Advances in Cell-Mediated Drug Delivery for Dermatologic Diseases: Mechanisms and Current Applications
Abstract
1. Introduction
2. Method
3. The Skin
4. Skin Disease and Traditional Routes of Drug Delivery
5. Burden of Disease
6. Economic Burden
7. Barriers to Traditional Drug Delivery Methods and Their Limitations
7.1. Topical and Transdermal Delivery
7.2. Oral Systemic Delivery
7.3. Injectable Systemic Delivery
7.4. Intralesional and Local Injectable Delivery
7.5. Skin Biochemistry and Immune Surveillance as Cross-Cutting Barriers
7.6. Implication for Therapy Design
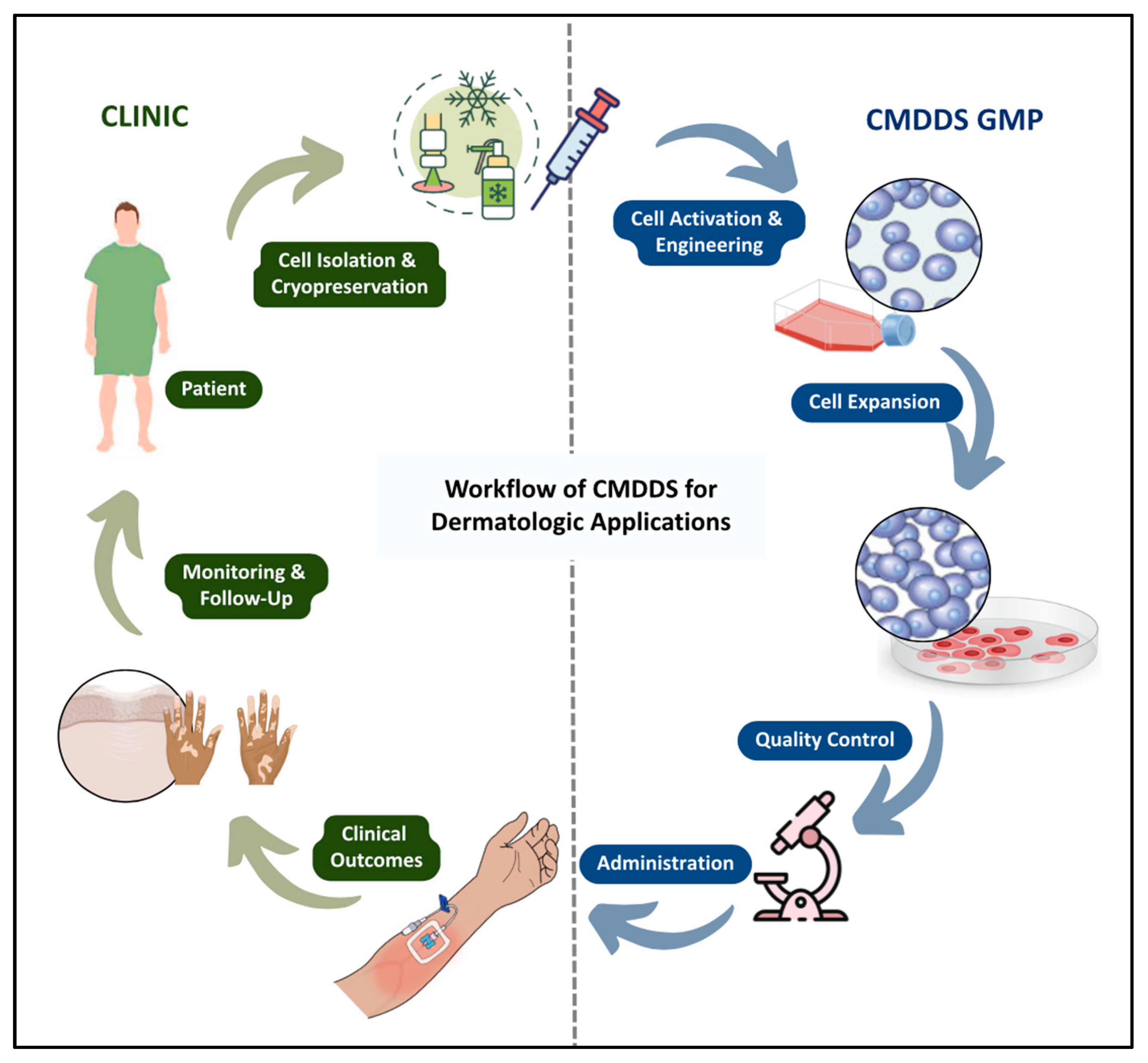
8. Development of Cell-Mediated Drug Delivery and Their Evolution
8.1. Integration of Nanotechnology and Advances in Genetic Engineering
8.2. Expanding Cell Sources and Acellular Derivatives
8.3. Current Trends and Future Refinements
8.4. 3D Bioprinting and Tissue Engineering in CMDDS
9. In Vivo Cells Used for Cell-Mediated Drug Deliveries
9.1. Macrophages
9.2. Neutrophils
9.3. Dendritic Cells
9.4. Mesenchymal Stem Cells
9.5. Induced Pluripotent Stem Cells
9.6. Keratinocytes
9.6.1. Epidermolysis Bullosa as a Clinical Example
9.6.2. Epidermal–Dermal Composite Cell Sheets
9.7. Fibroblasts
10. Biological Mechanisms of Action of CMDDS
10.1. Homing and Targeted Migration to Diseased Tissue
10.2. Cellular Internalization of Therapeutic Payloads
10.3. Payload Release via Secretion and Cell Fusion
10.4. Immunomodulatory Effects at Target Sites
10.5. Pharmacokinetics and Pharmacodynamics in Dermatologic CMDDS
10.6. Tracking and Monitoring CMDDS In Vivo
11. In Vitro Engineering and Enhancement Methods
11.1. Passive Loading via Endocytosis and Phagocytosis
11.2. Electroporation for Enhanced Intracellular Delivery
11.3. Nanoparticle Surface Conjugation and Intracellular Loading
11.4. Cell Surface Modification for Targeting Specificity
11.5. Genetic Engineering of Carrier Cells
11.6. Exosome Engineering for Cell-Free Therapeutic Delivery
11.7. Comparative Summary of In Vitro Engineering Approaches
12. Dermatological Applications of CMDDS
12.1. Acne Vulgaris
12.2. Psoriasis
12.3. Eczema
12.4. Melanoma
12.5. Vitiligo
12.6. Hidradenitis Suppurativa
12.7. Cutaneous T-Cell Lymphoma
12.8. Wound Healing and Scars
| Cell-Based/Derived Modality | Dermatological Application | Therapeutic Payload | Development Stage | Reference |
|---|---|---|---|---|
| Autologous gene-corrected keratinocyte sheets | Junctional/recessive dystrophic EB (ex vivo–corrected epidermal stem cells, re-grafted) | Functional gene/protein via engrafted keratinocytes | Clinical case series; phase 3 programs | [82,83,84] |
| Autologous epidermal/dermal cell sheets | Chronic wounds, burns, ulcers (living skin equivalents) | Paracrine pro-healing factors; ECM | Clinical practice; phase 2/3 trials | [85,86] |
| Autologous fibroblast injections | Atrophic acne scars and rhytids (dermal fibroblast suspensions) | ECM proteins; trophic factors | Approved product history; niche clinical use | [89,90,91,92] |
| Melanocyte–keratinocyte cell transplantation (MKTP/MKCT) | Stable vitiligo repigmentation | Functional melanocytes (melanin production) | Specialized centers; long-term follow-up | [158,159,160] |
| Mesenchymal stromal/stem cells (MSC) | AD, psoriasis, scars, radiation injury, wounds (local or IV) | Immunomodulatory secretome; anti-inflammatory cytokines | Phase 1/2a; expanding preclinical base | [70,71,72] |
| MSC-derived extracellular vesicles (exosomes) | AD and chronic wounds (topical/intradermal) | miRNA/siRNA, proteins, lipids (cell-free therapy) | Preclinical to phase 1 | [73,74,75] |
| Dendritic-cell–targeted skin vaccination (incl. microneedles) | Cutaneous immunization; melanoma/viral antigens | Antigen/genes to skin APCs (Langerhans/DCs) | Clinical vaccinology; near-clinical oncology | [64,65,66,67] |
| Adoptive T-cell therapies (TILs/engineered T cells) | Cutaneous melanoma; CTCL | Cell-delivered cytotoxicity to tumor | Established in melanoma; phase 2 in CTCL | [146,147,148,149] |
| Leukocyte-mimicking/leukocyte-hitchhiking nanoparticles | Psoriasis/AD models (neutrophil/macrophage membrane–coated NPs carrying MTX/tacrolimus) | Small-molecule immunomodulators with inflamed-skin homing | Robust preclinical; emerging translational data | [34,47] |
| Platelet- or RBC-membrane–coated nanoparticles | Melanoma; infected wounds (damage-site tropism) | Chemo/photothermal agents; antibiotics | Preclinical (oncology/wound focus) | [47,48] |
| “Backpack” cargo on living immune cells | Inflammation-homing macrophages carrying drug-loaded micro-disks | Localized anti-inflammatories/antibiotics | Preclinical proofs-of-concept | [49,50] |
| Probiotic/commensal live biotherapeutics | AD and acne (Roseomonas mucosa, Staph. hominis, ammonia-oxidizers) | In situ AMPs, sphingolipids, nitric oxide | Phase 1/2 signals; ongoing trials | [141,142,143,144] |
| Engineered skin microbes | Designed Staph. epidermidis secreting therapeutic proteins (antipruritics, AMPs) | On-skin biologics/peptides | Preclinical; first-in-human pathways | [140] |
| Bacteriophage/oncolytic virus via cells | Intratumoral melanoma therapy; phage for acne biofilms | Genes/cytotoxic payloads to target cells | Clinical (oncolytic viruses); preclinical (phage) | [152,153,154,155] |
| Cell-derived vesicles targeting keratinocytes/DCs (siRNA) | Psoriasis/AD models (EV-siRNA to epithelial–immune axis) | Nucleic acids (gene silencing) | Preclinical dermatology | [134,135,136] |
| Platelet-rich plasma (PRP) | Chronic wounds, acne scars, androgenetic alopecia, photoaging, vitiligo | Growth factors (e.g., PDGF, VEGF, TGF-β), cytokines for regeneration and anti-inflammation | Clinical practice; multiple trials with variable standardization | [169,170,171] |
| Granulocyte transfusions | Severe skin/soft tissue infections in neutropenic patients (e.g., fungal, bacterial) | Antimicrobial peptides, phagocytic cells for immune response | Specialized clinical use; mixed efficacy in trials | [172,173,174] |
13. Challenges in Translating CMDDS to Clinical Practice
13.1. Autoimmune Complications
13.2. Complications of Production of Specialized Cells
13.3. Regulatory Requirement Restraints
13.4. Good Manufacturing Practices Requirements for Cell Products
14. Future Directions
15. Ethical and Societal Considerations
15.1. Accessibility and Equity
15.2. Autologous vs. Allogeneic Cell Sourcing Ethics
15.3. Pediatric and Vulnerable Populations
15.4. Global Disparities
15.5. Societal Implications and Public Engagement
16. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMDDS | Cell-Mediated Drug Delivery Systems |
| DDS | Drug Delivery Systems |
| MSC | Mesenchymal Stem Cell(s) |
| iPSC | Induced Pluripotent Stem Cell(s) |
| EV | Extracellular Vesicle(s) |
| NET | Neutrophil Extracellular Trap |
| ROS | Reactive Oxygen Species |
| DC | Dendritic Cell(s) |
| APC | Antigen-Presenting Cell |
| MHC | Major Histocompatibility Complex |
| Treg | Regulatory T Cell |
| CTLA-4 | Cytotoxic T-Lymphocyte Associated Protein 4 |
| IL | Interleukin (e.g., IL-10, IL-8) |
| TGF-β | Transforming Growth Factor Beta |
| PGE2 | Prostaglandin E2 |
| IDO | Indoleamine 2,3-Dioxygenase |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| ICAM-1 | Intracellular Adhesion Molecule 1 |
| LTB4 | Leukotriene B4 |
| FDA | Food and Drug Administration |
| EMA | European Medicines Agency |
References
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef]
- Prieložná, J.; Mikušová, V.; Mikuš, P. Advances in the delivery of anticancer drugs by nanoparticles and chitosan-based nanoparticles. Int. J. Pharm. X 2024, 8, 100281. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Xia, G.; Adilijiang, N.; Li, Y.; Hou, Z.; Fan, Z.; Li, J. Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy. Pharmaceutics 2023, 15, 2233. [Google Scholar] [CrossRef]
- Yousef, H.; Alhajj, M.; Fakoya, A.O.; Okpaleke, O.; Khanna, S.; Sharma, S. Anatomy, Skin (Integument), Epidermis. [Updated 8 June 2024]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470464 (accessed on 27 September 2025).
- Lopez-Ojeda, W.; Pandey, A.; Alhajj, M.; Oakley, A.M. Anatomy, Skin (Integument) [Updated 17 October 2022]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441980 (accessed on 27 September 2025).
- Brown, T.M.; Krishnamurthy, K. Histology, Dermis. [Updated 14 November 2022]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK535346 (accessed on 27 September 2025).
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef]
- Bezie, Z.; Deboch, B.; Ayele, D.; Workeneh, D.; Haile, M.; Mulugeta, G.; Belay, G.; Sewhunegn, A.; Mohammed, A. Common skin diseases. Int. Dev. 2005, 13, 200–204. [Google Scholar]
- Paige, D. Skin disease. In Clinical Medicine, 5th ed.; Kumar, P., Clark, M., Eds.; W.B. Saunders: Edinburgh, UK, 2002; pp. 1271–1320. [Google Scholar]
- Kazandjieva, J.; Tsankov, N. Unmet medical needs in chronic, non-communicable inflammatory skin diseases. Front. Med. 2022, 9, 875492. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, J.; Bai, B.; Song, G.; Zhang, J.; Yu, H.; Huang, S.; Wang, Z.; Lu, G. Topical drug delivery strategies for enhancing drug effectiveness by skin barriers, drug delivery systems and individualized dosing. Front. Pharmacol. 2024, 14, 1333986. [Google Scholar] [CrossRef]
- Stacey, S.K.; McEleney, M. Topical corticosteroids: Choice and application. Am. Fam. Physician 2021, 103, 337–343. [Google Scholar]
- Williamson, D.A.; Carter, G.P.; Howden, B.P. Current and Emerging Topical Antibacterials and Antiseptics: Agents, Action, and Resistance Patterns. Clin. Microbiol. Rev. 2017, 30, 827–860. [Google Scholar] [CrossRef]
- Hasan, N.; Nadaf, A.; Imran, M.; Jiba, U.; Sheikh, A.; Almalki, W.H.; Almujri, S.S.; Mohammed, Y.H.; Kesharwani, P.; Ahmad, F.J. Skin cancer: Understanding the journey of transformation from conventional to advanced treatment approaches. Mol. Cancer. 2023, 22, 168. [Google Scholar] [CrossRef]
- Li, D.; Chen, M.; Li, W.; Xu, X.; Li, Q. Global burden of viral skin diseases from 1990 to 2021, A systematic analysis for the global burden of disease study 2021. Front. Public Health 2025, 13, 1464372. [Google Scholar] [CrossRef]
- Yakupu, A.; Aimaier, R.; Yuan, B.; Chen, B.; Cheng, J.; Zhao, Y.; Peng, Y.; Dong, J.; Lu, S. The burden of skin and subcutaneous diseases: Findings from the Global Burden of Disease Study 2019. Front Public Health 2023, 11, 1145513. [Google Scholar] [CrossRef]
- Lim, H.W.; Collins, S.A.B.; Resneck, J.S., Jr.; Bolognia, J.L.; Hodge, J.A.; Rohrer, T.A.; Van Beek, M.J.; Margolis, D.J.; Sober, A.J.; Weinstock, M.A.; et al. The burden of skin disease in the United States. J. Am. Acad. Dermatol. 2017, 76, 958–972.e2. [Google Scholar] [CrossRef]
- Villacorta, R.; Teeple, A.; Lee, S.; Fakharzadeh, S.; Lucas, J.; McElligott, S. A multinational assessment of work-related productivity loss and indirect costs from a survey of patients with psoriasis. Br. J. Dermatol. 2020, 183, 548–558. [Google Scholar] [CrossRef]
- Chren, M.M. The contribution of health services research to improved dermatologic care. J Investig. Dermatol. 2012, 132 Pt 2, 1003–1007. [Google Scholar] [CrossRef]
- Qu, F.; Geng, R.; Liu, Y.; Zhu, J. Advanced nanocarrier- and microneedle-based transdermal drug delivery strategies for skin diseases treatment. Theranostics 2022, 12, 3372–3406. [Google Scholar] [CrossRef] [PubMed]
- Ballreich, J.M.; Gross, C.P.; Powe, N.R.; Anderson, G.F. Allocation of National Institutes of Health Funding by Disease Category in 2008 and 2019. JAMA Netw. Open 2021, 4, e2034890. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, E.; Balmert, S.C.; Carey, C.D.; Erdos, G.; Falo, L.D., Jr. Emerging skin-targeted drug delivery strategies to engineer immunity: A focus on infectious diseases. Expert. Opin. Drug Deliv. 2021, 18, 151–167. [Google Scholar] [CrossRef]
- Sanz-Nogués, C.; O’Brien, T. Current good manufacturing practice considerations for mesenchymal stromal cells as therapeutic agents. Biomater. Biosyst. 2021, 2, 100018. [Google Scholar] [CrossRef] [PubMed]
- Gorzelanny, C.; Mess, C.; Schneider, S.W.; Huck, V.; Brandner, J.M. Skin Barriers in Dermal Drug Delivery: Which Barriers Have to Be Overcome and How Can We Measure Them? Pharmaceutics. 2020, 12, 684. [Google Scholar] [CrossRef]
- Raina, N.; Rani, R.; Thakur, V.K.; Gupta, M. New Insights in Topical Drug Delivery for Skin Disorders: From a Nanotechnological Perspective. ACS Omega 2023, 8, 19145–19167. [Google Scholar] [CrossRef] [PubMed]
- Guy, R.H. Drug delivery to and through the skin. Drug Deliv. Transl. Res. 2024, 14, 2032–2040. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Roustit, M.; Blaise, S.; Cracowski, J.L. Trials and tribulations of skin iontophoresis in therapeutics. Br. J. Clin. Pharmacol. 2014, 77, 63–71. [Google Scholar] [CrossRef]
- Aldawood, F.K.; Andar, A.; Desai, S. A Comprehensive Review of Microneedles: Types, Materials, Processes, Characterizations and Applications. Polymers 2021, 13, 2815. [Google Scholar] [CrossRef]
