Enhanced Antiproliferative Activity of Docetaxel by Extremely Low Frequency Electromagnetic Fields in MCF-7 Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cell Culture
2.2.1. Docetaxel Treatment
2.2.2. EMF Exposure Setup
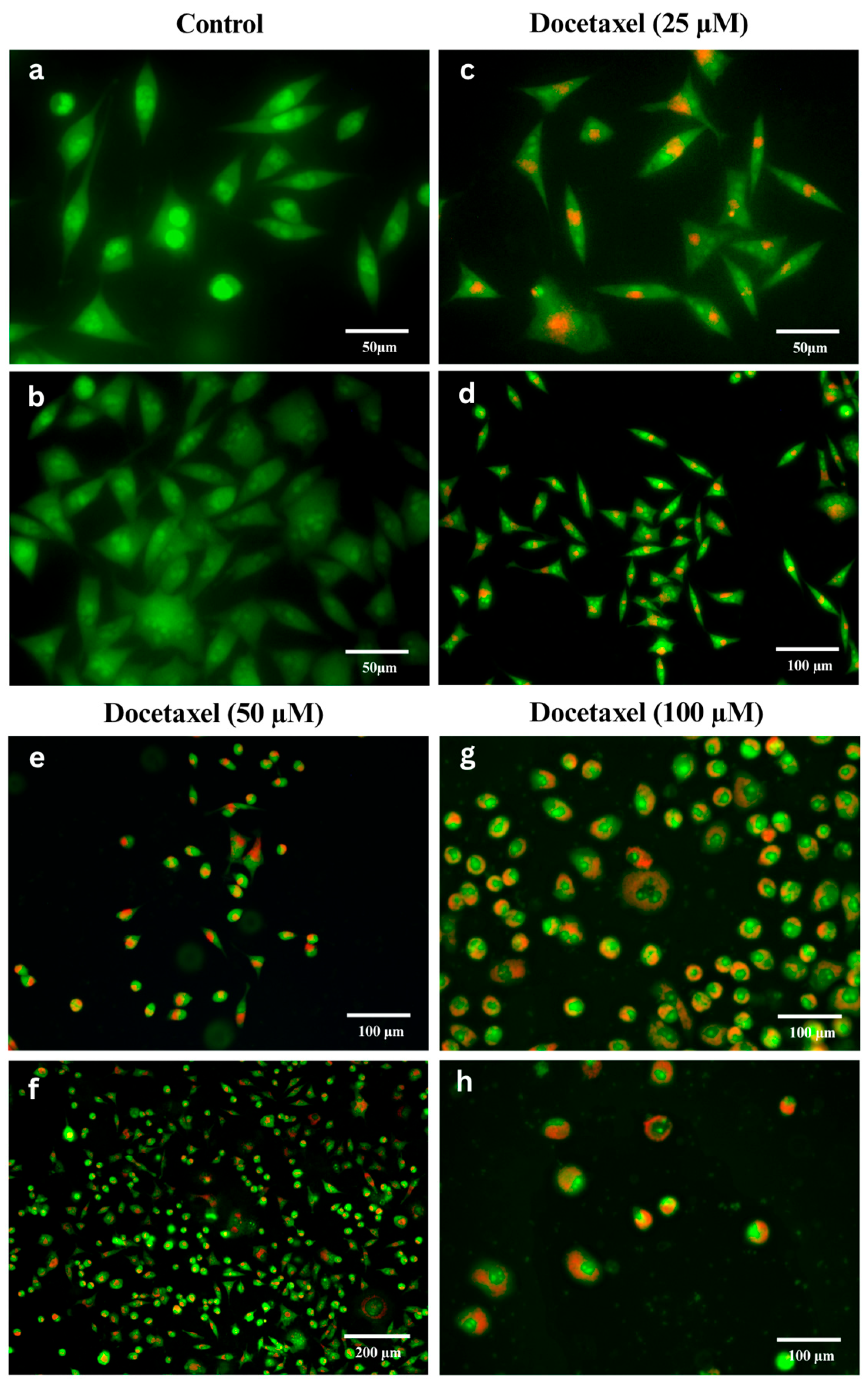
2.3. Acridine Orange/Propidium Iodide (AO/PI) Staining
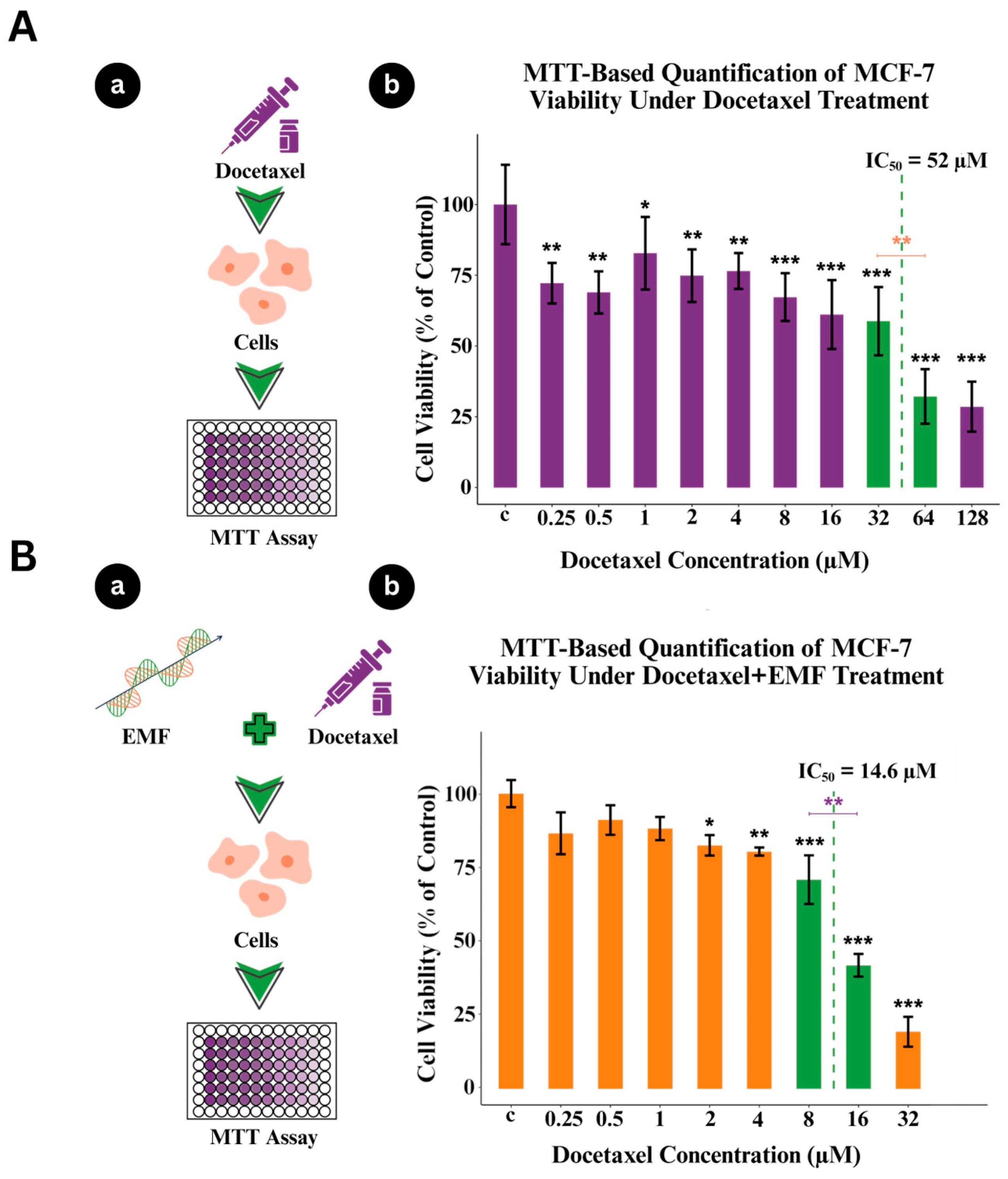
2.4. MTT Viability Assay
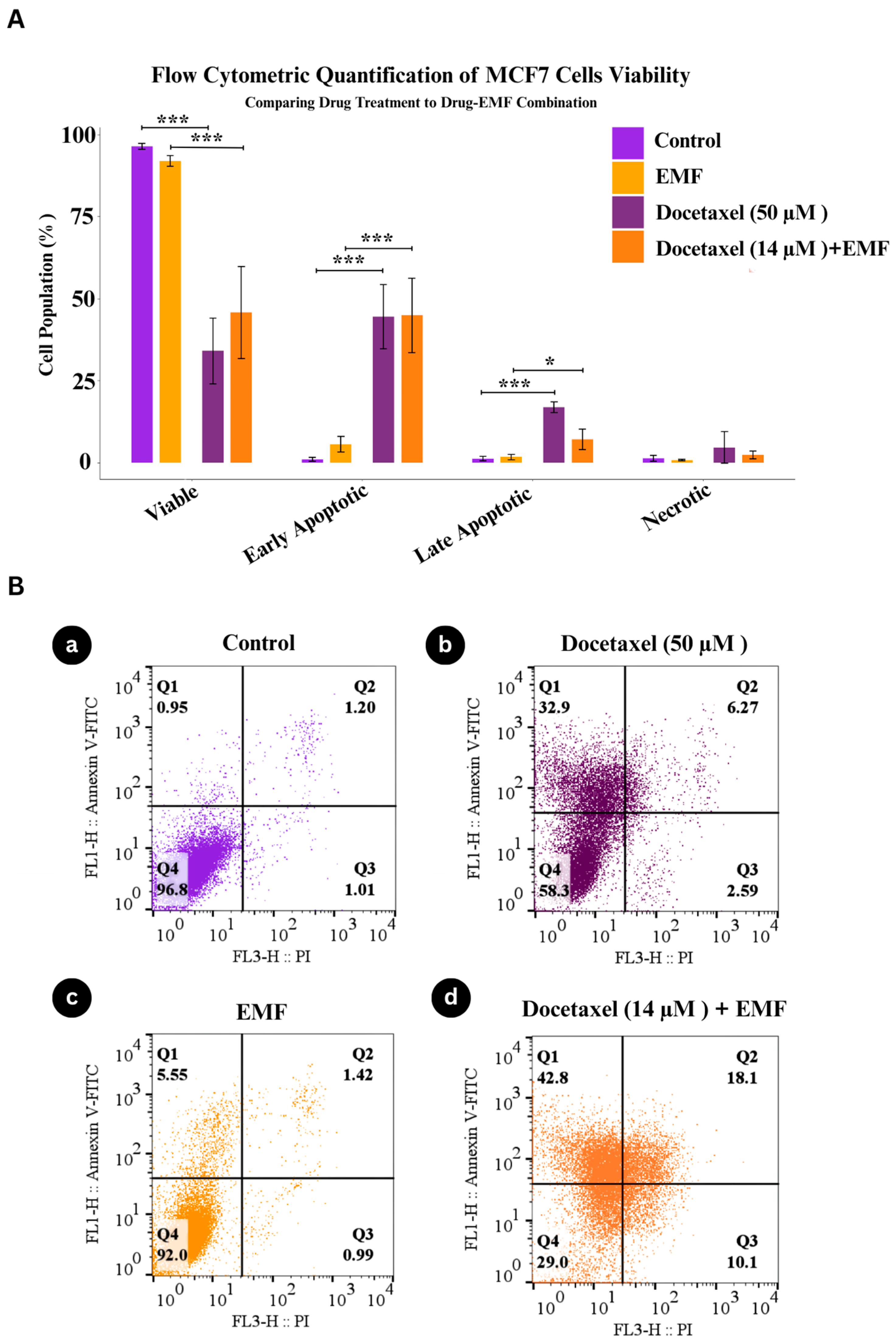
2.5. Annexin v/PI Apoptosis Flow Cytometry
2.6. Intracellular ROS Measurement via Flow Cytometry
2.7. Cell Cycle Analysis
2.8. Statistical Analysis
3. Results
3.1. Dose-Dependent Cell Death in MCF-7 Cells Induced by Docetaxel
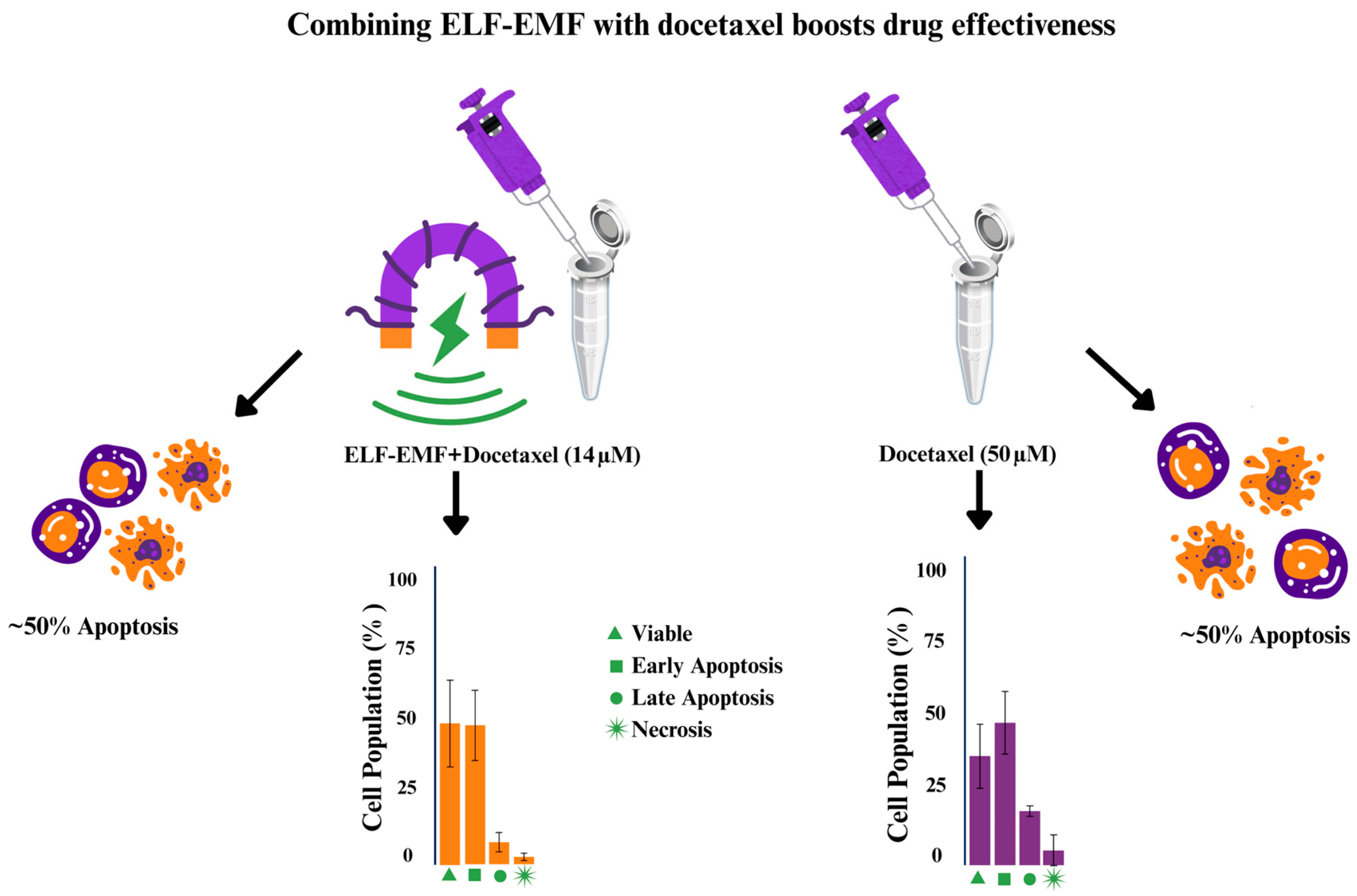
3.2. MCF-7 Cell Viability Under Combined Docetaxel and ELF-EMF Treatment
3.3. Apoptotic Response of MCF-7 Cells Under Combined Docetaxel and ELF-EMF Treatment
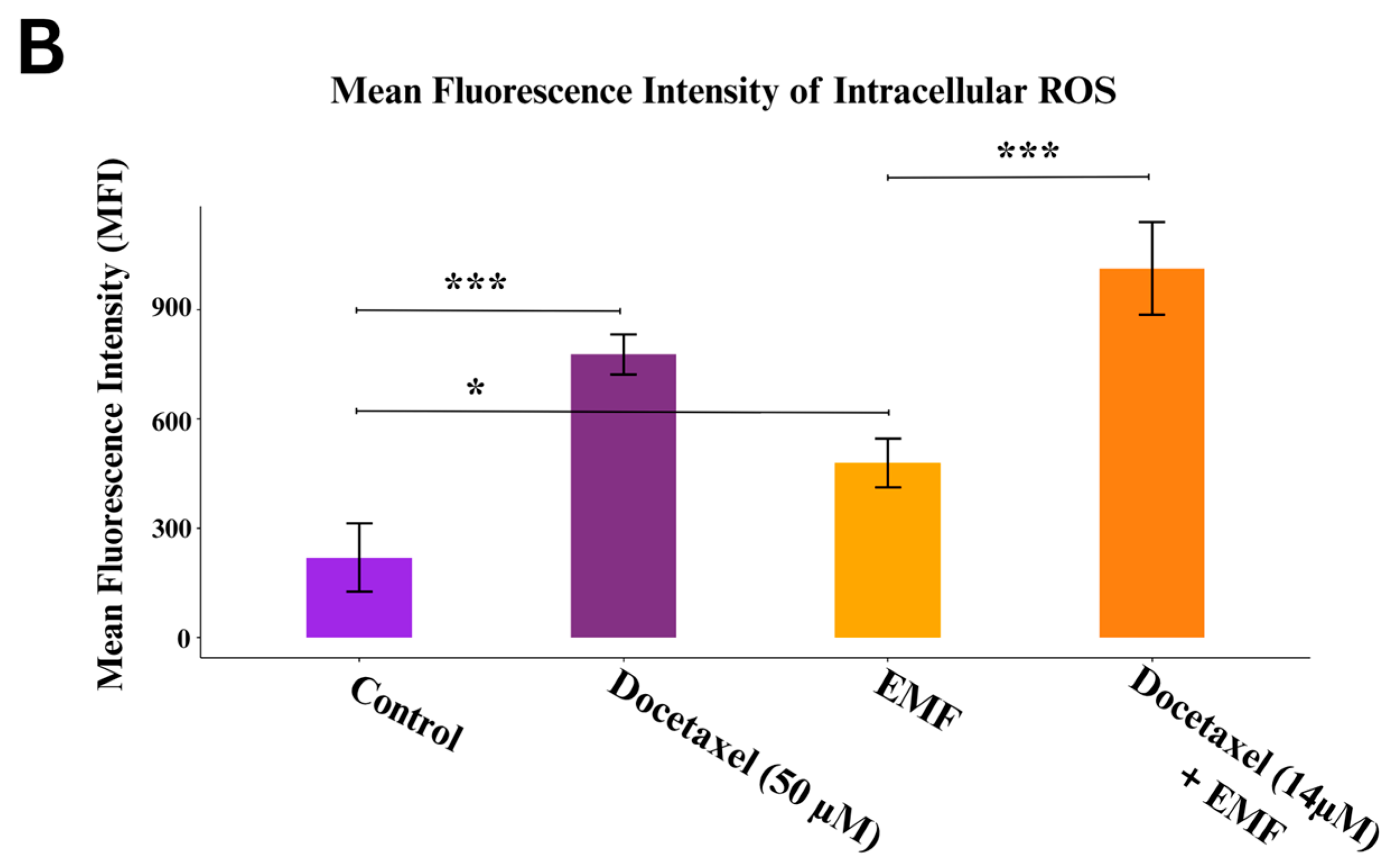
3.4. The Combined Effect of Docetaxel and ELF-EMF Stem from ROS Elevation
3.5. Docetaxel +ELF-EMF Induces G2/M Cell Cycle Arrest
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ELF | Electromagnetic field |
| ELF-EMF | Extremely Low-Frequency EMF |
| ROS | Reactive Oxygen Species |
| BCL-2 | B-cell lymphoma 2 |
| AO | Acridine Orange |
| PI | Propidium Iodide |
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| PBS | Phosphate-Buffered Saline |
| DMSO | Dimethyl sulfoxide |
References
- Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer?utm_source (accessed on 30 July 2025).[Green Version]
- Al-thoubaity, F.K. Molecular classification of breast cancer: A retrospective cohort study. Ann. Med. Surg. 2020, 49, 44–48. [Google Scholar] [CrossRef]
