The Convergence of Polymer Science and Predictive Modeling for Noninvasive Glucose Monitoring
Abstract
1. Introduction
2. The Technological Evolution of Glucose Monitoring
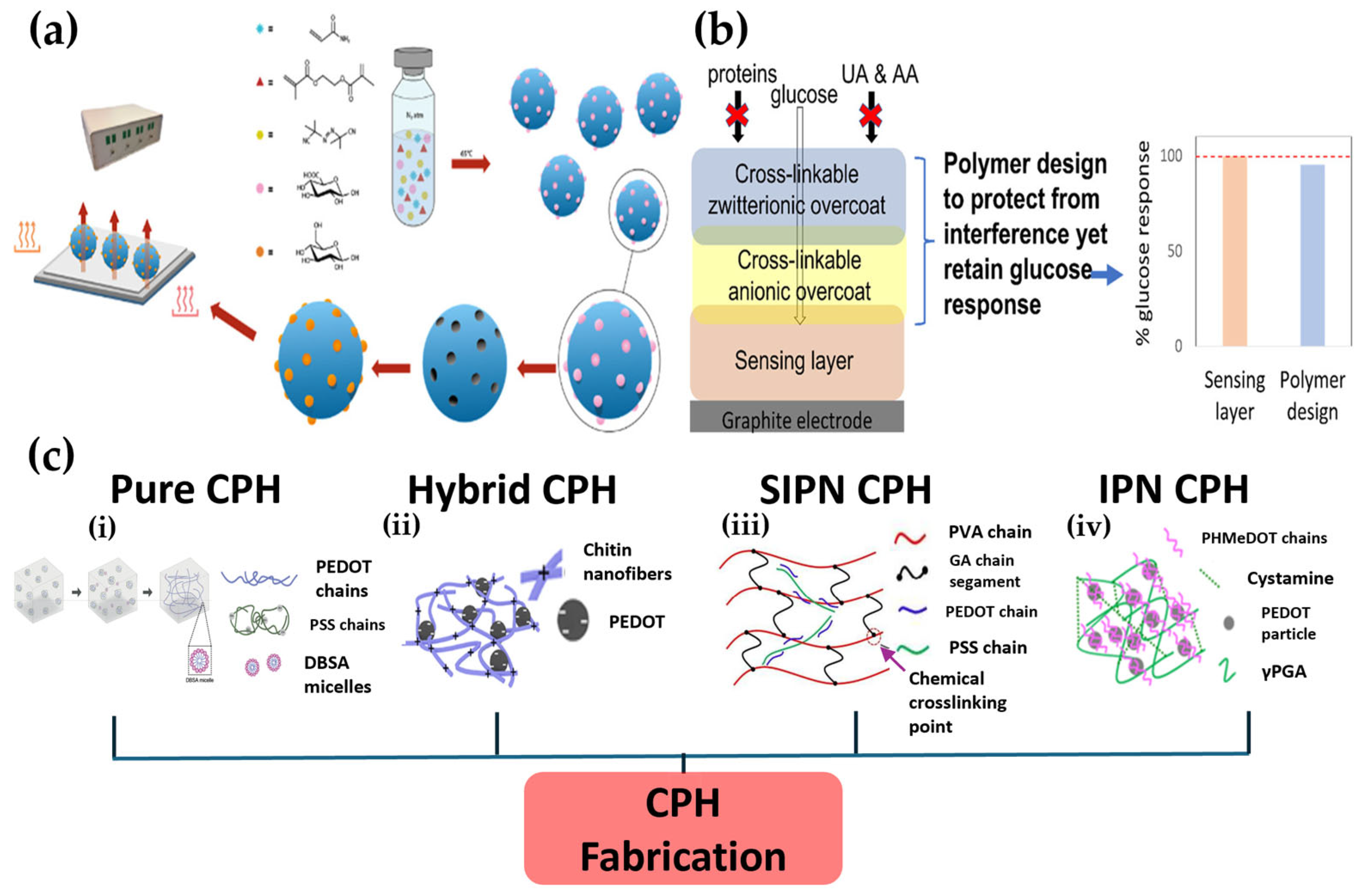
3. Advanced Polymer Platforms for Noninvasive Sensors
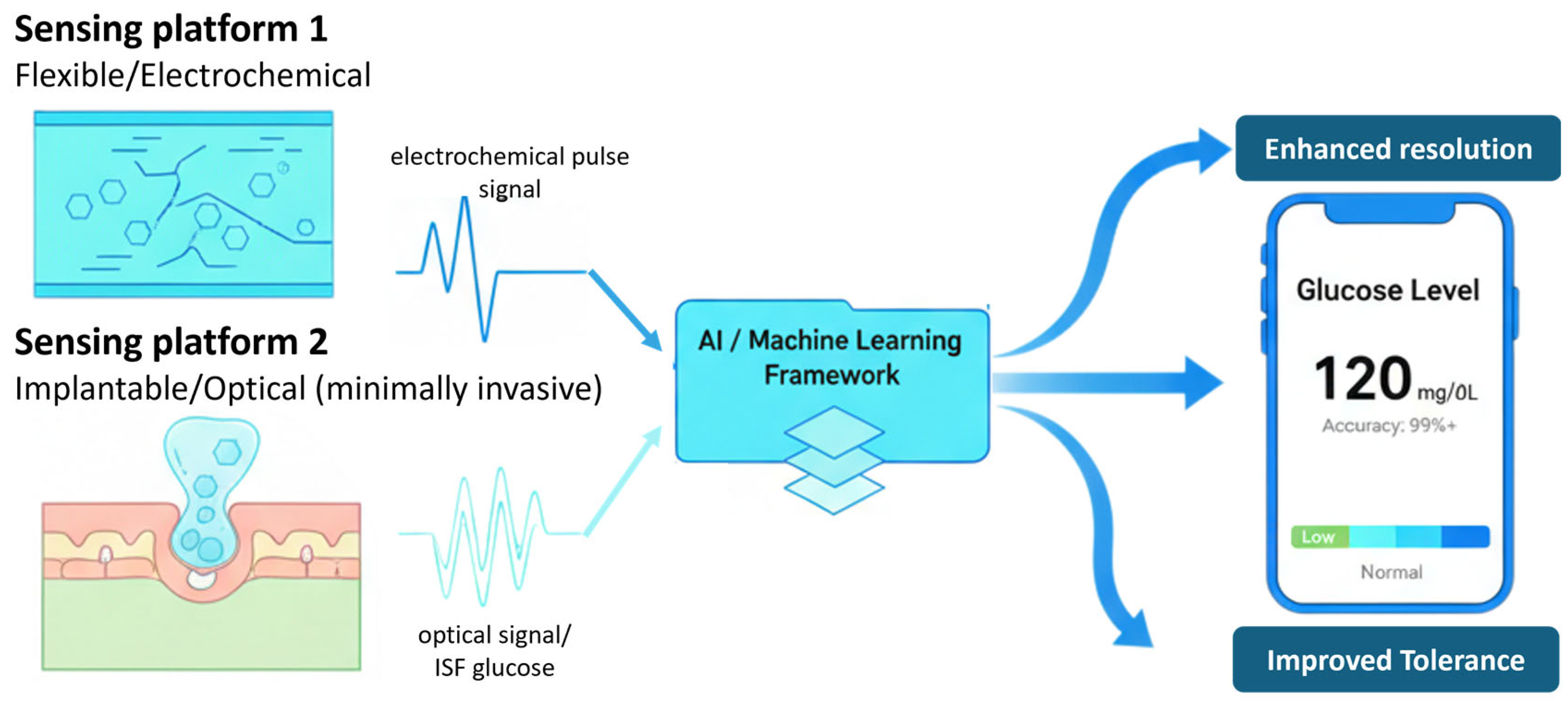
4. AI for Signal Processing and Prediction
5. Representative Recent Studies of Polymer–AI Integrated Glucose Monitoring
6. Challenges for Clinical Translation
7. Limitations and Regulatory Considerations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SMBG | Self-monitoring of blood glucose |
| CGM | Continuous glucose monitoring |
| ISF | Interstitial fluid |
| MIP | Molecularly imprinted polymer |
| AI | Artificial intelligence |
| WEBS | Wearable and implantable electrochemical biosensor |
| CPH | Conductive polymer hydrogel |
| AA | Ascorbic acid |
| UA | Uric acid |
| GOx | Glucose oxidase |
| RF | Random forest |
| SVR | Support vector regression |
| RNN | Recurrent neural network |
| LSTM | Long short-term memory |
| CNN | Convolutional neural network |
| PLI | Phosphorescence lifetime imager |
| PEGDA | Polyethylene glycol diacrylate |
| SNR | Signal-to-noise ratio |
| SWIR | Short-wave infrared |
| DPV | Differential pulse voltammetry |
| CEG | Clarke error grid |
| RMSE | Root mean square error |
| MAE | Mean absolute error |
| XGBoost | eXtreme gradient boosting |
| GDPR | General Data Protection Regulation |
| HIPAA | Health Insurance Portability and Accountability Act |
| XAI | Explainable artificial intelligence |
References
- Jeon, H.-J.; Kim, H.S.; Chung, E.; Lee, D.Y. Nanozyme-based colorimetric biosensor with a systemic quantification algorithm for noninvasive glucose monitoring. Theranostics 2022, 12, 6308. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-J.; Kim, S.; Park, S.; Jeong, I.-K.; Kang, J.; Kim, Y.R.; Lee, D.Y.; Chung, E. Optical assessment of tear glucose by smart biosensor based on nanoparticle embedded contact lens. Nano Lett. 2021, 21, 8933–8940. [Google Scholar] [CrossRef]
- Bloomgarden, Z.T. Cardiovascular disease, neuropathy, and retinopathy. Diabetes Care 2009, 32, e64–e68. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.S.; Khan, M.S.; Butler, J. The interplay between diabetes, cardiovascular disease, and kidney disease. ADA Clin. Compend. 2021, 2021, 13–18. [Google Scholar] [CrossRef]
- Caldara, M.; Lowdon, J.W.; Rogosic, R.; Arreguin-Campos, R.; Jimenez-Monroy, K.L.; Heidt, B.; Tschulik, K.; Cleij, T.J.; Diliën, H.; Eersels, K. Thermal detection of glucose in urine using a molecularly imprinted polymer as a recognition element. ACS Sens. 2021, 6, 4515–4525. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Chen, C. The Development, Characteristics, and Challenges of Biosensors: The Example of Blood Glucose Meters. Chemosensors 2025, 13, 300. [Google Scholar] [CrossRef]
