Development of Propofol-Encapsulated Liposomes and the Effect of Intranasal Administration on Bioavailability in Rabbits
Abstract
1. Introduction
2. Materials and Methods
2.1. Agents
2.2. Preparation of Propofol-Encapsulated Liposomes
2.3. Measurement of Propofol Concentrations
2.3.1. HPLC Conditions
2.3.2. Establishment of the Calibration Curve and Measurement of Propofol Concentrations
2.4. Animal Model
2.5. In Vivo Experiments with Intravenous Administration
2.6. In Vivo Experiments with Oral Administration
2.7. In Vivo Experiments with Intranasal Administration
2.8. Statistical Analysis
3. Results
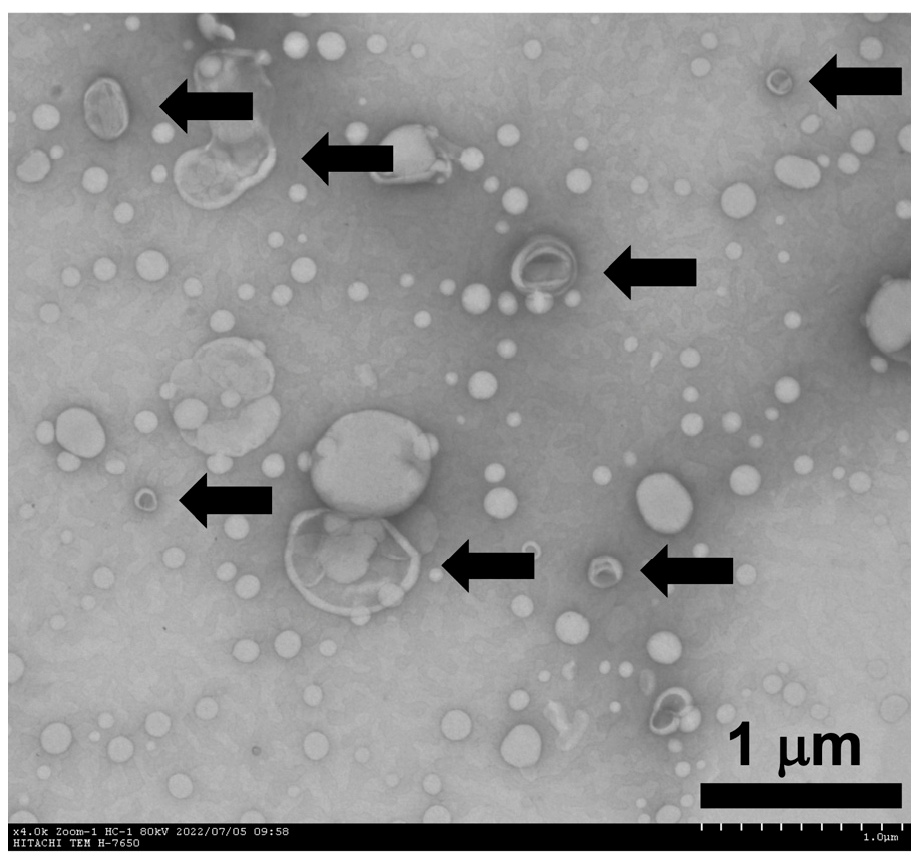
3.1. Characteristics of Propofol-Encapsulated Liposomes
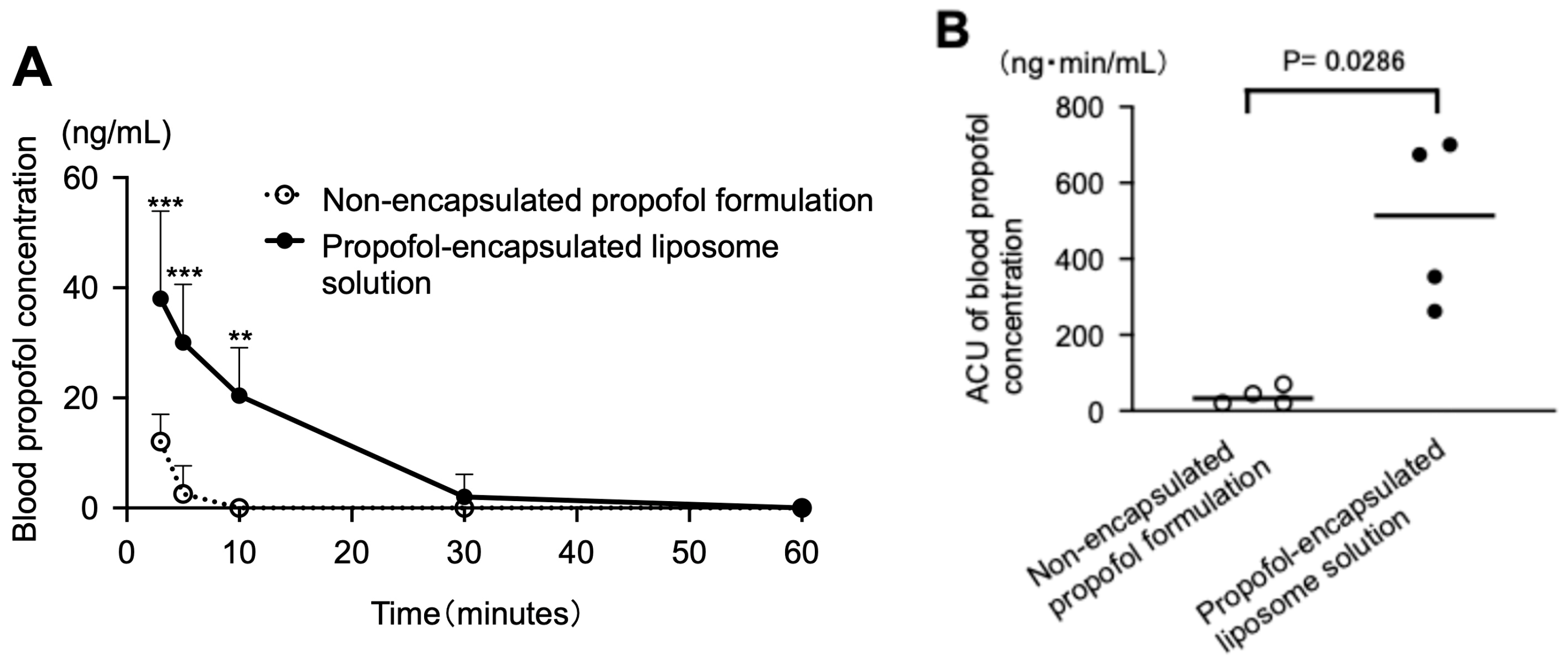
3.2. Blood Concentrations After Intravenous Administration of the Test Solutions
3.3. Blood Concentrations After Oral Administration of the Test Solutions
3.4. Blood Concentrations After Intranasal Administration of the Test Solutions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC0–60 | area under the blood concentration-time curve from 0 to 60 min |
| ANOVA | analysis of variance |
| CNS | central nervous system |
| BBB | blood–brain barrier |
| PCHL | L-α-phosphatidylcholine |
| DMPC | dimyristoylphosphatidylcholine |
| HPLC | high-performance liquid chromatography |
| TEM | transmission electron microscopy |
| IS | internal standard |
References
- Taléns-Visconti, R.; de Julián-Ortiz, J.V.; Vila-Busó, O.; Diez-Sales, O.; Nácher, A. Intranasal drug administration in Alzheimer-type dementia: Towards clinical applications. Pharmaceutics 2023, 15, 1399. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Li, J.; Ruan, H.; Xia, Q.; Hou, X.; Wang, Z.; Guo, T.; Zhu, C.; Feng, N.; Zhang, Y. Microneedle-mediated nose-to-brain drug delivery for improved Alzheimer’s disease treatment. J. Control. Release 2024, 366, 712–731. [Google Scholar] [CrossRef]
- Balagurusamy, B.; Ganesan, V.; Gopi, V.; Ilayaperumal, P. Liposomal and nanomaterial-based strategies for targeted Alzheimer’s disease therapy. ACS Omega ASAP 2025, ASAP. [Google Scholar] [CrossRef]
- Shu, W.; Jiang, Y.; Li, S.; Li, J.; Pan, X.; Wang, C.; Wang, H.; Yu, T. Developing intranasally administered electro-responsive nanodrugs for rapid epilepsy treatment. Nano Today 2025, 64, 102808. [Google Scholar] [CrossRef]
