Nanostructured Delivery Systems for Curcumin: Improving Bioavailability and Plaque-Targeting Efficacy in Atherosclerosis
Abstract
1. Introduction
2. Formation and Development of AS
3. Traditional Medicine for AS
3.1. Hypolipidemic Drug
3.2. Anti-Inflammatory Drugs
3.3. Antiplatelet Drugs
3.4. Anticoagulants
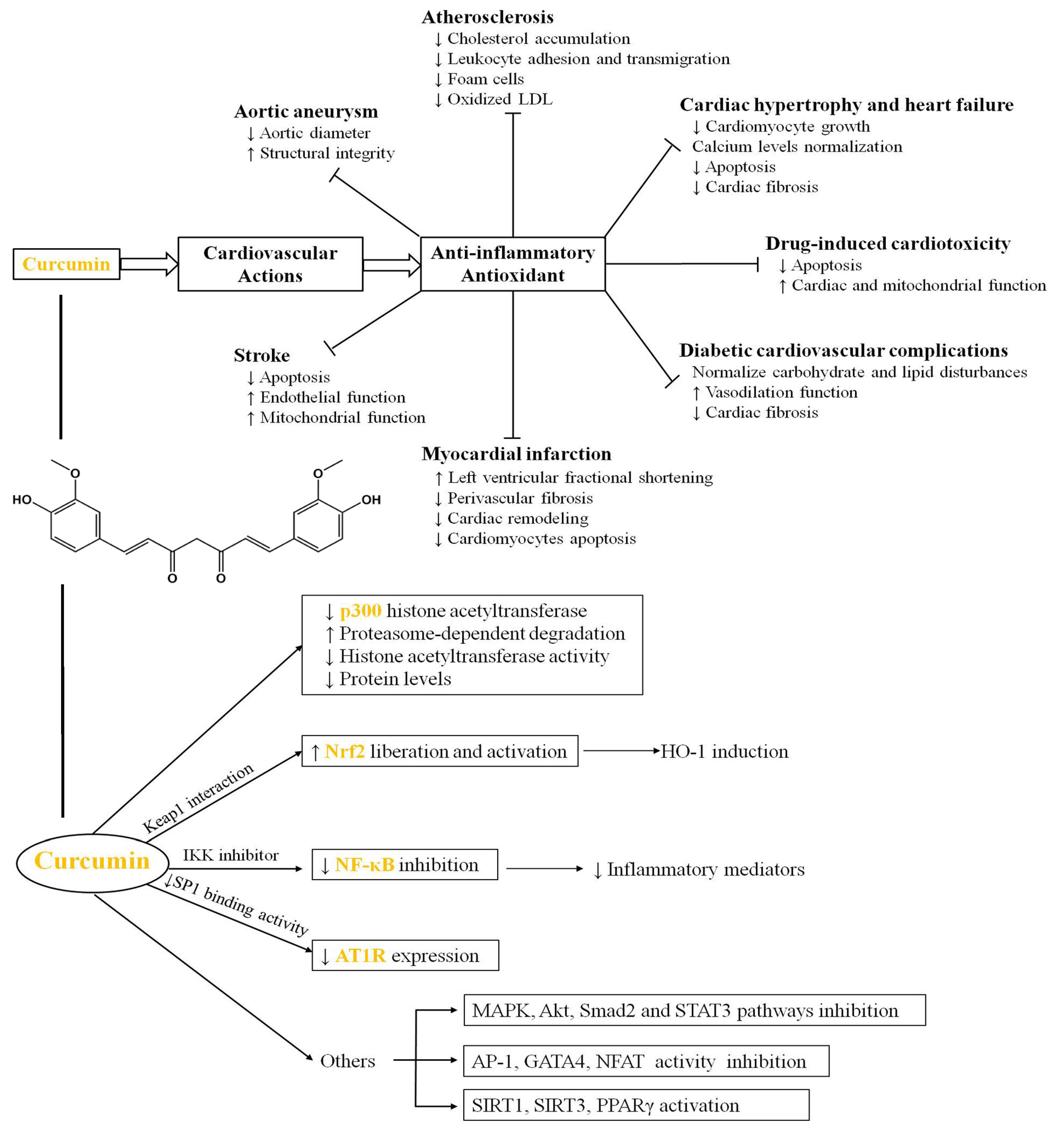
4. Cur in the Treatment of AS
4.1. Inhibition of the Inflammatory Response
4.2. ROS Scavenging and Antioxidant
4.3. Hypolipidemic
4.4. Lowering Blood Pressure
4.5. Other Properties of Cur
4.6. Clinical Research
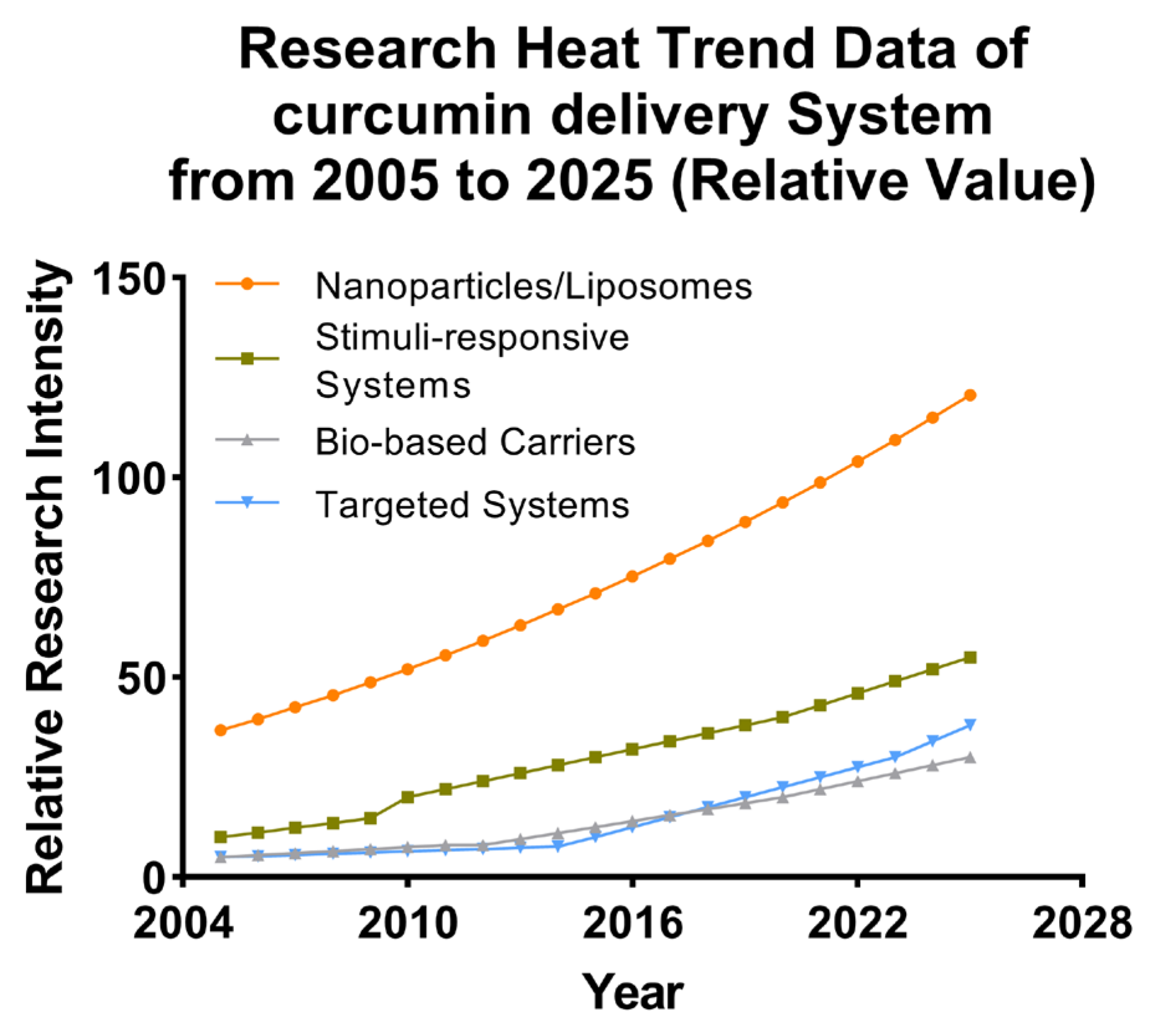
5. Nanostructured Delivery Systems for Cur
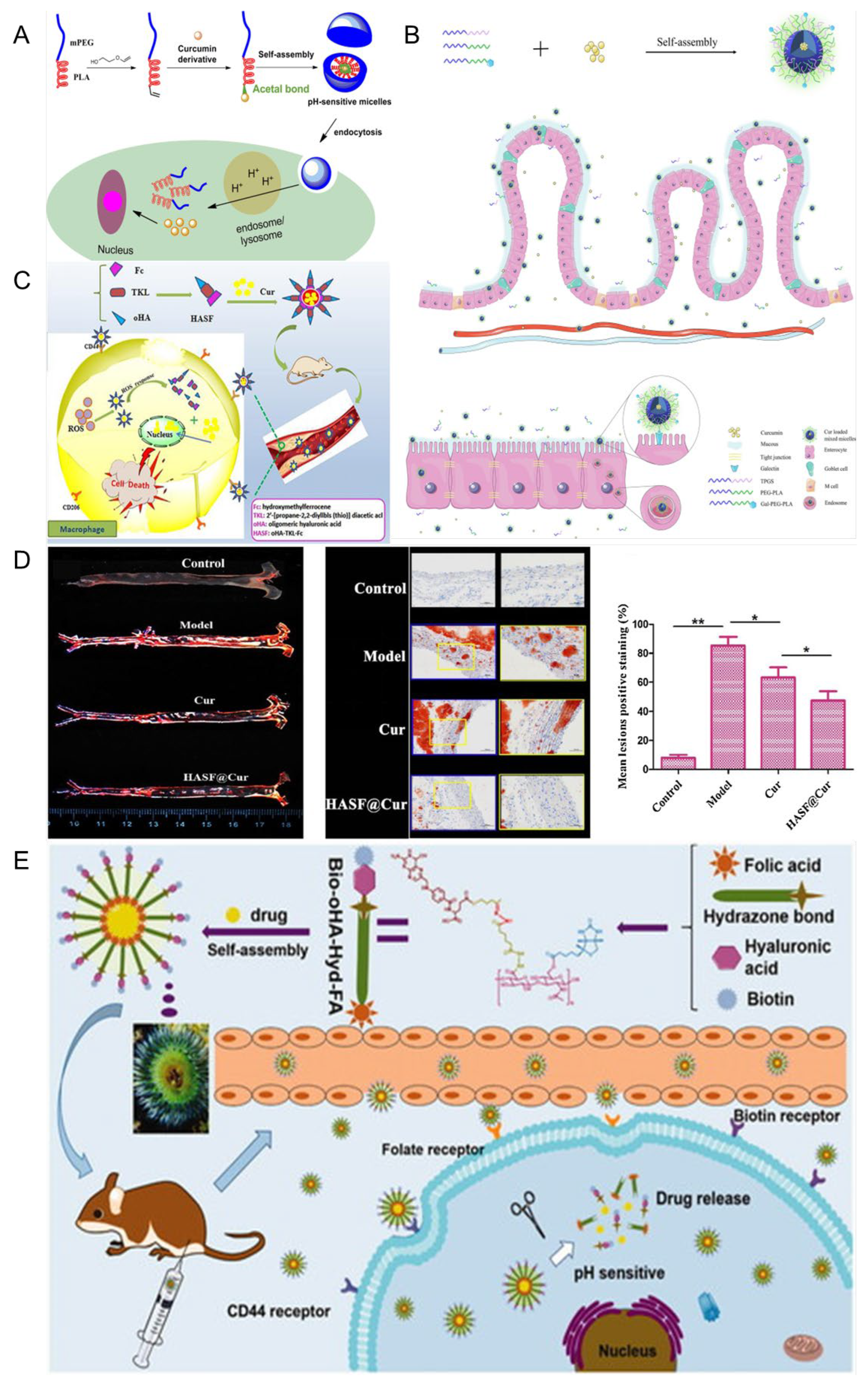
5.1. Polymeric Micelles
5.2. Liposome
5.3. Nanoparticles
5.4. Microspheres and Microcapsules
5.5. Hydrogel
5.6. Nanosuspension
5.7. Other Cur Delivery Platforms
6. The Limitations of Curcumin in Clinical Applications
7. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Xu, J.; Liu, G.; Di, C.; Zhao, X.; Li, X.; Li, Y.; Pang, N.; Yang, C.; et al. Engineered Nanoplatelets for Targeted Delivery of Plasminogen Activators to Reverse Thrombus in Multiple Mouse Thrombosis Models. Adv. Mater. 2020, 32, e1905145. [Google Scholar] [CrossRef] [PubMed]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Bairey Merz, C.N.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.; et al. The Lancet women and cardiovascular disease Commission: Reducing the global burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Vasan, R.S. Epidemiology of cardiovascular disease in young individuals. Nat. Rev. Cardiol. 2018, 15, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Joseph, P.G.; McKee, M.; Anand, S.S.; Teo, K.K.; Schwalm, J.D.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 2: Prevention and Treatment of Cardiovascular Disease. Circ. Res. 2017, 121, 695–710. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Y.; Zeng, Y.; Gao, G.F.; Liang, X.; Zhou, M.; Wan, X.; Yu, S.; Jiang, Y.; Naghavi, M.; et al. Rapid health transition in China, 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 381, 1987–2015. [Google Scholar] [CrossRef]
- Murray, C.J.; Richards, M.A.; Newton, J.N.; Fenton, K.A.; Anderson, H.R.; Atkinson, C.; Bennett, D.; Bernabé, E.; Blencowe, H.; Bourne, R.; et al. UK health performance: Findings of the Global Burden of Disease Study 2010. Lancet 2013, 381, 997–1020. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, J.; Wang, M.; Zhang, X.; Zhou, M. Epidemiology of cardiovascular disease in China: Current features and implications. Nat. Rev. Cardiol. 2019, 16, 203–212. [Google Scholar] [CrossRef]
- Turk-Adawi, K.; Sarrafzadegan, N.; Fadhil, I.; Taubert, K.; Sadeghi, M.; Wenger, N.K.; Tan, N.S.; Grace, S.L. Cardiovascular disease in the Eastern Mediterranean region: Epidemiology and risk factor burden. Nat. Rev. Cardiol. 2018, 15, 106–119. [Google Scholar] [CrossRef]
- Cohen Tervaert, J.W. Cardiovascular disease due to accelerated atherosclerosis in systemic vasculitides. Best. Pract. Res. Clin. Rheumatol. 2013, 27, 33–44. [Google Scholar] [CrossRef]
- Falk, E. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006, 47, C7–C12. [Google Scholar] [CrossRef]
- Ali, A.H.; Younis, N.; Abdallah, R.; Shaer, F.; Dakroub, A.; Ayoub, M.A.; Iratni, R.; Yassine, H.M.; Zibara, K.; Orekhov, A.; et al. Lipid-Lowering Therapies for Atherosclerosis: Statins, Fibrates, Ezetimibe and PCSK9 Monoclonal Antibodies. Curr. Med. Chem. 2021, 28, 7427–7445. [Google Scholar] [CrossRef]
- Paraskevas, K.I.; Gloviczki, P.; Antignani, P.L.; Comerota, A.J.; Dardik, A.; Davies, A.H.; Eckstein, H.H.; Faggioli, G.; Fernandes, E.F.J.; Fraedrich, G.; et al. Benefits and drawbacks of statins and non-statin lipid lowering agents in carotid artery disease. Prog. Cardiovasc. Dis. 2022, 73, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Niimi, M.; Watanabe, T.; Wang, Y.; Liang, J.; Fan, J. Treatment of atherosclerosis by traditional Chinese medicine: Questions and quandaries. Atherosclerosis 2018, 277, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Hamburg, N.M.; Creager, M.A. Contemporary Medical Management of Peripheral Artery Disease. Circ. Res. 2021, 128, 1868–1884. [Google Scholar] [CrossRef]
- Ding, N.; Sang, Y.; Chen, J.; Ballew, S.H.; Kalbaugh, C.A.; Salameh, M.J.; Blaha, M.J.; Allison, M.; Heiss, G.; Selvin, E.; et al. Cigarette Smoking, Smoking Cessation, and Long-Term Risk of 3 Major Atherosclerotic Diseases. J. Am. Coll. Cardiol. 2019, 74, 498–507. [Google Scholar] [CrossRef]
