Structural and Functional Insights into Viral and Fungal Proteins Involved in Chronic Inflammation and Their Biologic Treatments
Abstract
1. Introduction
2. Viral Proteins Associated with Chronic Inflammation: Structural and Functional Analyses
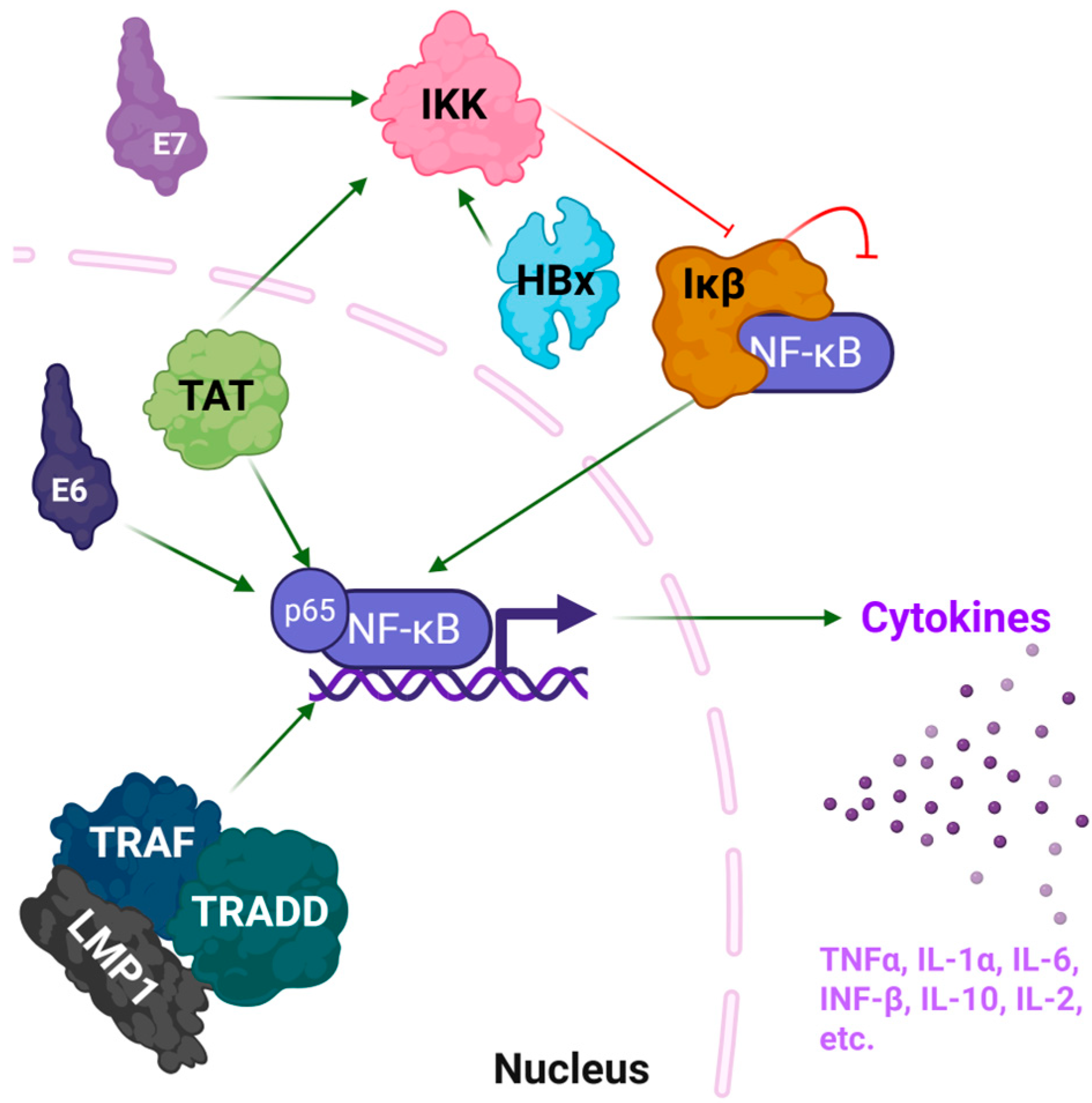
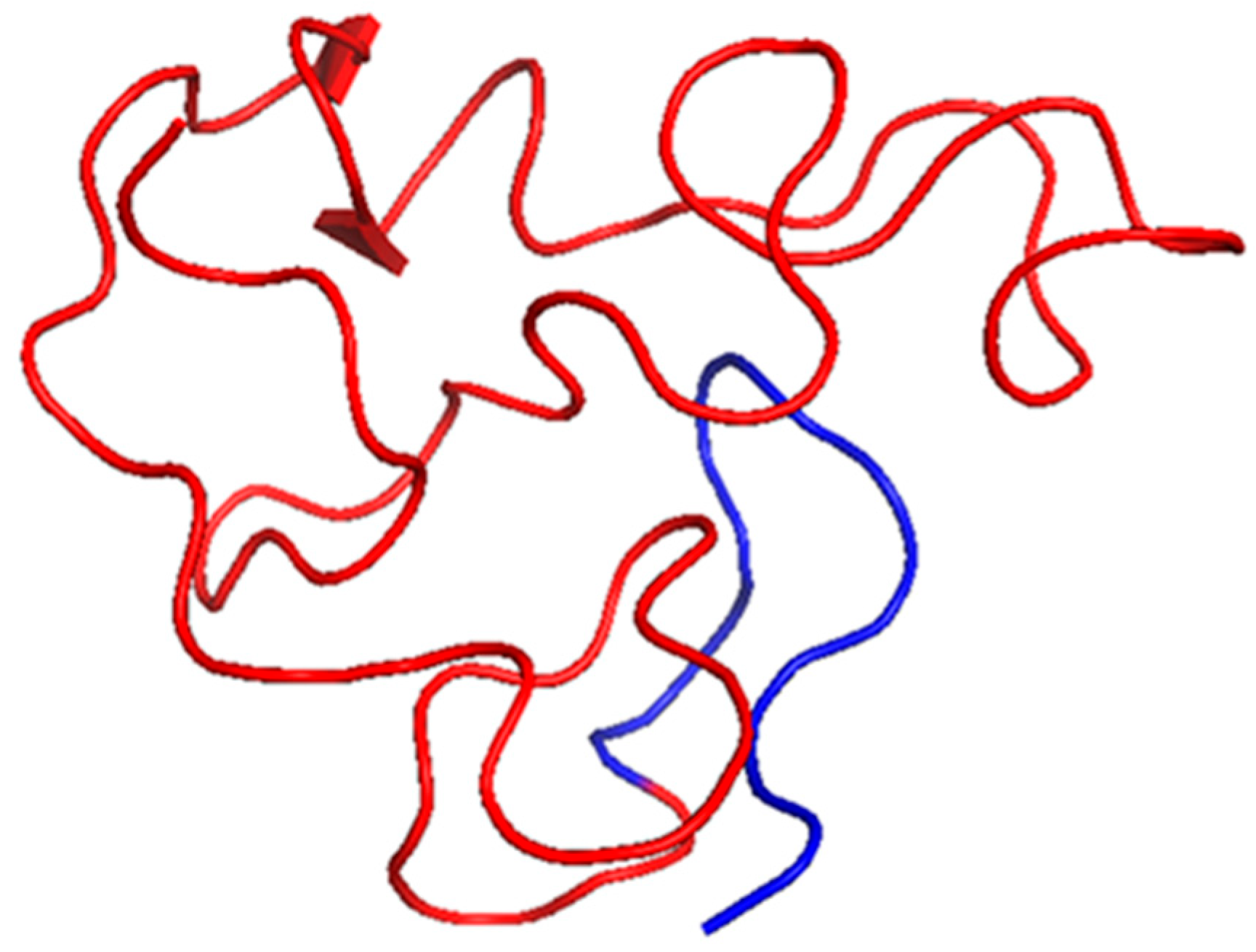
2.1. HIV-1 Tat
2.2. HBV X Protein
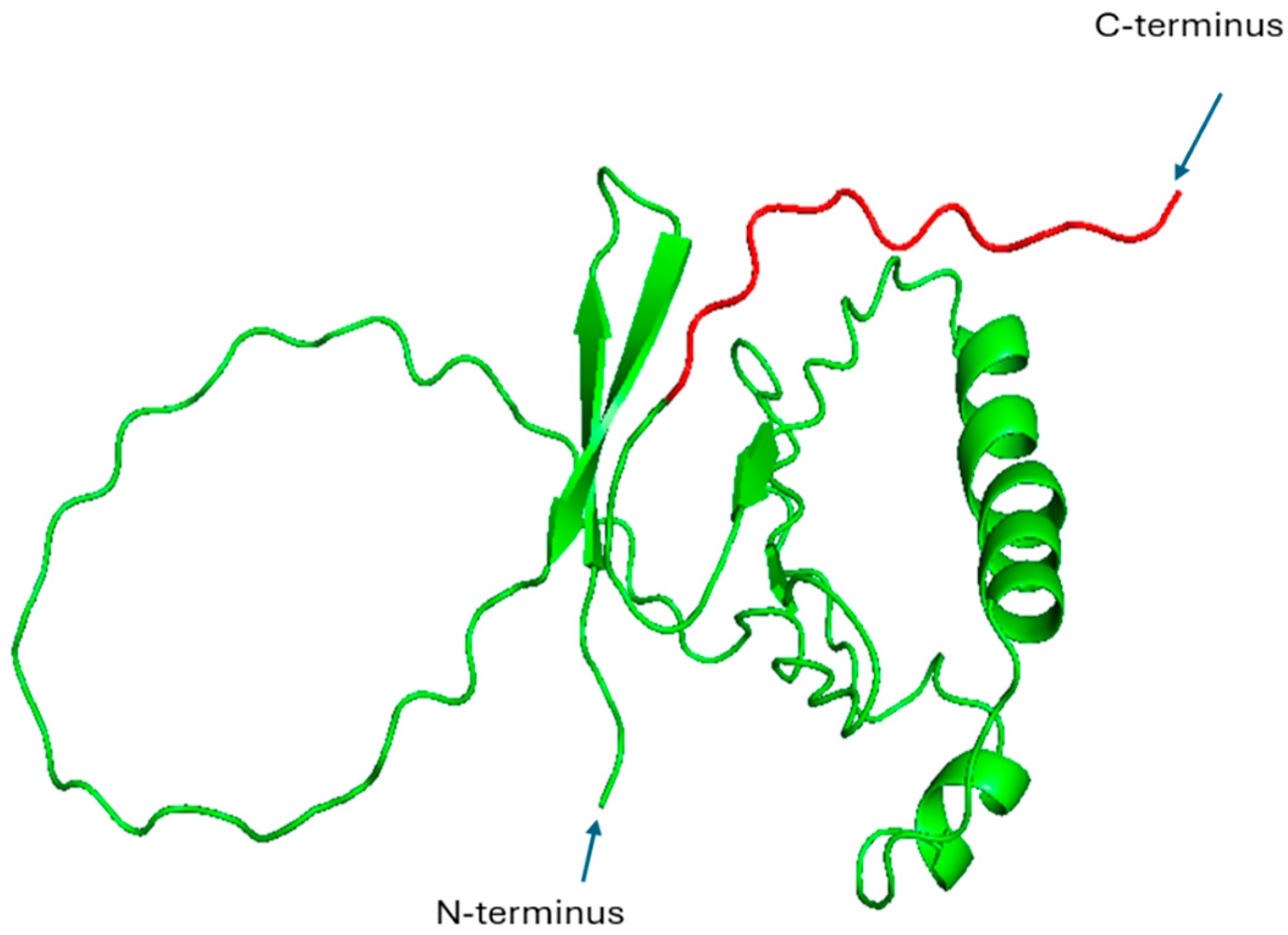
2.3. HPV E6 and E7 Proteins
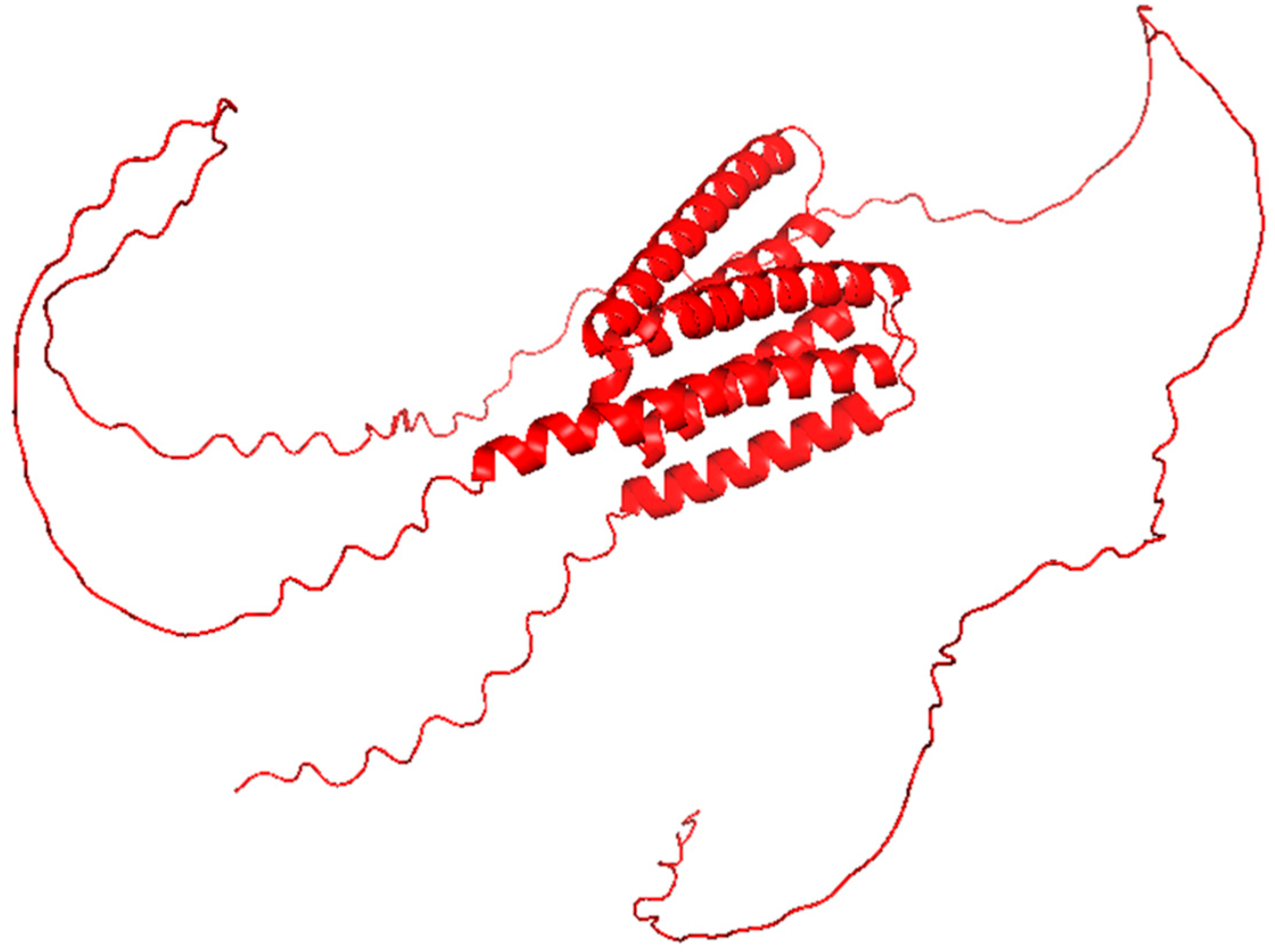
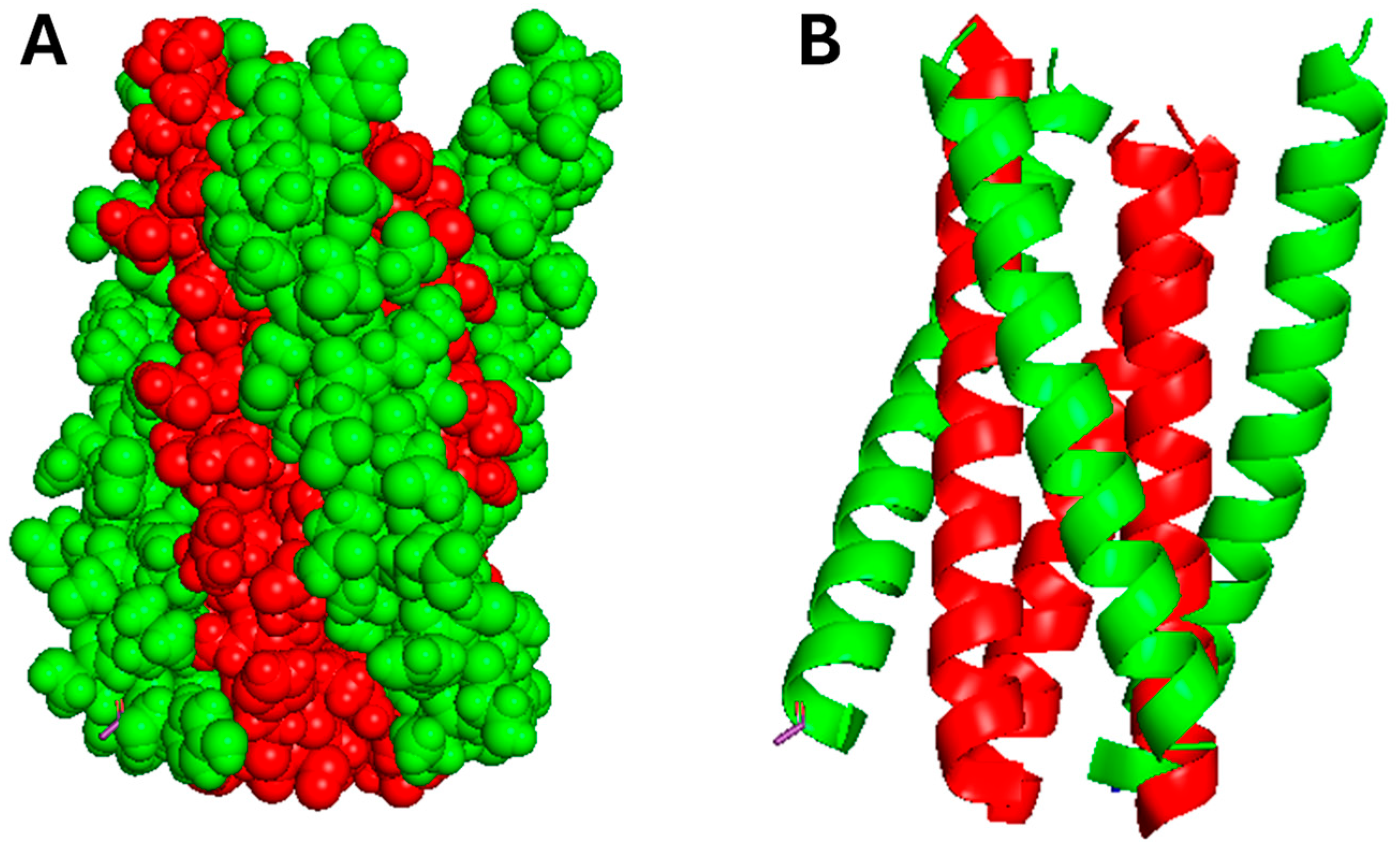
2.4. EBV LMP1
3. Fungal Proteins Involved in Chronic Inflammation: Structural and Functional Insights
3.1. Saps from C. Albicans
| Fungal Protein | Species | Mechanism of Inflammation | Structural Features | PDB Code | Binding Motif or Interface | Ref. |
|---|---|---|---|---|---|---|
| Sap2 | C. albicans | TLR4, Dectin-1; NLRP3 inflammasome activation; cytokine induction (IL-1β, IL-6, TNF-α) | Pepsin-like fold; active-site cleft | 1EAG | Active-site Asp residues + substrate-binding loops mediate TLR engagement | [53] |
| Asp f1 | A. fumigatus | TLR2/TLR4 activation; Th2 response; eosinophilic inflammation | Conserved α/β ribotoxin topology | 1JBS | Ribotoxin loop regions interact with TLR extracellular domains | [54] |
| Chitin | Multiple | Dectin-1, TLR9, NOD receptor engagement; IL-17 production; granuloma formation | β-sandwich fold (modelled from bacterial CBP21) | 2BEM | Glucan-binding surface recognized by Dectin-1 CRD | [55] |
| Als3 | C. albicans | Binds E-cadherin and N-cadherin, triggers NF-κB and MAPK signaling | β barrel of the N-terminus | 4LE8 | β barrel of the N-terminus binds to the host cadherin receptors. | [56] |
3.2. Asp f1 of A. fumigatus
3.3. Chitin and Chitin-Binding Proteins
3.4. Agglutinin-like Sequence Protein
4. Biologics Targeting Viral and Fungal Inflammation
5. Limitations of Current Biologics and the Potential of Conjugated Therapeutic Proteins
6. Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABPA | Allergic Bronchopulmonary Aspergillosis |
| ADC | Antibody–Drug Conjugate |
| AFP | Antifungal Peptide |
| AVP | Antiviral Peptide |
| CBP | Chitin-Binding Protein |
| CTAR | C-Terminal Activation Region (e.g., in LMP1) |
| DAMP | Damage-Associated Molecular Pattern |
| EBV | Epstein–Barr Virus |
| ECM | Extracellular Matrix |
| FDA | Food and Drug Administration (USA) |
| HBV | Hepatitis B Virus |
| HBx | Hepatitis B virus X protein |
| HCV | Hepatitis C Virus |
| HIV | Human Immunodeficiency Virus |
| HPV | Human Papillomavirus |
| IKK | IκB Kinase |
| IL | Interleukin (e.g., IL-1β, IL-6) |
| LMP1 | Latent Membrane Protein 1 (EBV) |
| LPS | Lipopolysaccharide |
| mAb | Monoclonal Antibody |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NLRP3 | NOD-, LRR- and Pyrin Domain-Containing Protein 3 (inflammasome) |
| PAMP | Pathogen-Associated Molecular Pattern |
| PDB | Protein Data Bank |
| PRR | Pattern Recognition Receptor |
| Rb | Retinoblastoma protein |
| Sap | Secreted Aspartyl Protease |
| Tat | Trans-Activator of Transcription (HIV-1) |
| TLR | Toll-Like Receptor |
| TNF-α | Tumor Necrosis Factor Alpha |
| TNFR | Tumor Necrosis Factor Receptor |
| TRAF | TNF Receptor-Associated Factor |
| TRADD | TNFR1-Associated Death Domain Protein |
| VIRIP | Virus-Inhibitory Peptide |
References
- Wen, Y.; Zhu, Y.; Zhang, C.; Yang, X.; Gao, Y.; Li, M.; Yang, H.; Liu, T.; Tang, H. Chronic inflammation, cancer development and immunotherapy. Front. Pharmacol. 2022, 13, 1040163. [Google Scholar] [CrossRef]
- Ren, D.; Ye, X.; Chen, R.; Jia, X.; He, X.; Tao, J.; Jin, T.; Wu, S.; Zhang, H. Activation and evasion of inflammasomes during viral and microbial infection. Cell. Mol. Life Sci. 2025, 82, 56. [Google Scholar] [CrossRef] [PubMed]
- Tugizov, S. HIV-1 Tat-induced disruption of epithelial junctions and epithelial-mesenchymal transition of oral and genital epithelial cells lead to increased invasiveness of neoplastic cells and the spread of herpes simplex virus and cytomegalovirus. Front. Immunol. 2025, 16, 1541532. [Google Scholar] [CrossRef] [PubMed]
- Lien, K.; Mayer, W.; Herrera, R.; Padilla, N.T.; Cai, X.; Lin, V.; Pholcharoenchit, R.; Palefsky, J.; Tugizov, S.M. HIV-1 Proteins gp120 and Tat Promote Epithelial-Mesenchymal Transition and Invasiveness of HPV-Positive and HPV-Negative Neoplastic Genital and Oral Epithelial Cells. Microbiol. Spectr. 2022, 10, e03622. [Google Scholar] [CrossRef] [PubMed]
- Floettmann, J.E.; Eliopoulos, A.G.; Jones, M.; Young, L.S.; Rowe, M. Epstein–Barr virus latent membrane protein-1 (LMP1) signalling is distinct from CD40 and involves physical cooperation of its two C-terminus functional regions. Oncogene 1998, 17, 2383–2392. [Google Scholar] [CrossRef]
- Das, B.; Sarkar, C.; Rawat, V.S.; Kalita, D.; Deka, S.; Agnihotri, A. Promise of the NLRP3 inflammasome inhibitors in in vivo disease models. Molecules 2021, 26, 4996. [Google Scholar] [CrossRef]