- Awad, R.; Avital, A.; Sosnik, A. Polymeric nanocarriers for nose-to-brain drug delivery in neurodegenerative diseases and neurodevelopmental disorders. Acta Pharm. Sin. B 2023, 13, 1866–1886. [Google Scholar] [CrossRef]
- Saha, P.; Kathuria, H.; Pandey, M.M. Intranasal nanotherapeutics for brain targeting and clinical studies in Parkinson’s disease. J. Control. Release 2023, 358, 293–318. [Google Scholar] [CrossRef]
- Vieira, D.B.; Gamarra, L.F. Getting into the brain: Liposome-based strategies for effective drug delivery across the blood-brain barrier. Int. J. Nanomedicine 2016, 11, 5381–5414. [Google Scholar] [CrossRef]
- Ozceylan, O.; Sezgin-Bayindir, Z. Current overview on the use of nanosized drug delivery systems in the treatment of neurodegenerative diseases. ACS Omega 2024, 9, 35223–35242. [Google Scholar] [CrossRef]
- Ma, R.; Li, Y.; Su, Y.; Chen, P.; Xie, S.; Tan, W.; Liu, X. Lipid nanoparticles: A delicate nucleic acid delivery system to be further explored. Nano Today 2025, 61, 102586. [Google Scholar] [CrossRef]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Recent advances in intranasal liposomes for drug, gene, and vaccine delivery. Pharmaceutics 2023, 15, 207. [Google Scholar] [CrossRef]
- Pires, A.; Fortuna, A.; Alves, G.; Falcão, A. Intranasal drug delivery: How, why and what for? J. Pharm. Pharm. Sci. 2009, 12, 288–311. [Google Scholar] [CrossRef]
- Mathias, N.; Hussain, M. Non-invasive systemic drug delivery: Developability considerations for alternate routes of administration. J. Pharm. Sci. 2010, 99, 1–20. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Blumer, J.L. Clinical pharmacology of midazolam in infants and children. Clin. Pharmacokinet. 1998, 35, 37–47. [Google Scholar] [CrossRef]
- Kochs, E.; Hoffman, W.E.; Werner, C.; Thomas, C.; Albrecht, R.F.; Schulte am Esch, J. The effects of propofol on brain electrical activity, neurologic outcome, and neuronal damage following incomplete ischemia in rats. Anesthesiology 1992, 76, 245–252. [Google Scholar] [CrossRef]
- Hanamoto, H.; Boku, A.; Sugimura, M.; Oyamaguchi, A.; Inoue, M.; Niwa, H. Premedication with midazolam in intellectually disabled dental patients: Intramuscular or oral administration? a retrospective study. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e470–e476. [Google Scholar] [CrossRef]
