Burst-Free Sustained Release of Proteins from Thermal Gelling Polymer Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. The Synthesis of the PLGA–PEG–PLGA Tri-Block Copolymer
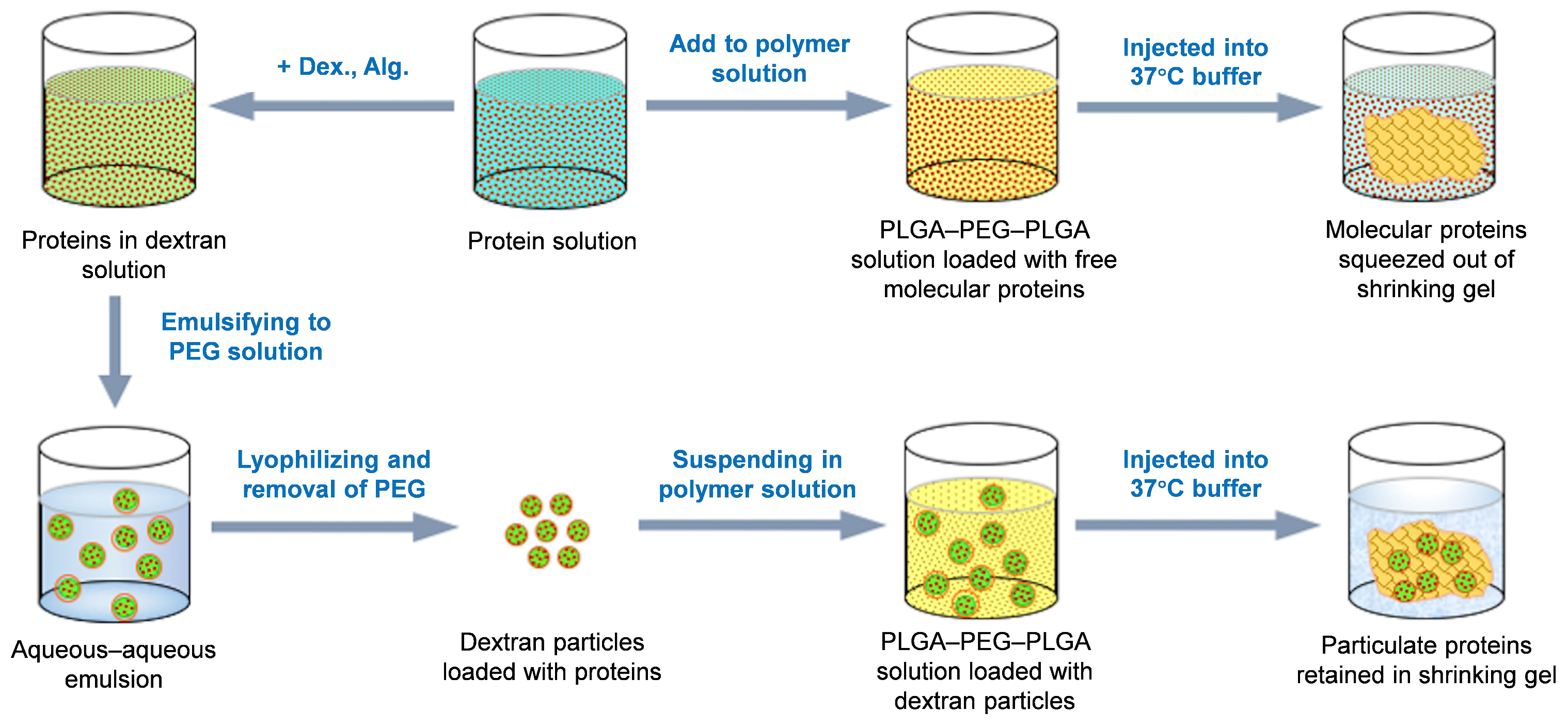
2.4. Preparation of the Protein-Loaded DEX Particles
2.5. Suspending Protein-Loaded Dextran Particles in the PLGA–PEG–PLGA Solution
2.6. Determination of Phase Transition Temperature
2.7. Confocal Imaging of the Polymer Gelling Process
2.8. Protein Activity Assessment Throughout the Formulation Process and In Vitro Release
2.9. In Vitro Release Profile Assays for the Proteins from the PLGA–PEG–PLGA Thermal Gel Formulations
2.10. In Vivo Release Profiles After Subcutaneous Injection of the PLGA–PEG–PLGA Thermal Gel Formulation
3. Results and Discussion
3.1. Insolubility of Polysaccharide Particles in the Aqueous PLGA–PEG–PLGA Solution
3.2. Retention of Polysaccharide Particles During the Polymer Gelling Process
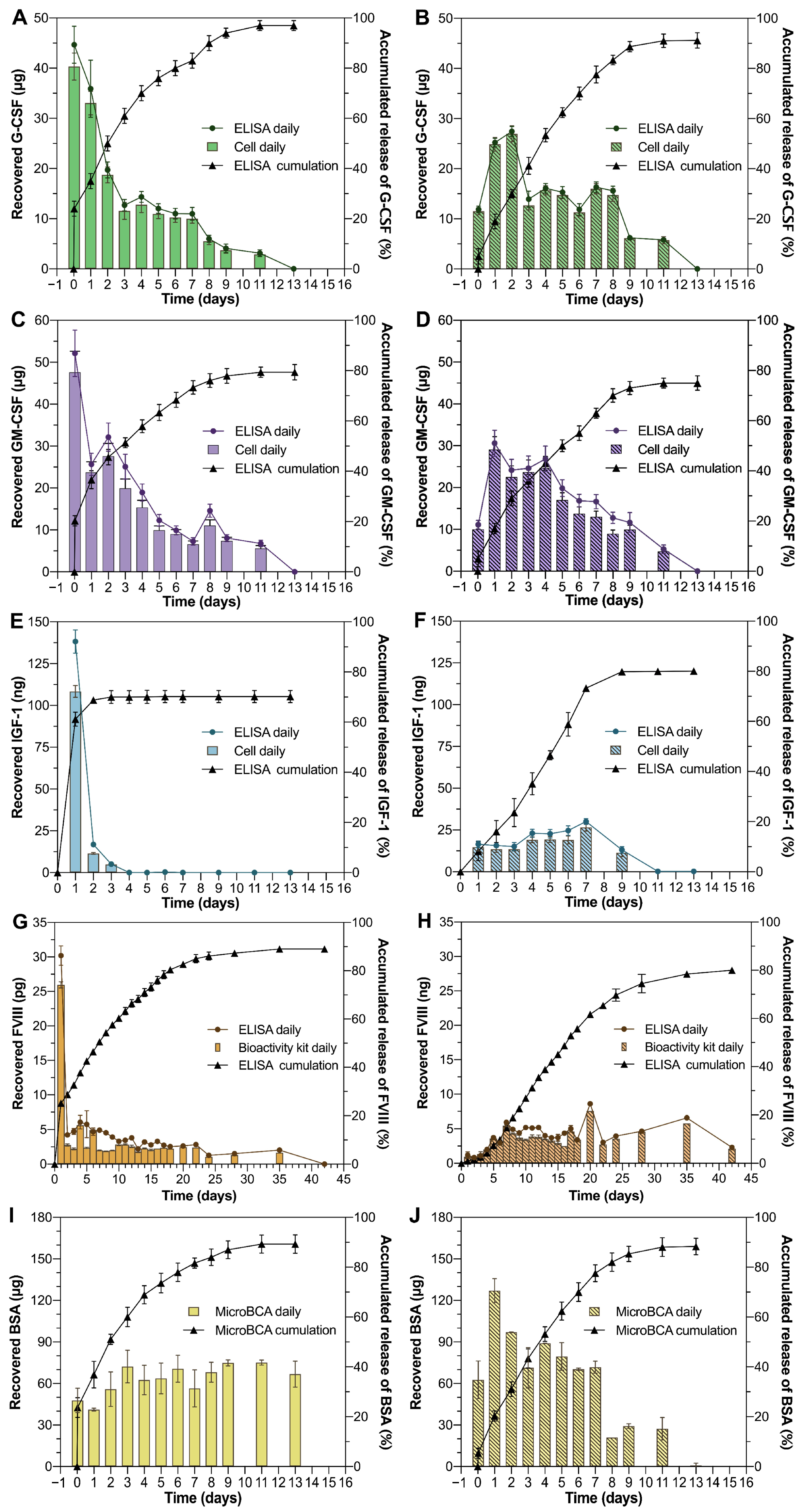
3.3. Preservation of Protein Activity During the Formulation Processes and Release Process
3.4. In Vitro Release Kinetics of Proteins from Thermal Gelling PLGA–PEG–PLGA
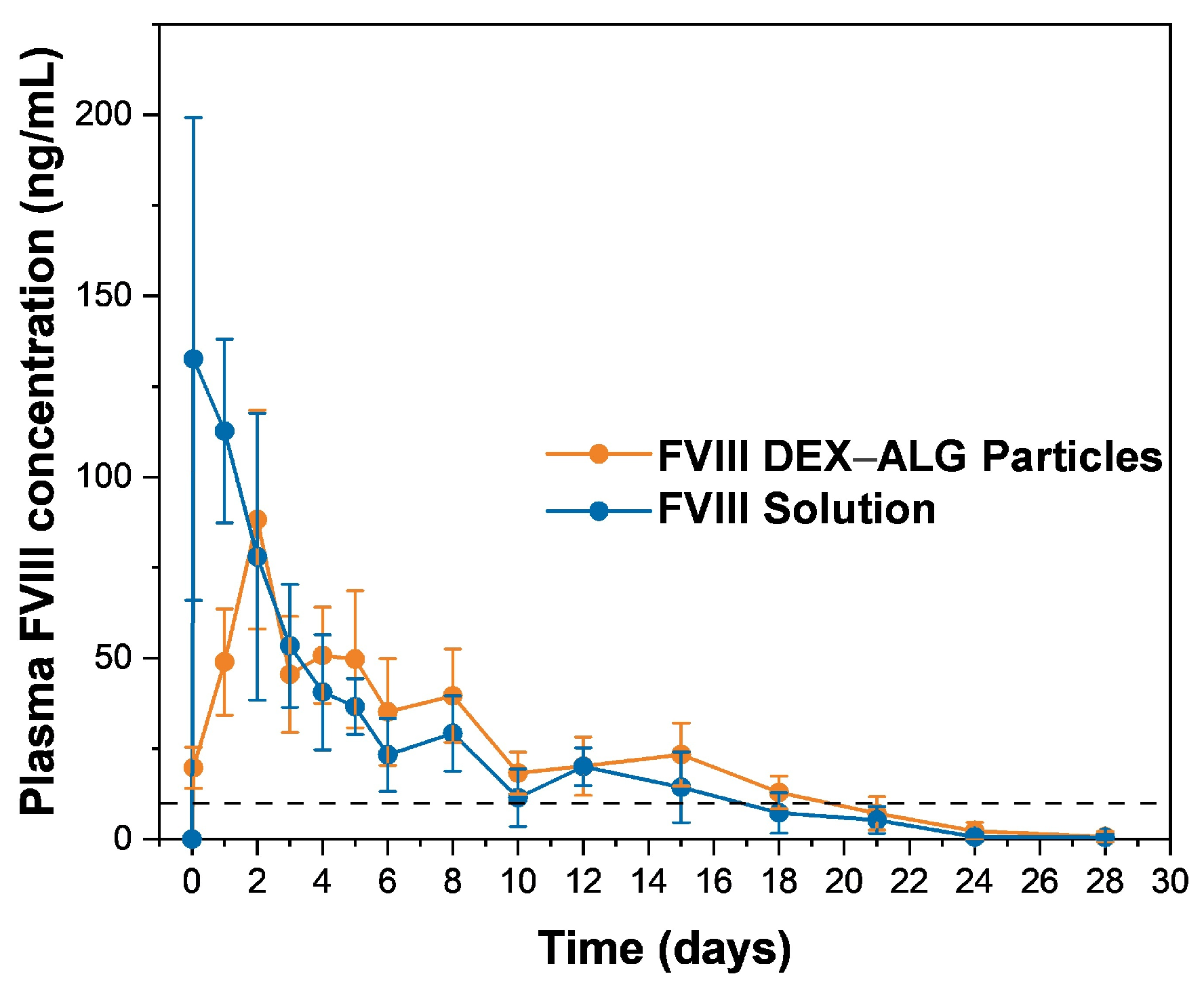
3.5. In Vivo Release Performance of FVIII from Thermal Gelling PLGA–PEG–PLGA
3.6. Feasibility of the Particle-Aided Thermal Gelling Polymer Depot as a Practical Dosage Form
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PEG | Polyethylene glycol |
| PLGA | Poly(lactic-co-glycolic acid) |
| DEX | Dextran |
| ALG | Sodium alginate |
| G-CSF | Granulocyte-colony stimulating factor |
| GM-CSF | Granulocyte-macrophage colony stimulating factor |
| BSA | Bovine serum albumin |
| IGF | Insulin-like growth factor |
References