- Brito, S.; Baek, M.; Bin, B.H. Skin Structure, Physiology, and Pathology in Topical and Transdermal Drug Delivery. Pharmaceutics 2024, 16, 1403. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Heliyon 2022, 8, e08938. [Google Scholar] [CrossRef]
- Azman, M.; Sabri, A.H.; Anjani, Q.K.; Mustaffa, M.F.; Hamid, K.A. Intestinal Absorption Study: Challenges and Absorption Enhancement Strategies in Improving Oral Drug Delivery. Pharmaceuticals 2022, 15, 975. [Google Scholar] [CrossRef]
- Marushchak, O.; Yakubov, R.; Yakubov, R.; Goldenberg, G. Review on Novel Oral Therapies for Psoriasis. J. Clin. Aesthet. Dermatol. 2021, 14, 55–63. [Google Scholar]
- Kulchar, R.J.; Singh, R.; Ding, S.; Alexander, E.; Leong, K.W.; Daniell, H. Delivery of biologics: Topical administration. Biomaterials. 2023, 302, 122312. [Google Scholar] [CrossRef]
- McNeil, M.M.; DeStefano, F. Vaccine-associated hypersensitivity. J. Allergy Clin. Immunol. 2018, 141, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Sakhiya, J.; Sakhiya, D.; Kaklotar, J.; Hirapara, B.; Purohit, M.; Bhalala, K.; Daruwala, F.; Dudhatra, N. Intralesional agents in dermatology: Pros and cons. J. Cutan. Aesthet. Surg. 2021, 14, 285–295. [Google Scholar] [CrossRef]
- Cîrstea, N.; Radu, A.; Vesa, C.; Radu, A.F.; Bungau, A.F.; Tit, D.M.; Cseppento, C.D.N.; Tarce, A.G.; Bungau, S.G. Current insights on treatment adherence in prevalent dermatological conditions and strategies to optimize adherence rates. Cureus 2024, 16, e69764. [Google Scholar] [CrossRef]
- Dhinsa, H.; McGuinness, A.E.; Ferguson, N.N. Successful treatment of corticosteroid-induced cutaneous atrophy and dyspigmentation with intralesional saline in the setting of keloids. JAAD Case Rep. 2021, 16, 116–119. [Google Scholar] [CrossRef]
- Telaprolu, K.C.; Grice, J.E.; Mohammed, Y.H.; Roberts, M.S. Human Skin Drug Metabolism: Relationships between Methyl Salicylate Metabolism and Esterase Activities in IVPT Skin Membranes. Metabolites 2023, 13, 934. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, T.; Wu, X.; Yuan, H.; Wei, Y.; Xiao, Y. The role of the cytochrome P450 superfamily in the skin. Expert. Rev. Mol. Med. 2024, 26, e15. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Gendelman, H.E.; Kabanov, A.V. Cell-mediated drug delivery. Expert. Opin. Drug Deliv. 2011, 8, 415–433. [Google Scholar] [CrossRef]
- Kim, M.; Shin, M.; Zhao, Y.; Ghosh, M.; Son, Y.O. Transformative Impact of Nanocarrier-Mediated Drug Delivery: Overcoming Biological Barriers and Expanding Therapeutic Horizons. Small Sci. 2024, 4, 2400280. [Google Scholar] [CrossRef]
- Su, S.; MKang, P. Recent Advances in Nanocarrier-Assisted Therapeutics Delivery Systems. Pharmaceutics 2020, 12, 837. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Xin, M.; Kahkoska, A.R.; Wang, J.; Gu, Z. Cell-drug conjugates. Nat. Biomed. Eng. 2024, 8, 1347–1365. [Google Scholar] [CrossRef]
- Parodi, A.; Quattrocchi, N.; Van de Ven, A.L.; Chiappini, C.; Evangelopoulos, M.; Martinez, J.O.; Brown, B.S.; Khaled, S.Z.; Yazdi, I.K.; Enzo, M.; et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat. Nanotechnol. 2013, 8, 61–68. [Google Scholar] [CrossRef]
- Li, H.; Jin, K.; Luo, M.; Wang, X.; Zhu, X.; Liu, X.; Jiang, T.; Zhang, Q.; Wang, S.; Pang, Z. Size dependency of circulation and biodistribution of biomimetic nanoparticles: Red blood cell membrane–coated nanoparticles. Cells 2019, 8, 881. [Google Scholar] [CrossRef]
- Doshi, N.; Swiston, A.J.; Gilbert, J.B.; Alcaraz, M.L.; Cohen, R.E.; Rubner, M.F.; Mitragotri, S. Cell-based drug delivery devices using phagocytosis-resistant backpacks. Adv. Mater. 2011, 23, H105–H109. [Google Scholar] [CrossRef]
- Northcutt, L.A.; Hetrick, E.M.; Arora, P.S.; Gupta, A.S. Cellular ‘backpacks’ for macrophage-mediated drug delivery in inflammation. Adv. Drug Deliv. Rev. 2024, 207, 115385. [Google Scholar]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; El Andaloussi, S.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Odeh-Couvertier, V.Y.; Dwarshuis, N.J.; Colonna, M.B.; Levine, B.L.; Edison, A.S.; Kotanchek, T.; Roy, K.; Torres-Garcia, W. Predicting T-cell quality during manufacturing through an artificial intelligence-based integrative multiomics analytical platform. Bioeng. Transl. Med. 2022, 7, e10282. [Google Scholar] [CrossRef]
- Choudhury, M.; Deans, A.J.; Candland, D.R.; Deans, T.L. Advancing cell therapies with artificial intelligence and synthetic biology. Curr. Opin. Biomed. Eng. 2025, 34, 100580. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.; Zhang, W.; Xia, Y.; Wu, P.; Yang, M.; Jin, R.; Xia, S.; Wang, J.; You, C.; Han, C.; et al. 3D bioprinting for skin tissue engineering: Current status and perspectives. J. Tissue Eng. 2021, 12, 20417314211028574. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Kilian, D.; Ramos Mejia, D.; Rios, R.J.; Ali, A.; Heilshorn, S.C. Diffusion-Based 3D Bioprinting Strategies. Adv. Sci. 2024, 11, e2306470. [Google Scholar] [CrossRef]
- Bo, T.; Pascucci, E.; Capuani, S.; Campa-Carranza, J.N.; Franco, L.; Farina, M.; Secco, J.; Becchi, S.; Cavazzana, R.; Joubert, A.L.; et al. 3D bioprinted mesenchymal stem cell laden scaffold enhances subcutaneous vascularization for delivery of cell therapy. Biomed. Microdevices 2024, 26, 29. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Qian, Z.M. Macrophage based drug delivery: Key challenges and strategies. Bioact. Mater. 2024, 38, 55–72. [Google Scholar] [CrossRef]
- Choi, A.; Javius-Jones, K.; Hong, S.; Park, H. Cell-Based Drug Delivery Systems with Innate Homing Capability as a Novel Nanocarrier Platform. Int. J. Nanomed. 2023, 18, 509–525. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophils at the crossroads of innate and adaptive immunity. J. Leukoc. Biol. 2020, 108, 377–396. [Google Scholar] [CrossRef]
- Herro, R.; Grimes, H.L. The diverse roles of neutrophils from protection to pathogenesis. Nat. Immunol. 2024, 25, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Giza, H.M.; Bozzacco, L. Unboxing dendritic cells: Tales of multi-faceted biology and function. Immunology 2021, 164, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, M.F.; Masten, B.J. Dendritic cells: Immune regulators in health and disease. Physiol. Rev. 2002, 82, 97–130. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, J.; Huang, Y.; Zhang, X.; Zhang, G.; Kong, S.; Gao, J.; Zhang, X.; Ding, B. Drug Delivery Systems Based on Dendritic-Cell-Derived Exosomes. Pharmaceutics 2025, 17, 326. [Google Scholar] [CrossRef]
