Revolutionizing Drug Delivery: The Impact of Advanced Materials Science and Technology on Precision Medicine
Abstract
1. Introduction
- ▪
- Simulating nanoparticle breakdown in physiological conditions, ensuring ideal drug release kinetics and clearance rates.
- ▪
- Identifying degradation pathways that produce biocompatible byproducts, reducing toxicity risks.
- ▪
- Customizing nanocarrier formulations based on patient-specific metabolism and disease states, contributing to precision medicine.
- ▪
- Optimizing material composition to enhance biodegradability without compromising stability and drug-loading efficiency.
2. Emerging Materials in Drug Delivery
2.1. Nanomaterials
2.2. Hydrogels
2.2.1. Injectable Hydrogels
2.2.2. Responsive Hydrogels
2.3. Bioresponsive Polymers
2.3.1. pH-Responsive Polymers
- ▪
- Poly(acrylic acid) (PAA) and poly(methacrylic acid) (PMAA) exhibit pH-dependent swelling, making them ideal for oral drug delivery systems that bypass gastric degradation and release drugs in the intestines.
- ▪
- Poly(β-amino esters) (PBAEs) and poly(N-vinyl imidazole) (PVI) degrade under acidic conditions, facilitating tumor-targeted drug release.
- ▪
- Chitosan, a naturally derived cationic polysaccharide, remains soluble in acidic environments but forms a gel at physiological pH, making it suitable for mucosal and gastrointestinal drug delivery.
2.3.2. Temperature-Responsive Polymers
- ▪
- Poly(N-isopropylacrylamide) (PNIPAM) is a well-known thermosensitive polymer that exhibits a lower critical solution temperature (LCST), around 32–35 °C, making it ideal for localized drug delivery in rheumatoid arthritis and postoperative pain management.
- ▪
- Pluronic® block copolymers (e.g., Poloxamer 407), composed of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO), form hydrogels at body temperature, making them suitable for sustained protein or peptide delivery.
- ▪
- Gelatin-based thermosensitive hydrogels, crosslinked with genipin or transglutaminase, have been explored for biodegradable tissue scaffolds and wound healing applications.
2.3.3. Enzyme-Responsive Polymers
- ▪
- Matrix metalloproteinase (MMP)-responsive polymers, such as PEGylated gelatin or polycaprolactone-based hydrogels, are degraded by MMP-2 and MMP-9, enzymes that are overexpressed in tumors and inflammatory sites, leading to selective drug release in cancerous tissues.
- ▪
- Hyaluronidase-sensitive nanocarriers, composed of hyaluronic acid (HA)-conjugated polymers, degrade in response to tumor-associated hyaluronidase, facilitating targeted delivery of anticancer drugs.
- ▪
- Trypsin- and chymotrypsin-responsive hydrogels, based on peptide crosslinked dextran or poly(ethylene glycol) (PEG), have been designed for enzyme-triggered drug release in digestive disorders.
2.4. Integration and Future Prospects
3. Clinical Applications and Case Studies
3.1. Oncology
3.1.1. Nanocarrier-Based Chemotherapy
3.1.2. Multifunctional Platforms
3.1.3. Overcoming Multidrug Resistance (MDR)
3.2. Chronic Disease Management
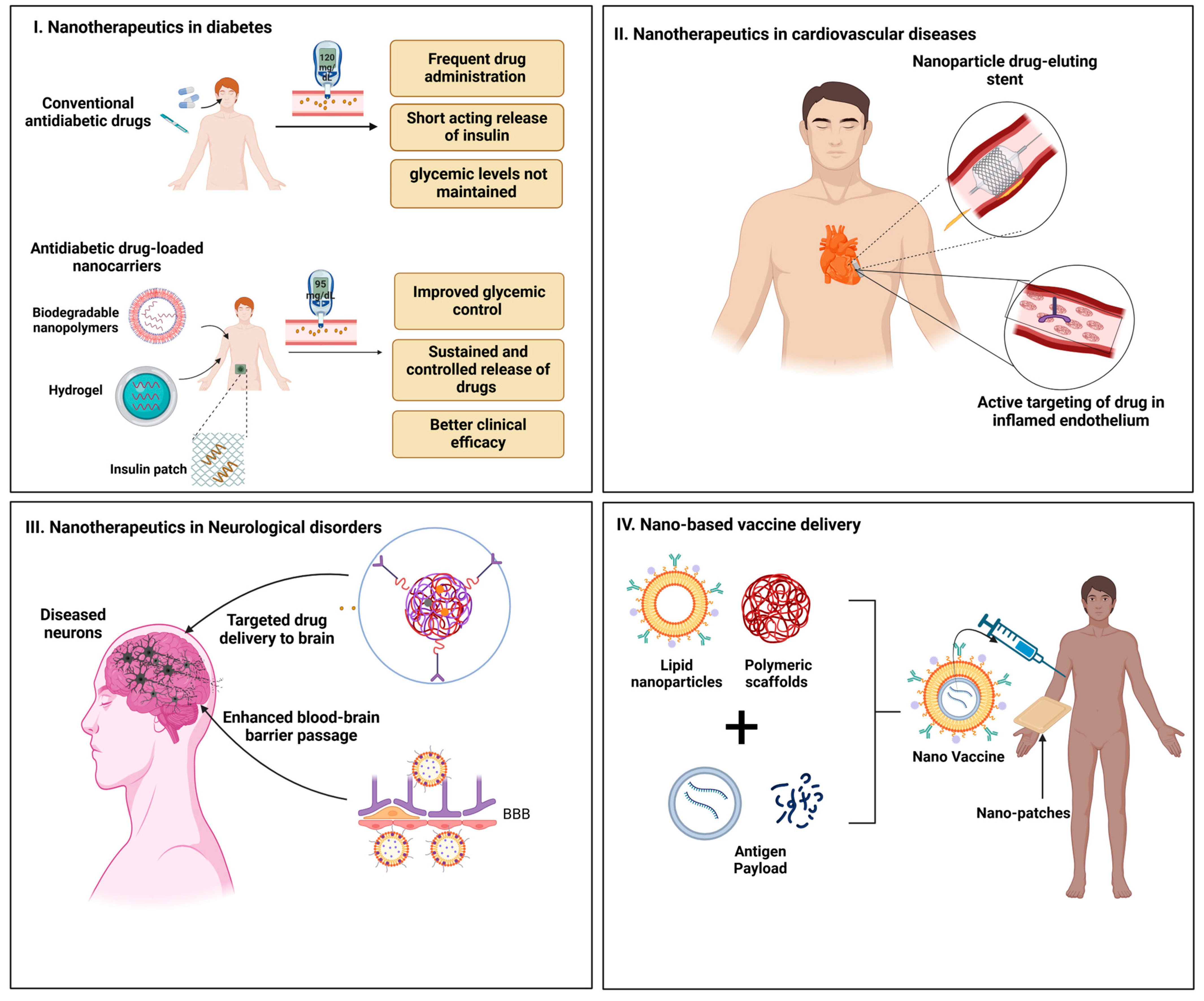
3.2.1. Diabetes Management
3.2.2. Cardiovascular Disorders
3.2.3. Neurological Disorders
3.3. Vaccine Delivery
3.3.1. Lipid Nanoparticles (LNPs)
3.3.2. Polymeric Scaffolds
3.3.3. Microneedle Patches
3.3.4. Adjuvant Systems
4. Translational Challenges
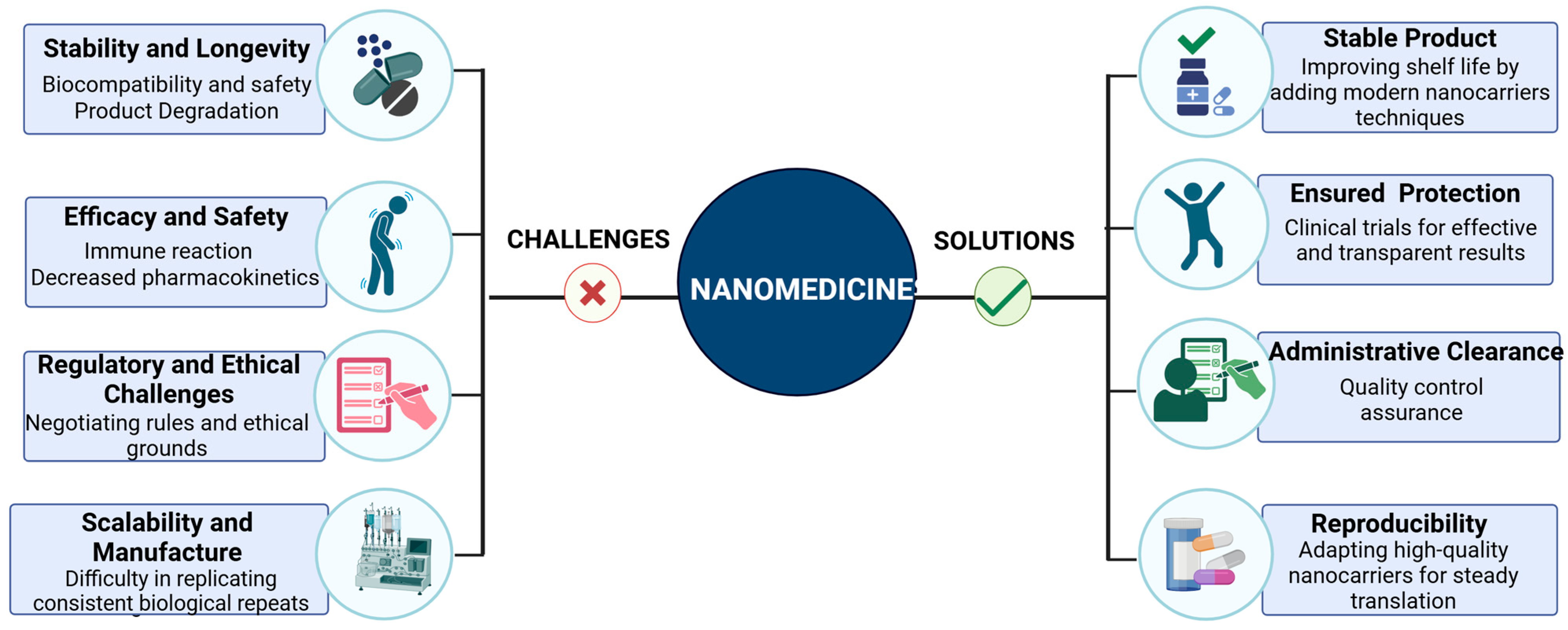
4.1. Biocompatibility and Safety
4.1.1. Immune Reactions
4.1.2. Degradation Products
4.1.3. Chronic Exposure Risks
4.2. Scalability and Manufacturing
4.2.1. Cost of Production
4.2.2. Batch-to-Batch Variability
4.2.3. Stability of Colloidal Suspensions
4.2.4. Scalable Manufacturing Technologies
4.3. Regulatory Considerations
4.3.1. Lack of Established Guidelines
4.3.2. Clinical Validation
4.3.3. Material Characterization Standards
4.4. Integration and Future Perspectives
5. Future Directions
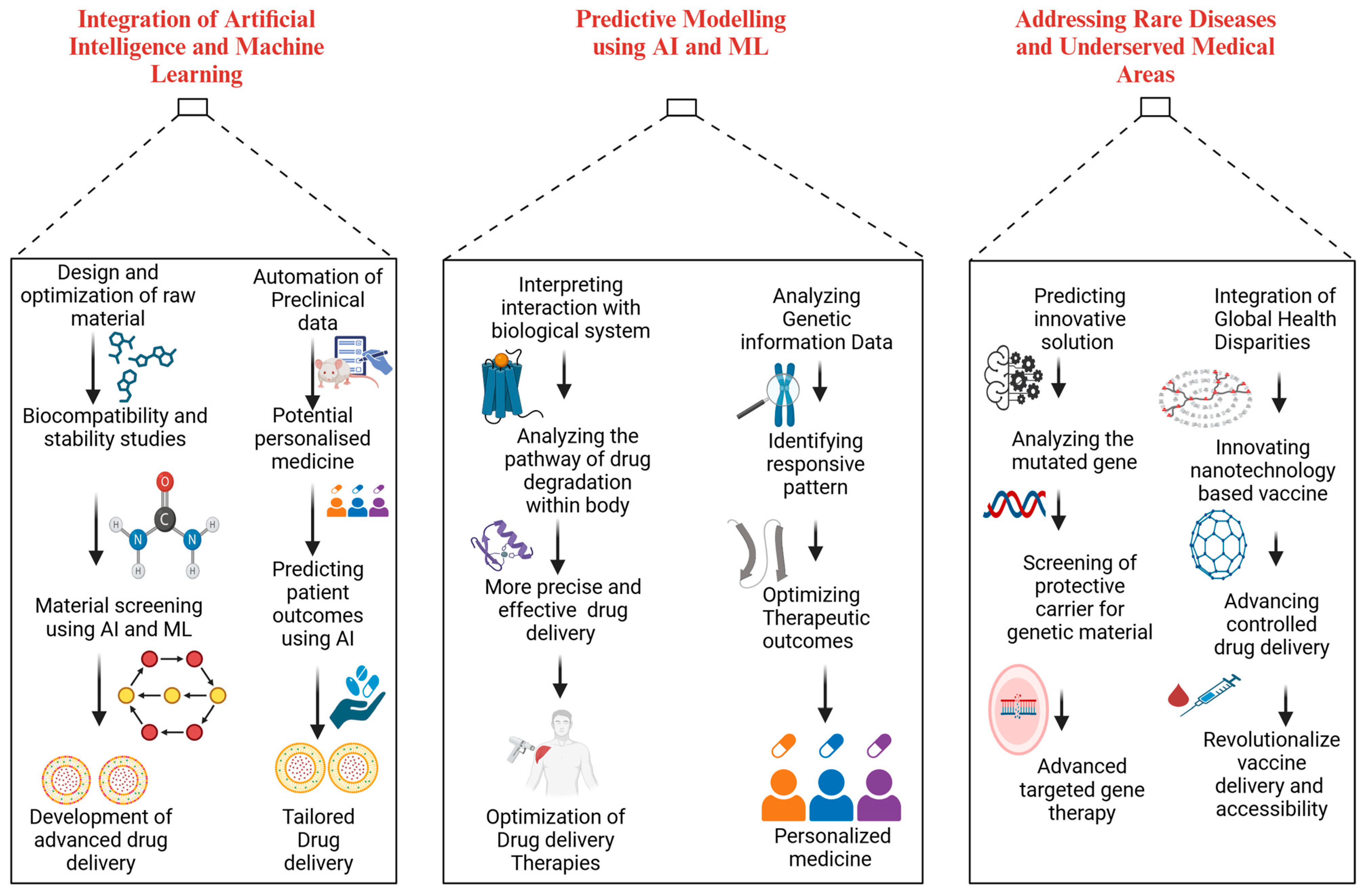
5.1. Integration of Artificial Intelligence and Machine Learning
5.1.1. Predictive Modeling
5.1.2. Personalized Medicine
5.2. Expansion to Rare Diseases
5.2.1. Gene and Cell Therapy
5.2.2. Orphan Drug Development
5.3. Exploration of Underserved Areas
5.3.1. Global Vaccine Accessibility
5.3.2. Aging-Related Therapies
5.4. Future Prospects and the Integration of Closely Linked Areas
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adepu, S.; Ramakrishna, S. Controlled drug delivery systems: Current status and future directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The smart drug delivery system and its clinical potential. Theranostics 2016, 6, 1306. [Google Scholar] [CrossRef]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Langer, R.; Jia, X. Nanostructured materials for applications in drug delivery and tissue engineering. J. Biomater. Sci. Polym. Ed. 2007, 18, 241–268. [Google Scholar] [CrossRef]
- Qayyum, I.; Rehman, F.U.; Zahra, M.; Batool, K.; Shoukat, W.; Arshad, S.; Zada, Z. Progressive innovations in advanced functional materials for emerging bio-electronics, drugs sensing and healthcare. J. Drug Alcohol. Res. 2023, 12, 5. [Google Scholar]
- Chen, Z.; Wang, X.; Zhao, N.; Chen, H.; Guo, G. Advancements in pH-responsive nanocarriers: Enhancing drug delivery for tumor therapy. Expert Opin. Drug Deliv. 2023, 20, 1623–1642. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-responsive polymeric nanocarriers for drug delivery, imaging, and theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Milcovich, G.; Lettieri, S.; Antunes, F.E.; Medronho, B.; Fonseca, A.C.; Coelho, J.F.; Marizza, P.; Perrone, F.; Farra, R.; Dapas, B.; et al. Recent advances in smart biotechnology: Hydrogels and nanocarriers for tailored bioactive molecules depot. Adv. Colloid Interface Sci. 2017, 249, 163–180. [Google Scholar] [CrossRef]