- Wu, F.; Jin, T. Polymer-based sustained-release dosage forms for protein drugs, challenges, and recent advances. AAPS PharmSciTech 2008, 9, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Putney, S.D.; Burke, P.A. Improving protein therapeutics with sustained-release formulations. Nat. Biotechnol. 1998, 16, 153–157. [Google Scholar] [CrossRef]
- Langer, R.; Folkman, J. Polymers for the sustained release of proteins and other macromolecules. Nature 1976, 263, 797–800. [Google Scholar] [CrossRef]
- Li, W.; Tang, J.; Lee, D.; Tice, T.R.; Schwendeman, S.P.; Prausnitz, M.R. Clinical translation of long-acting drug delivery formulations. Nat. Rev. Mater. 2022, 7, 406–420. [Google Scholar] [CrossRef]
- Ma, G. Microencapsulation of protein drugs for drug delivery: Strategy, preparation, and applications. J. Control. Release 2014, 193, 324–340. [Google Scholar] [CrossRef]
- Hu, M.; Jiang, Y.B.; Jian, H.L.; Song, G.J. Preparation of Zein Microparticles Loaded with Astaxanthin by a Supercritical Carbon Dioxide Anti-solvent Process. Mod. Food Sci. Technol. 2017, 33, 139–145. [Google Scholar]
- Khodaverdi, E.; Tafaghodi, M.; Beizaei, S.; Abnous, K.; Alibolandi, M.; Hadizadeh, F. Preparation and characterisation of PLGA-PEG-PLGA nanospheres prepared with a new thermogelling method for insulin delivery. J. Chem. Pharm. Res. 2013, 5, 311–319. [Google Scholar]
- Sutter, M.; Siepmann, J.; Hennink, W.; Jiskoot, W. Recombinant gelatin hydrogels for the sustained release of proteins. J. Control. Release 2007, 119, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Zentner, G.; Rathi, R.; Shih, C.; McRea, J.; Seo, M.-H.; Oh, H.; Rhee, B.; Mestecky, J.; Moldoveanu, Z.; Morgan, M.; et al. Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J. Control. Release 2001, 72, 203–215. [Google Scholar] [CrossRef]
- Hegyi, H.; Gerstein, M. The Relationship between Protein Structure and Function: A Comprehensive Survey with Application to the Yeast Genome. J. Mol. Biol. 1999, 288, 147–164. [Google Scholar] [CrossRef]
