Bis-Oxadiazole Assemblies as NO-Releasing Anticancer Agents
Abstract
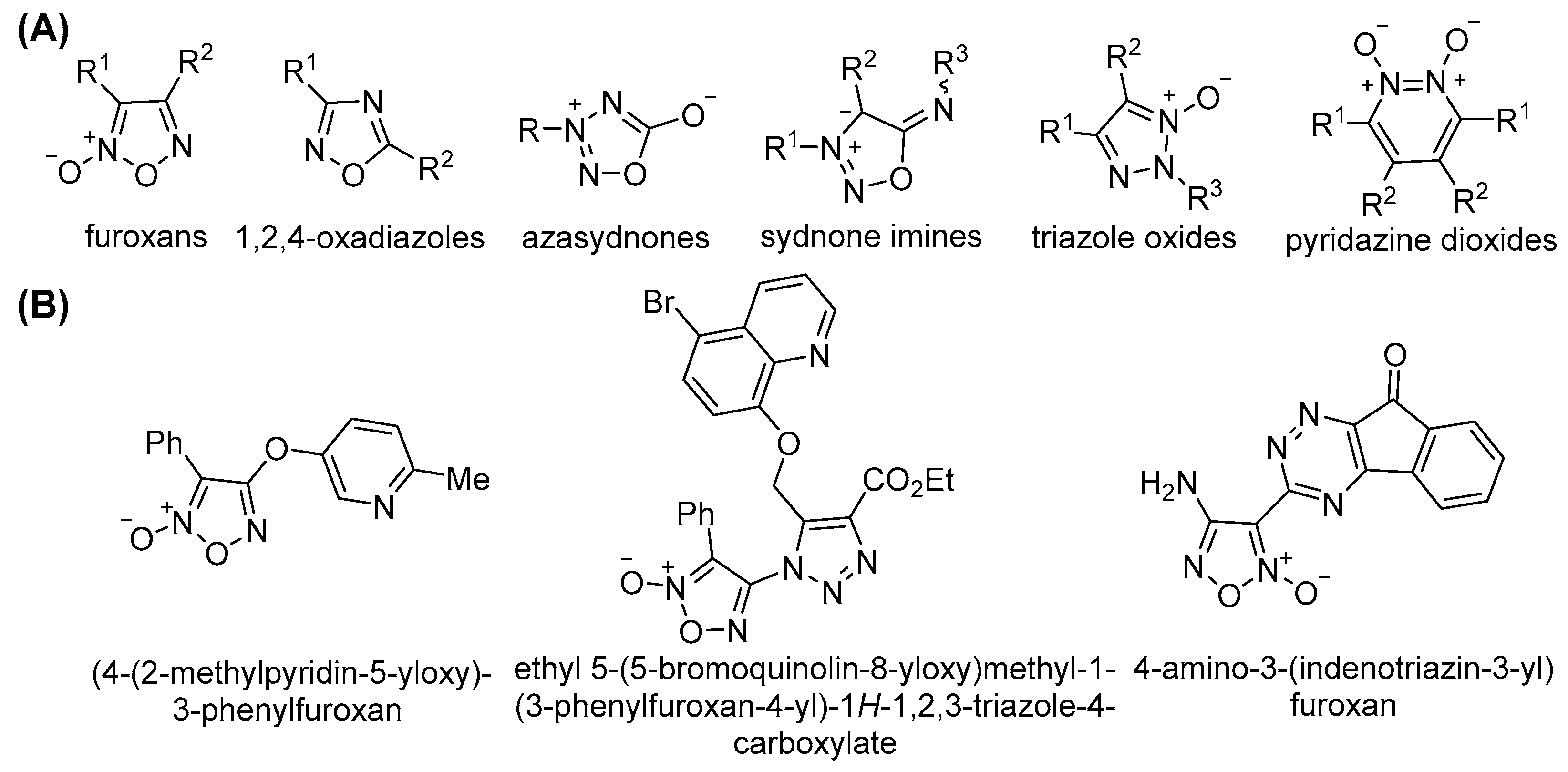
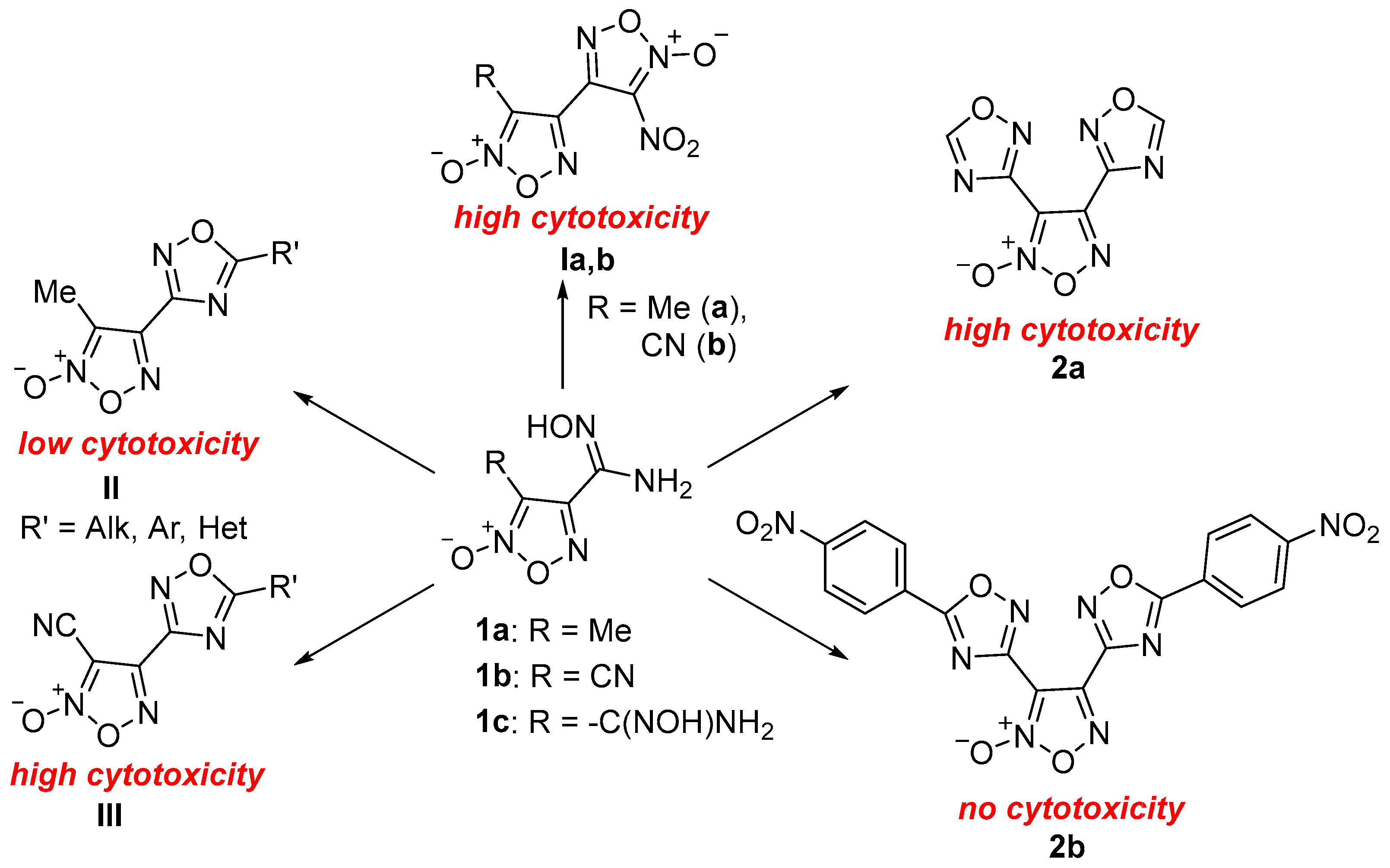
1. Introduction
2. Results and Discussion
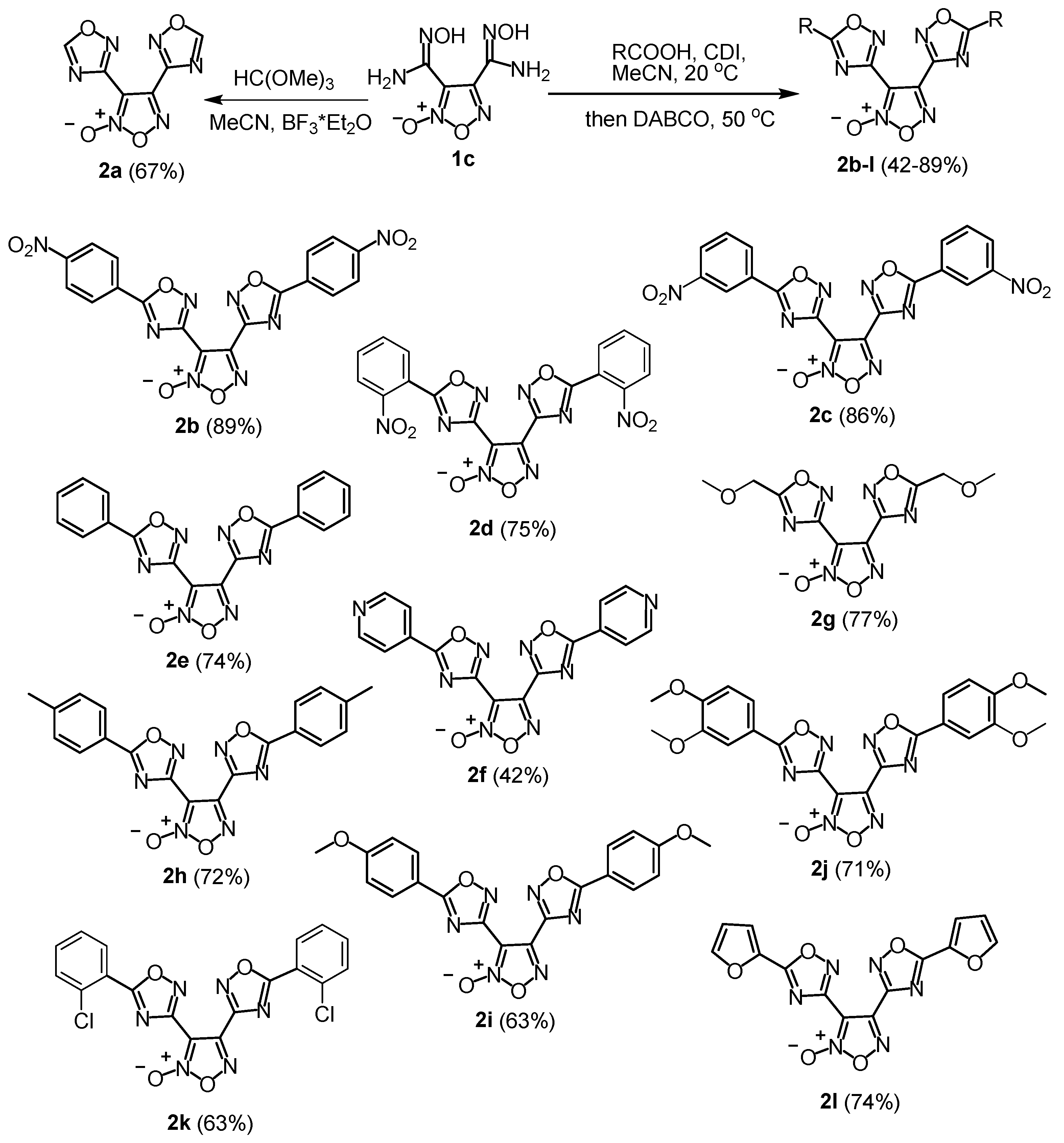
2.1. Synthesis
2.2. X-Ray Crystallography and Density-Functional Theory Calculations
2.3. NO Release
2.4. Anticancer Activity
2.5. Investigation of the In Vitro Mechanism of Action
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Smith, J.M.; Dixon, J.A.; deGruyter, J.N.; Baran, P.S. Alkyl Sulfinates: Radical Precursors Enabling Drug Discovery Miniperspective. J. Med. Chem. 2019, 62, 2256–2264. [Google Scholar] [CrossRef]
- Krska, S.W.; DiRocco, D.A.; Dreher, S.D.; Shevlin, M. The Evolution of Chemical High-Throughput Experimentation To Address Challenging Problems in Pharmaceutical Synthesis. Acc. Chem. Res. 2017, 50, 2976–2985. [Google Scholar] [CrossRef]
- Hansen, E.C.; Pedro, D.J.; Wotal, A.C.; Gower, N.J.; Nelson, J.D.; Caron, S.; Weix, D.J. New ligands for nickel catalysis from diverse pharmaceutical heterocycle libraries. Nat. Chem. 2016, 8, 1126–1130. [Google Scholar] [CrossRef]
- Hilton, M.C.; Zhang, X.; Boyle, B.T.; Alegre-Requena, J.V.; Paton, R.S.; McNally, A. Heterobiaryl synthesis by contractive C–C coupling via P(V) intermediates. Science 2018, 362, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Delost, M.D.; Qureshi, M.H.; Smith, D.T.; Njardarson, J.T. A Survey of the Structures of US FDA Approved Combination Drugs. J. Med. Chem. 2019, 62, 4265–4311. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef]
- Serafim, R.A.M.; Pernichelle, F.G.; Ferreira, E.I. The latest advances in the discovery of nitric oxide hybrid drug compounds. Expert Opin. Drug. Discov. 2017, 12, 941–953. [Google Scholar] [CrossRef]