- Sack, M.N. Mitochondrial fidelity and metabolic agility control immune cell fate and function. J. Clin. Investig. 2018, 128, 3651–3661. [Google Scholar] [CrossRef]
- Hinz, M.; Scheidereit, C. The IκB kinase complex in NF-κB regulation and beyond. EMBO Rep. 2014, 15, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Lien, K.; Mayer, W.; Herrera, R.; Rosbe, K.; Tugizov, S.M. HIV-1 proteins gp120 and tat induce the epithelial–mesenchymal transition in oral and genital mucosal epithelial cells. PLoS ONE 2019, 14, e0226343. [Google Scholar] [CrossRef]
- Mahomed, S. Broadly neutralizing antibodies for HIV prevention: A comprehensive review and future perspectives. Clin. Microbiol. Rev. 2024, 37, e0015222. [Google Scholar] [CrossRef]
- Riddler, S.A.; Zheng, L.; Durand, C.M.; Ritz, J.; Koup, R.A.; Ledgerwood, J.; Bailer, R.T.; Koletar, S.L.; Eron, J.J.; Keefer, M.C. Randomized clinical trial to assess the impact of the broadly neutralizing HIV-1 monoclonal antibody VRC01 on HIV-1 persistence in individuals on effective ART. Open Forum Infect. Dis. 2018, 5, ofy242. [Google Scholar] [CrossRef]
- Gunst, J.D.; Pahus, M.H.; Rosás-Umbert, M.; Lu, I.-N.; Benfield, T.; Nielsen, H.; Johansen, I.S.; Mohey, R.; Østergaard, L.; Klastrup, V. Early intervention with 3BNC117 and romidepsin at antiretroviral treatment initiation in people with HIV-1: A phase 1b/2a, randomized trial. Nat. Med. 2022, 28, 2424–2435. [Google Scholar] [CrossRef] [PubMed]
- Mahomed, S.; Pillay, K.; Hassan-Moosa, R.; Galvão, B.P.G.V.; Burgers, W.A.; Moore, P.L.; Rose-Abrahams, M.; Williamson, C.; Garrett, N. Clinical trials of broadly neutralizing monoclonal antibodies in people living with HIV—A review. AIDS Res. Ther. 2025, 22, 44. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Gong, X.; Li, X.; Jiang, Y.; Deng, S.; Tang, J.; Ge, H.; Wu, C.; Tang, H.; Wang, G.; et al. Dectin-1 aggravates neutrophil inflammation through caspase-11/4-mediated macrophage pyroptosis in asthma. Respir. Res. 2024, 25, 119. [Google Scholar] [CrossRef]
- Sa, M.; da Silva, M.; Ball, B.; Geddes-McAlister, J. Revealing the dynamics of fungal disease with proteomics. Mol. Omics 2025, 21, 173–184. [Google Scholar] [CrossRef]
- Lohse, M.B.; Gulati, M.; Craik, C.S.; Johnson, A.D.; Nobile, C.J. Combination of antifungal drugs and protease inhibitors prevent Candida albicans biofilm formation and disrupt mature biofilms. Front. Microbiol. 2020, 11, 1027. [Google Scholar] [CrossRef]
- Xiao, Q.; Liu, Y.; Shu, X.; Li, Y.; Zhang, X.; Wang, C.; He, S.; Li, J.; Li, T.; Liu, T.; et al. Molecular mechanisms of viral oncogenesis in haematological malignancies: Perspectives from metabolic reprogramming, epigenetic regulation and immune microenvironment remodeling. Exp. Hematol. Oncol. 2025, 14, 69. [Google Scholar] [CrossRef]
- Fiume, G.; Vecchio, E.; De Laurentiis, A.; Trimboli, F.; Palmieri, C.; Pisano, A.; Falcone, C.; Pontoriero, M.; Rossi, A.; Scialdone, A.; et al. Human immunodeficiency virus-1 Tat activates NF-κB via physical interaction with IκB-α and p65. Nucleic Acids Res. 2012, 40, 3548–3562. [Google Scholar] [CrossRef]
- Dandekar, D.H.; Ganesh, K.N.; Mitra, D. HIV-1 Tat directly binds to NFκB enhancer sequence: Role in viral and cellular gene expression. Nucleic Acids Res. 2004, 32, 1270–1278. [Google Scholar] [CrossRef]
- Péloponèse, J.-M., Jr.; Grégoirea, C.; Opia, S.; Esquieua, D.; Sturgisa, J.; Lebrunb, É.; Meursc, É.; Colletted, Y.; Olived, D.; Aubertine, A.-M. 1H-13 C nuclear magnetic resonance assignment and structural characterization of HIV-1 Tat protein. Comptes Rendus L’académie Sci.-Ser. III-Sci. Vie 2000, 323, 883–894. [Google Scholar]
- You, H.; Qin, S.; Zhang, F.; Hu, W.; Li, X.; Liu, D.; Kong, F.; Pan, X.; Zheng, K.; Tang, R. Regulation of pattern-recognition receptor signaling by HBX during hepatitis B virus infection. Front. Immunol. 2022, 13, 829923. [Google Scholar] [CrossRef]
- Schollmeier, A.; Glitscher, M.; Hildt, E. Relevance of HBx for hepatitis B virus-associated pathogenesis. Int. J. Mol. Sci. 2023, 24, 4964. [Google Scholar] [CrossRef]
- Zanier, K.; Charbonnier, S.; Sidi, A.O.M.h.O.; McEwen, A.G.; Ferrario, M.G.; Poussin-Courmontagne, P.; Cura, V.; Brimer, N.; Babah, K.O.; Ansari, T. Structural basis for hijacking of cellular LxxLL motifs by papillomavirus E6 oncoproteins. Science 2013, 339, 694–698. [Google Scholar] [CrossRef]
- Ohlenschläger, O.; Seiboth, T.; Zengerling, H.; Briese, L.; Marchanka, A.; Ramachandran, R.; Baum, M.; Korbas, M.; Meyer-Klaucke, W.; Dürst, M. Solution structure of the partially folded high-risk human papilloma virus 45 oncoprotein E7. Oncogene 2006, 25, 5953–5959. [Google Scholar] [CrossRef]
- Šimičić, P.; Batović, M.; Stojanović Marković, A.; Židovec-Lepej, S. Deciphering the role of Epstein–Barr virus latent membrane protein 1 in immune modulation: A multifaced Signalling perspective. Viruses 2024, 16, 564. [Google Scholar] [CrossRef]