- Vingerhoeds, M.; Harmsen, P. Proteins: Versatile Materials for Encapsulation. In Fundamentals of Cell Immobilisation Biotechnology; Springer: Dordrecht, The Netherlands, 2004; Volume 8, pp. 73–102. [Google Scholar]
- Zeng, Y.; Chen, J.; Li, Y.; Huang, J.; Huang, Z.; Huang, Y.; Pan, X.; Wu, C. Thermo-sensitive gel in glaucoma therapy for enhanced bioavailability: In vitro characterization, in vivo pharmacokinetics and pharmacodynamics study. Life Sci. 2018, 212, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, H.; Gu, D.; Sun, H.; Chen, K.; Tan, G.; Pan, W. A Novel Carbon Dots/Thermo-Sensitive In Situ Gel for a Composite Ocular Drug Delivery System: Characterization, Ex-Vivo Imaging and In Vivo Evaluation. Int. J. Mol. Sci. 2021, 22, 993. [Google Scholar] [CrossRef]
- Anand, R.; Vallooran, J. Polypeptides: PASylation and XTEN. In Engineering of Biomaterials for Drug Delivery Systems; Woodhead Publishing: Sawston, UK, 2018; pp. 299–315. [Google Scholar]
- Matthes, K.; Mino-Kenudson, M.; Sahani, D.V.; Holalkere, N.; Fowers, K.D.; Rathi, R.; Brugge, W.R. EUS-guided injection of paclitaxel (OncoGel) provides therapeutic drug concentrations in the porcine pancreas (with video). Gastrointest. Endosc. 2007, 65, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Duvall, G.A.; Tarabar, D.; Seidel, R.H.; Elstad, N.L.; Fowers, K.D. Phase 2: A dose-escalation study of OncoGel (ReGel/paclitaxel), a controlled-release formulation of paclitaxel, as adjunctive local therapy to external-beam radiation in patients with inoperable esophageal cancer. Anti-Cancer Drugs 2009, 20, 89–95. [Google Scholar] [CrossRef]
- Chan, P.S.; Xian, J.W.; Li, Q.; Chan, C.W.; Leung, S.; To, K. Biodegradable Thermosensitive PLGA-PEG-PLGA Polymer for Non-irritating and Sustained Ophthalmic Drug Delivery. Aaps J. 2019, 21, 59. [Google Scholar] [CrossRef]
