Development of an Intelligent Tablet Press Machine for the In-Line Detection of Defective Tablets Using Machine Learning and Deep Learning Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tablet Manufacturing and Testing Method
2.2.1. Morphological and Physical Characteristics of MF Granules
2.2.2. Preparation of MF-Loaded Granules via Wet Granulation and Tablet Compression
2.2.3. Research Procedure
2.2.4. Preparation of MF-Loaded Tablets
2.2.5. QC Test
Compaction Force Required to Fracture the MF-Loaded Tablets
Friability Test
2.3. Defect Prediction Method and System Configuration Using ML and DL
2.3.1. ML and DL Model
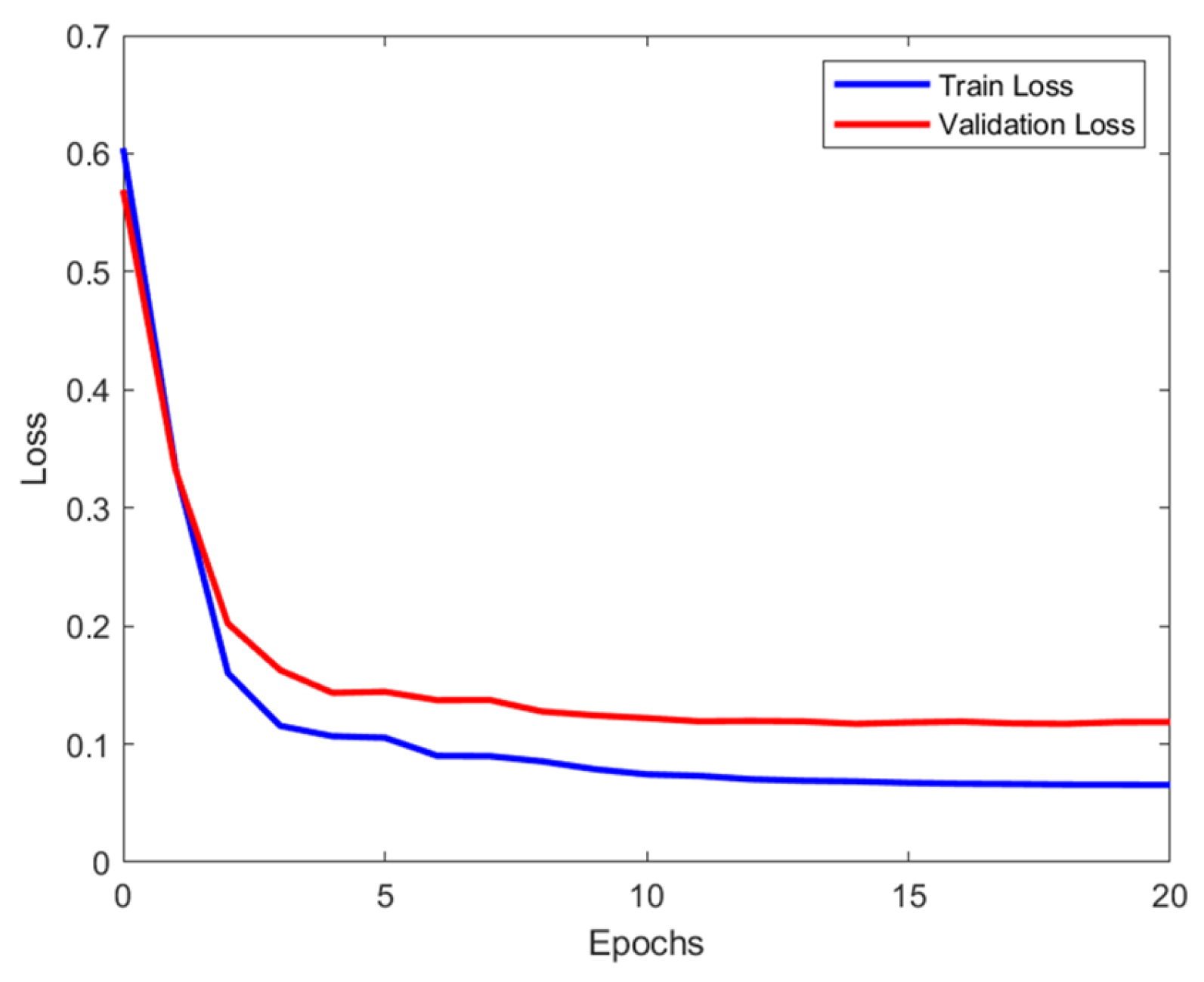
2.3.2. Hyperparameter Optimization of RF and ANN Models
2.3.3. ROC-AUC Confidence Interval
2.3.4. Feature Importance Analysis
2.3.5. Evaluation of the Results
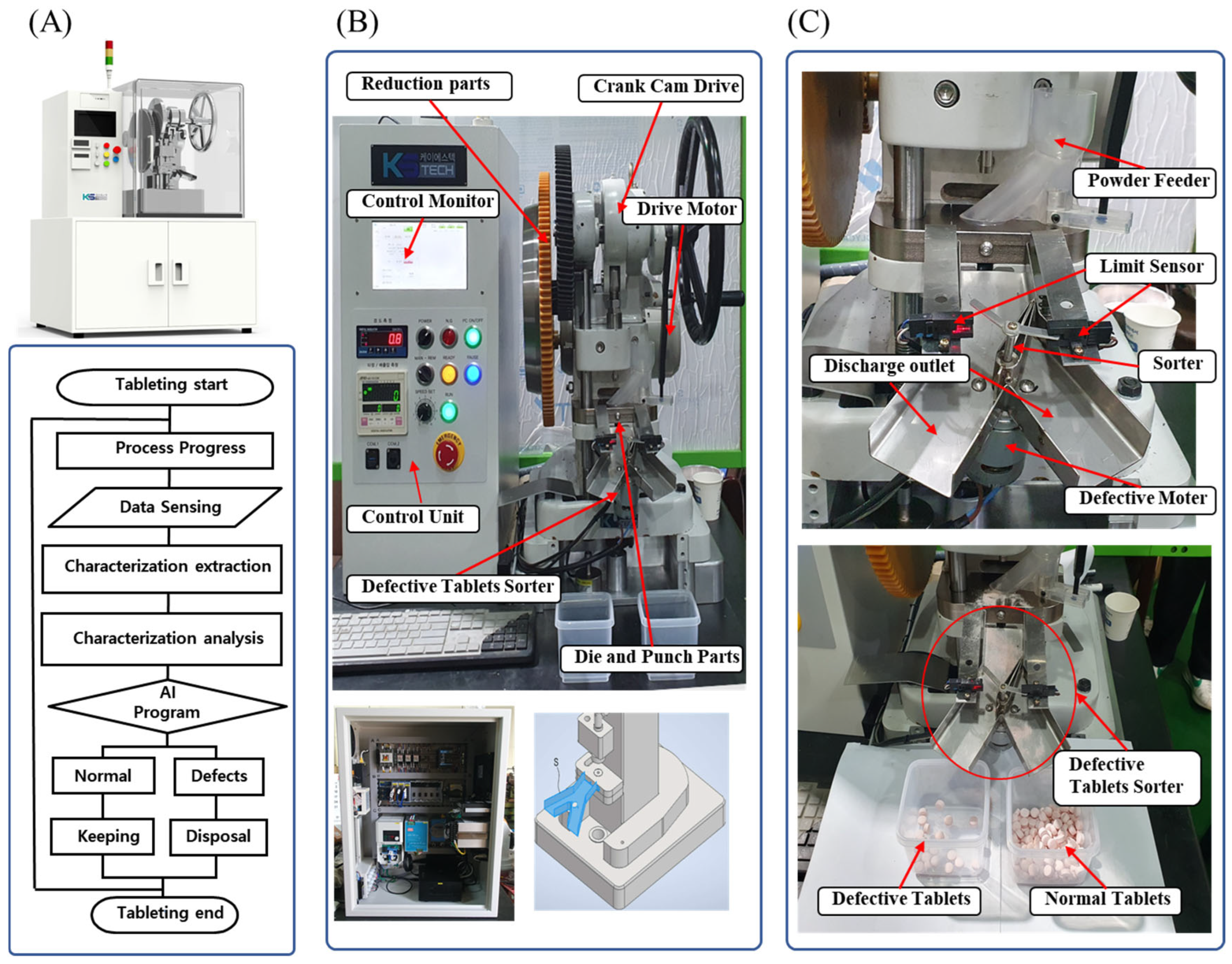
2.3.6. Configuration of the Defective Tablet Sorting Device
3. Results
3.1. Tablet Defect Prediction Using RF and ANN
3.1.1. Data Preparation
3.1.2. Criteria for Determining Tablet Defect
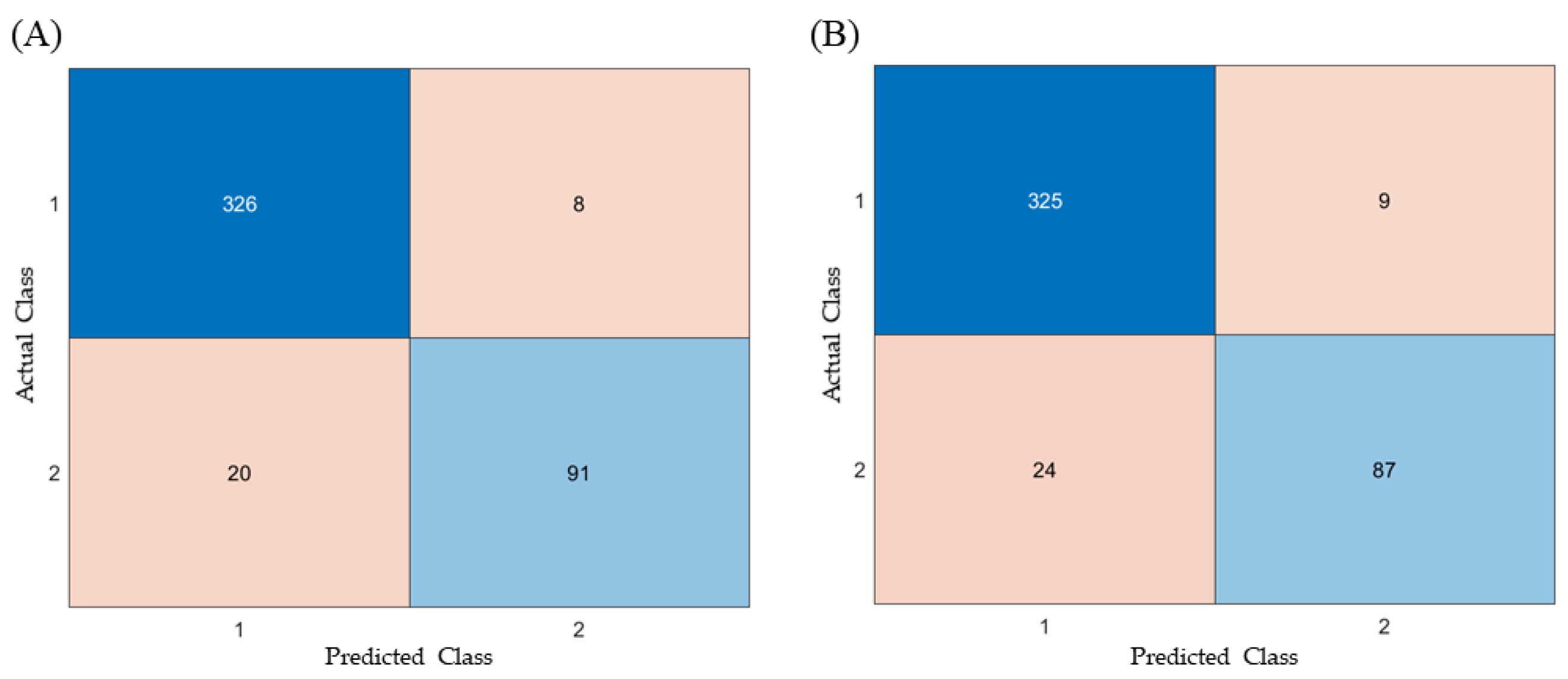
3.1.3. Tablet Defect Prediction Results
3.1.4. Feature Importance Analysis of Factors Influencing Tablet Defects
3.2. Verification of the In-Line TPM Application for the Detection of Defective Tablets
3.2.1. Design and Manufacture of TPM for the Detection of Defective Tablets
3.2.2. Verification of the TPM Application for the Sorting of Defective Tablets
3.3. Practical Advantages and Applicability of the Proposed In-Line QC System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TPM | Tablet Press Machine |
| PAT | Process Analytical Technology |
| TBF | Tablet Breaking Force |
| MF | Metformin HCl |
| RF | Random Forest |
| ANN | Artificial Neural Network |
| ML | Machine Learning |
