Hierarchical Delivery of Anti-Inflammatory Compound and Stem Cells for Chronic Wounds
Abstract
1. Introduction
2. Materials and Methods
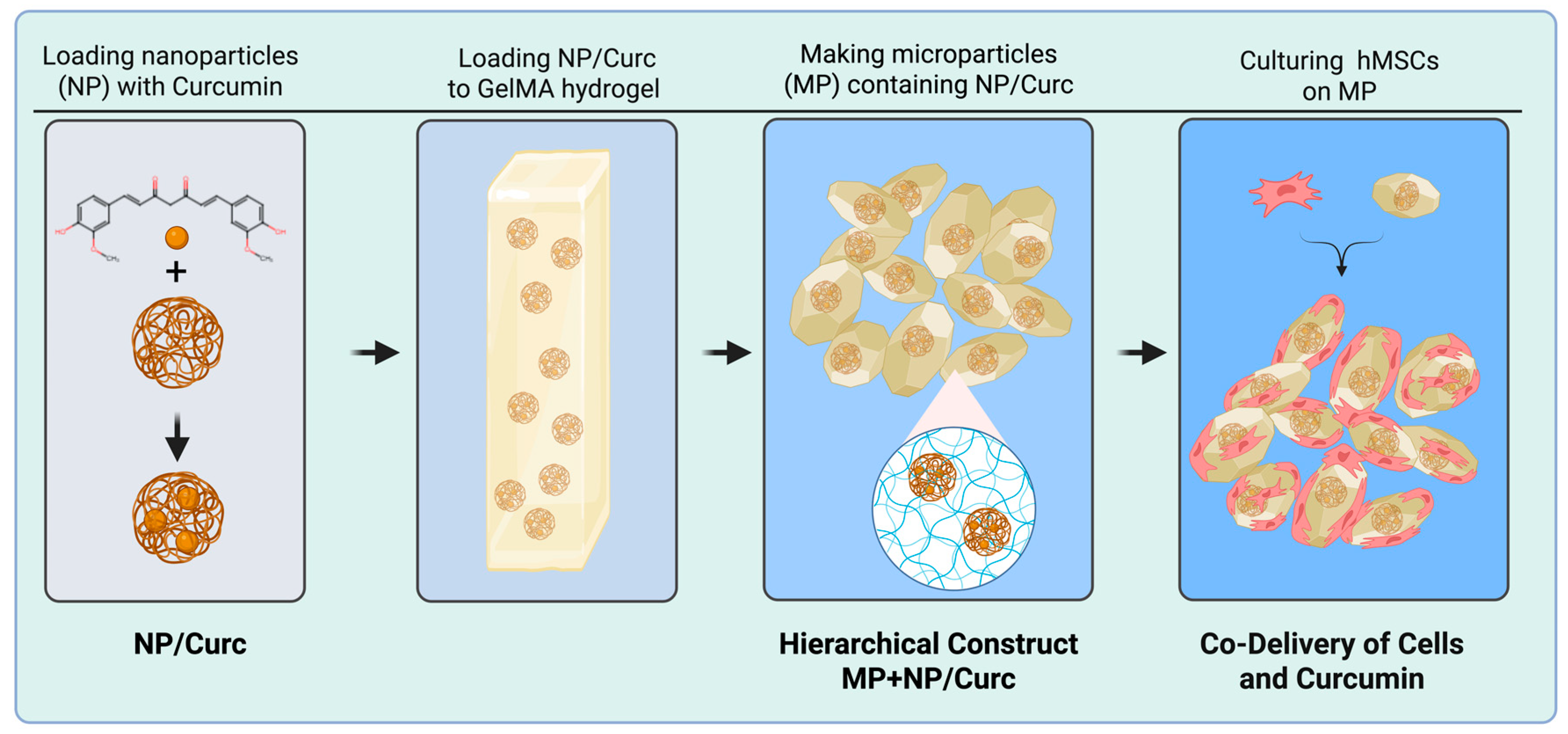
2.1. Synthesis of Gelatin Nanoparticles
2.2. Loading Nanoparticles with Curcumin
2.3. Synthesizing Curcumin MPs
