Advancing Personalized Medicine Through FDM 3D Printing: Ketoprofen Tablets with Customizable Drug Release Profiles and In Silico Simulation
Abstract
1. Introduction
2. Materials an Methods
2.1. Materials
2.2. Methods
2.2.1. Filament Fabrication via HME
2.2.2. Filament Images
2.2.3. Filament Scanning Electron Microscopy (SEM)
2.2.4. Filament Quality Control
2.2.5. Filament Tensile Strength
2.2.6. Filament Dissolution Studies
2.2.7. Tablet Design and Dimensions
2.2.8. Selection of Dose
2.2.9. Images
2.2.10. Tablet Quality Control
2.2.11. Tablet In Vitro Studies
2.2.12. Comparison with Market Tablets
2.2.13. Tablet Micro-CT Scan
2.2.14. Tablet Attenuated Total Reflection Fourier-Transform Infrared (ATR-FTIR) Spectroscopy
2.2.15. Tablet Scanning Electron Microscopy (SEM)
2.2.16. Tablet XRD (X-RAY Diffractometry)
2.2.17. Tablet Thermogravimetric Analysis (TGA)
2.2.18. Tablet Differential Scanning Calorimetry (DSC)
2.2.19. In Silico Simulation
2.2.20. Statistical Analysis
3. Results and Discussion
3.1. Filament Fabrication via HME
3.2. Filament Images
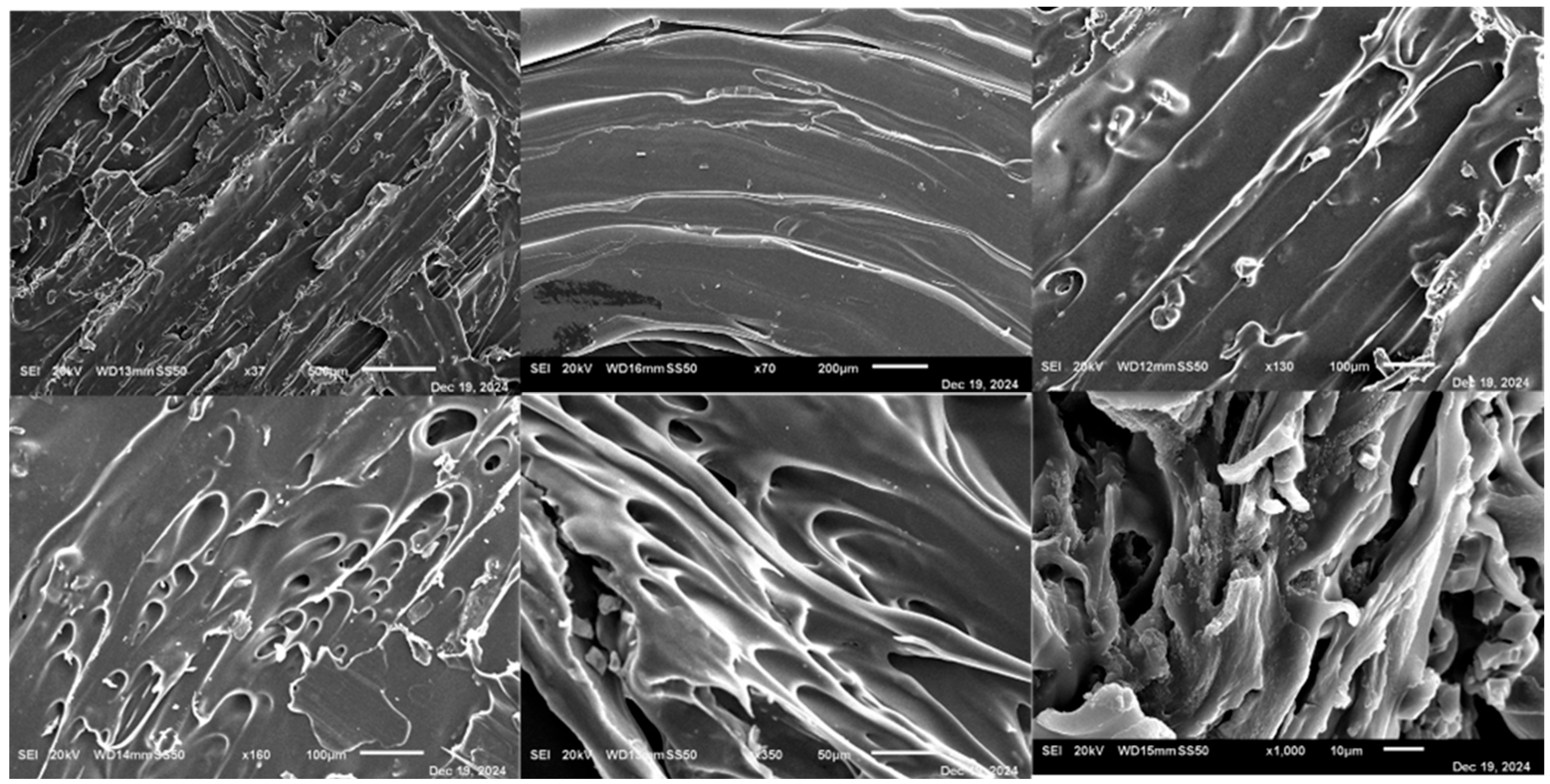
3.3. Filament SEM
3.4. Filament Quality Control
3.5. Filament Tensile Strength
3.6. Filament Dissolution
3.7. Selection of Filaments
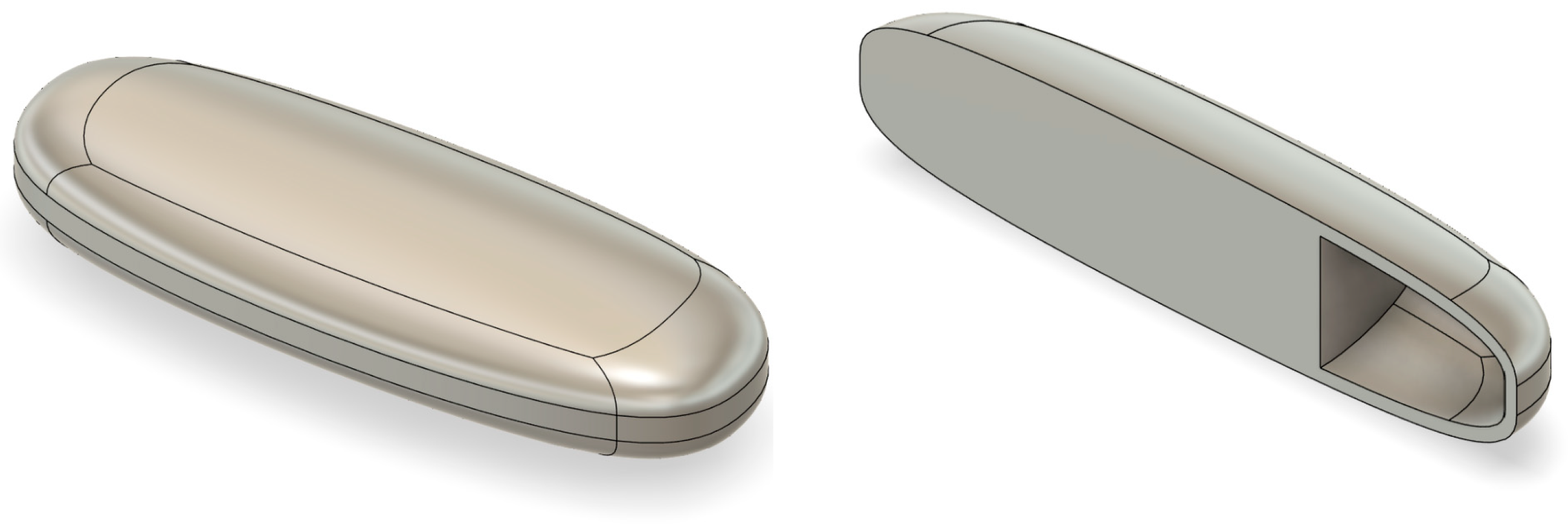
3.8. Design Dimensions and Printing Parameters of 3D-Printed Tablets
3.9. Tablet Images
3.10. Tablet Quality Control
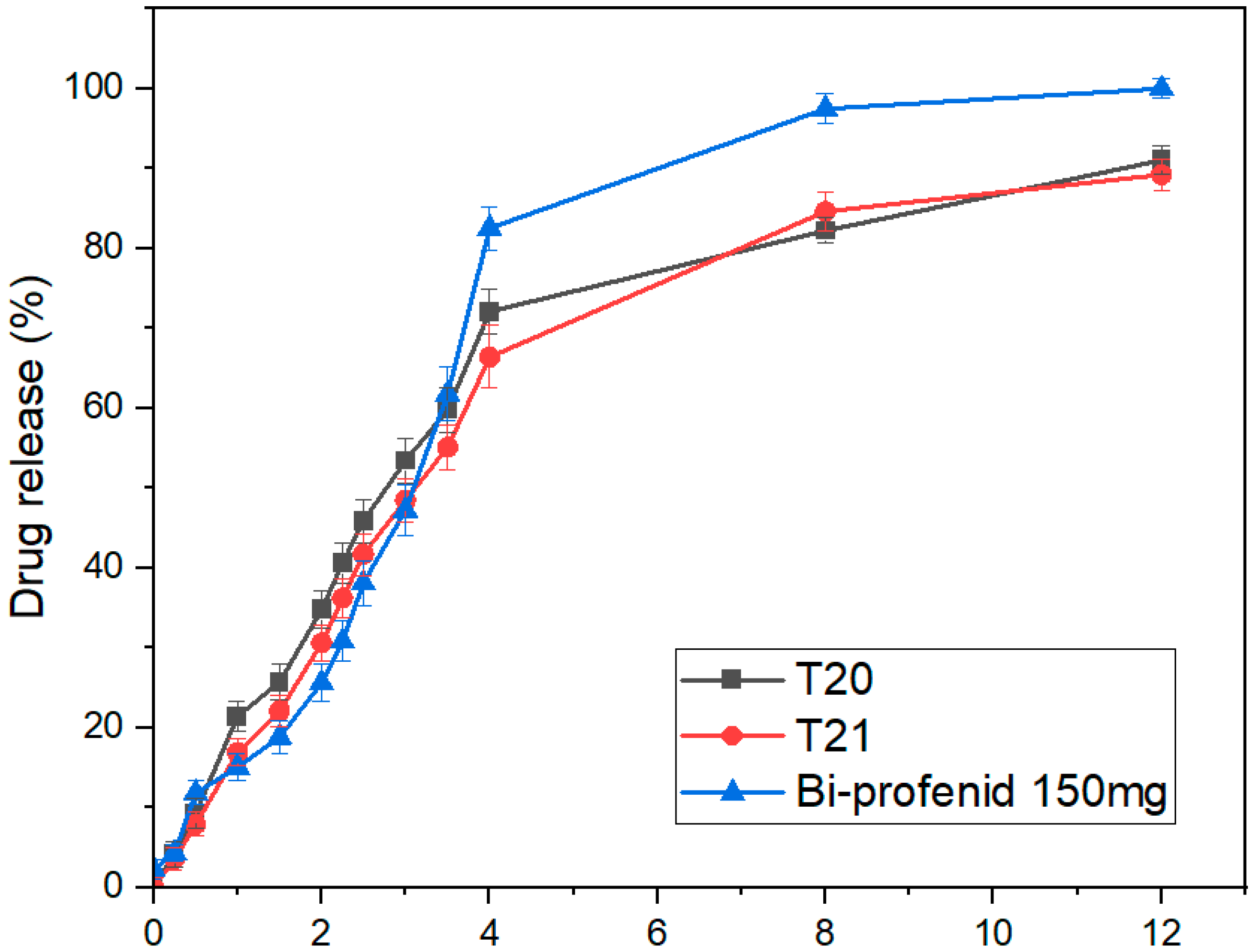
3.11. Tablet In Vitro Dissolution
