± 0.02 | 0.24 ± 0.01 | 0.26 ± 002 | 0.22 ± 0.01 | 0.27 ± 0.01 | 0.23 ± 0.01 |
| Kaempferol | 2.67 ± 0.35 | 2.14 ± 0.08 | 2.28 ± 0.04 | 2.27 ± 0.15 | 2.13 ± 0.18 | 2.71 ± 0.30 | 2.45 ± 0.11 |
| 3,4-Dihydroxy-phenylacetic acid | 1.71 ± 0.11 | 0.58 ± 0.01 | 0.62 ± 0.01 | 0.85 ± 0.02 | 0.71 ± 0.02 | 0.64 ± 0.03 | 0.60 ± 0.02 |
| Isorhamnetin | 1.08 ± 0.18 | 0.74 ± 0.01 | 0.80 ± 0.01 | 0.80 ± 0.01 | 0.82 ± 0.03 | 0.99 ± 0.02 | 0.81 ± 0.01 |
| Rutin | 28.23 ± 0.42 | 4.76 ± 0.06 | 5.02 ± 0.22 | 5.35 ± 0.10 | 4.85 ± 0.26 | 5.55 ± 0.20 | 5.33 ± 0.15 |
| Hyperoside | 0.42 ± 0.05 | 0.12 ± 0.00 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.12 ± 0.02 | 0.15 ± 0.01 | 0.13 ± 0.00 |
| 4.5-Dicaffeoylquinic acid | 3.06 ± 0.31 | 1.82 ± 0.14 | 1.88 ± 0.12 | 2.20 ± 0.22 | 2.33 ± 0.14 | 2.29 ± 0.17 | 2.26 ± 0.07 |
| 3.5-Dicaffeylquinic acid | 4.86 ± 0.27 | 3.67 ± 0.05 | 3.99 ± 0.42 | 4.24 ± 0.29 | 3.93 ± 0.14 | 4.71 ± 0.18 | 4.74 ± 0.18 |
| 3.4-Dicaffeylquinic acid | 25.42 ± 0.53 | 21.25 ± 0.04 | 24.23 ± 2.14 | 25.44 ± 2.39 | 21.78 ± 2.38 | 28.11 ±1.15 | 28.45 ± 2.53 |
| Hydroxycinnamic acids (chlorogenic acid equivalents, spectrophotometry), % | 5.34 ± 0.42 | 4.93 ± 0.36 | 2.73 ± 0.26 | 4.34 ± 0.17 | 5.07 ± 0.32 | 3.22 ± 0.29 | 4.42 ± 0.21 |
| Flavonoids (rutin equivalents, spectrophotometry), % | 9.68 ± 0.14 | 5.18 ± 0.36 | 6.07 ± 0.47 | 6.51 ± 0.13 | 7.20 ± 0.06 | 7.55 ± 0.11 | 6.72 ± 0.15 |
| Total phenolic compounds (gallic acid equivalents, spectrophotometry), % | 11.56 ± 0.28 | 7.61 ± 0.22 | 7.19 ± 0.50 | 7.28 ± 0.09 | 7.68 ± 0.24 | 7.34 ± 0.19 | 7.88 ± 0.37 |
| Compound | Content in the Dry Extract, mg/g | ||||||
|---|---|---|---|---|---|---|---|
| S [19] | S-Phe | S-Arg | S-Gly | S-β-Ala | S-Lys | S-Val | |
| Alanine | 2.09 ± 0.07 | 1.71 ± 0.13 | 1.61 ± 0.06 | 1.95 ± 0.09 | 1.89 ± 0.32 | 1.63 ± 0.19 | 2.02 ± 0.15 |
| Arginine | 1.72 ± 0.05 | 4.45 ± 0.31 | 91.67 ± 3.78 | 8.01 ± 0.37 | 2.23 ± 0.24 | 4.23 ± 0.24 | 2.03 ± 0.12 |
| Aspartic acid | 2.26 ± 0.08 | 2.05 ± 0.20 | 2.02 ± 0.22 | 2.16 ± 0.13 | 1.59 ± 0.22 | 1.85 ± 0.07 | 2.14 ± 0.22 |
| Glutamic acid | 2.01 ± 0.11 | 1.75 ± 0.05 | 1.65 ± 0.14 | 1.78 ± 0.07 | 1.56 ± 0.10 | 1.60 ± 0.02 | 1.74 ± 0.02 |
| Glycine | 0.32 ± 0.04 | 0.32 ± 0.01 | 0.99 ± 0.08 | 92.67 ± 6.40 | 0.51 ± 0.05 | 0.28 ± 0.07 | 0.38 ± 0.07 |
| Histidine | 1.12 ± 0.03 | 0.88 ± 0.04 | 0.05 ± 0.00 | 0.76 ± 0.11 | 1.10 ± 0.01 | 1.26 ± 0.07 | 1.21 ± 0.09 |