- Mohammedi, K.; Woodward, M.; Hirakawa, Y.; Zoungas, S.; Colagiuri, S.; Hamet, P.; Harrap, S.; Poulter, N.; Matthews, D.R.; Marre, M.; et al. Presentations of major peripheral arterial disease and risk of major outcomes in patients with type 2 diabetes: Results from the ADVANCE-ON study. Cardiovasc. Diabetol. 2016, 15, 129. [Google Scholar] [CrossRef]
- Chung, E.J.; Tirrell, M. Recent Advances in Targeted, Self-Assembling Nanoparticles to Address Vascular Damage Due to Atherosclerosis. Adv. Healthc. Mater. 2015, 4, 2408–2422. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Lahori, D.; Namit, A.F.; Paxton, Z.; Ratna, N.; Thornton, D.; Ramana, K.V. Curcumin in Inflammatory Complications: Therapeutic Applications and Clinical Evidence. Int. J. Mol. Sci. 2025, 26, 9366. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lv, Q.; Liu, X.a.; Ye, X.; Cao, L.; Wang, M.; Li, J.; Yang, Y.; Li, L.; Wang, S. Natural compounds from botanical drugs targeting mTOR signaling pathway as promising therapeutics for atherosclerosis: A review. Front. Pharmacol. 2023, 14, 1083875. [Google Scholar] [CrossRef]
- Gu, H.-F.; Li, H.-Z.; Tang, Y.-L.; Tang, X.-Q.; Zheng, X.-L.; Liao, D.-F. Nicotinate-Curcumin Impedes Foam Cell Formation from THP-1 Cells through Restoring Autophagy Flux. PLoS ONE 2016, 11, e0154820. [Google Scholar] [CrossRef]
- Riccardi, G.; Giosuè, A.; Calabrese, I.; Vaccaro, O. Dietary recommendations for prevention of atherosclerosis. Cardiovasc. Res. 2022, 118, 1188–1204. [Google Scholar] [CrossRef]
- Raitakari, O.; Pahkala, K.; Magnussen, C.G. Prevention of atherosclerosis from childhood. Nat. Rev. Cardiol. 2022, 19, 543–554. [Google Scholar] [CrossRef]
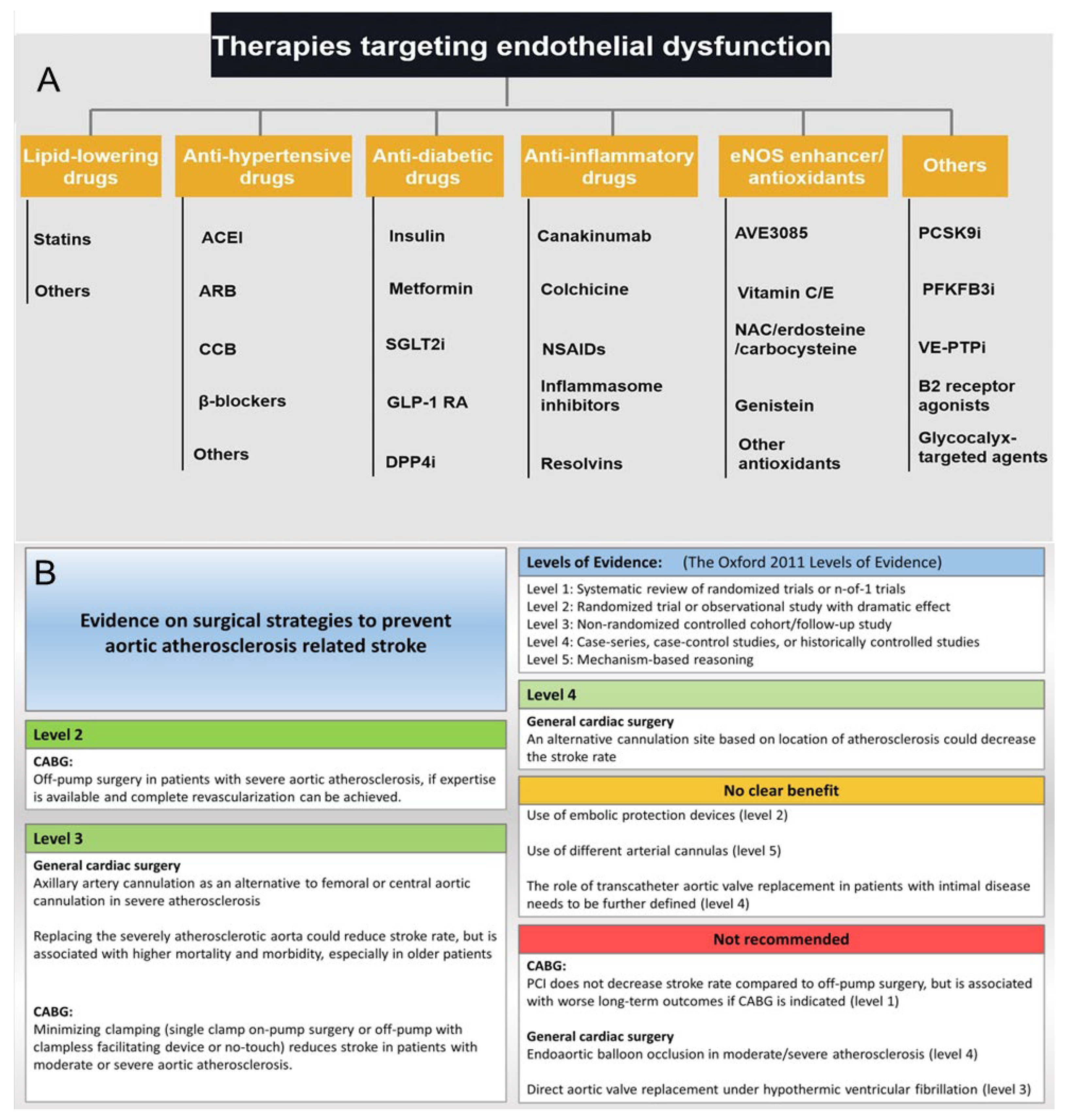
- Knol, W.G.; Budde, R.P.J.; Mahtab, E.A.F.; Bekkers, J.A.; Bogers, A. Intimal aortic atherosclerosis in cardiac surgery: Surgical strategies to prevent embolic stroke. Eur. J. Cardiothorac. Surg. 2021, 60, 1259–1267. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- De Mol, J.; De Korte, D.H.; Smit, V.; Kuiper, J.; Foks, A.C. Rapamycin reduces atherosclerotic plaque inflammation in aged mice. Cardiovasc. Res. 2024, 120, cvae088.204. [Google Scholar] [CrossRef]
- Al-kuraishy, H.M.; Sulaiman, G.M.; Mohsin, M.H.; Mohammed, H.A.; Dawood, R.A.; Albuhadily, A.K.; Al-Gareeb, A.I.; Albukhaty, S.; Abomughaid, M.M. Targeting of AMPK/MTOR signaling in the management of atherosclerosis: Outmost leveraging. Int. J. Biol. Macromol. 2025, 309, 142933. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Yue, K.; Zhong, W.; Zhang, G.; Wang, L.; Zhang, H.; Zhang, X. Delivery of rapamycin by biomimetic peptide nanoparticles targeting oxidized low-density lipoprotein in atherosclerotic plaques. Biomater. Sci. 2024, 12, 4181–4193. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Lin, J.; Zhao, Y.; Wang, X.; Lv, Q.; Li, W.; Tang, R.; Fu, G. Plaque-Specific Adhesive Balloons Coated with Calcium Phosphate Nanoparticles Loaded with Rapamycin for Atherosclerosis Therapy. Adv. Funct. Mater. 2024, 34, 2315317. [Google Scholar] [CrossRef]
- Zhou, Z.; Lan, H.; Tan, H.; Wang, Y.; Chen, W.; Batur, S.; Fu, C.; Kong, L.; Yang, C.; Niu, B.; et al. ROS-responsive biomimetic nanovesicles to plaque microenvironment in targeted therapy of atherosclerosis. Nano Today 2024, 59, 102530. [Google Scholar] [CrossRef]
- Blum, A. HMG-CoA reductase inhibitors (statins), inflammation, and endothelial progenitor cells-New mechanistic insights of atherosclerosis. Biofactors 2014, 40, 295–302. [Google Scholar] [CrossRef]
- Masana, L.; Plana, N.; Andreychuk, N.; Ibarretxe, D. Lipid lowering combination therapy: From prevention to atherosclerosis plaque treatment. Pharmacol. Res. 2023, 190, 106738. [Google Scholar] [CrossRef]
- Okopień, B.; Bułdak, Ł.; Bołdys, A. Benefits and risks of the treatment with fibrates--a comprehensive summary. Expert. Rev. Clin. Pharmacol. 2018, 11, 1099–1112. [Google Scholar] [CrossRef]
- Lampsas, S.; Xenou, M.; Oikonomou, E.; Pantelidis, P.; Lysandrou, A.; Sarantos, S.; Goliopoulou, A.; Kalogeras, K.; Tsigkou, V.; Kalpis, A.; et al. Lipoprotein(a) in Atherosclerotic Diseases: From Pathophysiology to Diagnosis and Treatment. Molecules 2023, 28, 969. [Google Scholar] [CrossRef]