- Farah, F.H.; Farah, F. Nanocarriers as delivery systems for therapeutics agents. Int. J. Pharm. Sci. Res. 2019, 10, 3487–3507. [Google Scholar]
- Thang, N.H.; Chien, T.B.; Cuong, D.X. Polymer-based hydrogels applied in drug delivery: An overview. Gels 2023, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Patil, A.S.; Gadad, A.P.; Dandagi, P.M. Mono and multi-stimuli responsive polymers: Application as intelligent nano-drug delivery systems. In Nanopharmaceutical Advanced Delivery Systems; Scrivener Publishing LLC: Beverly, MA, USA, 2021; pp. 237–265. [Google Scholar]
- Zhou, M.; Wen, K.; Bi, Y.; Lu, H.; Chen, J.; Hu, Y.; Chai, Z. The application of stimuli-responsive nanocarriers for targeted drug delivery. Curr. Top. Med. Chem. 2017, 17, 2319–2334. [Google Scholar] [CrossRef]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 215. [Google Scholar] [CrossRef] [PubMed]
- Onzi, G.; Guterres, S.S.; Pohlmann, A.R.; Frank, L.A. Passive targeting and the enhanced permeability and retention (EPR) effect. In The ADME encyclopedia: A Comprehensive guide on Biopharmacy and Pharmacokinetics; Springer: Cham, Switzerland, 2021; pp. 1–13. [Google Scholar]
- Abdolahinia, E.D.; Barati, G.; Ranjbar-Navazi, Z.; Kadkhoda, J.; Islami, M.; Hashemzadeh, N.; Dizaj, S.M.; Sharifi, S. Application of nanogels as drug delivery systems in multicellular spheroid tumor model. J. Drug Deliv. Sci. Technol. 2022, 68, 103109. [Google Scholar] [CrossRef]
- Harwansh, R.K.; Mishra, S.; Mazumder, R.; Deshmukh, R.; Rahman, A. Recent Updates on Transdermal Drug Delivery Approaches for the Management of Gout and its Clinical Perspective. Curr. Pharm. Biotechnol. 2024, 25, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Throat, S.; Bhattacharya, S. Macromolecular Poly (N-isopropylacrylamide)(PNIPAM) in Cancer Treatment and Beyond: Applications in Drug Delivery, Photothermal Therapy, Gene Delivery and Biomedical Imaging. Adv. Polym. Technol. 2024, 2024, 1444990. [Google Scholar] [CrossRef]
- Kashyap, S.; Kirtania, S.; Banerjee, S. Smart Biomaterials for Thermal Regulation in Biomedical Applications. In Engineering Materials for Efficient Energy Storage and Conversion; IGI Global: Hershey, PA, USA, 2024; pp. 377–418. [Google Scholar]
- Singh, R.; Jadhav, K.; Vaghasiya, K.; Ray, E.; Shukla, R.; Verma, R.K. New generation smart drug delivery systems for rheumatoid arthritis. Curr. Pharm. Des. 2023, 29, 984–1001. [Google Scholar] [CrossRef]
- Subhan, M.A.; Torchilin, V.P. Biocompatible polymeric nanoparticles as promising candidates for drug delivery in cancer treatment. In Handbook of Polymer and Ceramic Nanotechnology; Springer: Cham, Switzerland, 2021; pp. 855–872. [Google Scholar]
- Zhu, Y.; Liao, L. Applications of nanoparticles for anticancer drug delivery: A review. J. Nanosci. Nanotechnol. 2015, 15, 4753–4773. [Google Scholar] [CrossRef]
- Acharya, G.; Hasan, N.; Yoo, J.-W.; Lee, C.H. Hormone therapy and delivery strategies against cardiovascular diseases. Curr. Pharm. Biotechnol. 2017, 18, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Bhat, B.B.; Mehta, C.H.; Suresh, A.; Velagacherla, V.; Nayak, U.Y. Controlled release technologies for chronotherapy: Current status and future perspectives. Curr. Pharm. Des. 2023, 29, 1069–1091. [Google Scholar] [CrossRef]
- Shaker, L.M.; Al-Amiery, A.A.; Kadhum, A.A.H. Advances in Drug Delivery Systems: A Mini-Review. Al-Ameed J. Med. Res. Health Sci. 2023, 1, 3. [Google Scholar] [CrossRef]
- Pilkington, E.H.; Suys, E.J.; Trevaskis, N.L.; Wheatley, A.K.; Zukancic, D.; Algarni, A.; Al-Wassiti, H.; Davis, T.P.; Pouton, C.W.; Kent, S.J. From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021, 131, 16–40. [Google Scholar] [CrossRef]
- Wilson, B.; Geetha, K.M. Lipid nanoparticles in the development of mRNA vaccines for COVID-19. J. Drug Deliv. Sci. Technol. 2022, 74, 103553. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef]
- Pandey, R.K.; Prajapati, V.K. Molecular and immunological toxic effects of nanoparticles. Int. J. Biol. Macromol. 2018, 107, 1278–1293. [Google Scholar] [CrossRef] [PubMed]
- Aundhia, C.; Parmar, G.; Talele, C.; Shah, N.; Talele, D. Impact of artificial intelligence on drug development and delivery. In Current Topics in Medicinal Chemistry; Bentham Science Publishers: Sharjah, United Arab Emirates, 2024. [Google Scholar]
- Gholap, A.D.; Uddin, M.J.; Faiyazuddin, M.; Omri, A.; Gowri, S.; Khalid, M. Advances in Artificial Intelligence in Drug Delivery and Development: A Comprehensive Review. Comput. Biol. Med. 2024, 178, 108702. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial intelligence in pharmaceutical technology and drug delivery design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef]