- Si, M.; Xia, Y.; Cong, M.; Wang, D.; Hou, Y.; Ma, H. In situ Co-Delivery of Doxorubicin and Cisplatin by Injectable Thermosensitive Hydrogels for Enhanced Osteosarcoma Treatment. Int. J. Nanomed. 2022, 17, 1309–1322. [Google Scholar] [CrossRef]
- Bromberg, L.; Ron, E. Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery. Adv. Drug. Deliver. Rev. 1998, 31, 197–221. [Google Scholar] [CrossRef] [PubMed]
- Santoveña, A.; Monzón, C.; Alvarez-Lorenzo, C.; del Rosario, C.; Delgado, A.; Evora, C.; Concheiro, A.; Llabres, M.; Fariña, J. Structure-performance relationships of temperature-responsive PLGA-PEG-PLGA gels for sustained release of BMP-2. J. Pharm. Sci. 2017, 106, 3353–3362. [Google Scholar] [CrossRef]
- Wang, Y. Programmable hydrogels. Biomaterials 2018, 178, 663–680. [Google Scholar] [CrossRef]
- Nascimento, M.H.M.; Franco, M.; Yokaichiya, F.; de Paula, E.; Lombello, C.; De Araujo, D. Hyaluronic acid in Pluronic F-127/F-108 hydrogels for postoperative pain in arthroplasties: Influence on physico-chemical properties and structural requirements for sustained drug-release. Int. J. Biol. Macromol. 2018, 111, 1245–1254. [Google Scholar] [CrossRef]
- Jin, T.; Wu, F.; Yuan, W. Polysaccharide Microparticles Containing Biological Agents: Their Preparation and Applications. U.S. Patent 8,932,633, 13 January 2015. [Google Scholar]
- Jin, T.; Geng, Y.; Wu, F.; Yuan, W. Sustained-Release System for EPO and GM-CSF. PCT Patent WO2007CN02962, 16 October 2007. [Google Scholar]
- Wu, F.; Dai, L.; Geng, L.; Zhu, H.; Jin, T. Practically feasible production of sustained-release microspheres of granulocyte-macrophage colony-stimulating factor (rhGM-CSF). J. Control. Release 2017, 259, 195–202. [Google Scholar] [CrossRef]