- Wang, J.; Wu, S.-G. Breast Cancer: An Overview of Current Therapeutic Strategies, Challenge, and Perspectives. Breast Cancer Targets Ther. 2023, 15, 721–730. [Google Scholar] [CrossRef]
- Kasgri, K.A.; Abazari, M.; Badeleh, S.M.; Badeleh, K.M.; Peyman, N. Comprehensive Review of Breast Cancer Consequences for the Patients and Their Coping Strategies: A Systematic Review. Cancer Control J. Moffitt Cancer Cent. 2024, 31, 10732748241249355. [Google Scholar] [CrossRef]
- Duan, Y.; Wu, X.; Gong, Z.; Guo, Q.; Kong, Y. Pathological impact and medical applications of electromagnetic field on melanoma: A focused review. Front. Oncol. 2022, 12, 857068. [Google Scholar] [CrossRef]
- Ramazi, S.; Salimian, M.; Allahverdi, A.; Kianamiri, S.; Abdolmaleki, P. Synergistic cytotoxic effects of an extremely low-frequency electromagnetic field with doxorubicin on MCF-7 cell line. Sci. Rep. 2023, 13, 8844. [Google Scholar] [CrossRef]
- Bergandi, L.; Lucia, U.; Grisolia, G.; Granata, R.; Gesmundo, I.; Ponzetto, A.; Paolucci, E.; Borchiellini, R.; Ghigo, E.; Silvagno, F. The extremely low frequency electromagnetic stimulation selective for cancer cells elicits growth arrest through a metabolic shift. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2019, 1866, 1389–1397. [Google Scholar] [CrossRef]
- Yousefian, B.; Firoozabadi, S.M.; Mokhtari-Dizaji, M. Magnetoporation: New Method for Permeabilization of Cancerous Cells to Hydrophilic Drugs. J. Biomed. Phys. Eng. 2022, 12, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Perera, G.T.; Nguyen, T.H.P.; Dekiwadia, C.; Wandiyanto, J.V.; Sbarski, I.; Bazaka, O.; Bazaka, K.; Crawford, R.J.; Croft, R.J.; Ivanova, E.P. Exposure to high-frequency electromagnetic field triggers rapid uptake of large nanosphere clusters by pheochromocytoma cells. Int. J. Nanomed. 2018, 13, 8429–8442. [Google Scholar] [CrossRef]
