Sustained Intraocular Pressure Reduction Using Bisoprolol-Loaded PLGA Nanoparticles: A Promising Strategy for Enhanced Ocular Delivery with Reduced GFAP Expression Indicative of Lower Glial Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. High-Performance Liquid Chromatography Analysis for Bisoprolol Hemifumarate
2.2.2. Formulation of Bisoprolol Hemifumarate-Loaded PLGA Nanoparticles
2.2.3. Experimental Design
2.2.4. Physicochemical Characterization of BSP-PLGA Nanoparticle Formulations
Particle Size (PS) and Polydispersity Index (PDI), Zeta Potential Determination
- Particle size and Polydispersity Index
- Zeta potential
- Entrapment Efficiency Determination
Optimization of Bisoprolol Hemifumarate Loaded with PLGA Nanoparticles
2.2.5. In-Vitro Characterization of the Optimum Formula
Transmission Electron Microscopy
Atomic Force Microscopy
Fourier-Transformed Infrared Spectroscopy
Differential Scanning Calorimetry
X-Ray Diffraction Spectroscopy
In-Vitro Drug Release Analysis
2.2.6. Short-Term Stability of Bisoprolol Hemifumarate Loaded with PLGA Nanoparticles
2.2.7. Ex-Vivo Analysis
Confocal Laser Scanning Microscopy Analysis
2.3. In Vivo Analysis
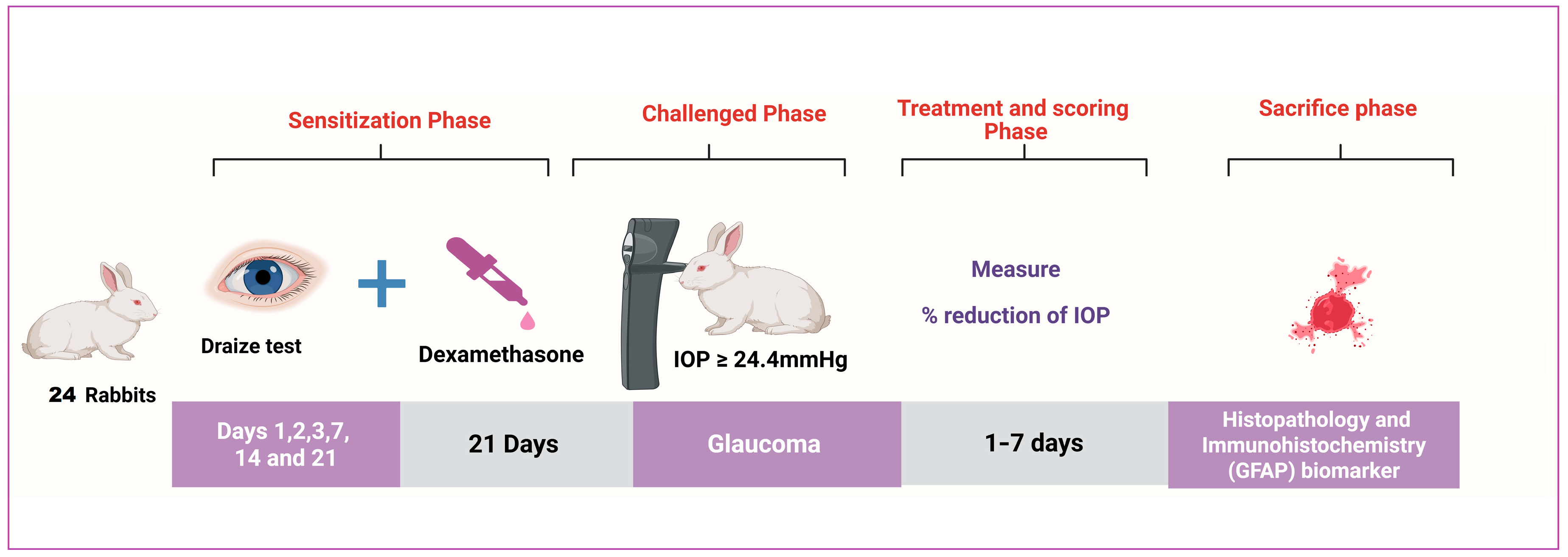
2.3.1. Animals
2.3.2. Ocular Irritancy Test (Draize Test)
2.3.3. Induction of Glaucoma by Using a Glucocorticoid
2.4. Histopathological Examination
2.5. Immuno-Histochemistry
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of Bisoprolol Hemifumarate Loaded with PLGA Nanoparticles
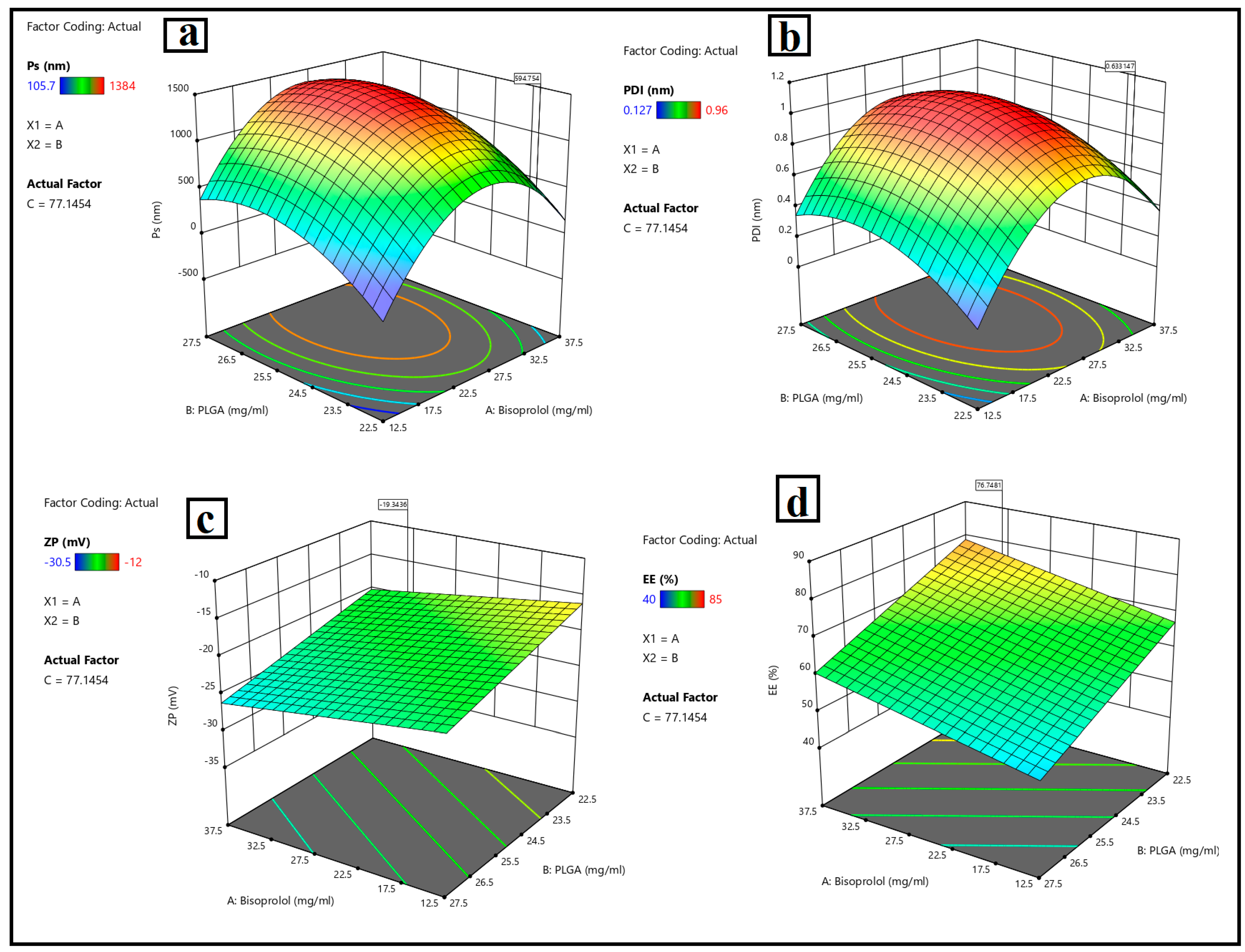
3.1.1. Particle Size, Polydispersity Index, Zeta Potential and Entrapment Efficiency Analysis
- Particle size analysis
- Polydispersity index assessment
- Zeta potential assessment
- Entrapment Efficiency assessment
3.1.2. Optimization of BSP-PLGA Nanoparticles
3.2. In Vitro Characterization of the Optimum Formula
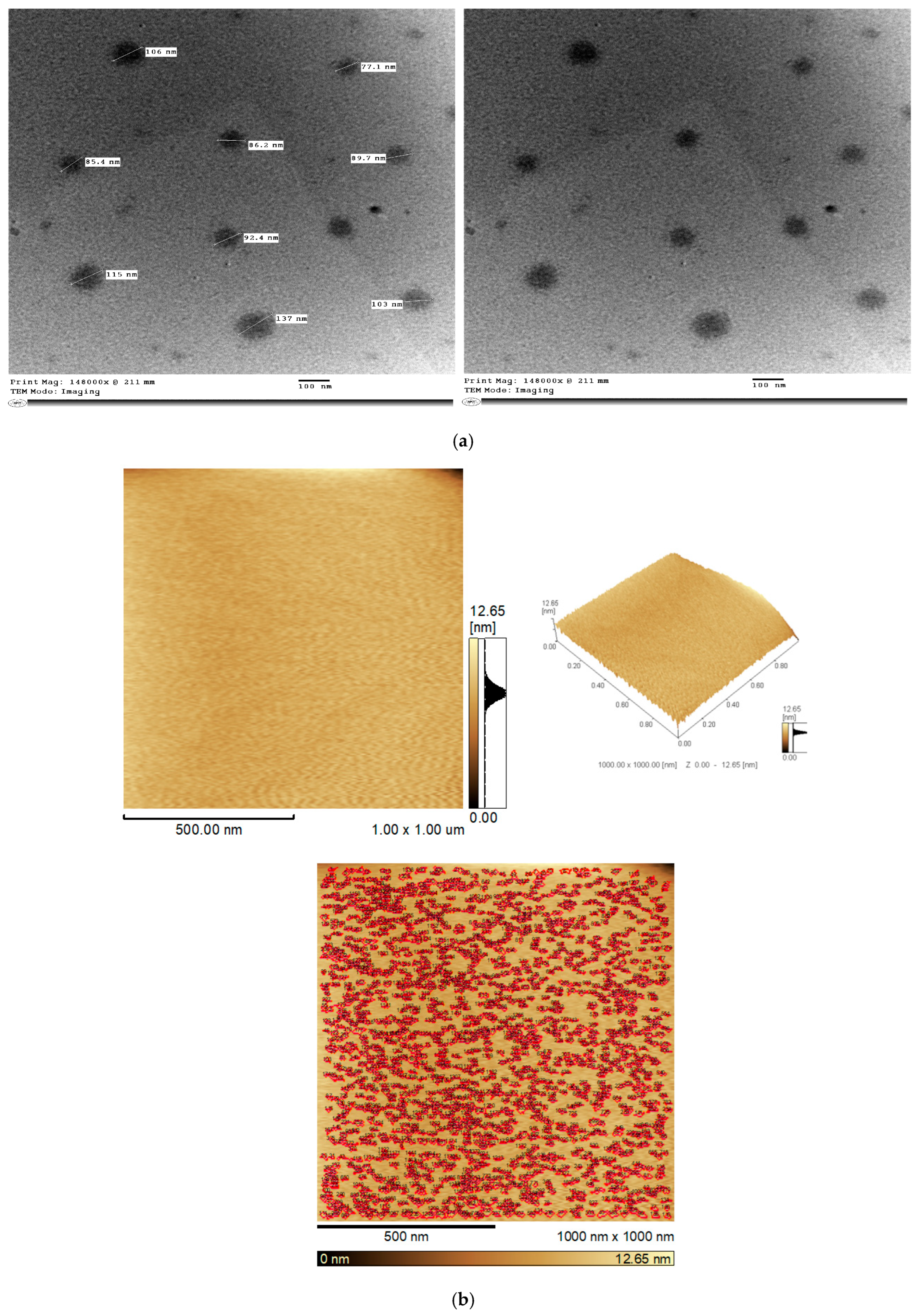
3.2.1. Transmission Electron Microscopy
3.2.2. Atomic Force Microscopy
3.2.3. Fourier Transformed Infrared Spectroscopy
3.2.4. Differential Scanning Calorimetry
3.2.5. X-Ray Diffraction Spectroscopy
3.2.6. In Vitro Release Analysis
3.3. Short-Term Stability of BSP-NPs
3.4. Ex-Vivo Analysis
Confocal Laser Scanning Microscopy Analysis (CLSM)
3.5. In Vivo Analysis