| DL | Deep Learning |
| QC | Quality Control |
| TPI | Terahertz Pulsed Imaging |
| NIR | Near-Infrared |
| mAb | Monoclonal Antibody |
| CQA | Critical Quality Attributes |
| LOD | Loss On Drying |
| HR | Hausner Ratio |
| CI | Compressibility Index |
| TD | Tapped Density |
| MgST | Magnesium Stearate |
| TP | True Positive |
| FP | False Positive |
| TN | True Negative |
| FN | False Negative |
| AUC | Area Under the Curve |
| ReLU | Rectified Linear Unit |
References
- U.S. Food and Drug Administration. Guidance for Industry on Quality Systems Approach to Pharmaceutical Current Good Manufacturing Practice Regulations. Fed. Regist. 2006, 71, 57980–57981. [Google Scholar]
- De Beer, T.; Burggraeve, A.; Fonteyne, M.; Saerens, L.; Remon, J.P.; Vervaet, C. Near infrared and Raman spectroscopy for the in-process monitoring of pharmaceutical production processes. Int. J. Pharm. 2011, 417, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; O’Connor, T.F.; Yang, X.; Cruz, C.N.; Chatterjee, S.; Madurawe, R.D.; Moore, C.M.; Yu, L.X.; Woodcock, J. Modernizing pharmaceutical manufacturing: From batch to continuous production. J. Pharm. Innov. 2015, 10, 191–199. [Google Scholar] [CrossRef]
- Sinka, I.C.; Motazedian, F.; Cocks, A.C.F.; Pitt, K.G. The effect of processing parameters on pharmaceutical tablet properties. Powder Technol. 2009, 189, 276–284. [Google Scholar]
- U.S. Food and Drug Administration. Guidance for Industry: PAT—A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance; U.S. Department of Health and Human Services: Washington, DC, USA, 2004.
- Haaser, M.; Naelapää, K.; Gordon, K.C.; Pepper, M.; Rantanen, J.; Strachan, C.J.; Taday, P.F.; Zeitler, J.A.; Rades, T. Evaluating the effect of coating equipment on tablet film quality using terahertz pulsed imaging. Eur. J. Pharm. Biopharm. 2013, 85, 1095–1102. [Google Scholar] [CrossRef]