- Sengupta, S.; Balla, V.K. A review on the use of magnetic fields and ultrasound for non-invasive cancer treatment. J. Adv. Res. 2018, 14, 97–111. [Google Scholar] [CrossRef]
- Shi, K.; Peng, X.; Xu, T.; Lin, Z.; Sun, M.; Li, Y.; Xian, Q.; Xiao, T.; Chen, S.; Xie, Y.; et al. Precise Electromagnetic Modulation of the Cell Cycle and Its Applications in Cancer Therapy. Int. J. Mol. Sci. 2025, 26, 4445. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.-H.; Kim, B.; Kim, S.H.; Jung, B.C.; Lee, Y.; Kim, Y.S. Pulsed electromagnetic field potentiates etoposide-induced MCF-7 cell death. BMB Rep. 2022, 55, 148–153. [Google Scholar] [CrossRef]
- Lai, H. Exposure to Static and Extremely-Low Frequency Electromagnetic Fields and Cellular Free Radicals. Electromagn. Biol. Med. 2019, 38, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Baharara, J.; Hosseini, N.; Farzin, T.R. Extremely low frequency electromagnetic field sensitizes cisplatin-resistant human ovarian adenocarcinoma cells via P53 activation. Cytotechnology 2016, 68, 1403–1413. [Google Scholar] [CrossRef]
- Lucia, U.; Bergandi, L.; Grisolia, G.; Fino, D.; Mareschi, K.; Marini, E.; Niclot, A.G.S.B.; Tirtei, E.; Asaftei, S.D.; Fagioli, F.; et al. The exposure to extremely low frequency electromagnetic-fields inhibits the growth and potentiates the sensitivity to chemotherapy of bidimensional and tridimensional human osteosarcoma models. Biomed. Pharmacother. 2024, 177, 117162. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, F.A.; Wheeler, R. The immunopharmacology of paclitaxel (Taxol®), docetaxel (Taxotere®), and related agents. Int. Immunopharmacol. 2003, 3, 1699–1714. [Google Scholar] [CrossRef]
