In Vitro Antifungal Efficacy of Blue-Light Photodynamic Therapy with Curcumin and Riboflavin Formulation Activated by 450 nm Diode Laser Against Candida albicans Biofilm on Titanium Implants
Abstract
1. Introduction
1.1. Background and Epidemiology
1.2. Role of Candida albicans
1.3. Rationale for aPDT
1.4. Objectives
2. Materials and Methods
2.1. Reference Microbial Strains
2.2. Laser and Photosensitizer
2.3. Experimental Design
- GC (Growth Control): No treatment applied.
- CHX: Treated with 1% chlorhexidine gluconate gel for 2 min.
- RIB: Treated with riboflavin gel (Xlinker Gel) for 20 min in the dark.
- CUR: Treated with curcumin gel (Curcumin-Gel 95+) for 20 min in the dark.
- QBX: Treated with QroxB2 gel for 20 min in the dark.
- L: Exposed to laser light only (450 nm, 400 mW, 2 min per surface), without any photosensitizer.
- L + RIB: Treated with riboflavin gel for 20 min, rinsed with 0.9% NaCl, and then irradiated as above.
- L + CUR: Treated with curcumin gel for 20 min, rinsed with 0.9% NaCl, and then irradiated as above.
- L + QBX: Treated with QroxB2 for 20 min, rinsed with 0.9% NaCl, and then irradiated as above.
2.4. Microbiological Analysis
2.5. Nanocarrier Characterization
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Significance of the Results
4.2. Clinical Relevance
4.3. Limitations of This Study
4.4. Implications and Translational Potential
4.5. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naemi, R.; Barikani, H.R.; Shahmoradi, L. Dental implant quality registries and databases, A systematic review. J. Educ. Health Promot. 2021, 10, 214. [Google Scholar] [CrossRef]
- Ting, M.; Suzuki, J.B. Peri-Implantitis. Dent. J. 2024, 12, 251. [Google Scholar] [CrossRef]
- Sanz, M.; Noguerol, B.; Sanz-Sánchez, I.; Hämmerle, C.H.F.; Schliephake, H.; Renouard, F.; Sicilia, A.; Steering Committee; Cordaro, L.; Jung, R.; et al. European Association for Osseointegration Delphi Study on the Trends in Implant Dentistry in Europe for the Year 2030. Clin. Oral Implant. Res. 2019, 30, 476–486. [Google Scholar] [CrossRef]