- Bryan, N.S. Natural Product Chemistry for Nitric Oxide Based Therapeutics. Isr. J. Chem. 2019, 59, 414–419. [Google Scholar] [CrossRef]
- Huang, Z.; Fu, J.; Zhang, Y. Nitric Oxide Donor-Based Cancer Therapy: Advances and Prospects. J. Med. Chem. 2017, 60, 7617–7635. [Google Scholar] [CrossRef]
- Wang, P.G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A.J. Nitric Oxide Donors: Chemical Activities and Biological Applications. Chem. Rev. 2002, 102, 1091–1134. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Zhilin, E.S. Recent Advances in the Synthesis and Biomedical Applications of Heterocyclic NO-Donors. Molecules 2021, 26, 5705. [Google Scholar] [CrossRef]
- Ranadive, S.M.; Eugene, A.R.; Dillon, G.; Nicholson, W.T.; Joyner, M.J. Comparison of the vasodilatory effects of sodium nitroprusside vs. nitroglycerin. J. Appl. Physiol. 2017, 123, 402–406. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Makhova, N.N. Molecular Hybridization Tools in the Development of Furoxan-Based NO-Donor Prodrugs. ChemMedChem 2017, 12, 622–638. [Google Scholar] [CrossRef] [PubMed]
- Makhova, N.N.; Fershtat, L.L. Recent advances in the synthesis and functionalization of 1,2,5-oxadiazole 2-oxides. Tetrahedron Lett. 2018, 59, 2317–2326. [Google Scholar] [CrossRef]
- Zhilin, E.S.; Polkovnichenko, M.S.; Ananyev, I.V.; Fershtat, L.L.; Makhova, N.N. Novel Arylazo-1,2,5-oxadiazole Photoswitches: Synthesis, Photoisomerization and Nitric Oxide Releasing Properties. ChemPhotoChem 2020, 4, 5346–5354. [Google Scholar] [CrossRef]
- Chaplygin, D.A.; Gorbunov, Y.K.; Fershtat, L.L. Ring Distortion Diversity-Oriented Approach to Fully Substituted Furoxans and Isoxazoles. Asian J. Org. Chem. 2021, 10, 2644–2653. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Teslenko, F.E. Five-Membered Hetarene N-Oxides: Recent Advances in Synthesis and Reactivity. Synthesis 2021, 53, 3673–3682. [Google Scholar] [CrossRef]
