Cyclodextrin: Dual Functions as a Therapeutic Agent and Nanocarrier for Regulating Cholesterol Homeostasis in Atherosclerosis
Abstract
1. Introduction
2. Atherosclerosis and Cholesterol Metabolism
2.1. Overview of Cholesterol Metabolism
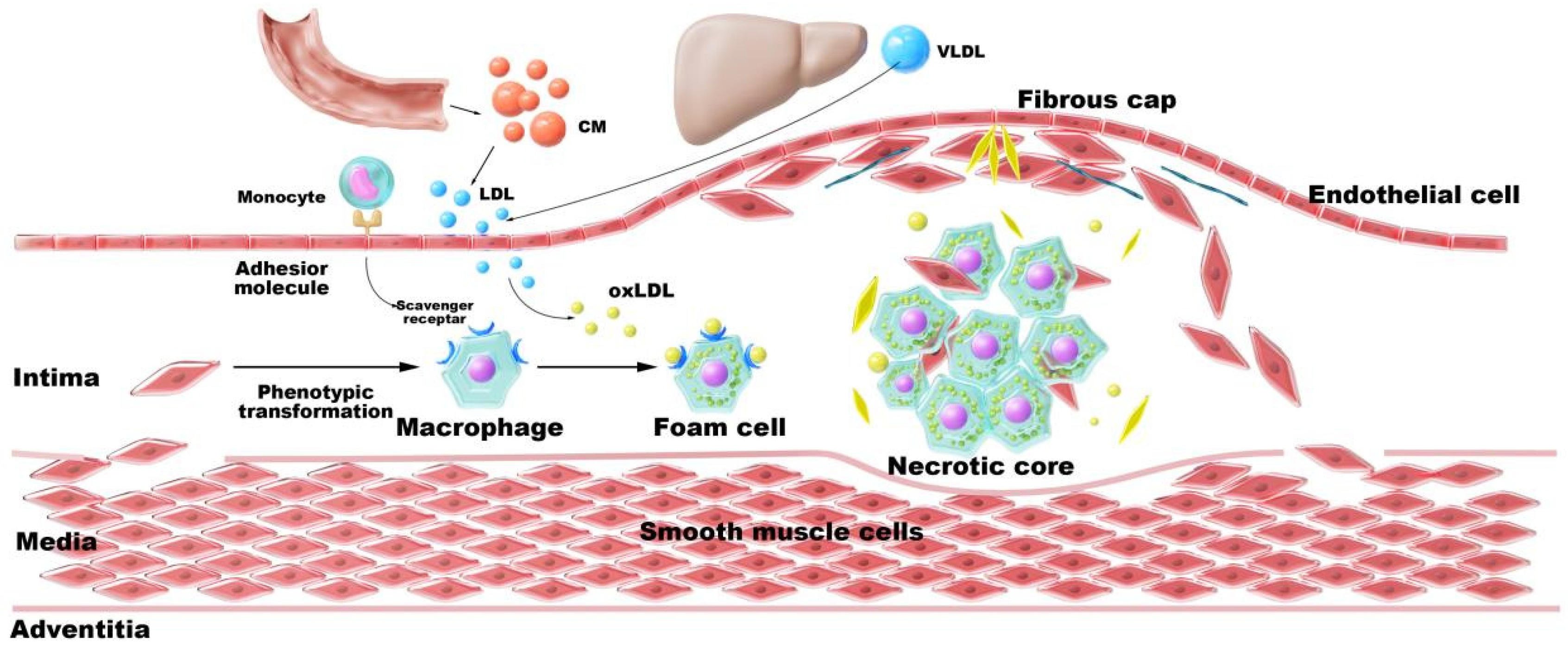
2.2. Cholesterol Metabolic Disorders Drive Atherosclerosis
2.2.1. Dysregulation of Cholesterol Homeostasis
2.2.2. Cholesterol Crystals
2.2.3. Impaired Cholesterol Efflux
3. CD and Cholesterol
3.1. Host–Guest Recognition
3.2. CD in Atherosclerosis
3.3. CD Derivatives
4. Construction Strategies for CD-NDDS
4.1. Further Research on Host–Guest Recognition
4.2. Covalent Bonding
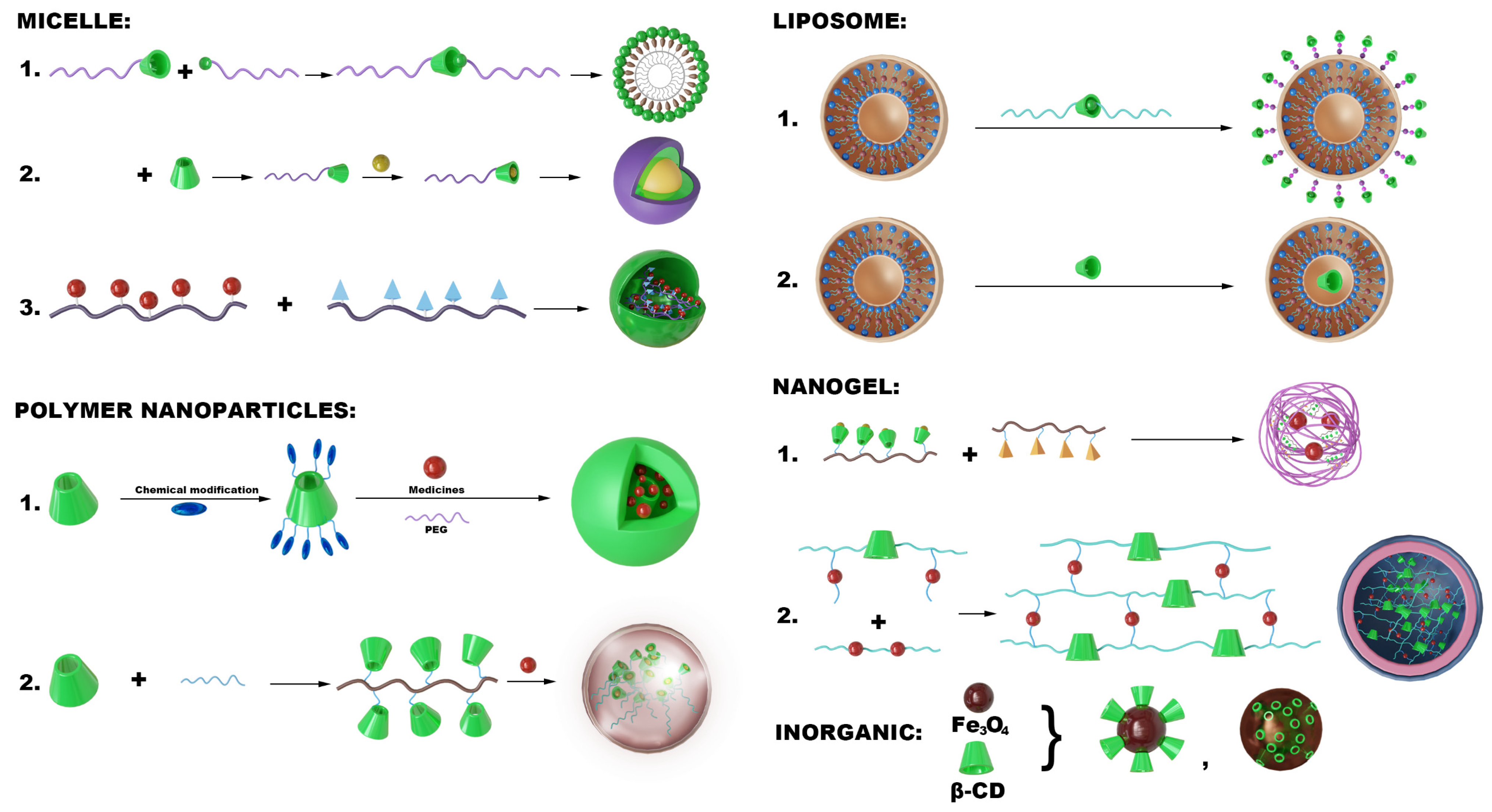
5. Structure of CD-NDDS in AS
5.1. Micelles
5.2. Polymeric Nanoparticles
5.3. Lipid Nanoparticles
5.4. Nanogels
5.5. Inorganic/Metal Hybrid CD Systems
6. Design of CD-NDDS Targeting for AS
6.1. RGD Modification
6.2. HA Modification
6.3. Cell Membrane Modification
7. Applications of CD-NDDS in the Therapy of AS
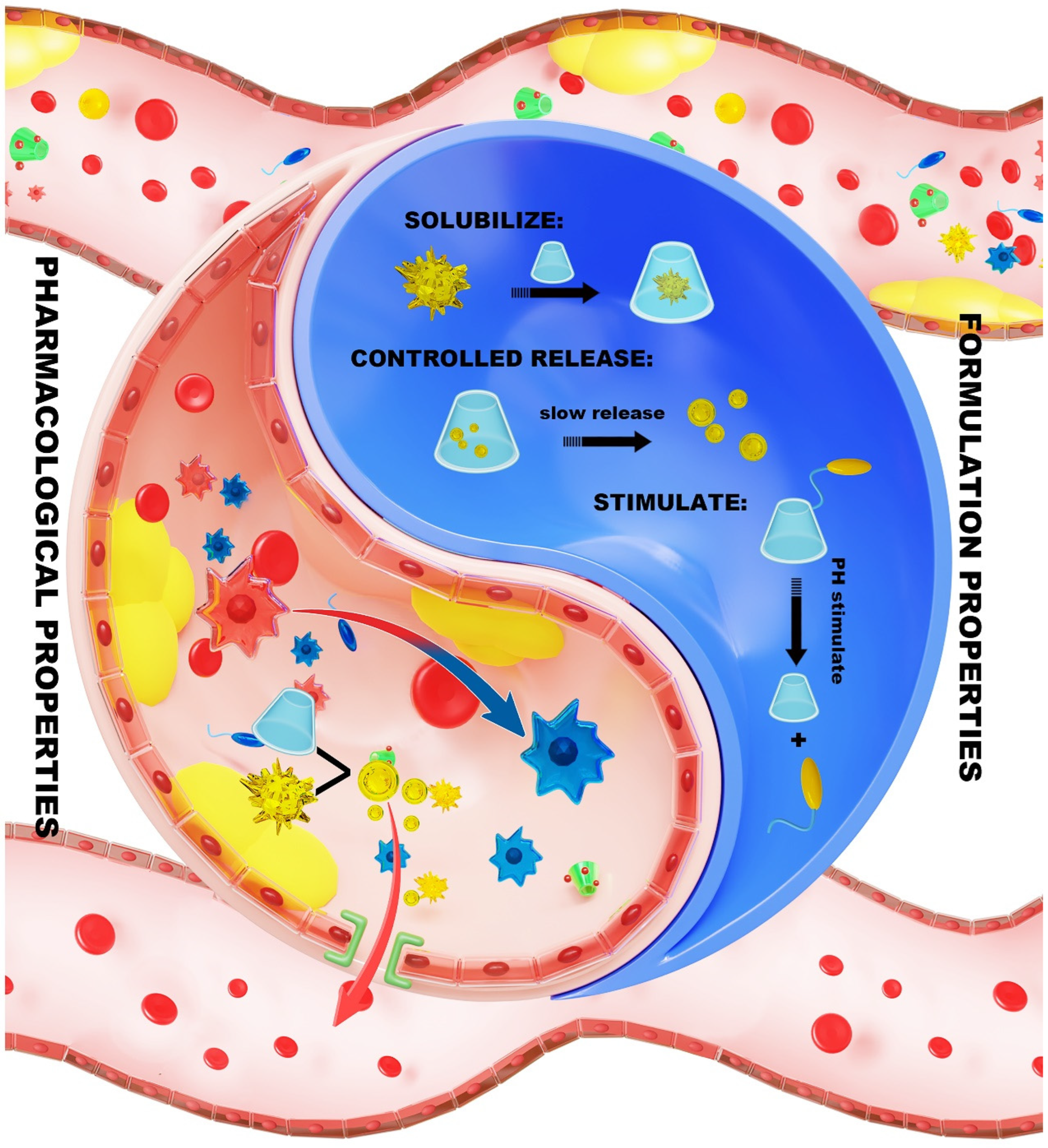
7.1. Formulation Properties of CD Within the NDDS
7.1.1. Solubilization
7.1.2. Controlled Release
7.1.3. Stimulus Response
7.2. Pharmacological Properties of CD in the NDDS
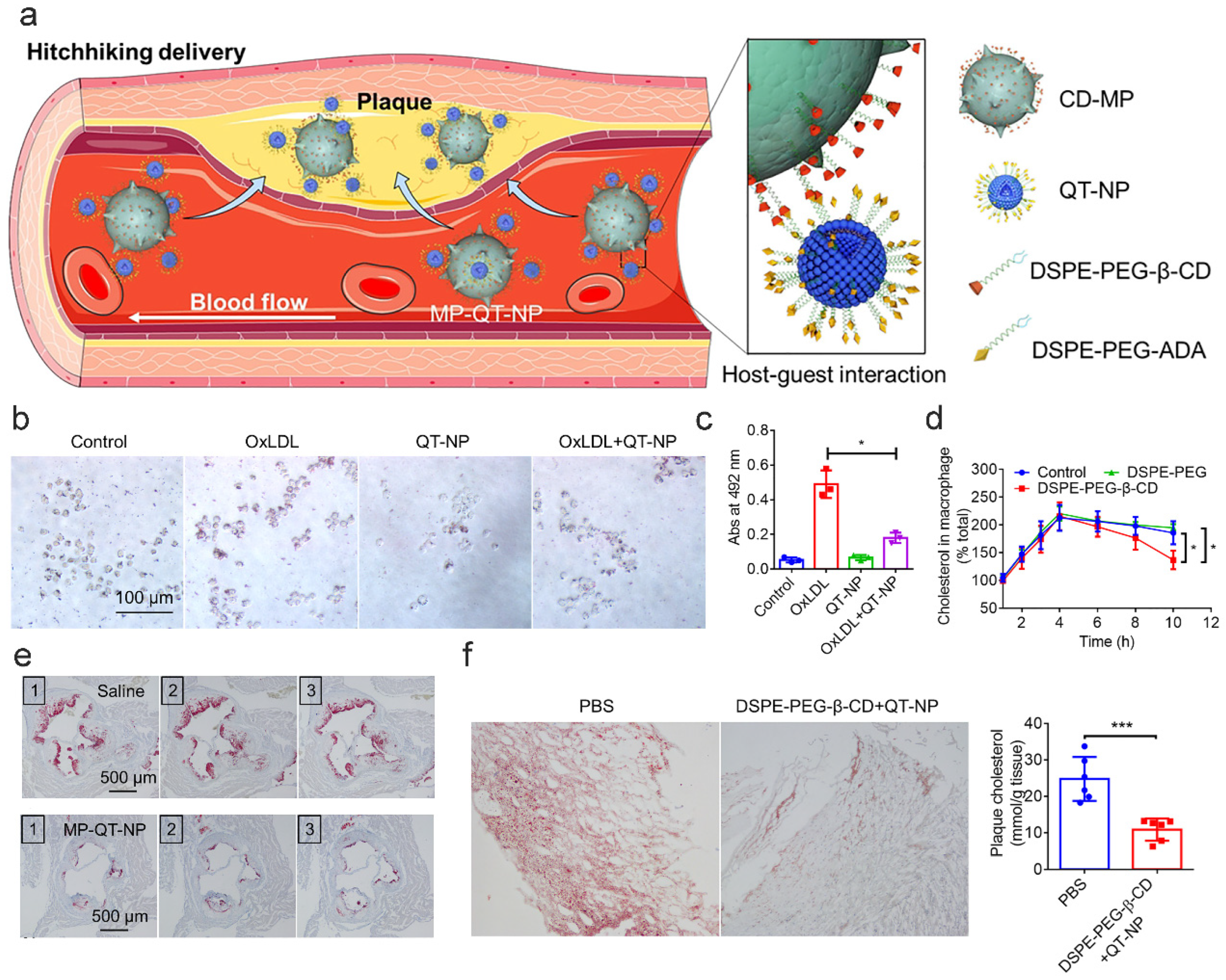
7.2.1. Dissolving CCs
7.2.2. Promoting Cholesterol Efflux
7.2.3. Alleviating Inflammatory Microenvironments
8. Challenges and Perspectives
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CD | Cyclodextrin |
| AS | Atherosclerosis |
| Chol | Cholesterol |
| LDL | Low-density lipoprotein |
| CCs | Chol crystals |
| CD-nano | Cyclodextrin nanodelivery systems |
| NDDS | Novel drug delivery systems |
| LDL-C | Low-density lipoprotein cholesterol |
| ABCA1 | ATP-binding cassette transporter A1 |
| ABCG1 | ATP-binding cassette transporter G1 |
| β-CD | β-Cyclodextrin |
| HP-β-CD | Hydroxypropyl β-Cyclodextrin |
| Me-β-CD | Methylated β-Cyclodextrin |
| MTX | Methotrexate |
| MTX NPs | Methotrexate nanoparticles |
| NP3 ST | β-CD-anchored discoidal recombinant high-density lipoprotein |
| HA | Hyaluronic acid |
| ROS | Reactive oxygen species |
| rHDL | reconstituted high-density lipoprotein |
| LXR | Liver X receptors |
| CYP27A1 | Cytochrome P450 27A1 |
References
- Rybarczyk, A.; Formanowicz, D.; Radom, M.; Formanowicz, P. Cholesterol Metabolism Pathways Disturbances in Atherosclerosis—Analyses Using Stochastic Petri Net-Based Model. Appl. Sci. 2023, 13, 6149. [Google Scholar] [CrossRef]
- Janoudi, A.; Shamoun, F.E.; Kalavakunta, J.K.; Abela, G.S. Cholesterol crystal induced arterial inflammation and destabilization of atherosclerotic plaque. Eur. Heart J. 2015, 37, 1959–1967. [Google Scholar] [CrossRef]
- Attiq, A.; Afzal, S.; Ahmad, W.; Kandeel, M. Hegemony of inflammation in atherosclerosis and coronary artery disease. Eur. J. Pharmacol. 2024, 966, 176338. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Guo, D.; Sun, L.; Chi, M.; Ma, X.; Jin, J. Crosstalk between lipid metabolism and macrophages in atherosclerosis: Therapeutic potential of natural products. Front. Cardiovasc. Med. 2025, 12, 1529924. [Google Scholar] [CrossRef]
- Almeida, S.O.; Budoff, M. Effect of statins on atherosclerotic plaque. Trends Cardiovasc. Med. 2019, 29, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Gao, N.; Zhu, J.; Wang, H.; Huang, X.; Zhang, Y.; Chen, F.; Ke, Q.; Meng, Q. Cyclodextrins-amphiphile molecules supramolecular self-assemblies: Mechanisms, characterization, and applications in advanced functional materials. Adv. Colloid Interface Sci. 2025, 346, 103676. [Google Scholar] [CrossRef] [PubMed]
- Makieła, D.; Janus-Zygmunt, I.; Górny, K.; Gburski, Z. Investigation of the influence of β-cyclodextrin on cholesterol lodgement—A molecular dynamics simulation study. J. Mol. Liq. 2018, 262, 451–459. [Google Scholar] [CrossRef]
- Krabicová, I.; Khazaei Monfared, Y.; Caldera, F.; Mahmoudian, M.; Hobbs, C.; Santalucia, R.; Appleton, S.L.; Matencio, A.; Zakeri-Milani, P.; Trotta, F. Leveraging Cholesterol-Functionalized Cyclodextrin Nanosponges for Enhanced Drug Delivery in Cancer Cells. Int. J. Mol. Sci. 2025, 26, 1213. [Google Scholar] [CrossRef]
- Deinavizadeh, M.; Kiasat, A.R.; Hooshmand, N.; Labouta, H.I.; Shafiei, M.; Sabaeian, M.; Mirzajani, R.; Zahraei, S.M.; Makvandi, P.; El-Sayed, M.A. Near-Infrared/pH Dual-Responsive Nanosponges Encapsulating Gold Nanorods for Synergistic Chemo-phototherapy of Lung Cancer. ACS Appl. Nano Mater. 2023, 6, 16332–16342. [Google Scholar] [CrossRef]
- Chen, H.; Xing, C.; Lei, H.; Yan, B.; Zhang, H.; Tong, T.; Guan, Y.; Kang, Y.; Pang, J. ROS-driven supramolecular nanoparticles exhibiting efficient drug delivery for chemo/Chemodynamic combination therapy for Cancer treatment. J. Control. Release 2024, 368, 637–649. [Google Scholar] [CrossRef]
- Zimmer, S.; Grebe, A.; Bakke, S.S.; Bode, N.; Halvorsen, B.; Ulas, T.; Skjelland, M.; De Nardo, D.; Labzin, L.I.; Kerksiek, A.; et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci. Transl. Med. 2016, 8, 333ra50. [Google Scholar] [CrossRef]
- Kim, H.; Han, J.; Park, J.-H. Cyclodextrin polymer improves atherosclerosis therapy and reduces ototoxicity. J. Control. Release 2020, 319, 77–86. [Google Scholar] [CrossRef]
- Mehta, S.; Bongcaron, V.; Nguyen, T.K.; Jirwanka, Y.; Maluenda, A.; Walsh, A.P.G.; Palasubramaniam, J.; Hulett, M.D.; Srivastava, R.; Bobik, A.; et al. An Ultrasound-Responsive Theranostic Cyclodextrin-Loaded Nanoparticle for Multimodal Imaging and Therapy for Atherosclerosis. Small 2022, 18, 2200967. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, Y.; Shao, W.; Kankala, R.K.; Chen, A. β-Cyclodextrin-based nanoassemblies for the treatment of atherosclerosis. Regen. Biomater. 2024, 11, rbae071. [Google Scholar] [CrossRef]
- Mahjoubin-Tehran, M.; Kovanen, P.T.; Xu, S.; Jamialahmadi, T.; Sahebkar, A. Cyclodextrins: Potential therapeutics against atherosclerosis. Pharmacol. Ther. 2020, 214, 107620. [Google Scholar] [CrossRef] [PubMed]
- Gylling, H. Cholesterol metabolism and its implications for therapeutic interventions in patients with hypercholesterolaemia. Int. J. Clin. Pract. 2004, 58, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Martini, C.; Pallottini, V. Cholesterol: From feeding to gene regulation. Genes Nutr. 2007, 2, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Espenshade, P.J.; Hughes, A.L. Regulation of sterol synthesis in eukaryotes. Annu. Rev. Genet. 2007, 41, 401–427. [Google Scholar] [CrossRef]
- The Photochemical Formation of Vitamin D in the Skin. Nutr. Rev. 1984, 42, 341–343. [CrossRef]
- Ness, G.C. Physiological feedback regulation of cholesterol biosynthesis: Role of translational control of hepatic HMG-CoA reductase and possible involvement of oxylanosterols. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 667–673. [Google Scholar] [CrossRef]
- Xue, S.; Su, Z.; Liu, D. Immunometabolism and immune response regulate macrophage function in atherosclerosis. Ageing Res. Rev. 2023, 90, 101993. [Google Scholar] [CrossRef]
- Marx, J.L. Atherosclerosis: The Cholesterol Connection. Science 1976, 194, 711–755. [Google Scholar] [CrossRef]
- Aguilar-Ballester, M.; Herrero-Cervera, A.; Vinue, A.; Martinez-Hervas, S.; Gonzalez-Navarro, H. Impact of Cholesterol Metabolism in Immune Cell Function and Atherosclerosis. Nutrients 2020, 12, 2021. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, Y.; Ren, C.; Liang, X.; Li, N. Palladium Hydride Nanopocket Cubes and Their H2-Therapy Function in Amplifying Inhibition of Foam Cells to Attenuate Atherosclerosis. Adv. Funct. Mater. 2021, 31, 2104892. [Google Scholar] [CrossRef]