- Pulit-Penaloza, J.; Esser, E.S.; Vassilieva, E.V.; Lee, J.W.; Taherbhai, M.T.; Pollack, B.P.; Prausnitz, M.R.; Compans, R.W.; Skountzou, I. A protective role of murine langerin+ cells in immune responses to cutaneous vaccination with microneedle patches. Sci. Rep. 2014, 4, 6094. [Google Scholar] [CrossRef]
- Zaric, M.; Lyubomska, O.; Touzelet, O.; Poux, C.; Al-Zahrani, S.; Fay, F.; Wallace, L.; Terhorst, D.; Malissen, B.; Henri, S.; et al. Skin dendritic cell targeting via microneedle arrays laden with antigen-encapsulated PLGA nanoparticles induces efficient antitumor and antiviral immune responses. ACS Nano 2013, 7, 2042–2055. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xu, B.; Xu, J.; Shou, D.; Liu, E.; Gao, J.; Liang, W.; Huang, Y. Microneedle-assisted dendritic cell-targeted nanoparticles for transcutaneous DNA immunization. Polym. Chem. 2015, 6, 373–379. [Google Scholar] [CrossRef]
- Marshall, S.; Sahm, L.J.; Moore, A.C. The success of microneedle-mediated vaccine delivery into skin. Hum. Vaccin. Immunother. 2016, 12, 2975–2983. [Google Scholar] [CrossRef]
- Tran, C.; Damaser, M.S. Stem cells as drug delivery methods: Application of stem cell secretome for regeneration. Adv. Drug Deliv. Rev. 2015, 82–83, 1–11. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, Q.; Tam, P.K.H. Immunomodulatory Mechanisms of Mesenchymal Stem Cells and Their Potential Clinical Applications. Int. J. Mol. Sci. 2022, 23, 10023. [Google Scholar] [CrossRef]
- Seo, H.M.; Lew, B.L.; Lee, Y.W.; Son, S.W.; Park, C.O.; Park, Y.L.; Baek, J.O.; Shin, M.K.; Kim, D.H.; Lee, D.H.; et al. Phase 1/2 trials of human bone marrow-derived clonal mesenchymal stem cells for treatment of adults with moderate to severe atopic dermatitis. J. Allergy Clin. Immunol. 2024, 154, 965–973. [Google Scholar] [CrossRef]
- Shin, H.T.; Lee, S.H.; Yoon, H.S.; Heo, J.H.; Lee, S.B.; Byun, J.W.; Shin, J.; Cho, Y.K.; Chung, E.; Jeon, M.S.; et al. Long-term efficacy and safety of intravenous injection of clonal mesenchymal stem cells derived from bone marrow in five adults with moderate to severe atopic dermatitis. J. Dermatol. 2021, 48, 1236–1242. [Google Scholar] [CrossRef]
- He, K.; Zang, J.; Ren, T.; Feng, S.; Liu, M.; Zhang, X.; Sun, W.; Chu, J.; Xu, D.; Liu, F. Therapeutic potential and mechanisms of mesenchymal stem cell and mesenchymal stem cell-derived extracellular vesicles in atopic dermatitis. J. Inflamm. Res. 2024, 17, 5783–5800. [Google Scholar] [CrossRef]
- Park, D.; Kim, J.H.; Yang, H.; Ji, Y.; Yoo, J.; Kim, J.; Bang, O.Y. Mesenchymal stem cell-derived extracellular vesicles exert Th1-mediated anti-inflammatory effects via miR-146a/NF-κB pathway: Comparison with dupilumab in a mouse model of atopic dermatitis. Stem Cell Res. Ther. 2025, 16, 496. [Google Scholar] [CrossRef]
- Kim, S.W.; Lim, K.M.; Cho, S.G.; Ryu, B.; Kim, C.Y.; Park, S.Y.; Jang, K.; Jung, J.H.; Park, C.; Choi, C.; et al. Efficacy of allogeneic and xenogeneic exosomes for the treatment of canine atopic dermatitis: A pilot study. Animals 2024, 14, 282. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.S.; Kim, S.B.; Kim, S.; Rhee, B.; Yoon, J.; Lee, J.W. Canine mesenchymal-stem-cell-derived extracellular vesicles attenuate atopic dermatitis. Animals 2023, 13, 2215. [Google Scholar] [CrossRef]
- Taheri, B.; Soleimani, M.; Fekri Aval, S.; Esmaeili, E.; Bazi, Z.; Zarghami, N. Induced pluripotent stem cell-derived extracellular vesicles: A novel approach for cell-free regenerative medicine. J. Cell. Physiol. 2019, 234, 8455–8464. [Google Scholar] [CrossRef]
- Jiang, Z.; Han, Y.; Cao, X. Induced pluripotent stem cell (iPSCs) and their application in immunotherapy. Cell. Mol. Immunol. 2014, 11, 17–24. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Behdarvand Dehkordi, F.; Chehelgerdi, M.; Kabiri, H.; Salehian-Dehkordi, H.; Abdolvand, M.; Salmanizadeh, S.; Rashidi, M.; Niazmand, A.; Ahmadi, S.; et al. Exploring the promising potential of induced pluripotent stem cells in cancer research and therapy. Mol. Cancer 2023, 22, 189. [Google Scholar] [CrossRef]
- Wang, X.; Zinkel, S.; Polonsky, K.; Fuchs, E. Transgenic studies with a keratin promoter-driven growth hormone transgene: Prospects for gene therapy. Proc. Natl. Acad. Sci. USA 1997, 94, 219–226. [Google Scholar] [CrossRef]
- Cao, T.; Wang, X.J.; Roop, D.R. Regulated cutaneous gene delivery: The skin as a bioreactor. Hum. Gene Ther. 2000, 11, 2297–2300. [Google Scholar] [CrossRef]
- Sawamura, D.; Akiyama, M.; Shimizu, H. Direct injection of naked DNA and cytokine transgene expression: Implications for keratinocyte gene therapy. Clin. Exp. Dermatol. 2002, 27, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Mavilio, F.; Pellegrini, G.; Ferrari, S.; Di Nunzio, F.; Di Iorio, E.; Recchia, A.; Maruggi, G.; Ferrari, G.; Provasi, E.; Bonini, C.; et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat. Med. 2006, 12, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, T.; Rothoeft, T.; Teig, N.; Bauer, J.W.; Pellegrini, G.; De Rosa, L.; Scaglione, D.; Reichelt, J.; Klausegger, A.; Kneisz, D.; et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature 2017, 551, 327–332. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Phase 3, Open-Label Clinical Trial of EB-101 for the Treatment of Recessive Dystrophic Epidermolysis Bullosa (RDEB). ClinicalTrials.gov Identifier: NCT04227106. Sponsored by Abeona Therapeutics, Inc. Last Updated 5 December 2022. Available online: https://clinicaltrials.gov/study/NCT04227106 (accessed on 27 September 2025).
- Vecin, N.M.; Kirsner, R.S. Skin substitutes as treatment for chronic wounds: Current and future directions. Front. Med. 2023, 10, 1154567. [Google Scholar] [CrossRef]
- MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Perisic, T.; Zhang, Z.; Foehr, P.; Hopfner, U.; Klutz, K.; Burgkart, R.H.; Slobodianski, A.; Goeldner, M.; Machens, H.G.; Schilling, A.F. Biodegradable poly (lactic acid-co-glycolic acid) scaffolds as carriers for genetically-modified fibroblasts. PLoS ONE 2017, 12, e0174860. [Google Scholar] [CrossRef]
- Nasiri, G.; Azarpira, N.; Alizadeh, A.; Goshtasbi, S.; Tayebi, L. Shedding light on the role of keratinocyte-derived extracellular vesicles on skin-homing cells. Stem Cell Res. Ther. 2020, 11, 421. [Google Scholar] [CrossRef]
- Kersten Compliance Services, LLC. LAVIV® (azficel-T): Biologics License Application (BLA) STN 125348 Summary Report; Kersten Compliance Services, LLC: Dobbs Ferry, NY, USA, 2011; Available online: https://kerstencompliance.com/sites/default/files/resources/laviv.pdf (accessed on 27 September 2025).