- Stebletsova, I.A.; Larin, A.A.; Ananyev, I.V.; Fershtat, L.L. Regioselective Synthesis of NO-Donor (4-Nitro-1,2,3-triazolyl)furoxans via Eliminative Azide–Olefin Cycloaddition. Molecules 2023, 28, 6969. [Google Scholar] [CrossRef]
- Vaidya, A.; Jain, S.; Prashantha Kumar, B.R.; Singh, S.K.; Kashaw, S.K.; Agrawal, R.K. Synthesis of 1,2,4-oxadiazole derivatives: Anticancer and 3D QSAR studies. Monatsh. Chem. 2020, 151, 385–395. [Google Scholar] [CrossRef]
- Shamsi, F.; Hasan, P.; Queen, A.; Hussain, A.; Khan, P.; Zeya, B.; King, H.M.; Rana, S.; Garrison, J.; Alajmi, M.F.; et al. Synthesis and SAR studies of novel 1,2,4-oxadiazole-sulfonamide based compounds as potential anticancer agents for colorectal cancer therapy. Bioorg. Chem. 2020, 98, 103754. [Google Scholar] [CrossRef] [PubMed]
- Zhilin, E.S.; Bystrov, D.M.; Ananyev, I.V.; Fershtat, L.L.; Makhova, N.N. Straightforward Access to the Nitric Oxide Donor Azasydnone Scaffold by Cascade Reactions of Amines. Chem. Eur. J. 2019, 25, 14284–14289. [Google Scholar] [CrossRef] [PubMed]
- Zhilin, E.S.; Ustyuzhanina, N.E.; Fershtat, L.L.; Nifantiev, N.E.; Makhova, N.N. Antiaggregant effects of (1,2,5-oxadiazolyl)azasydnone ring assemblies as novel antiplatelet agents. Chem. Biol. Drug Des. 2022, 100, 1017–1024. [Google Scholar] [CrossRef]
- Shuvaev, A.D.; Zhilin, E.S.; Fershtat, L.L. NOBF4-Mediated Assembly of the Sydnone Imine Scaffold in the Synthesis of Double Nitric Oxide Donors. Synthesis 2023, 55, 1863–1874. [Google Scholar] [CrossRef]