- Kupka, J.R.; König, J.; Al-Nawas, B.; Sagheb, K.; Schiegnitz, E. How far can we go? A 20-year meta-analysis of dental implant survival rates. Clin. Oral Investig. 2024, 28, 541. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, É.B.S.; Romandini, M.; Sadilina, S.; Sant’Ana, A.C.P.; Sanz, M. Microbiota associated with peri-implantitis, A systematic review with meta-analyses. Clin. Oral Implant. Res. 2023, 34, 1176–1187. [Google Scholar] [CrossRef]
- Berglundh, T.; Mombelli, A.; Schwarz, F.; Derks, J. Etiology, Pathogenesis and Treatment of Peri-Implantitis: A European Perspective. Periodontol. 2000, 2024; Online ahead of print. [Google Scholar]
- Schwarz, F.; Becker, K.; Rahn, S.; Hegewald, A.; Pfeffer, K.; Henrich, B. Real-Time PCR Analysis of Fungal Organisms and Bacterial Species at Peri-Implantitis Sites. Int. J. Implant Dent. 2015, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- de Lafuente-Ibáñez Mendoza, I.; Cayero-Garay, A.; Quindós-Andrés, G.; Aguirre-Urizar, J.M. A systematic review on the implication of Candida in peri-implantitis. Int. J. Implant Dent. 2021, 7, 73. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L. Peri-Implant Mucositis and Peri-Implantitis, Key Features and Differences. Br. Dent. J. 2024, 236, 791–794. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions, Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S286–S291. [Google Scholar] [CrossRef] [PubMed]
- Froum, S.J.; De la Torre, E.G.; Rosen, P.S. Peri-implant mucositis. Int. J. Periodontics Restor. Dent. 2019, 39, 2. [Google Scholar] [CrossRef]
- Barootchi, S.; Ravidà, A.; Tavelli, L.; Wang, H.L. Nonsurgical Treatment for Peri-Implant Mucositis, A Systematic Review and Meta-Analysis. Int. J. Oral Implant 2020, 13, 123–139. [Google Scholar]
- Verket, A.; Koldsland, O.C.; Bunaes, D.; Lie, S.A.; Romandini, M. Non-Surgical Therapy of Peri-Implant Mucositis—Mechanical/Physical Approaches: A Systematic Review. J. Clin. Periodontol. 2023, 50 (Suppl. 26), 135–145. [Google Scholar] [CrossRef]