- Vella-Zarb, L.; Dinnebier, R.E.; Baisch, U. The Devil is in the Detail: A Rare H-Bonding Motif in New Forms of Docetaxel. Cryst. Growth Des. 2013, 13, 4402–4410. [Google Scholar] [CrossRef]
- Hashemi, M.; Zandieh, M.A.; Talebi, Y.; Rahmanian, P.; Shafiee, S.S.; Nejad, M.M.; Babaei, R.; Sadi, F.H.; Rajabi, R.; Abkenar, Z.O.; et al. Paclitaxel and docetaxel resistance in prostate cancer: Molecular mechanisms and possible therapeutic strategies. Biomed. Pharmacother. 2023, 160, 114392. [Google Scholar] [CrossRef]
- Ogura, T.; Tanaka, Y.; Tamaki, H.; Harada, M. Docetaxel induces Bcl-2- and pro-apoptotic caspase-independent death of human prostate cancer DU145 cells. Int. J. Oncol. 2016, 48, 2330–2338. [Google Scholar] [CrossRef] [PubMed]
- De Porras, V.R.; Wang, X.C.; Palomero, L.; Marin-Aguilera, M.; Solé-Blanch, C.; Indacochea, A.; Jimenez, N.; Bystrup, S.; Bakht, M.; Conteduca, V.; et al. Taxane-induced Attenuation of the CXCR2/BCL-2 Axis Sensitizes Prostate Cancer to Platinum-based Treatment. Eur. Urol. 2021, 79, 722–733. [Google Scholar] [CrossRef]
- Nehmé, A.; Varadarajan, P.; Sellakumar, G.; Gerhold, M.; Niedner, H.; Zhang, Q.; Lin, X.; Christen, R.D. Modulation of docetaxel-induced apoptosis and cell cycle arrest by all- trans retinoic acid in prostate cancer cells. Br. J. Cancer 2001, 84, 1571–1576. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Mirzaei, S.; Hashemi, F.; Zarrabi, A.; Zabolian, A.; Saleki, H.; Sharifzadeh, S.O.; Soleymani, L.; Daneshi, S.; Hushmandi, K.; et al. New insight towards development of paclitaxel and docetaxel resistance in cancer cells: EMT as a novel molecular mechanism and therapeutic possibilities. Biomed. Pharmacother. 