- Titenkova, K.; Shuvaev, A.D.; Teslenko, F.E.; Zhilin, E.S.; Fershtat, L.L. Empowering Strategies of Electrochemical N-N Bond Forming Reactions: Direct Access to Previously Neglected 1,2,3-Triazole 1-Oxides. Green Chem. 2023, 25, 6686–6693. [Google Scholar] [CrossRef]
- Kulikov, A.S.; Epishina, M.A.; Zhilin, E.S.; Shuvaev, A.D.; Fershtat, L.L.; Makhova, N.N. Design and synthesis of pyrazolo[3,4-d]pyridazine 5,6-dioxides as novel NO-donors. Mendeleev Commun. 2021, 31, 42–45. [Google Scholar] [CrossRef]
- Cerecetto, H.; Porcal, W. Pharmacological properties of furoxans and benzofuroxans: Recent developments. Mini-Rev. Med. Chem. 2005, 5, 57–71. [Google Scholar] [CrossRef]
- Boiani, M.; Cerecetto, H.; Gonzalez, M.; Risso, M.; Olea-Azar, C.; Piro, O.E.; Castellano, E.E.; de Cerain, A.L.; Ezpeleta, O.; Monge-Vega, A. 1,2,5-Oxadiazole N-oxide derivatives as potential anti-cancer agents: Synthesis and biological evaluation. Part IV. Eur. J. Med. Chem. 2001, 36, 771–782. [Google Scholar] [CrossRef]
- Shi, Y.-M.; Wang, J.; Yan, H. The current scenario of furoxan hybrids with anticancer potential. J. Heterocycl. Chem. 2023, 60, 1651–1665. [Google Scholar] [CrossRef]
- Youssef, M.A.; Matsubara, R. Recent progress in synthesis and application of furoxan. RSC Adv. 2023, 13, 5228–5248. [Google Scholar] [CrossRef]
- Sinha, B.K.; Perera, L.; Cannon, R.E. NCX-4040, a Unique Nitric Oxide Donor, Induces Reversal of Drug-Resistance in Both ABCB1- and ABCG2-Expressing Multidrug Human Cancer Cells. Cancers 2021, 13, 1680. [Google Scholar] [CrossRef] [PubMed]