- Kim, S.; Jeon, H.-J.; Park, S.; Lee, D.Y.; Chung, E. Tear glucose measurement by reflectance spectrum of a nanoparticle embedded contact lens. Sci. Rep. 2020, 10, 8254. [Google Scholar] [CrossRef]
- Park, S.; Nam, D.Y.; Jeon, H.-J.; Han, J.H.; Jang, D.; Hwang, J.; Park, Y.-S.; Han, Y.-G.; Choy, Y.B.; Lee, D.Y. Chromophoric cerium oxide nanoparticle-loaded sucking disk-type strip sensor for optical measurement of glucose in tear fluid. Biomater. Res. 2023, 27, 135. [Google Scholar] [CrossRef]
- Sankhala, D.; Sardesai, A.U.; Pali, M.; Lin, K.-C.; Jagannath, B.; Muthukumar, S.; Prasad, S. A machine learning-based on-demand sweat glucose reporting platform. Sci. Rep. 2022, 12, 2442. [Google Scholar] [CrossRef]
- Shi, J.; Fernández-García, R.; Gil, I. Sensor technologies for non-invasive blood glucose monitoring. Sensors 2025, 25, 3591. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.M.C.; Forlenza, G.P.; Prioleau, T.O.; Zhou, X. Noninvasive glucose sensing in vivo. Sensors 2023, 23, 7057. [Google Scholar] [CrossRef]
- Chan, P.Z.; Jin, E.; Jansson, M.; Chew, H.S.J. AI-based noninvasive blood glucose monitoring: Scoping review. J. Med. Internet Res. 2024, 26, e58892. [Google Scholar] [CrossRef] [PubMed]
- Dey, K.; Santra, T.S.; Tseng, F.G. Advancements in Glucose Monitoring: From Traditional Methods to Wearable Sensors. Appl. Sci. 2025, 15, 2523. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, X.; Chen, W.; Chen, Q.; Kong, L.; Li, P. A systematic review of Continuous Glucose Monitoring Sensors: Principles, Core Technologies and Performance Evaluation. Sens. Actuators Rep. 2025, 10, 100361, Erratum in Sens. Actuators Rep. 2025, 10, 100381. https://doi.org/10.1016/j.snr.2025.100381. [Google Scholar] [CrossRef]
- Morales-Dopico, L.; MacLeish, S.A. Expanding the horizon of continuous glucose monitoring into the future of pediatric medicine. Pediatr. Res. 2024, 96, 1464–1474. [Google Scholar] [CrossRef]
- Mistry, S.; Tonyushkina, K.N.; Benavides, V.C.; Choudhary, A.; Huerta-Saenz, L.; Patel, N.S.; Mahmud, F.H.; Libman, I.; Sperling, M.A. A centennial review of discoveries and advances in diabetes: Children and youth. Pediatr. Diabetes 2022, 23, 926–943. [Google Scholar] [CrossRef]
- Montagnana, M.; Caputo, M.; Giavarina, D.; Lippi, G. Overview on self-monitoring of blood glucose. Clin. Chim. Acta 2009, 402, 7–13. [Google Scholar] [CrossRef]
- Clarke, S.; Foster, J. A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br. J. Biomed. Sci. 2012, 69, 83–93. [Google Scholar] [CrossRef]
- Fiedorova, K.; Augustynek, M.; Kubicek, J.; Kudrna, P.; Bibbo, D. Review of present method of glucose from human blood and body fluids assessment. Biosens. Bioelectron. 2022, 211, 114348. [Google Scholar] [CrossRef]