- Weiss, R.A.; Weiss, M.A.; Beasley, K.L.; Vrcek, I. Successful treatment of depressed, distensible acne scars using autologous fibroblasts: A multi-site, prospective, double-blind, placebo-controlled clinical trial. Dermatol. Surg. 2013, 39, 1226–1236. [Google Scholar] [CrossRef]
- Weiss, R.A.; Weiss, M.A.; Beasley, K.L.; Munavalli, G. Autologous cultured fibroblast injection for facial contour deformities: A prospective, placebo-controlled, phase III clinical trial. Dermatol. Surg. 2007, 33, 263–268. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. LAVIV (azficel-T)—BLA 125348. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/laviv (accessed on 26 September 2025).
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Serbina, N.V.; Pamer, E.G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006, 7, 311–317. [Google Scholar] [CrossRef]
- Torchilin, V. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef]
- Underhill, D.M.; Goodridge, H.S. Information processing during phagocytosis. Nat. Rev. Immunol. 2012, 12, 492–502. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106 Pt A, 148–156. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Lai, R.C.; Yeo, R.W.; Tan, K.H.; Lim, S.K. Exosomes for drug delivery—A novel application for the mesenchymal stem cell. Biotechnol. Adv. 2013, 31, 543–551. [Google Scholar] [CrossRef]
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.H. Nanotubular highways for intercellular organelle transport. Science 2004, 303, 1007–1010. [Google Scholar] [CrossRef]
- Haddadzadegan, S.; Dorkoosh, F.; Bernkop-Schnürch, A. Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Adv. Drug Deliv. Rev. 2022, 182, 114097. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Najar, M.; Raicevic, G.; Fayyad-Kazan, H.; Bron, D.; Toungouz, M.; Lagneaux, L. Mesenchymal stromal cells and immunomodulation: A gathering of regulatory immune cells. Cytotherapy 2016, 18, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, R.; Liu, X.; Ding, Q.; Wu, S.; Li, C.; Lin, Y.; Ye, Y.; Zhong, Z.; Zhou, M. Recent advances in macrophage-mediated drug delivery systems. Int. J. Nanomedicine 2021, 16, 2703–2714. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 2015, 219, 396–405. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.; Chen, Y.; Xu, Y.; Peng, J. Cell-based drug delivery systems and their in vivo fate. Adv. Drug Deliv. Rev. 2022, 187, 114394. [Google Scholar] [CrossRef] [PubMed]
- Charoenphun, P.; Meszaros, L.K.; Chuamsaamarkkee, K.; Sharif-Paghaleh, E.; Ballinger, J.R.; Ferris, T.J.; Went, M.J.; Mullen, G.E.D.; Blower, P.J. [^89Zr]Oxinate4 for long-term in vivo cell tracking by positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 278–287. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xuan, S.; Port, M.; Idee, J.M. Recent advances in superparamagnetic iron oxide nanoparticles for cellular imaging and targeted therapy research. Curr. Pharm. Des. 2013, 19, 6575–6593. [Google Scholar] [CrossRef]
- Shin, K.O.; Ha, D.H.; Kim, J.O.; Crumrine, D.A.; Meyer, J.M.; Wakefield, J.S.; Lee, Y.; Kim, B.; Kim, S.; Kim, H.K.; et al. Exosomes from Human Adipose Tissue-Derived Mesenchymal Stem Cells Promote Epidermal Barrier Repair by Inducing de Novo Synthesis of Ceramides in Atopic Dermatitis. Cells 2020, 9, 680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oh, N.; Park, J.H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9 (Suppl. S1), 51–63. [Google Scholar] [CrossRef]
- Gehl, J. Electroporation: Theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 2003, 177, 437–447. [Google Scholar] [CrossRef]
- Pang, L.; Zhang, C.; Qin, J.; Han, L.; Li, R.; Hong, C.; He, H.; Wang, J. A novel strategy to achieve effective drug delivery: Exploit cells as carriers combined with nanoparticles. Drug Deliv. 2017, 24, 83–91. [Google Scholar] [CrossRef]
- Stephan, M.T.; Irvine, D.J. Enhancing Cell therapies from the Outside In: Cell Surface Engineering Using Synthetic Nanomaterials. Nano Today 2011, 6, 309–325. [Google Scholar] [CrossRef]
- Stephan, M.T.; Moon, J.J.; Um, S.H.; Bershteyn, A.; Irvine, D.J. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat. Med. 2010, 16, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, M.S.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef]
- Milošević, N.; Rütter, M.; David, A. Endothelial Cell Adhesion Molecules- (un)Attainable Targets for Nanomedicines. Front. Med. Technol. 2022, 4, 846065. [Google Scholar] [CrossRef]
- Scarfò, I.; Ormhøj, M.; Frigault, M.J.; Castano, A.P.; Lorrey, S.; Bouffard, A.A.; van Scoyk, A.; Rodig, S.J.; Shay, A.J.; Aster, J.C.; et al. Anti-CD37 chimeric antigen receptor T cells are active against B- and T-cell lymphomas. Blood 2018, 132, 1495–1506, Erratum in Blood 2018, 132, 2527. [Google Scholar] [CrossRef]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Porada, C.D.; Almeida-Porada, G. Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 1156–1166. [Google Scholar] [CrossRef]
- Lee, D.E.; Ayoub, N.; Agrawal, D.K. Mesenchymal stem cells and cutaneous wound healing: Novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res. Ther. 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liang, Y.; Xia, M.; Tian, X.; Chen, Z.; Lin, L.; Liang, J.; Liu, Y. Serum exosomal microRNA profiling reveals a down-regulation of hsa-miR-124-3p in patients with severe acne. Front. Immunol. 2025, 16, 1554811. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.Y.; Chua, K.H.; Tan, E.W.; Goh, B.H. Therapeutic Potential of Mesenchymal Stem Cells in Psoriasis. Cell Biochem. Biophys. 2025, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.K.; Mohamed, A.H.; Amer Alsaiari, A.; Olegovich Bokov, D.; Ali Patel, A.; Al Abdulmonem, W.; Shafie, A.; Adnan Ashour, A.; Azhar Kamal, M.; Ahmad, F.; et al. The role of mesenchymal stem cells in the treatment and pathogenesis of psoriasis. Cytokine 2024, 182, 156699. [Google Scholar] [CrossRef]
- Lu, C.H.; Lai, C.Y.; Yeh, D.W.; Liu, Y.L.; Su, Y.W.; Hsu, L.C.; Chang, C.H.; Catherine Jin, S.L.; Chuang, T.H. Involvement of M1 Macrophage Polarization in Endosomal Toll-Like Receptors Activated Psoriatic Inflammation. Mediat. Inflamm. 2018, 2018, 3523642. [Google Scholar] [CrossRef]