- Almenrader, N.; Passariello, M.; Coccetti, B.; Haiberger, R.; Pietropaoli, P. Premedication in children: A comparison of oral midazolam and oral clonidine. Pediatr. Anesth. 2007, 17, 1143–1149. [Google Scholar] [CrossRef]
- Maeda, S.; Tomayasu, Y.; Higuchi, H.; Ishii-Maruhama, M.; Yamane, A.; Yabuki, A.; Honda, Y.; Egusa, M.; Miyawaki, T. Independent factors affecting recovery time after sedation in patients with intellectual disabilities. Open Dent. J. 2015, 9, 146–149. [Google Scholar] [CrossRef]
- Vanlersberghe, C.; Camu, F. Propofol. In Modern Anesthetics; Schüttler, J., Schwilden, H., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2008; Volume 182, pp. 227–252. [Google Scholar] [CrossRef]
- Hughes, M.A.; Glass, P.S.; Jacobs, J.R. Context-sensitive half-time in multicompartment pharmacokinetic models for intravenous anesthetic drugs. Anesthesiology 1992, 76, 334–341. [Google Scholar] [CrossRef]
- Raoof, A.A.; Augustijns, P.F.; Verbeeck, R.K. In vivo assessment of intestinal, hepatic, and pulmonary first pass metabolism of propofol in the rat. Pharm. Res. 1996, 13, 891–895. [Google Scholar] [CrossRef]
- Tomoyasu, Y.; Yasuda, T.; Maeda, S.; Higuchi, H.; Miyawaki, T. Liposome-encapsulated midazolam for oral administration. J. Liposome Res. 2011, 21, 166–172. [Google Scholar] [CrossRef]
- Nishioka, Y.; Lu, Y.; Higuchi, H.; Miyake, S.; Fujimoto, M.; Hamaoka-Inoue, M.; Tanimura, H.; Ujita, H.; Maeda, S.; Miyawaki, T. PEGylation of liposome-encapsulated midazolam does not improve the bioavailability of midazolam when administered orally. BMC Pharmacol. Toxicol. 2025, 26, 166. [Google Scholar] [CrossRef]
- Almeida, B.; Nag, O.K.; Rogers, K.E.; Delehanty, J.B. Recent progress in bioconjugation strategies for liposome-mediated drug delivery. Molecules 2020, 25, 5672. [Google Scholar] [CrossRef]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef]
- Ujita, H. The Development of Liposome-Encapsulated Propofol and Pharmacokinetics Following Its Administration in Rabbits. Ph.D. Thesis, Okayama University, Okayama, Japan, 2023. Available online: https://ousar.lib.okayama-u.ac.jp/files/public/6/65382/20230609101154665321/K0006821_fulltext.pdf (accessed on 15 September 2025).