- Ciemins, E.; Coon, P.; Sorli, C. An analysis of data management tools for diabetes self-management: Can smart phone technology keep up? J. Diabetes Sci. Technol. 2010, 4, 958–960. [Google Scholar] [CrossRef]
- Böhm, A.-K.; Jensen, M.L.; Sørensen, M.R.; Stargardt, T. Real-world evidence of user engagement with mobile health for diabetes management: Longitudinal observational study. JMIR MHealth uHealth 2020, 8, e22212. [Google Scholar] [CrossRef]
- Kulzer, B.; Freckmann, G.; Ziegler, R.; Schnell, O.; Glatzer, T.; Heinemann, L. Nocturnal hypoglycemia in the era of continuous glucose monitoring. J. Diabetes Sci. Technol. 2024, 18, 1052–1060. [Google Scholar] [CrossRef]
- Lin, M.; Chen, T.; Fan, G. Current status and influential factors associated with adherence to self-monitoring of blood glucose with type 2 diabetes mellitus patients in grassroots communities: A cross-sectional survey based on information-motivation-behavior skills model in China. Front. Endocrinol. 2023, 14, 1111565. [Google Scholar] [CrossRef] [PubMed]
- Ang, E.; Lee, Z.X.; Moore, S.; Nana, M. Flash glucose monitoring (FGM): A clinical review on glycaemic outcomes and impact on quality of life. J. Diabetes Complicat. 2020, 34, 107559. [Google Scholar] [CrossRef] [PubMed]
- Ajjan, R.A.; Battelino, T.; Cos, X.; Del Prato, S.; Philips, J.-C.; Meyer, L.; Seufert, J.; Seidu, S. Continuous glucose monitoring for the routine care of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2024, 20, 426–440. [Google Scholar] [CrossRef]
- Acciaroli, G.; Vettoretti, M.; Facchinetti, A.; Sparacino, G. Calibration of minimally invasive continuous glucose monitoring sensors: State-of-the-art and current perspectives. Biosensors 2018, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Matuleviciene, V.; Joseph, J.I.; Andelin, M.; Hirsch, I.B.; Attvall, S.; Pivodic, A.; Dahlqvist, S.; Klonoff, D.; Haraldsson, B.; Lind, M. A clinical trial of the accuracy and treatment experience of the Dexcom G4 sensor (Dexcom G4 system) and Enlite sensor (guardian REAL-time system) tested simultaneously in ambulatory patients with type 1 diabetes. Diabetes Technol. Ther. 2014, 16, 759–767. [Google Scholar] [CrossRef]
- Bailey, T.; Bode, B.W.; Christiansen, M.P.; Klaff, L.J.; Alva, S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol. Ther. 2015, 17, 787–794. [Google Scholar] [CrossRef]
- Domingo-Lopez, D.A.; Lattanzi, G.; Schreiber, L.H.; Wallace, E.J.; Wylie, R.; O’Sullivan, J.; Dolan, E.B.; Duffy, G.P. Medical devices, smart drug delivery, wearables and technology for the treatment of Diabetes Mellitus. Adv. Drug Deliv. Rev. 2022, 185, 114280. [Google Scholar] [CrossRef]
- Bai, J.; Liu, D.; Tian, X.; Wang, Y.; Cui, B.; Yang, Y.; Dai, S.; Lin, W.; Zhu, J.; Wang, J. Coin-sized, fully integrated, and minimally invasive continuous glucose monitoring system based on organic electrochemical transistors. Sci. Adv. 2024, 10, eadl1856. [Google Scholar] [CrossRef]