- Shu, F.; Chen, J.; Ma, X.; Fan, Y.; Yu, L.; Zheng, W.; Amrein, M.W.; Xia, T.; Shi, Y. Cholesterol Crystal-Mediated Inflammation Is Driven by Plasma Membrane Destabilization. Front. Immunol. 2018, 9, 1163. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; He, N.; Wang, Z.; Fu, X.; Aung, L.H.H.; Liu, Y.; Li, M.; Cho, J.Y.; Yang, Y.; Yu, T. Functional roles and mechanisms of ginsenosides from Panax ginseng in atherosclerosis. J. Ginseng Res. 2021, 45, 22–31. [Google Scholar] [CrossRef]
- Lin, P.; Ji, H.H.; Li, Y.J.; Guo, S.D. Macrophage Plasticity and Atherosclerosis Therapy. Front. Mol. Biosci. 2021, 8, 679797. [Google Scholar] [CrossRef] [PubMed]
- Falk, E. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006, 47, C7–C12. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Pedro-Botet, J.; Climent, E.; Benaiges, D. Atherosclerosis and inflammation. New therapeutic approaches. Med. Clín. (Engl. Ed.) 2020, 155, 256–262. [Google Scholar] [CrossRef]
- Abela, G.S.; Aziz, K. Cholesterol crystals cause mechanical damage to biological membranes: A proposed mechanism of plaque rupture and erosion leading to arterial thrombosis. Clin. Cardiol. 2005, 28, 413–420. [Google Scholar] [CrossRef]
- Boren, J.; Packard, C.J.; Binder, C.J. Apolipoprotein B-containing lipoproteins in atherogenesis. Nat. Rev. Cardiol. 2025, 22, 399–413. [Google Scholar] [CrossRef]
- Sekimoto, T.; Koba, S.; Mori, H.; Arai, T.; Matsukawa, N.; Sakai, R.; Yokota, Y.; Sato, S.; Tanaka, H.; Masaki, R.; et al. Impact of small dense low-density lipoprotein cholesterol on cholesterol crystals in patients with acute coronary syndrome: An optical coherence tomography study. J. Clin. Lipidol. 2022, 16, 438–446. [Google Scholar] [CrossRef]
- Ye, D.; Lammers, B.; Zhao, Y.; Meurs, I.; Van Berkel, T.J.; Van Eck, M. ATP-binding cassette transporters A1 and G1, HDL metabolism, cholesterol efflux, and inflammation: Important targets for the treatment of atherosclerosis. Curr. Drug Targets 2011, 12, 647–660. [Google Scholar] [CrossRef]
- Yvan-Charvet, L.; Wang, N.; Tall, A.R. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li-Hawkins, J.; Hammer, R.E.; Berge, K.E.; Horton, J.D.; Cohen, J.C.; Hobbs, H.H. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Investig. 2002, 110, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Hammer, R.E.; Li-Hawkins, J.; von Bergmann, K.; Lutjohann, D.; Cohen, J.C.; Hobbs, H.H. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl. Acad. Sci. USA 2002, 99, 16237–16242. [Google Scholar] [CrossRef]
- Bajaj, A.; Xie, D.; Cedillo-Couvert, E.; Charleston, J.; Chen, J.; Deo, R.; Feldman, H.I.; Go, A.S.; He, J.; Horwitz, E.; et al. Abstract P120: Relationship of Lipoproteins With Cardiovascular Disease in Persons With Chronic Kidney Disease. Circulation 2017, 135 (Suppl. S1), AP120. [Google Scholar] [CrossRef]
- Kral, M.; Döring, Y.; Weber, C. Early intermittent cholesterol exposure accelerates atherosclerosis: An oscillating amplifier calling for preemptive control. Signal Transduct. Target. Ther. 2024, 9, 313. [Google Scholar] [CrossRef]
- Zhao, M.J.; Xiao, M.L.; Tan, Q.; Ji, J.J.; Lu, F. Cumulative residual cholesterol predicts the risk of cardiovascular disease in the general population aged 45 years and older. Lipids Health Dis. 2024, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.Y.; Zhu, L.; Lei, L.; Li, H.F.; Cong, R.; Hou, J.F.; Zhao, J.H.; Li, P.W.; Tang, Y.W.; Su, Z.G.; et al. Lesional Macrophage-Targeted Nanomedicine Regulating Cholesterol Homeostasis for the Treatment of Atherosclerosis. Adv. Mater. 2025, 37, 2502581. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Tumminello, G.; Fichtner, I.; Corsini, A.; Santos, R.D.; Carugo, S.; Ruscica, M. PCSK9 and Coronary Artery Plaque-New Opportunity or Red Herring? Curr. Atheroscler. Rep. 2024, 26, 589–602. [Google Scholar] [CrossRef] [PubMed]
- López, C.A.; de Vries, A.H.; Marrink, S.J. Molecular mechanism of cyclodextrin mediated cholesterol extraction. PLoS Comput. Biol. 2011, 7, e1002020. [Google Scholar] [CrossRef]
- Dai, Y.; Zhong, J.; Li, J.; Liu, X.; Wang, Y.; Qin, X. Interaction mechanism of cholesterol/β-cyclodextrin complexation by combined experimental and computational approaches. Food Hydrocoll. 2022, 130, 107725. [Google Scholar] [CrossRef]
- Gong, L.; Wu, F.; Yang, W.; Huang, C.; Li, W.; Wang, X.; Wang, J.; Tang, T.; Zeng, H. Unraveling the hydrophobic interaction mechanisms of hydrocarbon and fluorinated surfaces. J. Colloid Interface Sci. 2023, 635, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Presti, F.T.; Pace, R.J.; Chan, S.I. Cholesterol-phospholipid interaction in membranes. 2. Stoichiometry and molecular packing of cholesterol-rich domains. Biochemistry 1982, 21, 3831–3835. [Google Scholar] [CrossRef]
- Huang, C.H. A structural model for the cholesterol-phosphatidylcholine complexes in bilayer membranes. Lipids 1977, 12, 348–356. [Google Scholar] [CrossRef]
- Sopic, M.; Vilne, B.; Gerdts, E.; Trindade, F.; Uchida, S.; Khatib, S.; Wettinger, S.B.; Devaux, Y.; Magni, P. Multiomics tools for improved atherosclerotic cardiovascular disease management. Trends Mol. Med. 2023, 29, 983–995. [Google Scholar] [CrossRef]
- Tracz, J.; Luczak, M. Applying Proteomics and Integrative “Omics” Strategies to Decipher the Chronic Kidney Disease-Related Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 7492. [Google Scholar] [CrossRef]
- Zhang, L.; Zou, W.; Hu, Y.; Wu, H.; Gao, Y.; Zhang, J.; Zheng, J. Maternal high-calorie diet feeding programs hepatic cholesterol metabolism and Abca1 promoter methylation in the early life of offspring. J. Nutr. Biochem. 2023, 122, 109449. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, S.; Guo, Z.; Xing, D.; Chen, W. The crosstalk of ABCA1 and ANXA1: A potential mechanism for protection against atherosclerosis. Mol. Med. 2020, 26, 84. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Calvo, R.; Kumari, A.; Sable, R.V.; Fang, Y.; Tao, D.; Hu, X.; Castle, S.G.; Nahar, S.; Li, D.; et al. Fatty Acid Derivatization and Cyclization of the Immunomodulatory Peptide RP-182 Targeting CD206high Macrophages Improve Antitumor Activity. Mol. Cancer Ther. 2024, 23, 1827–1841. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Orekhov, N.A.; Glanz, V.Y.; Sukhorukov, V.N.; Pleshko, E.M.; Orekhov, A.N. Role of ABCA1 in Atherosclerosis: Novel Mutations and Potential Plant-derived Therapies. Curr. Med. Chem. 2025, 32, 2069–2092. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, A.; Zhu, L.; Yang, X.; Fang, G.; Tang, B. Cyclodextrin-based ocular drug delivery systems: A comprehensive review. Coord. Chem. Rev. 2023, 476, 214919. [Google Scholar] [CrossRef]
- Khatoon, H.; Faudzi, S.M.M.; Sohajda, T. Mechanisms and Therapeutic Applications of β-Cyclodextrin in Drug Solubilisation and Delivery Systems. Chem. Biodivers. 2025, e00359. [Google Scholar] [CrossRef]
- Zhang, W.-X.; Zhou, J.-F.; Xu, H.-X.; Wang, W.-J.; Chen, J.-G.; Zhang, Q.-F. Enhanced solubility, stability, and bioavailability of astilbin via inclusion complexes formation with methyl-β-cyclodextrin. Colloids Surf. Physicochem. Eng. Asp. 2025, 726, 138088. [Google Scholar] [CrossRef]
- Liang, B.; Hao, J.; Zhu, N.; Han, L.; Song, L.; Hong, H. Formulation of nitrendipine/hydroxypropyl-β-cyclodextrin inclusion complex as a drug delivery system to enhance the solubility and bioavailability by supercritical fluid technology. Eur. Polym. J. 2023, 187, 111880. [Google Scholar] [CrossRef]