- Lim, K.-H.; Choi, H.S.; Park, Y.K.; Park, E.-S.; Shin, G.C.; Kim, D.H.; Ahn, S.H.; Kim, K.-H. HBx-induced NF-κB signaling in liver cells is potentially mediated by the ternary complex of HBx with p22-FLIP and NEMO. PLoS ONE 2013, 8, e57331. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, X.; Deng, Y.; Wang, D.; Li, H. Unfolding HBx for an epigenetic switch of HBV cccDNA minichromosome. Protein Cell 2025, 16, 753–763. [Google Scholar] [CrossRef]
- Letafati, A.; Taghiabadi, Z.; Zafarian, N.; Tajdini, R.; Mondeali, M.; Aboofazeli, A.; Chichiarelli, S.; Saso, L.; Jazayeri, S.M. Emerging paradigms: Unmasking the role of oxidative stress in HPV-induced carcinogenesis. Infect. Agents Cancer 2024, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Wang, L.; Zuo, L.; Gao, S.; Jiang, X.; Han, Y.; Lin, J.; Peng, M.; Wu, N.; Tang, Y.; et al. HPV E6/E7: Insights into their regulatory role and mechanism in signaling pathways in HPV-associated tumor. Cancer Gene Ther. 2024, 31, 9–17. [Google Scholar] [CrossRef] [PubMed]
- James, M.A.; Lee, J.H.; Klingelhutz, A.J. Human papillomavirus type 16 E6 activates NF-kappaB, induces cIAP-2 expression, and protects against apoptosis in a PDZ binding motif-dependent manner. J. Virol. 2006, 80, 5301–5307. [Google Scholar] [CrossRef]
- Carrillo-Beltrán, D.; Muñoz, J.P.; Guerrero-Vásquez, N.; Blanco, R.; León, O.; de Souza Lino, V.; Tapia, J.C.; Maldonado, E.; Dubois-Camacho, K.; Hermoso, M.A.; et al. Human Papillomavirus 16 E7 promotes EGFR/PI3K/AKT1/NRF2 signaling pathway contributing to PIR/NF-κB activation in oral cancer cells. Cancers 2020, 12, 1904. [Google Scholar] [CrossRef]
- Suarez, I.; Trave, G. Structural insights in multifunctional papillomavirus oncoproteins. Viruses 2018, 10, 37. [Google Scholar] [CrossRef]
- Braspenning, J.; Marchini, A.; Albarani, V.; Levy, L.; Ciccolini, F.; Cremonesi, C.; Ralston, R.; Gissmann, L.; Tommasino, M. The CXXC Zn binding motifs of the human papillomavirus type 16 E7 oncoprotein are not required for its in vitro transforming activity in rodent cells. Oncogene 1998, 16, 1085–1089. [Google Scholar] [CrossRef]
- Spardy, N.; Covella, K.; Cha, E.; Hoskins, E.E.; Wells, S.I.; Duensing, A.; Duensing, S. Human papillomavirus 16 E7 oncoprotein attenuates DNA damage checkpoint control by increasing the proteolytic turnover of claspin. Cancer research 2009, 69, 7022–7029. [Google Scholar] [CrossRef]
- Eliopoulos, A.G.; Young, L.S. LMP1 structure and signal transduction. Semin. Cancer Biol. 2001, 11, 435–444. [Google Scholar] [CrossRef]
- Kieser, A.; Sterz, K.R. The latent membrane protein 1 (LMP1). In Epstein Barr Virus Volume 2: One Herpes Virus: Many Diseases; Springer: Cham, Switzerland, 2015; pp. 119–149. [Google Scholar]
- Huang, X.; Zhang, M.; Zhang, Z. The role of LMP1 in Epstein-Barr virus-associated gastric cancer. Curr. Cancer Drug Targets 2024, 24, 127–141. [Google Scholar] [CrossRef]
- Stuckey, P.V.; Santiago-Tirado, F.H. Fungal mechanisms of intracellular survival: What can we learn from bacterial pathogens? Infect. Immun. 2023, 91, e0043422. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Lin, G.; Langdon, W.Y.; Tao, L.; Zhang, J. Regulation of C-type lectin receptor-mediated antifungal immunity. Front. Immunol. 2018, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Hatinguais, R.; Willment, J.A.; Brown, G.D. PAMPs of the fungal cell wall and mammalian PRRs. In The Fungal Cell Wall: An Armour and a Weapon for Human Fungal Pathogens; Springer: Cham, Switzerland, 2020; pp. 187–223. [Google Scholar]
- Behnen, J.; Köster, H.; Neudert, G.; Craan, T.; Heine, A.; Klebe, G. Experimental and computational active site mapping as a starting point to fragment-based lead discovery. ChemMedChem 2012, 7, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Bruno, V.M.; Shetty, A.C.; Yano, J.; Fidel, P.L.; Noverr, M.C.; Peters, B.M. Transcriptomic Analysis of Vulvovaginal Candidiasis Identifies a Role for the NLRP3 Inflammasome. mBio 2015, 6, e00182-15. [Google Scholar] [CrossRef]
- Camilli, G.; Griffiths, J.S.; Ho, J.; Richardson, J.P.; Naglik, J.R. Some like it hot: Candida activation of inflammasomes. PLoS Pathog. 2020, 16, e1008975. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.O.; Montano, D.E.; Zelante, T.; Dietschmann, A.; Gresnigt, M.S. Inflammatory cytokine signalling in vulvovaginal candidiasis: A hot mess driving immunopathology. Oxf. Open Immunol. 2024, 5, iqae010. [Google Scholar] [CrossRef]
- De Lorenzo, G.; Cervone, F. Plant immunity by damage-associated molecular patterns (DAMPs). Essays Biochem. 2022, 66, 459–469. [Google Scholar] [CrossRef]
- Harris, F.M.; Mou, Z. Damage-associated molecular patterns and systemic signaling. Phytopathology 2024, 114, 308–327. [Google Scholar] [CrossRef]