| Isoleucine | 0.87 ± 0.04 | 1.08 ± 0.05 | 0.45 ± 0.03 | 0.60 ± 0.18 | 0.67 ± 0.08 | 0.25 ± 0.05 | 0.67 ± 0.08 |
| Leucine | 0.79 ± 0.02 | 1.24 ± 0.12 | 0.71 ± 0.02 | 0.83 ± 0.06 | 0.75 ± 0.07 | 0.67 ± 0.03 | 2.18 ± 0.14 |
| Lysine | 1.31 ± 0.05 | 0.98 ± 0.12 | 0.84 ± 0.10 | 6.78 ± 0.33 | 2.29 ± 0.13 | 231.10 ± 11.61 | 3.60 ± 0.11 |
| Phenylalanine | 1.54 ± 0.06 | 135.51 ± 10.47 | 0.65 ± 0.02 | 0.55 ± 0.01 | 0.49 ± 0.03 | 0.41 ± 0.02 | 0.56 ± 0.06 |
| Proline | 7.32 ± 0.07 | 6.60 ± 0.14 | 6.79 ± 0.27 | 7.80 ± 0.23 | 7.00 ± 0.10 | 6.88 ± 0.13 | 9.16 ± 0.09 |
| Serine | 1.76 ± 0.02 | 1.65 ± 0.10 | 1.50 ± 0.04 | 1.81 ± 0.10 | 1.46 ± 0.09 | 1.48 ± 0.05 | 1.60 ± 0.12 |
| Threonine | 0.75 ± 0.03 | 0.64 ± 0.09 | 0.54 ± 0.16 | 0.78 ± 0.43 | 0.51 ± 0.21 | 0.88 ± 0.05 | 0.63 ± 0.27 |
| Valine | 0.95 ± 0.04 | 1.60 ± 0.20 | 0.49 ± 0.05 | 0.60 ± 0.02 | 0.55 ± 0.04 | 0.99 ± 0.04 | 85.87 ± 3.81 |
| β-Alanine | - | - | - | - | 127.63 ± 4.75 | ||
| Molecules | COX-1 | COX-2 | ||||
|---|---|---|---|---|---|---|
| Affinity DG 1 | EDoc 2 | Ki 3 | Affinity DG 1 | EDoc 2 | Ki 3 | |
| Quercetin | −8.8 | −4.81 | 297.39 μM | −8.8 | −5.63 | 75.03 μM |
| Isorhamnetin | −8.6 | −5.11 | 179.54 μM | −8.9 | −4.48 | 516.88 μM |
| Kaempferol | −9.2 | −5.28 | 134.94 μM | −8.7 | −6.07 | 35.27 μM |
| Chlorogenic acid | −7.5 | −1.85 | 43.74 mM | −7.1 | −2.09 | 29.57 mM |
| Neochlorogenic acid | −8.6 | −3.57 | 2.42 mM | −7.4 | −4.10 | 994.77 μM |
| 4,5-Dicaffeoylquinic acid | −7.7 | +7.51 | - | −7.3 | −0.99 | 188.10 mM |
| 3,5-Dicaffeoylquinic acid | −9.0 | +2.68 | - | −6.4 | −0.67 | 323.17 mM |
| 3,4-Dicaffeoylquinic acid | −9.0 | +0.98 | - | −7.1 | −0.96 | 198.68 mM |
| Caffeic acid | −7.5 | −4.78 | 314.41 μM | −7.1 | −3.53 | 2.60 mM |
| Quinic acid | −6.1 | −1.65 | 61.23 mM | −6.1 | −1.40 | 93.74 mM |
| Celecoxib | −10.9 | −8.65 | 453.15 nM | −11.9 | −9.87 | 58.02 nM |
| Group of Animals | , n = 6 | |||
|---|---|---|---|---|
| Before the Experiment Begins | 3 Days | 7 Days | 14 Days | |
| 1 (Extract S) | 21.95 ± 0.75 | 22.50 ± 0.71 | 23.15 ± 0.88 * | 23.83 ± 0.85 * |
| 2 (Extract S-Phe) | 23.40 ± 0.88 | 23.82 ± 0.86 | 24.43 ± 0.91 | 25.08 ± 0.82 * |
| 3 (Extract S-Arg) | 20.32 ± 1.06 | 20.87 ± 1.14 | 21.28 ± 1.05 | 21.90 ± 1.13 * |
| 4 (Extract S-Gly) | 23.02 ± 0.96 | 23.33 ± 1.00 | 23.88 ± 1.01 | 24.53 ± 1.06 * |
| 5 (Extract S-Ala) | 20.87 ± 0.96 | 21.28 ± 0.89 | 21.82 ± 0.95 | 22.50 ± 0.80 * |
