Chitosan-Coated Liposomes for Intranasal Delivery of Ghrelin: Enhancing Bioavailability to the Central Nervous System
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Chitosan-Coated Liposomes
2.2.1. Preparation of Liposomes
2.2.2. Chitosan Coating
2.3. Physicochemical Characterization of Liposomes
2.3.1. Morphology
2.3.2. Size, Size Distribution, Zeta Potential, and pH
2.3.3. Determination of Encapsulation Efficiency
2.4. Stability Studies
2.5. In Vitro Cell Viability
2.5.1. Cell Culture
2.5.2. Cell Treatment
2.5.3. Cell Viability Assay
2.6. Ex Vivo Mucoadhesion and Permeation
2.6.1. Preparation of Porcine Nasal Mucosal Membrane
2.6.2. Mucoadhesion Assessment
2.6.3. Transmucosal Permeation
2.7. In Vivo Brain Biodistribution Study
2.7.1. Animal Care and Handling
2.7.2. Preparation of Brain Homogenate
2.7.3. Ghrelin Determination in Brain Homogenates by HPLC-UV
2.7.4. Intranasal Administration for Brain Bioavailability
3. Results and Discussion
3.1. Physicochemical Parameters of the Formulations
3.1.1. Morphological Analysis
3.1.2. Analysis of Size, Distribution, and Zeta Potential
3.1.3. Encapsulation Efficiency
3.2. Formulations Stability
3.3. Cell Viability Assessment
3.4. Ex Vivo Mucoadhesion and Permeation Data
3.4.1. Mucoadhesion Properties
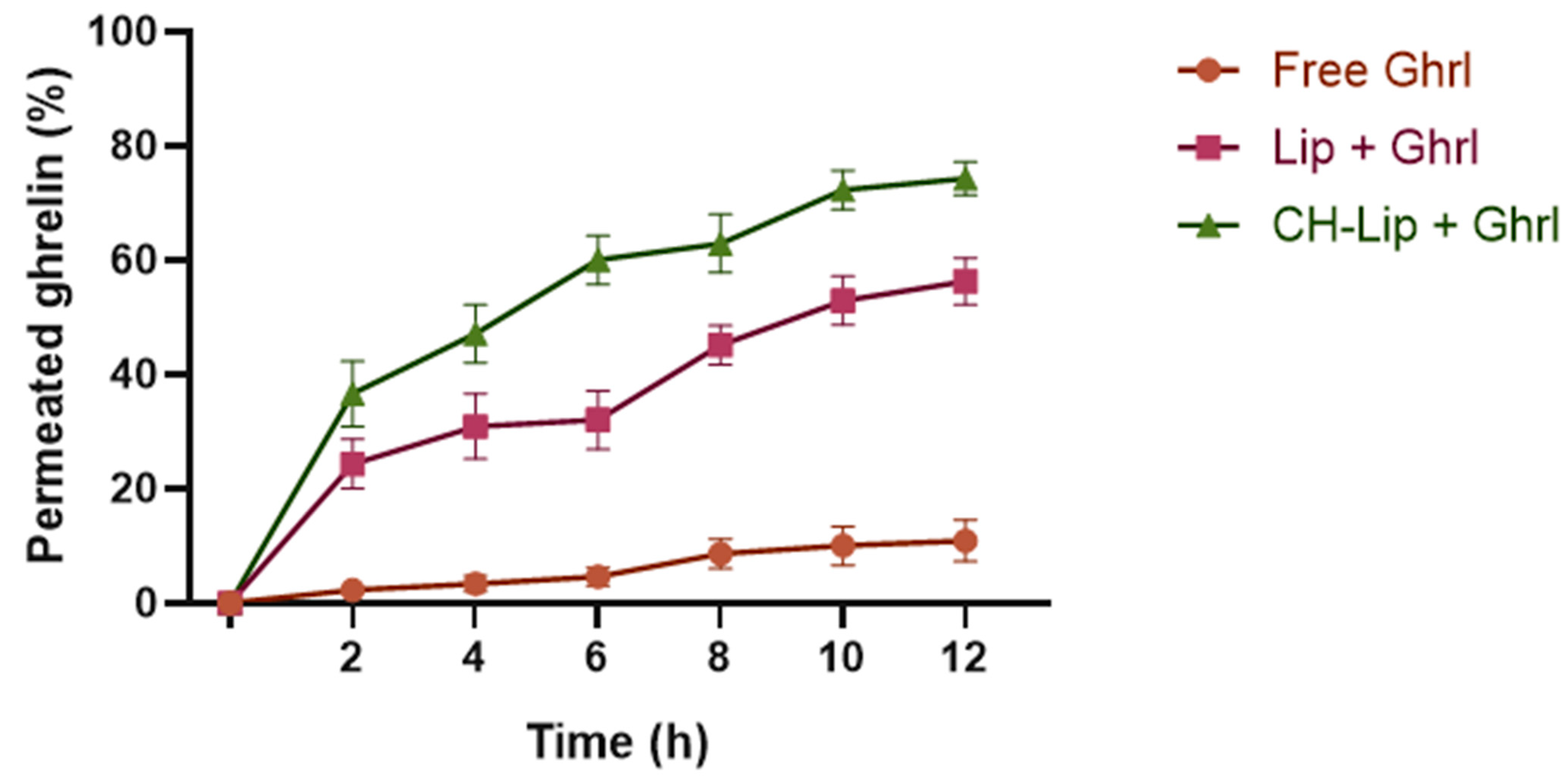
3.4.2. Transmucosal Permeation Profile
3.5. Brain Biodistribution Findings
3.5.1. Ghrelin Quantification in Brain Homogenate
3.5.2. Brain Bioavailability After Intranasal Administration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muscaritoli, M.; Anker, S.D.; Argilés, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus Definition of Sarcopenia, Cachexia and Pre-Cachexia: Joint Document Elaborated by Special Interest Groups (SIG) “ Cachexia-Anorexia in Chronic Wasting Diseases” and “ Nutrition in Geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Anthony, T.G.; Ayres, J.S.; Biffi, G.; Brown, J.C.; Caan, B.J.; Cespedes Feliciano, E.M.; Coll, A.P.; Dunne, R.F.; Goncalves, M.D.; et al. Cachexia: A Systemic Consequence of Progressive, Unresolved Disease. Cell 2023, 186, 1824–1845. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Sakuma, K. Pathophysiology of Cachexia and Characteristics of Dysphagia in Chronic Diseases. Asia Pac. J. Oncol. Nurs. 2022, 9, 100120. [Google Scholar] [CrossRef]
- Rausch, V.; Sala, V.; Penna, F.; Porporato, P.E.; Ghigo, A. Understanding the Common Mechanisms of Heart and Skeletal Muscle Wasting in Cancer Cachexia. Oncogenesis 2021, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Dudhat, K.; Vanpariya, M.; Sah, R.K. Cachexia: Unraveling Its Complex Pathophysiology and Novel Therapeutic Approaches. Curr. Aging Sci. 2025, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Szlachcic, A.; Ptak-Belowska, A.; Brzozowski, T. Physical Activity, Exerkines, and Their Role in Cancer Cachexia. Int. J. Mol. Sci. 2025, 26, 8011. [Google Scholar] [CrossRef]