- Dawoud, A.M.; Saied, S.A.; Torayah, M.M.; Ramadan, A.E.; Elaskary, S.A. Antifungal susceptibility and virulence determinants profile of candida species isolated from patients with candidemia. Sci. Rep. 2024, 14, 11597. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.S.; Braga-Silva, L.A.; Gonçalves, D.S.; Ramos, L.S.; Oliveira, S.S.C.; Souza, L.O.P.; Oliveira, V.S.; Lins, R.D.; Pinto, M.R.; Muñoz, J.E.; et al. Repositioning Lopinavir, an HIV Protease Inhibitor, as a Promising Antifungal Drug: Lessons Learned from Candida albicans—In Silico, In Vitro and In Vivo Approaches. J. Fungi 2021, 7, 424. [Google Scholar] [CrossRef]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef]
- Abad-Zapatero, C.; Goldman, R.; Muchmore, S.W.; Hutchins, C.; Stewart, K.; Navaza, J.; Payne, C.D.; Ray, T.L. Structure of a secreted aspartic protease from C. albicans complexed with a potent inhibitor: Implications for the design of antifungal agents. Protein Sci. 1996, 5, 640–652. [Google Scholar] [CrossRef]
- Cutfield, S.; Dodson, E.; Anderson, B.; Moody, P.; Marshall, C.; Sullivan, P.; Cutfield, J. The crystal structure of a major secreted aspartic proteinase from Candida albicans in complexes with two inhibitors. Structure 1995, 3, 1261–1271. [Google Scholar] [CrossRef]
- Yang, X.; Gérczei, T.; Glover, L.; Correll, C.C. Crystal structures of restrictocin–inhibitor complexes with implications for RNA recognition and base flipping. Nat. Struct. Biol. 2001, 8, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Vaaje-Kolstad, G.; Houston, D.R.; Riemen, A.H.; Eijsink, V.G.; Van Aalten, D.M. Crystal structure and binding properties of the Serratia marcescens chitin-binding protein CBP21. J. Biol. Chem. 2005, 280, 11313–11319. [Google Scholar] [CrossRef] [PubMed]
- Phan, Q.T.; Myers, C.L.; Fu, Y.; Sheppard, D.C.; Yeaman, M.R.; Welch, W.H.; Ibrahim, A.S.; Edwards, J.E., Jr.; Filler, S.G. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007, 5, e64. [Google Scholar] [CrossRef]
- Banerjee, B.; Kurup, V.; Greenberger, P.; Johnson, B.; Fink, J. Cloning and expression of Aspergillus fumigatus allergen Asp f 16 mediating both humoral and cell-mediated immunity in allergic bronchopulmonary aspergillosis (ABPA). Clin. Exp. Allergy 2001, 31, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Sun, Y.; Zhang, Y. The Triad of Pathogenesis in Allergic Bronchopulmonary Aspergillosis: Interactions Among Aspergillus fumigatus, Epithelium, and Immunity. Int. Arch. Allergy Immunol. 2025. [Google Scholar] [CrossRef]
- Gross, O.; Gewies, A.; Finger, K.; Schäfer, M.; Sparwasser, T.; Peschel, C.; Förster, I.; Ruland, J. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 2006, 442, 651–656. [Google Scholar] [CrossRef]
- Mata-Martínez, P.; Bergón-Gutiérrez, M.; del Fresno, C. Dectin-1 signaling update: New perspectives for trained immunity. Front. Immunol. 2022, 13, 812148. [Google Scholar] [CrossRef]
- Hu, C.; Sun, L.; Hu, Y.; Lu, D.; Wang, H.; Tang, S. Functional characterization of the NF-kappaB binding site in the human NOD2 promoter. Cell. Mol. Immunol. 2010, 7, 288–295. [Google Scholar] [CrossRef]
- Lenardon, M.D.; Munro, C.A.; Gow, N.A. Chitin synthesis and fungal pathogenesis. Curr. Opin. Microbiol. 2010, 13, 416–423. [Google Scholar] [CrossRef]
- Wagener, J.; Malireddi, R.K.S.; Lenardon, M.D.; Köberle, M.; Vautier, S.; MacCallum, D.M.; Biedermann, T.; Schaller, M.; Netea, M.G.; Kanneganti, T.-D.; et al. Fungal Chitin Dampens Inflammation Through IL-10 Induction Mediated by NOD2 and TLR9 Activation. PLoS Pathog. 2014, 10, e1004050. [Google Scholar] [CrossRef]
- Fadel, F.; Zhao, Y.; Cousido-Siah, A.; Ruiz, F.X.; Mitschler, A.; Podjarny, A. X-ray crystal structure of the full length human chitotriosidase (CHIT1) reveals features of its chitin binding domain. PLoS ONE 2016, 11, e0154190. [Google Scholar] [CrossRef]
- Smith, P.J.; Ortiz-Soto, M.E.; Roth, C.; Barnes, W.J.; Seibel, J.; Urbanowicz, B.R.; Pfrengle, F. Enzymatic synthesis of artificial polysaccharides. ACS Sustain. Chem. Eng. 2020, 8, 11853–11871. [Google Scholar] [CrossRef]
- Moyes, D.L.; Richardson, J.P.; Naglik, J.R. Candida albicans-epithelial interactions and pathogenicity mechanisms: Scratching the surface. Virulence 2015, 6, 338–346. [Google Scholar] [CrossRef]
- Zhou, T.; Solis, N.V.; Marshall, M.; Yao, Q.; Pearlman, E.; Filler, S.G.; Liu, H. Fungal Als proteins hijack host death effector domains to promote inflammasome signaling. Nat. Commun. 2025, 16, 1562. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.R.; König, A.; Hube, B.; Gaffen, S.L. Candida albicans-epithelial interactions and induction of mucosal innate immunity. Curr. Opin. Microbiol. 2017, 40, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Salgado, P.S.; Yan, R.; Taylor, J.D.; Burchell, L.; Jones, R.; Hoyer, L.L.; Matthews, S.J.; Simpson, P.J.; Cota, E. Structural basis for the broad specificity to host-cell ligands by the pathogenic fungus Candida albicans. Proc. Natl. Acad. Sci. USA 2011, 108, 15775–15779. [Google Scholar] [CrossRef]