- De Beer, T.R.; Vercruysse, P.; Burggraeve, A.; Quinten, T.; Ouyang, J.; Zhang, X.; Vervaet, C.; Remon, J.P.; Baeyens, W.R. In-line and real-time process monitoring of a freeze-drying process using Raman and NIR spectroscopy as complementary process analytical technology (PAT) tools. J. Pharm. Sci. 2009, 98, 3430–3446. [Google Scholar] [CrossRef]
- De Beer, T.R.; Bodson, C.; Dejaegher, B.; Walczak, B.; Vercruysse, P.; Burggraeve, A.; Lemos, A.; Delattre, L.; Vander Heyden, Y.; Remon, J.P.; et al. Raman spectroscopy as a process analytical technology (PAT) tool for the in-line monitoring and understanding of a powder blending process. J. Pharm. Biomed. Anal. 2008, 48, 772–779. [Google Scholar] [CrossRef]
- Nitika, N.; Keerthiveena, B.; Thakur, G.; Rathore, A.S. Convolutional neural networks guided Raman spectroscopy as a process analytical technology (PAT) tool for monitoring and simultaneous prediction of monoclonal antibody charge variants. Pharm. Res. 2024, 41, 463–479. [Google Scholar] [CrossRef]
- Kim, S.H.; Han, S.H.; Seo, D.W.; Kang, M.J. Evaluation of prediction models for the capping and breaking force of tablets using machine learning tools in wet granulation commercial-scale pharmaceutical manufacturing. Pharmaceuticals 2024, 18, 23. [Google Scholar] [CrossRef]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- Kim, S.H.; Kook, J.H.; Seo, D.W.; Kang, M.J. The effect of compression pressure on the first-layer surface roughness and delamination of metformin and evogliptin bilayer and trilayer tablets. Pharmaceuticals 2023, 16, 1523. [Google Scholar] [CrossRef]