- Liñares, A.; Sanz-Sánchez, I.; Dopico, J.; Molina, A.; Blanco, J.; Montero, E. Efficacy of adjunctive measures in the non-surgical treatment of peri-implantitis: A systematic review. J. Clin. Periodontol. 2023, 50 (Suppl. 26), 224–243. [Google Scholar] [CrossRef]
- Giok, K.C.; Veettil, S.K.; Menon, R.K. Comparative effectiveness of interventions for the treatment of peri-implantitis, A systematic review with network meta-analysis. J. Prosthet. Dent. 2024, in press. [CrossRef]
- Cheng, J.; Chen, L.; Tao, X.; Qiang, X.; Li, R.; Ma, J.; Shi, D.; Qiu, Z. Efficacy of surgical methods for peri-implantitis: A systematic review and network meta-analysis. BMC Oral Health 2023, 23, 227. [Google Scholar] [CrossRef]
- Wiench, R.; Nowicka, J.; Pajączkowska, M.; Kuropka, P.; Skaba, D.; Kruczek-Kazibudzka, A.; Kuśka-Kiełbratowska, A.; Grzech-Leśniak, K. Influence of Incubation Time on Ortho-Toluidine Blue Mediated Antimicrobial Photodynamic Therapy Directed against Selected Candida Strains: An In Vitro Study. Int. J. Mol. Sci. 2021, 22, 10971. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, R.; Nikparto, N.; Gharibpour, F.; Pourhajibagher, M.; Bahador, A. Antimicrobial Photodynamic Therapy for Managing the Peri-Implant Mucositis and Peri-Implantitis: A Systematic Review of Randomized Clinical Trials. Photodiagn. Photodyn. Ther. 2024, 45, 103990. [Google Scholar] [CrossRef]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Kubizna, M.; Dawiec, G.; Wiench, R. Efficacy of Curcumin-Mediated Antimicrobial Photodynamic Therapy on Candida spp.—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 8136. [Google Scholar] [CrossRef]
- Ali, H.M.; Karam, K.; Khan, T.; Wahab, S.; Ullah, S.; Sadiq, M. Reactive Oxygen Species Induced Oxidative Damage to DNA, Lipids, and Proteins of Antibiotic-Resistant Bacteria by Plant-Based Silver Nanoparticles. 3 Biotech 2023, 13, 414. [Google Scholar] [CrossRef] [PubMed]
- Dantas, A.d.S.; Day, A.; Ikeh, M.; Kos, I.; Achan, B.; Quinn, J. Oxidative Stress Responses in the Human Fungal Pathogen, Candida albicans. Biomolecules 2015, 5, 142–165. [Google Scholar] [CrossRef] [PubMed]