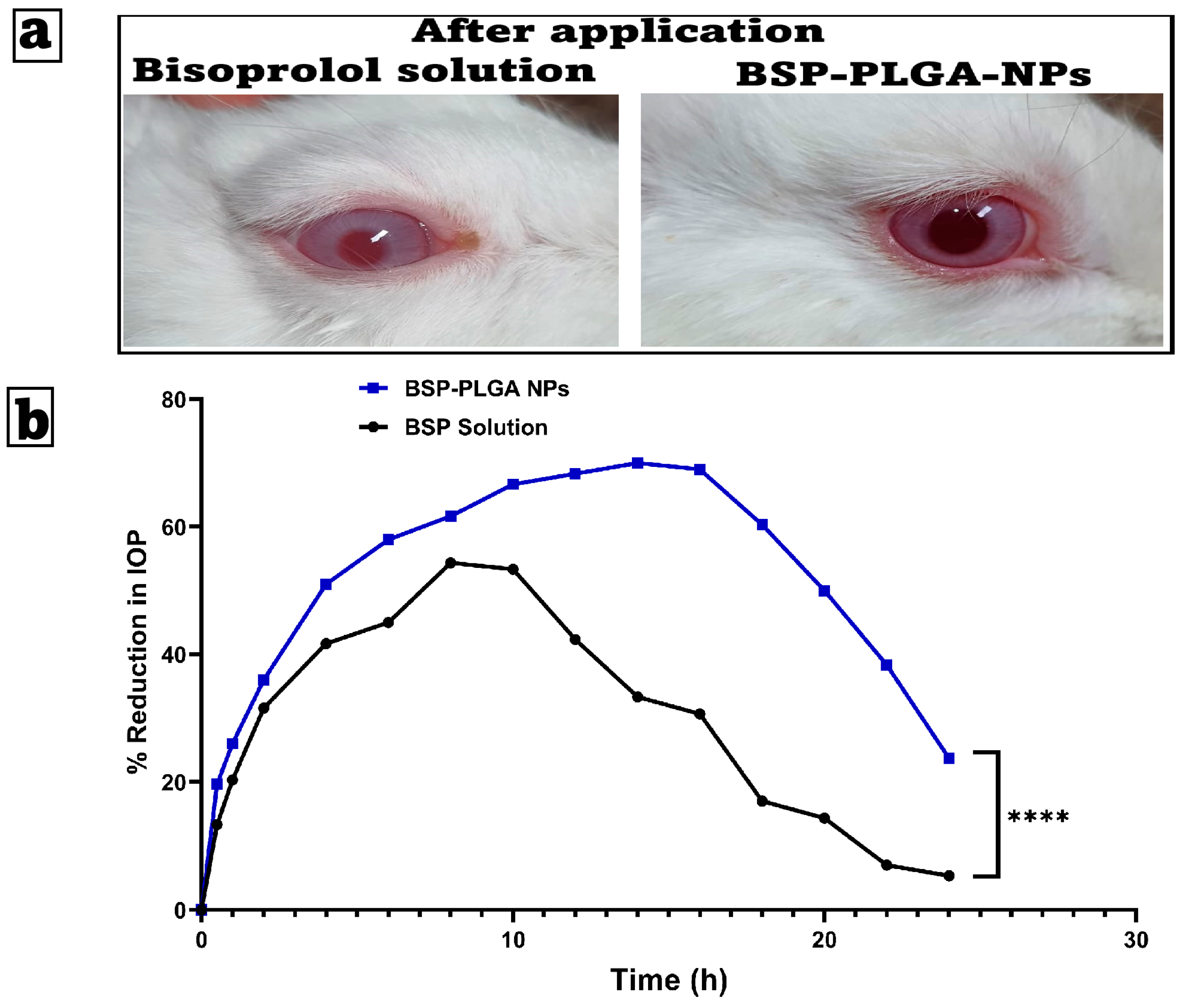
3.5.1. Ocular Irritancy Test (Draize Test)
3.5.2. Pharmacodynamic Analysis
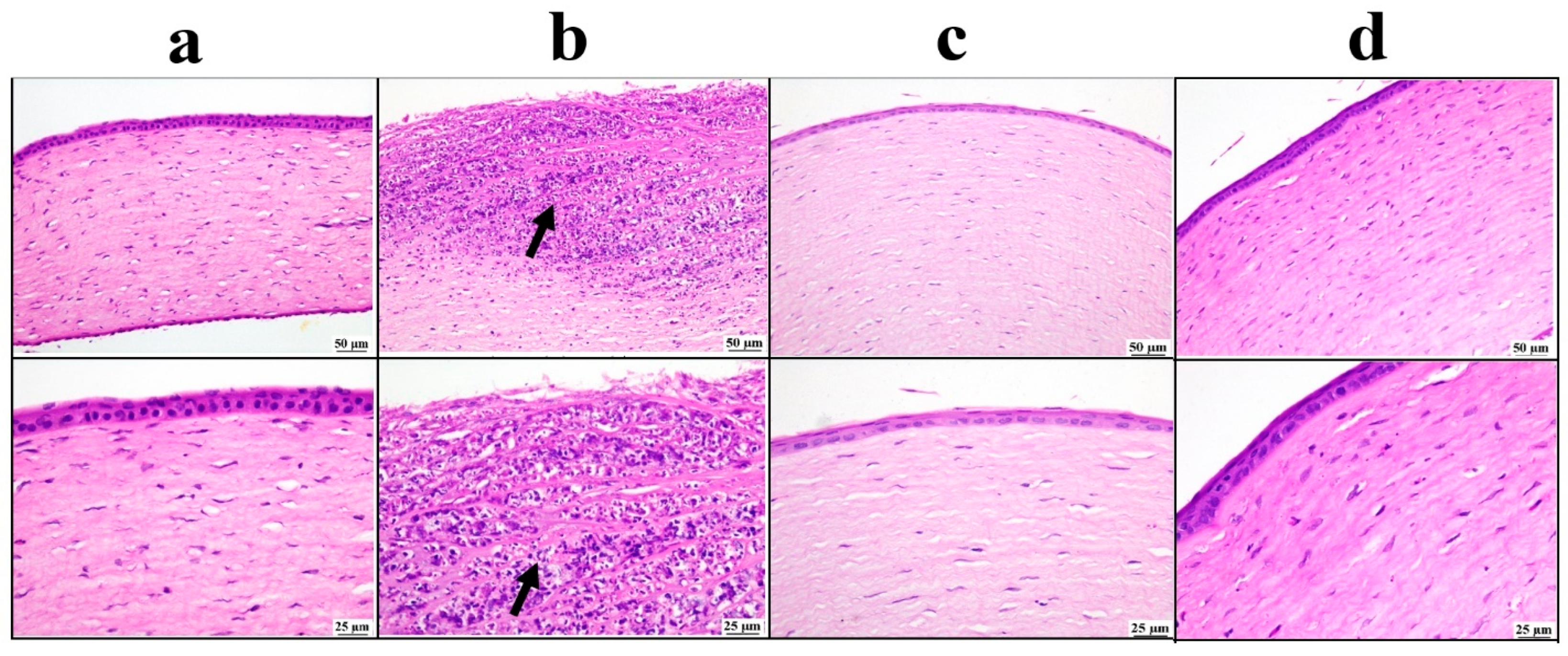
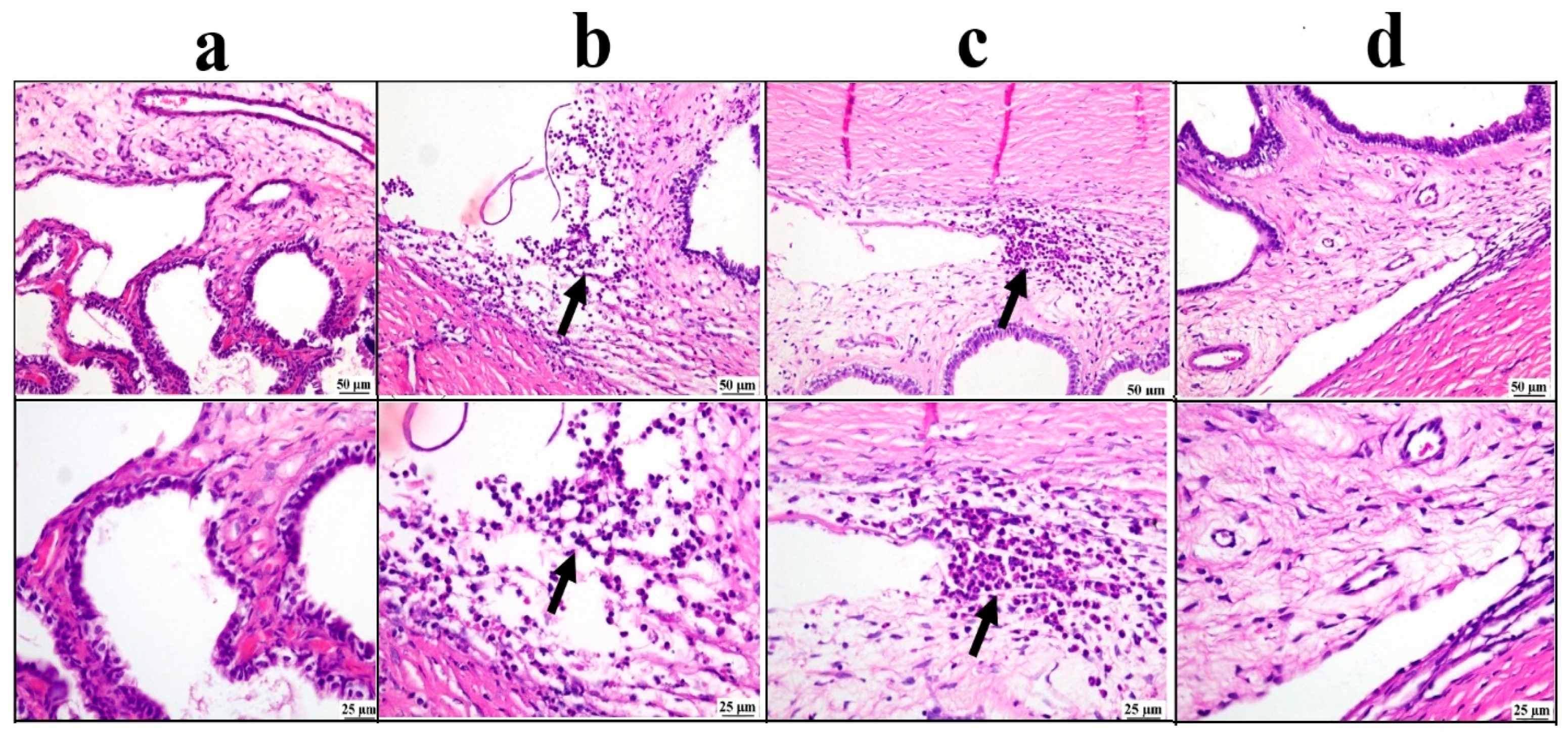
3.5.3. Histopathological Examination
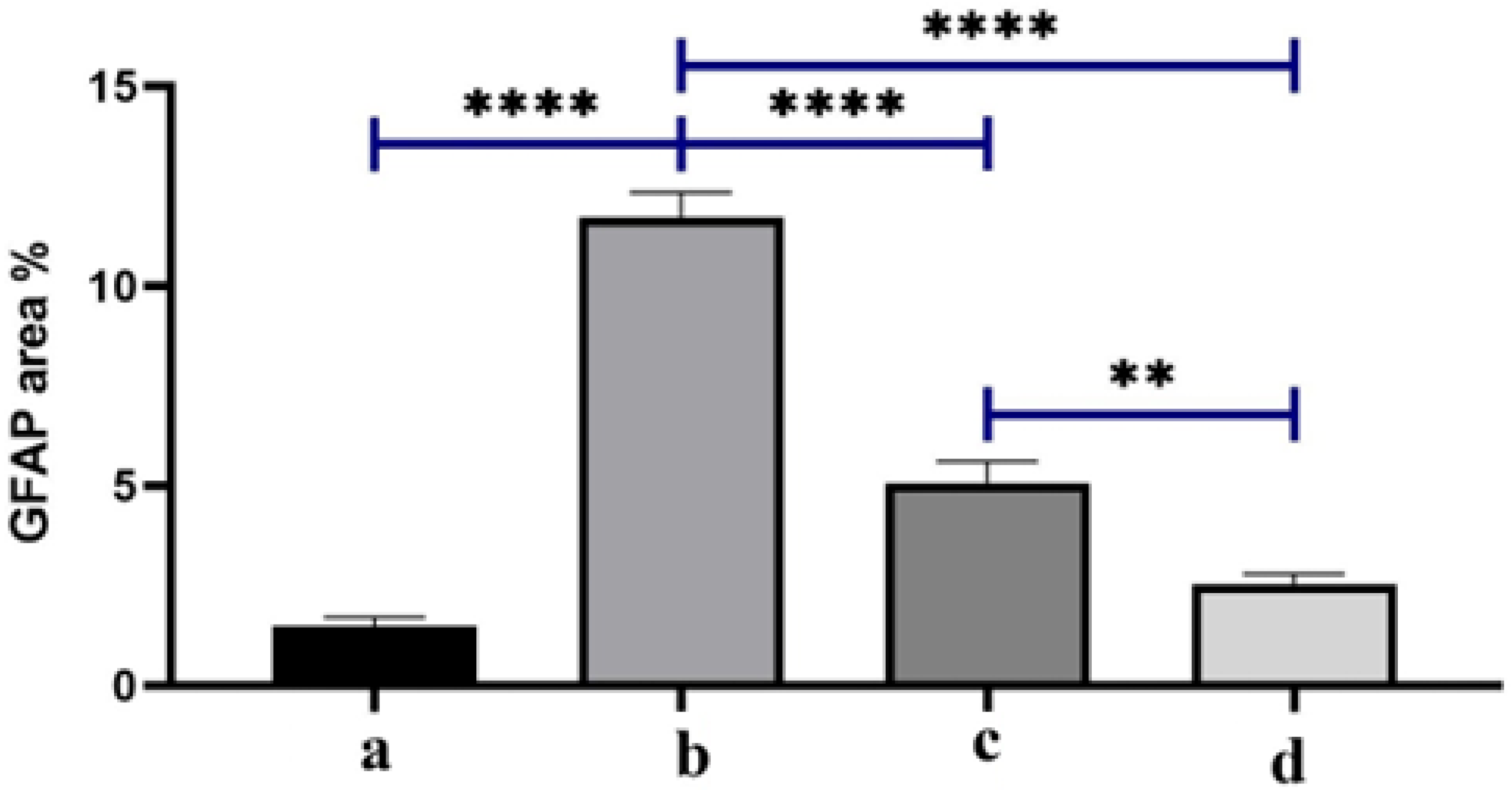
3.6. Immuno-Histochemistry (IHC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EE% | entrapment efficiency |
| PS | particle size |
| PDI | polydispersity index |
| ZP | zeta potential |
| O.F | optimized formula |
| NPs | nanoparticles |
| PLGA | poly lactic co -glycolic acid |
| BSP | bisoprolol |
| DSC | differential scanning calorimetry |
| IHC | immuno-histochemistry |
| TEM | transmission electron micrograph |
| AFM | atomic force microscopy |
| FTIR | Fourier transform infrared spectroscopy |
| XRD | X-ray diffraction |
| CLSM | confocal laser scanning microscopy |
| GFAP | glial fibrillary acidic protein |
References
- World Health Organization. World Report on Vision; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Ako-Adounvo, A.-M.; Karla, P.K. Preparation and In Vitro Testing of Brinzolamide-Loaded Poly Lactic-Co-Glycolic Acid (PLGA) Nanoparticles for Sustained Drug Delivery. J. Clin. Transl. Ophthalmol. 2024, 2, 1–14. [Google Scholar] [CrossRef]
- Alkhatib, A.W. Glaucoma: Types, Risk Factors, Detection, and Management. Scholars Acad. J. Biosci. 2023, 11, 207–211. [Google Scholar] [CrossRef]
- Warsi, M.H.; Anwar, M.; Garg, V.; Jain, G.K.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K. Dorzolamide-Loaded PLGA/Vitamin E TPGS Nanoparticles for Glaucoma Therapy: Pharmacoscintigraphy Study and Evaluation of Extended Ocular Hypotensive Effect in Rabbits. Colloids Surf. B Biointerfaces 2014, 122, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Lambuk, L.; Suhaimi, N.A.A.; Sadikan, M.Z.; Jafri, A.J.A.; Ahmad, S.; Nasir, N.A.A.; Uskoković, V.; Kadir, R.; Mohamud, R. Nanoparticles for the Treatment of Glaucoma-Associated Neuroinflammation. Eye Vis. 2022, 9, 26. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.; Chen, B.; Li, Y.; Jiang, B. Prevalence of Primary Angle Closure Glaucoma in the Last 20 Years: A Meta-Analysis and Systematic Review. Front. Med. 2021, 7, 624179. [Google Scholar] [CrossRef]