- Pieretti, J.C.; Pelegrino, M.T.; Nascimento, M.H.M.; Tortella, G.R.; Rubilar, O.; Seabra, A.B. Small molecules for great solutions: Can nitric oxide-releasing nanomaterials overcome drug resistance in chemotherapy? Biochem. Pharmacol. 2020, 176, 113740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Rong, Y.; Zheng, J.; Yang, C.; Chen, Y.; Wang, J.; Wei, G. Design, synthesis and biological evaluation of novel nitric oxide-donating podophyllotoxin derivatives as potential antiproliferative agents against multi-drug resistant leukemia cells. RSC Adv. 2018, 8, 34266–34274. [Google Scholar] [CrossRef]
- Kulikov, A.S.; Larin, A.A.; Fershtat, L.L.; Anikina, L.V.; Pukhov, S.A.; Klochkov, S.G.; Struchkova, M.I.; Romanova, A.A.; Ananyev, I.V.; Makhova, N.N. Synthesis, structural characterization and cytotoxic activity of heterocyclic compounds containing the furoxan ring. Arkivoc 2017, 2017, 250–268. [Google Scholar] [CrossRef]
- Pukhov, S.A.; Anikina, L.V.; Larin, A.A.; Fershtat, L.L.; Kulikov, A.S.; Makhova, N.N. Hetarylfuroxans: Cytotoxic eff ect and induction of apoptosis in chronic myeloid leukemia K562 cells. Russ. Chem. Bull. Int. Ed. 2019, 68, 158–162. [Google Scholar] [CrossRef]
- Stebletsova, I.A.; Larin, A.A.; Matnurov, E.M.; Ananyev, I.V.; Babak, M.V.; Fershtat, L.L. Exploring the anticancer potential of NO-donor oxadiazole assemblies against malignant pleural mesothelioma. Pharmaceutics 2025, 17, 230. [Google Scholar] [CrossRef]
- Larin, A.A.; Fershtat, L.L.; Ustyuzhanina, N.E.; Gening, M.L.; Nifantiev, N.E.; Makhova, N.N. New hybrid furoxan structures with antiaggregant activity. Mendeleev Commun. 2018, 28, 595–597. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Fershtat, L.L.; Gening, M.L.; Nifantiev, N.E.; Makhova, N.N. Antiaggregant activity of water-soluble furoxans. Mendeleev Commun. 2018, 28, 49–51. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Fershtat, L.L.; Gening, M.L.; Nifantiev, N.E.; Makhova, N.N. New insight into the antiaggregant activity of furoxans. Mendeleev Commun. 2016, 26, 513–515. [Google Scholar] [CrossRef]