- Ben-Aissa, S.; Tripathy, S.; Cass, A.E.G. Implantable Electrochemical Biosensors: Challenges, Strategies, and Applications. Curr. Opin. Electrochem. 2025, 54, 101745. [Google Scholar] [CrossRef]
- Pullano, S.A.; Greco, M.; Bianco, M.G.; Foti, D.; Brunetti, A.; Fiorillo, A.S. Glucose biosensors in clinical practice: Principles, limits and perspectives of currently used devices. Theranostics 2022, 12, 493. [Google Scholar] [CrossRef]
- Lielpetere, A.; Jayakumar, K.; Leech, D.; Schuhmann, W. Cross-linkable polymer-based multi-layers for protecting electrochemical glucose biosensors against uric acid, ascorbic acid, and biofouling interferences. ACS Sens. 2023, 8, 1756–1765. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, J.; Paulo-Mirasol, S.; Estrany, F.; Torras, J. Recent progress in biomedical sensors based on conducting polymer hydrogels. ACS Appl. Bio Mater. 2023, 6, 1720–1741. [Google Scholar] [CrossRef] [PubMed]
- Seong, H.; Lee, H.-B.; Park, K. Glucose binding to molecularly imprinted polymers. J. Biomater. Sci. Polym. Ed. 2002, 13, 637–649. [Google Scholar] [CrossRef][Green Version]
- Thirumalai, D.; Santhamoorthy, M.; Kim, S.-C.; Lim, H.-R. Conductive polymer-based hydrogels for wearable electrochemical biosensors. Gels 2024, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y. Review of Noninvasive Continuous Glucose Monitoring in Diabetics. ACS Sens. 2023, 8, 3659–3679. [Google Scholar] [CrossRef]
- Park, S.; Gil, M.-S.; Im, H.; Moon, Y.-S. Measurement noise recommendation for efficient Kalman filtering over a large amount of sensor data. Sensors 2019, 19, 1168. [Google Scholar] [CrossRef]
- Hoss, U.; Budiman, E.S. Factory-calibrated continuous glucose sensors: The science behind the technology. Diabetes Technol. Ther. 2017, 19, S-44–S-50. [Google Scholar] [CrossRef]
- Corcione, E.; Pfezer, D.; Hentschel, M.; Giessen, H.; Tarín, C. Machine learning methods of regression for plasmonic nanoantenna glucose sensing. Sensors 2021, 22, 7. [Google Scholar] [CrossRef]
- Zimmerman, N.; Presto, A.A.; Kumar, S.P.; Gu, J.; Hauryliuk, A.; Robinson, E.S.; Robinson, A.L. A machine learning calibration model using random forests to improve sensor performance for lower-cost air quality monitoring. Atmos. Meas. Tech. 2018, 11, 291–313. [Google Scholar] [CrossRef]
- Liu, K.; Li, L.; Ma, Y.; Jiang, J.; Liu, Z.; Ye, Z.; Liu, S.; Pu, C.; Chen, C.; Wan, Y. Machine learning models for blood glucose level prediction in patients with diabetes mellitus: Systematic review and network meta-analysis. JMIR Med. Inform. 2023, 11, e47833. [Google Scholar] [CrossRef]
- Paucar, I.R.; Yactayo-Arias, C.; Andrade-Arenas, L. Random Forest Model Based on Machine Learning for Early Detection of Diabetes. Int. J. Adv. Comput. Sci. Appl. 2025, 16, 1051. [Google Scholar] [CrossRef]