- Rahić, O.; Tucak, A.; Omerović, N.; Sirbubalo, M.; Hindija, L.; Hadžiabdić, J.; Vranić, E. Novel Drug Delivery Systems Fighting Glaucoma: Formulation Obstacles and Solutions. Pharmaceutics 2021, 13, 28. [Google Scholar] [CrossRef]
- Casson, R.J.; Chidlow, G.; Wood, J.P.M.; Crowston, J.G.; Goldberg, I. Definition of Glaucoma: Clinical and Experimental Concepts. Clin. Exp. Ophthalmol. 2012, 40, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Varano, G.P.; Adornetto, A.; Nucci, C.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Retinal Ganglion Cell Death in Glaucoma: Exploring the Role of Neuroinflammation. Eur. J. Pharmacol. 2016, 787, 134–142. [Google Scholar] [CrossRef]
- Arranz-Romera, A.; Davis, B.M.; Bravo-Osuna, I.; Esteban-Pérez, S.; Molina-Martínez, I.T.; Shamsher, E.; Ravindran, N.; Guo, L.; Cordeiro, M.F.; Herrero-Vanrell, R. Simultaneous Co-Delivery of Neuroprotective Drugs from Multi-Loaded PLGA Microspheres for the Treatment of Glaucoma. J. Control. Release 2019, 297, 26–38. [Google Scholar] [CrossRef] [PubMed]
- del Amo, E.M. Topical Ophthalmic Administration: Can a Drug Instilled onto the Ocular Surface Exert an Effect at the Back of the Eye? Front. Drug Deliv. 2022, 2, 954771. [Google Scholar] [CrossRef]
- Wang, T.; Cao, L.; Jiang, Q.; Zhang, T. Topical Medication Therapy for Glaucoma and Ocular Hypertension. Front. Pharmacol. 2021, 12, 749858. [Google Scholar] [CrossRef]
- Vaneev, A.; Tikhomirova, V.; Chesnokova, N.; Popova, E.; Beznos, O.; Kost, O.; Klyachko, N. Nanotechnology for Topical Drug Delivery to the Anterior Segment of the Eye. Int. J. Mol. Sci. 2021, 22, 12368. [Google Scholar] [CrossRef]
- Elhabal, S.F.; El-Nabarawi, M.; Elrefai, M.F.M.; Teaima, M.H.; Shoela, M.S.; Khamis, G.M.; Faheem, A.M.; Kholeif, N.A.; Sanad, M.T. Nano-Spanlastics-Loaded Dissolving Microneedle Patches for Ketotifen Fumarate: Advanced Strategies for Allergic Conjunctivitis Treatment and Molecular Insights. Drug Deliv. Transl. Res. 2025, 15, 3161–3184. [Google Scholar] [CrossRef]