- Hu, L.D.; Liu, Y.; Tang, X.; Zhang, Q. Preparation and in vitro/in vivo evaluation of sustained-release metformin hydrochloride pellets. Eur. J. Pharm. Biopharm. 2006, 64, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Won, D.H.; Park, H.; Ha, E.S.; Kim, H.H.; Jang, S.W.; Kim, M.S. Optimization of bilayer tablet manufacturing process for fixed-dose combination of sustained-release high-dose drug and immediate-release low-dose drug based on quality by design (QbD). Int. J. Pharm. 2021, 605, 120838. [Google Scholar] [CrossRef] [PubMed]
- Awais, M.; Belhaouari, S.B.; Kassoul, K. Graphical insight: Revolutionizing seizure detection with EEG representation. Biomedicines 2024, 12, 1283. [Google Scholar] [CrossRef] [PubMed]
- Parmar, A.; Katariya, R.; Patel, V. A review on random forest: An ensemble classifier. In Proceedings of the International Conference on Intelligent Data Communication Technologies and Internet of Things (ICICI) 2018, Coimbatore, India, 7–8 August 2018; Volume 758, p. 763. [Google Scholar] [CrossRef]
- Mäki-Lohiluoma, E.; Säkkinen, N.; Palomäki, M.; Winberg, O.; Ta, H.X.; Heikkinen, T.; Kiljunen, E.; Kauppinen, A. Use of machine learning in prediction of granule particle size distribution and tablet tensile strength in commercial pharmaceutical manufacturing. Int. J. Pharm. 2021, 609, 121146. [Google Scholar] [CrossRef]
- Probst, P.; Wright, M.N.; Boulesteix, A.L. Hyperparameters and tuning strategies for random forest. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2019, 9, e1301. [Google Scholar] [CrossRef]
- Taherdoost, H. Deep learning and neural networks: Decision-making implications. Symmetry 2023, 15, 1723. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, X.; Liu, B.; Gao, M. Solving partial differential equations using deep learning and physical constraints. Appl. Sci. 2020, 10, 5917. [Google Scholar] [CrossRef]