- Ghzaiel, I.; Sassi, K.; Zarrouk, A.; Ghosh, S.; Dias, I.H.K.; Nury, T.; Ksila, M.; Essadek, S.; Tahri Joutey, M.; Brahmi, F.; et al. Sources of 7-ketocholesterol, metabolism and inactivation strategies: Food and biomedical applications. Redox Exp. Med. 2022, 2022, R40–R56. [Google Scholar] [CrossRef]
- Szente, L.; Singhal, A.; Domokos, A.; Song, B. Cyclodextrins: Assessing the Impact of Cavity Size, Occupancy, and Substitutions on Cytotoxicity and Cholesterol Homeostasis. Molecules 2018, 23, 1228. [Google Scholar] [CrossRef] [PubMed]
- Jelínková, K.; Kovačević, J.; Wrzecionková, E.; Prucková, Z.; Rouchal, M.; Dastychová, L.; Vícha, R. Binding study on 1-adamantylalkyl(benz)imidazolium salts to cyclodextrins and cucurbit[n]urils. New J. Chem. 2020, 44, 7071–7079. [Google Scholar] [CrossRef]
- Younis, M.; Ahmad, S.; Atiq, A.; Amjad Farooq, M.; Huang, M.H.; Abbas, M. Recent Progress in Azobenzene-Based Supramolecular Materials and Applications. Chem. Rec. 2023, 23, e202300126. [Google Scholar] [CrossRef]
- Deng, J.; Liu, X.; Shi, W.; Cheng, C.; He, C.; Zhao, C. Light-Triggered Switching of Reversible and Alterable Biofunctionality via β-Cyclodextrin/Azobenzene-Based Host–Guest Interaction. ACS Macro Lett. 2014, 3, 1130–1133. [Google Scholar] [CrossRef]
- Ben Doudou, B.; Chen, J.; Vivet, A.; Poilâne, C. Ab initio study of chemical bond interactions between covalently functionalized carbon nanotubes via amide, ester and anhydride linkages. Solid State Sci. 2016, 53, 56–62. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Wang, X.; Xu, J.; Luo, Q.; Liu, J. Self-assembled nanostructures from C60-containing supramolecular complex: Its stimuli-responsive reversible transition and biological antioxidative capacity. New J. Chem. 2011, 35, 2632. [Google Scholar] [CrossRef]
- Yang, J.; Ye, H.J.; Xiang, H.M.; Zhou, X.; Wang, P.Y.; Liu, S.S.; Yang, B.X.; Yang, H.B.; Liu, L.W.; Yang, S. Photo-Stimuli Smart Supramolecular Self-Assembly of Azobenzene/β-Cyclodextrin Inclusion Complex for Controlling Plant Bacterial Diseases. Adv. Funct. Mater. 2023, 33, 2303206. [Google Scholar] [CrossRef]
- Sabapathy, R.C.; Bhattacharyya, S.; Cleland, W.E.; Hussey, C.L. Host–Guest Complexation in Self-Assembled Monolayers: Inclusion of a Monolayer-Anchored Cationic Ferrocene-Based Guest by Cyclodextrin Hosts. Langmuir 1998, 14, 3797–3807. [Google Scholar] [CrossRef]
- Deng, Z.; Guo, Y.; Zhao, X.; Ma, P.X.; Guo, B. Multifunctional Stimuli-Responsive Hydrogels with Self-Healing, High Conductivity, and Rapid Recovery through Host–Guest Interactions. Chem. Mater. 2018, 30, 1729–1742. [Google Scholar] [CrossRef]
- Almawash, S.; Mohammed, A.M.; El Hamd, M.A.; Osman, S.K. Injectable Hydrogels Based on Cyclodextrin/Cholesterol Inclusion Complexation and Loaded with 5-Fluorouracil/Methotrexate for Breast Cancer Treatment. Gels 2023, 9, 326. [Google Scholar] [CrossRef]
- Rodell, C.B.; Mealy, J.E.; Burdick, J.A. Supramolecular Guest–Host Interactions for the Preparation of Biomedical Materials. Bioconjugate Chem. 2015, 26, 2279–2289. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Non-covalent interactions in the synthesis of coordination compounds: Recent advances. Coord. Chem. Rev. 2017, 345, 54–72. [Google Scholar] [CrossRef]
- Brewster, R.E.; Teresa, B.F.; Schuh, M.D. Inclusion complexes of 6-bromo-2-naphthol (guest) and α-cyclodextrin (host): Thermodynamics of the binary complex and first-reported dynamics of a triplet-state guest/host2 complex. J. Phys. Chem. A 2003, 107, 10521–10526. [Google Scholar] [CrossRef]
- Sadaquat, H.; Akhtar, M.; Nazir, M.; Ahmad, R.; Alvi, Z.; Akhtar, N. Biodegradable and biocompatible polymeric nanoparticles for enhanced solubility and safe oral delivery of docetaxel: In vivo toxicity evaluation. Int. J. Pharm. 2021, 598, 120363. [Google Scholar] [CrossRef]
- Yoon, D.Y.; Kim, J.-C. In vivo lifetime and anti-cancer efficacy of doxorubicin-loaded nanogels composed of cinnamoyl poly (β-cyclodextrin) and cinnamoyl Pluronic F127. J. Biomater. Sci. Polym. Ed. 2017, 28, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, P.X. Host–guest interactions mediated nano-assemblies using cyclodextrin-containing hydrophilic polymers and their biomedical applications. Nano Today 2010, 5, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yang, Z.; Fu, X.; Yung, B.C.; Yang, J.; Mao, Z.; Shao, L.; Hua, B.; Liu, Y.; Zhang, F.; et al. Polyrotaxane-based supramolecular theranostics. Nat. Commun. 2018, 9, 766. [Google Scholar] [CrossRef]
- Hernandez-Montelongo, J.; Naveas, N.; Degoutin, S.; Tabary, N.; Chai, F.; Spampinato, V.; Ceccone, G.; Rossi, F.; Torres-Costa, V.; Manso-Silvan, M.; et al. Porous silicon-cyclodextrin based polymer composites for drug delivery applications. Carbohydr. Polym. 2014, 110, 238–252. [Google Scholar] [CrossRef]
- Kost, B.; Brzeziński, M.; Socka, M.; Baśko, M.; Biela, T. Biocompatible Polymers Combined with Cyclodextrins: Fascinating Materials for Drug Delivery Applications. Molecules 2020, 25, 3404. [Google Scholar] [CrossRef] [PubMed]
- Kharazmi, A.; Ghorbani-Vaghei, R.; Khazaei, A.; Karakaya, I.; Karimi-Nami, R. A cost-efficient method for green synthesis of novel derivatives lower-rim-connected bisresorcinarene macrocycles in large-scale by sodium p-styrenesulfonate. Curr. Res. Green Sustain. Chem. 2024, 8, 100396. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Winterton, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, É.; Crini, G. Water-insoluble β-cyclodextrin–epichlorohydrin polymers for removal of pollutants from aqueous solutions by sorption processes using batch studies: A review of inclusion mechanisms. Prog. Polym. Sci. 2018, 78, 1–23. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Wei, Z.; Cui, Q.; Yang, X.; Yang, Y.; Zhang, X. Cyclodextrin Nano-Assemblies Enabled Robust, Highly Stretchable, and Healable Elastomers with Dynamic Physical Network. Adv. Funct. Mater. 2022, 33, 2210441. [Google Scholar] [CrossRef]
- Leavy, O. Dissolving cholesterol to unclog arteries. Nat. Rev. Immunol. 2016, 16, 275. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhao, F.; Zhang, D.; Li, J. Hyaluronic acid conjugated β-cyclodextrin-oligoethylenimine star polymer for CD44-targeted gene delivery. Int. J. Pharm. 2015, 483, 169–179. [Google Scholar] [CrossRef]
- Panel, N.; Sun, Y.J.; Fuentes, E.J.; Simonson, T. A Simple PB/LIE Free Energy Function Accurately Predicts the Peptide Binding Specificity of the Tiam1 PDZ Domain. Front. Mol. Biosci. 2017, 4, 65. [Google Scholar] [CrossRef]
- Pellecchia, M. Abstract 20: Targeting difficult targets with lysine-covalent ligands: Expanding the druggable space for covalent drugs. Clin. Cancer Res. 2020, 26, 20. [Google Scholar] [CrossRef]
- Shah, D.A.; Murdande, S.B.; Dave, R.H. A Review: Pharmaceutical and Pharmacokinetic Aspect of Nanocrystalline Suspensions. J. Pharm. Sci. 2016, 105, 10–24. [Google Scholar] [CrossRef]
- Lee, J.; Min An, J.; Kim, J.; Bang, E.-K.; Kim, D. A hybrid formulation of porous silicon nanoparticle with carboxymethyl cellulose for enhanced drug loading. Mater. Lett. 2024, 371, 136929. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Dang, L.H.; Le, H.K.; Ngan, L.T.; Tran, N.Q.; Park, K.D.; Le Thi, P. Injectable hyaluronic acid–cyclodextrin-based hydrogels for localized and sustained release of anticancer drugs. Macromol. Res. 2024, 32, 777–788. [Google Scholar] [CrossRef]