- Blum, D.; de Wolf-Linder, S.; Oberholzer, R.; Brändle, M.; Hundsberger, T.; Strasser, F. Natural Ghrelin in Advanced Cancer Patients with Cachexia, a Case Series. J. Cachexia Sarcopenia Muscle 2021, 12, 506–516, Erratum in J. Cachexia Sarcopenia Muscle 2022, 13, 2261. https://doi.org/10.1002/jcsm.12884. [Google Scholar] [CrossRef]
- Kerr, H.L.; Krumm, K.; Lee, I.; Anderson, B.; Christiani, A.; Strait, L.; Breckheimer, B.A.; Irwin, B.; Jiang, A.; Rybachok, A.; et al. EXT418, a Novel Long-Acting Ghrelin, Mitigates Lewis Lung Carcinoma Induced Cachexia in Mice. J. Cachexia Sarcopenia Muscle 2023, 14, 1337–1348. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, P.; Zhao, L.; Chen, S. Acylated and Unacylated Ghrelin Relieve Cancer Cachexia in Mice through Multiple Mechanisms. Chin. J. Physiol. 2020, 63, 195–203. [Google Scholar] [CrossRef]
- Jiao, Z.T.; Luo, Q. Molecular Mechanisms and Health Benefits of Ghrelin: A Narrative Review. Nutrients 2022, 14, 4191. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.S.; Yennurajalingam, S. Prokinetics and Ghrelin for the Management of Cancer Cachexia Syndrome. Ann. Palliat. Med. 2019, 8, 80–85. [Google Scholar] [CrossRef]
- Julien, M.; Kay, R.G.; Delhanty, P.J.D.; Allas, S.; Granata, R.; Barton, C.; Constable, S.; Ghigo, E.; Van Der Lely, A.J.; Abribat, T. In Vitro and in Vivo Stability and Pharmacokinetic Profile of Unacylated Ghrelin (UAG) Analogues. Eur. J. Pharm. Sci. 2012, 47, 625–635. [Google Scholar] [CrossRef]
- Rosenfeld, R.G.; Bakker, B. Compliance and Persistence in Pediatric and Adult Patients Receiving Growth Hormone Therapy. Endocr. Pract. 2008, 14, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Alabsi, W.; Eedara, B.B.; Encinas-Basurto, D.; Polt, R.; Mansour, H.M. Nose-to-Brain Delivery of Therapeutic Peptides as Nasal Aerosols. Pharmaceutics 2022, 14, 1870. [Google Scholar] [CrossRef]
- Bahadur, S.; Pathak, K. Physicochemical and Physiological Considerations for Efficient Nose-to-Brain Targeting. Expert Opin. Drug Deliv. 2012, 9, 19–31. [Google Scholar] [CrossRef]
- Salade, L.; Wauthoz, N.; Vermeersch, M.; Amighi, K.; Goole, J. Chitosan-Coated Liposome Dry-Powder Formulations Loaded with Ghrelin for Nose-to-Brain Delivery. Eur. J. Pharm. Biopharm. 2018, 129, 257–266, Erratum in Eur. J. Pharm. Biopharm. 2018, 131, 151. https://doi.org/10.1016/j.ejpb.2018.08.005. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, X.; Yu, S.; Gong, G.; Shu, H. Research Progress in Brain-Targeted Nasal Drug Delivery. Front. Aging Neurosci. 2024, 15, 1341295. [Google Scholar] [CrossRef]
- Bangham, A.D. Properties and Uses of Lipid Vesicles: An Overview. Ann. N. Y. Acad. Sci. 1978, 308, 2–7. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Östh, K.; Grasjö, J.; Björk, E. A New Method for Drug Transport Studies on Pig Nasal Mucosa Using a Horizontal Ussing Chamber. J. Pharm. Sci. 2002, 91, 1259–1273. [Google Scholar] [CrossRef] [PubMed]