| 6 (Extract S-Lys) | 23.20 ± 1.17 | 23.62 ± 1.12 | 24.22 ± 1.15 | 24.85 ± 1.13 * |
| 7 (Extract S-Val) | 22.27 ± 0.61 | 22.70 ± 0.55 | 23.27 ± 0.54 * | 23.88 ± 0.49 * |
| Intact animals (water purified) | 18.97 ± 0.55 | 19.50 ± 0.57 | 19.88 ± 0.49 * | 20.52 ± 0.39 * |
| Group of Animals | , n = 6 | ||
|---|---|---|---|
| Liver | Heart | Kidneys | |
| 1 (Extract S) | 1.31 ± 0.032 | 0.11 ± 0.009 | 0.28 ± 0.020 |
| 2 (Extract S-Phe) | 1.33 ± 0.069 | 0.12 ± 0.007 | 0.32 ± 0.017 |
| 3 (Extract S-Arg) | 1.30 ± 0.039 | 0.09 ± 0.004 | 0.29 ± 0.015 |
| 4 (Extract S-Gly) | 1.28 ± 0.054 | 0.10 ± 0.009 | 0.28 ± 0.031 |
| 5 (Extract S-Ala) | 1.26 ± 0.028 | 0.10 ± 0.005 | 0.27 ± 0.020 |
| 6 (Extract S-Lys) | 1.32 ± 0.034 | 0.10 ± 0.008 | 0.31 ± 0.025 |
| 7 (Extract S-Val) | 1.28 ± 0.031 | 0.11 ± 0.013 | 0.28 ± 0.020 |
| Intact animals (water purified) | 1.25 ± 0.020 | 0.09 ± 0.012 | 0.27 ± 0.025 |
| Group of Animals | , n = 6 | ||
|---|---|---|---|
| ALT, µmol/h·mL | AST, µmol/h·mL | de Ritis Ratio | |
| 1 (Extract S) | 0.26 ± 0.031 | 0.30 ± 0.035 | 1.15 |
| 2 (Extract S-Phe) | 0.29 ± 0.029 | 0.32 ± 0.046 | 1.10 |
| 3 (Extract S-Arg) | 0.26 ± 0.034 | 0.30 ± 0.042 | 1.20 |
| 4 (Extract S-Gly) | 0.27 ± 0.033 | 0.34 ± 0.039 | 1.26 |
| 5 (Extract S-Ala) | 0.25 ± 0.043 | 0.31 ± 0.024 | 1.24 |
| 6 (Extract S-Lys) | 0.30 ± 0.054 | 0.32 ± 0.038 | 1.07 |
| 7 (Extract S-Val) | 0.28 ± 0.039 | 0.31 ± 0.040 | 1.11 |
| Intact animals (purified water) | 0.29 ± 0.023 | 0.31 ± 0.033 | 1.09 |
| Microorganisms | Control Ethanol 40% | S | S-Phe | S-Arg | S-Gly | S-Ala | S-Lys | S-Val | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Clinical Material | Resistance | ||||||||
| Staphylococcus aureus | Pharynx | BSSA | growth | 13.68 ± 0.39 | 11.31 ± 0.50 | growth | 10.34 ± 1.00 | 13.77 ± 0.32 | 10.58 ± 0.19 | 11.78 ± 0.74 |
| Staphylococcus aureus | Wound | BSSA, MLs | growth | 13.09 ± 0.64 | 11.44 ± 0.28 | growth | 10.51 ± 0.28 | 13.37 ± 0.50 | 11.38 ± 1.46 | 11.62 ± 0.74 |
| Enterococcus faecalis | Urethra | Tet, FQin | growth | growth | growth | 13.79 ± 0.49 | 12.55 ± 1.11 | 12.09 ± 0.56 | 0 | 11.86 ± 0.71 |
| β-hemolytic Streptococcus pyogenes | Pharynx | S | 14.60 ± 2.28 | growth | growth | 18.18 ± 0.69 | growth | growth | growth | growth |
| α-hemolytic Streptococcus anginosus | Pharynx | AMO, Tet, MLs | 15.51 ± 1.28 | growth | growth | growth | growth | growth | growth | growth |
