From Vineyard to Hydrogel: Antioxidant, Anti-Inflammatory, and Regenerative Potential of Grape Skin Extract in Diabetic Wound Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Grape Skin Extract (GSE)
2.2. Preparation of Hydrogels Loaded with GSE (HG+GSE)
2.3. In Vivo Assessment of Safety and Wound Healing Potential of HG+GSE
2.3.1. Ethics Statement
2.3.2. Study Animals
2.3.3. Acute Dermal Irritation Assessment
- BG group—rats treated with the base gel without GSE,
- GSE group—rats treated with the gel with incorporated GSE (HG+GSE).
2.3.4. Induction of Diabetes Mellitus
2.3.5. Induction of Excision Wounds
2.3.6. Treatment Protocol
- Negative control (NC)—included rats with wounds that were left untreated;
- Positive control (PC)—included rats with wounds treated with a cream containing 1% silver sulfadiazine;
- Hydrogel base (HG)—included rats with wounds treated with a hydrogel without grape skin extract;
- Hydrogel enriched with GSE (HG + GSE)—included rats with wounds treated with a hydrogel with incorporated grape skin extract.
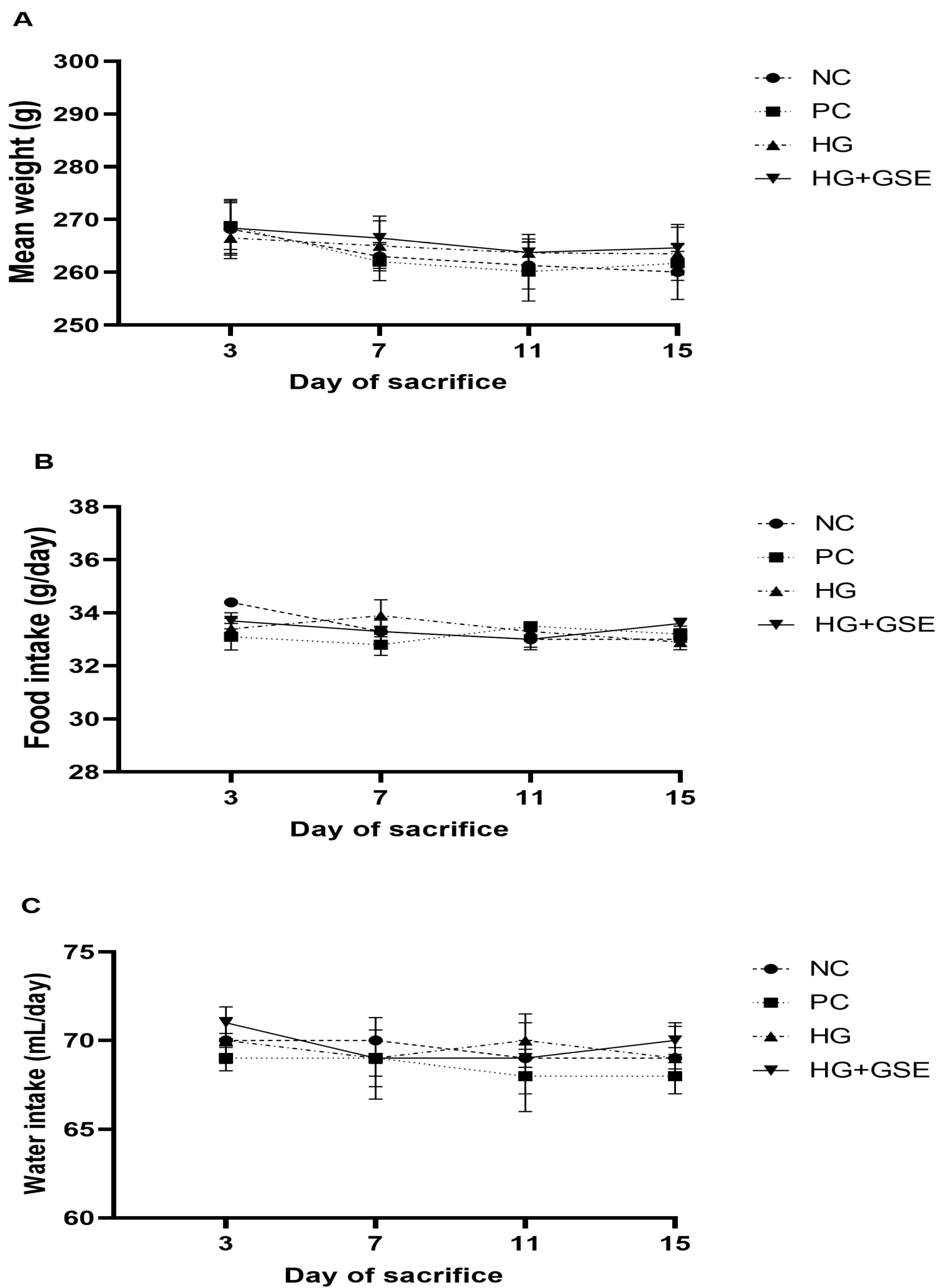
2.3.7. Monitoring of Food and Water Consumption and Body Weight Changes in Rats
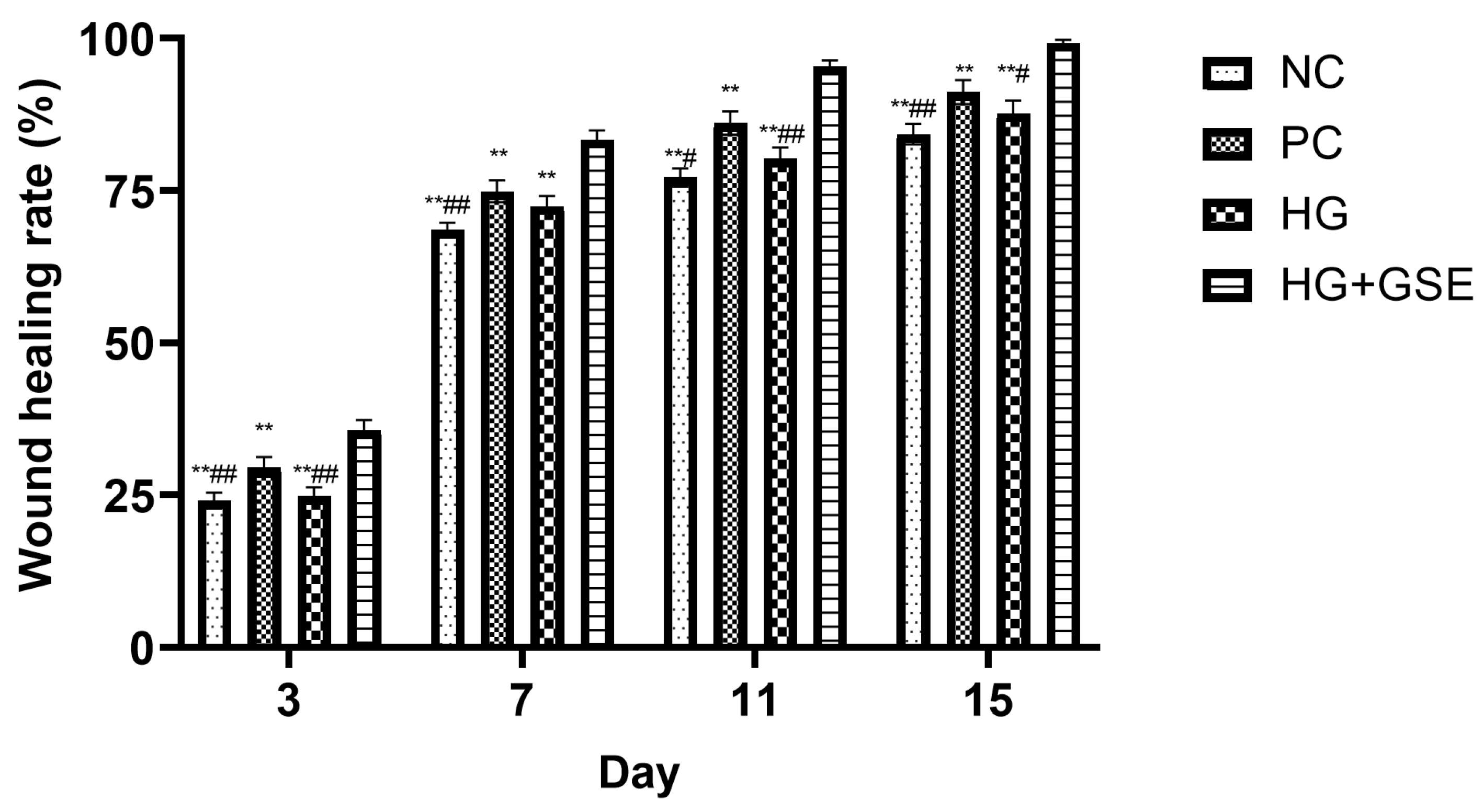
2.3.8. Wound Contraction Estimation
2.4. Biochemical Parameters
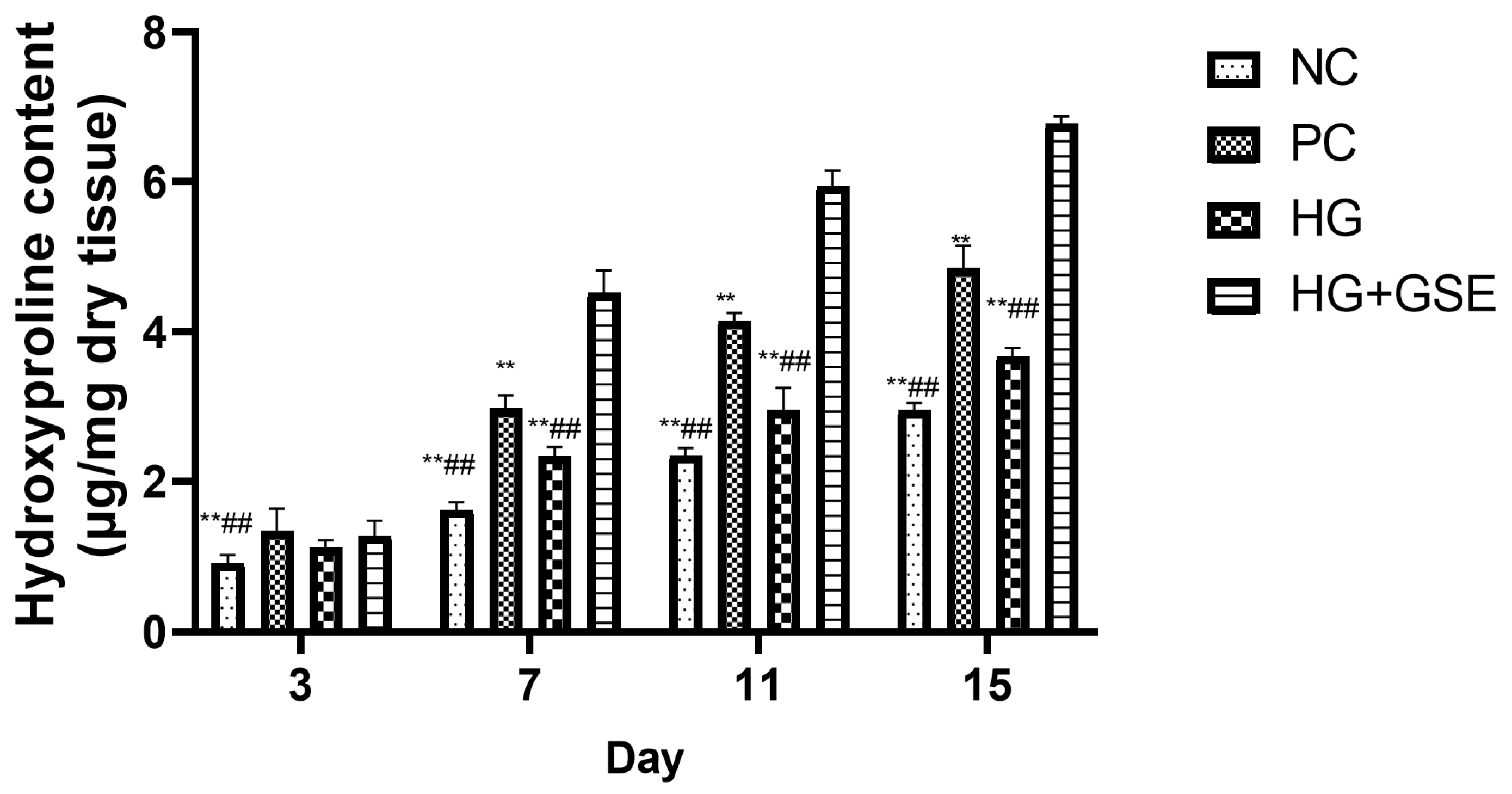
2.4.1. Hydroxyproline Estimation
2.4.2. Estimation of Tissue Redox Parameters
2.4.3. Estimation of System Inflammatory Markers
2.5. Histological Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Skin Tolerability of HG+GSE in Rats
3.2. Therapeutic Effects of HG+GSE on Diabetic Wound Healing
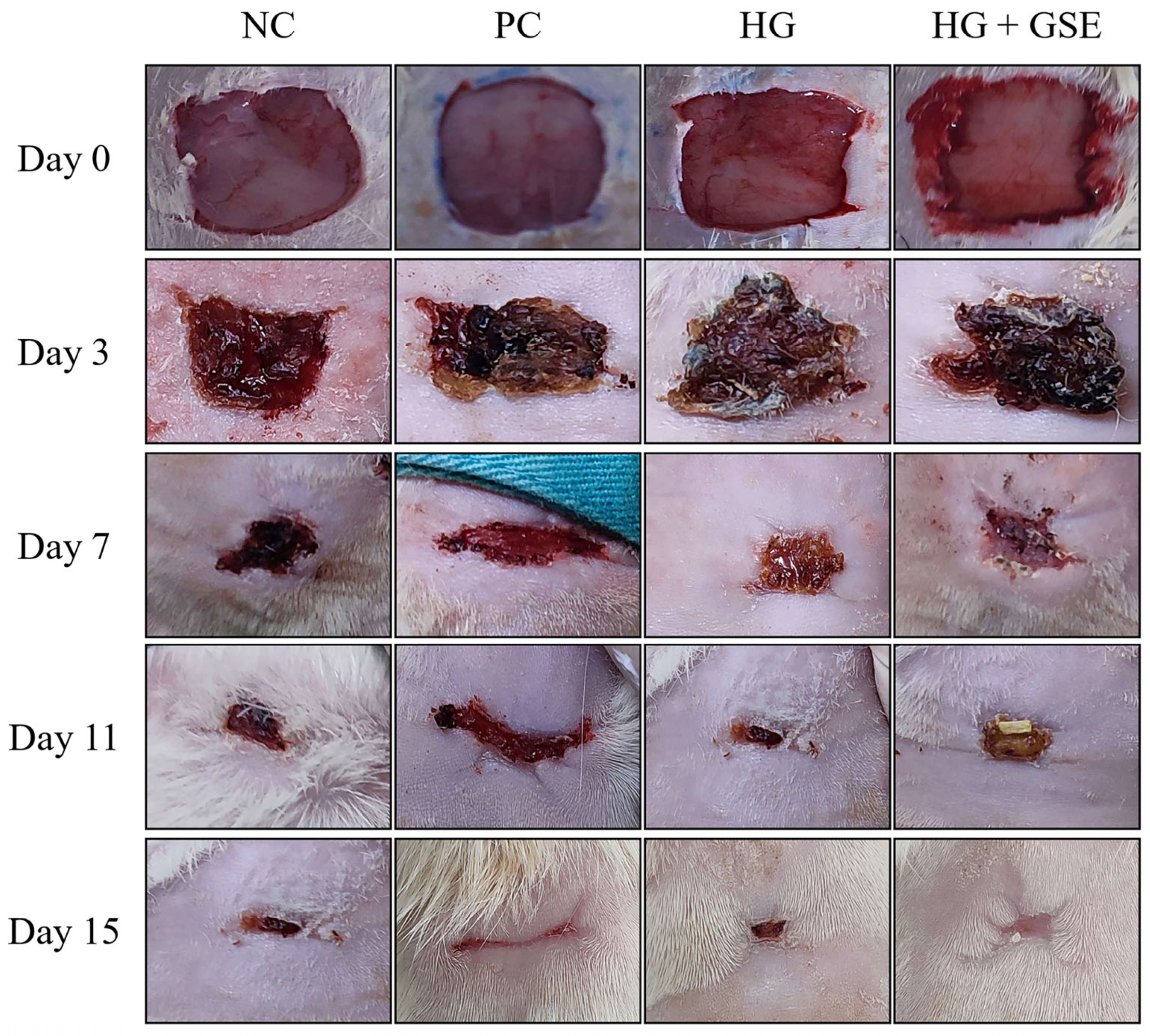
3.2.1. Wound Healing Progression Following HG+GSE Treatment
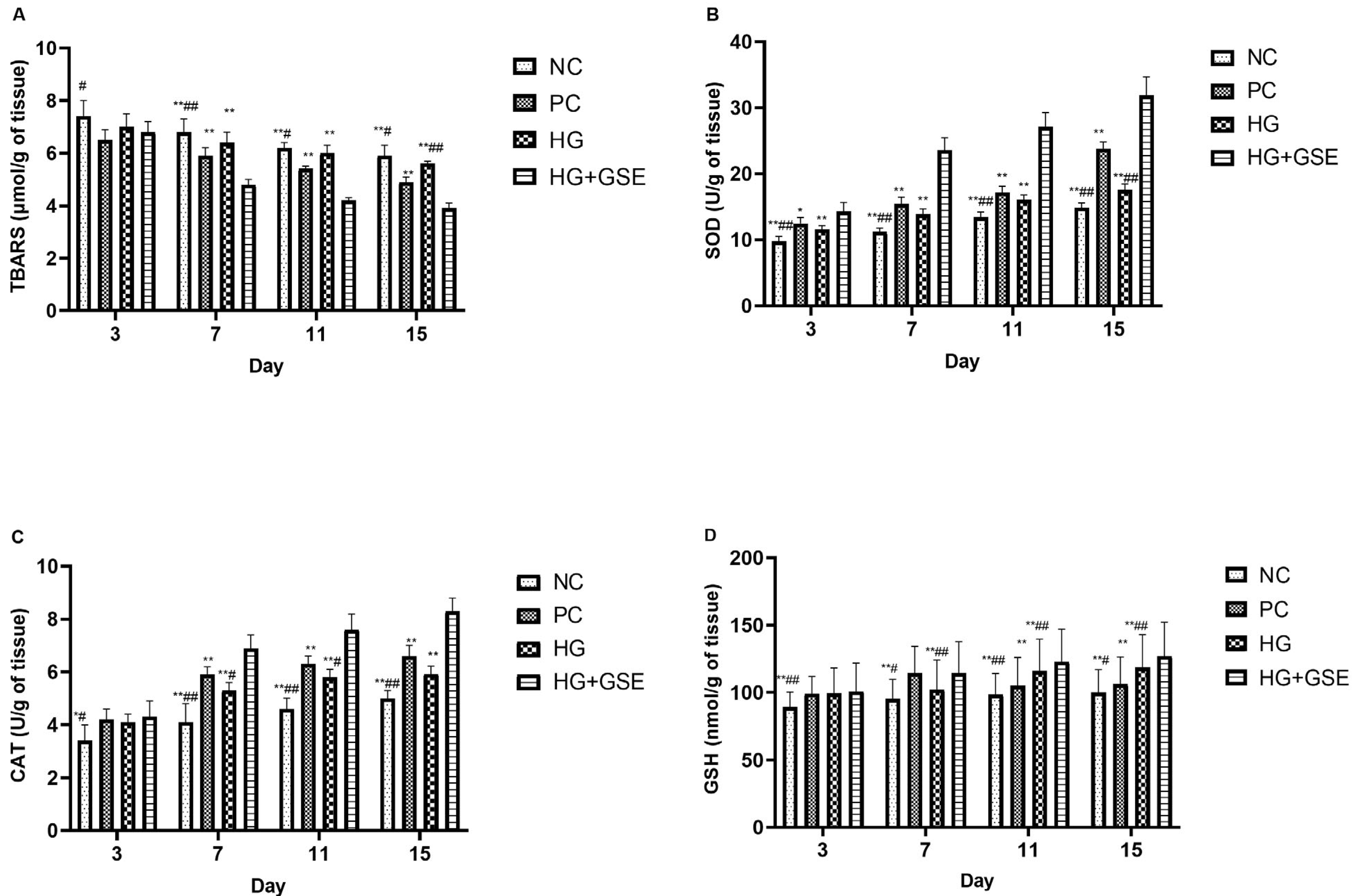
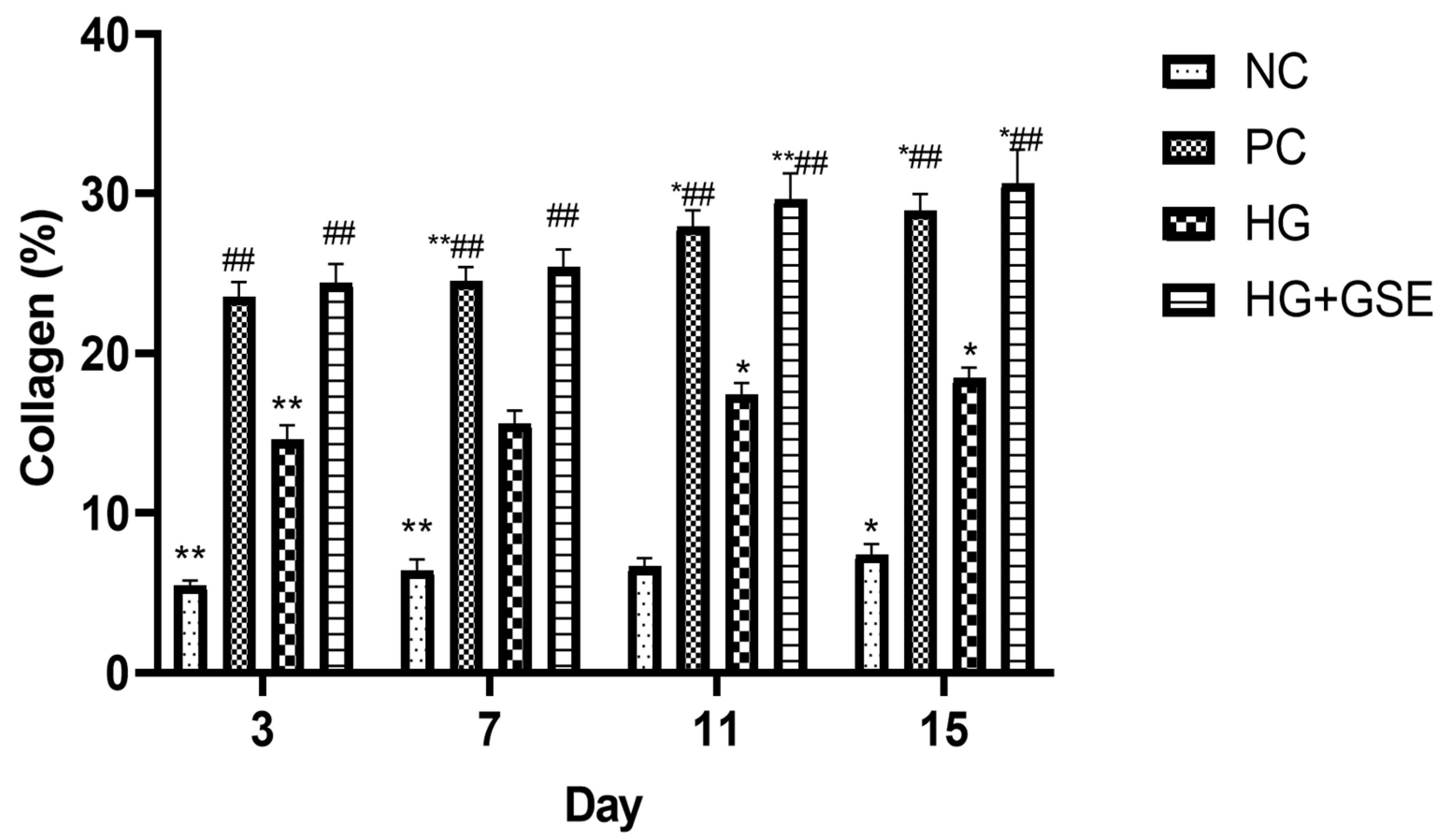
3.2.2. Molecular Indicators of Healing: Collagen Synthesis, Oxidative Balance, and Inflammation
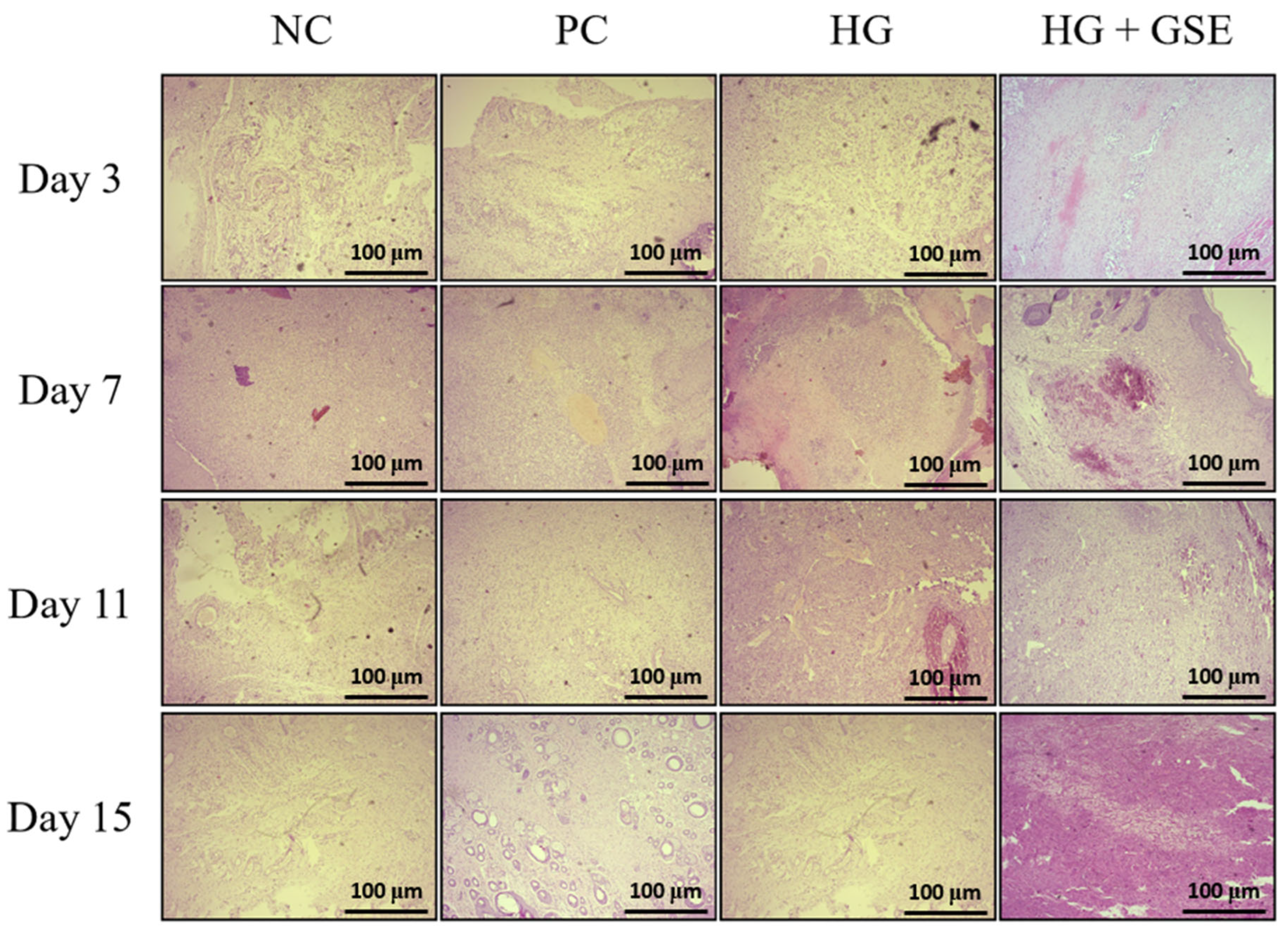
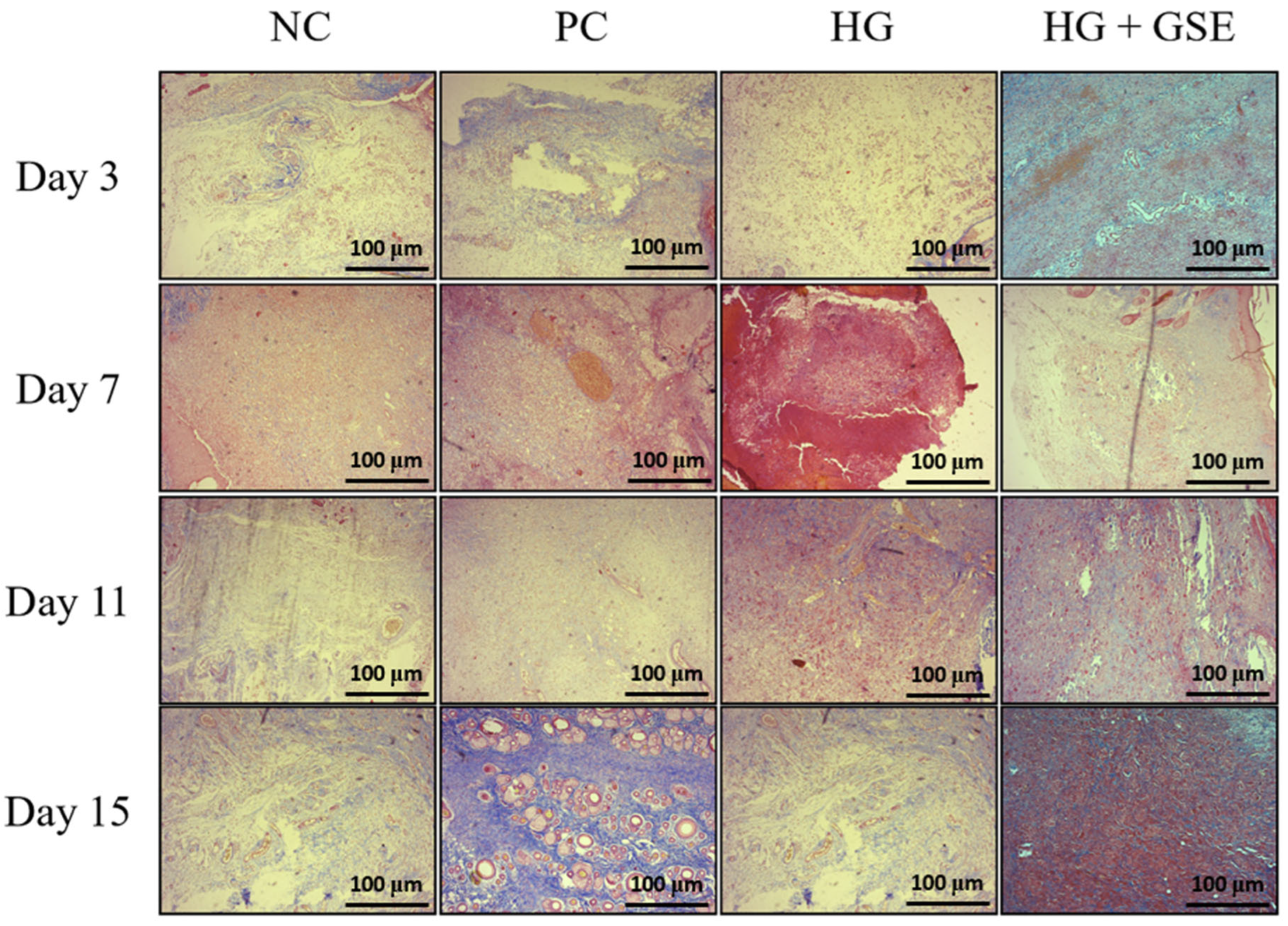