- Wadell, C.; Björk, E.; Camber, O. Nasal Drug Delivery—Evaluation of an in Vitro Model Using Porcine Nasal Mucosa. Eur. J. Pharm. Sci. 1999, 7, 197–206. [Google Scholar] [CrossRef]
- Staes, E.; Rozet, E.; Učakar, B.; Hubert, P.; Préat, V. Validation of a Method for the Quantitation of Ghrelin and Unacylated Ghrelin by HPLC. J. Pharm. Biomed. Anal. 2010, 51, 633–639. [Google Scholar] [CrossRef]
- Yanagihara, S.; Kitayama, Y.; Yuba, E.; Harada, A. Preparing Size-Controlled Liposomes Modified with Polysaccharide Derivatives for PH-Responsive Drug Delivery Applications. Life 2023, 13, 2158. [Google Scholar] [CrossRef] [PubMed]
- Doskocz, J.; Dałek, P.; Przybyło, M.; Trzebicka, B.; Foryś, A.; Kobyliukh, A.; Iglič, A.; Langner, M. The Elucidation of the Molecular Mechanism of the Extrusion Process. Materials 2021, 14, 4278. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, M.; Liu, Z.; Wang, Y.; Chen, L.; Guo, J.; Zhang, W.; Zhang, Y.; Yu, C.; Bie, T.; et al. Transnasal-Brain Delivery of Nanomedicines for Neurodegenerative Diseases. Front. Drug Deliv. 2023, 3, 1247162. [Google Scholar] [CrossRef]
- Eugster, R.; Luciani, P. Liposomes: Bridging the Gap from Lab to Pharmaceuticals. Curr. Opin. Colloid Interface Sci. 2025, 75, 101875. [Google Scholar] [CrossRef]
- Moslehi, M.; Ali Reza Mortazavi, S.; Azadi, A.; Fateh, S.; Hamidi, M.; Mohsen Foroutan, S. Preparation, Optimization and Characterization of Chitosan-Coated Liposomes for Solubility Enhancement of Furosemide: A Model BCS IV Drug. Iran. J. Pharm. Res. 2020, 19, 366. [Google Scholar] [CrossRef]
- Filippov, S.K.; Khusnutdinov, R.; Murmiliuk, A.; Inam, W.; Zakharova, L.Y.; Zhang, H.; Khutoryanskiy, V.V. Dynamic Light Scattering and Transmission Electron Microscopy in Drug Delivery: A Roadmap for Correct Characterization of Nanoparticles and Interpretation of Results. Mater. Horiz. 2023, 10, 5354–5370. [Google Scholar] [CrossRef] [PubMed]
- Németh, Z.; Csóka, I.; Semnani Jazani, R.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef] [PubMed]
- Salade, L.; Wauthoz, N.; Deleu, M.; Vermeersch, M.; De Vriese, C.; Amighi, K.; Goole, J. Development of Coated Liposomes Loaded with Ghrelin for Nose-to-Brain Delivery for the Treatment of Cachexia. Int. J. Nanomed. 2017, 12, 8531–8543. [Google Scholar] [CrossRef]
- Vieira, D.B.; Gamarra, L.F. Getting into the Brain: Liposome-Based Strategies for Effective Drug Delivery across the Blood–Brain Barrier. Int. J. Nanomed. 2016, 11, 5381–5414. [Google Scholar] [CrossRef]
- Shirnoush, N.; Emamifar, A.; Davati, N. Preparation and Characteristics Evaluation of Chitosan-Coated Nanoliposomes Containing Ferrous Sulfate. Sci. Rep. 2025, 15, 19161. [Google Scholar] [CrossRef]
- Tan, F.; Li, H.; Zhang, K.; Xu, L.; Zhang, D.; Han, Y.; Han, J. Sodium Alginate/Chitosan-Coated Liposomes for Oral Delivery of Hydroxy-α-Sanshool: In Vitro and In Vivo Evaluation. Pharmaceutics 2023, 15, 2010. [Google Scholar] [CrossRef]