- Liu, J.M.; Jin, Q.X.; Fujimoto, M.; Li, F.F.; Jin, L.B.; Yu, R.; Yan, G.H.; Zhu, L.H.; Meng, F.P.; Zhang, Q.G.; et al. Dihydroartemisinin alleviates imiquimod-induced psoriasis-like skin lesion in mice involving modulation of IL-23/Th17 axis. Front. Pharmacol. 2021, 12, 704481. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, J.; Shi, P.; Su, D.; Cheng, X.; Yi, W.; Yan, J.; Chen, H.; Cheng, F. Small extracellular vesicles derived from MSCs have immunomodulatory effects to enhance delivery of ASO-210 for psoriasis treatment. Front. Cell Dev. Biol. 2022, 10, 842813. [Google Scholar] [CrossRef]
- Luliano, M.; Grimaldi, L.; Rosa, P.; Scibetta, S.; Bernardini, N.; Proietti, I.; Tolino, E.; Skroza, N.; Potenza, C.; Mangino, G.; et al. Extracellular vesicles in psoriasis: From pathogenesis to possible roles in therapy. Front. Immunol. 2024, 15, 1360618. [Google Scholar] [CrossRef]
- Lo Sicco, C.; Reverberi, D.; Balbi, C.; Ulivi, V.; Principi, E.; Pascucci, L.; Becherini, P.; Bosco, M.C.; Varesio, L.; Franzin, C.; et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: Endorsement of macrophage polarization. Stem Cells Transl. Med. 2017, 6, 1018–1028. [Google Scholar] [CrossRef]
- Ubanako, P.; Mirza, S.; Ruff, P.; Penny, C. Exosome-mediated delivery of siRNA molecules in cancer therapy: Triumphs and challenges. Front. Mol. Biosci. 2024, 11, 1447953. [Google Scholar] [CrossRef]
- Ha, D.H.; Kim, H.; Lee, J.; Kwon, H.H.; Park, G.H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Yin, P.; Liang, C.; Zhao, X.; Wen, D.; Tan, Y. Secretome of human umbilical cord mesenchymal stem cell maintains skin homeostasis by regulating multiple skin physiological function. Cell Tissue Res. 2023, 391, 111–125. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, M.; Ma, K.; Li, H.; Ran, M.; Yang, S.; Yang, Y.; Fu, X.; Yang, S. Therapeutic effects of mesenchymal stem cells and their derivatives in common skin inflammatory diseases: Atopic dermatitis and psoriasis. Front. Immunol. 2023, 14, 1092668. [Google Scholar] [CrossRef]
- Chen, Y.E.; Bousbaine, D.; Veinbachs, A.; Atabakhsh, K.; Dimas, A.; Yu, V.K.; Zhao, A.; Enright, N.J.; Nagashima, K.; Belkaid, Y.; et al. Engineered skin bacteria induce antitumor T cell responses against melanoma. Science 2023, 380, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Myles, I.A.; Earland, N.J.; Anderson, E.D.; Moore, I.N.; Kieh, M.D.; Williams, K.W.; Saleem, A.; Fontecilla, N.M.; Welch, P.A.; Darnell, D.A.; et al. First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight 2018, 3, e120608. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Hata, T.R.; Tong, Y.; Cheng, J.Y.; Shafiq, F.; Butcher, A.M.; Salem, S.S.; Brinton, S.L.; Rudman Spergel, A.K.; Johnson, K.; et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat. Med. 2021, 27, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Gallo, R.L.; Shafiq, F.; Tong, Y.; Chun, K.; Butcher, A.M.; Cheng, J.Y.; Hata, T.R. Use of autologous bacteriotherapy to treat Staphylococcus aureus in patients with atopic dermatitis: A randomized double-blind clinical trial. JAMA Dermatol. 2021, 157, 978–982. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Targeted Microbiome Transplant in Atopic Dermatitis. ClinicalTrials.gov Identifier: NCT03151148. Available online: https://clinicaltrials.gov/study/NCT03151148 (accessed on 27 September 2025).
- Tsai, K.K.; Komanduri, K.V. Tumor-Infiltrating Lymphocyte Therapy for the Treatment of Metastatic Melanoma. Am. J. Clin. Dermatol. 2025, 26, 733. [Google Scholar] [CrossRef]
- Rohaan, M.W.; van den Berg, J.H.; Kvistborg, P.; Haanen, J.B.A.G. Tumor-infiltrating lymphocyte therapy or ipilimumab in advanced melanoma. N. Engl. J. Med. 2022, 387, 2113–2125. [Google Scholar] [CrossRef]
- Mehta, A.; Motavaf, M.; Nebo, I.; Luyten, S.; Osei-Opare, K.D.; Gru, A.A. Advancements in melanoma treatment: A review of PD-1 inhibitors, T-VEC, mRNA vaccines, and tumor-infiltrating lymphocyte therapy in an evolving landscape of immunotherapy. J. Clin. Med. 2025, 14, 1200. [Google Scholar] [CrossRef]
- Soares da Silva, L.M.; de Brito Gomes, E.S.; Vieira, J.H.; de Aguiar, M.P.; Moreira da Silva, S.F.; Michelin, M.A. Efficacy of treatment with tumor-infiltrating lymphocytes as adoptive cell therapy: An integrative review. Einstein 2024, 22, eRW0935. [Google Scholar] [CrossRef]
- Amaria, R.; Knisely, A.; Vining, D.; Kopetz, S.; Overman, M.J.; Javle, M.; Antonoff, M.B.; Tzeng, C.W.D.; Wolff, R.A.; Pant, S.; et al. Efficacy and safety of autologous tumor-infiltrating lymphocytes in recurrent or refractory ovarian cancer, colorectal cancer, and pancreatic ductal adenocarcinoma. J. Immunother. Cancer 2024, 12, e006822. [Google Scholar] [CrossRef]
- Babajani, A.; Soltani, P.; Jamshidi, E.; Farjoo, M.H.; Niknejad, H. Recent Advances on Drug-Loaded Mesenchymal Stem Cells With Anti-neoplastic Agents for Targeted Treatment of Cancer. Front. Bioeng. Biotechnol. 2020, 8, 748. [Google Scholar] [CrossRef]
- Bommareddy, P.K.; Patel, A.; Hossain, S.; Kaufman, H.L. Talimogene Laherparepvec (T-VEC) and Other Oncolytic Viruses for the Treatment of Melanoma. Am. J. Clin. Dermatol. 2017, 18, 1–15. [Google Scholar] [CrossRef]
- Zhang, T.; Jou, T.H.; Hsin, J.; Wang, Z.; Huang, K.; Ye, J.; Yin, H.; Xing, Y. Talimogene laherparepvec (T-VEC): A review of the recent advances in cancer therapy. J. Clin. Med. 2023, 12, 1098. [Google Scholar] [CrossRef] [PubMed]
- Chesney, J.A.; Puzanov, I.; Collichio, F.A.; Singh, P.; Milhem, M.M.; Glaspy, J.; Hamid, O.; Ross, M.; Friedlander, P.; Garbe, C.; et al. Talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone for advanced melanoma: 5-year final analysis of a multicenter, randomized, open-label, phase II trial. J. Immunother. Cancer 2023, 11, e006270. [Google Scholar] [CrossRef]
- Natarelli, N.; Gahoonia, N.; Sivamani, R.K. Bacteriophages and the microbiome in dermatology: The role of the phageome and a potential therapeutic strategy. Int. J. Mol. Sci. 2023, 24, 2695. [Google Scholar] [CrossRef] [PubMed]
- Golembo, M.; Puttagunta, S.; Rappo, U.; Weinstock, E.; Engelstein, R.; Gahali-Sass, I.; Moses, A.; Kario, E.; Ben-Dor Cohen, E.; Nicenboim, J.; et al. Development of a topical bacteriophage gel targeting Cutibacterium acnes for acne-prone skin and results of a phase 1 cosmetic randomized clinical trial. Skin. Health Dis. 2022, 2, e93. [Google Scholar] [CrossRef] [PubMed]