- Armaly, M.F.; Krueger, D.E.; Maunder, L.; Becker, B.; Hetherington, J., Jr.; Kolker, A.E.; Levene, R.Z.; Maumenee, A.E.; Pollack, I.P.; Shaffer, R.N. Biostatistical Analysis of the Collaborative Glaucoma Study: I. Summary Report of the Risk Factors for Glaucomatous Visual-Field Defects. Arch. Ophthalmol. 1980, 98, 2163–2171. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.J.; Nguyen, D.D.; Lai, J.Y. Dually Functional Hollow Ceria Nanoparticle Platform for Intraocular Drug Delivery: A Push beyond the Limits of Static and Dynamic Ocular Barriers toward Glaucoma Therapy. Biomaterials 2020, 243, 119961. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Yao, C.H.; Lue, S.J.; Yang, C.J.; Su, Y.H.; Huang, C.C.; Lai, J.Y. Amination-Mediated Nano Eye-Drops with Enhanced Corneal Permeability and Effective Burst Release for Acute Glaucoma Treatment. Chem. Eng. J. 2023, 451, 138620. [Google Scholar] [CrossRef]
- Marques, D.R.; Dos Santos, L.A. Analysis of Poly(Lactic-co-Glycolic Acid)/Poly(Isoprene) Polymeric Blend for Application as Biomaterial. Polímeros 2013, 23, 579–584. [Google Scholar] [CrossRef]
- Warsi, M.H. Development and Optimization of Vitamin E TPGS Based PLGA Nanoparticles for Improved and Safe Ocular Delivery of Ketorolac. J. Drug Deliv. Sci. Technol. 2021, 61, 102121. [Google Scholar] [CrossRef]
- Marquina, S.; Ozgul, M.; Robertson-Brown, K.; Kenney, M.C. A Review on PLGA Particles as a Sustained Drug-Delivery System and Its Effect on the Retina. Exp. Eye Res. 2023, 235, 109626. [Google Scholar] [CrossRef] [PubMed]
- Logan, A.; Pucker, A.D.; Franklin, Q.; McGwin, G.; Hogan, C.; Kelley, L.R.; Christensen, M.; Brafford, R.; Lievens, C. Determining Initial Ocular Comfort Differences between 0.7% Olopatadine and 0.035% Ketotifen Fumarate. Contact Lens Anterior Eye 2023, 46, 101769. [Google Scholar] [CrossRef]
- Datta, D.; Roy, G.; Garg, P.; Venuganti, V.V.K. Ocular Delivery of Cyclosporine A Using Dissolvable Microneedle Contact Lens. J. Drug Deliv. Sci. Technol. 2022, 70, 103211. [Google Scholar] [CrossRef]
- Wang, T.; Ling, Q.; Shen, B.; Jia, X. The Strong Correlation between Visual Function Improvement and Retinal Microcirculation Enhancement in Glaucoma. Front. Med. 2025, 12, 1537741. [Google Scholar] [CrossRef]
- Heijl, A.; Cristina Leske, M.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M. Reduction of Intraocular Pressure and Glaucoma Progression Results From the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef]