2021, 141, 111824. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Hussain, M.; Sarkar, F.H. Regulation of Microtubule, Apoptosis, and Cell Cycle-Related Genes by Taxotere in Prostate Cancer Cells Analyzed by Microarray. Neoplasia 2004, 6, 158–167. [Google Scholar] [CrossRef]
- Caraglia, M. Docetaxel induces p53-dependent apoptosis and synergizes with farnesyl transferase inhibitor r115777 in human epithelial cancer cells. Front. Biosci. 2005, 10, 2566–2575. [Google Scholar] [CrossRef]
- Geng, C.-X.; Zeng, Z.-C.; Wang, J.-Y. Docetaxel inhibits SMMC-7721 human hepatocellular carcinoma cells growth and induces apoptosis. World J. Gastroenterol. WJG 2003, 9, 696–700. [Google Scholar] [CrossRef]
- Mohamed, A.F.; Nasr, M.; Amer, M.E.; Abuamara, T.M.M.; Abd-Elhay, W.M.; Kaabo, H.F.; Matar, E.E.R.; El Moselhy, L.E.; Gomah, T.A.; Deban, M.A.E.-F.; et al. Anticancer and antibacterial potentials induced post short-term exposure to electromagnetic field and silver nanoparticles and related pathological and genetic alterations: In vitro study. Infect. Agents Cancer 2022, 17, 4. [Google Scholar] [CrossRef]
- Vadalà, M.; Morales-Medina, J.C.; Vallelunga, A.; Palmieri, B.; Laurino, C.; Iannitti, T. Mechanisms and therapeutic effectiveness of pulsed electromagnetic field therapy in oncology. Cancer Med. 2016, 5, 3128–3139. [Google Scholar] [CrossRef] [PubMed]
- Cameron, I.L.; Markov, M.S.; Hardman, W.E. Optimization of a therapeutic electromagnetic field (EMF) to retard breast cancer tumor growth and vascularity. Cancer Cell Int. 2014, 14, 125. [Google Scholar] [CrossRef]