- Ashique, S.; Garg, A.; Hussain, A.; Farid, A.; Kumar, P.; Taghizadeh-Hesary, F. Nanodelivery systems: An efficient and target-specific approach for drug-resistant cancers. Cancer Med. 2023, 12, 18797–18825. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, Q.; Duan, T.; Hu, R.; Tang, J. The fate of nanoparticles in vivo and the strategy of designing stealth nanoparticle for drug delivery. Curr. Drug Targets 2021, 22, 922–946. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Kang, Y.; Huang, K.; Gu, Z.; Wu, J. Advances in long-circulating drug delivery strategy. Curr. Drug Metab. 2018, 19, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, P.; Haddadi, A. Pharmacokinetic consequences of PLGA nanoparticles in docetaxel drug delivery. Pharm. Nanotechnol. 2017, 5, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Mozar, F.S.; Chowdhury, E.H. Impact of PEGylated nanoparticles on tumor targeted drug delivery. Curr. Pharm. Des. 2018, 24, 3283–3296. [Google Scholar] [CrossRef] [PubMed]
- Sofou, S.; Sgouros, G. Antibody-targeted liposomes in cancer therapy and imaging. Expert Opin. Drug Deliv. 2008, 5, 189–204. [Google Scholar] [CrossRef]
- Srinivasarao, M.; Galliford, C.V.; Low, P.S. Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat. Rev. Drug Discov. 2015, 14, 203–219. [Google Scholar] [CrossRef]
- Srinivasarao, M.; Low, P.S. Ligand-targeted drug delivery. Chem. Rev. 2017, 117, 12133–12164. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and limitations of dendrimers in biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef]
- Lyu, Z.; Ding, L.; Tintaru, A.; Peng, L. Self-assembling supramolecular dendrimers for biomedical applications: Lessons learned from poly (amidoamine) dendrimers. Acc. Chem. Res. 2020, 53, 2936–2949. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Medina, S.H.; El-Sayed, M.E. Dendrimers as carriers for delivery of chemotherapeutic agents. Chem. Rev. 2009, 109, 3141–3157. [Google Scholar] [CrossRef] [PubMed]
- Chis, A.A.; Dobrea, C.M.; Rus, L.-L.; Frum, A.; Morgovan, C.; Butuca, A.; Totan, M.; Juncan, A.M.; Gligor, F.G.; Arseniu, A.M. Dendrimers as non-viral vectors in gene-directed enzyme prodrug therapy. Molecules 2021, 26, 5976. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wang, M.; Wang, C.; Wang, Y.; Sun, C.; Zeng, Z.; Cui, H.; Zhao, X. Application of non-viral vectors in drug delivery and gene therapy. Polymers 2021, 13, 3307. [Google Scholar] [CrossRef]
- Tarach, P.; Janaszewska, A. Recent advances in preclinical research using PAMAM dendrimers for cancer gene therapy. Int. J. Mol. Sci. 2021, 22, 2912. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Singh, J.; Ilyas, R.A.; Asyraf, M.R.M.; Razman, M.R. Critical review of biodegradable and bioactive polymer composites for bone tissue engineering and drug delivery applications. Polymers 2021, 13, 2623. [Google Scholar] [CrossRef]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Lipid-based nanoparticles for drug delivery systems. In Characterization and Biology of Nanomaterials for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–76. [Google Scholar]
- Perinelli, D.R.; Cespi, M.; Bonacucina, G.; Palmieri, G.F. PEGylated polylactide (PLA) and poly (lactic-co-glycolic acid)(PLGA) copolymers for the design of drug delivery systems. J. Pharm. Investig. 2019, 49, 443–458. [Google Scholar] [CrossRef]
- Pala, R.; Zeng, Y.; Pattnaik, S.; Busi, S.; Alomari, N.; Nauli, S.M.; Liu, G. Functionalized silver nanoparticles for sensing, molecular imaging and therapeutic applications. Curr. Nanomed. (Former. Recent Pat. Nanomed.) 2018, 8, 234–250. [Google Scholar] [CrossRef]
- Attia, M.F.; Wallyn, J.; Anton, N.; Vandamme, T.F. Inorganic nanoparticles for X-ray computed tomography imaging. Crit. Rev.™ Ther. Drug Carr. Syst. 2018, 35, 391–431. [Google Scholar] [CrossRef]
- Kwiatkowska, A.; Drabik, M.; Lipko, A.; Grzeczkowicz, A.; Stachowiak, R.; Marszalik, A.; Granicka, L.H. Composite membrane dressings system with metallic nanoparticles as an antibacterial factor in wound healing. Membranes 2022, 12, 215. [Google Scholar] [CrossRef]
- Sakthi Devi, R.; Girigoswami, A.; Siddharth, M.; Girigoswami, K. Applications of gold and silver nanoparticles in theranostics. Appl. Biochem. Biotechnol. 2022, 194, 4187–4219. [Google Scholar] [CrossRef]
- Jindal, M.; Nagpal, M.; Singh, M.; Aggarwal, G.; Dhingra, G.A. Gold nanoparticles-boon in cancer theranostics. Curr. Pharm. Des. 2020, 26, 5134–5151. [Google Scholar] [CrossRef]
- Rahman, M. Magnetic resonance imaging and iron-oxide nanoparticles in the era of personalized medicine. Nanotheranostics 2023, 7, 424. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandit, S.; Balusamy, S.R.; Madhusudanan, M.; Singh, H.; Amsath Haseef, H.M.; Mijakovic, I. Advanced Nanomaterials for Cancer Therapy: Gold, Silver, and Iron Oxide Nanoparticles in Oncological Applications. Adv. Healthc. Mater. 2024, 14, 2403059. [Google Scholar] [CrossRef] [PubMed]
- Raka, S.; Belemkar, S.; Bhattacharya, S. Hybrid Nanoparticles for Cancer Theranostics: A Critical Review on Design, Synthesis, and Multifunctional Capabilities. In Current Medicinal Chemistry; Bentham Science Publishers: Sharjah, United Arab Emirates, 2025. [Google Scholar]
- Zhang, J.; Zhou, J.; Tang, L.; Ma, J.; Wang, Y.; Yang, H.; Wang, X.; Fan, W. Custom-Design of Multi-Stimuli-Responsive Degradable Silica Nanoparticles for Advanced Cancer-Specific Chemotherapy. Small 2024, 20, 2400353. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Minko, T. Targeted nanoparticle-based diagnostic and treatment options for pancreatic cancer. Cancers 2024, 16, 1589. [Google Scholar] [CrossRef]
- Ray, A.; Mandal, A.; Joseph, M.; K Mitra, A. Recent patents on nanoparticles and nanoformulations for cancer therapy. Recent Pat. Drug Deliv. Formul. 2016, 10, 11–23. [Google Scholar] [CrossRef]