- Lovrić, M.; Horner, D.; Chen, L.; Brustad, N.; Schoos, A.M.M.; Lasky-Su, J.; Chawes, B.; Rasmussen, M.A. Vertical Metabolome Transfer from Mother to Child: An Explainable Machine Learning Method for Detecting Metabolomic Heritability. Metabolites 2024, 14, 136. [Google Scholar] [CrossRef]
- Zhang, S. Advanced Imaging Techniques for Point-Measurement Analysis of Pharmaceutical Materials. Available online: https://docs.lib.purdue.edu/open_access_dissertations/1854 (accessed on 15 February 2025).
- Jiao, Y.; Du, P. Performance measures in evaluating machine learning-based bioinformatics predictors for classifications. Quant. Biol. 2016, 4, 320–330. [Google Scholar] [CrossRef]
- Huang, M.; Zhao, H.; Chen, Y. Research on SAR image quality evaluation method based on improved Harris Hawk optimization algorithm and XGBoost. Sci. Rep. 2024, 14, 28364. [Google Scholar] [CrossRef]
- Pumhirunroj, B.; Littidej, P.; Boonmars, T.; Artchayasawat, A.; Prasertsri, N.; Khamphilung, P.; Sangpradid, S.; Buasri, N.; Uttha, T.; Slack, D. Spatial predictive modeling of liver fluke Opisthorchis viverrine (OV) infection under the mathematical models in hexagonal symmetrical shapes using machine learning-based forest classification regression. Symmetry 2024, 16, 1067. [Google Scholar] [CrossRef]
- Kobraei, K.; Baradaran, M.; Sadeghi, S.M.; Masumshah, R.; Eslahchi, C. ADEP: A novel approach based on discriminator-enhanced encoder-decoder architecture for accurate prediction of adverse effects in polypharmacy. arXiv 2024. [Google Scholar] [CrossRef]
- USP Standard No. 1216; Tablet Friability. The United States Pharmacopeial Convention: North Bethesda, MD, USA, 2019. Available online: https://online.uspnf.com/uspnf/document/1_GUID-BE725528-5F2D-4F87-8184-2EACA45448F9_2_en-US?source=Quick%20Search&highlight=friability (accessed on 2 March 2025).