- Gonçalves, J.J.; Melo, B.L.; Pouso, M.R.; Correia, I.J.; de Melo-Diogo, D. Dual-crosslinked injectable in situ forming Alginate/CaCl2/Pluronic F127/α-Cyclodextrin hydrogels incorporating Doxorubicin and graphene-based nanomaterials for cancer chemo-photothermal therapy. J. Drug Deliv. Sci. Technol. 2025, 114, 107520. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, C.; Allcock, H.R. Injectable and Biodegradable Supramolecular Hydrogels by Inclusion Complexation between Poly(organophosphazenes) and α-Cyclodextrin. Macromolecules 2013, 46, 2715–2724. [Google Scholar] [CrossRef]
- Saitani, E.-M.; Selianitis, D.; Pippa, N.; Pispas, S.; Valsami, G. Cyclodextrins-block copolymer drug delivery systems: From design and development to preclinical studies. Nanotechnol. Rev. 2024, 13, 20230204. [Google Scholar] [CrossRef]
- Fülöp, Z.; Kurkov, S.V.; Nielsen, T.T.; Larsen, K.L.; Loftsson, T. Self-assembly of cyclodextrins: Formation of cyclodextrin polymer based nanoparticles. J. Drug Deliv. Sci. Technol. 2012, 22, 215–221. [Google Scholar] [CrossRef]
- Gharib, R.; Greige-Gerges, H.; Fourmentin, S.; Charcosset, C.; Auezova, L. Liposomes incorporating cyclodextrin–drug inclusion complexes: Current state of knowledge. Carbohydr. Polym. 2015, 129, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Hu, Y.; He, W.; Xu, Y.; Zhan, A.; Chen, K.; Liu, M.; Xiao, X.; Xu, X.; Feng, Q.; et al. An injectable and self-healing hydrogel with dual physical crosslinking for in-situ bone formation. Mater. Today Bio 2023, 19, 100558. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, M.; Li, J.; Cheng, B.; Wang, C.; Dong, H.; Shen, D. Tunable Synthesis of Sub-20 nm Gold Nanoparticles Capping with Cyclodextrin for Host–Guest Molecular Recognition on Nano-Surface. Part. Part. Syst. Charact. 2023, 40, 2300095. [Google Scholar] [CrossRef]
- Isenbügel, K.; Ritter, H.; Branscheid, R.; Kolb, U. Nanoparticle Vesicles Through Self Assembly of Cyclodextrin- and Adamantyl-Modified Silica. Macromol. Rapid Commun. 2010, 31, 2121–2126. [Google Scholar] [CrossRef]
- Ishida, Y.; Sakata, H.; Achalkumar, A.S.; Yamada, K.; Matsuoka, Y.; Iwahashi, N.; Amano, S.; Saigo, K. Cover Picture: Guest-Responsive Covalent Frameworks by the Cross-Linking of Liquid-Crystalline Salts: Tuning of Lattice Flexibility by the Design of Polymerizable Units. Chem. Eur. J. 2011, 17, 14693. [Google Scholar] [CrossRef]
- Su, Y.; Dang, J.; Zhang, H.; Zhang, Y.; Tian, W. Supramolecular Host–Guest Interaction-Enhanced Adjustable Drug Release Based on β-Cyclodextrin-Functionalized Thermoresponsive Porous Polymer Films. Langmuir 2017, 33, 7393–7402. [Google Scholar] [CrossRef]
- Huang, J.; Ren, L.; Zhu, H.; Chen, Y. Hydrophilic Block Copolymer Aggregation in Solution Induced by Selective Threading of Cyclodextrins. Macromol. Chem. Phys. 2006, 207, 1764–1772. [Google Scholar] [CrossRef]
- Petitjean, M.; García-Zubiri, I.X.; Isasi, J.R. History of cyclodextrin-based polymers in food and pharmacy: A review. Environ. Chem. Lett. 2021, 19, 3465–3476. [Google Scholar] [CrossRef]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Recent Advances in Host–Guest Self-Assembled Cyclodextrin Carriers: Implications for Responsive Drug Delivery and Biomedical Engineering. Adv. Funct. Mater. 2020, 30, 1909049. [Google Scholar] [CrossRef]
- Pandey, A. Cyclodextrin-based nanoparticles for pharmaceutical applications: A review. Environ. Chem. Lett. 2021, 19, 4297–4310. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Li, J.; Zhong, Y.; Wu, S.; Yan, M.; Ni, S.; Zhang, K.; Wang, G.; Qu, K.; et al. Biomimetic nanoparticles to enhance the reverse cholesterol transport for selectively inhibiting development into foam cell in atherosclerosis. J. Nanobiotechnol. 2023, 21, 307. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, F.; Wu, Y.; Hou, S.; Xue, L.; Su, Z.; Zhang, C. Poly-β-cyclodextrin Supramolecular Nanoassembly with a pH-Sensitive Switch Removing Lysosomal Cholesterol Crystals for Antiatherosclerosis. Nano Lett. 2021, 21, 9736–9745. [Google Scholar] [CrossRef] [PubMed]
- Gillella, S.; Divyanjali, M.; Rishitha, S.; Amzad, S.K.; Reddy, U.; Girish, C.; Apparao, C.H. Polymeric nanoparticles—A review. J. Innov. Appl. Pharm. Sci. JIAPS 2024, 9, 25–31. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; Cecchi, M.; Bragagni, M.; Almeida, A. Development of a new delivery system consisting in ‘drug—in cyclodextrin—in PLGA nanoparticles’. J. Microencapsul. 2010, 27, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Tejashwini, D.M.; Harini, H.V.; Nagaswarupa, H.P.; Naik, R.; Deshmukh, V.V.; Basavaraju, N. An in-depth exploration of eco-friendly synthesis methods for metal oxide nanoparticles and their role in photocatalysis for industrial dye degradation. Chem. Phys. Impact 2023, 7, 100355. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, Y.; Li, G.; Yang, S.; Chu, F. Lignin-Based Hollow Nanoparticles for Controlled Drug Delivery: Grafting Preparation Using β-Cyclodextrin/Enzymatic-Hydrolysis Lignin. Nanomaterials 2019, 9, 997. [Google Scholar] [CrossRef]
- Ou, L.-C.; Zhong, S.; Ou, J.-S.; Tian, J.-W. Application of targeted therapy strategies with nanomedicine delivery for atherosclerosis. Acta Pharmacol. Sin. 2020, 42, 10–17. [Google Scholar] [CrossRef]
- Li, Y.-N.; Shi, X.; Sun, D.; Han, S.; Zou, Y.; Wang, L.; Yang, L.; Li, Y.; Shi, Y.; Guo, J.; et al. Delivery of melarsoprol using folate-targeted PEGylated cyclodextrin-based nanoparticles for hepatocellular carcinoma. Int. J. Pharm. 2023, 636, 122791. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Xiao, F.; Song, L.; Sun, B.; Sun, D.; Chu, D.; Wang, L.; Han, S.; Yu, Z.; O’Driscoll, C.M.; et al. A folate-targeted PEGylated cyclodextrin-based nanoformulation achieves co-delivery of docetaxel and siRNA for colorectal cancer. Int. J. Pharm. 2021, 606, 120888. [Google Scholar] [CrossRef]
- Han, X.; Li, Z.; Sun, J.; Luo, C.; Li, L.; Liu, Y.; Du, Y.; Qiu, S.; Ai, X.; Wu, C.; et al. Stealth CD44-targeted hyaluronic acid supramolecular nanoassemblies for doxorubicin delivery: Probing the effect of uncovalent pegylation degree on cellular uptake and blood long circulation. J. Control. Release 2015, 197, 29–40. [Google Scholar] [CrossRef]
- Metcalf, C.A.; Svenson, S.; Hwang, J.; Tripathi, S.; Gangal, G.; Kabir, S.; Lazarus, D.; Cole, R.; Sweryda-Krawiec, B.; Shum, P.; et al. Discovery of a Novel Cabazitaxel Nanoparticle–Drug Conjugate (CRLX522) with Improved Pharmacokinetic Properties and Anticancer Effects Using a β-Cyclodextrin–PEG Copolymer Based Delivery Platform. J. Med. Chem. 2019, 62, 9541–9559. [Google Scholar] [CrossRef]
- Yu, J.; Ma, Y.; Zhang, X.; Wang, S.; Zhou, L.; Liu, X.; Li, L.; Liu, L.; Song, H.; Luo, Y.; et al. β-Cyclodextrin and Hyaluronic Acid-Modified Targeted Nanodelivery System for Atherosclerosis Prevention. ACS Appl. Mater. Interfaces 2024, 16, 35421–35437. [Google Scholar] [CrossRef]
- Singh, P.; Chen, Y.; Tyagi, D.; Wu, L.; Ren, X.; Feng, J.; Carrier, A.; Luan, T.; Tang, Y.; Zhang, J.; et al. β-Cyclodextrin-grafted hyaluronic acid as a supramolecular polysaccharide carrier for cell-targeted drug delivery. Int. J. Pharm. 2021, 602, 120602. [Google Scholar] [CrossRef]