- Almawash, S.; Osman, S.K.; Mustafa, G.; El Hamd, M.A. Current and future prospective of injectable hydrogels—Design challenges and limitations. Pharmaceuticals 2022, 15, 371. [Google Scholar] [CrossRef]
- Liao, J.; Timoshenko, A.B.; Cordova, D.J.; Astudillo Potes, M.D.; Gaihre, B.; Liu, X.; Elder, B.D.; Lu, L.; Tilton, M. Propelling Minimally Invasive Tissue Regeneration With Next-Era Injectable Pre-Formed Scaffolds. Adv. Mater. 2024, 36, 2400700. [Google Scholar] [CrossRef]
- Sua, P.; Nowaczykc, G.; Wanga, W. Injectable bioactive materials for myocardial regeneration. In Bioactive Materials for Soft Tissue Regeneration; Elsevier: Amsterdam, The Netherlands, 2024; p. 115. [Google Scholar]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C. Self-healing injectable hydrogels for tissue regeneration. Chem. Rev. 2022, 123, 834–873. [Google Scholar] [CrossRef]
- Lee, J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res. 2018, 22, 27. [Google Scholar] [CrossRef]
- Poustchi, F.; Amani, H.; Ahmadian, Z.; Niknezhad, S.V.; Mehrabi, S.; Santos, H.A.; Shahbazi, M.A. Combination therapy of killing diseases by injectable hydrogels: From concept to medical applications. Adv. Healthc. Mater. 2021, 10, 2001571. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Nan, D.; Jin, H.; Qu, X. Recent advances of injectable hydrogels for drug delivery and tissue engineering applications. Polym. Test. 2020, 81, 106283. [Google Scholar] [CrossRef]
- Solanki, R.; Bhatia, D. Stimulus-responsive hydrogels for targeted cancer therapy. Gels 2024, 10, 440. [Google Scholar] [CrossRef]
- Hu, S.; Zhao, R.; Shen, Y.; Lyu, B. Revolutionizing drug delivery: The power of stimulus-responsive nanoscale systems. Chem. Eng. J. 2024, 496, 154265. [Google Scholar] [CrossRef]
- Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive hydrogels and advances in their application in disease therapy. Polymers 2022, 14, 2379. [Google Scholar] [CrossRef] [PubMed]
- AlSawaftah, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. pH-responsive nanocarriers in cancer therapy. Polymers 2022, 14, 936. [Google Scholar] [CrossRef]
- Yi, Y.-H.; Chen, G.; Gong, S.; Han, L.-Z.; Gong, T.-L.; Wang, Y.-X.; Xu, W.-H.; Jin, X. Injectable temperature-sensitive hydrogel loaded with IL-36Ra for the relief of osteoarthritis. ACS Biomater. Sci. Eng. 2023, 9, 1672–1681. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.; Kim, M.S. Intra-articular hydrogel formulation prolongs the in vivo stability of Toll-like receptor antagonistic peptides for rheumatoid arthritis treatment. J. Control. Release 2024, 372, 467–481. [Google Scholar] [CrossRef]
- Ramli, I.; Cheriet, T.; Posadino, A.M.; Giordo, R.; Zayed, H.; Eid, A.H.; Pintus, G. Potential therapeutic targets of resveratrol in the prevention and treatment of pulmonary fibrosis. Front. Biosci. 2023, 28, 198. [Google Scholar] [CrossRef]
- Unsoy, G.; Gunduz, U. Smart drug delivery systems in cancer therapy. Curr. Drug Targets 2018, 19, 202–212. [Google Scholar] [CrossRef]
- Xia, W.; Tao, Z.; Zhu, B.; Zhang, W.; Liu, C.; Chen, S.; Song, M. Targeted delivery of drugs and genes using polymer nanocarriers for cancer therapy. Int. J. Mol. Sci. 2021, 22, 9118. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Zheng, G. Improving accessibility of EPR-insensitive tumor phenotypes using EPR-adaptive strategies: Designing a new perspective in nanomedicine delivery. Theranostics 2019, 9, 8091. [Google Scholar] [CrossRef]
- Eetezadi, S. Nanomedicines and Combination Therapy of Doxorubicin and Olaparib for Treatment of Ovarian Cancer. ProQuest Dissertations & Thesis, University of Toronto (Canada), Toronto, ON, Canada, 2016. [Google Scholar]
- Wang, C.; Xu, J.; Zhang, Y.; Nie, G. Emerging nanotechnological approaches to regulating tumor vasculature for cancer therapy. J. Control. Release 2023, 362, 647–666. [Google Scholar] [CrossRef] [PubMed]
- Kalita, P. The Role of Nanoparticles in Pharmaceuticals: A Comprehensive. Preprints 2025. [Google Scholar] [CrossRef]
- Soni, N.; Tekade, M.; Kesharwani, P.; Bhattacharya, P.; Maheshwari, R.; Dua, K.; Hansbro, P.M.; Kumar Tekade, R. Recent advances in oncological submissions of dendrimer. Curr. Pharm. Des. 2017, 23, 3084–3098. [Google Scholar] [CrossRef] [PubMed]
- Yanamandala, N.; Achalla, P.K.; Dubey, S.K. Polymeric micelles and dendrimer drug delivery. In Nanocosmetics: Delivery Approaches, Applications and Regulatory Aspects; CRC Press: Boca Raton, FL, USA, 2023; pp. 205–219. [Google Scholar]
- Crintea, A.; Motofelea, A.C.; Șovrea, A.S.; Constantin, A.-M.; Crivii, C.-B.; Carpa, R.; Duțu, A.G. Dendrimers: Advancements and potential applications in cancer diagnosis and treatment—An overview. Pharmaceutics 2023, 15, 1406. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, H.; Govindaraju, T. Dendrimer architectonics to treat cancer and neurodegenerative diseases with implications in theranostics and personalized medicine. ACS Appl. Bio Mater. 2021, 4, 1115–1139. [Google Scholar] [CrossRef]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef]
- Ghafari, Y.; Asefnejad, A.; Ogbemudia, D.O. Gold Nanoparticles in Biomedicine: Advancements in Cancer Therapy, Drug Delivery, Diagnostics, and Tissue Regeneration. Sci. Hypotheses 2024, 1, 21–35. [Google Scholar] [CrossRef]
- Al-Thani, A.N.; Jan, A.G.; Abbas, M.; Geetha, M.; Sadasivuni, K.K. Nanoparticles in cancer theragnostic and drug delivery: A comprehensive review. Life Sci. 2024, 352, 122899. [Google Scholar] [CrossRef]