| Streptococcus pneumoniae | Sputum | S | growth | growth | growth | growth | growth | growth | growth | growth |
| Streptococcus pneumoniae | Sputum | b-Lac, Tet | growth | growth | growth | growth | growth | growth | growth | growth |
| E. coli | Wound | S | growth | growth | growth | growth | growth | growth | growth | growth |
| E. coli | Wound | S | growth | growth | growth | growth | growth | growth | growth | growth |
| E. coli | Wound | AMO Tet, FQin | growth | growth | growth | growth | growth | growth | growth | growth |
| E. coli hly+ | Faeces | AMO, MLs | growth | growth | growth | growth | growth | growth | growth | growth |
| Acinetobacter baumani | Sputum | ESbL | growth | growth | growth | growth | growth | growth | growth | growth |
| Pseudomonas aureginosa | Wound | ESbL | growth | growth | growth | growth | growth | growth | growth | growth |
| Candida albicans | Oral cavity | FCZ-R | growth | growth | growth | growth | growth | growth | growth | growth |
| Candida albicans | Sputum | FCZ-R | 10.54 ± 0.62 | growth | growth | growth | growth | growth | growth | growth |
| Candida albicans | Urine | FCZ-R | growth | growth | growth | growth | growth | growth | growth | growth |
| Candida albicans | Oral cavity | FCZ-S | growth | growth | growth | growth | growth | growth | growth | growth |
| Candida lusitaniae | Oral cavity | FCZ-R | growth | growth | growth | growth | growth | growth | growth | growth |
| Candida lipolytica | Oral cavity | FCZ-S | growth | growth | growth | growth | growth | growth | growth | growth |
| Group of Animals | Dose, mg/100 g | , n = 7 | ||
|---|---|---|---|---|
| in 1 h | in 1 h | in 5 h | ||
| 1 (Extract S) | 10 | 18.33 ± 4.21 */# | 22.89 ± 5.23 * | 16.57 ± 4.46 */# |
| 2 (Extract S-Phe) | 10 | 25.51 ± 6.80 | 22.06 ± 4.12 * | 15.93 ± 3.73 */# |
| 3 (Extract S-Arg) | 10 | 19.86 ± 3.07 * | 22.17 ± 5.17 * | 18.00 ± 2.89 */# |
| 4 (Extract S-Gly) | 10 | 24.75 ± 6.84 * | 18.30 ± 4.25 */# | 19.05 ± 4.17 */# |
| 5 (Extract S-Ala) | 10 | 22.79 ± 6.21 * | 21.29 ± 6.09 * | 21.60 ± 5.91 */# |
| 6 (Extract S-Lys) | 10 | 21.37 ± 4.79 * | 29.30 ± 5.19 * | 25.78 ± 6.51 * |
| 7 (Extract S-Val) | 10 | 20.40 ± 5.48 * | 24.15 ± 7.10 * | 27.10 ± 6.69 * |
| 8 (Sodium diclofenac) | 0.8 | 20.17 ± 3.18 * | 19.86 ± 3.08 * | 21.97 ± 4.13 * |
| 9 (Quercetin) | 0.5 | 24.22 ± 4.55 | 28.49 ± 6.06 * | 32.43 ± 5.76 */** |