- MP: 10% GelMA w/v, 0.05% LAP w/v (freshly prepared and kept in dark)
- MP + Curc: 10% GelMA w/v, 0.05% LAP w/v, 100 µM curcumin
- MP + NP/Curc: 10% GelMA w/v, 0.05% LAP w/v, 100 µM curcumin, 3.84 mg/mL gelatin NPs)
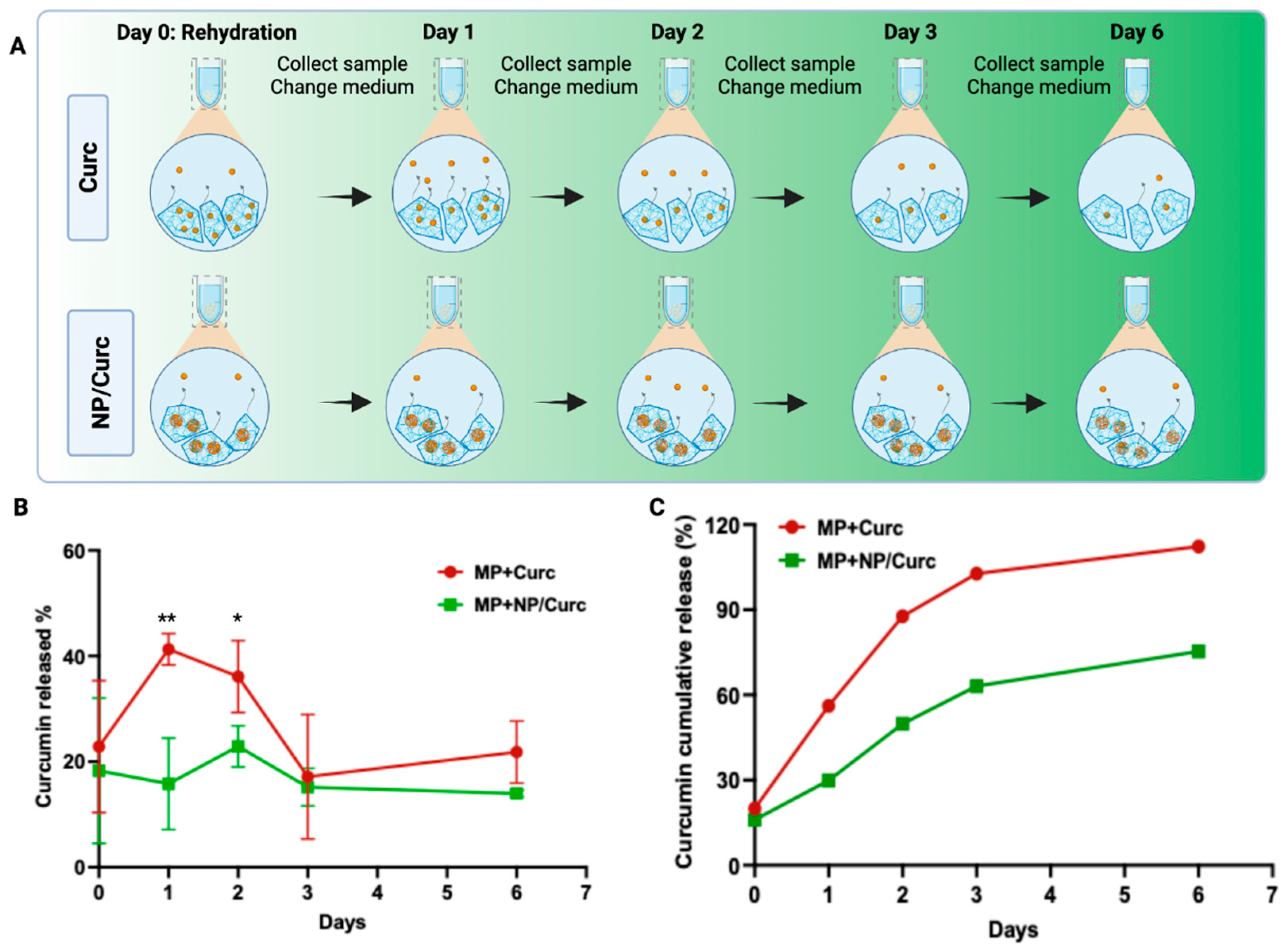