- Naresh, M.; Nagaraju, V.S.; Kollem, S.; Kumar, J.; Peddakrishna, S. Non-invasive glucose prediction and classification using NIR technology with machine learning. Heliyon 2024, 10, e28720. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, X.; Liao, C. AI-Driven Wearable Bioelectronics in Digital Healthcare. Biosensors 2025, 15, 410. [Google Scholar] [CrossRef]
- Belfarsi, E.A.; Flores, H.; Valero, M. Reliable Noninvasive Glucose Sensing via CNN-Based Spectroscopy. arXiv 2025, arXiv:2506.13819. [Google Scholar] [CrossRef]
- Sadeghi, P.; Noroozizadeh, S.; Alshawabkeh, R.; Sun, N.X. Machine Learning-Driven D-Glucose Prediction Using a Novel Biosensor for Non-Invasive Diabetes Management. Biosensors 2025, 15, 152. [Google Scholar] [CrossRef]
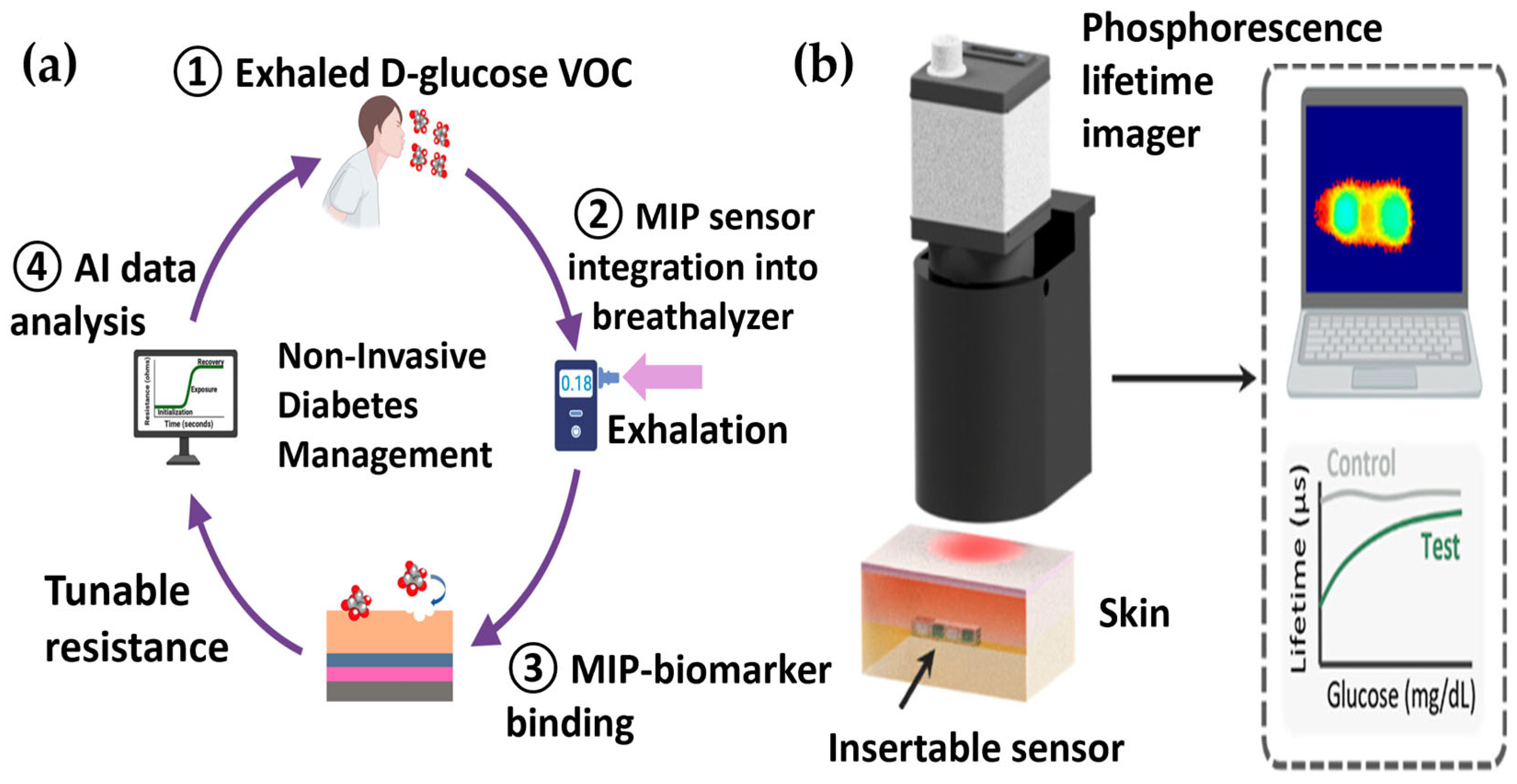
- Goncharov, A.; Gorocs, Z.; Pradhan, R.; Ko, B.; Ajmal, A.; Rodriguez, A.; Baum, D.; Veszpremi, M.; Yang, X.; Pindrys, M. Insertable glucose sensor using a compact and cost-effective phosphorescence lifetime imager and machine learning. ACS Nano 2024, 18, 23365–23379. [Google Scholar] [CrossRef]
- Pal, A.; Biswas, S.; Chaudhury, K.; Das, S. A frugal machine-intelligent paper sensor for quantification of glucose through standalone desktop application: A computational and experimental approach. Chem. Eng. J. 2024, 496, 154138. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Shen, J.; Zhou, Z.; Zhu, G. Research on differential pulse voltammetry detection method for low concentration glucose based on machine learning model. Int. J. Electrochem. Sci. 2024, 19, 100479. [Google Scholar] [CrossRef]
- Sabatini, A.; Cenerini, C.; Vollero, L.; Pau, D. Calibrating glucose sensors at the edge: A stress generation model for tiny ML drift compensation. BioMedInformatics 2024, 4, 1519–1530. [Google Scholar] [CrossRef]
- Caldara, M.; Kulpa, J.; Lowdon, J.W.; Cleij, T.J.; Diliën, H.; Eersels, K.; Grinsven, B.v. Recent advances in molecularly imprinted polymers for glucose monitoring: From fundamental research to commercial application. Chemosensors 2023, 11, 32. [Google Scholar] [CrossRef]
- Johnston, L.; Wang, G.; Hu, K.; Qian, C.; Liu, G. Advances in biosensors for continuous glucose monitoring towards wearables. Front. Bioeng. Biotechnol. 2021, 9, 733810. [Google Scholar] [CrossRef] [PubMed]
- Khor, S.M.; Choi, J.; Won, P.; Ko, S.H. Challenges and strategies in developing an enzymatic wearable sweat glucose biosensor as a practical point-of-care monitoring tool for type II diabetes. Nanomaterials 2022, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Qi, Y.; Wang, W.; Tian, X.; Wang, J.; Xu, L.; Zhai, X. Future horizons in diabetes: Integrating AI and personalized care. Front. Endocrinol. 2025, 16, 1583227. [Google Scholar] [CrossRef]
- Jin, X.; Liu, C.; Xu, T.; Su, L.; Zhang, X. Artificial intelligence biosensors: Challenges and prospects. Biosens. Bioelectron. 2020, 165, 112412. [Google Scholar] [CrossRef]