- Baudouin, C.; Kolko, M.; Melik-Parsadaniantz, S.; Messmer, E.M. Inflammation in Glaucoma: From the Back to the Front of the Eye, and Beyond. Prog. Retin. Eye Res. 2021, 83, 100916. [Google Scholar] [CrossRef]
- Bessone, C.D.V.; Akhlaghi, S.P.; Tártara, L.I.; Quinteros, D.A.; Loh, W.; Allemandi, D.A. Latanoprost-Loaded Phytantriol Cubosomes for the Treatment of Glaucoma. Eur. J. Pharm. Sci. 2021, 160, 105748. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.S.; Ravi, P.R.; Khan, M.S.; Mahajan, R.R.; Szeleszczuk, Ł. Nebivolol Polymeric Nanoparticles-Loaded In Situ Gel for Effective Treatment of Glaucoma: Optimization, Physicochemical Characterization, and Pharmacokinetic and Pharmacodynamic Evaluation. Nanomaterials 2024, 14, 1347. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Sun, C.; Li, T.; Wang, J. Inhibition of AGTR1 Attenuates Cell Proliferation after Glaucoma Filtration Surgery via NF-ΚB Pathway-Mediated G0/G1-Phase Cell Cycle Arrest. Exp. Cell Res. 2025, 447, 114514. [Google Scholar] [CrossRef] [PubMed]
- Stamper, R.L.; Wigginton, S.A.; Higginbotham, E.J. Primary Drug Treatment for Glaucoma: Beta-Blockers Versus Other Medications I. Individualize Initial Therapy II. Choosing Beta-Blockers for Initial Medical Therapy for Glaucoma I. Individualize Initial Therapy. Surv. Ophthalmol. 2002, 47, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Yuki, K.; Matsuura, Y.; Yaginuma, M.; Negishi, T.; Shinjoh, M. Secondary Angle-Closure Glaucoma Associated With Congenital Acorea That Developed Into Endophthalmitis After Glaucoma Drainage Implant Surgery: A Case Report. Cureus 2025, 17, e92358. [Google Scholar] [CrossRef]
- Bhagat, P.; Singhania, M.; Navare, S.; Mazumdar, M.; Paul, C.; Shet, S.; VKS, K.; Nicholsan, A.; Bansal, N.; Jain, P. Efficacy and Safety of a Fixed-Dose Combination of Brinzolamide 1%/Timolol 0.5% vs. Dorzolamide 2%/Timolol 0.5% in Indian Patients with Primary Open-Angle Glaucoma or Ocular Hypertension: A Randomized Phase 3 Study. Cureus 2024, 16, e73599. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, L.; Xu, X.; Wei, X.; Wen, L.; Xie, Q. Therapeutic Effect of Beta-Blocker in Patients with Traumatic Brain Injury: A Systematic Review and Meta-Analysis. J. Crit. Care 2017, 41, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Zangbar, B.; Khalil, M.; Rhee, P.; Joseph, B.; Kulvatunyou, N.; Tang, A.; Friese, R.S.; O’Keeffe, T. Metoprolol Improves Survival in Severe Traumatic Brain Injury Independent of Heart Rate Control. J. Surg. Res. 2016, 200, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Goyagi, T.; Horiguchi, T.; Nishikawa, T.; Tobe, Y.; Masaki, Y. Neuroprotective Effects of Selective Beta-1 Adrenoceptor Antagonists, Landiolol and Esmolol, on Transient Forebrain Ischemia in Rats; a Dose–Response Study. Brain Res. 2012, 1461, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Nana Wandji, B.; Bacq, N.; Ehongo, A. Efficacy and Safety of Rho Kinase Inhibitors vs. Beta-Blockers in Primary Open-Angle Glaucoma: A Systematic Review with Meta-Analysis. J. Clin. Med. 2024, 13, 1747. [Google Scholar] [CrossRef]
- Perry, C.M.; McGavin, J.K.; Culy, C.R.; Ibbotson, T. Latanoprost: An Update of Its Use in Glaucoma and Ocular Hypertension. Drugs Aging 2003, 20, 597–630. [Google Scholar] [CrossRef]
- Islam, S.; Ahmed, M.M.S.; Islam, M.A.; Hossain, N.; Chowdhury, M.A. Advances in Nanoparticles in Targeted Drug Delivery–A Review. Results Surf. Interfaces 2025, 19, 100529. [Google Scholar] [CrossRef]
- Li, S.; Chen, L.; Fu, Y. Nanotechnology-Based Ocular Drug Delivery Systems: Recent Advances and Future Prospects. J. Nanobiotechnol. 2023, 21, 232. [Google Scholar] [CrossRef]
- Mahdy, N.K.; El-Mosallamy, A.E.M.K.; Mohamed, E.A.; Teaima, M.H.; El-Said Azzazy, H.M.; El-Nabarawi, M.; Elhabal, S.F. Carvedilol-Loaded Propolis Nanoparticles Embedded in Gel-Casted Film and 3D Electrospun Nanofiber Film—An in Vivo Study to Enhance the Bioavailability via the Intranasal Route. J. Drug Deliv. Sci. Technol. 2025, 112, 107254. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable Polymeric Nanoparticles as Drug Delivery Devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Al-Zuhairy, S.A.K.S.; Elhabal, S.F.; Mohamed Elrefai, M.F.; Hababeh, S.; Nelson, J.; Fady, M.; Elzohairy, N.A.; Ewedah, T.M.; Mousa, I.S.; Hamdan, A.M.E. Polylactic-Co-Glycolic Acid/Alginate/Neem Oil-Reduced Graphene Oxide as a PH-Sensitive Nanocarrier for Hesperidin Drug Delivery: Antimicrobial and Acute Otitis Media Assessments. Pharmaceuticals 2025, 18, 381. [Google Scholar] [CrossRef]