- Hamzavi, I.H.; Ganesan, A.K.; Mahmoud, B.H.; Weiss, E.; Ahmed, A.M.; Robinson, D.; Goldman, M.P.; Munavalli, G.; Kahn, S.A.; Huang, V.; et al. Effective and durable repigmentation for stable vitiligo: A randomized within-subject controlled trial assessing treatment with autologous skin cell suspension transplantation. J. Am. Acad. Dermatol. 2024, 91, 1104–1112. [Google Scholar] [CrossRef]
- Zavala, G.; Sandoval, C.; Meza, D.; Contreras, R.; Gubelin, W.; Khoury, M. Differentiation of adipose-derived stem cells to functional CD105neg CD73low melanocyte precursors guided by defined culture condition. Stem Cell Res. Ther. 2019, 10, 249. [Google Scholar] [CrossRef]
- Ramos, M.G.; Ramos, D.G.; Gontijo, G.; Ramos, C.G.; Rocha, T.N.; Rocha, R.H. Non-cultured melanocyte/keratinocyte transplantation for the treatment of stable vitiligo on the face: Report of two cases. An. Bras. Dermatol. 2013, 88, 811–813. [Google Scholar] [CrossRef]
- Nuntawisuttiwong, P.; Yothachai, P.; Paringkarn, T.; Chaiyabutr, C.; Wongpraparut, C.; Silpa-Archa, N. Sustained Repigmentation in Vitiligo and Leukodermas Using Melanocyte–Keratinocyte Transplantation: 7 Years of Data. Clin. Cosmet. Investig. Dermatol. 2024, 17, 1467–1478. [Google Scholar] [CrossRef] [PubMed]
- Silpa-Archa, N.; Griffith, J.L.; Huggins, R.H.; Henderson, M.D.; Kerr, H.A.; Jacobsen, G.; Mulekar, S.V.; Lim, H.W.; Hamzavi, I.H. Long-term follow-up of patients undergoing autologous noncultured melanocyte-keratinocyte transplantation for vitiligo and other leukodermas. J. Am. Acad. Dermatol. 2017, 77, 318–327. [Google Scholar] [CrossRef]
- Nowak-Liduk, A.; Kitala, D.; Ochała-Gierek, G.; Łabuś, W.; Bergler-Czop, B.; Pietrauszka, K.; Niemiec, P.; Szyluk, K.; Gierek, M. Hidradenitis suppurativa: An interdisciplinary problem in dermatology, gynecology, and surgery—Pathogenesis, comorbidities, and current treatments. Life 2023, 13, 1895. [Google Scholar] [CrossRef] [PubMed]
- Dagenet, C.B.; Atluri, S.; Ma, E.; Tong, L.; Tran, K.A.; Hekmatjah, J.; Masson, R.; Hsiao, J.L.; Shi, V.Y. Adherence to hidradenitis suppurativa treatment. Am. J. Clin. Dermatol. 2024, 25, 585–594. [Google Scholar] [CrossRef]
- Hristov, A.C.; Tejasvi, T.; AWilcox, R. Cutaneous T-cell lymphomas: 2021 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2021, 96, 1313–1328. [Google Scholar] [CrossRef]
- Knoedler, S.; Broichhausen, S.; Guo, R.; Dai, R.; Knoedler, L.; Kauke-Navarro, M.; Diatta, F.; Pomahac, B.; Machens, H.G.; Jiang, D.; et al. Fibroblasts-the cellular choreographers of wound healing. Front. Immunol. 2023, 14, 1233800. [Google Scholar] [CrossRef]
- Guillamat-Prats, R. The Role of MSC in Wound Healing, Scarring and Regeneration. Cells 2021, 10, 1729. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.C.; Rodrigues, M.; Nuschke, A.; Johnson, Z.I.; Whaley, D.; Stolz, D.; Newsome, J.; Wells, A. Multipotent stromal cells/mesenchymal stem cells and fibroblasts combine to minimize skin hypertrophic scarring. Stem Cell Res. Ther. 2017, 8, 193. [Google Scholar] [CrossRef]
- Jo, C.; Choi, Y.J.; Lee, T.J. Therapeutic Potential of Stem Cell-Derived Exosomes in Skin Wound Healing. Biomimetics 2025, 10, 546. [Google Scholar] [CrossRef]
- Han, S.K.; Choi, K.J.; Kim, W.K. Clinical application of fresh fibroblast allografts for the treatment of diabetic foot ulcers: A pilot study. Plast. Reconstr. Surg. 2004, 114, 1783–1789. [Google Scholar] [CrossRef]
- Manole, C.G.; Soare, C.; Ceafalan, L.C.; Voiculescu, V.M. Platelet-rich plasma in dermatology: New insights on the cellular mechanism of skin repair and regeneration. Life 2023, 14, 40. [Google Scholar] [CrossRef]
- Vladulescu, D.; Scurtu, L.G.; Simionescu, A.A.; Scurtu, F.; Popescu, M.I.; Simionescu, O. Platelet-rich plasma (PRP) in dermatology: Cellular and molecular mechanisms of action. Biomedicines 2023, 12, 7. [Google Scholar] [CrossRef]
- Liu, M.; Ding, J.; Peng, Y.; Fang, J.; Zhao, M.; Zhang, W.; Chen, H.; Zhang, J.; Peng, H.; Wang, Q. Platelet-rich plasma-contained drug delivery systems to treat orthopedic injuries. Int. J. Pharm. X 2025, 10, 100372. [Google Scholar] [CrossRef] [PubMed]
- West, K.A.; Gea-Banacloche, J.; Stroncek, D.; Kadri, S.S. Granulocyte transfusions in the management of invasive fungal infections. Br. J. Haematol. 2017, 177, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Price, T.H.; Boeckh, M.; Harrison, R.W.; McCullough, J.; Ness, P.M.; Strauss, R.G.; Nichols, W.G.; Hamza, T.H.; Cushing, M.M.; King, K.E.; et al. Efficacy of transfusion with granulocytes from G-CSF/dexamethasone-treated donors in neutropenic patients with infection. Blood 2015, 126, 2153–2161. [Google Scholar] [CrossRef]
- Michelen, Y.; Kantarjian, H.M.; Ravandi, F.; Pemmaraju, N.; Kadia, T.M.; Konopleva, M.; Verstovsek, S.; Daver, N.G.; Garcia-Manero, G.; Krause, H.; et al. Granulocyte transfusions in patients with skin and soft tissue infections and leukemia: Are they useful? J. Clin. Oncol. 2019, 37 (Suppl. 15), 7022. [Google Scholar] [CrossRef]
- Mei, R.; Wan, Z.; Yang, C.; Shen, X.; Wang, R.; Zhang, H.; Yang, R.; Li, J.; Song, Y.; Su, H. Advances and clinical challenges of mesenchymal stem cell therapy. Front. Immunol. 2024, 15, 1421854. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, Z.; Jolly, K.J. Myeloid cell-mediated drug delivery: From nanomedicine to cell therapy. Adv. Drug Deliv. Rev. 2023, 197, 114827. [Google Scholar] [CrossRef]
- Fernández-Santos, M.E.; Garcia-Arranz, M.; Andreu, E.J.; García-Hernández, A.M.; López-Parra, M.; Villarón, E.; Sepúlveda, P.; Fernández-Avilés, F.; García-Olmo, D.; Prosper, F.; et al. Optimization of Mesenchymal Stromal Cell (MSC) Manufacturing Processes for a Better Therapeutic Outcome. Front. Immunol. 2022, 13, 918565. [Google Scholar] [CrossRef]
- Chao, C.J.; Zhang, E.; Zhao, Z. Engineering cells for precision drug delivery: New advances, clinical translation, and emerging strategies. Adv. Drug Deliv. Rev. 2023, 197, 114840. [Google Scholar] [CrossRef]
- Agrawal, S.; Vaidya, S.; Patel, J.; Jirvankar, P.; Gurjar, P. Challenges and pathways in regulating next-gen biological therapies. Curr. Pharm. Biotechnol. 2025, 26. [Google Scholar] [CrossRef]
- Weng, C.F.; Dong, J.Y.; Lin, S.F.; Jiang, A.L.; Cheng, Y.L.; Chang, L.C. Analysis of cellular and gene therapy product reviews in the United States. Regul. Toxicol. Pharmacol. RTP 2025, 162, 105885. [Google Scholar] [CrossRef]