3.12. Comparison with Market Tablets
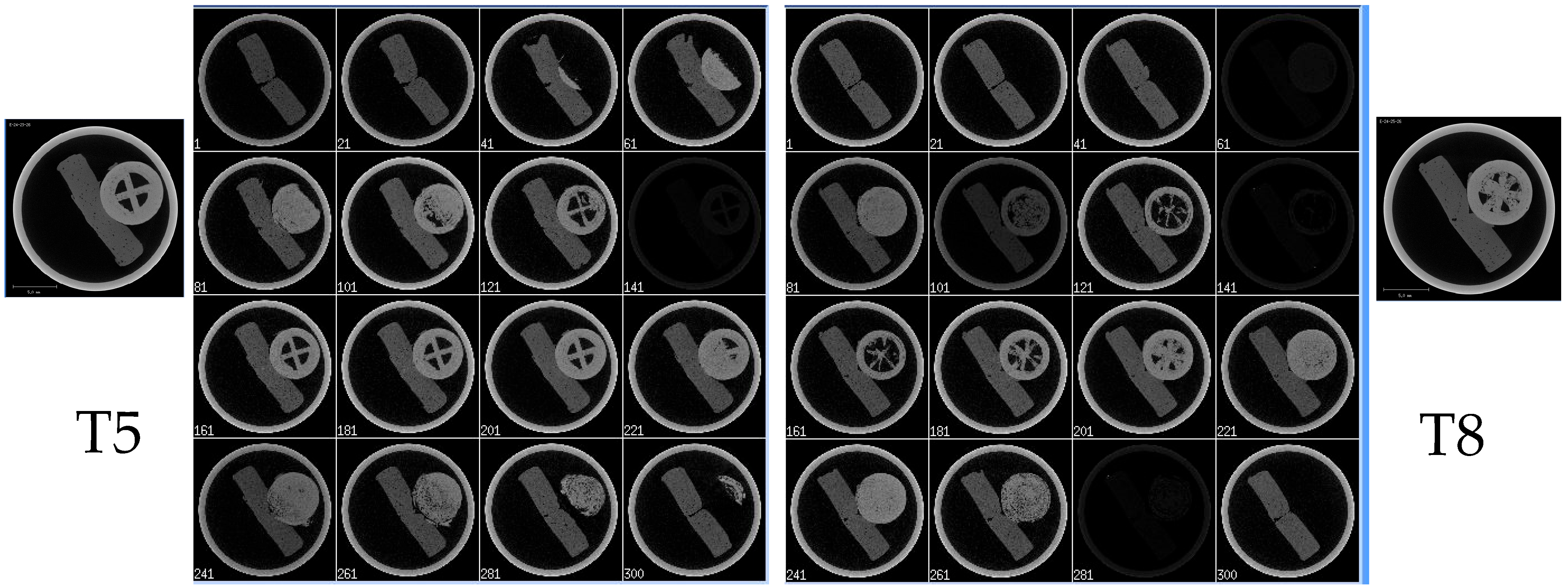
3.13. Micro-CT
3.14. Attenuated Total Reflection Fourier-Transform Infrared (ATR-FTIR) Spectroscopy
3.15. Scanning Electron Microscopy (SEM)
3.16. XRD
3.17. Thermogravimetric Analysis (TGA)
3.18. Differential Scanning Calorimetry (DSC)
3.19. In Silico Simulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3DP | Three-Dimensional Printing |
| AM | Additive Manufacturing |
| API | Active Pharmaceutical Ingredient |
| ATR-FTIR | Attenuated Total Reflection Fourier Transform Infrared |
| BV | Bone Volume |
| DSC | Differential Scanning Calorimetry |
| FDM | Fused Deposition Modeling |
| HCl | Hydrochloric Acid |
| HME | Hot-Melt Extrusion |
| HPMC | Hydroxypropyl Methylcellulose |
| HPC | Hydroxypropyl Cellulose |
| IR | Immediate Release |
| IVIVC | In Vitro–In Vivo Correlation |
| KIR | Kollicoat® IR |
| NSAID | Non-Steroidal Anti-Inflammatory Drug |
| PVA | Polyvinyl Alcohol |
| PVP | Polyvinylpyrrolidone |
| SD | Standard Deviation |
| SEM | Scanning Electron Microscopy |
| SLS | Sodium Lauryl Sulfate |
| SR | Sustained Release |
| TGA | Thermogravimetric Analysis |
| Tmax | Time to Maximum Plasma Concentration |
| Cmax | Maximum Plasma Concentration |
| AUC | Area Under the Curve |
| USP | United States Pharmacopeia |
| XRD | X-ray Diffraction |
References