- Ishii-Maruhama, M.; Higuchi, H.; Nakanou, M.; Honda-Wakasugi, Y.; Yabuki-Kawase, A.; Maeda, S.; Miyawaki, T. In vitro changes in the proportion of protein-unbound-free propofol induced by valproate. J. Anesth. 2018, 32, 688–693. [Google Scholar] [CrossRef]
- Baker, M.T.; Naguib, M. Propofol: The challenges of formulation. Anesthesiology 2005, 103, 860–876. [Google Scholar] [CrossRef]
- Sebel, P.S.; Lowdon, J.D. Propofol: A new intravenous anesthetic. Anesthesiology 1989, 71, 260–277. [Google Scholar] [CrossRef]
- Mirtallo, M.J.; Dasta, J.F.; Kleinschmidt, K.C.; Varon, J. State of the art review: Intravenous fat emulsions: Current applications, safety profile, and clinical implications. Ann. Pharmacother. 2010, 44, 688–700. [Google Scholar] [CrossRef]
- Favetta, P.; Degoute, C.S.; Perdrix, J.P.; Dufresne, C.; Boulieu, R.; Guitton, J. Propofol metabolites in man following propofol induction and maintenance. Br. J. Anaesth. 2002, 88, 653–658. [Google Scholar] [CrossRef]
- Sahinovic, M.M.; Struys, M.M.R.F.; Absalom, A.R. Clinical pharmacokinetics and pharmacodynamics of propofol. Clin. Pharmacokinet. 2018, 57, 1539–1558. [Google Scholar] [CrossRef]
- Shioya, N.; Ishibe, Y.; Shibata, S.; Makabe, H.; Kan, S.; Matsumoto, N.; Takahashi, G.; Yamada, Y.; Endo, S. Green urine discoloration due to propofol infusion: A case report. Case Rep. Emerg. Med. 2011, 2011, 242514. [Google Scholar] [CrossRef]
- Veroli, P.; O’Kelly, B.; Bertrand, F.; Trouvin, J.H.; Farinotti, R.; Ecoffey, C. Extrahepatic metabolism of propofol in man during the anhepatic phase of orthotopic liver transplantation. Br. J. Anaesth. 1992, 68, 183–186. [Google Scholar] [CrossRef]
- Takizawa, D.; Sato, E.; Hiraoka, H.; Tomioka, A.; Yamamoto, K.; Horiuchi, R.; Goto, F. Changes in apparent systemic clearance of propofol during transplantation of living related donor liver. Br. J. Anaesth. 2005, 95, 643–647. [Google Scholar] [CrossRef]
- Raoof, A.A.; van Obbergh, L.J.; de Ville de Goyet, J.; Verbeeck, R.K. Extrahepatic glucuronidation of propofol in man: Possible contribution of gut wall and kidney. Eur. J. Clin. Pharmacol. 1996, 50, 91–96. [Google Scholar] [CrossRef]
- Desai, M.P.; Labhasetwar, V.; Walter, E.; Levy, R.J.; Amidon, G.L. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm. Res. 2005, 10, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.P.; Labhasetwar, V.; Amidon, G.L.; Levy, R.J. Gastrointestinal uptake of biodegradable microparticles: Effect of particle size. Pharm. Res. 1996, 13, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Khan, D.R. The treatment of breast cancer using liposome technology. J. Drug Deliv. 2012, 2012, 212965. [Google Scholar] [CrossRef]
- Ochoa-Sánchez, C.; Rodríguez-León, E.; Iñiguez-Palomares, R.; César Rodríguez-Beas, C. Brief comparison of the efficacy of cationic and anionic liposomes as nonviral delivery systems. ACS Omega 2024, 9, 46664–46678. [Google Scholar] [CrossRef]
- Costa, C.; Moreira, J.N.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J. Control. Release 2019, 295, 187–200. [Google Scholar] [CrossRef]