2.4. Release and Quantification of Curcumin from MPs
2.5. Culturing Human Mesenchymal Stem Cells on GelMA Microparticles
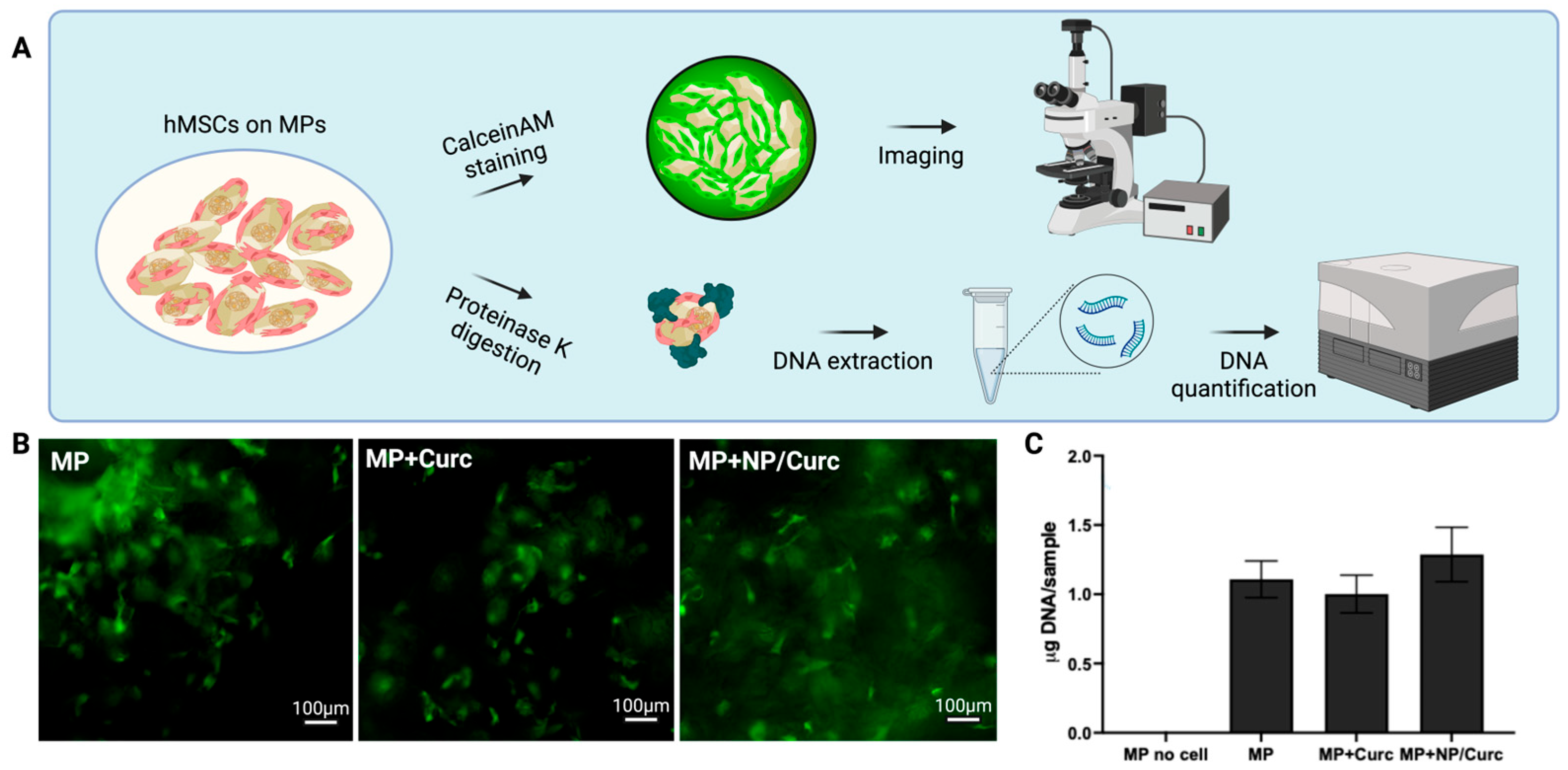
2.6. Calcein AM Staining
2.7. DNA Quantification of hMSCs Cultured on MPs
2.8. Differentiation and Activation of Human Monocyte Cell Line THP-1