- Mustafa, Y.L.; Keirouz, A.; Leese, H.S. Molecularly imprinted polymers in diagnostics: Accessing analytes in biofluids. J. Mater. Chem. B 2022, 10, 7418–7449. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Dilien, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K. MIPs for commercial application in low-cost sensors and assays—An overview of the current status quo. Sens. Actuators B Chem. 2020, 325, 128973. [Google Scholar] [CrossRef]
- Rokhmat, L.S.K.; Irkham; Zuliska, S.; Adisyahputra; Ridwan, Y.S.; Fauzia, R.P.; Ramdani, P.R.; Hartati, Y.W. Molecularly imprinted polymer technology for electrochemical detection of diabetes-related biomarkers. Sens. Actuators Rep. 2025, 10, 100353. [Google Scholar] [CrossRef]
- Erdem, A.; Senturk, H.; Karakus, M. Molecularly imprinted polymer-based sensors: Design and advances in the analysis of DNA and protein. Talanta Open 2025, 12, 100507. [Google Scholar] [CrossRef]
- Noor, A.A.; Manzoor, A.; Mazhar Qureshi, M.D.; Qureshi, M.A.; Rashwan, W. Unveiling Explainable AI in Healthcare: Current Trends, Challenges, and Future Directions. WIREs Data Min. Knowl. Discov. 2025, 15, e70018. [Google Scholar] [CrossRef]
- Sadeghi, Z.; Alizadehsani, R.; Cifci, M.A.; Kausar, S.; Rehman, R.; Mahanta, P.; Bora, P.K.; Almasri, A.; Alkhawaldeh, R.S.; Hussain, S.; et al. A review of Explainable Artificial Intelligence in healthcare. Comput. Electr. Eng. 2024, 118, 109370. [Google Scholar] [CrossRef]
- Räz, T.; Pahud De Mortanges, A.; Reyes, M. Explainable AI in medicine: Challenges of integrating XAI into the future clinical routine. Front. Radiol. 2025, 5, 1627169. [Google Scholar] [CrossRef] [PubMed]
- Fraser, H.S.; Celi, L.A.; Cellini, J.; Charpignon, M.-L.; Dee, E.C.; Dernoncourt, F.; Eber, R.; Mitchell, W.G.; Moukheiber, L.; Schirmer, J.; et al. Sources of bias in artificial intelligence that perpetuate healthcare disparities—A global review. PLoS Digit. Health 2022, 1, e0000022. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Good Machine Learning Practice for Medical Device Development: Guiding Principles. Available online: https://www.fda.gov/medical-devices/software-medical-device-samd/good-machine-learning-practice-medical-device-development-guiding-principles (accessed on 14 October 2025).
| Polymer Type | Working Mechanism | Key Advantages | Challenges | Representative Applications | Ref. |
|---|---|---|---|---|---|
| MIPs | Forms nano-cavities complementary to template molecules | High stability, low cost, a synthetic alternative to enzymes | Difficulty in mass production of uniform particles, template bleeding incomplete removal | Noninvasive sensors (urine/breath), food analysis, environmental monitoring | [5] |