- Antognazza, M.R.; Abdel Aziz, I.; Lodola, F. Use of Exogenous and Endogenous Photomediators as Efficient ROS Modulation Tools, Results and Perspectives for Therapeutic Purposes. Oxid. Med. Cell. Longev. 2019, 2019, 2867516. [Google Scholar] [CrossRef]
- Xie, L.; Ji, X.; Zhang, Q.; Wei, Y. Curcumin Combined with Photodynamic Therapy, Promising Therapies for the Treatment of Cancer. Biomed. Pharmacother. 2022, 146, 112567. [Google Scholar] [CrossRef]
- Kah, G.; Chandran, R.; Abrahamse, H. Curcumin a Natural Phenol and Its Therapeutic Role in Cancer and Photodynamic Therapy: A Review. Pharmaceutics 2023, 15, 639. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Fekrazad, R.; Zhang, L.; Jiang, X.; He, G.; Wen, X. Polyphenolic Natural Products as Photosensitizers for Antimicrobial Photodynamic Therapy: Recent Advances and Future Prospects. Front. Immunol. 2023, 14, 1275859. [Google Scholar] [CrossRef] [PubMed]
- Buchovec, I.; Vyčaitė, E.; Badokas, K.; Sužiedelienė, E.; Bagdonas, S. Application of Antimicrobial Photodynamic Therapy for Inactivation of Acinetobacter baumannii Biofilms. Int. J. Mol. Sci. 2022, 24, 722. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Pavarina, A.C.; Carmello, J.C.; Machado, A.L.; Brunetti, I.L.; Bagnato, V.S. Susceptibility of clinical isolates of Candida to photodynamic effects of curcumin. Lasers Surg. Med. 2011, 43, 927–934. [Google Scholar] [CrossRef]
- Kanpittaya, K.; Teerakapong, A.; Morales, N.P.; Hormdee, D.; Priprem, A.; Weera-archakul, W.; Damrongrungruang, T. Inhibitory Effects of Erythrosine/Curcumin Derivatives/Nano-Titanium Dioxide-Mediated Photodynamic Therapy on Candida albicans. Molecules 2021, 26, 2405. [Google Scholar] [CrossRef]
- Morelato, L.; Budimir, A.; Smojver, I.; Katalinić, I.; Vuletić, M.; Ajanović, M.; Gabrić, D. A Novel Technique for Disinfection Treatment of Contaminated Dental Implant Surface Using 0.1% Riboflavin and 445 nm Diode Laser—An In Vitro Study. Bioengineering 2022, 9, 308. [Google Scholar] [CrossRef]