- Toshev, K.; Endekovska, I.; Kostovska, M.; Angelovska, V.; Stojanovska, N.A. Impact of qualitative formulation variables on critical quality attributes of tablets with fast disintegration. Maced. Pharm. Bull. 2022, 68, 229–230. [Google Scholar] [CrossRef]
- Dube, L.; Verster, T. Interpretability of the random forest model under class imbalance. Data Sci. Finance Econ. 2024, 4, 446–468. [Google Scholar] [CrossRef]
- Patere, S.N.; Kapadia, C.J.; Nagarsenker, M.S. Influence of formulation factors and compression force on release profile of sustained release metoprolol tablets using Compritol® 888ATO as lipid excipient. Indian J. Pharm. Sci. 2015, 77, 620–625. [Google Scholar]
- Tanino, T.; Aoki, Y.; Furuya, Y.; Sato, K.; Takeda, T.; Mizuta, T. Occurrence of capping due to insufficient air escape during tablet compression and a method to prevent it. Chem. Pharm. Bull. 1995, 43, 1772–1779. [Google Scholar] [CrossRef]
- Uzunović, A.; Vranić, E. Effect of magnesium stearate concentration on dissolution properties of ranitidine hydrochloride coated tablets. Bosn. J. Basic Med. Sci. 2007, 7, 279–283. [Google Scholar] [CrossRef]


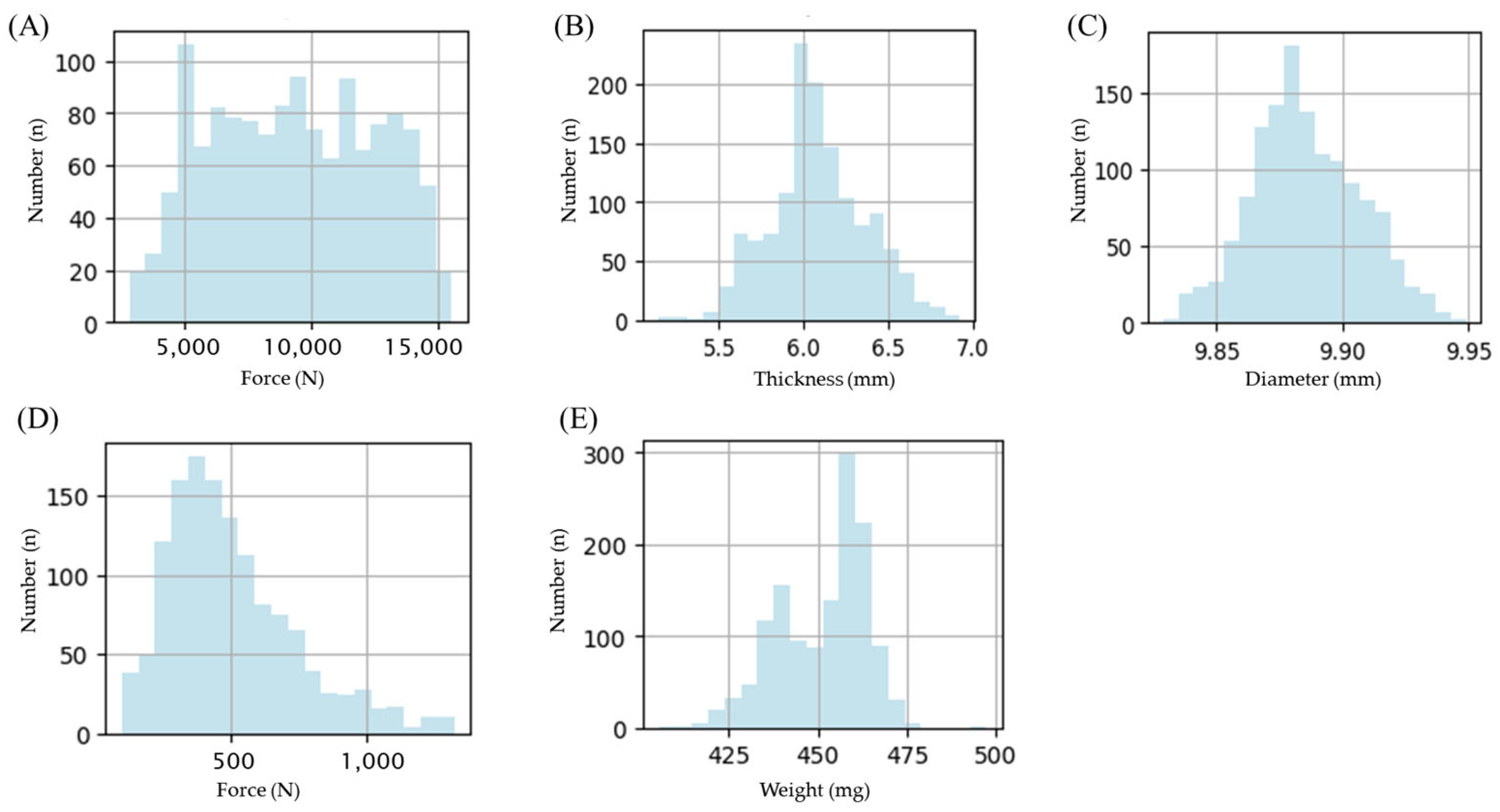





| Samples | MF-Loaded Granules |
|---|---|
| LOD (%) 1 | 0.74 ± 0.09 |
| BD (g/mL) 1 | 0.43 ± 0.01 |