- Ito, M.K. Long-chain omega-3 fatty acids, fibrates and niacin as therapeutic options in the treatment of hypertriglyceridemia: A review of the literature. Atherosclerosis 2015, 242, 647–656. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- González, L.; Bulnes, J.F.; Orellana, M.P.; Muñoz Venturelli, P.; Martínez Rodriguez, G. The Role of Colchicine in Atherosclerosis: From Bench to Bedside. Pharmaceutics 2022, 14, 1395. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Bharadwaj, D.; Prasad, G.; Grechko, A.V.; Sazonova, M.A.; Orekhov, A.N. Anti-Inflammatory Therapy for Atherosclerosis: Focusing on Cytokines. Int. J. Mol. Sci. 2021, 22, 7016. [Google Scholar] [CrossRef] [PubMed]
- Fijałkowski, Ł.; Skubiszewska, M.; Grześk, G.; Koech, F.K.; Nowaczyk, A. Acetylsalicylic Acid-Primus Inter Pares in Pharmacology. Molecules 2022, 27, 8412. [Google Scholar] [CrossRef]
- Hiatt, W.R.; Bonaca, M.P.; Patel, M.R.; Nehler, M.R.; Debus, E.S.; Anand, S.S.; Capell, W.H.; Brackin, T.; Jaeger, N.; Hess, C.N.; et al. Rivaroxaban and Aspirin in Peripheral Artery Disease Lower Extremity Revascularization: Impact of Concomitant Clopidogrel on Efficacy and Safety. Circulation 2020, 142, 2219–2230. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef]
- Piran, S.; Schulman, S. Treatment of bleeding complications in patients on anticoagulant therapy. Blood 2019, 133, 425–435. [Google Scholar] [CrossRef]
- Schuliga, M. The inflammatory actions of coagulant and fibrinolytic proteases in disease. Mediat. Inflamm. 2015, 2015, 437695. [Google Scholar] [CrossRef]
- Shen, C.Y.; Jiang, J.G.; Yang, L.; Wang, D.W.; Zhu, W. Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: Pharmacological mechanisms and implications for drug discovery. Br. J. Pharmacol. 2017, 174, 1395–1425. [Google Scholar] [CrossRef]
- Chainoglou, E.; Hadjipavlou-Litina, D. Curcumin in Health and Diseases: Alzheimer’s Disease and Curcumin Analogues, Derivatives, and Hybrids. Int. J. Mol. Sci. 2020, 21, 1975. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Amjad, M.W.; Hussain, F.; Ahmed, T.A.; Khan, S. Exploring recent developments to improve antioxidant, anti-inflammatory and antimicrobial efficacy of curcumin: A review of new trends and future perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1316–1326. [Google Scholar] [CrossRef]
- Ruan, H.; Huang, Q.; Wan, B.; Yang, M. Curcumin alleviates lipopolysaccharides-induced inflammation and apoptosis in vascular smooth muscle cells via inhibition of the NF-κB and JNK signaling pathways. Inflammopharmacology 2022, 30, 517–525. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Grechko, A.V.; Orekhova, V.A.; Chegodaev, Y.S.; Wu, W.-K.; Orekhov, A.N. Oxidative Stress and Antioxidants in Atherosclerosis Development and Treatment. Biology 2020, 9, 60. [Google Scholar] [CrossRef]
- Soltani, S.; Boozari, M.; Cicero, A.F.G.; Jamialahmadi, T.; Sahebkar, A. Effects of phytochemicals on macrophage cholesterol efflux capacity: Impact on atherosclerosis. Phytother. Res. 2021, 35, 2854–2878. [Google Scholar] [CrossRef]
- Gutsche, L.C.; Dörfler, J.; Hübner, J. Curcumin as a complementary treatment in oncological therapy: A systematic review. Eur. J. Clin. Pharmacol. 2025, 81, 1–33. [Google Scholar] [CrossRef]
- Li, C.; Kang, B.; Zhang, T.; Gu, H.; Song, P.; Chen, J.; Wang, X.; Xu, B.; Zhao, W.; Zhang, J. Dietary Pattern and Dietary Energy from Fat Associated with Sarcopenia in Community-Dwelling Older Chinese People: A Cross-Sectional Study in Three Regions of China. Nutrients 2020, 12, 3689. [Google Scholar] [CrossRef]
- Nair, V.V.; Cabrera, P.; Ramírez-Lecaros, C.; Jara, M.O.; Brayden, D.J.; Morales, J.O. Buccal delivery of small molecules and biologics: Of mucoadhesive polymers, films, and nanoparticles—An update. Int. J. Pharm. 2023, 636, 122789. [Google Scholar] [CrossRef]
- Hao, M.; Zhang, C.; Wang, T.; Hu, H. Pharmacological effects, formulations, and clinical research progress of curcumin. Front. Pharmacol. 2025, 16, 1509045. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yang, T.; Yang, K.; Yu, G.; Li, J.; Xiang, W.; Chen, H. Curcumin and Curcuma longa Extract in the Treatment of 10 Types of Autoimmune Diseases: A Systematic Review and Meta-Analysis of 31 Randomized Controlled Trials. Front. Immunol. 2022, 13, 896476. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sureda, A.; Devkota, H.P.; Pittalà, V.; Barreca, D.; Silva, A.S.; Tewari, D.; Xu, S.; Nabavi, S.M. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol. Adv. 2020, 38, 107343. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, K.; Fang, R.; Liu, J.; Liu, M.; Ma, J.; Wang, H.; Ding, M.; Wang, X.; Song, Y.; et al. Nanotechnological strategies to increase the oxygen content of the tumor. Front. Pharmacol. 2023, 14, 1140362. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bai, Y.; Li, Y.; Li, X.; Luo, K. Reprogramming the immunosuppressive tumor microenvironment through nanomedicine: An immunometabolism perspective. eBioMedicine 2024, 107, 105301. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Mishra, S.; Chaturvedi, R.; Pandey, A. Therapeutic potential of curcumin in cardiovascular disease: Targeting atherosclerosis pathophysiology. Biomed. Pharmacother. 2025, 190, 118412. [Google Scholar] [CrossRef] [PubMed]