- Goyanes, A.; Wang, J.; Buanz, A.; Martínez-Pacheco, R.; Telford, R.; Gaisford, S.; Basit, A.W. 3D Printing of Medicines: Engineering Novel Oral Devices with Unique Design and Drug Release Characteristics. Mol. Pharm. 2015, 12, 4077–4084. [Google Scholar] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [PubMed]
- Arafat, B.; Wojsz, M.; Isreb, A.; Forbes, R.T.; Isreb, M.; Ahmed, W.; Arafat, T.; Alhnan, M.A. Tablet fragmentation without a disintegrant: A novel design approach for accelerating disintegration and drug release from 3D printed cellulosic tablets. Eur. J. Pharm. Sci. 2018, 118, 191–199. [Google Scholar]
- Goyanes, A.; Robles Martinez, P.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar]
- Agoram, B.; Woltosz, W.S.; Bolger, M.B. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. In Advanced Drug Delivery Reviews; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Jamei, M.; Dickinson, G.L.; Rostami-Hodjegan, A. A framework for assessing inter-individual variability in pharmacokinetics using virtual human populations and integrating general knowledge of physical chemistry, biology, anatomy, physiology and genetics: A tale of “bottom-up” vs. “top-down” recognition of covariates. Drug Metab. Pharmacokinet. 2009, 24, 53–75. [Google Scholar]
- Solanki, N.G.; Tahsin, M.; Shah, A.V.; Serajuddin, A.T.M. Formulation of 3D Printed Tablet for Rapid Drug Release by Fused Deposition Modeling: Screening Polymers for Drug Release, Drug-Polymer Miscibility and Printability. J. Pharm. Sci. 2018, 107, 390–401. [Google Scholar]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-Melt Extrusion: From Theory to Application in Pharmaceutical Formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar]