- Singh, P.; Wu, L.; Ren, X.; Zhang, W.; Tang, Y.; Chen, Y.; Carrier, A.; Zhang, X.; Zhang, J. Hyaluronic-acid-based β-cyclodextrin grafted copolymers as biocompatible supramolecular hosts to enhance the water solubility of tocopherol. Int. J. Pharm. 2020, 586, 119542. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, K.; Ye, Y.; Wang, L.; Chu, L.; Tan, H. Preparation of Chitosan Immobilized with Β-Cd at C6 Position by a Nucleophilic Displacement Reaction. Acta Polym. Sin. 2009, 7, 712–716. [Google Scholar] [CrossRef]
- Lu, C.Y.; Church, D.C.; Learn, G.D.; Pokorski, J.K.; von Recum, H.A. Modified Cyclodextrin Microparticles to Improve PMMA Drug Delivery Without Mechanical Loss. Macromol. Biosci. 2021, 21, 2000328. [Google Scholar] [CrossRef]
- Li, G.; Song, S.; Guo, L.; Ma, S. Self-assembly of thermo- and pH-responsive poly(acrylic acid)-b-poly(N-isopropylacrylamide) micelles for drug delivery. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 5028–5035. [Google Scholar] [CrossRef]
- Liu, P. Cyclodextrins as versatile supramolecular building block in nanoscale drug delivery systems for precise tumor chemotherapy. Chin. Chem. Lett. 2025, 36, 111406. [Google Scholar] [CrossRef]
- Sarabandi, K.; Rafiee, Z.; Khodaei, D.; Jafari, S.M. Encapsulation of food ingredients by nanoliposomes. In Lipid-Based Nanostructures for Food Encapsulation Purposes; Academic Press: Amsterdam, The Netherlands, 2019; pp. 347–404. [Google Scholar] [CrossRef]
- Maboudi, A.H.; Lotfipour, M.H.; Rasouli, M.; Azhdari, M.H.; MacLoughlin, R.; Bekeschus, S.; Doroudian, M. Micelle-based nanoparticles with stimuli-responsive properties for drug delivery. Nanotechnol. Rev. 2024, 13, 20230218. [Google Scholar] [CrossRef]
- Li, Y.; Liao, J.; Xiong, L.; Xiao, Z.; Ye, F.; Wang, Y.; Chen, T.; Huang, L.; Chen, M.; Chen, Z.-S.; et al. Stepwise targeted strategies for improving neurological function by inhibiting oxidative stress levels and inflammation following ischemic stroke. J. Control. Release 2024, 368, 607–622. [Google Scholar] [CrossRef]
- He, J.; Zhang, W.; Zhou, X.; Xu, F.; Zou, J.; Zhang, Q.; Zhao, Y.; He, H.; Yang, H.; Liu, J. Reactive oxygen species (ROS)-responsive size-reducible nanoassemblies for deeper atherosclerotic plaque penetration and enhanced macrophage-targeted drug delivery. Bioact. Mater. 2023, 19, 115–126. [Google Scholar] [CrossRef]
- He, J.; Zhou, X.; Xu, F.; He, H.; Ma, S.; Liu, X.; Zhang, M.; Zhang, W.; Liu, J. Anchoring β-CD on simvastatin-loaded rHDL for selective cholesterol crystals dissolution and enhanced anti-inflammatory effects in macrophage/foam cells. Eur. J. Pharm. Biopharm. 2022, 174, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, V.; Nivetha, R.P.; Tamilanban, T.; Narayanan, J.; Vetriselvan, S.; Fuloria, N.K.; Chinni, S.V.; Sekar, M.; Fuloria, S.; Wong, L.S.; et al. Nanogels as novel drug nanocarriers for CNS drug delivery. Front. Mol. Biosci. 2023, 10, 1232109. [Google Scholar] [CrossRef]
- Garg, A.; Shah, K.; Chauhan, C.S.; Agrawal, R. Ingenious nanoscale medication delivery system: Nanogel. J. Drug Deliv. Sci. Technol. 2024, 92, 105289. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Li, X.; Chen, J.; Yu, Z.; Li, X.; Sun, C.; Hu, L.; Wu, M.; Liu, L. Polydatin protects against atherosclerosis by activating autophagy and inhibiting pyroptosis mediated by the NLRP3 inflammasome. J. Ethnopharmacol. 2023, 309, 116304. [Google Scholar] [CrossRef] [PubMed]
- Shoukat, H.; Pervaiz, F.; Rehman, S.; Akram, F.; Noreen, S.; Khan, K.U.; Basit, A.; Ashraf, M.A. Development, in vitro and in vivo evaluation of β-cyclodextrin/Polyvinyl alcohol-co-poly (2-acrylamide-2-methylpropane sulfonic acid) cross-linked hybrid IPN-nanogels to improve the dissolution and absorption of anti-hyperlipidemic drug. Polym. Plast. Technol. Mater. 2023, 62, 1945–1967. [Google Scholar] [CrossRef]
- Roy, I.; Stoddart, J.F. Cyclodextrin Metal–Organic Frameworks and Their Applications. Acc. Chem. Res. 2021, 54, 1440–1453. [Google Scholar] [CrossRef]
- Patel, H.A.; Islamoglu, T.; Liu, Z.; Nalluri, S.K.M.; Samanta, A.; Anamimoghadam, O.; Malliakas, C.D.; Farha, O.K.; Stoddart, J.F. Noninvasive Substitution of K+ Sites in Cyclodextrin Metal–Organic Frameworks by Li+ Ions. J. Am. Chem. Soc. 2017, 139, 11020–11023. [Google Scholar] [CrossRef]
- Li, S.; Gao, H.; Wang, H.; Zhao, X.; Pan, D.; Pacheco-Fernández, I.; Ma, M.; Liu, J.; Hirvonen, J.; Liu, Z.; et al. Tailored polysaccharide entrapping metal-organic framework for RNAi therapeutics and diagnostics in atherosclerosis. Bioact. Mater. 2025, 43, 376–391. [Google Scholar] [CrossRef]
- Ge, X.; Liu, Y.; Zhao, X.; Nafady, A.; Bhattacharya, G.; Mai, J.; Al-Enizi, A.M.; Pettigrew, R.I.; Ma, S. Advances in metal-organic frameworks for cardiovascular therapy: From structural design to preclinical applications. Coord. Chem. Rev. 2025, 544, 216971. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Khalid, G.M.; Selmin, F.; Minghetti, P.; Cilurzo, F. Trends in the production methods of orodispersible films. Int. J. Pharm. 2020, 576, 118963. [Google Scholar] [CrossRef]
- Das, M.K.; Sarma, A.; Deka, T. Polydopamine-Based Simple and Versatile Surface Modification of Polymeric Nano Drug Carriers. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2019; pp. 369–389. [Google Scholar] [CrossRef]
- Zhuang, J.; Holay, M.; Park, J.H.; Fang, R.H.; Zhang, J.; Zhang, L. Nanoparticle Delivery of Immunostimulatory Agents for Cancer Immunotherapy. Theranostics 2019, 9, 7826–7848. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, R.; Liu, C.; Pan, L.; Qi, Y.; Cheng, J.; Guo, J.; Jia, Y.; Ding, J.; Zhang, J.; et al. A pH/ROS dual-responsive and targeting nanotherapy for vascular inflammatory diseases. Biomaterials 2020, 230, 119605. [Google Scholar] [CrossRef]
- Kang, H.; Zhang, K.; Wong, D.S.H.; Han, F.; Li, B.; Bian, L. Near-infrared light-controlled regulation of intracellular calcium to modulate macrophage polarization. Biomaterials 2018, 178, 681–696. [Google Scholar] [CrossRef]
- Xu, Z. CRISPR/Cas9-mediated silencing of CD44: Unveiling the role of hyaluronic acid-mediated interactions in cancer drug resistance. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 397, 2849–2876. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, X.; Shen, H.; Dong, J.; Rong, S.; Cai, W.; Zhang, R. CD44-targeted melanin-based nanoplatform for alleviation of ischemia/reperfusion-induced acute kidney injury. J. Control. Release 2024, 368, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Sun, C.; Cai, Z.; Li, Y.; Liu, W.; Luan, Y.; Wang, C. Effective therapy of advanced breast cancer through synergistic anticancer by paclitaxel and P-glycoprotein inhibitor. Mater. Today Bio 2024, 26, 101029. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.; Uthaman, S.; Park, I.-K. Cell Membrane-Camouflaged Nanoparticles: A Promising Biomimetic Strategy for Cancer Theragnostics. Polymers 2018, 10, 983. [Google Scholar] [CrossRef]
- Kraynak, C.A.; Huang, W.; Bender, E.C.; Wang, J.-L.; Hanafy, M.S.; Cui, Z.; Suggs, L.J. Apoptotic body-inspired nanoparticles target macrophages at sites of inflammation to support an anti-inflammatory phenotype shift. Int. J. Pharm. 2022, 618, 121634. [Google Scholar] [CrossRef]
- Gao, C.; Liu, C.; Chen, Q.; Wang, Y.; Kwong, C.H.T.; Wang, Q.; Xie, B.; Lee, S.M.Y.; Wang, R. Cyclodextrin-mediated conjugation of macrophage and liposomes for treatment of atherosclerosis. J. Control. Release 2022, 349, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, Z.; Hu, C.; Maitz, M.F.; Yang, L.; Luo, R.; Wang, Y. Platelet Membrane-Coated Nanocarriers Targeting Plaques to Deliver Anti-CD47 Antibody for Atherosclerotic Therapy. Research 2022, 2022, 9845459. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Liu, K.; Liu, T.; Song, N.; Dai, W.; Du, D.; Li, X.; Peng, Y.; Meng, Y. Macrophage membrane-coated biomimetic magnetic nanoparticle loaded with interleukin 10 as potential candidate towards anti-atherosclerotic therapy: Characterization and in vitro studies. J. Nanoparticle Res. 2023, 25, 183. [Google Scholar] [CrossRef]