- Javid, H.; Oryani, M.A.; Rezagholinejad, N.; Hashemzadeh, A.; Karimi-Shahri, M. Unlocking the potential of RGD-conjugated gold nanoparticles: A new frontier in targeted cancer therapy, imaging, and metastasis inhibition. J. Mater. Chem. B 2024, 12, 10786–10817. [Google Scholar] [CrossRef]
- Kiessling, F.; Fokong, S.; Koczera, P.; Lederle, W.; Lammers, T. Ultrasound microbubbles for molecular diagnosis, therapy, and theranostics. J. Nucl. Med. 2012, 53, 345–348. [Google Scholar] [CrossRef]
- Liu, M.; Dasgupta, A.; Koczera, P.; Schipper, S.; Rommel, D.; Shi, Y.; Kiessling, F.; Lammers, T. Drug loading in poly (butyl cyanoacrylate)-based polymeric microbubbles. Mol. Pharm. 2020, 17, 2840–2848. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Jafari, K.; Naghib, S.M.; Mozafari, M. Cisplatin-based Liposomal Nanocarriers for Drug Delivery in Lung Cancer Therapy: Recent Progress and Future Outlooks. Curr. Pharm. Des. 2024, 30, 2850–2881. [Google Scholar] [CrossRef] [PubMed]
- Salatin, S.; Azarfarin, M.; Farjami, A.; Hamidi, S. The simultaneous use of nanovesicles and magnetic nanoparticles for cancer targeting and imaging. Ther. Deliv. 2024, 16, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Yuqian, W.; Renqi, H.; Shufan, F.; Ran, M. Advances in nanocarriers for targeted drug delivery and controlled drug release. Chin. J. Nat. Med. 2024, 23, 1–21. [Google Scholar]
- Wahengbam, G.S.; Nirmal, S.; Nandwana, J.; Kar, S.; Kumari, V.; Mishra, R.; Singh, A. Polymeric Nanoparticles Revolutionizing Brain Cancer Therapy: A Comprehensive Review of Strategies and Advances. Crit. Rev.™ Ther. Drug Carr. Syst. 2025, 42, 73–106. [Google Scholar] [CrossRef]
- Xiong, R.; Zhu, X.; Zhao, J.; Ling, G.; Zhang, P. Nanozymes-Mediated Cascade Reaction System for Tumor-Specific Diagnosis and Targeted Therapy. Small Methods 2024, 8, 2301676. [Google Scholar] [CrossRef]
- Nyamba, I.; Sombie, C.B.; Yabre, M.; Zime-Diawara, H.; Yameogo, J.; Ouedraogo, S.; Lechanteur, A.; Semde, R.; Evrard, B. Pharmaceutical approaches for enhancing solubility and oral bioavailability of poorly soluble drugs. Eur. J. Pharm. Biopharm. 2024, 204, 114513. [Google Scholar] [CrossRef]
- Kang, W.; Xu, Z.; Lu, H.; Liu, S.; Li, J.; Ding, C.; Lu, Y. Advances in biomimetic nanomaterial delivery systems: Harnessing nature’s inspiration for targeted drug delivery. J. Mater. Chem. B 2024, 12, 7001–7019. [Google Scholar] [CrossRef]
- Yu, T.; Xu, Q.; Chen, X.; Deng, X.; Chen, N.; Kou, M.T.; Huang, Y.; Guo, J.; Xiao, Z.; Wang, J. Biomimetic nanomaterials in myocardial infarction treatment: Harnessing bionic strategies for advanced therapeutics. Mater. Today Bio 2024, 25, 100957. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Fu, Q.; Lu, Z.; Jin, Q.; Jin, T.; Zhang, M. Ginsenoside Rg3 combined with near-infrared photothermal reversal of multidrug resistance in breast cancer MCF-7/ADR cells. Food Sci. Nutr. 2024, 12, 5750–5761. [Google Scholar] [CrossRef]
- Xiang, Y.; Chen, Q.; Nan, Y.; Liu, M.; Xiao, Z.; Yang, Y.; Zhang, J.; Ying, X.; Long, X.; Wang, S.; et al. Nitric Oxide-Based Nanomedicines for Conquering TME Fortress: Say “NO” to Insufficient Tumor Treatment. Adv. Funct. Mater. 2024, 34, 2312092. [Google Scholar] [CrossRef]
- Poltronieri, P.; Joardar, S. Unravelling the Interplay between Biomolecular Condensates and RNA in Cancer and Diseases. J. Biol. Regul. Homeost. Agents 2024, 38, 5627–5652. [Google Scholar] [CrossRef]
- Uppalapati, S.S.; Guha, L.; Kumar, H.; Mandoli, A. Nanotechnological Advancements For The Theranostic Intervention In Anaplastic Thyroid Cancer: Current Perspectives And Future Direction. Curr. Cancer Drug Targets 2024, 24, 245–270. [Google Scholar] [CrossRef]
- Ertas, Y.N.; Ertas, D.; Erdem, A.; Segujja, F.; Dulchavsky, S.; Ashammakhi, N. Diagnostic, Therapeutic, and Theranostic Multifunctional Microneedles. Small 2024, 20, 2308479. [Google Scholar] [CrossRef]
- Khattak, S.; Ullah, I.; Sohail, M.; Akbar, M.U.; Rauf, M.A.; Ullah, S.; Shen, J.; Xu, H.T. Endogenous/exogenous stimuli-responsive smart hydrogels for diabetic wound healing. Aggregate 2024, 6, e688. [Google Scholar] [CrossRef]
- Yu, H.; Gao, R.; Liu, Y.; Fu, L.; Zhou, J.; Li, L. Stimulus-Responsive Hydrogels as Drug Delivery Systems for Inflammation Targeted Therapy. Adv. Sci. 2024, 11, 2306152. [Google Scholar] [CrossRef]
- Caturano, A.; Nilo, R.; Nilo, D.; Russo, V.; Santonastaso, E.; Galiero, R.; Rinaldi, L.; Monda, M.; Sardu, C.; Marfella, R. Advances in nanomedicine for precision insulin delivery. Pharmaceuticals 2024, 17, 945. [Google Scholar] [CrossRef]
- Sabbagh, F.; Muhamad, I.I.; Niazmand, R.; Dikshit, P.K.; Kim, B.S. Recent progress in polymeric non-invasive insulin delivery. Int. J. Biol. Macromol. 2022, 203, 222–243. [Google Scholar] [CrossRef]
- Yao, Y.; Ji, K.; Wang, Y.; Gu, Z.; Wang, J. Materials and carriers development for glucose-responsive insulin. Acc. Mater. Res. 2022, 3, 960–970. [Google Scholar] [CrossRef]
- Ma, Q.; Bian, L.; Zhao, X.; Tian, X.; Yin, H.; Wang, Y.; Shi, A.; Wu, J. Novel glucose-responsive nanoparticles based on p-hydroxyphenethyl anisate and 3-acrylamidophenylboronic acid reduce blood glucose and ameliorate diabetic nephropathy. Mater. Today Bio 2022, 13, 100181. [Google Scholar] [CrossRef]
- Behzadifar, S.; Barras, A.; Plaisance, V.; Pawlowski, V.; Szunerits, S.; Abderrahmani, A.; Boukherroub, R. Polymer-Based Nanostructures for Pancreatic Beta-Cell Imaging and Non-Invasive Treatment of Diabetes. Pharmaceutics 2023, 15, 1215. [Google Scholar] [CrossRef] [PubMed]