- Han, Q.; Chen, R.; Wang, F.; Chen, S.; Sun, X.; Guan, X.; Yang, Y.; Peng, B.; Pan, X.; Li, J.; et al. Pre-exposure to 50 Hz-electromagnetic fields enhanced the antiproliferative efficacy of 5-fluorouracil in breast cancer MCF-7 cells. PLoS ONE 2018, 13, e0192888. [Google Scholar] [CrossRef] [PubMed]
- Trebuňová, M.; Laputková, G.; Géci, I.; Andrašina, I.; Sabo, J. Enhancement of docetaxel-treated MCF-7 cell death by 900-MHz radiation. Open Life Sci. 2013, 8, 357–365. [Google Scholar] [CrossRef]
- Woo, S.-H.; Jung, B.C.; Kim, J.-Y.; Lee, Y.-H.; Kim, Y.S. PEMF Potentiates Doxorubicin-induced Late G2 Arrest in MDA-MB-231 Breast Cancer Cells. Anticancer Res. 2024, 44, 2837–2846. [Google Scholar] [CrossRef] [PubMed]
- Storch, K.; Dickreuter, E.; Artati, A.; Adamski, J.; Cordes, N. BEMER Electromagnetic Field Therapy Reduces Cancer Cell Radioresistance by Enhanced ROS Formation and Induced DNA Damage. PLoS ONE 2016, 11, e0167931. [Google Scholar] [CrossRef] [PubMed]
- Vesal, M.; Mahdavian, R.; Mortazavi, S.M.R.; Vaezi, Z.; Allahverdi, A.; Naderi-Manesh, H. Exquisite targeted delivery via Cerasome–Herceptin in HER2-positive human breast cancer cells. J. Drug Deliv. Sci. Technol. 2025, 112, 107224. [Google Scholar] [CrossRef]
- Fard, A.E.; Tavakoli, M.B.; Salehi, H.; Emami, H. Synergetic effects of Docetaxel and ionizing radiation reduced cell viability on MCF-7 breast cancer cell. Appl. Cancer Res. 2017, 37, 29. [Google Scholar] [CrossRef]
- Sukumar, V.K.; Tai, Y.K.; Chan, C.W.; Iversen, J.N.; Wu, K.Y.; Fong, C.H.H.; Lim, J.S.J.; Franco-Obregón, A. Brief Magnetic Field Exposure Stimulates Doxorubicin Uptake into Breast Cancer Cells in Association with TRPC1 Expression: A Precision Oncology Methodology to Enhance Chemotherapeutic Outcome. Cancers 2024, 16, 3860. [Google Scholar] [CrossRef]