3.2.3. Structural Evaluation of Skin Repair via Histological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nirenjen, S.; Narayanan, J.; Tamilanban, T.; Subramaniyan, V.; Chitra, V.; Fuloria, N.K.; Wong, L.S.; Ramachawolran, G.; Sekar, M.; Gupta, G.; et al. Exploring the contribution of pro-inflammatory cytokines to impaired wound healing in diabetes. Front. Immunol. 2023, 14, 1216321. [Google Scholar] [CrossRef]
- Nasra, S.; Pramanik, S.; Oza, V.; Kansara, K.; Kumar, A. Advancements in wound management: Integrating nanotechnology and smart materials for enhanced therapeutic interventions. Discov. Nano 2024, 19, 159. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Tao, S.; Wang, Q.; Ma, P.Q.; Li, Z.B.; Wu, Y.-L.; Li, D.-W. Research advances in smart responsive-hydrogel dressings with potential clinical diabetic wound healing properties. Mil. Med. Res. 2023, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Aswathy, S.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef] [PubMed]
- Satchanska, G.; Davidova, S.; Petrov, P. Natural and synthetic polymers for biomedical and environmental applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, Ł.; Kuś, M.; Jurak, J.; Rybka, M.; Kuczeriszka, M.; Stradczuk-Mazurek, M.; Konop, M. Biomedical potential of alginate wound dressings—From preclinical studies to clinical applications: A review. Int. J. Biol. Macromol. 2025, 260, 142908. [Google Scholar] [CrossRef]
- Mogha, P.; Iyer, S.; Majumder, A. Extracellular matrix protein gelatin provides higher expansion, reduces size heterogeneity, and maintains cell stiffness in a long-term culture of mesenchymal stem cells. Tissue Cell 2023, 80, 101969. [Google Scholar] [CrossRef]
- Maikovych, O.; Pasetto, P.; Nosova, N.; Kudina, O.; Ostapiv, D.; Samaryk, V.; Varvarenko, S. Functional properties of gelatin–alginate hydrogels for use in chronic wound healing applications. Gels 2025, 11, 174. [Google Scholar] [CrossRef]