- Farah, N.; Lim, C.W.; Chin, V.K.; Chong, P.P.; Basir, R.; Yeo, W.W.Y.; Tay, S.T.; Choo, S.; Lee, T.Y. Photoactivated riboflavin inhibits planktonic and biofilm growth of Candida albicans and non-albicans Candida species. Microb. Pathog. 2024, 191, 106665. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.P.; Rosseti, I.B.; Carvalho, M.L.; da Silva, B.G.M.; Alberto-Silva, C.; Costa, M.S. Photodynamic Antimicrobial Chemotherapy (PACT), using Toluidine blue O inhibits the viability of biofilm produced by Candida albicans at different stages of development. Photodiagn. Photodyn. Ther. 2018, 21, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms, development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef]
- Albuquerque Garcia, B.; Dias Panariello, B.H.; de Freitas-Pontes, K.M.; Duarte, S. Candida biofilm matrix as a resistance mechanism against photodynamic therapy. Photodiagn. Photodyn. Ther. 2021, 36, 102525. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, M.; Mertas, A.; Kuśka-Kiełbratowska, A.; Fiegler-Rudol, J.; Bobela, E.; Cisowska, M.; Morawiec, T.; Skaba, D.; Wiench, R. In Vitro Evaluation of Candida spp. and Staphylococcus aureus Sensitivity to 450 nm Diode Laser-Mediated Antimicrobial Photodynamic Therapy with Curcumin and Riboflavin. Int. J. Mol. Sci. 2025, 26, 5645. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Barazy, R.; Alafif, H.; Achour, H.; Al-Aloul, A.; Tolibah, Y.A. Can antimicrobial photodynamic therapy serve as an effective adjunct protocol for disinfecting the necrotic root canal system? A randomized controlled study. BDJ Open 2024, 10, 53. [Google Scholar] [CrossRef]
- Warakomska, A.; Fiegler-Rudol, J.; Kubizna, M.; Skaba, D.; Wiench, R. The Role of Photodynamic Therapy Mediated by Natural Photosensitisers in the Management of Peri-Implantitis: A Systematic Review. Pharmaceutics 2025, 17, 443. [Google Scholar] [CrossRef]