- Halawa, M.; ElSayed, R.M.R.; Aderibigbe, T.; Newman, P.M.; Reid, B.E.; Carabetta, V.J. Biosimilars Targeting Pathogens: A Comprehensive Review of Their Role in Bacterial, Fungal, Parasitic, and Viral Infections. Pharmaceutics 2025, 17, 581. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Drummond, R.A.; Hohl, T.M. Immune responses to human fungal pathogens and therapeutic prospects. Nat. Rev. Immunol. 2023, 23, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Kwaku, G.N.; Jensen, K.N.; Simaku, P.; Floyd, D.J.; Saelens, J.W.; Reardon, C.M.; Ward, R.A.; Basham, K.J.; Hepworth, O.W.; Vyas, T.D.; et al. Extracellular vesicles from diverse fungal pathogens induce species-specific and endocytosis-dependent immunomodulation. PLoS Pathog. 2025, 21, e1012879. [Google Scholar] [CrossRef]
- Xiao, Q.; Liu, Y.; Li, T.; Wang, C.; He, S.; Zhai, L.; Yang, Z.; Zhang, X.; Wu, Y.; Liu, Y. Viral oncogenesis in cancer: From mechanisms to therapeutics. Signal Transduct. Target. Ther. 2025, 10, 151. [Google Scholar] [CrossRef]
- Iuliano, M.; Mangino, G.; Chiantore, M.V.; Zangrillo, M.S.; Accardi, R.; Tommasino, M.; Fiorucci, G.; Romeo, G. Human Papillomavirus E6 and E7 oncoproteins affect the cell microenvironment by classical secretion and extracellular vesicles delivery of inflammatory mediators. Cytokine 2018, 106, 182–189. [Google Scholar] [CrossRef]
- Deckers, J.; Anbergen, T.; Hokke, A.M.; de Dreu, A.; Schrijver, D.P.; de Bruin, K.; Toner, Y.C.; Beldman, T.J.; Spangler, J.B.; de Greef, T.F. Engineering cytokine therapeutics. Nat. Rev. Bioeng. 2023, 1, 286–303. [Google Scholar] [CrossRef]
- Maleki, M.S.M.; Sardari, S.; Alavijeh, A.G.; Madanchi, H. Recent patents and FDA-approved drugs based on antiviral peptides and other peptide-related antivirals. Int. J. Pept. Res. Ther. 2022, 29, 5. [Google Scholar] [CrossRef]
- Yi, A.; Fochtman, C.; Rizzo, C.; Jacobs, A. Inhibition of HIV entry by targeting the envelope transmembrane subunit gp41. Curr. HIV Res. 2016, 14, 283–294. [Google Scholar] [CrossRef]
- Jing, S.; Zhao, Q.; Debnath, A.K. Peptide and non-peptide HIV fusion inhibitors. Curr. Pharm. Des. 2002, 8, 563–580. [Google Scholar] [CrossRef]
- Münch, J.; Ständker, L.; Forssmann, W.-G.; Kirchhoff, F. Discovery of modulators of HIV-1 infection from the human peptidome. Nat. Rev. Microbiol. 2014, 12, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Dong, T.; Chen, X.; Tong, B.; Qian, X.; Che, J.; Cheng, Y. Pharmacokinetics of sifuvirtide in treatment-naive and treatment-experienced HIV-infected patients. J. Pharm. Sci. 2014, 103, 4038–4047. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.; Yao, X.; Zhang, C.; Cai, L.; Cui, S.; Wang, Y.; He, Y. Biophysical property and broad anti-HIV activity of albuvirtide, a 3-maleimimidopropionic acid-modified peptide fusion inhibitor. PLoS ONE 2012, 7, e32599. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.; Traboni, C.; Penchala, S.D.; Bouliotis, G.; Doyle, N.; Libri, V.; Khoo, S.; Ashby, D.; Weber, J.; Nicosia, A.; et al. A first-in-human study of the novel HIV-fusion inhibitor C34-PEG(4)-Chol. Sci. Rep. 2017, 7, 9447. [Google Scholar] [CrossRef]
- Angell, Y.M.; Hartsock, W.J.; Reichart, T.M. Albuvirtide (Aikening), A gp41 Analog as an HIV-1 Fusion Inhibitor. In Current Drug Synthesis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 101–118. [Google Scholar]
- Münch, J.; Ständker, L.; Adermann, K.; Schulz, A.; Schindler, M.; Chinnadurai, R.; Pöhlmann, S.; Chaipan, C.; Biet, T.; Peters, T.; et al. Discovery and Optimization of a Natural HIV-1 Entry Inhibitor Targeting the gp41 Fusion Peptide. Cell 2007, 129, 263–275. [Google Scholar] [CrossRef]
- Forssmann, W.-G.; The, Y.-H.; Stoll, M.; Adermann, K.; Albrecht, U.; Tillmann, H.-C.; Barlos, K.; Busmann, A.; Canales-Mayordomo, A.; Giménez-Gallego, G. Short-term monotherapy in HIV-infected patients with a virus entry inhibitor against the gp41 fusion peptide. Sci. Transl. Med. 2010, 2, 63re3. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, J.; Zhang, F.; Xu, Y.; Yu, Y.; Liang, W.; Li, Q. Effectiveness of combination therapy with ISA101 vaccine for the treatment of human papillomavirus-induced cervical cancer. Front. Oncol. 2022, 12, 990877. [Google Scholar] [CrossRef]
- Kong, A.H.; Hesselink, M.S.K.; Aguilera, B.; Adkins, D.; Even, C.; Fayette, J.; Muzaffar, J.; Visscher, S.; Melief, C.J.; Hooftman, L.W. Phase 2 study of ISA101b (peltopepimut-S) and cemiplimab in patients with advanced HPV16+ oropharyngeal cancer who failed anti-PD1 therapy. J. Clin. Oncol. 2023, 41, 6028. [Google Scholar] [CrossRef]