- Lopes, J.; Madureira, J.; Margaça, F.; Cabo, V.S. Grape pomace: A review of its bioactive phenolic compounds, health benefits, and applications. Molecules 2025, 30, 362. [Google Scholar] [CrossRef]
- Avdović, E.; Dimić, D.; Nakarada, Đ.; Simijonović, D.; Jovičić Milić, S.; Marković, K.; Grujović, M.; Antonijević, M.; Ćirić, A.; Milenković, D.; et al. Evaluation of bioactive properties of ultrasound-assisted extracts from Prokupac grape skins for functional foods. Antioxidants 2025, 14, 733. [Google Scholar] [CrossRef]
- Avdović, E.; Antonijević, M.; Simijonović, D.; Grujović, M.; Marković, K.; Milenković, D.; Ćirić, A.; Marković, Z. Ultrasound-assisted extraction of bioactive phenolics from Marselan and Shiraz grape skins: A step toward circular economy in food and pharmaceutical industry. LWT 2025, 224, 117817. [Google Scholar] [CrossRef]
- Ajit, A.; Vishnu, A.; Varkey, P. Incorporation of grape seed extract towards wound care product development. 3 Biotech 2021, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Bradić, J.; Petrović, A.; Kočović, A.; Ugrinovic, V.; Popovic, S.; Ciric, A.; Markovic, Z.; Avdović, E. Development and optimization of grape skin extract-loaded gelatin–alginate hydrogels: Assessment of antioxidant and antimicrobial properties. Pharmaceutics 2025, 17, 790. [Google Scholar] [CrossRef] [PubMed]
- Pilipenko, N.; Goncalves, O.H.; Bona, E.; Fernandes, I.P.; Pinto, J.A.; Sorita, G.D.; Leimann, F.V.; Barreiro, M.F. Tailoring swelling of alginate–gelatin hydrogel microspheres by crosslinking with calcium chloride combined with transglutaminase. Carbohydr. Polym. 2019, 223, 115035. [Google Scholar] [CrossRef] [PubMed]
- Draize, J. Appraisal of the Safety of Chemicals in Foods, Drugs and Cosmetics; The Association of Food and Drug Officials of the United States: Austin, TX, USA, 1959; pp. 49–51. [Google Scholar]