- Icart, L.P.; Souza, F.G., Jr.; Lima, L.M.T. Polymeric microparticle systems for modified release of glucagon-like-peptide-1 receptor agonists. J. Microencapsul. 2021, 38, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Adwani, G.; Bharti, S.; Kumar, A. Engineered nanoparticles in non-invasive insulin delivery for precision therapeutics of diabetes. Int. J. Biol. Macromol. 2024, 275, 133437. [Google Scholar] [CrossRef]
- Wen, Y.; Li, Y.; Yang, R.; Chen, Y.; Shen, Y.; Liu, Y.; Liu, X.; Zhang, B.; Li, H. Biofunctional coatings and drug-coated stents for restenosis therapy. Mater. Today Bio 2024, 29, 101259. [Google Scholar] [CrossRef]
- Zong, J.; He, Q.; Liu, Y.; Qiu, M.; Wu, J.; Hu, B. Advances in the development of biodegradable coronary stents: A translational perspective. Mater. Today Bio 2022, 16, 100368. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Mitra, I.; Goodman, S.B.; Kumar, M.; Bose, S. Improving biocompatibility for next generation of metallic implants. Prog. Mater. Sci. 2023, 133, 101053. [Google Scholar] [CrossRef]
- Pan, C.; Liu, X.; Hong, Q.; Chen, J.; Cheng, Y.; Zhang, Q.; Meng, L.; Dai, J.; Yang, Z.; Wang, L. Recent advances in surface endothelialization of the magnesium alloy stent materials. J. Magnes. Alloys 2023, 11, 48–77. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, Y.; Yang, L.; Wu, Y.; Chen, N.; Li, M.; Li, Y.; Wan, H.; Fu, D.; Luo, R.; et al. A polyphenol-network-mediated coating modulates inflammation and vascular healing on vascular stents. ACS Nano 2022, 16, 6585–6597. [Google Scholar] [CrossRef]
- Mahajan, K.; Bhattacharya, S. The Advancement and Obstacles in Improving the Stability of Nanocarriers for Precision Drug Delivery in the Field of Nanomedicine. Curr. Top. Med. Chem. 2024, 24, 686–721. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; M Kang, P. Recent advances in nanocarrier-assisted therapeutics delivery systems. Pharmaceutics 2020, 12, 837. [Google Scholar] [CrossRef]
- Nikolić, D.D.; Filipović, N. Use Case: Stent Biodegradation Modeling. In In Silico Clinical Trials for Cardiovascular Disease: A Finite Element and Machine Learning Approach; Springer: Cham, Switzerland, 2024; pp. 303–334. [Google Scholar]
- Parvin, N.; Joo, S.W.; Mandal, T.K. Biodegradable and Stimuli-Responsive Nanomaterials for Targeted Drug Delivery in Autoimmune Diseases. J. Funct. Biomater. 2025, 16, 24. [Google Scholar] [CrossRef]
- Nag, S.; Mohanto, S.; Ahmed, M.G.; Subramaniyan, V. “Smart” stimuli-responsive biomaterials revolutionizing the theranostic landscape of inflammatory arthritis. Mater. Today Chem. 2024, 39, 102178. [Google Scholar] [CrossRef]
- Filipczak, N.; Yalamarty, S.S.K.; Li, X.; Khan, M.M.; Parveen, F.; Torchilin, V. Lipid-based drug delivery systems in regenerative medicine. Materials 2021, 14, 5371. [Google Scholar] [CrossRef]
- Maher, R.; Moreno-Borrallo, A.; Jindal, D.; Mai, B.T.; Ruiz-Hernandez, E.; Harkin, A. Intranasal polymeric and lipid-based nanocarriers for CNS drug delivery. Pharmaceutics 2023, 15, 746. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.; Patil, A. Nanotechnology-Based Approaches for Parkinson’s Disease: Progress in Drug Delivery and Regenerative Medicine. J. Drug Deliv. Biother. 2024, 1, 48–57. [Google Scholar]
- Mohi-Ud-Din, R.; Mir, R.H.; Wani, T.U.; Shah, A.J.; Banday, N.; Pottoo, F.H. Berberine in the treatment of neurodegenerative diseases and nanotechnology enabled targeted delivery. Comb. Chem. High Throughput Screen. 2022, 25, 616–633. [Google Scholar] [CrossRef]
- Panghal, A.; Flora, S. Nano-based approaches for the treatment of neuro-immunological disorders: A special emphasis on multiple sclerosis. Discov. Nano 2024, 19, 171. [Google Scholar] [CrossRef]
- Mottaqi, M.; Langarizadeh, M.A.; Molaali, N.; Rezaei, M.; Ameri, A.; Forootanfar, H. The synthesis, application and therapeutic perspectives of medicinal plants-based solid lipid nanoparticles: A comprehensive review. Adv. Tradit. Med. 2024, 25, 69–105. [Google Scholar] [CrossRef]
- Gupta, J.; Sharma, G. Nanogel: A versatile drug delivery system for the treatment of various diseases and their future perspective. Drug Deliv. Transl. Res. 2024, 15, 455–482. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, V.; Nivetha, R.; Tamilanban, T.; Narayanan, J.; Vetriselvan, S.; Fuloria, N.K.; Chinni, S.V.; Sekar, M.; Fuloria, S.; Wong, L.S.; et al. Nanogels as novel drug nanocarriers for CNS drug delivery. Front. Mol. Biosci. 2023, 10, 1232109. [Google Scholar] [CrossRef]
- Collins, M.N.; Zamboni, F.; Serafin, A.; Escobar, A.; Stepanian, R.; Culebras, M.; Reis, R.L.; Oliveira, J.M. Emerging scaffold-and cellular-based strategies for brain tissue regeneration and imaging. Vitr. Models 2022, 1, 129–150. [Google Scholar] [CrossRef]
- Chauhan, A.; Alam, M.A.; Kaur, A.; Malviya, R. Advancements and utilizations of scaffolds in tissue engineering and drug delivery. Curr. Drug Targets 2023, 24, 13–40. [Google Scholar] [PubMed]
- Li, Q.; Shao, X.; Dai, X.; Guo, Q.; Yuan, B.; Liu, Y.; Jiang, W. Recent trends in the development of hydrogel therapeutics for the treatment of central nervous system disorders. NPG Asia Mater. 2022, 14, 14. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Bao, N.S.; Van Vo, G. Advances in hydrogel-based drug delivery systems for Parkinson’s disease. Neurochem. Res. 2022, 47, 2129–2141. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Sharma, M.; Bani, K.S.; Gupta, S.K. Advances in neuronal regeneration: Hydrogel-based delivery systems loaded with extracellular vesicles in modulating neural impulses and tissue repair. Eur. Polym. J. 2024, 220, 113457. [Google Scholar] [CrossRef]