- Zamaraev, A.; Zhivotovsky, B.; Kopeina, G. Viral infections: Negative regulators of apoptosis and oncogenic factors. Biochemistry 2020, 85, 1191–1201. [Google Scholar] [CrossRef]
- Giehler, F.; Ostertag, M.S.; Sommermann, T.; Weidl, D.; Sterz, K.R.; Kutz, H.; Moosmann, A.; Feller, S.M.; Geerlof, A.; Biesinger, B.; et al. Epstein-Barr virus-driven B cell lymphoma mediated by a direct LMP1-TRAF6 complex. Nat. Commun. 2024, 15, 414. [Google Scholar] [CrossRef] [PubMed]
- Ndour, P.A.; Brocqueville, G.; Ouk, T.S.; Goormachtigh, G.; Morales, O.; Mougel, A.; Bertout, J.; Melnyk, O.; Fafeur, V.; Feuillard, J.; et al. Inhibition of latent membrane protein 1 impairs the growth and tumorigenesis of latency II Epstein-Barr virus-transformed T cells. J. Virol. 2012, 86, 3934–3943. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.G.; Felipe, M.S. Candida albicans and antifungal peptides. Infect. Dis. Ther. 2023, 12, 2631–2648. [Google Scholar] [CrossRef]
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral candidiasis: A disease of opportunity. J. Fungi 2020, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Memariani, M.; Memariani, H. Antifungal properties of cathelicidin LL-37: Current knowledge and future research directions. World J. Microbiol. Biotechnol. 2024, 40, 34. [Google Scholar] [CrossRef]
- Grönberg, A.; Mahlapuu, M.; Ståhle, M.; Whately-Smith, C.; Rollman, O. Treatment with LL-37 is safe and effective in enhancing healing of hard-to-heal venous leg ulcers: A randomized, placebo-controlled clinical trial. Wound Repair Regen. 2014, 22, 613–621. [Google Scholar] [CrossRef]
- Cieślik, M.; Bagińska, N.; Górski, A.; Jończyk-Matysiak, E. Human β-defensin 2 and its postulated role in modulation of the immune response. Cells 2021, 10, 2991. [Google Scholar] [CrossRef]
- Koeninger, L.; Armbruster, N.S.; Brinch, K.S.; Kjaerulf, S.; Andersen, B.; Langnau, C.; Autenrieth, S.E.; Schneidawind, D.; Stange, E.F.; Malek, N.P. Human β-defensin 2 mediated immune modulation as treatment for experimental colitis. Front. Immunol. 2020, 11, 93. [Google Scholar] [CrossRef]
- Ul Haq, I.; Maryam, S.; Shyntum, D.Y.; Khan, T.A.; Li, F. Exploring the frontiers of therapeutic breadth of antifungal peptides: A new avenue in antifungal drugs. J. Ind. Microbiol. Biotechnol. 2024, 51, kuae018. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Sprute, R.; Arastehfar, A.; Perfect, J.R.; Lass-Flörl, C.; Bellmann, R.; Prattes, J.; Thompson III, G.R.; Wiederhold, N.P.; Al Obaidi, M.M. Invasive candidiasis: Investigational drugs in the clinical development pipeline and mechanisms of action. Expert Opin. Investig. Drugs 2022, 31, 795–812. [Google Scholar] [CrossRef]
- Duncan, V.; Smith, D.; Simpson, L.; Lovie, E.; Katvars, L.; Berge, L.; Robertson, J.; Smith, S.; Munro, C.; Mercer, D.; et al. Preliminary Characterization of NP339, a Novel Polyarginine Peptide with Broad Antifungal Activity. Antimicrob. Agents Chemother. 2021, 65, e0234520. [Google Scholar] [CrossRef]
- Sánchez-Robles, E.M.; Girón, R.; Paniagua, N.; Rodríguez-Rivera, C.; Pascual, D.; Goicoechea, C. Monoclonal antibodies for chronic pain treatment: Present and future. Int. J. Mol. Sci. 2021, 22, 10325. [Google Scholar] [CrossRef]
- Abubakar, S.D.; Ihim, S.A.; Farshchi, A.; Maleknia, S.; Abdullahi, H.; Sasaki, T.; Azizi, G. The role of TNF-α and anti-TNF-α agents in the immunopathogenesis and management of immune dysregulation in primary immunodeficiency diseases. Immunopharmacol. Immunotoxicol. 2022, 44, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Levitz, S.M. Host control of fungal infections: Lessons from basic studies and human cohorts. Annu. Rev. Immunol. 2018, 36, 157–191. [Google Scholar] [CrossRef]
- Yang, C.; Liu, M. Tocilizumab in treatment for patients with COVID-19. JAMA Intern. Med. 2021, 181, 1017–1018. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Kida, H.; Yoshida, M.; Tomita, T.; Fujii, M.; Ihara, S.; Goya, S.; Tachibana, I.; Kawase, I. Recurrent allergic bronchopulmonary aspergillosis in a patient with rheumatoid arthritis treated with etanercept and tocilizumab. Mod. Rheumatol. 2011, 21, 660–664. [Google Scholar] [CrossRef]
- Broman, N.; Feuth, T.; Vuorinen, T.; Valtonen, M.; Hohenthal, U.; Löyttyniemi, E.; Hirvioja, T.; Jalava-Karvinen, P.; Marttila, H.; Nordberg, M.; et al. Early administration of tocilizumab in hospitalized COVID-19 patients with elevated inflammatory markers; COVIDSTORM-a prospective, randomized, single-centre, open-label study. Clin. Microbiol. Infect. 2022, 28, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Dinarello, C.A. Anakinra therapy for non-cancer inflammatory diseases. Front. Pharmacol. 2018, 9, 1157. [Google Scholar] [CrossRef] [PubMed]
- Teresa Cruz, M.; Ferreira, I.; Liberal, J.; Martins, J.D.; Silva, A.; Miguel Neves, B. Inflammasome in dendritic cells immunobiology: Implications to diseases and therapeutic strategies. Curr. Drug Targets 2017, 18, 1003–1018. [Google Scholar] [CrossRef]