- Della Schiava, N.; Lermusiaux, P. Comments and question on “Selective inhibition of endothelial NF-κB signaling attenuates chronic intermittent hypoxia-induced atherosclerosis in mice”. Atherosclerosis 2018, 272, 247. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Abdollahi, E.; Nikfar, B.; Chaichian, S.; Ekhlasi-Hundrieser, M. Curcumin as a potential modulator of M1 and M2 macrophages: New insights in atherosclerosis therapy. Heart Fail. Rev. 2019, 24, 399–409. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Sreedhar, R.; Giridharan, V.V.; Afrin, R.; Harima, M.; Miyashita, S.; Hara, M.; Suzuki, K.; et al. Curcumin alleviates renal dysfunction and suppresses inflammation by shifting from M1 to M2 macrophage polarization in daunorubicin induced nephrotoxicity in rats. Cytokine 2016, 84, 1–9. [Google Scholar] [CrossRef]
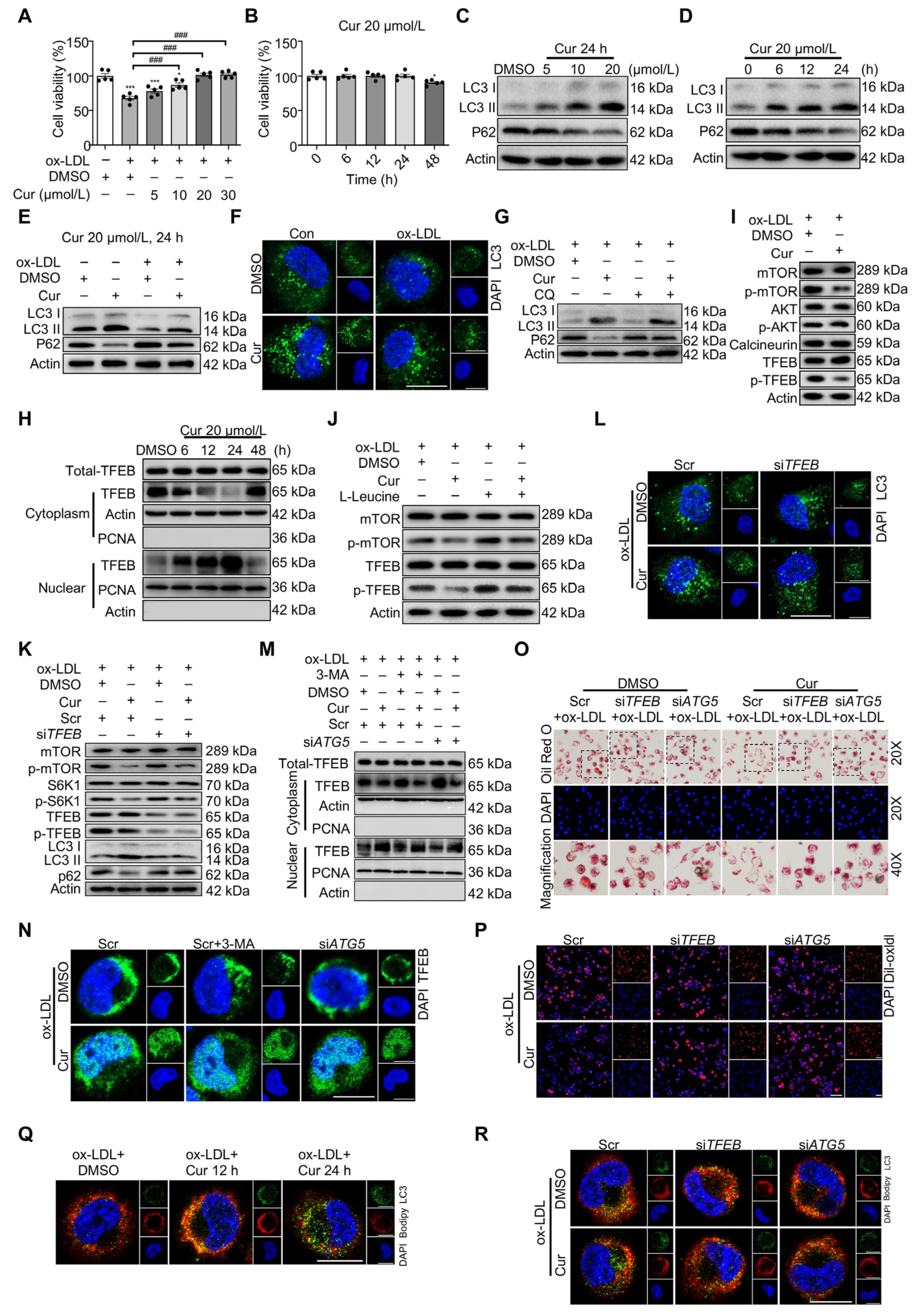
- Li, X.; Zhu, R.; Jiang, H.; Yin, Q.; Gu, J.; Chen, J.; Ji, X.; Wu, X.; Fu, H.; Wang, H.; et al. Autophagy enhanced by curcumin ameliorates inflammation in atherogenesis via the TFEB-P300-BRD4 axis. Acta Pharm. Sin. B 2022, 12, 2280–2299. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Guo, Q.; Li, X.; Tang, T.; Li, C.; Wang, H.; Sun, Y.; Feng, Q.; Ma, C.; Gao, C.; et al. Curcumin Suppresses IL-1β Secretion and Prevents Inflammation through Inhibition of the NLRP3 Inflammasome. J. Immunol. 2018, 200, 2835–2846. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Maguire, E.M.; Pearce, S.W.A.; Xiao, Q. Foam cell formation: A new target for fighting atherosclerosis and cardiovascular disease. Vasc. Pharmacol. 2019, 112, 54–71. [Google Scholar] [CrossRef]
- Wang, B.; Tang, X.; Yao, L.; Wang, Y.; Chen, Z.; Li, M.; Wu, N.; Wu, D.; Dai, X.; Jiang, H.; et al. Disruption of USP9X in macrophages promotes foam cell formation and atherosclerosis. J. Clin. Investig. 2022, 132, e154217. [Google Scholar] [CrossRef]
- Berger, R.G.; Lunkenbein, S.; Ströhle, A.; Hahn, A. Antioxidants in food: Mere myth or magic medicine? Crit. Rev. Food Sci. Nutr. 2012, 52, 162–171. [Google Scholar] [CrossRef]
- Rajaram, S.; Jones, J.; Lee, G.J. Plant-Based Dietary Patterns, Plant Foods, and Age-Related Cognitive Decline. Adv. Nutr. 2019, 10, S422–S436. [Google Scholar] [CrossRef]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef]
- Ravindran, J.; Subbaraju, G.V.; Ramani, M.V.; Sung, B.; Aggarwal, B.B. Bisdemethylcurcumin and structurally related hispolon analogues of curcumin exhibit enhanced prooxidant, anti-proliferative and anti-inflammatory activities in vitro. Biochem. Pharmacol. 2010, 79, 1658–1666. [Google Scholar] [CrossRef]
- Liu, C.; Rokavec, M.; Huang, Z.; Hermeking, H. Curcumin activates a ROS/KEAP1/NRF2/miR-34a/b/c cascade to suppress colorectal cancer metastasis. Cell Death Differ. 2023, 30, 1771–1785. [Google Scholar] [CrossRef]
- Kim, J.S.; Oh, J.M.; Choi, H.; Kim, S.W.; Kim, S.W.; Kim, B.G.; Cho, J.H.; Lee, J.; Lee, D.C. Activation of the Nrf2/HO-1 pathway by curcumin inhibits oxidative stress in human nasal fibroblasts exposed to urban particulate matter. BMC Complement. Med. Ther. 2020, 20, 101. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T.; Samini, F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed. Pharmacother. 2017, 87, 223–229. [Google Scholar] [CrossRef]
- Halasz, G.; Piepoli, M.F. Focus on atherosclerosis and lipids. Eur. J. Prev. Cardiol. 2020, 27, 1571–1574. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Malhotra, P.; Ma, K.; Singla, A.; Hedroug, O.; Saksena, S.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. SREBP2 mediates the modulation of intestinal NPC1L1 expression by curcumin. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G148–G155. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zou, J.; Zhang, S.; Li, X.; Lu, M. Hypocholesterolemic Activity of Curcumin Is Mediated by Down-regulating the Expression of Niemann-Pick C1-like 1 in Hamsters. J. Agric. Food Chem. 2017, 65, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Zhang, S.; Li, P.; Zheng, X.; Feng, D. Supplementation with curcumin inhibits intestinal cholesterol absorption and prevents atherosclerosis in high-fat diet-fed apolipoprotein E knockout mice. Nutr. Res. 2018, 56, 32–40. [Google Scholar] [CrossRef]