- Kadappu, P.; Jonnagaddala, J.; Liaw, S.-T.; Cochran, B.J.; Rye, K.-A.; Ong, K.L. Statin Prescription Patterns and Associations with Subclinical Inflammation. Medicina 2022, 58, 1096. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Inflammation in Atherosclerosis. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef]
- Borràs, C.; Rotllan, N.; Griñán, R.; Santos, D.; Solé, A.; Dong, C.; Zhao, Q.; Llorente-Cortes, V.; Mourín, M.; Soto, B.; et al. Restoring cholesterol efflux in vascular smooth muscle cells transitioning into foam cells through Liver X receptor activation. Biomed. Pharmacother. 2025, 188, 118178. [Google Scholar] [CrossRef]
- Vijayan, V.; Uthaman, S.; Park, I.-K. Cell Membrane Coated Nanoparticles: An Emerging Biomimetic Nanoplatform for Targeted Bioimaging and Therapy. In Biomimetic Medical Materials: From Nanotechnology to 3D Bioprinting; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 45–59. [Google Scholar] [CrossRef]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef]
- Li, J.; Cao, Y.; Zhang, X.; An, M.; Liu, Y. The Application of Nano-drug Delivery System With Sequential Drug Release Strategies in Cancer Therapy. Am. J. Clin. Oncol. 2023, 46, 459–473. [Google Scholar] [CrossRef]
- Giri, B.R.; Yang, H.S.; Song, I.-S.; Choi, H.-G.; Cho, J.H.; Kim, D.W. Alternative Methotrexate Oral Formulation: Enhanced Aqueous Solubility, Bioavailability, Photostability, and Permeability. Pharmaceutics 2022, 14, 2073. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Liu, K.; Peng, Y.; Dai, W.; Du, D.; Li, X.; Liu, T.; Song, N.; Meng, Y. Research progress on the therapeutic effects of nanoparticles loaded with drugs against atherosclerosis. Cardiovasc. Drugs Ther. 2023, 38, 977–997. [Google Scholar] [CrossRef]
- Chen, Z.; Chu, Z.; Jiang, Y.; Xu, L.; Qian, H.; Wang, Y.; Wang, W. Recent advances on nanomaterials for antibacterial treatment of oral diseases. Mater. Today Bio 2023, 20, 100635. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.F.; Chen, W.H.; Lei, Q.; Qiu, W.X.; Liu, Y.X.; Cheng, Y.J.; Zhang, X.Z. A Triple-Collaborative Strategy for High-Performance Tumor Therapy by Multifunctional Mesoporous Silica-Coated Gold Nanorods. Adv. Funct. Mater. 2016, 26, 4339–4350. [Google Scholar] [CrossRef]
- Abishek Jung, P.; Feng, H.; Lixia, H.; Lin, X.; Guang, Y. Supramolecular hydrogels based on poly (ethylene glycol)-poly (lactic acid) block copolymer micelles and α-cyclodextrin for potential injectable drug delivery system. Carbohydr. Polym. 2018, 194, 69–79. [Google Scholar] [CrossRef]
- Hu, Q.D.; Tang, G.P.; Chu, P.K. Cyclodextrin-based host-guest supramolecular nanoparticles for delivery: From design to applications. Acc. Chem. Res. 2014, 47, 2017–2025. [Google Scholar] [CrossRef]
- Jiang, K.; Wu, W.; Xie, M.; He, H.; Sun, R. Fabrication of Hyaluronic Acid-Targeted Supramolecular Delivery Platform with pH and ROS-Responsive Drug Release. Macromol. Rapid Commun. 2025, 46, e2500201. [Google Scholar] [CrossRef]
- An, L.; Dang, J.; Hu, C.; Zhu, C. Physicochemical Properties and Gastric Mucosa Irritation of Cantharidin-hydroxypropyl-β-cyclodextrin Inclusion Complex. Chin. Herb. Med. 2012, 4, 224–229. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, T.; Li, Y.; Chen, L.; Xu, Y.; Chi, X.; Yu, S.; Wang, W.; Liu, D.; Zhu, B.; et al. Biocompatible hydrophobic cross-linked cyclodextrin-based metal-organic framework as quercetin nanocarrier for enhancing stability and controlled release. Food Chem. 2024, 448, 139167. [Google Scholar] [CrossRef]
- Karuppiah, N.; Kaufman, P.B.; Kapustka, S.A.; Sharma, A. Use of Cyclodextrin-Cholesterol Complex as a Primary Standard in Cholesterol Analysis. Microchem. J. 1993, 47, 325–329. [Google Scholar] [CrossRef]
- Nataly, S.; Ana, R.; Nicolás, Y.; Erika, L.; Boris, C.; Simón, G.; Josep, S.; Paul, J.; Marcelo, J.K. Photothermally Controlled Methotrexate Release System Using β-Cyclodextrin and Gold Nanoparticles. Nanomaterials 2018, 8, 985. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, S.; Wang, M.; Liu, Q.; Jing, X.; Cai, X. One-pot preparations of cyclodextrin polymer-entrapped nano zero-valent iron for the removal of p-nitrophenol in water. Chem. Eng. J. 2022, 431, 133370. [Google Scholar] [CrossRef]
- Xu, H.; She, P.Y.; Ma, B.X.; Zhao, Z.Y.; Li, G.C.; Wang, Y.B. ROS responsive nanoparticles loaded with lipid-specific AIEgen for atherosclerosis-targeted diagnosis and bifunctional therapy. Biomaterials 2022, 288, 121734. [Google Scholar] [CrossRef]
- Kim, H.; Kumar, S.; Kang, D.-W.; Jo, H.; Park, J.-H. Affinity-Driven Design of Cargo-Switching Nanoparticles to Leverage a Cholesterol-Rich Microenvironment for Atherosclerosis Therapy. ACS Nano 2020, 14, 6519–6531. [Google Scholar] [CrossRef]
- Hironori, I.; Kohsaku, K.; Masato, S.; Yoshitaka, T.; Jonathan, P.H.; Katsuhiko, A. β-Cyclodextrin-crosslinked alginate gel for patient-controlled drug delivery systems: Regulation of host–guest interactions with mechanical stimuli. J. Mater. Chem. B 2013, 1, 2155. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Liu, Y.H.; Liu, Y. Cyclodextrin-Based Multistimuli-Responsive Supramolecular Assemblies and Their Biological Functions. Adv. Mater. 2019, 32, 1806158. [Google Scholar] [CrossRef]
- Alboni, S.; Secco, V.; Papotti, B.; Vilella, A.; Adorni, M.P.; Zimetti, F.; Schaeffer, L.; Tascedda, F.; Zoli, M.; Leblanc, P.; et al. Hydroxypropyl-β-Cyclodextrin Depletes Membrane Cholesterol and Inhibits SARS-CoV-2 Entry into HEK293T-ACEhi Cells. Pathogens 2023, 12, 647. [Google Scholar] [CrossRef]
- Fong, E.M.; Kinzie, J.S.D.; Christopherson, A.; Al-Hussieni, J.K.; Ye, K.; Vinay, A.; Mooney, R.; Johal, M.S. A Hydrophobic Goldilocks Zone for Cyclodextrin-Lipid-Membrane Interactions: Implications of Drug Hydrophobicity on Kinetics of Cholesterol Removal from Lipid Membranes. Langmuir 2025, 41, 15909–15917. [Google Scholar] [CrossRef]
- Rao, M.R.P.; Chaudhari, J.; Trotta, F.; Caldera, F. Investigation of Cyclodextrin-Based Nanosponges for Solubility and Bioavailability Enhancement of Rilpivirine. AAPS PharmSciTech 2018, 19, 2358–2369. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, T.; Liu, S.; Li, J.; Huang, G.; Yang, H.-H. Bifunctional magnetic nanoparticles for efficient cholesterol detection and elimination via host-guest chemistry in real samples. Biosens. Bioelectron. 2018, 120, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Cirri, M.; Bragagni, M.; Mennini, N.; Mura, P. Development of a new delivery system consisting in “drug–in cyclodextrin–in nanostructured lipid carriers” for ketoprofen topical delivery. Eur. J. Pharm. Biopharm. 2012, 80, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Hadadian, M.; Allahyari, R.; Mahdavi, B.; Rezaei-Seresht, E. Design, characterization, and in vitro evaluation of magnetic carboxymethylated β-cyclodextrin as a pH-sensitive carrier system for amantadine delivery: A novel approach for targeted drug delivery. RSC Adv. 2025, 15, 446–459. [Google Scholar] [CrossRef]