- Bohn, H.; Brendel, J.; Martorana, P.A.; Schönafinger, K. Cardiovascular actions of the furoxan CAS 1609, a novel nitric oxide donor. Br. J. Pharmacol. 1995, 114, 1605. [Google Scholar] [CrossRef]
- Ispikoudi, M.; Amvrazis, M.; Kontogiorgis, C.; Koumbis, A.E.; Litinas, K.E.; Hadjipavlou-Litina, D.; Fylaktakidou, K.C. Convenient synthesis and biological profile of 5-amino-substituted 1,2,4-oxadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 5635–5645. [Google Scholar] [CrossRef]
- Youssif, B.G.M.; Mohamed, M.F.A.; Al-Sanea, M.M.; Moustafa, A.H.; Abdelhamid, A.A.; Gomaa, H.A.M. Novel aryl carboximidamides and 3-aryl-1,2,4-oxadiazoles analogues of naproxen as dual selective COX-2/15-LOX inhibitors: Design, synthesis and Docking studies. Bioorg. Chem. 2019, 85, 577–584. [Google Scholar] [CrossRef]
- Kotsiou, O.S.; Gourgoulianis, K.I.; Zarogiannis, S.G. The role of nitric oxide in pleural disease. Respir. Med. 2021, 179, 106350. [Google Scholar] [CrossRef] [PubMed]
- Soini, Y.; Kahlos, K.; Puhakka, A.; Lakari, E.; Säily, M.; Pääkkö, P.; Kinnula, V. Expression of inducible nitric oxide synthase in healthy pleura and in malignant mesothelioma. Br. J. Cancer 2000, 83, 880–886. [Google Scholar] [CrossRef]
- Sorensen, J.B.; Frank, H.; Palshof, T. Cisplatin and vinorelbine first-line chemotherapy in non-resectable malignant pleural mesothelioma. Br. J. Cancer 2008, 99, 44–50. [Google Scholar] [CrossRef][Green Version]
- Brown, G.C. Nitric oxide and mitochondrial respiration. Biochim. Biophys. Acta (BBA) Bioenerg. 1999, 1411, 351–369. [Google Scholar] [CrossRef]
- Peterson, L.A. Reactive Metabolites in the Biotransformation of Molecules Containing a Furan Ring. Chem. Res. Toxicol. 2013, 26, 6–25. [Google Scholar] [CrossRef] [PubMed]