- Li, X.; Xiulan, S. Multifunctional smart hydrogels: Potential in tissue engineering and cancer therapy. J. Mater. Chem. B 2018, 6, 4714–4730. [Google Scholar] [CrossRef] [PubMed]
- Kamali, H.; Khodaverdi, E.; Hadizadeh, F. Ring-opening polymerization of PLGA-PEG-PLGA triblock copolymer in supercritical carbon dioxide. J. Supercrit. Fluid. 2018, 137, 9–15. [Google Scholar] [CrossRef]
- Křížek Oborná, J.; Mravcová, L.; Michlovská, L.; Vojtova, L.; Vávrová, M. The effect of PLGA-PEG-PLGA modification on the sol-gel transition and degradation properties. Express. Polym. Lett. 2016, 10, 361–372. [Google Scholar] [CrossRef]
- Mohajeri, S.; Yaghoubi, S.; Abdollahi, E.; Tekie, F.; Kamali, H.; Khodaverdi, E.; Hadizadeh, F. In-vivo study of naltrexone hydrochloride release from an in-situ forming PLGA-PEG-PLGA system in the rabbit. J. Drug Deliv. Sci. Technol. 2016, 36, 156–160. [Google Scholar] [CrossRef]
- Michlovská, L.; Vojtova, L.; Humpa, O.; Kucerik, J.; Zidek, J.; Jancar, J. Hydrolytic stability of end-linked hydrogels from PLGA-PEG-PLGA macromonomers terminated by α,ω-itaconyl groups. RSC Adv. 2016, 6, 16808–16816. [Google Scholar] [CrossRef]
- Soni, V.; Pandey, V.; Asati, S.; Gour, V.; Tekade, R.K. Biodegradable block copolymers and their applications for drug delivery. In Basic Fundamentals of Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 401–447. [Google Scholar]
- Qiao, M.; Chen, D.; Ma, X.; Liu, Y. Injectable biodegradable temperature-responsive PLGA-PEG-PLGA copolymers: Synthesis and effect of copolymer composition on the drug release from the copolymer-based hydrogels. Int. J. Pharm. 2005, 294, 103–112. [Google Scholar] [CrossRef]
- Liow, S.S.; Karim, A.; Loh, X.J. Biodegradable thermogelling polymers for biomedical applications. MRS Bull. 2016, 41, 557–566. [Google Scholar] [CrossRef]