- Kantor, T.G. Ketoprofen: A Review of Its Pharmacologic and Clinical Properties. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1986, 6, 93–102. [Google Scholar]
- Pyteraf, J.; Jamróz, W.; Kurek, M.; Szafraniec-Szczęsny, J.; Kramarczyk, D.; Jurkiewicz, K.; Knapik-Kowalczuk, J.; Tarasiuk, J.; Wroński, S.; Paluch, M.; et al. How to obtain the maximum properties flexibility of 3d printed ketoprofen tablets using only one drug-loaded filament? Molecules 2021, 26, 3106. [Google Scholar]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar]
- Zhang, J.; Yang, W.; Vo, A.Q.; Feng, X.; Ye, X.; Kim, D.W.; Repka, M.A. Hydroxypropyl methylcellulose-based controlled release dosage by melt extrusion and 3D printing: Structure and drug release correlation. Carbohydr. Polym. 2017, 177, 49–57. [Google Scholar] [CrossRef] [PubMed]
- The United States Pharmacopeial Convention. 〈1151〉 Pharmaceutical Dosage Forms; The United States Pharmacopeia: Rockville, MD, USA, 2023. [Google Scholar]
- ASTM International. Standard Test Method for Tensile Properties of Thin Plastic Sheeting, D882-10; Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2010; Volume 87, Available online: https://www.wewontech.com/testing-standards/190125019.pdf (accessed on 27 October 2025).