- Aydin, Y.; Yilmaz, B.; Dikbasan, Y.U.; Orta-Yilmaz, B. Assessment of the oxidative damage and apoptotic pathway related to furan cytotoxicity in cultured mouse Leydig cells. Toxicol. Res. 2023, 12, 400–407. [Google Scholar] [CrossRef]
- Bruker. APEX-III; Bruker AXS Inc.: Madison, WI, USA, 2018. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenium microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Cryst. Struct. Commun. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Perdew, J.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]








| Compound | NO Released (%) a | Fold Change | ||||
|---|---|---|---|---|---|---|
| 1 h | 24 h | 48 h | 24 h/1 h | 48 h/1 h | 48 h/24 h | |
| 1b b | 70 ± 5 | 81 ± 4 | 84 ± 10 | 1.2 | 1.2 | 1.0 |
| 1c | 75 ± 9 | 81 ± 5 | 82 ± 7 | 1.1 | 1.1 | 1.0 |
| 2a | 73 ± 6 | 131 ± 1 | 144 ± 1 | 1.8 | 2.0 | 1.1 |
| 2b | 38 ± 3 | 46 ± 1 | 48 ± 2 | 1.2 | 1.3 | 1.0 |
| 2c | 37 ± 1 | 49 ± 2 | 63 ± 2 | 1.3 | 1.7 | 1.3 |
| 2d | 54 ± 14 | 69 ± 14 | 72 ± 13 | 1.3 | 1.3 | 1.0 |
| 2e | 51 ± 10 | 61 ± 12 | 64 ± 2 | 1.2 | 1.3 | 1.1 |
| 2f | 71 ± 15 | 94 ± 15 | 103 ± 14 | 1.3 | 1.5 | 1.1 |
| 2g | 75 ± 7 | 112± 1 | 125 ± 1 | 1.5 | 1.7 | 1.1 |
| 2h | 37 ± 5 | 52 ± 1 | 56 ± 1 | 1.4 | 1.5 | 1.1 |
| 2i | 75 ± 1 | 78 ± 5 | 89 ± 3 | 1.0 | 1.2 | 1.1 |
| 2j | 35 ± 2 | 37 ± 3 | 39 ± 5 | 1.1 | 1.1 | 1.1 |
| 2k | 37 ± 5 | 41 ± 8 | 43 ± 7 | 1.1 | 1.2 | 1.0 |
| 2l | 52 ± 3 | 70 ± 5 | 73 ± 8 | 1.3 | 1.4 | 1.0 |
| CAS-1609 b | 27 ± 3 | 68 ± 6 | 73 ± 6 | 2.5 | 2.7 | 1.1 |
| CHF-2363 b | 26 ± 10 | 71 ± 4 | 77 ± 9 | 2.7 | 3.0 | 1.1 |
| Compound | IC50, μM a | SFJU77 b | ||
|---|---|---|---|---|
| AB1 | JU77 | MRC-5 | ||
| 2a | 11.7 ± 2.8 | 8.6 ± 1.3 | 16.4 ± 3.4 | 1.9 |