- Nikolić, M.; Andjić, M.; Bradić, J.; Kočović, A.; Tomović, M.; Samanović, A.M.; Jakovljevic, V.; Veselinovic, M.; Capo, I.; Krstonosic, V.; et al. Topical application of Siberian pine essential oil formulations enhance diabetic wound healing. Pharmaceutics 2023, 15, 2437. [Google Scholar] [CrossRef]
- Alsareii, S.; Ahmad, J.; Umar, A.; Ahmad, M.; Shaikh, I. Enhanced in vivo wound healing efficacy of a novel piperine-containing bioactive hydrogel in excision wound rat model. Molecules 2023, 28, 545. [Google Scholar] [CrossRef]
- G/Giorgis, S.G.; Ambikar, D.; Tsegaw, A.; Belayneh, Y.M. Wound healing activity of 80% methanolic crude extract and solvent fractions of the leaves of Justicia schimperiana (Hochst. ex Nees) T. Anderson (Acanthaceae) in mice. J. Exp. Pharmacol. 2022, 14, 167–183. [Google Scholar] [CrossRef]
- Chen, W.; Liou, S.; Tzeng, T.; Lee, S.; Liu, I. Wound repair and anti-inflammatory potential of Lonicera japonica in excision wound-induced rats. BMC Complement. Altern. Med. 2012, 12, 226. [Google Scholar] [CrossRef]
- Jović, J.J.; Sretenović, J.; Jović, N.; Rudić, J.; Živković, V.; Srejović, I.; Mihajlovic, K.; Draginic, N.; Andjic, M.; Milinkovic, M.; et al. Cardiovascular properties of the androgen-induced PCOS model in rats: The role of oxidative stress. Oxidative Med. Cell. Longev. 2021, 2021, 8862878. [Google Scholar] [CrossRef]
- Bancroft, J.; Gamble, M. Theory and Practice of Histological Techniques, 6th ed.; Churchill Livingstone, Elsevier: London, UK, 2008. [Google Scholar]
- Vuletić, M.; Jakovljević, V.; Živanović, S.; Papić, M.; Papić, M.; Mladenović, R.; Živković, V.; Srejović, I.; Jeremić, J.; Anđić, M.; et al. The evaluation of healing properties of Galium verum-based oral gel in aphthous stomatitis in rats. Molecules 2022, 27, 4680. [Google Scholar] [CrossRef]