- Domingo-Espin, M.; Puigoriol-Forcada, J.M.; Garcia-Granada, A.A.; Llumà, J.; Borros, S.; Reyes, G. Mechanical property characterization and simulation of fused deposition modeling Polycarbonate parts. Mater. Des. 2015, 83, 670–677. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Li, H.; Ou, Z.; Yang, G. 3D printed tablets with internal scaffold structure using ethyl cellulose to achieve sustained ibuprofen release. Eur. J. Pharm. Sci. 2018, 115, 11–18. [Google Scholar] [CrossRef]
- Li Chew, S.; de Mohac, L.M.; Raimi-Abraham, B.T. 3d-printed solid dispersion drug products. Pharmaceutics 2019, 11, 672. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.M.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef]
- Tagami, T.; Fukushige, K.; Ogawa, E.; Hayashi, N.; Ozeki, T. 3D printing factors important for the fabrication of polyvinylalcohol filament-based tablets. Biol. Pharm. Bull. 2017, 40, 357–364. [Google Scholar] [CrossRef]
- FDA. FDA Inactive Ingredient Database (IID). Sodium Lauryl Sulfate; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2025.
- Spritam-A new formulation of levetiracetam for epilepsy. Med. Lett. Drugs Ther. 2016, 58. Available online: https://secure.medicalletter.org/TML-article-1497b (accessed on 27 October 2025).
- Goyanes, A.; Buanz, A.B.M.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef]
- Fuenmayor, E.; Forde, M.; Healy, A.V.; Devine, D.M.; Lyons, J.G.; McConville, C.; Major, I. Material considerations for fused-filament fabrication of solid dosage forms. Pharmaceutics 2018, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Sadia, M.; Sośnicka, A.; Arafat, B.; Isreb, A.; Ahmed, W.; Kelarakis, A.; A Alhnan, M. Adaptation of pharmaceutical excipients to FDM 3D printing for the fabrication of patient-tailored immediate release tablets. Int. J. Pharm. 2016, 513, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Molavi, F.; Hamishehkar, H.; Nokhodchi, A. Impact of tablet shape on drug dissolution rate through immediate released tablets. Adv. Pharm. Bull. 2020, 10, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.C.; Lin, R.P.; Liu, J.P. Statistical evaluations of dissolution similarity. Stat. Sin. 1999, 9, 1011–1027. [Google Scholar]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Tiţa, D.; Fuliaş, A.; Tiţa, B. Thermal stability of ketoprofen—Active substance and tablets. J. Therm. Anal. Calorim. 2011, 105, 501–508. [Google Scholar] [CrossRef]
- Bogdan, U.T.A.; Marian, E.; Rusu, G.; Bandur, G.; Tita, D. Effects of experimental conditions on the thermal behaviour of some non-steroidal anti-inflammatory drugs. Rev. Chim. 2013, 64, 1390–1394. [Google Scholar]
- Dahan, A.; Miller, J.M.; Amidon, G.L. Prediction of solubility and permeability class membership: Provisional BCS classification of the world’s top oral drugs. AAPS J. 2009, 11, 740–746. [Google Scholar] [CrossRef] [PubMed]




