- Illum, L. Nasal drug delivery—possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198. [Google Scholar] [CrossRef]
- Spampinato, M.D.; Costanzini, A.; Giorgio, R.D.; Passaro, A.; Realdon, N.; Bortolotti, F.; Banella, S.; Colombo, G. Ex vivo propofol permeation across nasal mucosa: A proof-of-concept study for outpatient light sedation via nasal route. ADMET DMPK 2024, 12, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Damitz, R.; Chauhan, A.; Gravenstein, N. Propofol emulsion-free drug concentration is similar between batches and stable over time. Rom. J. Anaesth. Intensiv. Care 2016, 23, 5–6. [Google Scholar] [CrossRef]
- Arumugam, K.; Subramanian, G.S.; Mallayasamy, S.R.; Averineni, R.K.; Reddy, M.S.; Udupa, N. A study of rivastigmine liposomes for delivery into the brain through intranasal route. Acta Pharm. 2008, 58, 287–297. [Google Scholar] [CrossRef]
- Mistry, A.; Stolnik, S.; Illum, L. Nanoparticles for direct nose-to-brain delivery of drugs. Int. J. Pharm. 2009, 379, 146–157. [Google Scholar] [CrossRef]
- Kuo, C.K.; Hanioka, N.; Hoshikawa, Y.; Oguri, K.; Yoshimura, H. Species difference of site-selective glucuronidation of morphine. J. Pharmacobio-Dyn. 1991, 14, 187–193. [Google Scholar] [CrossRef]
- Shafer, S.L. The pharmacology of anesthetic drugs in elderly patients. Anesthesiol. Clin. N. Am. 2000, 18, 1–29. [Google Scholar] [CrossRef]
- Jensen, G.M.; Ashvar, C.S.; Bunte, S.W.; Barzak, C.D.; Bunch, T.H.; Fahrner, R.L.; Hu, N.; Kennavane, J.; Pham, H.; Skenes, C.; et al. A liposomal dispersion formulation of propofol: Formulation, pharmacokinetics, stability, and identification of an oxidative degradant. Theor. Chem. Acc. 2008, 119, 291–296. [Google Scholar] [CrossRef]
- Laffleur, F.; Bauer, B. Progress in nasal drug delivery systems. Int. J. Pharm. 2021, 607, 120994. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ujita, H.; Higuchi, H.; Nishioka, Y.; Miyake, S.; Sato, R.; Miyawaki, T. Development of Propofol-Encapsulated Liposomes and the Effect of Intranasal Administration on Bioavailability in Rabbits. Pharmaceutics 2025, 17, 1446. https://doi.org/10.3390/pharmaceutics17111446
Ujita H, Higuchi H, Nishioka Y, Miyake S, Sato R, Miyawaki T. Development of Propofol-Encapsulated Liposomes and the Effect of Intranasal Administration on Bioavailability in Rabbits. Pharmaceutics. 2025; 17(11):1446. https://doi.org/10.3390/pharmaceutics17111446
Chicago/Turabian StyleUjita, Hitomi, Hitoshi Higuchi, Yukiko Nishioka, Saki Miyake, Riko Sato, and Takuya Miyawaki. 2025. "Development of Propofol-Encapsulated Liposomes and the Effect of Intranasal Administration on Bioavailability in Rabbits" Pharmaceutics 17, no. 11: 1446. https://doi.org/10.3390/pharmaceutics17111446
APA StyleUjita, H., Higuchi, H., Nishioka, Y., Miyake, S., Sato, R., & Miyawaki, T. (2025). Development of Propofol-Encapsulated Liposomes and the Effect of Intranasal Administration on Bioavailability in Rabbits. Pharmaceutics, 17(11), 1446. https://doi.org/10.3390/pharmaceutics17111446