- Sharma, M.; Manoharlal, R.; Puri, N.; Prasad, R. Antifungal curcumin induces reactive oxygen species and triggers an early apoptosis but prevents hyphae development by targeting the global repressor TUP1 in Candida albicans. Biosci. Rep. 2010, 30, 391–404. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Lei, J.; Huang, J.; Xin, C.; Liu, F.; Zhang, J.; Xie, Y.; Mao, Y.; Chen, W.; Song, Z. Riboflavin Targets the Cellular Metabolic and Ribosomal Pathways of Candida albicans In Vitro and Exhibits Efficacy against Oropharyngeal Candidiasis. Microbiol. Spectr. 2023, 11, e0380122. [Google Scholar] [CrossRef] [PubMed]
- Sandai, D.; Yin, Z.; Selway, L.; Stead, D.; Walker, J.; Leach, M.D.; Bohovych, I.; Ene, I.V.; Kastora, S.; Budge, S.; et al. The evolutionary rewiring of ubiquitination targets has reprogrammed the regulation of carbon assimilation in the pathogenic yeast Candida albicans. mBio 2012, 3, e00495-12. [Google Scholar] [CrossRef]
- Dai, T.; Gupta, A.; Murray, C.K.; Vrahas, M.S.; Tegos, G.P.; Hamblin, M.R. Blue light for infectious diseases, Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist. Updat. 2012, 15, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Tomb, R.M.; Maclean, M.; Coia, J.E.; MacGregor, S.J.; Anderson, J.G. Inactivation of Streptomyces phage φC31 by 405 nm light, Requirement for exogenous photosensitizers? Viruses 2014, 6, 2866–2878. [Google Scholar]
- Zhang, Y.; Zhu, Y.; Chen, J.; Wang, Y.; Sherwood, M.E.; Murray, C.K.; Vrahas, M.S.; Hooper, D.C.; Hamblin, M.R.; Dai, T. Antimicrobial Blue Light Inactivation of Candida albicans, In Vitro and In Vivo Studies. Virulence 2016, 7, 536–545. [Google Scholar] [CrossRef]
- Jusuf, S.; Zhan, Y.; Zhang, M.; Alexander, N.J.; Viens, A.; Mansour, M.K.; Cheng, J.X. Blue Light Deactivation of Catalase Suppresses Candida Hyphae Development through Lipogenesis Inhibition. Photochem. Photobiol. 2023, 99, 936–946. [Google Scholar] [CrossRef]
- Durantini, E.N. New Insights into the Antimicrobial Blue Light Inactivation of Candida albicans. Virulence 2016, 7, 493–494. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Chen, H.-F.; Yeh, Y.-C.; Xue, Y.-P.; Lan, C.-Y. The Transcription Factor Sfp1 Regulates the Oxidative Stress Response in Candida albicans. Microorganisms 2019, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Kielbik, A.; Wawryka, P.; Przystupski, D.; Rossowska, J.; Szewczyk, A.; Saczko, J.; Kulbacka, J.; Chwiłkowska, A. Effects of Photosensitization of Curcumin in Human Glioblastoma Multiforme Cells. In Vivo 2019, 33, 1857–1864. [Google Scholar] [CrossRef]