| Filament Formulation | Polymer Type | Drug Loading (% w/w) | Extrusion Temperature (°C) | Screw Speed (rpm) | Additives |
|---|---|---|---|---|---|
| F1 | Kollicoat® IR | 10% | 160 | 30 | |
| F2 | Kollicoat® IR | 30% | 160 | 30 | |
| F3 | Kollicoat® IR | 50% | 160 | 30 | |
| F4 | Kollicoat® IR | 30% | 180 | 30 | |
| F5 | Kollicoat® IR | 30% | 160 | 50 | |
| F6 | Kollicoat® IR | 30% | 160 | 30 | 1% Sorbitol + 1% SLS |
| F7 | Kollicoat® IR | 30% | 160 | 30 | 1% Sorbitol + 1% Tween |
| F8 | Kollicoat® IR | 30% | 160 | 30 | 1% Sorbitol |
| F9 | PVA | 30% | 160 | 30 | 1% Sorbitol |
| F10 | Kollidon® SR | 30% | 160 | 30 | 1% Sorbitol |
| F11 | Ethyl cellulose | 30% | 160 | 30 | 1% Sorbitol |
| F12 | HPMC 2600–5600 cP | 30% | 160 | 30 | |
| F13 | HPMC 2600–5600 cP | 30% | 160 | 30 | 1% Sorbitol |
| F14 | HPMC 2600–5600 cP | 30% | 160 | 30 | 1% Sorbitol + 1%SLS |
| F15 | HPMC 2600–5600 cP | 30% | 160 | 30 | 1% Sorbitol + 1% Tween |
| Filament | Mean Weight (g) ± SD | Mean Diameter (mm) ± SD | Mean Actual Drug Content (%) ± SD |
|---|---|---|---|
| F1 | 0.097 ± 0.0032 | 1.704 ± 0.012 | 95.14 ± 2.15 |
| F2 | 0.1032 ± 0.0028 | 1.702 ± 0.014 | 94.15 ± 2.05 |
| F3 | 0.102 ± 0.0041 | 1.707 ± 0.015 | 82.47 ± 3.62 |
| F4 | 0.103 ± 0.0030 | 1.698 ± 0.018 | 90.12 ± 2.48 |
| F5 | 0.102 ± 0.0033 | 1.714 ± 0.016 | 92.14 ± 2.71 |
| F6 | 0.101 ± 0.0035 | 1.707 ± 0.011 | 100.92 ± 1.93 |
| F7 | 0.1056 ± 0.0042 | 1.7077 ± 0.014 | 93.24 ± 2.37 |
| F8 | 0.10397 ± 0.0036 | 1.7011 ± 0.012 | 102.11 ± 2.15 |
| F9 | 0.109 ± 0.0034 | 1.703 ± 0.013 | 99.60 ± 2.12 |
| F10 | 0.142 ± 0.0040 | 1.819 ± 0.021 | 89.95 ± 2.68 |
| F11 | 0.1236 ± 0.0037 | 1.550 ± 0.019 | 77.11 ± 4.01 |
| F12 | 0.1622 ± 0.0035 | 1.735 ± 0.016 | 97.52 ± 2.10 |
| F13 | 0.1625 ± 0.0034 | 1.732 ± 0.015 | 99.32 ± 1.92 |
| F14 | 0.1622 ± 0.0033 | 1.732 ± 0.017 | 96.31 ± 2.25 |
| F15 | 0.1623 ± 0.0031 | 1.734 ± 0.013 | 99.19 ± 1.89 |
| Formulation | Mean Break Force (N) | Mean Break Stress (N/mm2) | Mean Break Stroke (mm) | Mean Break Strain (%) | Mean Break Time (s) | Mean Max Force (N) |
|---|---|---|---|---|---|---|
| F1 | 37.11 ± 1.43 | 47.25 ± 1.88 | 5.10 ± 0.44 | 5.10 ± 0.44 | 20.38 ± 1.15 | 50.06 ± 1.77 |
| F2 | 31.35 ± 0.44 | 41.18 ± 0.63 | 7.91 ± 0.39 | 7.91 ± 0.39 | 19.62 ± 1.04 | 39.50 ± 0.53 |
| F3 | 3.85 ± 0.28 | 2.33 ± 0.21 | 0.94 ± 0.08 | 1.14 ± 0.09 | 11.32 ± 0.91 | 4.43 ± 0.22 |
| F4 | 26.68 ± 0.19 | 33.98 ± 0.74 | 49.80 ± 0.34 | 49.80± 0.34 | 34.14± 0.24 | 34.40 ± 0.84 |
| F5 | 3.88 ± 0.29 | 4.94 ± 0.32 | 5.20 ± 0.42 | 5.20 ± 0.42 | 26.79 ± 1.10 | 4.24 ± 0.27 |
| F6 | 79.65 ± 2.14 | 100.15 ± 3.22 | 3.89 ± 0.36 | 3.89 ± 0.36 | 15.56 ± 0.98 | 84.04 ± 2.11 |
| F7 | 25.66 ± 1.13 | 8.17 ± 0.74 | 33.55 ± 2.61 | 67.09 ± 3.88 | 402.56 ± 6.21 | 45.82 ± 1.88 |
| F8 | 56.15 ± 1.76 | 71.49 ± 2.10 | 6.37 ± 0.50 | 6.37 ± 0.50 | 25.49 ± 1.16 | 81.69 ± 2.35 |