- Aderibigbe, B.; Buyana, B. Alginate in wound dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Cao, H.; Wang, J.; Hao, Z.; Zhao, D. Gelatin-based biomaterials and gelatin as an additive for chronic wound repair. Front. Pharmacol. 2024, 15, 1398939. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.M.; Ramachandran, V.; Mohanasundaram, T.; Obilineni, I. Study on acute dermal irritation of mangiferin hydrogel in rabbits. TWIST 2024, 19, 219–221. Available online: https://twistjournal.net/twist/article/view/318 (accessed on 1 August 2025).
- Masson-Meyers, D.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Landén, N.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Kandhare, A.; Alam, J.; Patil, M.; Sinha, A.; Bodhankar, S. Wound healing potential of naringin ointment formulation via regulating the expression of inflammatory, apoptotic and growth mediators in experimental rats. Pharm. Biol. 2016, 54, 419–432. [Google Scholar] [CrossRef]
- Yen, J.; Chio, W.; Chuang, C.; Yang, H.; Huang, S. Improved wound healing by naringin associated with MMP and the VEGF pathway. Molecules 2022, 27, 1695. [Google Scholar] [CrossRef]
- Raina, N.; Haque, S.; Tuli, H.S.; Jain, A.; Slama, P.; Gupta, M. Optimization and characterization of a novel antioxidant naringenin-loaded hydrogel for encouraging re-epithelization in chronic diabetic wounds: A preclinical study. ACS Omega 2023, 8, 34995–35011. [Google Scholar] [CrossRef]
- Yang, H.; Xu, H.; Lv, D.; Li, S.; Rong, Y.; Wang, Z.; Wang, P.; Cao, X.; Li, X.; Xu, Z.; et al. The naringin/carboxymethyl chitosan/sodium hyaluronate/silk fibroin scaffold facilitates the healing of diabetic wounds by restoring the ROS-related dysfunction of vascularization and macrophage polarization. Int. J. Biol. Macromol. 2024, 260, 129348. [Google Scholar] [CrossRef]
- Fan, R.; Pan, T.; Zhu, A.L.; Zhang, M.H. Anti-inflammatory and anti-arthritic properties of naringenin via attenuation of NF-κB and activation of the heme oxygenase (HO)-1/related factor 2 pathway. Pharmacol. Rep. 2017, 69, 1021–1029. [Google Scholar] [CrossRef]
- Gil, M.; Kim, Y.; Hong, S.; Lee, K. Naringin decreases TNF-α and HMGB1 release from LPS-stimulated macrophages and improves survival in a CLP-induced sepsis mice. PLoS ONE 2016, 11, e0164186. [Google Scholar] [CrossRef]