- Bułdak, R.J.; Polaniak, R.; Bułdak, Ł.; Żwirska-Korczala, K.; Skonieczna, M.; Monsiol, A.; Kukla, M.; Duława-Bułdak, A.; Birkner, E. Short-term exposure to 50 Hz ELF-EMF alters the cisplatin-induced oxidative response in AT478 murine squamous cell carcinoma cells. Bioelectromagnetics 2012, 33, 641–651. [Google Scholar] [CrossRef]
- Sanie-Jahromi, F.; Saadat, I.; Saadat, M. Effects of extremely low frequency electromagnetic field and cisplatin on mRNA levels of some DNA repair genes. Life Sci. 2016, 166, 41–45. [Google Scholar] [CrossRef]
- Hernández-Vargas, H.; Palacios, J.; Moreno-Bueno, G. Molecular profiling of docetaxel cytotoxicity in breast cancer cells: Uncoupling of aberrant mitosis and apoptosis. Oncogene 2007, 26, 2902–2913. [Google Scholar] [CrossRef] [PubMed]
- Berchem, G.J.; Bosseler, M.; Mine, N.; Avalosse, B. Nanomolar range docetaxel treatment sensitizes MCF-7 cells to chemotherapy induced apoptosis, induces G2M arrest and phosphorylates bcl-2. Anticancer Res. 1999, 19, 535–540. [Google Scholar]
- Masoudi-Khoram, N.; Abdolmaleki, P. Effects of repeated exposure to 50 Hz electromagnetic field on breast cancer cells. Electromagn. Biol. Med. 2022, 41, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Elexpuru-Zabaleta, M.; Lazzarini, R.; Tartaglione, M.F.; Piva, F.; Ciarapica, V.; Busilacchi, E.M.; Poloni, A.; Valentino, M.; Santarelli, L.; Bracci, M. A 50 Hz magnetic field influences the viability of breast cancer cells 96 h after exposure. Mol. Biol. Rep. 2023, 50, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lv, Y. Magnetic Fields as Biophysical Modulators of Anticancer Drug Action. Magnetochemistry 2025, 11, 89. [Google Scholar] [CrossRef]