- Laye, C.; McClements, D.J.; Weiss, J. Formation of Biopolymer-Coated Liposomes by Electrostatic Deposition of Chitosan. J. Food Sci. 2008, 73, N7–N15. [Google Scholar] [CrossRef]
- Keller, L.A.; Merkel, O.; Popp, A. Intranasal Drug Delivery: Opportunities and Toxicologic Challenges during Drug Development. Drug Deliv. Transl. Res. 2022, 12, 735–757. [Google Scholar] [CrossRef]
- Kang, B.R.; Park, J.S.; Ryu, G.R.; Jung, W.J.; Choi, J.S.; Shin, H.M. Effect of Chitosan Coating for Efficient Encapsulation and Improved Stability under Loading Preparation and Storage Conditions of Bacillus Lipopeptides. Nanomaterials 2022, 12, 4189. [Google Scholar] [CrossRef] [PubMed]
- Frigaard, J.; Liaaen Jensen, J.; Kanli Galtung, H.; Hiorth, M. Stability and Cytotoxicity of Biopolymer-Coated Liposomes for Use in the Oral Cavity. Int. J. Pharm. 2023, 645, 123407. [Google Scholar] [CrossRef]
- Syama, K.; Jakubek, Z.J.; Chen, S.; Zaifman, J.; Tam, Y.Y.C.; Zou, S. Development of Lipid Nanoparticles and Liposomes Reference Materials (II): Cytotoxic Profiles. Sci. Rep. 2022, 12, 18071. [Google Scholar] [CrossRef] [PubMed]
- Schwendener, R.A. Liposomes in Biology and Medicine. Adv. Exp. Med. Biol. 2007, 620, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.T.S.; Sheikh, Z.; Fathi, A.; Maleknia, S.; Oveissi, F.; Abrams, T.; Knox, W.; Casettari, L.; Tiboni, M.; Suman, J.; et al. Exploring Intranasal Delivery of Peptide and Protein Nanoparticles by a Thermoresponsive Hydrogel. J. Drug Deliv. Sci. Technol. 2025, 110, 107070. [Google Scholar] [CrossRef]
- Gil-Gonzalo, R.; Durante-Salmerón, D.A.; Pouri, S.; Doncel-Pérez, E.; Alcántara, A.R.; Aranaz, I.; Acosta, N. Chitosan-Coated Liposome Formulations for Encapsulation of Ciprofloxacin and Etoposide. Pharmaceutics 2024, 16, 1036. [Google Scholar] [CrossRef]
- Rogatsky, E.; Stein, D. Evaluation of Matrix Effect and Chromatography Efficiency: New Parameters for Validation of Method Development. J. Am. Soc. Mass Spectrom. 2005, 16, 1757–1759. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Chu, L.; Jiao, H.; Lin, L.; Liu, Y.; Chen, G.; Zou, L.; Wang, X.; Di, X. A Highly Sensitive and Rapid LC-MS/MS Method for Quantification of Bexarotene in Mouse Plasma and Brain Tissue: Application to Mice Pharmacokinetic Study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1189, 123025. [Google Scholar] [CrossRef]
- Poelman, R.; Le May, M.V.; Schéle, E.; Stoltenborg, I.; Dickson, S.L. Intranasal Delivery of a Ghrelin Mimetic Engages the Brain Ghrelin Signaling System in Mice. Endocrinology 2025, 166, bqae166. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Utami, R.N.; Qin, Y.; Liam-Or, R.; Sreedharan, J.; Davies, J.S.; Al-Jamal, K.T. Nose-to-Brain Delivery of Acyl-Ghrelin Peptide Gold Nanoconjugates for Treatment of Neurodegenerative Diseases. Small 2025, 21, e04517. [Google Scholar] [CrossRef]