- Shin, S.K.; Ha, T.Y.; McGregor, R.A.; Choi, M.S. Long-term curcumin administration protects against atherosclerosis via hepatic regulation of lipoprotein cholesterol metabolism. Mol. Nutr. Food Res. 2011, 55, 1829–1840. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, Y.; Zhang, X.; Aa, J.; Wang, G.; Xie, Y. Curcumin regulates endogenous and exogenous metabolism via Nrf2-FXR-LXR pathway in NAFLD mice. Biomed. Pharmacother. 2018, 105, 274–281. [Google Scholar] [CrossRef]
- Yang, J.; Zou, J.; Mai, H.; Hong, T.; Liu, H.; Feng, D. Curcumin protects against high-fat diet-induced nonalcoholic simple fatty liver by inhibiting intestinal and hepatic NPC1L1 expression via down-regulation of SREBP-2/HNF1α pathway in hamsters. J. Nutr. Biochem. 2023, 119, 109403. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.; Sekhon-Loodu, S.; Mantso, T.; Panayiotidis, M.I. Phytochemicals in regulating fatty acid β-oxidation: Potential underlying mechanisms and their involvement in obesity and weight loss. Pharmacol. Ther. 2016, 165, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Lone, J.; Choi, J.H.; Kim, S.W.; Yun, J.W. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J. Nutr. Biochem. 2016, 27, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Hurtubise, J.; McLellan, K.; Durr, K.; Onasanya, O.; Nwabuko, D.; Ndisang, J.F. The Different Facets of Dyslipidemia and Hypertension in Atherosclerosis. Curr. Atheroscler. Rep. 2016, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, W.; Li, M.; Ren, H.; Chen, C.; Wang, J.; Wang, W.E.; Yang, J.; Zeng, C. Curcumin Exerts its Anti-hypertensive Effect by Down-regulating the AT1 Receptor in Vascular Smooth Muscle Cells. Sci. Rep. 2016, 6, 25579. [Google Scholar] [CrossRef]
- Zhuang, X.D.; Liao, L.Z.; Dong, X.B.; Hu, X.; Guo, Y.; Du, Z.M.; Liao, X.X.; Wang, L.C. Design, synthesis, and antihypertensive activity of curcumin-inspired compounds via ACE inhibition and vasodilation, along with a bioavailability study for possible benefit in cardiovascular diseases. Drug Des. Devel Ther. 2016, 10, 129–139. [Google Scholar] [CrossRef]
- Li, H.B.; Xu, M.L.; Du, M.M.; Yu, X.J.; Bai, J.; Xia, W.J.; Dai, Z.M.; Li, C.X.; Li, Y.; Su, Q.; et al. Curcumin ameliorates hypertension via gut-brain communication in spontaneously hypertensive rat. Toxicol. Appl. Pharmacol. 2021, 429, 115701. [Google Scholar] [CrossRef]
- Keihanian, F.; Saeidinia, A.; Bagheri, R.K.; Johnston, T.P.; Sahebkar, A. Curcumin, hemostasis, thrombosis, and coagulation. J. Cell Physiol. 2018, 233, 4497–4511. [Google Scholar] [CrossRef]
- Kim, D.C.; Ku, S.K.; Bae, J.S. Anticoagulant activities of curcumin and its derivative. BMB Rep. 2012, 45, 221–226. [Google Scholar] [CrossRef]
- Hu, M.; Peng, X.; Shi, S.; Wan, C.; Cheng, C.; Yu, X. Dialdehyde xanthan gum and curcumin synergistically crosslinked bioprosthetic valve leaflets with anti-thrombotic, anti-inflammatory and anti-calcification properties. Carbohydr. Polym. 2023, 310, 120724. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and Type 2 Diabetes Mellitus: Prevention and Treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef]
- Thota, R.N.; Acharya, S.H.; Garg, M.L. Curcumin and/or omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance and blood lipids in individuals with high risk of type 2 diabetes: A randomised controlled trial. Lipids Health Dis. 2019, 18, 31. [Google Scholar] [CrossRef]
- Balakumar, P.; Venkatesan, K.; Abdulla Khan, N.; Raghavendra, N.M.; Venugopal, V.; Bharathi, D.R.; Fuloria, N.K. Mechanistic insights into the beneficial effects of curcumin on insulin resistance: Opportunities and challenges. Drug Discov. Today 2023, 28, 103627. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Liu, Y.; Luo, X.; Lei, W.; Xie, L. Antiviral and virucidal effects of curcumin on transmissible gastroenteritis virus in vitro. J. Gen. Virol. 2020, 101, 1079–1084. [Google Scholar] [CrossRef]