- Lu, Y.; Cheng, D.; Niu, B.; Wang, X.; Wu, X.; Wang, A. Properties of Poly (Lactic-Co-Glycolic Acid) and Progress of Poly (Lactic-Co-Glycolic Acid)-Based Biodegradable Materials in Biomedical Research. Pharmaceuticals 2023, 16, 454. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A Unique Polymer for Drug Delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Jain, R.A. The Manufacturing Techniques of Various Drug Loaded Biodegradable Poly(Lactide-Co-Glycolide) (PLGA) Devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Chen, Y.; Pei, Y.; Wang, W.; Zhu, Z.; Zheng, Z.; Yang, L.; Sun, L. The Drug Release of PLGA-Based Nanoparticles and Their Application in Treatment of Gastrointestinal Cancers. Heliyon 2024, 10, e38165. [Google Scholar] [CrossRef]
- Costa, M.P.; Abdu, J.O.C.; Machado Resende Guedes, M.C.; Sarcinelli, M.A.; Fabri, R.L.; Pittella, F.; Macedo, G.C.; Vilela, F.M.P.; Rocha, H.V.A.; Tavares, G.D. Dexamethasone-Loaded Chitosan-Decorated PLGA Nanoparticles: A Step Forward in Attenuating the COVID-19 Cytokine Storm? Colloids Surf. B Biointerfaces 2025, 246, 114359. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, L.; Wang, J.; Gong, M.; Yuan, R.; Lu, J.; Xiao, X.; Liu, X. Effects of RAmb a 1-Loaded PLGA-PEG Nanoparticles in a Murine Model of Allergic Conjunctivitis. Molecules 2022, 27, 598. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Arribada, R.G.; Silva, J.O.; Silva-Cunha, A.; Townsend, D.M.; Ferreira, L.A.M.; Barros, A.L.B. In Vitro and In Vivo Effect of PH-Sensitive PLGA-TPGS-Based Hybrid Nanoparticles Loaded with Doxorubicin for Breast Cancer Therapy. Pharmaceutics 2022, 14, 2394. [Google Scholar] [CrossRef] [PubMed]
- Iester, M. Brinzolamide ophthalmic suspension: A review of its pharmacology and use in the treatment of open angle glaucoma and ocular hypertension. Clin. Ophthalmol. 2008, 2, 517–523. [Google Scholar] [CrossRef]
- Palermiti, A.; De Nicolò, A.; Manca, A.; Antonucci, M.; Mula, J.; Billi, M.; Cusato, J.; Carta, A.; Pappaccogli, M.; Ponsa, L.; et al. Development and Validation of a UHPLC-MS/MS Method for the Simultaneous Quantification of Candesartan and Bisoprolol Together with Other 16 Antihypertensive Drugs in Plasma Samples. J. Med. Chem. 2024, 67, 22124–22133. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Niu, Y.; Ning, C.; Qin, F.; Yan, C.; Lu, X. A Rapid and Sensitive Method for the Determination of Bisoprolol in Human Plasma by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry. Biomed. Chromatogr. 2025, 39, e70054. [Google Scholar] [CrossRef]