| CPHs | Provides a tissue-like electronic interface by combining electrical activity, flexibility | High biocompatibility, Stretchability, Self-healing capability, Tissue-mimicking properties | Limited strain-sensing range, functional degradation due to swelling, signal hysteresis | Wearable and implantable sensors, flexible electronic devices, drug delivery systems | [35] |
| Functional protective coatings | Protects the sensor from interfering substances and biofouling via electrostatic repulsion or the formation of a hydration layer | Enhanced in vivo stability, increased selectivity, extended sensor lifespan | Potential formation of an additional diffusion barrier, issues with the long-term durability of the coating | Implantable CGM sensors, stabilization of in vivo biosensors | [34] |
| AI Model | Main Applications | Input Data Types | Key Performance Metrics | Advantages/Disadvantages | Ref. |
|---|---|---|---|---|---|
| Ensemble methods (e.g., Random forest) | Predictive modeling, calibration, classification | Tabular feature data (e.g., clinical information, sensor readings) | Accuracy, F1-Score, AUC | Robust and resistant to overfitting/ Model interpretation can be complex | [44] |
| Support vector machine (SVM) | Predictive modeling, classification | Feature vectors, sensor signals | Accuracy, sensitivity, precision | Effective in high-dimensional spaces/ Can be computationally intensive for large datasets | [12] |
| Feedforward neural networks (ANN, MLP) | Glucose level estimation, classification | Feature vectors, multi-sensor inputs | Accuracy, RMSE, MAE | Excellent for modeling non-linear relationships/ Requires large datasets and significant hyperparameter tuning | [12] |
| Recurrent neural networks (RNN, LSTM) | Time-series forecasting (e.g., future glucose prediction) | Continuous glucose data, temporal sensor signals | RMSE, MAE | Specialized for learning temporal patterns/ May struggle with capturing long-term dependencies | [48] |
| Convolutional neural networks (CNN) | Noninvasive estimation (analysis of spatial data) | Spectroscopic images, thermal images | MAPE, clarke error grid (CEG) | Highly effective for feature extraction from grid-like data/ Difficult to apply directly to sequential time-series data | [47] |
| Category | Matrix | Performance | Clinical Relevance | Maturity | Limitation | Ref. |
|---|---|---|---|---|---|---|
| Commercial CGM (Dexcom G6/G7, Freestyle Libre 2/3) | ISF | Mard ≈ 9–10% (Dexcom G6); Libre Mard ≈ 9–15% (Study-dependent); Factory calibrated | Most readings in Clarke/Consensus zones A + B (>95%), acceptable for diabetes management | Commercial, large clinical use | ISF–blood time lag (~5 min); limited wear time (10–14 days); enzyme-based drift/biofouling | [24,25,28,32] |