- Sharma, P.; Kaur, N.; Shanavas, A. Targeting strategies using PLGA nanoparticles for efficient drug delivery. In Poly (Lactic-co-Glycolic Acid)(PLGA) Nanoparticles for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2023; pp. 123–151. [Google Scholar]
- Zhuo, Z.; Wang, J.; Luo, Y.; Zeng, R.; Zhang, C.; Zhou, W.; Guo, K.; Wu, H.; Sha, W.; Chen, H. Targeted extracellular vesicle delivery systems employing superparamagnetic iron oxide nanoparticles. Acta Biomater. 2021, 134, 13–31. [Google Scholar] [CrossRef]
- Sun, J. Development of Molecular Transporter Platforms for the Delivery of Clinically Relevant Antibiotics and Genes. Ph.D. Thesis, Stanford University, Stanford, CA, USA, 2024. [Google Scholar]
- Donnelly, R.F.; Hettie, K.S.; Chang, L.; Gendelman, H.E.; Kevadiya, B.D. Nanocarrier vaccines for SARS-CoV-2. Adv. Drug Deliv. Rev. 2021, 171, 215–239. [Google Scholar]
- Nalla, L.V.; Gajula, S.N.; Chavda, V.P. Nanoparticle-Based mRNA Vaccines: Are We One Step Closer to Targeted Cancer Therapy? In Nanocarrier Vaccines: Biopharmaceutics-Based Fast Track Development; Scrivener Publishing LLC: Beverly, MA, USA, 2024; pp. 275–303. [Google Scholar]
- Puccetti, M.; Pariano, M.; Schoubben, A.; Ricci, M.; Giovagnoli, S. Engineering carrier nanoparticles with biomimetic moieties for improved intracellular targeted delivery of mRNA therapeutics and vaccines. J. Pharm. Pharmacol. 2024, 76, 592–605. [Google Scholar] [CrossRef]
- Ramachandran, S.; Satapathy, S.R.; Dutta, T. Delivery strategies for mRNA vaccines. Pharm. Med. 2022, 36, 11–20. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, E.; Yang, X.; Luo, C.; Zi, G.; Wang, R.; Xu, Y.; Peng, B. Entrapment of lipid nanoparticles in peripheral endosomes but not lysosomes impairs intracellular trafficking and endosomal escape. Int. J. Pharm. 2025, 669, 125024. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, L.; Rossi, R.; Sechi, F.; Buonocore, D.; Sorrenti, M.; Perteghella, S.; Peviani, M.; Bonferoni, M.C. Effect of Lipid Nanoparticle Physico-Chemical Properties and Composition on Their Interaction with the Immune System. Pharmaceutics 2024, 16, 1521. [Google Scholar] [CrossRef] [PubMed]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of lipid nanoparticles for RNA delivery. Acc. Chem. Res. 2021, 55, 2–12. [Google Scholar] [CrossRef]
- Atella, V.; Scandizzo, P.L. The COVID-19 Disruption and the Global Health Challenge; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Kiaie, S.H.; Majidi Zolbanin, N.; Ahmadi, A.; Bagherifar, R.; Valizadeh, H.; Kashanchi, F.; Jafari, R. Recent advances in mRNA-LNP therapeutics: Immunological and pharmacological aspects. J. Nanobiotechnol. 2022, 20, 276. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ye, Z.; Huang, C.; Qiu, M.; Song, D.; Li, Y.; Xu, Q. Lipid nanoparticle-mediated lymph node–targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc. Natl. Acad. Sci. USA 2022, 119, e2207841119. [Google Scholar] [CrossRef]
- Bettini, E.A. Untangling the Adjuvanticity of mRNA Vaccines: Nucleoside-Modified mRNA and Lipid Nanoparticles Cooperate to Drive T Follicular Helper Cell Responses. Ph.D. Thesis, University of Pennsylvania, Philadelphia, PA, USA, 2024. [Google Scholar]
- Huang, L.; Huang, Z.; Zhang, Y.; Lin, C.; Zhao, Z.; Li, R.; Saw, P.E.; Xu, X. Advances in targeted delivery of mRNA into immune cells for enhanced cancer therapy. Theranostics 2024, 14, 5528. [Google Scholar] [CrossRef]
- Li, Z.Z.; Zhong, N.N.; Cao, L.M.; Cai, Z.M.; Xiao, Y.; Wang, G.R.; Liu, B.; Xu, C.; Bu, L.L. Nanoparticles targeting lymph nodes for cancer immunotherapy: Strategies and influencing factors. Small 2024, 20, 2308731. [Google Scholar] [CrossRef]
- Shin, H.E.; Han, J.H.; Park, J.D.; Park, M.; Han, J.; Kang, M.H.; Lee, J.S.; Park, C.G.; Park, J.; Kim, H.Y.; et al. Enhancing CAR-NK Cells Against Solid Tumors Through Chemical and Genetic Fortification with DOTAP-Functionalized Lipid Nanoparticles. Adv. Funct. Mater. 2024, 34, 2315721. [Google Scholar] [CrossRef]
- John, R.; Monpara, J.; Swaminathan, S.; Kalhapure, R. Chemistry and art of developing lipid nanoparticles for biologics delivery: Focus on development and scale-up. Pharmaceutics 2024, 16, 131. [Google Scholar] [CrossRef]
- Deyhimfar, R.; Izady, M.; Shoghi, M.; Kazazi, M.H.; Ghazvini, Z.F.; Nazari, H.; Fekrirad, Z.; Arefian, E. The clinical impact of mRNA therapeutics in the treatment of cancers, infections, genetic disorders, and autoimmune diseases. Heliyon 2024, 10, e26971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Tang, T.; Chen, Y.; Huang, X.; Liang, T. mRNA vaccines in disease prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Chavda, V.; Singh, T.R.R.; Thorat, N.D.; Vora, L.K. Cancer nanovaccines: Nanomaterials and clinical perspectives. Small 2024, 20, 2401631. [Google Scholar] [CrossRef]
- Liang, J.; Yao, L.; Liu, Z.; Chen, Y.; Lin, Y.; Tian, T. Nanoparticles in Subunit Vaccines: Immunological Foundations, Categories, and Applications. Small 2025, 21, 2407649. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.-W.; Gao, Y.; Feng, Z.; Mooney, D.J.; Mitragotri, S. Designing drug delivery systems for cell therapy. Nat. Rev. Bioeng. 2024, 2, 944–959. [Google Scholar] [CrossRef]