| F9 | 31.87 ± 1.24 | 40.57 ± 1.76 | 0.81 ± 0.07 | 0.81 ± 0.07 | 3.23 ± 0.20 | 32.17 ± 1.30 |
| F10 | 31.27 ± 0.91 | 40.35 ± 1.02 | 71.58 ± 1.40 | 71.58 ± 1.40 | 42.67 ± 2.32 | 39.27 ± 0.97 |
| F11 | 10.35 ± 0.40 | 13.18 ± 0.56 | 4.91 ± 0.35 | 4.91 ± 0.35 | 19.62 ± 0.92 | 10.50 ± 0.48 |
| F12 | 61.88 ± 0.52 | 80.40 ± 0.71 | 3.73 ± 0.48 | 3.73 ± 0.48 | 20.92 ± 1.15 | 3.59 ± 0.80 |
| F13 | 82.83 ± 3.24 | 108.01 ± 3.11 | 5.93 ± 0.75 | 5.93 ± 0.75 | 22.35 ± 0.92 | 102.77 ± 2.98 |
| F14 | 80.83 ± 2.34 | 108.01 ± 3.11 | 6.37 ± 0.52 | 6.37 ± 0.52 | 25.71 ± 1.22 | 82.77 ± 2.98 |
| F15 | 14.19 ± 0.38 | 4.97 ± 0.61 | 0.70 ± 0.63 | 0.70 ± 0.63 | 6.17 ± 2.04 | 14.85 ± 0.52 |
| PVA (MakerBot) | 41.74 ± 1.75 | 53.15 ± 2.13 | 1.43 ± 0.12 | 1.43 ± 0.12 | 5.71 ± 0.37 | 41.74 ± 1.75 |
| 3DP Tablet | Body Design Dimension x × y × z mm | Mean Time to Print Body/min |
|---|---|---|
| Immediate 50 mg | 7 × 7 × 4 | 2.75 ± 0.10 |
| Immediate 75 mg | 9 × 9 × 4 | 3 ± 0.12 |
| Immediate 100 mg | 9.25 × 9.25 × 5 | 3.75 ± 0.20 |
| Sustained | 10.5 × 10.5 × 5 | 5.5 ± 0.15 |
| Sustained Air | 11 × 11 × 5 | 6 ± 0.25 |
| Formulation | Special Design | Printing Pattern | Shell Number | Infill Density % | Printing Speed Mm/s | Temp °C | Accuracy /mm | Drug Amount /mg | Polymer |
|---|---|---|---|---|---|---|---|---|---|
| T1 | No air pocket | One wall | 1 | 1 | 30 | 180 | 0.2 | 100 | Kollicoat® IR |
| T2 | No air pocket | One wall | 5 | 1 | 30 | 180 | 0.2 | 100 | Kollicoat® IR |
| T3 | No air pocket | One wall | 10 | 1 | 30 | 180 | 0.2 | 100 | Kollicoat® IR |
| T4 | No air pocket | One wall | 1 | 25 | 30 | 180 | 0.2 | 100 | Kollicoat® IR |
| T5 | No air pocket | One wall | 5 | 25 | 30 | 180 | 0.2 | 100 | Kollicoat® IR |
| T6 | No air pocket | One wall | 10 | 25 | 30 | 180 | 0.2 | 100 | Kollicoat® IR |
| T7 | No air pocket | One wall | 1 | 50 | 30 | 180 | 0.2 | 100 | Kollicoat® IR |
| T8 | No air pocket | One wall | 5 | 50 | 30 | 180 | 0.2 | 100 | Kollicoat® IR |
| T9 | No air pocket | One wall | 10 | 50 | 30 | 180 | 0.2 | 100 | Kollicoat® IR |
| T10 | No air pocket | Tri hexa | 5 | 25 | 30 | 180 | 0.2 | 100 | Kollicoat® IR |
| T11 | No air pocket | Grid | 5 | 25 | 30 | 180 | 0.2 | 100 | Kollicoat® IR |
| T12 | No air pocket | One wall | 5 | 25 | 30 | 200 | 0.2 | 100 | Kollicoat® IR |
| T13 | No air pocket | One wall | 5 | 25 | 30 | 180 | 0.2 | 50 | Kollicoat® IR |
| T14 | No air pocket | One wall | 5 | 25 | 30 | 180 | 0.2 | 75 | Kollicoat® IR |
| T15 | No air pocket | Grid | 10 | 50 | 30 | 180 | 0.2 | 150 | HPMC 2600–5600 cP |
| T16 | No air pocket | Grid | 50 | 50 | 30 | 180 | 0.2 | 150 | HPMC 2600–5600 cP |
| T17 | No air pocket | Grid | 100 | 50 | 30 | 180 | 0.2 | 150 | HPMC 2600–5600 cP |
| T18 | No air pocket | Grid | 10 | 100 | 30 | 180 | 0.2 | 150 | HPMC 2600–5600 cP |