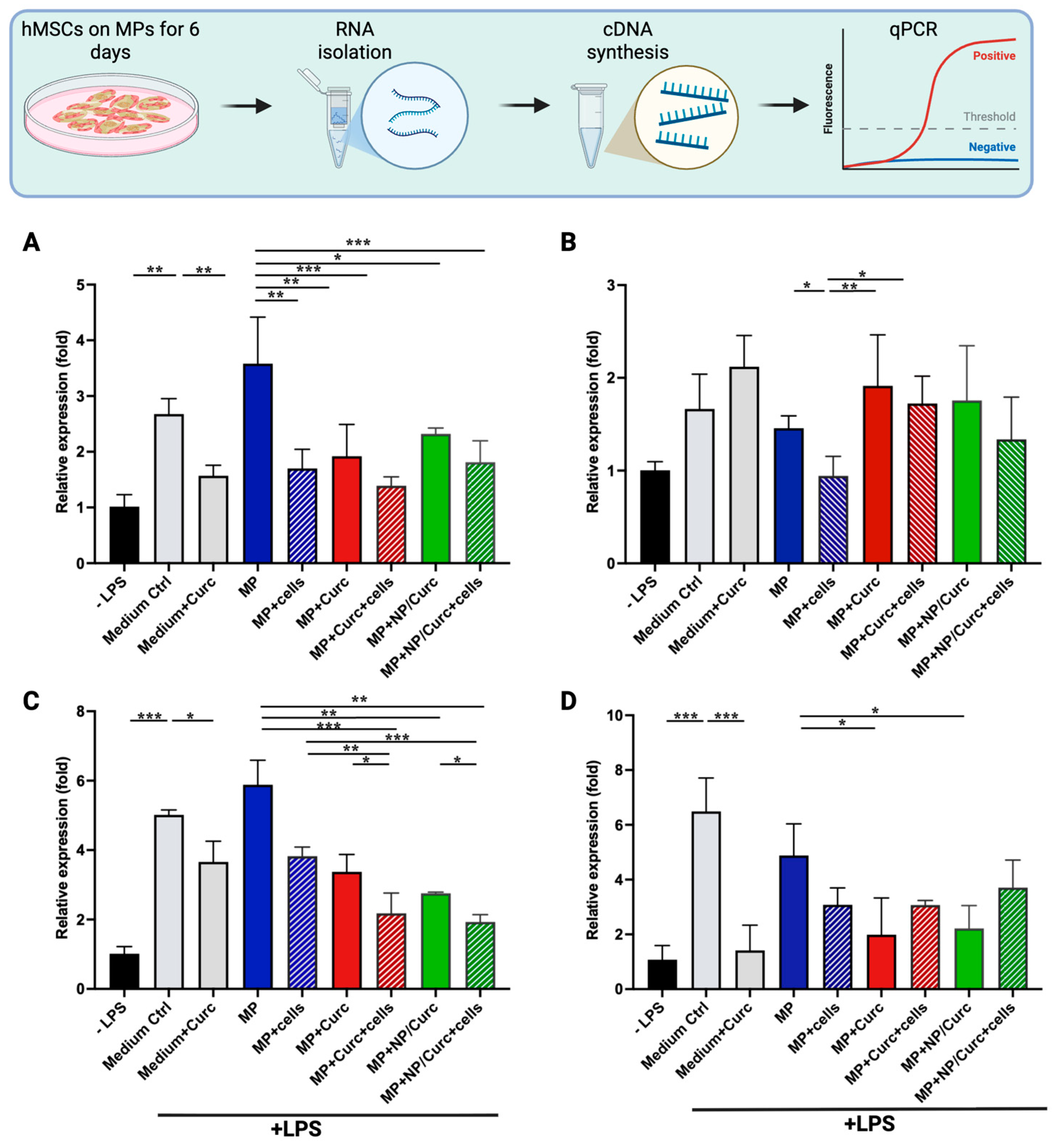
2.9. Quantification of Relative Expression of Inflammatory Markers by Quantitative PCR (qPCR)
2.10. Statistical Analysis
3. Results
3.1. Synthesis of NPs and MP Scaffold
- MP (blank): Microparticles containing no curcumin or nanoparticle carriers.