- de Luca, A.; Smeekens, S.P.; Casagrande, A.; Iannitti, R.; Conway, K.L.; Gresnigt, M.S.; Begun, J.; Plantinga, T.S.; Joosten, L.A.; van der Meer, J.W. IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 3526–3531. [Google Scholar] [CrossRef] [PubMed]
- Iannitti, R.G.; Napolioni, V.; Oikonomou, V.; De Luca, A.; Galosi, C.; Pariano, M.; Massi-Benedetti, C.; Borghi, M.; Puccetti, M.; Lucidi, V. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat. Commun. 2016, 7, 10791. [Google Scholar] [CrossRef]
- Yoshimori, M.; Shibayama, H.; Imadome, K.-I.; Kawano, F.; Ohashi, A.; Nishio, M.; Shimizu, N.; Kurata, M.; Fujiwara, S.; Arai, A. Antineoplastic and anti-inflammatory effects of bortezomib on systemic chronic active EBV infection. Blood Adv. 2021, 5, 1805–1815. [Google Scholar] [CrossRef]
- Cross, S.A.; Cook, D.R.; Chi, A.W.; Vance, P.J.; Kolson, L.L.; Wong, B.J.; Jordan-Sciutto, K.L.; Kolson, D.L. Dimethyl fumarate, an immune modulator and inducer of the antioxidant response, suppresses HIV replication and macrophage-mediated neurotoxicity: A novel candidate for HIV neuroprotection. J. Immunol. 2011, 187, 5015–5025. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Joosten, L.A.; Netea, M.G. The interplay between inflammasome activation and antifungal host defense. Immunol. Rev. 2015, 265, 172–180. [Google Scholar] [CrossRef]
- Diao, L.; Meibohm, B. Pharmacokinetics and pharmacokinetic–pharmacodynamic correlations of therapeutic peptides. Clin. Pharmacokinet. 2013, 52, 855–868. [Google Scholar] [CrossRef]
- Mochnáčová, E.; Bhide, K.; Kucková, K.; Jozefiaková, J.; Maľarik, T.; Bhide, M. Antimicrobial cyclic peptides effectively inhibit multiple forms of Borrelia and cross the blood-brain barrier model. Sci. Rep. 2025, 15, 6147. [Google Scholar] [CrossRef]
- Hermeling, S.; Crommelin, D.J.; Schellekens, H.; Jiskoot, W. Structure-immunogenicity relationships of therapeutic proteins. Pharm. Res. 2004, 21, 897–903. [Google Scholar] [CrossRef]
- Nellan, A.; McCully, C.M.L.; Cruz Garcia, R.; Jayaprakash, N.; Widemann, B.C.; Lee, D.W.; Warren, K.E. Improved CNS exposure to tocilizumab after cerebrospinal fluid compared to intravenous administration in rhesus macaques. Blood J. Am. Soc. Hematol. 2018, 132, 662–666. [Google Scholar] [CrossRef]
- Verma, S.; Goand, U.K.; Husain, A.; Katekar, R.A.; Garg, R.; Gayen, J.R. Challenges of peptide and protein drug delivery by oral route: Current strategies to improve the bioavailability. Drug Dev. Res. 2021, 82, 927–944. [Google Scholar] [CrossRef]
- Halawa, M.; Newman, P.M.; Aderibigbe, T.; Carabetta, V.J. Conjugated therapeutic proteins as a treatment for bacteria which trigger cancer development. iScience 2024, 27, 111029. [Google Scholar] [CrossRef]
- Yu, S.; Pearson, A.D.; Lim, R.K.; Rodgers, D.T.; Li, S.; Parker, H.B.; Weglarz, M.; Hampton, E.N.; Bollong, M.J.; Shen, J. Targeted delivery of an anti-inflammatory PDE4 inhibitor to immune cells via an antibody–drug conjugate. Mol. Ther. 2016, 24, 2078–2089. [Google Scholar] [CrossRef]
- Dixit, T.; Vaidya, A.; Ravindran, S. Therapeutic potential of antibody-drug conjugates possessing bifunctional anti-inflammatory action in the pathogenies of rheumatoid arthritis. Arthritis Res. Ther. 2024, 26, 216. [Google Scholar] [CrossRef] [PubMed]
- Boersma, B.; Poinot, H.; Pommier, A. Stimulating the antitumor immune response using immunocytokines: A preclinical and clinical overview. Pharmaceutics 2024, 16, 974. [Google Scholar] [CrossRef]
- Zhang, J.-F.; Xiong, H.-L.; Cao, J.-L.; Wang, S.-J.; Guo, X.-R.; Lin, B.-Y.; Zhang, Y.; Zhao, J.-H.; Wang, Y.-B.; Zhang, T.-Y. A cell-penetrating whole molecule antibody targeting intracellular HBx suppresses hepatitis B virus via TRIM21-dependent pathway. Theranostics 2018, 8, 549. [Google Scholar] [CrossRef] [PubMed]
- Boroumand, H.; Badie, F.; Mazaheri, S.; Seyedi, Z.S.; Nahand, J.S.; Nejati, M.; Baghi, H.B.; Abbasi-Kolli, M.; Badehnoosh, B.; Ghandali, M. Chitosan-based nanoparticles against viral infections. Front. Cell. Infect. Microbiol. 2021, 11, 643953. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Kim, J. Advances in the development of site-specific antibody-drug conjugation. Anti-Cancer Agents Med. Chem.-Anti-Cancer Agents 2015, 15, 828–836. [Google Scholar] [CrossRef]
- Walsh, S.J.; Bargh, J.D.; Dannheim, F.M.; Hanby, A.R.; Seki, H.; Counsell, A.J.; Ou, X.; Fowler, E.; Ashman, N.; Takada, Y. Site-selective modification strategies in antibody–drug conjugates. Chem. Soc. Rev. 2021, 50, 1305–1353. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A.; Chari, R.V. Antibody conjugate therapeutics: Challenges and potential. Clin. Cancer Res. 2011, 17, 6389–6397. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Vugmeyster, Y. Challenges and opportunities in absorption, distribution, metabolism, and excretion studies of therapeutic biologics. AAPS J. 2012, 14, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef]