- Ganguly, R.; Singh, S.; Jaiswal, K.; Kumar, R.; Pandey, A. Modulatory effect of caffeic acid in alleviating diabetes and associated complications. World J. Diabetes 2023, 14, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Feng, Y.; Zhao, J.; Sun, S.; Zheng, T.; Wang, J.; Chen, H.; Ye, H.; Lv, S.; Zhang, Y.; et al. Caffeic acid-mediated photodynamic multifunctional hyaluronic acid–gallic acid hydrogels with instant and enduring bactericidal potency accelerate bacterial infected wound healing. Int. J. Biol. Macromol. 2024, 282, 136877. [Google Scholar] [CrossRef] [PubMed]
- Song, H.S.; Park, T.W.; Sohn, U.D.; Shin, Y.K.; Choi, B.C.; Kim, C.J.; Sim, S.S. The effect of caffeic acid on wound healing in skin-incised mice. Korean J. Physiol. Pharmacol. 2008, 12, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Andjić, M.; Draginić, N.; Kočović, A.; Jeremić, J.; Vučićević, K.; Jeremić, N.; Krstonošić, V.; Božin, B.; Kladar, N.; Čapo, I.; et al. Immortelle essential oil-based ointment improves wound healing in a diabetic rat model. Biomed. Pharmacother. 2022, 150, 112941. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Z.; Zhou, S.; Shen, S.; Chen, W. The effect of a compound protein on wound healing and nutritional status. Evid.-Based Complement. Altern. Med. 2022, 2022, 4231516. [Google Scholar] [CrossRef]
- Yadav, E.; Singh, D.; Yadav, P.; Verma, A. Attenuation of dermal wounds via downregulating oxidative stress and inflammatory markers by protocatechuic acid rich n-butanol fraction of Trianthema portulacastrum Linn. in Wistar albino rats. Biomed. Pharmacother. 2017, 96, 86–97. [Google Scholar] [CrossRef]
- Nasrullah, M. Caffeic acid phenethyl ester loaded PEG–PLGA nanoparticles enhance wound healing in diabetic rats. Antioxidants 2023, 12, 60. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The role of oxidative stress and antioxidants in diabetic wound healing. Oxidative Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef]