| 10 (Control group) | - | 33.74 ± 6.73 | 47.33 ± 10.68 | 46.52 ± 11.45 |
| Group of Animals | Inflammatory Response Suppression Index, % | Total Anti-Inflammatory Activity, % | ||
|---|---|---|---|---|
| in 1 h | in 1 h | in 5 h | ||
| 1 (Extract S) | 45.67 | 51.64 | 64.37 | 53.89 |
| 2 (Extract S-Phe) | 24.37 | 53.41 | 65.75 | 47.84 |
| 3 (Extract S-Arg) | 41.15 | 53.17 | 61.30 | 51.87 |
| 4 (Extract S-Gly) | 26.65 | 61.34 | 59.09 | 49.03 |
| 5 (Extract S-Ala) | 32.46 | 55.03 | 53.56 | 47.02 |
| 6 (Extract S-Lys) | 36.65 | 38.10 | 44.58 | 39.78 |
| 7 (Extract S-Val) | 39.54 | 48.98 | 41.75 | 43.42 |
| 8 (Sodium diclofenac) | 40.26 | 58.05 | 52.77 | 50.36 |
| 9 (Quercetin) | 28.22 | 39.81 | 30.28 | 32.77 |
| Group of Animals | manimal, g | mliver, g | LMI, % |
|---|---|---|---|
| 1 (Intact animals) | 145.00 ± 11.64 | 4.59 ± 0.56 | 3.16 ± 0.26 |
| 2 (Control group, CCl4) | 153.29 ± 13.79 | 8.40 ± 1.19 | 5.45 ± 0.36 * |
| 3 (Extract S) | 188.29 ± 15.49 | 6.57 ± 0.89 | 3.63 ± 0.2 */** |
| 4 (Extract S-Phe) | 203.29 ± 19.74 | 7.79 ± 0.78 | 3.83 ± 0.14 */**/# |
| 5 (Extract S-Arg) | 186.57 ± 25.98 | 7.78 ± 1.80 | 4.15 ± 0.48 */**/# |
| 6 (Extract S-Gly) | 181.57 ± 15.49 | 8.09 ± 1.38 | 4.51 ± 0.98 */**/# |
| 7 (Extract S-Ala) | 220.00 ± 16.02 | 8.02 ± 0.60 | 3.60 ± 0.11 */** |
| 8 (Extract S-Lys) | 213.9 ± 23.68 | 7.64 ± 0.85 | 3.61 ± 0.44 ** |
| 9 (Extract S-Val) | 216.57 ± 11.54 | 7.26 ± 1.03 | 3.34 ± 0.37 ** |
| 10 (Silymarin) | 220.00 ± 11.94 | 7.93 ± 0.82 | 3.60 ± 0.22 */** |
| Group of Animals | Biochemical Indicators | |||
|---|---|---|---|---|
| Blood Serum | Liver Homogenate | |||
| ALT, μmol/h·mL | AST, μmol/h·mL | ALP, nmol/s·L | TBK-AP nmol/g | |
| 1 (Intact animals) | 1.55 ± 0.13 | 2.23 ± 0.28 | 1859.14 ± 177.67 | 18.63 ± 2.68 |
| 2 (Control group, CCl4) | 4.64 ± 0.31 * | 3.83 ± 0.24 * | 4913.29 ± 465.37 * | 51.60 ± 8.58 * |
| 3 (Extract S) | 2.78 ± 0.39 */**/# | 3.32 ± 0.47 */** | 3025.29 ± 442.29 */**/# | 29.72 ± 3.40 */**/# |
| 4 (Extract S-Phe) | 2.22 ± 0.36 */**/# | 2.69 ± 0.44 */**/# | 2783.86 ± 332.95 */**/# | 23.98 ± 5.69 **/# |
| 5 (Extract S-Arg) | 4.33 ± 0.50 * | 3.82 ± 0.25 * | 4366.86 ± 483.42 * | 47.40 ± 10.35 * |
| 6 (Extract S-Gly) | 4.30 ± 0.42 * | 3.75 ± 0.61 * | 4520.00 ± 741.14 * | 48.81 ± 5.86 */# |
| 7 (Extract S-Ala) | 3.84 ± 0.54 */** | 3.52 ± 0.51 * | 3354.86 ± 407.24 */**/# | 26.71 ± 7.69 */**/# |