| TD (g/mL) 1 | 0.48 ± 0.01 |
| HR 1,2 | 1.12 ± 0.02 |
| CI (%) 1,3 | 10.48 ± 1.90 |
| Particle size d0.1 (μm) 1,4 | 10.73 ± 0.25 |
| Particle size d0.5 (μm) 1,5 | 44.74 ± 2.19 |
| Particle size d0.9 (μm) 1,6 | 110.93 ± 4.23 |
| Span 1,7 | 2.24 ± 0.02 |
| Function | Ingredient | Contents (mg) |
|---|---|---|
| Active pharmaceutical ingredient | MF | 380 |
| Binder | PVP K30 | 15.2 |
| Lubricant | MgSt | 2.74–9.35 |
| Controlled release excipient | Carbomer 934P | 7.6 |
| Controlled release excipient | HPMC2208 | 38 |
| Controlled release excipient | Methacrylic acid copolymer | 15.2 |
| Colorant | Ferric oxide red | 0.3 |
| RF | ANN | |
|---|---|---|
| Accuracy | 0.9371 | 0.9258 |
| Precision | 0.9192 | 0.9061 |
| Recall | 0.820 | 0.784 |
| F1-Score | 0.867 | 0.841 |
| Fall-out | 0.024 | 0.027 |
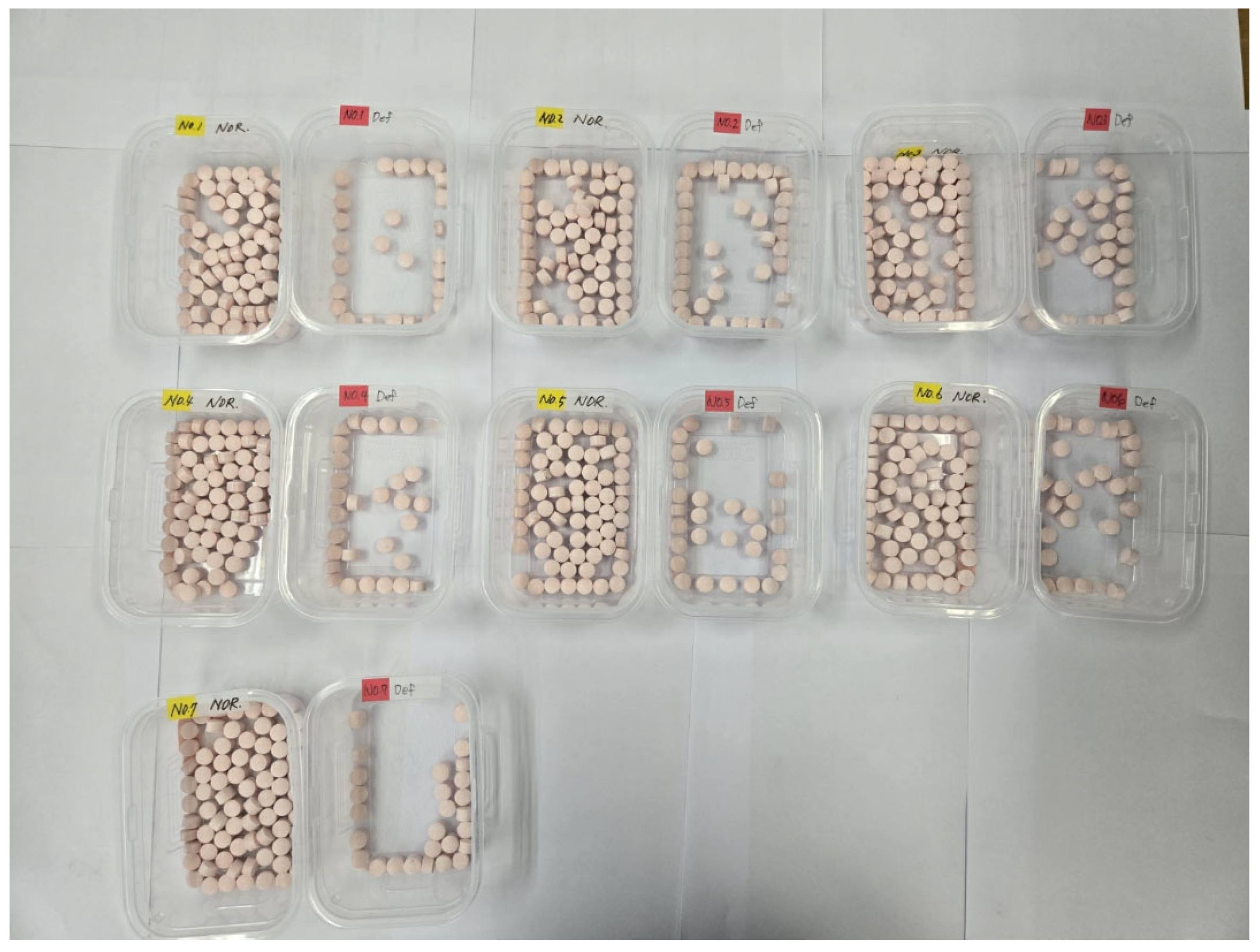
| No. | Number of Samples (a) | RF Model Program | TPM | Machine-Sorting Accuracy (%) (d) | Defect-Detection Accuracy for Model (%) (e) | Machine Defect-Detection Accuracy (%) (f) | ||
|---|---|---|---|---|---|---|---|---|
| Normal | Defective (b) | Normal | Defective (c) | |||||
| 1 | 100 | 75 | 25 | 74 | 26 | 98 | 93.71 | 91.83 |
| 2 | 100 | 62 | 38 | 62 | 38 | 100 | 93.70 | |
| 3 | 100 | 64 | 36 | 62 | 38 | 98 | 91.83 | |
| 4 | 100 | 71 | 29 | 71 | 29 | 100 | 93.70 | |