- Nakamura, H.; Kiyoshi, M.; Anraku, M.; Hashii, N.; Oda-Ueda, N.; Ueda, T.; Ohkuri, T. Glycosylation decreases aggregation and immunogenicity of adalimumab Fab secreted from Pichia pastoris. J. Biochem. 2021, 169, 435–443. [Google Scholar] [CrossRef]
- Pasut, G. Pegylation of biological molecules and potential benefits: Pharmacological properties of certolizumab pegol. BioDrugs 2014, 28, 15–23. [Google Scholar] [CrossRef]
- Cheng, H.-B.; Cheng, Y.; Wang, J.; Liu, H.; Zhang, K.; Wu, R.; Li, J.; Fang, Y.; Hu, R.; Jeong, H. Protein-based nanomedicines for cancer theranostics: From supramolecular self-assembly to AI-driven design and applications. Chem 2025, 11, 102624. [Google Scholar] [CrossRef]
- Alzhikeyeva, A.; Yazici, A.; Sultankulov, B. Enzyme Engineering and Its Applications in Cancer Therapies: A Review of Machine Learning Approaches. Preprints 2025. [Google Scholar] [CrossRef]
- Lorenc-KuKu, K. Cutting-edge AI tools revolutionizing scientific research in life sciences. BioTechnologia 2025, 106, 77. [Google Scholar] [CrossRef]


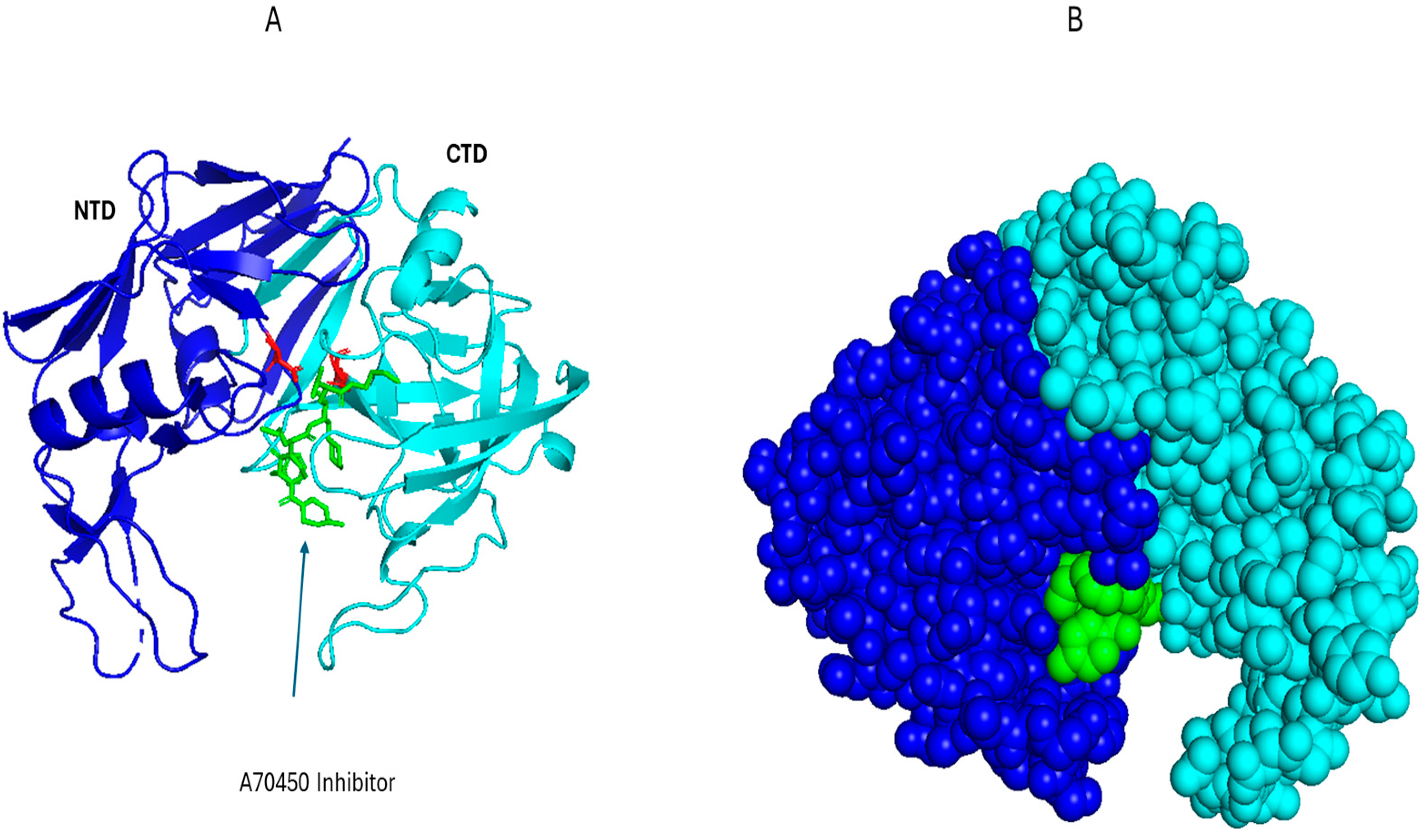
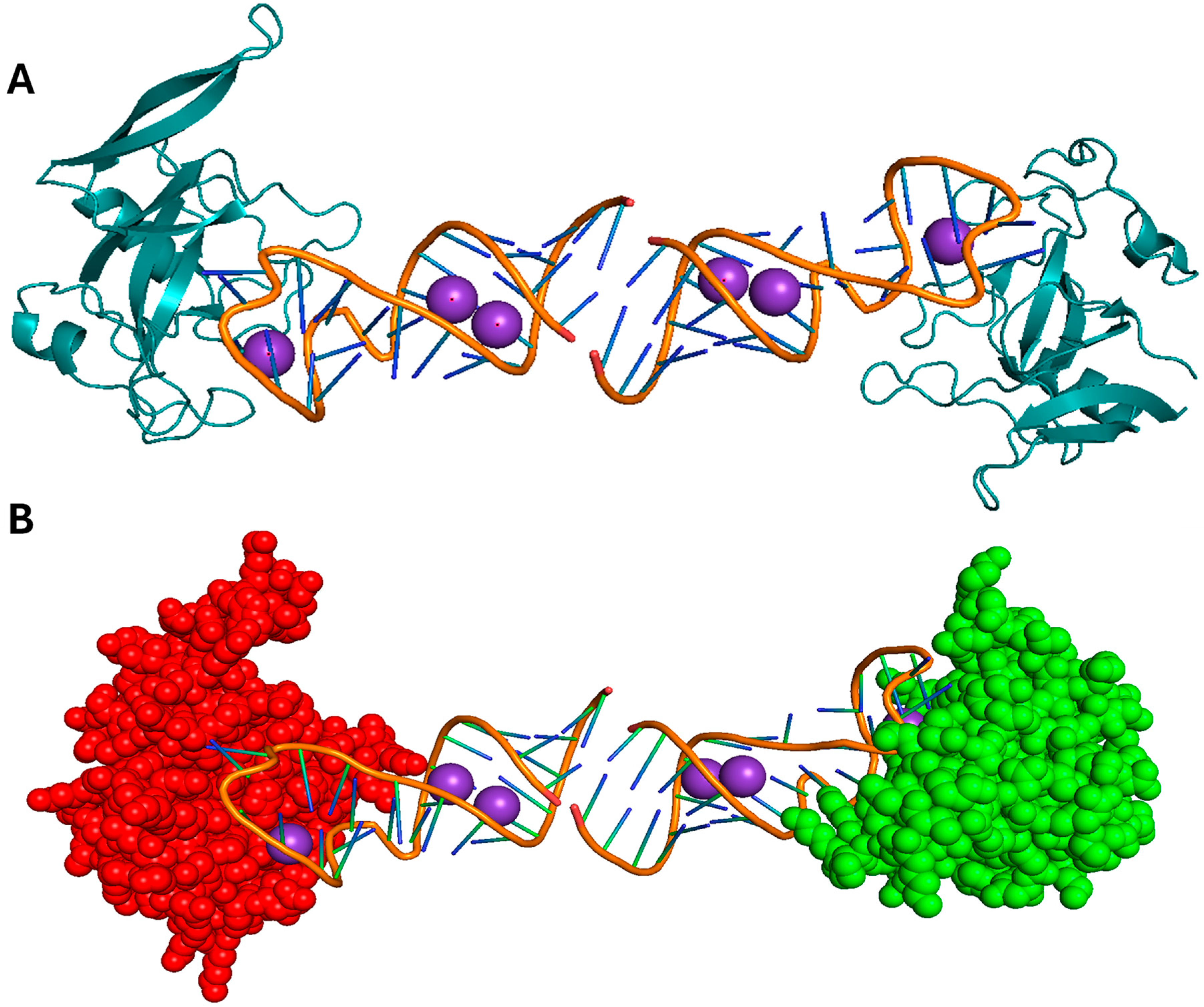

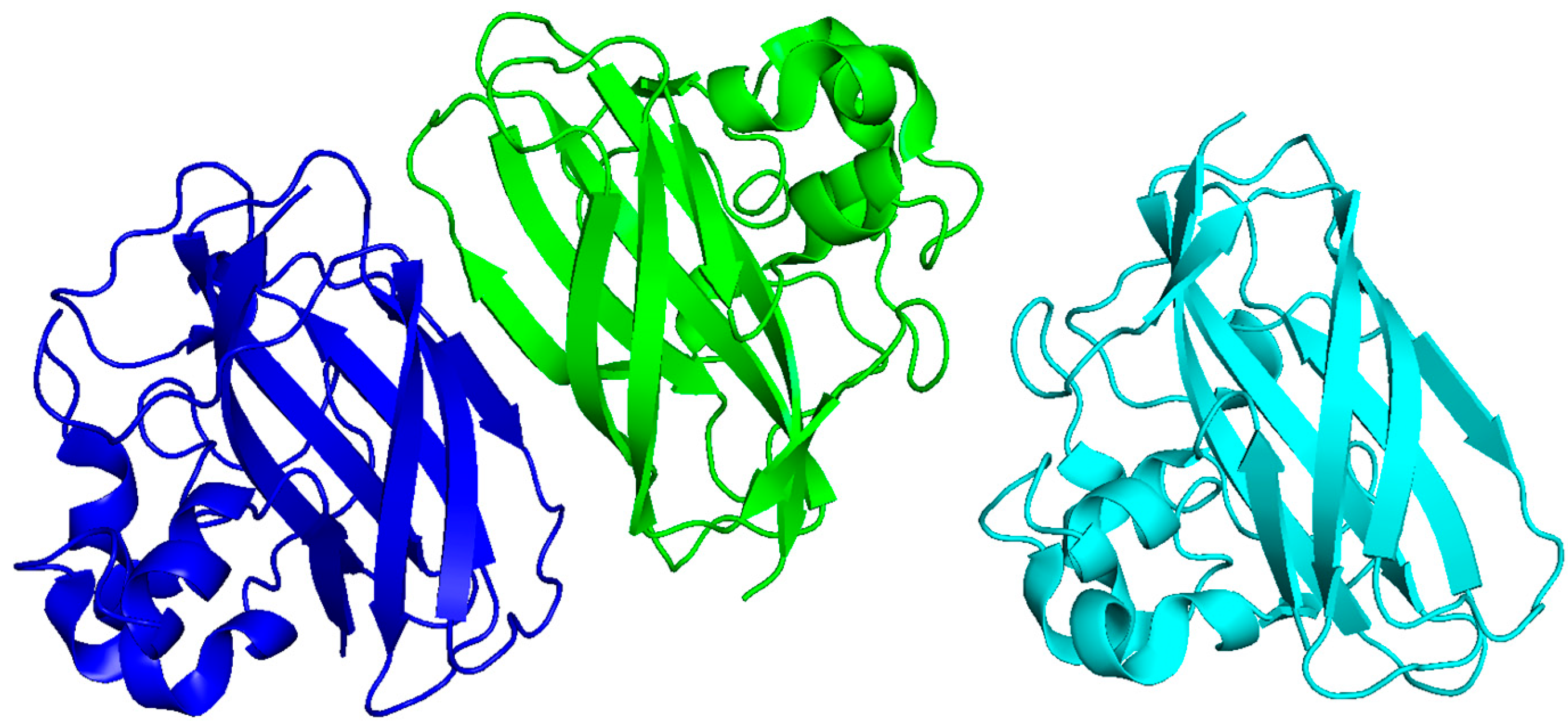
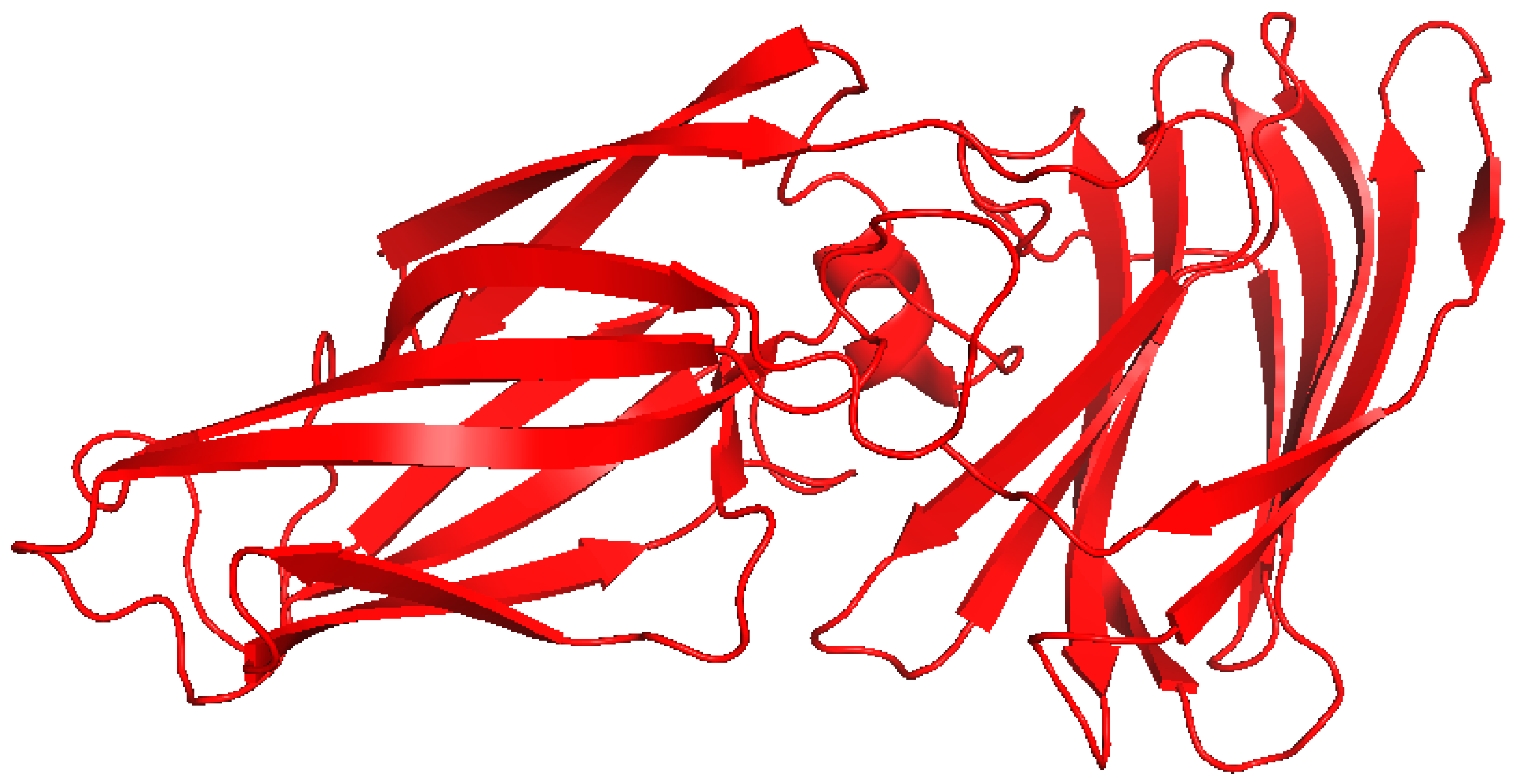
| Viral Protein | Virus | Mechanism of Inflammation | Structural Features | Known Motifs or Binding Sites | PDB | Refs. |
|---|---|---|---|---|---|---|
| Tat | HIV-1 | NF-κB activation; cytokine induction (IL-6, TNF-α, IL-1β) | Intrinsically disordered with transient helices | Interacts with cyclin T1 via core region (aa 49–57) | 1JFW | [22] |
| HBx | HBV | NF-κB activation via IKK complex; oxidative stress; cytokine upregulation | Multiple helical segments and a flexible C-terminal region | Interacts with IKKγ through C-terminal motifs | None 1 | [23,24] |
| E6 | HPV | Degrades p53, activates NF-κB and promotes cytokine expression | Two zinc-binding domains | Binds E6AP via two LxxLL motifs (aa 95–99) | 4GIZ | [25] |
| E7 | HPV | Inactivation of Rb; cell cycle dysregulation; NF-κB pathway stimulation | Zinc-binding C-terminal domain | Zinc-binding motif CxxC (aa 49 to 52) | 2EWL | [26] |
| LMP1 | EBV | Mimics TNFR; constitutive NF-κB activation; cytokine storm (IL-6, IL-8) | Predicted α-helical domains; partially disordered | C-terminal activation regions (CTAR1 and CTAR2) mediate TRAF binding | None 1 | [27] |
| Targeted Mechanism | Shared in | Therapeutic Biologics | Mechanism of Action |
|---|---|---|---|
| NF-κB pathway | Viruses, Fungi | Bortezomib, Anti-TNF mAbs | Inhibits IκB degradation, blocks TNF-driven activation |
| IL-6/IL-1 cytokine axis | Viruses, Fungi | Tocilizumab, Anakinra | IL-6R/IL-1R blockade to reduce cytokine storm |
| Major Challenge | Description | Proposed Solutions & Strategies | Refs. |
|---|---|---|---|
| Targeted Delivery & Bioavailability | Complex biological barriers at inflamed sites, e.g., thick mucus or altered vasculatures, impede the penetration and retention of large protein conjugates. | Development of advanced delivery systems:
| [110,111] |
| Immunogenicity & Rapid Degradation | Conjugates with non-human sequences or novel linkers can trigger immune responses that neutralize the drug. High protease activity in inflamed tissues leads to rapid degradation and reduced half-life. | Protein Engineering Techniques:
| [112,113] |
| Limitations of Computational Design | AI tools (e.g., AlphaFold) have constraints in predicting the behavior of flexible linkers, post-translational modifications, and complex physiological interactions. |
| [114,115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halawa, M.; Gallo, A.L.; Carabetta, V.J. Structural and Functional Insights into Viral and Fungal Proteins Involved in Chronic Inflammation and Their Biologic Treatments. Pharmaceutics 2025, 17, 1466. https://doi.org/10.3390/pharmaceutics17111466
Halawa M, Gallo AL, Carabetta VJ. Structural and Functional Insights into Viral and Fungal Proteins Involved in Chronic Inflammation and Their Biologic Treatments. Pharmaceutics. 2025; 17(11):1466. https://doi.org/10.3390/pharmaceutics17111466
Chicago/Turabian StyleHalawa, Mohamed, Alicia L. Gallo, and Valerie J. Carabetta. 2025. "Structural and Functional Insights into Viral and Fungal Proteins Involved in Chronic Inflammation and Their Biologic Treatments" Pharmaceutics 17, no. 11: 1466. https://doi.org/10.3390/pharmaceutics17111466
APA StyleHalawa, M., Gallo, A. L., & Carabetta, V. J. (2025). Structural and Functional Insights into Viral and Fungal Proteins Involved in Chronic Inflammation and Their Biologic Treatments. Pharmaceutics, 17(11), 1466. https://doi.org/10.3390/pharmaceutics17111466