- Zinger, A. Unleashing the potential of cell biomimetic nanoparticles: Strategies and challenges in their design and fabrication for therapeutic applications. J. Control. Release 2023, 358, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; An, Y.; Jia, W.; Wang, Y.; Wu, Y.; Zhen, Y.; Cao, J.; Gao, H. Macrophage-mimic shape changeable nanomedicine retained in tumor for multimodal therapy of breast cancer. J. Control. Release 2020, 321, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Frambach, S.J.C.M.; de Haas, R.; Smeitink, J.A.M.; Russel, F.G.M.; Schirris, T.J.J. Restoring cellular NAD(P)H levels by PPARα and LXRα stimulation to improve mitochondrial complex I deficiency. Life Sci. 2022, 300, 120571. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhong, Y.; Yan, M.; Ni, S.; Zhao, X.; Wu, S.; Wang, G.; Zhang, K.; Chi, Q.; Qin, X.; et al. Macrophage Membrane-Encapsulated Dopamine-Modified Poly Cyclodextrin Multifunctional Biomimetic Nanoparticles for Atherosclerosis Therapy. ACS Appl. Mater. Interfaces 2024, 16, 32027–32044. [Google Scholar] [CrossRef]
- He, J.; Yang, Y.; Zhou, X.; Zhang, W.; Liu, J. Shuttle/sink model composed of β-cyclodextrin and simvastatin-loaded discoidal reconstituted high-density lipoprotein for enhanced cholesterol efflux and drug uptake in macrophage/foam cells. J. Mater. Chem. B 2020, 8, 1496–1506. [Google Scholar] [CrossRef]
- Dai, Z.; Yang, H.; Yin, P.; Liu, X.; Zhang, L.; Dou, Y.; Sun, S. Applications of Cyclodextrin-Based Drug Delivery Systems in Inflammation-Related Diseases. Pharmaceutics 2025, 17, 378. [Google Scholar] [CrossRef]
- Despres, H.; Sunasee, R.; Carson, M.; Pacherille, A.; Nunez, K.; Ckless, K. Cell-based analysis of the immune and antioxidant response of the nanocarrier β-cyclodextrin conjugated with cellulose nanocrystals. Free Radic. Biol. Med. 2018, 128, S102. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, C.; Gong, Z.; Li, Z.; Ding, S. Ox-LDL induced endothelial progenitor cells oxidative stress via p38/Keap1/Nrf2 pathway. Res. Sq. 2021. preprint. [Google Scholar] [CrossRef]
- Gupta, D.; Lessard, S.; Moore, N.; Duan, J.; Nakamura, Y.; Yang, F.-C.; Hicks, A.; Light, D.R.; Krishnamoorthy, S. Genetic Activation of NRF2 By KEAP1 Inhibition Induces Fetal Hemoglobin Expression and Triggers Anti-Oxidant Stress Response in Erythroid Cells. Blood 2019, 134, 210. [Google Scholar] [CrossRef]
- Aiassa, V.; Zoppi, A.; Albesa, I.; Longhi, M.R. Inclusion complexes of chloramphenicol with β-cyclodextrin and aminoacids as a way to increase drug solubility and modulate ROS production. Carbohydr. Polym. 2015, 121, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, G.; Zhou, Z.; Gao, L.; Tao, Q. Sensitive complex micelles based on host-guest recognition from chitosan-graft-β-cyclodextrin for drug release. Int. J. Biol. Macromol. 2017, 105, 74–80. [Google Scholar] [CrossRef]
- Zaid, A.; Ariel, A. Harnessing anti-inflammatory pathways and macrophage nano delivery to treat inflammatory and fibrotic disorders. Adv. Drug Del. Rev. 2024, 207, 115204. [Google Scholar] [CrossRef]
- McCarthy, J.; O’Neill, M.J.; Bourre, L.; Walsh, D.; Quinlan, A.; Hurley, G.; Ogier, J.; Shanahan, F.; Melgar, S.; Darcy, R.; et al. Gene silencing of TNF-alpha in a murine model of acute colitis using a modified cyclodextrin delivery system. J. Control. Release 2013, 168, 28–34. [Google Scholar] [CrossRef]
- Ganesan, R.; Henkels, K.M.; Wrenshall, L.E.; Kanaho, Y.; Di Paolo, G.; Frohman, M.A.; Gomez-Cambronero, J. Oxidized LDL phagocytosis during foam cell formation in atherosclerotic plaques relies on a PLD2–CD36 functional interdependence. J. Leukoc. Biol. 2018, 103, 867–883. [Google Scholar] [CrossRef]
- Hwang, A.-R. Abstract 1157: Inhibition Of P90rsk Ameliorates Pdgf-bb-induced Vascular Smooth Muscle Cell Phenotypic Change and Neointimal Hyperplasia. Arterioscler. Thromb. Vasc. Biol. 2024, 44, A1157. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, J.; Bai, X.; Liu, B.; Liu, C.-j.; Xu, Q.; Zhu, Y.; Wang, N.; Kong, W.; Wang, X. ADAMTS-7 Mediates Vascular Smooth Muscle Cell Migration and Neointima Formation in Balloon-Injured Rat Arteries. Circ. Res. 2009, 104, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, M.; Zhou, Q.; Li, X. Recent developments of mesoporous silica nanoparticles in biomedicine. Emergent Mater. 2020, 3, 381–405. [Google Scholar] [CrossRef]
- Liu, X.; Ding, D.; Chen, G.-D.; Li, L.; Jiang, H.; Salvi, R. 2-Hydroxypropyl-β-cyclodextrin Ototoxicity in Adult Rats: Rapid Onset and Massive Destruction of Both Inner and Outer Hair Cells Above a Critical Dose. Neurotox. Res. 2020, 38, 808–823. [Google Scholar] [CrossRef] [PubMed]
- Roozbehi, S.; Dadashzadeh, S.; Mirshahi, M.; Sadeghizadeh, M.; Sajedi, R.H. Targeted anticancer prodrug therapy using dextran mediated enzyme–antibody conjugate and β-cyclodextrin-curcumin inclusion complex. Int. J. Biol. Macromol. 2020, 160, 1029–1041. [Google Scholar] [CrossRef]
- Roozbehi, S.; Dadashzadeh, S.; Sajedi, R.H. An enzyme-mediated controlled release system for curcumin based on cyclodextrin/cyclodextrin degrading enzyme. Enzym. Microb. Technol. 2021, 144, 109727. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Li, Y.; Zhang, S.; Fan, H.; Cheng, Z. CSH/SBE-β-CD nanoparticles: Controlled synthesis and application for loading and pH-responsive drug release. New J. Chem. 2022, 46, 13498–13503. [Google Scholar] [CrossRef]
- Wang, F.; Cai, X.; Su, Y.; Hu, J.; Wu, Q.; Zhang, H.; Xiao, J.; Cheng, Y. Reducing cytotoxicity while improving anti-cancer drug loading capacity of polypropylenimine dendrimers by surface acetylation. Acta Biomater. 2012, 8, 4304–4313. [Google Scholar] [CrossRef] [PubMed]
- Olteanu, A.A.; Aramă, C.-C.; Radu, C.; Mihăescu, C.; Monciu, C.-M. Effect of β-cyclodextrins based nanosponges on the solubility of lipophilic pharmacological active substances (repaglinide). J. Incl. Phenom. Macrocycl. Chem. 2014, 80, 17–24. [Google Scholar] [CrossRef]
- Kotsovilis, S.; Salagianni, M.; Varela, A.; Davos, C.H.; Galani, I.E.; Andreakos, E. Comprehensive Analysis of 1-Year-Old Female Apolipoprotein E-Deficient Mice Reveals Advanced Atherosclerosis with Vulnerable Plaque Characteristics. Int. J. Mol. Sci. 2024, 25, 1355. [Google Scholar] [CrossRef]
- Kiruba Daniel, S.C.G.; Julius, L.A.N.; Gorthi, S.S. Microfluidics based Handheld Nanoparticle Synthesizer. J. Clust. Sci. 2016, 28, 1201–1213. [Google Scholar] [CrossRef]
- Shelton, K.A.; Clarkson, T.B.; Kaplan, J.R. Nonhuman Primate Models of Atherosclerosis. In Nonhuman Primates in Biomedical Research: Diseases, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Tao, W.; Yurdagul, A., Jr.; Kong, N.; Li, W.; Wang, X.; Doran, A.C.; Feng, C.; Wang, J.; Islam, M.A.; Farokhzad, O.C.; et al. siRNA nanoparticles targeting CaMKIIγ in lesional macrophages improve atherosclerotic plaque stability in mice. Sci. Transl. Med. 2020, 12, eaay1063. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Gupta, V.; Zern, B.J.; Pan, D.; Zakrewsky, M.; Muzykantov, V.; Mitragotri, S. Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS Nano 2013, 7, 11129–11137. [Google Scholar] [CrossRef]
- Schleich, N.; Danhier, F.; Préat, V. Iron oxide-loaded nanotheranostics: Major obstacles to in vivo studies and clinical translation. J. Control. Release 2015, 198, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Wu, C.; Zheng, L.; Li, Y.; Yang, R.; Yuan, P.; Jiang, J.; Li, C.; Zhou, X. Advances in stimulus-responsive nanomedicine for treatment and diagnosis of atherosclerosis. Colloids Surf. B Biointerfaces 2025, 245, 114298. [Google Scholar] [CrossRef]