| 8 (Extract S-Lys) | 3.39 ± 0.56 */**/# | 2.95 ± 0.46 */**/# | 3148.29 ± 451.68 */**/# | 24.10 ± 4.44 */**/# |
| 9 (Extract S-Val) | 4.09 ± 0.40 */** | 3.57 ± 0.37 * | 3962.00 ± 435.33 */** | 34.22 ± 6.20 */** |
| 10 (Silymarin) | 4.27 ± 0.39 * | 3.67 ± 0.37 * | 4146.71 ± 610.78 */** | 37.82 ± 3.71 */** |
| The Amount of Extract (g) in the Printing Gel (10 g) | Viscosity, cP (22 ± 2 °C) | Surface Area of the 3D-Printed Lattices, mm2 | Spractical/ Stheoretical | Mass of Lattices, mg | Mass of Round-Shaped Discs, mg |
|---|---|---|---|---|---|
| 0.5 | 126,867 ± 4958 | 347.72 ± 19.56 | 1.07 | 176.4 ± 2.1 | 125.7 ± 1.1 |
| 1.0 | 125,233 ± 5132 | 354.36 ± 27.97 | 1.09 | 206.7 ± 2.7 | 148.0 ± 4.6 |
| 1.5 | 102,967 ± 1775 | 373.13 ± 29.34 | 1.15 | 220.3 ± 2.5 | 154.4 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koshovyi, O.; Hrytsyk, Y.; Perekhoda, L.; Suleiman, M.; Jakštas, V.; Žvikas, V.; Grytsyk, L.; Yurchyshyn, O.; Heinämäki, J.; Raal, A. Solidago canadensis L. Herb Extract, Its Amino Acids Preparations and 3D-Printed Dosage Forms: Phytochemical, Technological, Molecular Docking and Pharmacological Research. Pharmaceutics 2025, 17, 407. https://doi.org/10.3390/pharmaceutics17040407
Koshovyi O, Hrytsyk Y, Perekhoda L, Suleiman M, Jakštas V, Žvikas V, Grytsyk L, Yurchyshyn O, Heinämäki J, Raal A. Solidago canadensis L. Herb Extract, Its Amino Acids Preparations and 3D-Printed Dosage Forms: Phytochemical, Technological, Molecular Docking and Pharmacological Research. Pharmaceutics. 2025; 17(4):407. https://doi.org/10.3390/pharmaceutics17040407
Chicago/Turabian StyleKoshovyi, Oleh, Yurii Hrytsyk, Lina Perekhoda, Marharyta Suleiman, Valdas Jakštas, Vaidotas Žvikas, Lyubov Grytsyk, Oksana Yurchyshyn, Jyrki Heinämäki, and Ain Raal. 2025. "Solidago canadensis L. Herb Extract, Its Amino Acids Preparations and 3D-Printed Dosage Forms: Phytochemical, Technological, Molecular Docking and Pharmacological Research" Pharmaceutics 17, no. 4: 407. https://doi.org/10.3390/pharmaceutics17040407
APA StyleKoshovyi, O., Hrytsyk, Y., Perekhoda, L., Suleiman, M., Jakštas, V., Žvikas, V., Grytsyk, L., Yurchyshyn, O., Heinämäki, J., & Raal, A. (2025). Solidago canadensis L. Herb Extract, Its Amino Acids Preparations and 3D-Printed Dosage Forms: Phytochemical, Technological, Molecular Docking and Pharmacological Research. Pharmaceutics, 17(4), 407. https://doi.org/10.3390/pharmaceutics17040407