- MP + Curc (free curcumin): Microparticles created from a 10% GelMA w/v and 100 μM curcumin solution.
- MP + NP/Curc (nanoparticle-loaded curcumin): Microparticles created from a 10% GelMA w/v, 100 μM curcumin, and 3.7 mg/mL NPs solution.
3.2. Curcumin Release Profile
3.3. Viability of Cells on Microparticle Carriers
3.4. Anti-Inflammatory Effects of hMSCs and Curcumin Carried by GelMA Microparticles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sen, C.K. Human Wound and Its Burden: Updated 2022 Compendium of Estimates. Adv. Wound Care 2023, 12, 657–670. [Google Scholar] [CrossRef]
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic wounds. Nat. Rev. Dis. Primers 2022, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.J.; Hofstad, O.; Feldman, H.I. Association between renal failure and foot ulcer or lower-extremity amputation in patients with diabetes. Diabetes Care 2008, 31, 1331–1336. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr266. [Google Scholar] [CrossRef]
- McDermott, K.; Fang, M.; Boulton, A.J.M.; Selvin, E.; Hicks, C.W. Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care 2022, 46, 209–221. [Google Scholar] [CrossRef]
- Salenius, J.E.; Suntila, M.; Ahti, T.; Huhtala, H.; Vaalasti, A.; Salmi, T.T.; Kimpimaki, T. Long-term Mortality among Patients with Chronic Ulcers. Acta Derm. Venereol. 2021, 101, adv00455. [Google Scholar] [CrossRef] [PubMed]
- Sassoli, C.; Pini, A.; Chellini, F.; Mazzanti, B.; Nistri, S.; Nosi, D.; Saccardi, R.; Quercioli, F.; Zecchi-Orlandini, S.; Formigli, L. Bone Marrow Mesenchymal Stromal Cells Stimulate Skeletal Myoblast Proliferation through the Paracrine Release of VEGF. PLoS ONE 2012, 7, e37512. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-R.; Kim, J.-W.; Jun, H.-S.; Roh, J.Y.; Lee, H.-Y.; Hong, I.-S. Stem Cell Secretome and Its Effect on Cellular Mechanisms Relevant to Wound Healing. Mol. Ther. 2018, 26, 606–617. [Google Scholar] [CrossRef]
- Ozhava, D.; Bektas, C.; Lee, K.; Jackson, A.; Mao, Y. Human Mesenchymal Stem Cells on Size-Sorted Gelatin Hydrogel Microparticles Show Enhanced In Vitro Wound Healing Activities. Gels 2024, 10, 97. [Google Scholar] [CrossRef]
- Putra, A.; Suwiryo, Z.H.; Muhar, A.M.; Widyatmoko, A.; Rahmi, F.L. The Role of Mesenchymal Stem Cells in Regulating PDGF and VEGF during Pancreatic Islet Cells Regeneration in Diabetic Animal Model. Folia Med. 2021, 63, 875–883. [Google Scholar] [CrossRef]
- De Pieri, A.; Rochev, Y.; Zeugolis, D.I. Scaffold-free cell-based tissue engineering therapies: Advances, shortfalls and forecast. npj Regen. Med. 2021, 6, 18. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Sivaraj, D.; Chen, K.; Chattopadhyay, A.; Henn, D.; Wu, W.; Noishiki, C.; Magbual, N.J.; Mittal, S.; Mermin-Bunnell, A.M.; Bonham, C.A.; et al. Hydrogel Scaffolds to Deliver Cell Therapies for Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 660145. [Google Scholar] [CrossRef]
- Kurian, A.G.; Singh, R.K.; Patel, K.D.; Lee, J.H.; Kim, H.W. Multifunctional GelMA platforms with nanomaterials for advanced tissue therapeutics. Bioact. Mater. 2022, 8, 267–295. [Google Scholar] [CrossRef]