- Geng, Y.; Song, M.; Huang, B.; Lin, R.; Wu, S.; Lin, A. Safranal ameliorates atherosclerosis progression partly via repressing PI3K/Akt and NF-κB signaling pathways in ApoE (−/−) mice. J. Nat. Med. 2025, 79, 821–832. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ko, S.-H.; Shim, J.-S.; Kim, D.-D.; Cho, H.-J. Tumor Targeting and Lipid Rafts Disrupting Hyaluronic Acid-Cyclodextrin-Based Nanoassembled Structure for Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 36628–36640. [Google Scholar] [CrossRef]
- Mei, Y.; Tang, L.; Xiao, Q.; Zhang, Z.; Zhang, Z.; Zang, J.; Zhou, J.; Wang, Y.; Wang, W.; Ren, M. Reconstituted high density lipoprotein (rHDL), a versatile drug delivery nanoplatform for tumor targeted therapy. J. Mater. Chem. B 2021, 9, 612–633. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Atyabi, F.; Dinarvand, R. The significance of artificial intelligence in drug delivery system design. Adv. Drug Del. Rev. 2019, 151, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Boczar, D.; Michalska, K. A Review of Machine Learning and QSAR/QSPR Predictions for Complexes of Organic Molecules with Cyclodextrins. Molecules 2024, 29, 3159. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S. Exploring the Synergistic Potential of Artificial Intelligence and Machine Learning in Chemistry. Vidyodaya J. Sci. 2024, 27, 68–85. [Google Scholar] [CrossRef]
- Titilayo, O.; Adetunji, C.O. Artificial Intelligence and Deep Learning Process and Drug Discovery. In Health Technologies and Informatics; CRC Press: Boca Raton, FL, USA, 2025; pp. 102–108. [Google Scholar]





| Types of CDs | Carrier Types | Nano-Systems | Drugs | Targeting Mechanisms | Animal Model | Route of Administration | Formulation Properties of CDs | Pharmacological Properties of CDs | References |
|---|---|---|---|---|---|---|---|---|---|
| HP-β-CD | Micelle | MTX NPs | Methotrexate (antimetabolite anticancer drug) | Host–guest recognition | Male Sprague-Dawley rats | Oral gavage | Enhanced drug solubility, improved encapsulation | Dissolve cholesterol crystals | [152] |
| β-CD | Nanogels | 8-arm polyethylene glycol 20,000-CD | 5-FU (antimetabolite chemotherapy); MTX (antimetabolite antitumor drug) | Prostate cancer model | tail Vein injection | Sustained release and high loading efficiency | Improve local drug concentration, enhance anticancer efficacy, reduce systemic toxicity | [68] | |
| CD-NH2 | Polymeric Nanoparticle | poly-β-CD/pH-sensitive benzimidazole-modified dextran sulfate/spherical nucleic acid | pH-responsive | ApoE−/− mice (apolipoprotein E knockout mice) | Intravenous injection. | Stimuli-responsiveness, self-assembly stability, anti-hemolytic activity | Cholesterol-dissolving capacity, biocompatibility | [103] | |
| β-CD | Polymeric Nanoparticle | PLGA-NPs | Atorvastatin (hepitorin) | HA | ApoE−/− mice (apolipoprotein E gene knockout mice) | Intravenous injection | Enhanced drug solubility, sustained release | Enhance drug bioavailability, reduce systemic toxicity, and improve targeting efficiency | [153] |
| β-CD | Polymeric Nanoparticle | β-CD-CS | Broad-spectrum antibacterial agent | Chitosan-mediated biofilm adhesion | Enhanced drug solubility and loading capacity | Low toxicity and excellent biocompatibility | [154] | ||
| β-CD | Polymeric Nanoparticle | β-CD-PAA-PMMA | Broad-spectrum antibacterial agent | Surface charge-mediated targeting | sustained release | Good biocompatibility; low cytotoxicity | [154] | ||
| HP-β-CD | Polymeric Nanoparticle | CDNPs | Nile red (fluorescent dye), Indocyanine Green (near-infrared fluorescent dye) | (EPR) effect | ApoE−/− mice (apolipoprotein E gene knockout mice) | tail Vein injection | Solubilization, stability, sustained release | Cholesterol-dissolving capacity, biocompatibility | [13] |
| β-CD | Liposome | HA-Fc/NP3 ST | Simvastatin (statins) | HA | ApoE-deficient mice (apoprotein E gene knockout mice) | Tail vein injection | Solubilization | ROS-responsive, promote deep plaque penetration | [123] |
| β-CD-NH2 | Nanogels | shell-crosslinked nanoparticles (SCNPs) | Paclitaxel, Camptothecin (antitumor drug) | Host–guest recognition | HeLa cervical cancer xenograft model | Tail vein injection | Improved drug loading capacity and colloidal stability | Dissolve cholesterol crystals, confer redox responsiveness | [75] |
| β-CD | Inorganic Nanoparticle | MMSGNR-AlPcS4 | AlPcS4 + Pt(IV) prodrug (a complex of photosensitizer and platinum prodrug) | Lactobionic acid targeting ligand | BALB/c nude mice with HepG2 human hepatocellular carcinoma cells | Tail vein injection | Controlled release; sealing mesoporous channels to prevent drug leakage | Reduction-responsive | [155] |
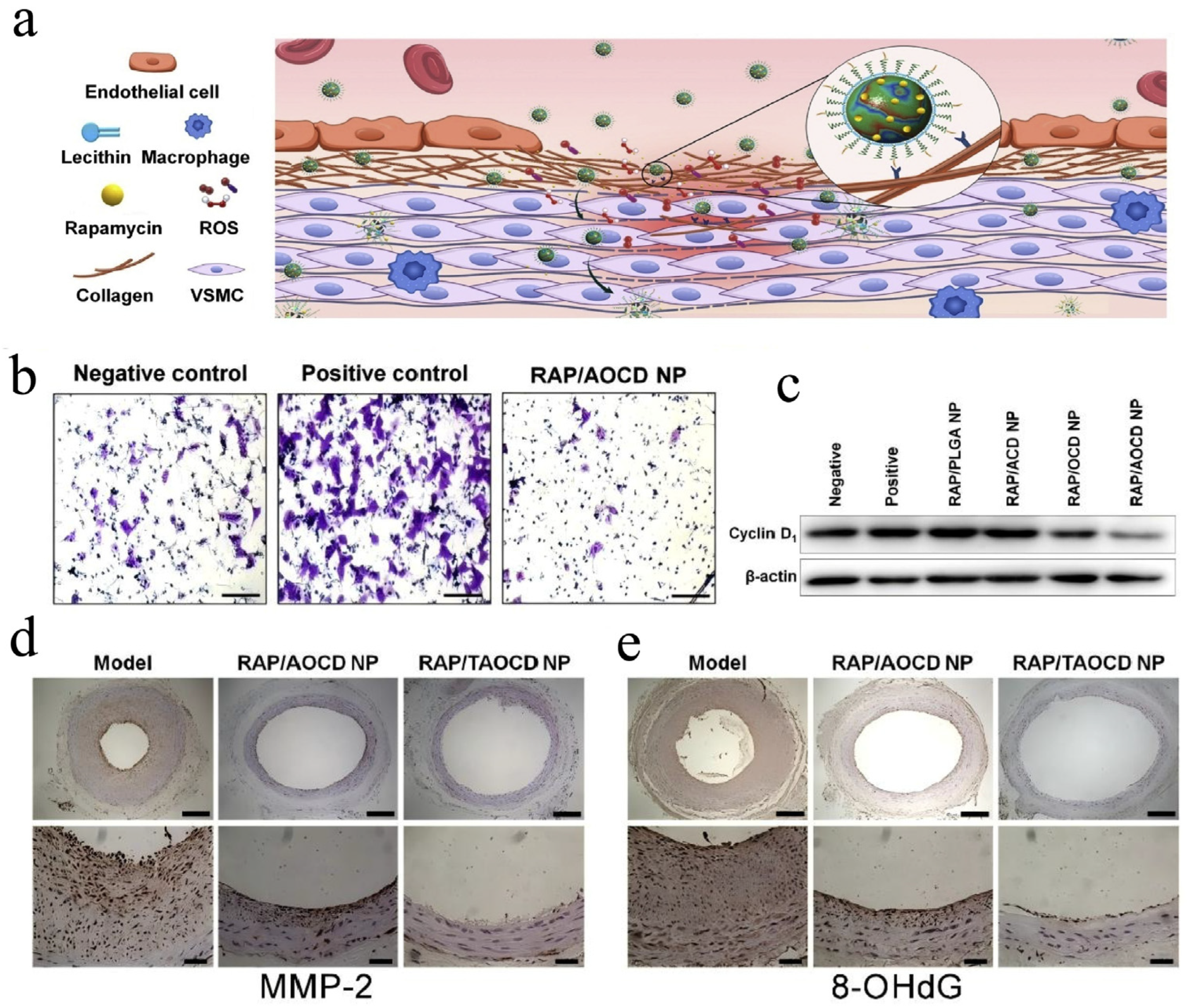
| β-CD | Polymeric Nanoparticle | AOCD NP, TAOCD NP | Rapamycin (mTOR inhibitor) | (EPR) effect | Carotid artery balloon injury induced vascular inflammation rats | Intravenous injection | Dual-responsive carrier materials: pH-sensitive (ACD component), ROS-sensitive (OCD component) | Improve drug-loading capacity | [136] |
| PH-CD, HA-CD | Polymeric Nanoparticle | dual-carrier nanoparticles (Double-NPs) | Epicatechin gallate (flavonoid compound) | HA | Superscale ApoE−/− mice (apolipoprotein E gene knockout mice) | Intraperitoneal injection | Enhanced drug stability, dual-carrier co-delivery, and low pH responsiveness | Alleviate inflammatory microenvironment; in vivo targeting of atherosclerotic plaques | [113] |
| β-CD | Micelle | MM@MTX NPs | MTX (immunosuppressant) | Cell membrane | ApoE−/− mice (apolipoprotein E gene knockout mice) | Tail vein injection | Host–guest recognition; controlled release | Promote cholesterol dissolution and efflux, inhibit foam cell formation | [102] |
| α-CD | Micelle | Poly(ethylene glycol)-polylactic acid | DOX (antitumor drug) | (EPR) effect | Controlled release | Solubilization-promoting, prolongs drug circulation time in vivo | [156] | ||
| β-CD | Nanogels | β-CD/Polyvinyl alcohol-co-poly(2-acrylamide-2-methylpropane sulfonic acid) cross-linked hybrid Interpenetrating polymer networks-nanogels | Rosuvastatin calcium, RST (statins) | (EPR) effect | New Zealand White Rabbit with high blood fat (1400–1500 g) | Oral administration | Improved stability; solubilization | Promote absorption; dissolve cholesterol | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Xu, Y.; Pu, S.; Guo, X.; Zhao, D.; Liu, Y.; Yang, Y.; Wang, C. Cyclodextrin: Dual Functions as a Therapeutic Agent and Nanocarrier for Regulating Cholesterol Homeostasis in Atherosclerosis. Pharmaceutics 2025, 17, 1496. https://doi.org/10.3390/pharmaceutics17111496
Cui H, Xu Y, Pu S, Guo X, Zhao D, Liu Y, Yang Y, Wang C. Cyclodextrin: Dual Functions as a Therapeutic Agent and Nanocarrier for Regulating Cholesterol Homeostasis in Atherosclerosis. Pharmaceutics. 2025; 17(11):1496. https://doi.org/10.3390/pharmaceutics17111496
Chicago/Turabian StyleCui, Hao, Yaqi Xu, Shulin Pu, Xue Guo, Danyu Zhao, Yuan Liu, Ye Yang, and Chengxiao Wang. 2025. "Cyclodextrin: Dual Functions as a Therapeutic Agent and Nanocarrier for Regulating Cholesterol Homeostasis in Atherosclerosis" Pharmaceutics 17, no. 11: 1496. https://doi.org/10.3390/pharmaceutics17111496
APA StyleCui, H., Xu, Y., Pu, S., Guo, X., Zhao, D., Liu, Y., Yang, Y., & Wang, C. (2025). Cyclodextrin: Dual Functions as a Therapeutic Agent and Nanocarrier for Regulating Cholesterol Homeostasis in Atherosclerosis. Pharmaceutics, 17(11), 1496. https://doi.org/10.3390/pharmaceutics17111496