- Aderibigbe, B.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Guaresti, O.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Gabilondo, N.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. β-Glycerol phosphate/genipin chitosan hydrogels: A comparative study of their properties and diclofenac delivery. Carbohydr. Polym. 2020, 248, 11681. [Google Scholar] [CrossRef]
- Ketabat, F.; Karkhaneh, A.; Mehdinavaz Aghdam, R.; Hossein Ahmadi Tafti, S. Injectable conductive collagen/alginate/polypyrrole hydrogels as a biocompatible system for biomedical applications. J. Biomater. Sci. Polym. Ed. 2017, 28, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Shang, L. A Stable Gel Containing Bromelain. CN Patent 201310686915.8, 13 December 2013. [Google Scholar]
- Dong, X.; Yuan, B.; Chen, B.; He, C.; Wang, J.; Chen, X. In vitro Antibacterial Activity of PLGA-PEG-PLGA Thermosensitive Hydrogels Loaded with Vancomycin. Chem. J. Chin. Univ. 2017, 38, 866–871. [Google Scholar]
- Gajendiran, M.; Divakar, S.; Raman, N.; Balasubramanian, S. In Vitro Drug Release Behavior, Mechanism and Antimicrobial Activity of Rifampicin Loaded Low Molecular Weight PLGA-PEG-PLGA Triblock Copolymeric Nanospheres. Curr. Drug Deliv. 2013, 10, 722–731. [Google Scholar] [CrossRef]
- Liow, S.S.; Dou, Q.; Kai, D.; Karim, A.; Zhang, K.; Xu, F.; Loh, X.J. Thermogels: In Situ Gelling Biomaterial. ACS Biomater. Sci. Eng. 2016, 2, 295–316. [Google Scholar] [CrossRef] [PubMed]
- Connelly, S.; Gardner, J.; Lyons, R.; McClelland, A.; Kaleko, M. Sustained expression of therapeutic levels of human factor VIII in mice. Blood 1996, 87, 4671–4677. [Google Scholar] [CrossRef]
- Fatouros, A.; Liden, Y.; Sjostrom, B. Recombinant factor VIII SQ—Stability of VIII: C in homogenates from porcine, monkey and human subcutaneous tissue. J. Pharm. Pharmacol. 2000, 52, 797–805. [Google Scholar] [CrossRef]