| 5 | 100 | 70 | 30 | 70 | 30 | 100 | 93.70 | |
| 6 | 100 | 63 | 37 | 63 | 37 | 100 | 93.70 | |
| 7 | 100 | 74 | 26 | 74 | 26 | 100 | 93.70 | |
| Average | 100 | 68.43 | 31.57 | 68 | 32 | 99.43 | 93.17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Han, S.H. Development of an Intelligent Tablet Press Machine for the In-Line Detection of Defective Tablets Using Machine Learning and Deep Learning Models. Pharmaceutics 2025, 17, 406. https://doi.org/10.3390/pharmaceutics17040406
Kim SH, Han SH. Development of an Intelligent Tablet Press Machine for the In-Line Detection of Defective Tablets Using Machine Learning and Deep Learning Models. Pharmaceutics. 2025; 17(4):406. https://doi.org/10.3390/pharmaceutics17040406
Chicago/Turabian StyleKim, Sun Ho, and Su Hyeon Han. 2025. "Development of an Intelligent Tablet Press Machine for the In-Line Detection of Defective Tablets Using Machine Learning and Deep Learning Models" Pharmaceutics 17, no. 4: 406. https://doi.org/10.3390/pharmaceutics17040406
APA StyleKim, S. H., & Han, S. H. (2025). Development of an Intelligent Tablet Press Machine for the In-Line Detection of Defective Tablets Using Machine Learning and Deep Learning Models. Pharmaceutics, 17(4), 406. https://doi.org/10.3390/pharmaceutics17040406