- Patel, K.; Ozhava, D.; Mao, Y. Expansion and Delivery of Human Chondrocytes on Gelatin-Based Cell Carriers. Gels 2025, 11, 199. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, C.; Zheng, H.; Meng, Z.; Heng, B.C.; Zhou, T.; Jiang, S.; Wei, Y. Superwettable and injectable GelMA-MSC microspheres promote cartilage repair in temporomandibular joints. Front. Bioeng. Biotechnol. 2022, 10, 1026911. [Google Scholar] [CrossRef]
- Winkler, P.; Mao, Y. Dual Delivery of Cells and Bioactive Molecules for Wound Healing Applications. Molecules 2025, 30, 1577. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Raina, N.; Wahi, A.; Goh, K.W.; Sharma, P.; Nagpal, R.; Jain, A.; Ming, L.C.; Gupta, M. Wound-Healing Effects of Curcumin and Its Nanoformulations: A Comprehensive Review. Pharmaceutics 2022, 14, 2288. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Rostamirad, A.; Seyyedebrahimi, S.; Meshkani, R. Curcumin ameliorates palmitate-induced inflammation in skeletal muscle cells by regulating JNK/NF-kB pathway and ROS production. Inflammopharmacology 2018, 26, 1265–1272. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [CrossRef] [PubMed]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef]
- Meng, Z.; Yan, C.; Deng, Q.; Gao, D.F.; Niu, X.L. Curcumin inhibits LPS-induced inflammation in rat vascular smooth muscle cells in vitro via ROS-relative TLR4-MAPK/NF-kappaB pathways. Acta Pharmacol. Sin. 2013, 34, 901–911. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Ravindran, J.; Subbaraju, G.V.; Ramani, M.V.; Sung, B.; Aggarwal, B.B. Bisdemethylcurcumin and structurally related hispolon analogues of curcumin exhibit enhanced prooxidant, anti-proliferative and anti-inflammatory activities in vitro. Biochem. Pharmacol. 2010, 79, 1658–1666. [Google Scholar] [CrossRef]
- Chainoglou, E.; Hadjipavlou-Litina, D. Curcumin analogues and derivatives with anti-proliferative and anti-inflammatory activity: Structural characteristics and molecular targets. Expert Opin. Drug Discov. 2019, 14, 821–842. [Google Scholar] [CrossRef]
- Liang, G.; Zhou, H.; Wang, Y.; Gurley, E.C.; Feng, B.; Chen, L.; Xiao, J.; Yang, S.; Li, X. Inhibition of LPS-induced production of inflammatory factors in the macrophages by mono-carbonyl analogues of curcumin. J. Cell. Mol. Med. 2009, 13, 3370–3379. [Google Scholar] [CrossRef] [PubMed]
- Kurien, B.T.; Singh, A.; Matsumoto, H.; Scofield, R.H. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay. Drug Dev. Technol. 2007, 5, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Weng, T.; Jin, R.; Yang, M.; Yu, M.; Zhang, W.; Wang, X.; Han, C. Curcumin-incorporated 3D bioprinting gelatin methacryloyl hydrogel reduces reactive oxygen species-induced adipose-derived stem cell apoptosis and improves implanting survival in diabetic wounds. Burn. Trauma 2022, 10, tkac001. [Google Scholar] [CrossRef]
- Bektas, C.; Mao, Y. Hydrogel Microparticles for Bone Regeneration. Gels 2024, 10, 28. [Google Scholar] [CrossRef]
- Scharstuhl, A.; Mutsaers, H.A.; Pennings, S.W.; Szarek, W.A.; Russel, F.G.; Wagener, F.A. Curcumin-induced fibroblast apoptosis and in vitro wound contraction are regulated by antioxidants and heme oxygenase: Implications for scar formation. J. Cell. Mol. Med. 2009, 13, 712–725. [Google Scholar] [CrossRef]