| No. | Formulation | Composition (w/w) | Method for Preparing DEX Particles |
|---|---|---|---|
| F1 | GM-CSF, PLGA–PEG–PLGA | 1/60 | / |
| F2 | GM-CSF, DEX, ALG, PLGA–PEG–PLGA | 1/5/1.875/78.75 | double aqueous emulsion |
| F3 | G-CSF, sucrose, PLGA–PEG–PLGA | 1/1/70 | / |
| F4 | G-CSF, sucrose, DEX, ALG, PLGA–PEG–PLGA | 1/1/5/1.875/88.75 | double aqueous emulsion |
| F5 | IGF-1, PLGA–PEG–PLGA | 1/6187.5 | / |
| F6 | IGF-1, DEX, ALG, PLGA–PEG–PLGA | 1/500/187.5/6187.5 | double aqueous emulsion |
| F7 | FVIII, PLGA–PEG–PLGA | 1/132.75 | / |
| F8 | FVIII, DEX, ALG, PLGA–PEG–PLGA | 1/10/3.75/132.75 | double aqueous emulsion |
| F9 | BSA, PLGA–PEG–PLGA | 1/60 | / |
| F10 | BSA, DEX, ALG, PLGA–PEG–PLGA | 1/5/1.875/78.75 | double aqueous emulsion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zou, X.; Du, Q.; Dong, X.; Chinta, U.K.; Yu, R.; Wu, F.; Jin, T. Burst-Free Sustained Release of Proteins from Thermal Gelling Polymer Solutions. Pharmaceutics 2025, 17, 376. https://doi.org/10.3390/pharmaceutics17030376
Zhang Y, Zou X, Du Q, Dong X, Chinta UK, Yu R, Wu F, Jin T. Burst-Free Sustained Release of Proteins from Thermal Gelling Polymer Solutions. Pharmaceutics. 2025; 17(3):376. https://doi.org/10.3390/pharmaceutics17030376
Chicago/Turabian StyleZhang, Yuxing, Xixi Zou, Qiran Du, Xiaotao Dong, Uday Kumar Chinta, Ruyue Yu, Fei Wu, and Tuo Jin. 2025. "Burst-Free Sustained Release of Proteins from Thermal Gelling Polymer Solutions" Pharmaceutics 17, no. 3: 376. https://doi.org/10.3390/pharmaceutics17030376
APA StyleZhang, Y., Zou, X., Du, Q., Dong, X., Chinta, U. K., Yu, R., Wu, F., & Jin, T. (2025). Burst-Free Sustained Release of Proteins from Thermal Gelling Polymer Solutions. Pharmaceutics, 17(3), 376. https://doi.org/10.3390/pharmaceutics17030376