| Compared Pairs | p-Value | ||
|---|---|---|---|
| Live | Apoptotic | Necrosis | |
| Control—Docetaxel (50 µM) | 0.0000984 | 0.0000798 | 0.4217 |
| ELF-EMF—Docetaxel (14 µM) +ELF-EMF | 0.00082 | 0.00058 | 0.8667 |
| Control—ELF-EMF | 0.9171 | 0.8646 | 0.9937 |
| Docetaxel (50 µM)—Docetaxel (14 µM) + EMF | −0.4027 | 0.5066 | 0.6972 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vesal, M.; Moazen Safaei, Y.; Ramazi, S.; Allahverdi, A.; Abdolmaleki, P.; Naderi-Manesh, H. Enhanced Antiproliferative Activity of Docetaxel by Extremely Low Frequency Electromagnetic Fields in MCF-7 Breast Cancer Cells. Pharmaceutics 2025, 17, 1505. https://doi.org/10.3390/pharmaceutics17121505
Vesal M, Moazen Safaei Y, Ramazi S, Allahverdi A, Abdolmaleki P, Naderi-Manesh H. Enhanced Antiproliferative Activity of Docetaxel by Extremely Low Frequency Electromagnetic Fields in MCF-7 Breast Cancer Cells. Pharmaceutics. 2025; 17(12):1505. https://doi.org/10.3390/pharmaceutics17121505
Chicago/Turabian StyleVesal, Maryam, Yasaman Moazen Safaei, Shahin Ramazi, Abdollah Allahverdi, Parviz Abdolmaleki, and Hossein Naderi-Manesh. 2025. "Enhanced Antiproliferative Activity of Docetaxel by Extremely Low Frequency Electromagnetic Fields in MCF-7 Breast Cancer Cells" Pharmaceutics 17, no. 12: 1505. https://doi.org/10.3390/pharmaceutics17121505
APA StyleVesal, M., Moazen Safaei, Y., Ramazi, S., Allahverdi, A., Abdolmaleki, P., & Naderi-Manesh, H. (2025). Enhanced Antiproliferative Activity of Docetaxel by Extremely Low Frequency Electromagnetic Fields in MCF-7 Breast Cancer Cells. Pharmaceutics, 17(12), 1505. https://doi.org/10.3390/pharmaceutics17121505