- Almohareb, T.; Alhamoudi, N.; Al Deeb, M.; Bin-Shuwaish, M.S.; Mokeem, S.A.; Shafqat, S.S.; Vohra, F.; Abduljabbar, T. Clinical efficacy of photodynamic therapy as an adjunct to mechanical debridement in the treatment of peri-implantitis with abscess. Photodiagn. Photodyn. Ther. 2020, 30, 101750. [Google Scholar] [CrossRef]
- Sosiawan, A.; Azhar, I.S.; Dhywinanda, D.E.; Jordana, J.; Salim, J.F.; Nugraha, A.P. The effectiveness of photodynamic therapy as an adjunct to mechanical debridement in peri-implantitis treatment. Indones. J. Dent. Med. 2022, 5, 62–65. [Google Scholar] [CrossRef]
- Dieckow, S.; Szafrański, S.P.; Grischke, J.; Qu, T.; Doll-Nikutta, K.; Steglich, M.; Yang, I.; Häussler, S.; Stiesch, M. Structure and Composition of Early Biofilms Formed on Dental Implants Are Complex, Diverse, Subject-Specific and Dynamic. NPJ Biofilms Microbiomes 2024, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Blank, E.; Grischke, J.; Winkel, A.; Eberhard, J.; Kommerein, N.; Doll, K.; Yang, I.; Stiesch, M. Evaluation of Biofilm Colonization on Multi-Part Dental Implants in a Rat Model. BMC Oral Health 2021, 21, 313. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wang, C.; Li, P.; Lu, H.; Li, A.; Xu, S. Role of Immune Dysregulation in Peri-Implantitis. Front. Immunol. 2024, 15, 1466417. [Google Scholar] [CrossRef] [PubMed]
- Strakas, D.; Franzen, R. The Blue Wavelengths in Laser Dentistry: A Review of Current Literature. Laser Dent. Sci. 2023, 7, 97–99. [Google Scholar] [CrossRef]
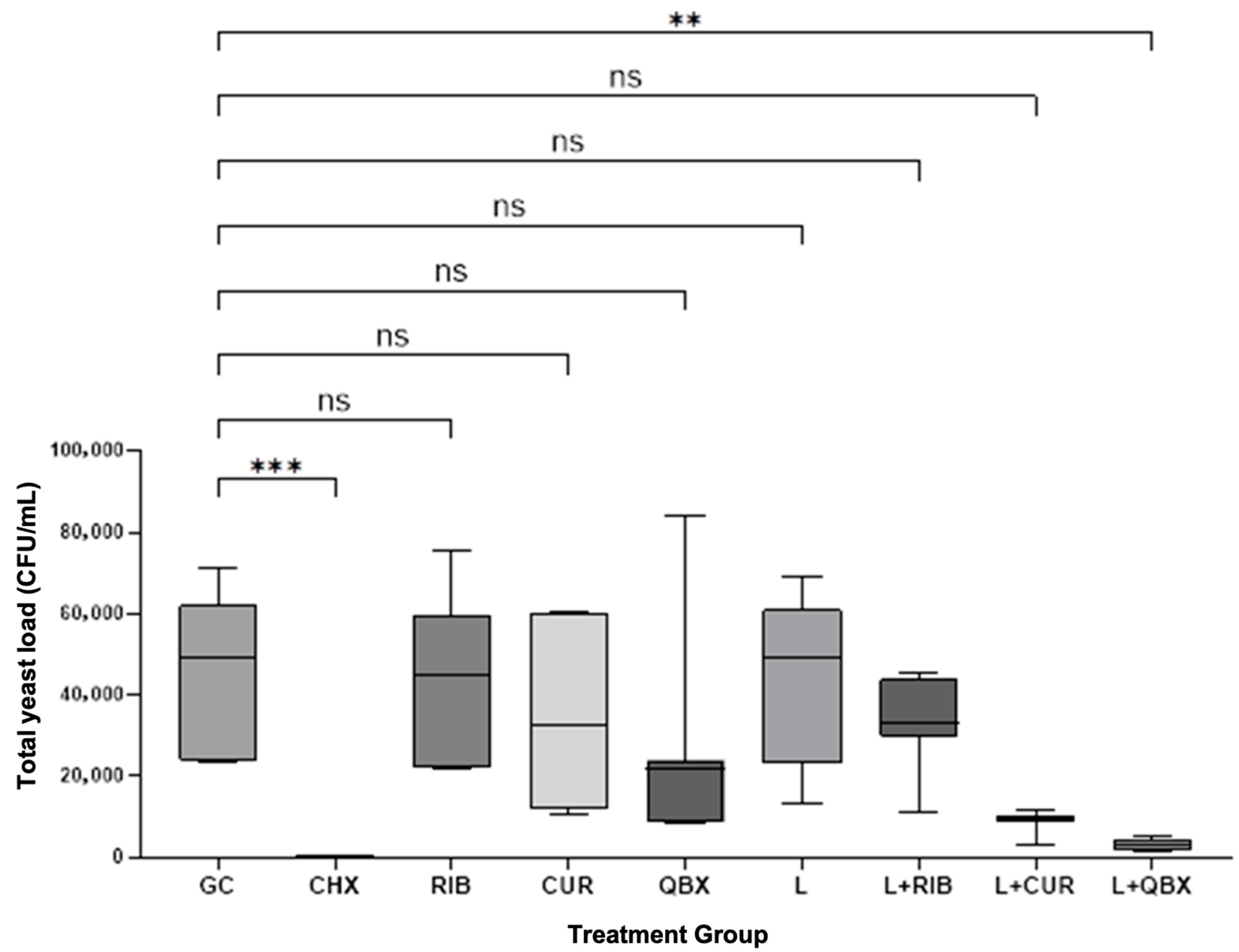
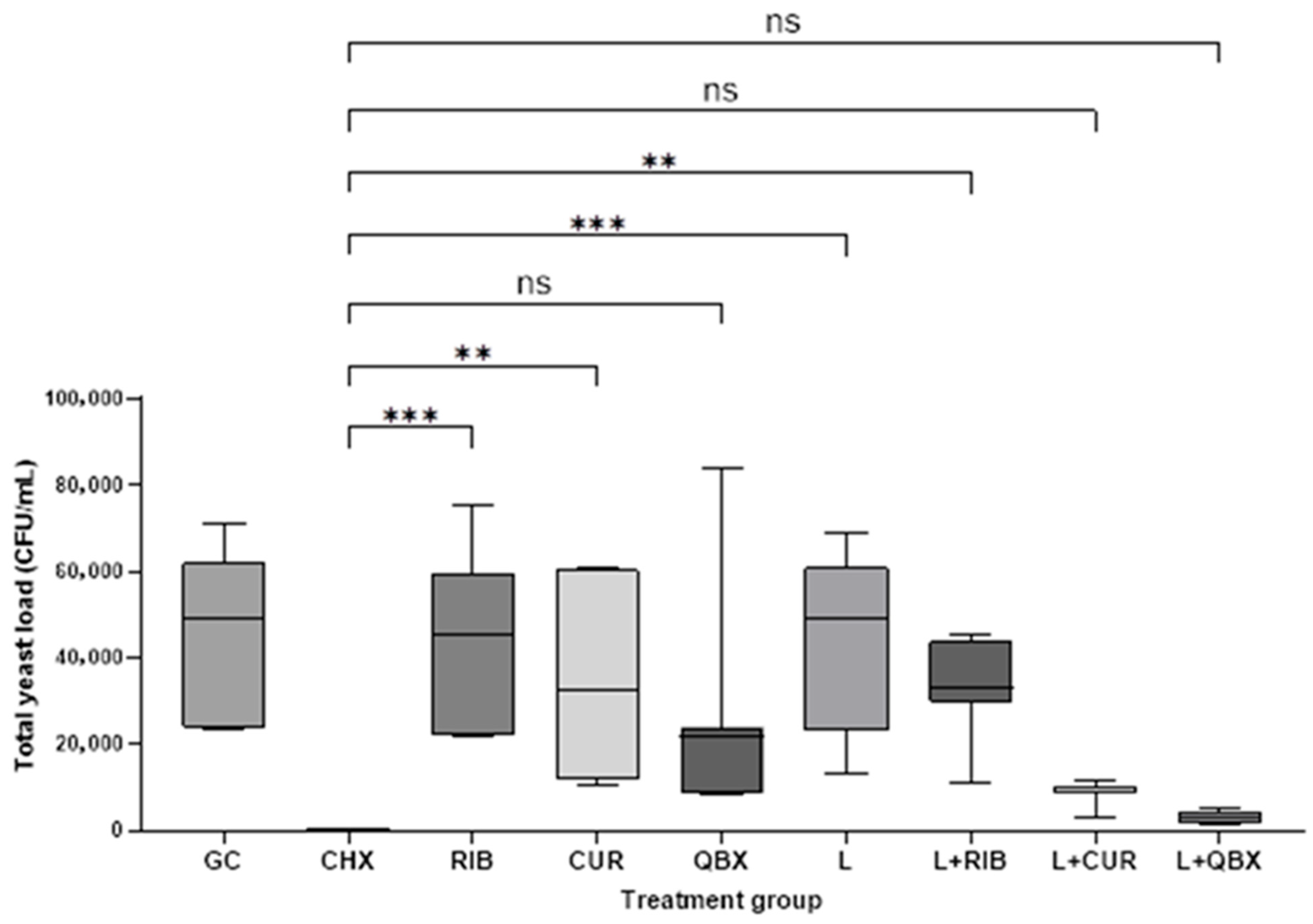
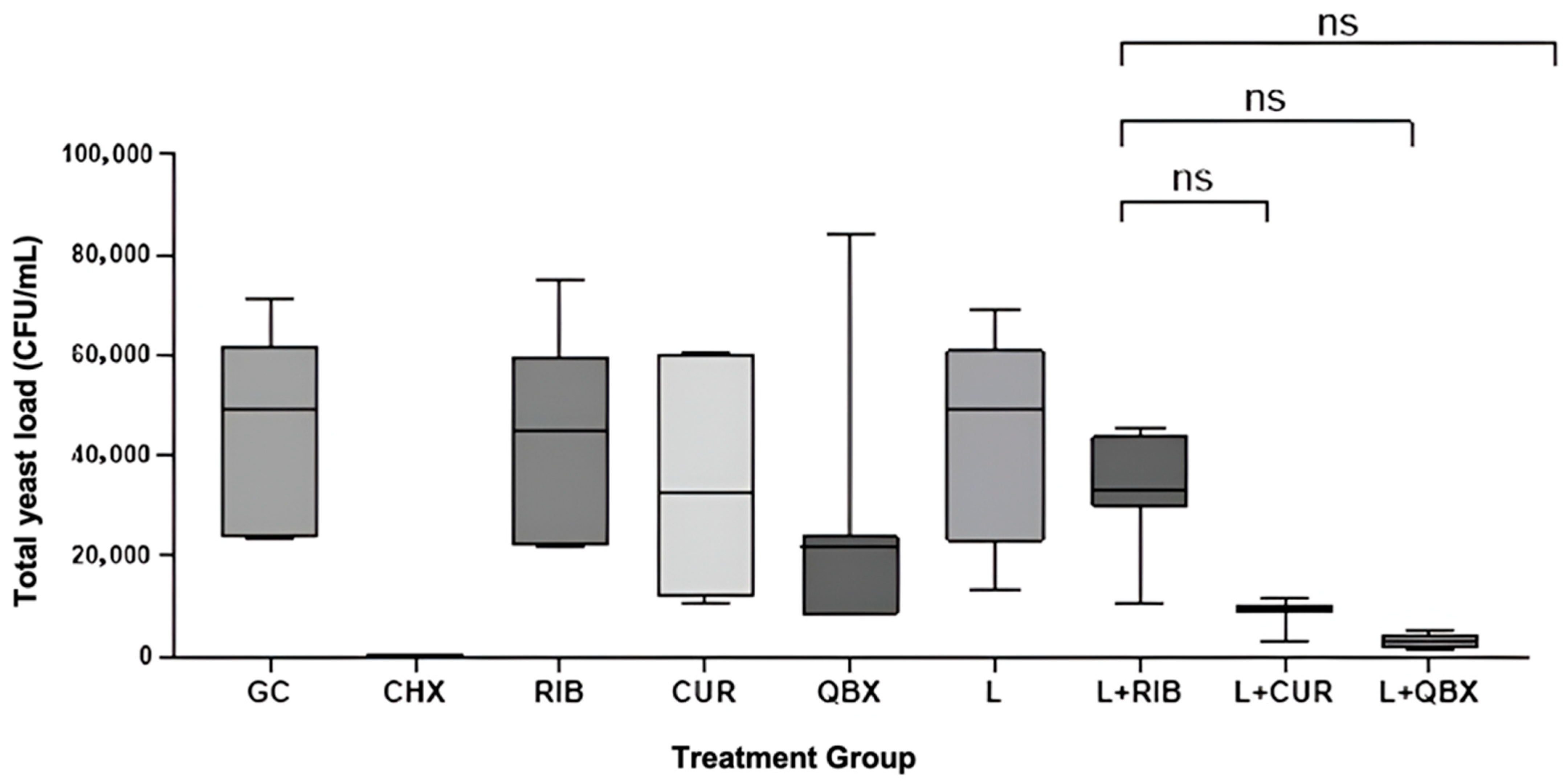
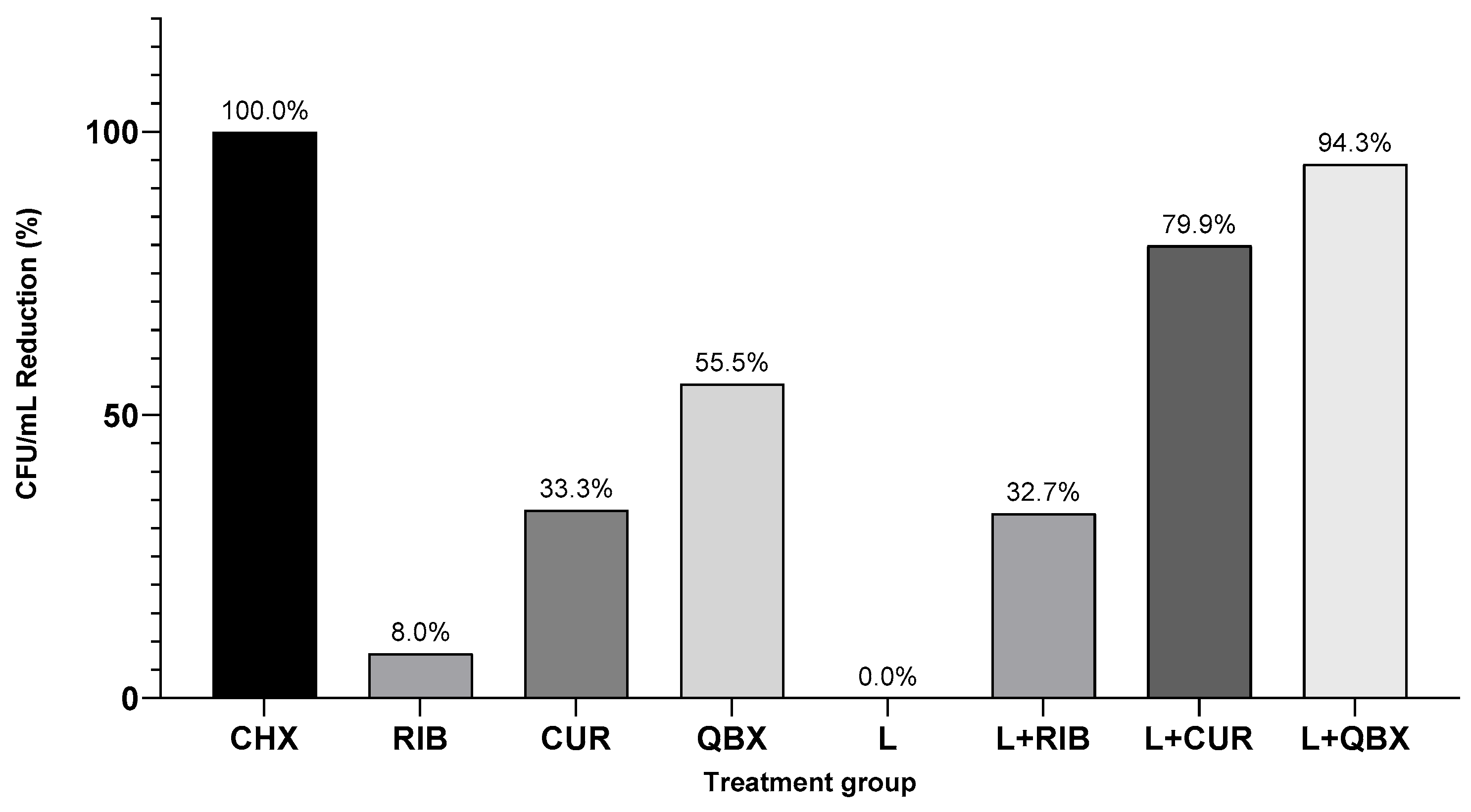
| Group | n | Mean | Standard Deviation | Median | Minimum | Maximum | Interquartile Range |
|---|---|---|---|---|---|---|---|
| GC | 7 | 44,085.7 | 20,062.9 | 49,000 | 23,600 | 71,000 | 38,100 |