- Pham, L.; Dang, L.H.; Truong, M.D.; Nguyen, T.H.; Le, L.; Le, V.T.; Nam, N.D.; Bach, L.G.; Nguyen, V.T.; Tran, N.Q. A dual synergistic of curcumin and gelatin on thermal-responsive hydrogel based on Chitosan-P123 in wound healing application. Biomed. Pharmacother. 2019, 117, 109183. [Google Scholar] [CrossRef] [PubMed]
- Mobaraki, M.; Bizari, D.; Soltani, M.; Khshmohabat, H.; Raahemifar, K.; Akbarzade Amirdehi, M. The Effects of Curcumin Nanoparticles Incorporated into Collagen-Alginate Scaffold on Wound Healing of Skin Tissue in Trauma Patients. Polymers 2021, 13, 4291. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Gupta, H. Formulation and comparative characterization of nanoparticles of curcumin using natural, synthetic and semi-synthetic polymers for wound healing. Life Sci. 2020, 253, 117588. [Google Scholar] [CrossRef] [PubMed]
- Madkhali, O.A. Drug Delivery of Gelatin Nanoparticles as a Biodegradable Polymer for the Treatment of Infectious Diseases: Perspectives and Challenges. Polymers 2023, 15, 4327. [Google Scholar] [CrossRef]
- Yu, S.H.; Kim, D.-Y.; Baek, Y.; Lee, H.G. Combination of nanoparticles and gelatin-genipin hydrogel enhances the antioxidant activity, stability, and release properties of curcumin. J. Food Eng. 2024, 365, 111814. [Google Scholar] [CrossRef]
- Attari, F.; Zahmatkesh, M.; Aligholi, H.; Mehr, S.E.; Sharifzadeh, M.; Gorji, A.; Mokhtari, T.; Khaksarian, M.; Hassanzadeh, G. Curcumin as a double-edged sword for stem cells: Dose, time and cell type-specific responses to curcumin. Daru 2015, 23, 33. [Google Scholar] [CrossRef]
- Shutava, T.G.; Balkundi, S.S.; Vangala, P.; Steffan, J.J.; Bigelow, R.L.; Cardelli, J.A.; O’Neal, D.P.; Lvov, Y.M. Layer-by-Layer-Coated Gelatin Nanoparticles as a Vehicle for Delivery of Natural Polyphenols. ACS Nano 2009, 3, 1877–1885. [Google Scholar] [CrossRef]
- Ozhava, D.; Winkler, P.; Mao, Y. Enhancing antimicrobial activity and reducing cytotoxicity of silver nanoparticles through gelatin nanoparticles. Nanomedicine 2024, 19, 199–211. [Google Scholar] [CrossRef]
- Kilic Bektas, C.; Zhang, W.; Mao, Y.; Wu, X.; Kohn, J.; Yelick, P.C. Self-Assembled Hydrogel Microparticle-Based Tooth-Germ Organoids. Bioengineering 2022, 9, 215. [Google Scholar] [CrossRef]
- Mao, Y.; Hoffman, T.; Dhall, S.; Singal, A.; Sathyamoorthy, M.; Danilkovitch, A.; Kohn, J. Endogenous viable cells in lyopreserved amnion retain differentiation potential and anti-fibrotic activity in vitro. Acta Biomater. 2019, 94, 330–339. [Google Scholar] [CrossRef]
- Mao, Y.; John, N.; Protzman, N.M.; Long, D.; Sivalenka, R.; Azimi, S.; Mirabile, B.; Pouliot, R.; Gosiewska, A.; Hariri, R.J.; et al. A tri-layer decellularized, dehydrated human amniotic membrane scaffold supports the cellular functions of human tenocytes in vitro. J. Mater. Sci. Mater. Med. 2023, 34, 37. [Google Scholar] [CrossRef]
- Lakshminarayanan, A.; Kannan, S.; Kuppusamy, M.K.; Sankaranarayanan, K.; Godla, U.; Punnoose, A.M. The effect of curcumin, catechin and resveratrol on viability, proliferation and cytotoxicity of human umbilical cord Wharton’s jelly derived mesenchymal stem cells. Tissue Cell 2025, 93, 102742. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Jin, Q.H.; Kim, H.K.; Na, J.Y.; Jin, C.; Seon, J.K. Anti-inflammatory effects of mesenchymal stem cell-conditioned media inhibited macrophages activation in vitro. Sci. Rep. 2022, 12, 4754. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Kell, D.B.; Pretorius, E. The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation. Chronic Stress 2022, 6, 24705470221076390. [Google Scholar] [CrossRef]