- Mrityunjaya, M.; Pavithra, V.; Neelam, R.; Janhavi, P.; Halami, P.M.; Ravindra, P.V. Immune-Boosting, Antioxidant and Anti-inflammatory Food Supplements Targeting Pathogenesis of COVID-19. Front. Immunol. 2020, 11, 570122. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the active substance of turmeric: Its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Deng, H.; Hwang, H.M. The current application of nanotechnology in food and agriculture. J. Food Drug Anal. 2019, 27, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Laux, P.; Tentschert, J.; Riebeling, C.; Braeuning, A.; Creutzenberg, O.; Epp, A.; Fessard, V.; Haas, K.H.; Haase, A.; Hund-Rinke, K.; et al. Nanomaterials: Certain aspects of application, risk assessment and risk communication. Arch. Toxicol. 2018, 92, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yu, B.; Cong, H.; Shen, Y. Delivery process and effective design of vectors for cancer therapy. J. Mater. Chem. B 2022, 10, 6896–6921. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Millican, R.; Sherwood, J.; Martin, S.; Jo, H.; Yoon, Y.S.; Brott, B.C.; Jun, H.W. Recent advances in nanomaterials for therapy and diagnosis for atherosclerosis. Adv. Drug Deliv. Rev. 2021, 170, 142–199. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Chauhan, P.N.; Noolvi, M.N.; Chaturvedi, K.; Ganguly, K.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric micelles: Basic research to clinical practice. Int. J. Pharm. 2017, 532, 249–268. [Google Scholar] [CrossRef]
- Petrov, P.D.; Davidova, S.; Satchanska, G. Polymer Micelles as Nanocarriers of Bioactive Peptides. Polymers 2025, 17, 1174. [Google Scholar] [CrossRef]
- Zheng, Y.; Oz, Y.; Gu, Y.; Ahamad, N.; Shariati, K.; Chevalier, J.; Kapur, D.; Annabi, N. Rational design of polymeric micelles for targeted therapeutic delivery. Nano Today 2024, 55, 102147. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Shang, H.; Sun, X.; An, K.; Zhang, Q.; Qiao, N. Preparation of pH/Light dual-responsive biocompatible polymer micelles: Application to curcumin delivery. J. Drug Deliv. Sci. Technol. 2023, 86, 104652. [Google Scholar] [CrossRef]
- Li, M.; Gao, M.; Fu, Y.; Chen, C.; Meng, X.; Fan, A.; Kong, D.; Wang, Z.; Zhao, Y. Acetal-linked polymeric prodrug micelles for enhanced curcumin delivery. Colloids Surf. B Biointerfaces 2016, 140, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Du, X.; Fu, M.; Khan, A.R.; Ji, J.; Liu, W.; Zhai, G. Galactosamine-modified PEG-PLA/TPGS micelles for the oral delivery of curcumin. Int. J. Pharm. 2021, 595, 120227. [Google Scholar] [CrossRef]
- Hou, X.; Lin, H.; Zhou, X.; Cheng, Z.; Li, Y.; Liu, X.; Zhao, F.; Zhu, Y.; Zhang, P.; Chen, D. Novel dual ROS-sensitive and CD44 receptor targeting nanomicelles based on oligomeric hyaluronic acid for the efficient therapy of atherosclerosis. Carbohydr. Polym. 2020, 232, 115787. [Google Scholar] [CrossRef]
- Liu, M.; Wang, B.; Guo, C.; Hou, X.; Cheng, Z.; Chen, D. Novel multifunctional triple folic acid, biotin and CD44 targeting pH-sensitive nano-actiniaes for breast cancer combinational therapy. Drug Deliv. 2019, 26, 1002–1016. [Google Scholar] [CrossRef]
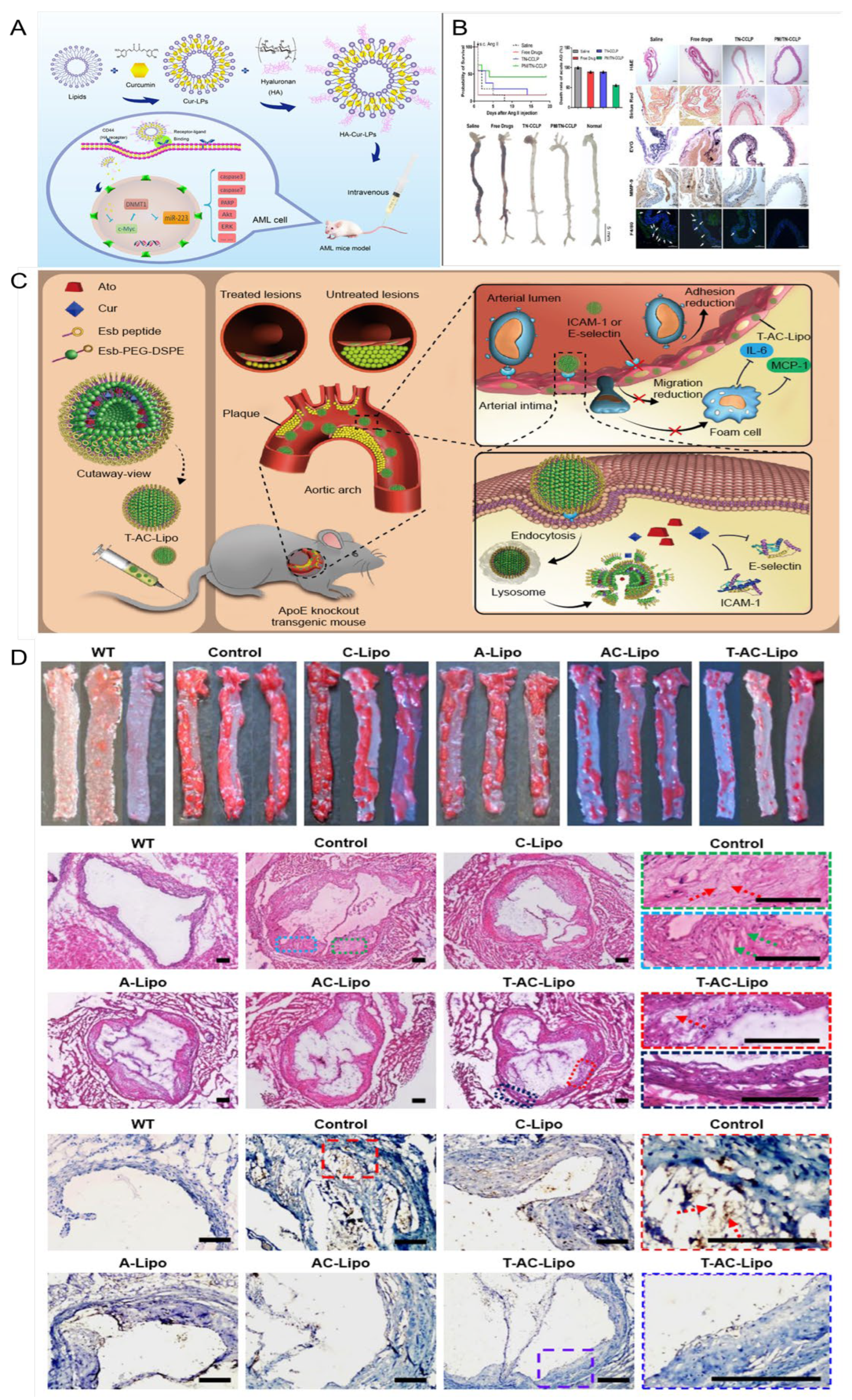
- Sun, D.; Zhou, J.K.; Zhao, L.; Zheng, Z.Y.; Li, J.; Pu, W.; Liu, S.; Liu, X.S.; Liu, S.J.; Zheng, Y.; et al. Novel Curcumin Liposome Modified with Hyaluronan Targeting CD44 Plays an Anti-Leukemic Role in Acute Myeloid Leukemia In Vitro and In Vivo. ACS Appl. Mater. Interfaces 2017, 9, 16857–16868. [Google Scholar] [CrossRef]
- Sebatana, R.; Kudzai, K.D.; Magura, A.; Mdlophane, A.; Zeevaart, J.R.; Sathekge, M.; Kahts, M.; Mdanda, S.; Witika, B.A. An Insight to Nanoliposomes as Smart Radiopharmaceutical Delivery Tools for Imaging Atherosclerotic Plaques: Positron Emission Tomography Applications. Pharmaceutics 2025, 17, 240. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Liu, X.; Widjaya, A.S.; Jiang, B.; Jiang, Y. Macrophage-biomimetic anti-inflammatory liposomes for homing and treating of aortic dissection. J. Control. Release 2021, 337, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiao, H.; Lin, C.; Sun, W.; Wu, T.; Wang, J.; Chen, B.; Chen, X.; Cheng, D. Synergistic effects of liposomes encapsulating atorvastatin calcium and curcumin and targeting dysfunctional endothelial cells in reducing atherosclerosis. Int. J. Nanomed. 2019, 14, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Kelley, W.J.; Safari, H.; Lopez-Cazares, G.; Eniola-Adefeso, O. Vascular-targeted nanocarriers: Design considerations and strategies for successful treatment of atherosclerosis and other vascular diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 909–926. [Google Scholar] [CrossRef]
- Jan, N.; Yehia, R.M.; Mohammed, M.; Ahmed, M.B.; Seedahmed, H.G.; Shah, H.; Badshah, S.F.; Shi, G. Cell membrane-coated nanoparticles: A new frontier in atherosclerosis therapy. Chem. Eng. J. 2025, 524, 169183. [Google Scholar] [CrossRef]