| T19 | No air pocket | Grid | 50 | 100 | 30 | 180 | 0.2 | 150 | HPMC 2600–5600 cP |
| T20 | No air pocket | Grid | 100 | 100 | 30 | 180 | 0.2 | 150 | HPMC 2600–5600 cP |
| T21 | Air pocket | Grid | 100 | 100 | 30 | 180 | 0.2 | 150 | HPMC 2600–5600 cP |
| Parameter | T5 (IR 100 mg) | T13 (IR 50 mg) | T14 (IR 75 mg) | T20 (SR 150 mg) | T21 (SR Air 150 mg) |
|---|---|---|---|---|---|
| Weight (g) Mean ± SD | 0.350 ± 0.006 | 0.170 ± 0.004 | 0.260 ± 0.005 | 0.520 ± 0.007 | 0.537 ± 0.008 |
| Diameter (mm) Mean ± SD | 9.23 ± 0.06 | 7.02 ± 0.04 | 9.03 ± 0.07 | 10.52 ± 0.08 | 11.03 ± 0.06 |
| Thickness (mm) Mean ± SD | 4.96 ± 0.03 | 4.04 ± 0.02 | 3.96 ± 0.04 | 5.02 ± 0.04 | 5.09 ± 0.03 |
| Hardness (kg) Mean ± SD | 4.49 ± 0.14 | 4.42 ± 0.12 | 4.56 ± 0.13 | 7.34 ± 0.16 | 7.39 ± 0.15 |
| Friability (%) Mean ± SD | 0.34 ± 0.03 | 0.28 ± 0.02 | 0.30 ± 0.03 | 0.21 ± 0.02 | 0.23 ± 0.01 |
| Disintegration Time (min) Mean ± SD | 28.46 ± 0.71 | 20.13 ± 0.68 | 22.47 ± 0.74 | N/A | N/A |
| Content Uniformity (%)Mean ± SD) | 99.5 ± 1.3 | 99.7 ± 1.17 | 100.75 ± 1.71 | 99.1 ± 1.6 | 98.0 ± 1.8 |
| Assay (%) Mean ± SD) | 99.5 ± 1.1 | 99.7 ± 1.3 | 100.8 ± 1.2 | 100.2 ± 1.5 | 98.0 ± 1.7 |
| Formulation | f1 (Difference Factor) | f2 (Similarity Factor) |
|---|---|---|
| T4 | 1.59 | 78.4 |
| T5 | 2.87 | 74.6 |
| Formulation | f1 (Difference Factor) | f2 (Similarity Factor) |
|---|---|---|
| T20 | 8.93 | 62.4 |
| T21 | 12.6 | 56.2 |
| Formulation | Dose (mg) | Tmax (h) | Cmax (ng/mL) | AUC (ng.h/mL) |
|---|---|---|---|---|
| T13 | 50 | 1.1 | 2600 | 5460 |
| T14 | 75 | 1.1 | 3800 | 9900 |
| T5 | 100 | 1.1 | 5400 | 13,200 |
| T20 | 150 | 4.0 | 2400 | 22,380 |
| T21 | 150 | 5.5 | 1850 | 21,610 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasin, H.K.A.; Al-Tabakha, M.M.; Chan, S.Y. Advancing Personalized Medicine Through FDM 3D Printing: Ketoprofen Tablets with Customizable Drug Release Profiles and In Silico Simulation. Pharmaceutics 2025, 17, 1495. https://doi.org/10.3390/pharmaceutics17111495
Yasin HKA, Al-Tabakha MM, Chan SY. Advancing Personalized Medicine Through FDM 3D Printing: Ketoprofen Tablets with Customizable Drug Release Profiles and In Silico Simulation. Pharmaceutics. 2025; 17(11):1495. https://doi.org/10.3390/pharmaceutics17111495
Chicago/Turabian StyleYasin, Haya Khader Ahmad, Moawia M. Al-Tabakha, and Siok Yee Chan. 2025. "Advancing Personalized Medicine Through FDM 3D Printing: Ketoprofen Tablets with Customizable Drug Release Profiles and In Silico Simulation" Pharmaceutics 17, no. 11: 1495. https://doi.org/10.3390/pharmaceutics17111495
APA StyleYasin, H. K. A., Al-Tabakha, M. M., & Chan, S. Y. (2025). Advancing Personalized Medicine Through FDM 3D Printing: Ketoprofen Tablets with Customizable Drug Release Profiles and In Silico Simulation. Pharmaceutics, 17(11), 1495. https://doi.org/10.3390/pharmaceutics17111495