- Vujčić, S.; Kotur-Stevuljević, J.; Vekić, J.; Perović-Blagojević, I.; Stefanović, T.; Ilić-Mijailović, S.; Uzelac, B.K.; Bosić, S.; Antonić, T.; Guzonjić, A.; et al. Oxidative stress and inflammatory biomarkers in patients with diabetic foot. Medicina 2022, 58, 1866. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The applications and mechanisms of superoxide dismutase in medicine, food, and cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.; Krieg, T.; Davidson, J. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Willenborg, S.; Eming, S.A. Macrophages—Sensors and effectors coordinating skin damage and repair. J. Dtsch. Dermatol. Ges. 2014, 12, 214–221. [Google Scholar] [CrossRef]
- Lin, Z.Q.; Kondo, T.; Ishida, Y.; Takayasu, T.; Mukaida, N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J. Leukoc. Biol. 2003, 73, 713–721. [Google Scholar] [CrossRef]
- Wetzler, C.; Kämpfer, H.; Stallmeyer, B.; Pfeilschifter, J.; Frank, S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: Prolonged persistence of neutrophils and macrophages during the late phase of repair. J. Investig. Dermatol. 2000, 115, 245–253. [Google Scholar] [CrossRef]
- Galkowska, H.; Olszewski, W.L.; Wojewodzka, U.; Rosinski, G.; Karnafel, W. Neurogenic factors in the impaired healing of diabetic foot ulcers. J. Surg. Res. 2006, 134, 252–258. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Koh, T.; DiPietro, L. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef]
- Liu, Y.; Min, D.; Bolton, T.; Nube, V.; Twigg, S.M.; Yue, D.K.; McLennan, S.V. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care 2009, 32, 117–119. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.M.; Prantl, L.; Landthaler, M.; Babilas, P. Wound healing in the 21st century. J. Am. Acad. Dermatol. 2010, 63, 866–881. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, P. Assessment of the histological state of the healing wound. Plast. Aesthet. Res. 2015, 2, 239–242. [Google Scholar] [CrossRef]