- Shi, Z.; Huang, J.; Chen, C.; Zhang, X.; Ma, Z.; Liu, Q. Lipid nanoparticles encapsulating curcumin for imaging and stabilization of vulnerable atherosclerotic plaques via phagocytic “eat-me” signals. J. Control. Release 2024, 373, 265–276. [Google Scholar] [CrossRef]
- Feng, Y.; Xiao, K.; He, Y.; Du, B.; Hong, J.; Yin, H.; Lu, D.; Luo, F.; Li, Z.; Li, J.; et al. Tough and biodegradable polyurethane-curcumin composited hydrogel with antioxidant, antibacterial and antitumor properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111820. [Google Scholar] [CrossRef]
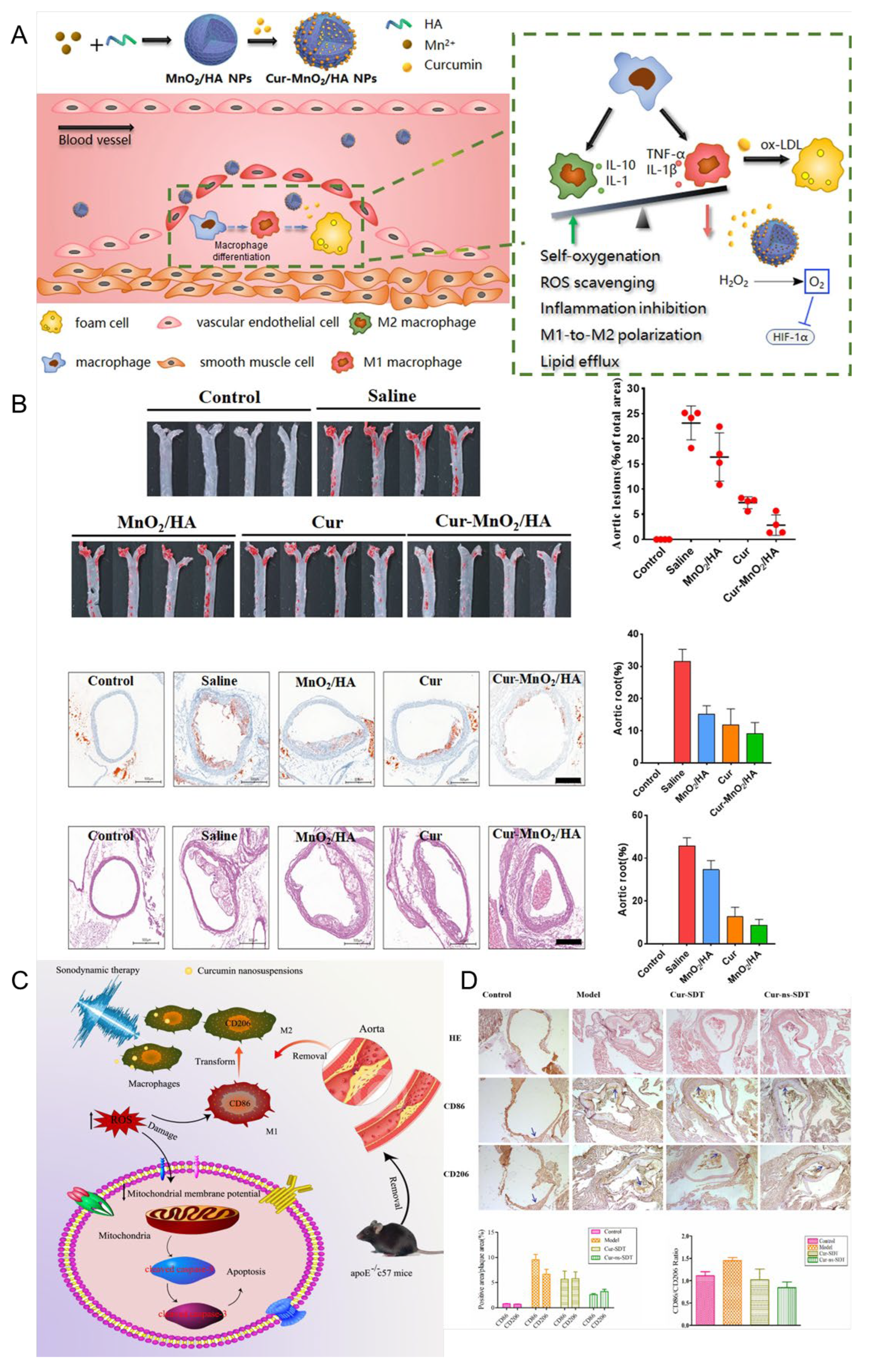
- Sun, W.; Xu, Y.; Yao, Y.; Yue, J.; Wu, Z.; Li, H.; Shen, G.; Liao, Y.; Wang, H.; Zhou, W. Self-oxygenation mesoporous MnO2 nanoparticles with ultra-high drug loading capacity for targeted arteriosclerosis therapy. J. Nanobiotechnol. 2022, 20, 88. [Google Scholar] [CrossRef]
- Deng, Y.; Deng, X.; Li, Y.; Tian, J.; Wu, M.; Tang, J.; Liang, X.; Yang, X.; He, X.; Liu, Y.; et al. Size-adjustable nanoparticles co-target macrophages and endothelial cells for enhanced atherosclerosis therapy. Colloids Surf. B Biointerfaces 2025, 255, 114952. [Google Scholar] [CrossRef]
- Yu, L.; Liang, Y.; Gao, L.; Chen, P.; Yu, Z.; Zhang, M.; Hinek, A.; Mao, S. Reconstruction of Postinfarcted Cardiac Functions Through Injection of Tanshinone IIA@ Reactive Oxygen Species-Sensitive Microspheres Encapsulated in a Thermoreversible Hydrogel. ENERGY Environ. Mater. 2024, 7, e12555. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, M.S. Advances in drug-loaded microspheres for targeted, controlled, and sustained drug delivery: Potential, applications, and future directions. Biomed. Pharmacother. 2025, 189, 118244. [Google Scholar] [CrossRef]
- Patra, D.; Sleem, F. A new method for pH triggered curcumin release by applying poly(L-lysine) mediated nanoparticle-congregation. Anal. Chim. Acta 2013, 795, 60–68. [Google Scholar] [CrossRef]
- Saranya, T.S.; Rajan, V.K.; Biswas, R.; Jayakumar, R.; Sathianarayanan, S. Synthesis, characterisation and biomedical applications of curcumin conjugated chitosan microspheres. Int. J. Biol. Macromol. 2018, 110, 227–233. [Google Scholar] [CrossRef]
- Duan, Y.; Lin, J.; Yue, J.; Li, Y.; Liao, J.; Sun, Y.; Wang, Q.; Duan, Y.; Li, Z. Temperature-sensitive hydrogel inhibits VEGFA-dependent neovascularization in atherosclerosis progression. Sci. China Mater. 2025, 68, 280–291. [Google Scholar] [CrossRef]
- Li, S.; Long, M.; Li, J.; Zhang, Y.; Feng, N.; Zhang, Z. Improved topical delivery of curcumin by hydrogels formed by composite carriers integrated with cyclodextrin metal-organic frameworks and cy clodextrin nanosponges. Int. J. Pharm. 2024, 8, 100310. [Google Scholar] [CrossRef]
- Hu, C.; Liu, W.; Long, L.; Wang, Z.; Zhang, W.; He, S.; Lu, L.; Fan, H.; Yang, L.; Wang, Y. Regeneration of infarcted hearts by myocardial infarction-responsive injectable hydrogels with combined anti-apoptosis, anti-inflammatory and pro-angiogenesis properties. Biomaterials 2022, 290, 121849. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cong, Z.; Gao, P.; Wang, Y. Nanosuspensions technology as a master key for nature products drug delivery and In vivo fate. Eur. J. Pharm. Sci. 2023, 185, 106425. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Kather, F.S.; Boddu, S.H.S.; Attimarad, M.; Nair, A.B. Nanosuspension Innovations: Expanding Horizons in Drug Delivery Techniques. Pharmaceutics 2025, 17, 136. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Jiang, J.; Zhang, C.; Zhao, M.; Chen, Z.; Wang, N.; Hu, D.; Liu, X.; Peng, H.; et al. Sonodynamic therapy in atherosclerosis by curcumin nanosuspensions: Preparation design, efficacy evaluation, and mechanisms analysis. Eur. J. Pharm. Biopharm. 2020, 146, 101–110. [Google Scholar] [CrossRef]
- Carvalho, T.; Ezazi, N.Z.; Correia, A.; Vilela, C.; Santos, H.A.; Freire, C.S.R. Gelatin-Lysozyme Nanofibrils Electrospun Patches with Improved Mechanical, Antioxidant, and Bioresorbability Properties for Myocardial Regeneration Applications. Adv. Funct. Mater. 2022, 32, 2113390. [Google Scholar] [CrossRef]
- Jahanmardi, Y.; Tavanaie, M.A.; Tehrani-Bagha, A.R. Curcumin release from blended polycaprolactone/polylactic acid electrospun nanofibrous meshes. J. Ind. Text. 2019, 50, 1065–1078. [Google Scholar] [CrossRef]
- Iqbal, N.; Bano, A.; Ahmed, T.; Nabeel, M.I.; Ullah, J.; Musharraf, S.G.; Saleem, I.; Malik, M.I. Fish gelatin nanoparticles: An effective carrier for curcumin delivery. J. Drug Deliv. Sci. Technol. 2025, 114, 107505. [Google Scholar] [CrossRef]