| CHX | 7 | 0.0 | 0.0 | 0 | 0 | 0 | 0 |
| RIB | 7 | 44,814.3 | 20,505.2 | 45,100 | 21,700 | 75,200 | 37,300 |
| CUR | 7 | 34,957.1 | 21,466.2 | 32,700 | 10,300 | 60,700 | 48,500 |
| QBX | 7 | 26,814.3 | 26,088.8 | 21,800 | 8300 | 84,000 | 15,500 |
| L | 7 | 42,257.1 | 21,465.0 | 49,000 | 13,300 | 69,000 | 38,000 |
| L + RIB | 7 | 32,935.7 | 11,377.1 | 33,000 | 10,850 | 45,500 | 14,000 |
| L + CUR | 7 | 8807.1 | 2687.7 | 9850 | 3100 | 11,500 | 1300 |
| L + QBX | 7 | 2828.6 | 1398.5 | 2800 | 1500 | 5000 | 2800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warakomska, A.; Kępa, M.; Fiegler-Rudol, J.; Latusek-Kotyczka, K.; Skaba, D.; Wiench, R. In Vitro Antifungal Efficacy of Blue-Light Photodynamic Therapy with Curcumin and Riboflavin Formulation Activated by 450 nm Diode Laser Against Candida albicans Biofilm on Titanium Implants. Pharmaceutics 2025, 17, 1437. https://doi.org/10.3390/pharmaceutics17111437
Warakomska A, Kępa M, Fiegler-Rudol J, Latusek-Kotyczka K, Skaba D, Wiench R. In Vitro Antifungal Efficacy of Blue-Light Photodynamic Therapy with Curcumin and Riboflavin Formulation Activated by 450 nm Diode Laser Against Candida albicans Biofilm on Titanium Implants. Pharmaceutics. 2025; 17(11):1437. https://doi.org/10.3390/pharmaceutics17111437
Chicago/Turabian StyleWarakomska, Aleksandra, Małgorzata Kępa, Jakub Fiegler-Rudol, Katarzyna Latusek-Kotyczka, Dariusz Skaba, and Rafał Wiench. 2025. "In Vitro Antifungal Efficacy of Blue-Light Photodynamic Therapy with Curcumin and Riboflavin Formulation Activated by 450 nm Diode Laser Against Candida albicans Biofilm on Titanium Implants" Pharmaceutics 17, no. 11: 1437. https://doi.org/10.3390/pharmaceutics17111437
APA StyleWarakomska, A., Kępa, M., Fiegler-Rudol, J., Latusek-Kotyczka, K., Skaba, D., & Wiench, R. (2025). In Vitro Antifungal Efficacy of Blue-Light Photodynamic Therapy with Curcumin and Riboflavin Formulation Activated by 450 nm Diode Laser Against Candida albicans Biofilm on Titanium Implants. Pharmaceutics, 17(11), 1437. https://doi.org/10.3390/pharmaceutics17111437