- El-Ferjani, R.; Ahmad, M.; Dhiyaaldeen, S.; Harun, F.W.; Ibrahim, M.Y.; Adam, H.; Yamin, B.M.; Al-Obaidi, M.M.J.; Al Batran, R. In vivo assessment of antioxidant and wound healing improvement of a new Schiff base derived Co (II) complex in rats. Sci. Rep. 2016, 6, 38748. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bradić, J.; Petrovic, A.; Joksimovic Jovic, J.; Simic, M.; Stankovic, V.; Matic, S.; Antonijević, M.; Avdovic, E.; Jakovljevic, V.; Kocovic, A. From Vineyard to Hydrogel: Antioxidant, Anti-Inflammatory, and Regenerative Potential of Grape Skin Extract in Diabetic Wound Repair. Pharmaceutics 2025, 17, 1464. https://doi.org/10.3390/pharmaceutics17111464
Bradić J, Petrovic A, Joksimovic Jovic J, Simic M, Stankovic V, Matic S, Antonijević M, Avdovic E, Jakovljevic V, Kocovic A. From Vineyard to Hydrogel: Antioxidant, Anti-Inflammatory, and Regenerative Potential of Grape Skin Extract in Diabetic Wound Repair. Pharmaceutics. 2025; 17(11):1464. https://doi.org/10.3390/pharmaceutics17111464
Chicago/Turabian StyleBradić, Jovana, Anica Petrovic, Jovana Joksimovic Jovic, Marko Simic, Vesna Stankovic, Sanja Matic, Marko Antonijević, Edina Avdovic, Vladimir Jakovljevic, and Aleksandar Kocovic. 2025. "From Vineyard to Hydrogel: Antioxidant, Anti-Inflammatory, and Regenerative Potential of Grape Skin Extract in Diabetic Wound Repair" Pharmaceutics 17, no. 11: 1464. https://doi.org/10.3390/pharmaceutics17111464
APA StyleBradić, J., Petrovic, A., Joksimovic Jovic, J., Simic, M., Stankovic, V., Matic, S., Antonijević, M., Avdovic, E., Jakovljevic, V., & Kocovic, A. (2025). From Vineyard to Hydrogel: Antioxidant, Anti-Inflammatory, and Regenerative Potential of Grape Skin Extract in Diabetic Wound Repair. Pharmaceutics, 17(11), 1464. https://doi.org/10.3390/pharmaceutics17111464