- Louis, M.J.; Balakrishnan, A.; Joseph, A.; Shanmughan, P.; Maliakel, B.; Illathu Madhavamenon, K. Two-Stage Supramolecular Self-Assembly-Directed Collagen-Peptide-Decorated Liposomal Complexes of Curcumin Microspheres with Enhanced Solubility and Bioavailability. ACS Omega 2023, 8, 26243–26252. [Google Scholar] [CrossRef]

| Delivery System | Advantages | Disadvantages | Therapeutic Efficiency |
|---|---|---|---|
| Polymeric micelles | Small size (~10–100 nm) enables passive targeting via the EPR effect in atherosclerotic plaques. The amphiphilic core–shell structure solubilizes hydrophobic curcumin (solubility ↑ 10–100-fold). Biodegradable polymers (e.g., PLGA and PEG-PLA) reduce systemic toxicity [112]. |
Low drug-loading efficiency (typically 5–15%).
Rapid dissociation in circulation (t1/2 < 6 h) may require frequent dosing. | Moderate efficiency: passive targeting improves plaque accumulation by 2–3-fold vs. free curcumin; inhibits macrophage foam cell formation [113]. |
| Liposomes | Biocompatible and biodegradable; mimics cell membranes for enhanced cellular uptake. Surface modification (e.g., ligand conjugation) enables active targeting (e.g., CD36 for macrophage targeting). Protects curcumin from enzymatic degradation [59]. |
Poor physical stability (prone to aggregation/fusion).
High production cost; batch-to-batch variability in size distribution. | High efficiency. |
| Nanoparticles | Versatile materials (e.g., solid lipid nanoparticles and silica NPs) allow for a tunable size (20–200 nm) and sustained release. High drug-loading capacity (up to 30% for lipid-based NPs). Enhanced penetration into atherosclerotic plaques via transcytosis [59]. | Potential immunogenicity (e.g., inorganic NPs may trigger cytokine release). Risk of burst release if not surface-modified [141]. | High efficiency: sustained release prolongs action (t1/2 > 24 h); reduces inflammation markers (TNF-α and IL-6) by 40–50%. |
|
Microspheres/ Microcapsules |
Sustained release profile (weeks to months) reduces dosing frequency.
Macrophage phagocytosis targeting (due to ~1–10 μm size) enhances plaque accumulation. | Their large size limits systemic circulation; cannot cross endothelial barriers efficiently. Their brittle structure may cause premature drug leakage [142]. | Low systemic efficiency: localized delivery reduces neointimal hyperplasia by 50% in rabbit models but has limited plaque penetration [132]. |
| Hydrogels | High water content mimics the extracellular matrix; biocompatible for in situ injection. Thermo/pH-responsive release (e.g., PLGA-PEG-PLGA hydrogels) matches the plaque microenvironment (low pH). Protects curcumin from oxidation [135]. | Their low degradation rate may lead to incomplete drug release. Poor mechanical strength in dynamic vascular environments [135]. | Moderate localized efficiency: in situ injection reduces plaque inflammation by 35% but requires surgical implantation. |
| Nanosuspensions | Simple preparation (no carrier needed); high drug loading (up to 90%). Small particle size (~200–500 nm) improves oral bioavailability (curcumin solubility ↑ 5–10-fold). Cost-effective for large-scale production [136]. | High surface energy causes aggregation in aqueous media. Limited targeting ability; rapid clearance by the reticuloendothelial system [137]. | Low efficiency: limited targeting; reduces plasma LDL by 15–20% but shows minimal plaque accumulation [137]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yu, T.; Zhang, C.; Yang, Z.; Yu, D.; He, B.; Liang, Y. Nanostructured Delivery Systems for Curcumin: Improving Bioavailability and Plaque-Targeting Efficacy in Atherosclerosis. Pharmaceutics 2025, 17, 1465. https://doi.org/10.3390/pharmaceutics17111465
Liu Y, Yu T, Zhang C, Yang Z, Yu D, He B, Liang Y. Nanostructured Delivery Systems for Curcumin: Improving Bioavailability and Plaque-Targeting Efficacy in Atherosclerosis. Pharmaceutics. 2025; 17(11):1465. https://doi.org/10.3390/pharmaceutics17111465
Chicago/Turabian StyleLiu, Yu, Tengfei Yu, Chao Zhang, Zhiyong Yang, Dahai Yu, Bin He, and Yan Liang. 2025. "Nanostructured Delivery Systems for Curcumin: Improving Bioavailability and Plaque-Targeting Efficacy in Atherosclerosis" Pharmaceutics 17, no. 11: 1465. https://doi.org/10.3390/pharmaceutics17111465
APA StyleLiu, Y., Yu, T., Zhang, C., Yang, Z., Yu, D., He, B., & Liang, Y. (2025). Nanostructured Delivery Systems for Curcumin: Improving Bioavailability and Plaque-Targeting Efficacy in Atherosclerosis. Pharmaceutics, 17(11), 1465. https://doi.org/10.3390/pharmaceutics17111465