- Qiu, Y.; Huang, S.; Peng, L.; Yang, L.; Zhang, G.; Liu, T.; Yan, F.; Peng, X. The Nasal–Brain Drug Delivery Route: Mechanisms and Applications to Central Nervous System Diseases. MedComm 2025, 6, e70213. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, C.; Huang, Y.; Ma, Y.; Song, Q.; Chen, H.; Jiang, G.; Gao, X. Intranasal Drug Delivery: The Interaction between Nanoparticles and the Nose-to-Brain Pathway. Adv. Drug Deliv. Rev. 2024, 207, 115196. [Google Scholar] [CrossRef]








| Experimental Group | Ghrelin Concentration (μg/mL) | Phosphatidylcholine Concentration (μg/mL) | Paraformaldehyde Concentration (mg/mL) |
|---|---|---|---|
| Viable Control | — | — | — |
| CH-Lip + Ghrl 0.5% | 0.35 | 50 | — |
| CH-Lip 0.5% | — | 50 | — |
| CH-Lip + Ghrl 1% | 0.7 | 100 | — |
| CH-Lip 1% | — | 100 | — |
| CH-Lip + Ghrl 2% | 1.4 | 200 | — |
| CH-Lip 2% | — | 200 | — |
| CH-Lip + Ghrl 3.5% | 2.45 | 350 | — |
| CH-Lip 3.5% | — | 350 | — |
| CH-Lip + Ghrl 5% | 3.5 | 500 | — |
| CH-Lip 5% | — | 500 | — |
| Non-viable Control | — | — | 40 |
| Parameters | Conditions |
|---|---|
| Equipment | Shimadzu Kyoto, Japan HPLC with UV/VIS detector (SPD-M 10A VP), injector SIL-10AD VP |
| Column | Purospher® (MerckMillipore, Darmstadt, Germany). STAR RP-18 endcapped (250 × 4.6 mm, 5 µm) |
| Column Temperature | 37 °C |
| Flow Rate | 0.95 mL/min |
| Injection Volume | 83 µL |
| Mobile Phase | A: water + 0.1% trifluoroacetic acid (TFA); B: acetonitrile + 0.1% TFA |
| Elution Gradient | 0 min: 88% A/12% B → 56.14 min: 48% A/52% B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Barros, C.T.; Alves, T.F.R.; Crescencio, K.M.M.; Asami, J.; Hausen, M.d.A.; Duek, E.A.d.R.; Chaud, M.V. Chitosan-Coated Liposomes for Intranasal Delivery of Ghrelin: Enhancing Bioavailability to the Central Nervous System. Pharmaceutics 2025, 17, 1493. https://doi.org/10.3390/pharmaceutics17111493
de Barros CT, Alves TFR, Crescencio KMM, Asami J, Hausen MdA, Duek EAdR, Chaud MV. Chitosan-Coated Liposomes for Intranasal Delivery of Ghrelin: Enhancing Bioavailability to the Central Nervous System. Pharmaceutics. 2025; 17(11):1493. https://doi.org/10.3390/pharmaceutics17111493
Chicago/Turabian Stylede Barros, Cecilia T., Thais F. R. Alves, Kessi M. M. Crescencio, Jessica Asami, Moema de A. Hausen, Eliana A. de R. Duek, and Marco V. Chaud. 2025. "Chitosan-Coated Liposomes for Intranasal Delivery of Ghrelin: Enhancing Bioavailability to the Central Nervous System" Pharmaceutics 17, no. 11: 1493. https://doi.org/10.3390/pharmaceutics17111493
APA Stylede Barros, C. T., Alves, T. F. R., Crescencio, K. M. M., Asami, J., Hausen, M. d. A., Duek, E. A. d. R., & Chaud, M. V. (2025). Chitosan-Coated Liposomes for Intranasal Delivery of Ghrelin: Enhancing Bioavailability to the Central Nervous System. Pharmaceutics, 17(11), 1493. https://doi.org/10.3390/pharmaceutics17111493