- Schindler, R.; Clark, B.D.; Dinarello, C.A. Dissociation between interleukin-1 beta mRNA and protein synthesis in human peripheral blood mononuclear cells. J. Biol. Chem. 1990, 265, 10232–10237. [Google Scholar] [CrossRef]
- Protzman, N.M.; Mao, Y.; Long, D.; Sivalenka, R.; Gosiewska, A.; Hariri, R.J.; Brigido, S.A. Placental-Derived Biomaterials and Their Application to Wound Healing: A Review. Bioengineering 2023, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, Y.; Wen, J.; Fan, G.; Wu, Y.; Zhang, C. A rapid and simple HPLC method for the determination of curcumin in rat plasma: Assay development, validation and application to a pharmacokinetic study of curcumin liposome. Biomed. Chromatogr. 2009, 23, 1201–1207. [Google Scholar] [CrossRef]
- Yagi, H.; Soto-Gutierrez, A.; Parekkadan, B.; Kitagawa, Y.; Tompkins, R.G.; Kobayashi, N.; Yarmush, M.L. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010, 19, 667–679. [Google Scholar] [CrossRef]
- Shu, F.; Thammasit, P.; Pruksaphon, K.; Nosanchuk, J.D.; Youngchim, S. Expression of Cytokine Profiles in Human THP-1 Cells during Phase Transition of Talaromyces marneffei. Pathogens 2022, 11, 1465. [Google Scholar] [CrossRef] [PubMed]
- Domaszewska-Szostek, A.; Krzyzanowska, M.; Siemionow, M. Cell-Based Therapies for Chronic Wounds Tested in Clinical Studies: Review. Ann. Plast. Surg. 2019, 83, e96–e109. [Google Scholar] [CrossRef] [PubMed]

| Markers | Genes | Function | Catalog Number of Primers |
|---|---|---|---|
| Glyceraldehyde-3-Phosphate Dehydrogenase | GAPDH | Housekeeping gene as an internal control | Hs_GAPDH_2_SG QT01192646 |
| Tumor Necrosis Factor alpha | TNF | Pro-inflammation | Hs_TNF_3_SG QT01079561 |
| Interleukin-1 beta | IL1B | Pro-inflammation | Hs_IL1B_1_SG QT00021385 |
| Interleukin-6 | IL6 | Pro-inflammation | Hs_IL6_1_SG QT00083720 |
| C-X-C Motif Chemokine Ligand 8 (Interleukin-8) | CXCL8 | Pro-inflammation | Hs_CXCL8_1_SG QT00000322 |
| C-C motif chemokine ligand 18 | CCL18 | Anti-inflammation | Hs_CCL18_1_SG QT00024066 |
| Glyceraldehyde-3-Phosphate Dehydrogenase | GAPDH | Housekeeping gene as an internal control | Hs_GAPDH_2_SG QT01192646 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winkler, P.; Zhang, R.; Mao, Y. Hierarchical Delivery of Anti-Inflammatory Compound and Stem Cells for Chronic Wounds. Pharmaceutics 2025, 17, 1549. https://doi.org/10.3390/pharmaceutics17121549
Winkler P, Zhang R, Mao Y. Hierarchical Delivery of Anti-Inflammatory Compound and Stem Cells for Chronic Wounds. Pharmaceutics. 2025; 17(12):1549. https://doi.org/10.3390/pharmaceutics17121549
Chicago/Turabian StyleWinkler, Petras, Ryan Zhang, and Yong Mao. 2025. "Hierarchical Delivery of Anti-Inflammatory Compound and Stem Cells for Chronic Wounds" Pharmaceutics 17, no. 12: 1549. https://doi.org/10.3390/pharmaceutics17121549
APA StyleWinkler, P., Zhang, R., & Mao, Y. (2025). Hierarchical Delivery of Anti-Inflammatory Compound and Stem Cells for Chronic Wounds. Pharmaceutics, 17(12), 1549. https://doi.org/10.3390/pharmaceutics17121549