- Goyal, P.; Malviya, R. Advancement in Polymer-based Carrier for DNA Vaccine. Curr. Pharm. Des. 2023, 29, 2062–2077. [Google Scholar] [CrossRef]
- Punchihewa, B.T.; Perera, A.; de Souza, F.M.; Gupta, R.K. Specialty Polymers for Biomedical Applications. In Specialty Polymers; CRC Press: Boca Raton, FL, USA, 2023; pp. 345–369. [Google Scholar]
- Sultana, A.; Zare, M.; Luo, H.; Ramakrishna, S. Surface engineering strategies to enhance the in situ performance of medical devices including atomic scale engineering. Int. J. Mol. Sci. 2021, 22, 11788. [Google Scholar] [CrossRef]
- Sapuan, S.; Harussani, M.; Ilyas, R.A. Advanced Composites: Applications for COVID-19 and Beyond; Springer Nature: Cham, Switzerland, 2024. [Google Scholar]
- Euliano, E.M.; Sklavounos, A.A.; Wheeler, A.R.; McHugh, K.J. Translating diagnostics and drug delivery technologies to low-resource settings. Sci. Transl. Med. 2022, 14, eabm1732. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Sabri, A.H.B.; Hutton, A.J.; Cárcamo-Martínez, Á.; Wardoyo, L.A.H.; Mansoor, A.Z.; Donnelly, R.F. Microarray patches for managing infections at a global scale. J. Control. Release 2023, 359, 97–115. [Google Scholar] [CrossRef]
- Parhi, R. Recent advances in microneedle designs and their applications in drug and cosmeceutical delivery. J. Drug Deliv. Sci. Technol. 2022, 75, 103639. [Google Scholar] [CrossRef]
- Al-Nimry, S.S.; Daghmash, R.M. Three dimensional printing and its applications focusing on microneedles for drug delivery. Pharmaceutics 2023, 15, 1597. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.S.; Saouaf, O.M.; Baillet, J.; Appel, E.A. Sustained delivery approaches to improving adaptive immune responses. Adv. Drug Deliv. Rev. 2022, 187, 114401. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.; Shah, S.A.; Gebhardt, T.; Jewell, C.M. Exploiting unique features of microneedles to modulate immunity. Adv. Mater. 2023, 35, 2302410. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Mao, Y.; Jiang, T.; Gao, B.; He, B. Structural design strategies of microneedle-based vaccines for transdermal immunity augmentation. J. Control. Release 2022, 351, 907–922. [Google Scholar] [CrossRef]
- Shah, S.A.; Oakes, R.S.; Jewell, C.M. Advancing immunotherapy using biomaterials to control tissue, cellular, and molecular level immune signaling in skin. Adv. Drug Deliv. Rev. 2024, 209, 115315. [Google Scholar] [CrossRef]
- Fan, J.; Jin, S.; Gilmartin, L.; Toth, I.; Hussein, W.M.; Stephenson, R.J. Advances in infectious disease vaccine adjuvants. Vaccines 2022, 10, 1120. [Google Scholar] [CrossRef]
- Cui, Y.; Ho, M.; Hu, Y.; Shi, Y. Vaccine adjuvants: Current status, research and development, licensing, and future opportunities. J. Mater. Chem. B 2024, 12, 4118–4137. [Google Scholar] [CrossRef]
- Zou, Y.; Kamoi, K.; Zong, Y.; Zhang, J.; Yang, M.; Ohno-Matsui, K. Vaccines and the Eye: Current Understanding of the Molecular and Immunological Effects of Vaccination on the Eye. Int. J. Mol. Sci. 2024, 25, 4755. [Google Scholar] [CrossRef]
- Li, Q.; Liu, X.; Yan, C.; Zhao, B.; Zhao, Y.; Yang, L.; Shi, M.; Yu, H.; Li, X.; Luo, K. Polysaccharide-Based Stimulus-Responsive Nanomedicines for Combination Cancer Immunotherapy. Small 2023, 19, 2206211. [Google Scholar] [CrossRef]
- de Moura, I.A.; Silva, A.J.D.; de Macêdo, L.S.; Invenção, M.d.C.V.; de Sousa, M.M.G.; de Freitas, A.C. Enhancing the effect of nucleic acid vaccines in the treatment of HPV-related cancers: An overview of delivery systems. Pathogens 2022, 11, 1444. [Google Scholar] [CrossRef]
- Dhamodharan, G.; Mohan, C.G. Machine learning models for predicting the activity of AChE and BACE1 dual inhibitors for the treatment of Alzheimer’s disease. Mol. Divers. 2022, 26, 1501–1517. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Luciano, F.C.; Anaya, B.J.; Ongoren, B.; Kara, A.; Molina, G.; Ramirez, B.I.; Sánchez-Guirales, S.A.; Simon, J.A.; Tomietto, G.; et al. Artificial intelligence (AI) applications in drug discovery and drug delivery: Revolutionizing personalized medicine. Pharmaceutics 2024, 16, 1328. [Google Scholar] [CrossRef] [PubMed]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Tanani, M.; Satyam, S.M.; Rabbani, S.A.; El-Tanani, Y.; Aljabali, A.A.A.; Al Faouri, I.; Rehman, A. Revolutionizing Drug Delivery: The Impact of Advanced Materials Science and Technology on Precision Medicine. Pharmaceutics 2025, 17, 375. https://doi.org/10.3390/pharmaceutics17030375
El-Tanani M, Satyam SM, Rabbani SA, El-Tanani Y, Aljabali AAA, Al Faouri I, Rehman A. Revolutionizing Drug Delivery: The Impact of Advanced Materials Science and Technology on Precision Medicine. Pharmaceutics. 2025; 17(3):375. https://doi.org/10.3390/pharmaceutics17030375
Chicago/Turabian StyleEl-Tanani, Mohamed, Shakta Mani Satyam, Syed Arman Rabbani, Yahia El-Tanani, Alaa A. A. Aljabali, Ibrahim Al Faouri, and Abdul Rehman. 2025. "Revolutionizing Drug Delivery: The Impact of Advanced Materials Science and Technology on Precision Medicine" Pharmaceutics 17, no. 3: 375. https://doi.org/10.3390/pharmaceutics17030375
APA StyleEl-Tanani, M., Satyam, S. M., Rabbani, S. A., El-Tanani, Y., Aljabali, A. A. A., Al Faouri, I., & Rehman, A. (2025). Revolutionizing Drug Delivery: The Impact of Advanced Materials Science and Technology on Precision Medicine. Pharmaceutics, 17(3), 375. https://doi.org/10.3390/pharmaceutics17030375