| Polymer-based sensing platforms (MIPs, multilayer coatings, CPHs) | Artificial plasma, artificial sweat, urine, saliva, serum | Coatings: low relative error vs. BSA/AA/UA (≈2–3%) and 77% signal retention after 12 h; MIPs: nM–µM LOD in noninvasive biofluids; CPHs: high sensitivity in sweat and ~30-day retention | No MARD/Clarke; goal is to extend in vivo lifetime by reducing biofouling/FBR; detection shown only in noninvasive biofluids | Lab-scale, Preclinical, prototype | MIP binding-site heterogeneity; unresolved long-term in vivo stability; low/evaporative sweat volume; scale-up difficulty | [31,33,34,52,54] |
| SWIR/optical sensing + CNN/RF | Skin/optical | Dual-mode processing reported “Clinically excellent” performance | No MARD/Clarke; only relative accuracy in noninvasive spectral setting | Feasibility, pilot | Instrument complexity; dual-signal dependence; skin/thickness variation needs compensation | [46] |
| Noninvasive EBC MIP sensor + DL | Exhaled breath condensate | Reported LOD 0.001 ppb: response ≈ 30 s | No MARD/Clarke; very low EBC glucose therefore not directly comparable to commercial CGM | Proof-of-concept | EBC collection reproducibility; unit inconsistency; no simultaneous human validation | [47] |
| Implantable PEGDA hydrogel CGM + DL alignment | Tissue/ISF (phantom) | Three-range glucose classification ≈ 89%; DL compensates reader misalignment | Range-level only; no MARD/Clarke; not CGM-equivalent yet | In vitro, phantom prototype | Alignment dependence; long-term in vivo/immunological effects not shown; possible high-glucose saturation | [48] |
| Paper-based electrochemical sensor + SVR | Spiked human serum | ML accuracy > 99%; LOD ≈ 100 nM; stable response for 50 days | No MARD/Clarke; validated for spot measurements, not continuous monitoring | Lab-scale | Low-cost sensor resolution limits; no continuous real-patient validation | [49] |
| Enzymatic DPV sensor + XGBoost | Prepared low-concentration glucose (sweat-target) | R2 > 0.92 with improved MAE/RMSE | No MARD/Clarke; preparatory step toward sweat-based sensing | Model-development | No real sweat samples; incomplete compensation for practical interferences | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Yun, H.-S.; Jeon, H.-J. The Convergence of Polymer Science and Predictive Modeling for Noninvasive Glucose Monitoring. Pharmaceutics 2025, 17, 1488. https://doi.org/10.3390/pharmaceutics17111488
Lee J-H, Yun H-S, Jeon H-J. The Convergence of Polymer Science and Predictive Modeling for Noninvasive Glucose Monitoring. Pharmaceutics. 2025; 17(11):1488. https://doi.org/10.3390/pharmaceutics17111488
Chicago/Turabian StyleLee, Ju-Hwan, Hong-Sik Yun, and Hee-Jae Jeon. 2025. "The Convergence of Polymer Science and Predictive Modeling for Noninvasive Glucose Monitoring" Pharmaceutics 17, no. 11: 1488. https://doi.org/10.3390/pharmaceutics17111488
APA StyleLee, J.-H., Yun, H.-S., & Jeon, H.-J. (2025). The Convergence of Polymer Science and Predictive Modeling for Noninvasive Glucose Monitoring. Pharmaceutics, 17(11), 1488. https://doi.org/10.3390/pharmaceutics17111488