| 2b | >25.0 d | >25.0 | >25 | - |
| 2c | 19.9 ± 4.3 | 7.3 ± 1.0 | 27.9 ± 4.6 | 3.8 |
| 2d | 11.2 ± 2.0 | 3.0 ± 0.4 | 16.8 ± 5.7 | 5.7 |
| 2e | 33.9 ± 3.4 | 10.5 ± 0.5 | 17.7 ± 3.8 | 1.6 |
| 2f | 8.5 ± 2.1 | 14.1 ± 4.1 | 29.9 ± 1.6 | 2.1 |
| 2g | 15.6 ± 3.5 | 19.5 ± 3.2 | 52.6 ± 6.7 | 2.8 |
| 2h | >25.0 | 16.0 ± 3.9 | >25.0 | >1.6 |
| 2i | >25.0 | >25.0 | >25.0 | - |
| 2j | 21.7 ± 4.5 | 19.7 ± 2.1 | 25.7 ± 8.7 | 1.3 |
| 2k | 15.5 ± 3.0 | 4.9 ± 1.2 | 7.8 ± 2.3 | 1.6 |
| 2l | 14.2 ± 2.1 | 5.0 ± 0.6 | 45.4 ± 14.2 | 9.0 |
| Cisplatin c | 5.8 ± 1.0 | 4.2 ± 0.5 | 3.1 ± 1.2 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matnurov, E.M.; Stebletsova, I.A.; Larin, A.A.; Arakelyan, J.; Ananyev, I.V.; Gushchin, A.L.; Fershtat, L.L.; Babak, M.V. Bis-Oxadiazole Assemblies as NO-Releasing Anticancer Agents. Pharmaceutics 2025, 17, 1494. https://doi.org/10.3390/pharmaceutics17111494
Matnurov EM, Stebletsova IA, Larin AA, Arakelyan J, Ananyev IV, Gushchin AL, Fershtat LL, Babak MV. Bis-Oxadiazole Assemblies as NO-Releasing Anticancer Agents. Pharmaceutics. 2025; 17(11):1494. https://doi.org/10.3390/pharmaceutics17111494
Chicago/Turabian StyleMatnurov, Egor M., Irina A. Stebletsova, Alexander A. Larin, Jemma Arakelyan, Ivan V. Ananyev, Artem L. Gushchin, Leonid L. Fershtat, and Maria V. Babak. 2025. "Bis-Oxadiazole Assemblies as NO-Releasing Anticancer Agents" Pharmaceutics 17, no. 11: 1494. https://doi.org/10.3390/pharmaceutics17111494
APA StyleMatnurov, E. M., Stebletsova, I. A., Larin, A. A., Arakelyan, J., Ananyev, I. V., Gushchin, A. L., Fershtat, L. L., & Babak, M. V. (2025). Bis-Oxadiazole Assemblies as NO-Releasing Anticancer Agents. Pharmaceutics, 17(11), 1494. https://doi.org/10.3390/pharmaceutics17111494