- Mozafari, N.; Mozafari, N.; Dehshahri, A.; Azadi, A. Knowledge Gaps in Generating Cell-Based Drug Delivery Systems and a Possible Meeting with Artificial Intelligence. Mol. Pharm. 2023, 20, 3757–3778. [Google Scholar] [CrossRef]
- Capponi, S.; Daniels, K.G. Harnessing the power of artificial intelligence to advance cell therapy. Immunol. Rev. 2023, 320, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Wolter, T.; Hu, Q. Biomaterial scaffolds for therapeutic immune cell engineering and delivery. J. Control. Release 2025, 386, 114072. [Google Scholar] [CrossRef] [PubMed]
- Facklam, A.L.; Volpatti, L.R.; Anderson, D.G. Biomaterials for Personalized Cell Therapy. Adv. Mater. 2020, 32, e1902005. [Google Scholar] [CrossRef]
- Gupta, R. The need for global access to biomedical innovations during pandemics. Nat. Biotechnol. 2021, 39, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Ayala Ceja, M.; Khericha, M.; Harris, C.M.; Puig-Saus, C.; Chen, Y.Y. CAR-T cell manufacturing: Major process parameters and next-generation strategies. J. Exp. Med. 2024, 221, e20230903. [Google Scholar] [CrossRef]
- Lonez, C.; Breman, E. Allogeneic CAR-T Therapy Technologies: Has the Promise Been Met? Cells 2024, 13, 146. [Google Scholar] [CrossRef]
- Askari, G.; Vajdi, M.; Jafari-Nasab, S.; Golpour-Hamedani, S. Ethical guidelines for human research on children and adolescents: A narrative review study. J. Res. Med. Sci. 2024, 29, 53. [Google Scholar] [CrossRef]
- Cornetta, K.; Bonamino, M.; Mahlangu, J.; Mingozzi, F.; Rangarajan, S.; Rao, J. Gene therapy access: Global challenges, opportunities, and views from Brazil, South Africa, and India. Mol. Ther. 2022, 30, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Aiyegbusi, O.L.; Macpherson, K.; Elston, L.; Myles, S.; Washington, J.; Sungum, N.; Briggs, M.; Newsome, P.N.; Calvert, M.J. Patient and public perspectives on cell and gene therapies: A systematic review. Nat. Commun. 2020, 11, 6265. [Google Scholar] [CrossRef] [PubMed]
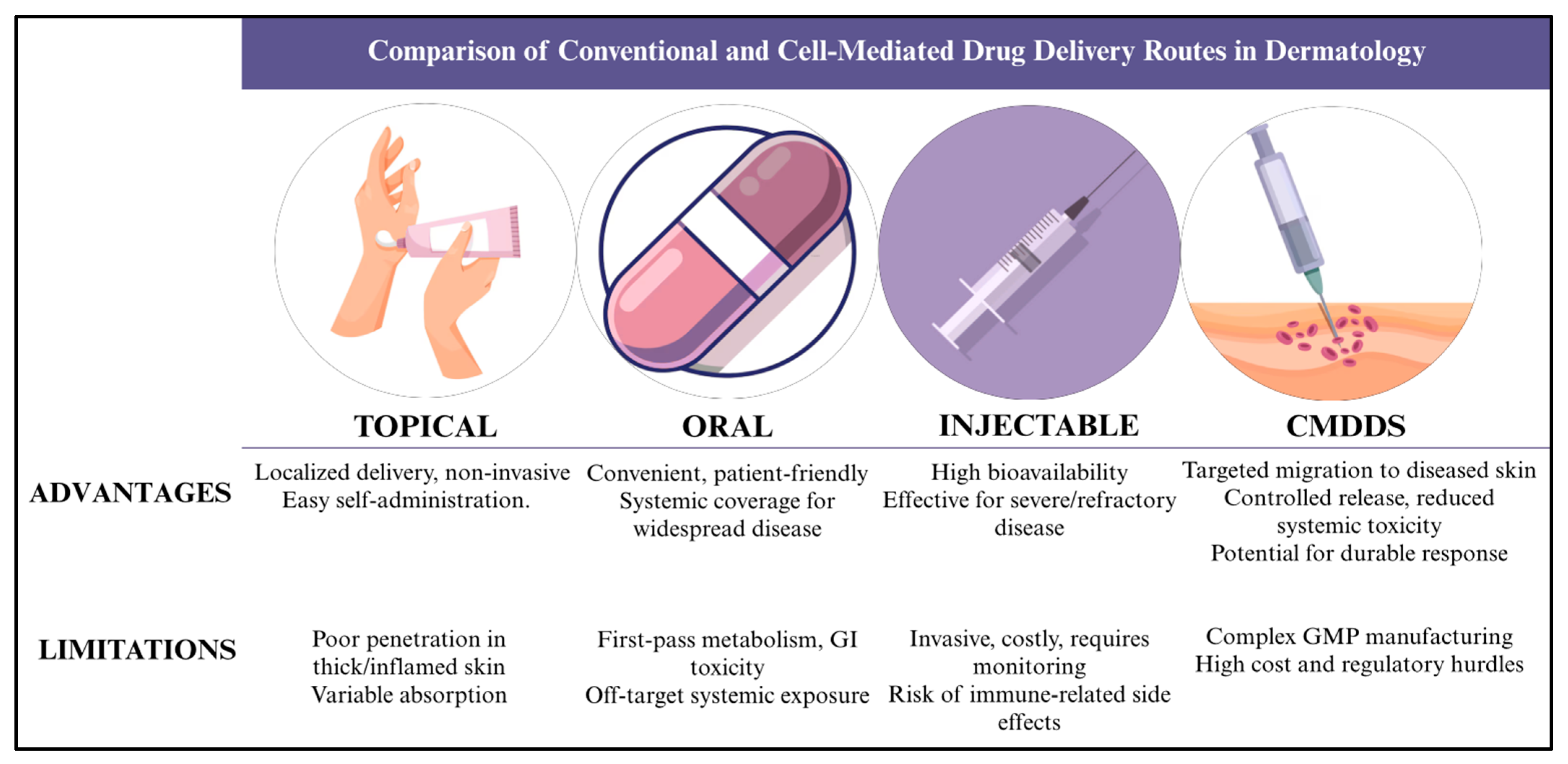
| Parameter | Topical | Oral | Injectable (Systemic) | Cell-Mediated Drug Delivery System |
|---|---|---|---|---|
| Target specificity | Low—relies on passive diffusion; limited to superficial lesions | Low—systemic distribution, minimal skin targeting | Medium—higher tissue but not skin-selective | High—uses cell homing to inflamed/malignant skin |
| Systemic toxicity | Low–Moderate; irritation possible | High—off-target organ toxicity common | Moderate; immune modulation risks | Low—minimizes off-target exposure |
| Penetration depth | Low; limited by stratum corneum | High (nonspecific) | High (nonspecific) | High (targeted to disease site) |
| Dosing frequency | High; frequent reapplication | Medium; once-daily to weekly | Low–Medium; every 2–8 weeks | Low; prolonged circulation time and controlled release |
| Cost | Low ($15–$30/tube) | Medium | High ($12,000–$70,000/yr) | High—variable (early development, potential cost reduction with scale) |
| Patient adherence | Moderate—frequent dosing reduces compliance | Variable—side effects limit use | Moderate—invasive administration impacts adherence | Potentially high—less frequent dosing, improved tolerability |
| Cell Type | Important Properties | Advantages | Limitations | Example Applications |
|---|---|---|---|---|
| Macrophages | Innate immune cells; phagocytic; home to inflammation via chemokines (CCL2, CXCL12) | Low immunogenicity; cross endothelial barriers; can carry nanoparticles | Potential pro-inflammatory activation; lifespan in vivo varies | Psoriasis, melanoma, chronic wounds |
| Neutrophils | First-responders to infection/injury; respond to IL-8, LTB4 | Rapid recruitment; efficient nanoparticle uptake without loss of migration | Short lifespan; risk of excessive inflammation | Bacterial skin infections, acute inflammatory flares |
| Dendritic cells | Antigen-presenting cells; initiate adaptive immunity | Potent immune modulation; can deliver antigens for immunotherapy | Complex ex vivo manipulation; limited scalability | Melanoma vaccines, autoimmune modulation |
| Mesenchymal stem cells | Multipotent stromal cells; strong immunomodulatory secretome | Low immunogenicity; promote regeneration; homing to injury | Donor variability; manufacturing scale limits | Psoriasis, eczema, wound healing |
| Induced pluripotent stem cells | Reprogrammed somatic cells; differentiate into many lineages | Patient-specific; scalable expansion | Regulatory and safety hurdles; tumorigenicity risk | Vitiligo (melanocyte regeneration), tissue repair |
| Keratinocytes | Main epidermal cells; can be genetically modified | Local protein delivery; autologous grafting | Limited to accessible lesions; genetic modification required | Gene therapy for genodermatoses, wound healing |
| Fibroblasts | Dermal ECM-producing cells | Localized sustained release; support tissue repair | Limited migration beyond injection site | Scar reduction, wound healing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shqair, L.; Draw, I.; Maya, T.; Bunick, C.G.; Akbarialiabad, H.; Schlesinger, T.; Damiani, G.; Ghannoum, M.; Grada, A. Advances in Cell-Mediated Drug Delivery for Dermatologic Diseases: Mechanisms and Current Applications. Pharmaceutics 2025, 17, 1438. https://doi.org/10.3390/pharmaceutics17111438
Shqair L, Draw I, Maya T, Bunick CG, Akbarialiabad H, Schlesinger T, Damiani G, Ghannoum M, Grada A. Advances in Cell-Mediated Drug Delivery for Dermatologic Diseases: Mechanisms and Current Applications. Pharmaceutics. 2025; 17(11):1438. https://doi.org/10.3390/pharmaceutics17111438
Chicago/Turabian StyleShqair, Lara, Iyla Draw, Tala Maya, Christopher G. Bunick, Hossein Akbarialiabad, Todd Schlesinger, Giovanni Damiani, Mahmoud Ghannoum, and Ayman Grada. 2025. "Advances in Cell-Mediated Drug Delivery for Dermatologic Diseases: Mechanisms and Current Applications" Pharmaceutics 17, no. 11: 1438. https://doi.org/10.3390/pharmaceutics17111438
APA StyleShqair, L., Draw, I., Maya, T., Bunick, C. G., Akbarialiabad, H., Schlesinger, T., Damiani, G., Ghannoum, M., & Grada, A. (2025). Advances in Cell-Mediated Drug Delivery for Dermatologic Diseases: Mechanisms and Current Applications. Pharmaceutics, 17(11), 1438. https://doi.org/10.3390/pharmaceutics17111438