- Al-Shoubki, A.A.; Teaima, M.H.M.; Abdelmonem, R.A.A.B.; El-Nabarawi, M.A.; Elhabal, S.F. Potential Application of Sucrose Acetate Isobutyrate, and Glyceryl Monooleate for Nanonization and Bioavailability Enhancement of Rivaroxaban Tablets. Pharm. Sci. Adv. 2024, 2, 100015. [Google Scholar] [CrossRef]
- Todaro, B.; Moscardini, A.; Luin, S. Pioglitazone-Loaded PLGA Nanoparticles: Towards the Most Reliable Synthesis Method. Int. J. Mol. Sci. 2022, 23, 2522. [Google Scholar] [CrossRef]
- Lee, D.A.; Higginbotham, E.J. Glaucoma and its treatment: A review. Am. J. Health-Syst. Pharm. 2005, 62, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Al-Shoubki, A.A.; Teaima, M.H.M.; Abdelmonem, R.A.A.B.; El-Nabarawi, M.A.; Elhabal, S.F. Sucrose Acetate Isobutyrate (SAIB) and Glyceryl Monooleate (GMO) Hybrid Nanoparticles for Bioavailability Enhancement of Rivaroxaban: An Optimization Study. Pharm. Dev. Technol. 2023, 28, 928–938. [Google Scholar] [CrossRef]
- Elhabal, S.F.; Farahat, M.S.; Teaima, M.H.; Elzohairy, N.A.; El-Nabarawi, M. Innovate Sodium Alginate Microneedle Patches Integrated with Soft Lidocaine Invasomes: Advanced Strategies for Oral Ulcerative Mucositis Treatment via TNF-α/NF-ΚB Pathways. Drug Deliv. Transl. Res. 2025, 1–26. [Google Scholar] [CrossRef]
- Zarif Attalla, K.; Hassan, D.H.; Teaima, M.H.; Yousry, C.; El-Nabarawi, M.A.; Said, M.A.; Elhabal, S.F. Enhanced Intranasal Delivery of Atorvastatin via Superparamagnetic Iron-Oxide-Loaded Nanocarriers: Cytotoxicity and Inflammation Evaluation and In Vivo, In Silico, and Network Pharmacology Study for Targeting Glioblastoma Management. Pharmaceuticals 2025, 18, 421. [Google Scholar] [CrossRef]
- Elhabal, S.F.; Al-Zuhairy, S.A.S.; El-Nabarawi, M.; Mohamed Elrefai, M.F.; Shoela, M.S.; Hababeh, S.; Nelson, J.; Abdel Khalek, M.A.; Fady, M.; Elzohairy, N.A.; et al. Enhancing Photothermal Therapy for Antibiofilm Wound Healing: Insights from Graphene Oxide-Cranberry Nanosheet Loaded Hydrogel in Vitro, in Silico, and in Vivo Evaluation. Int. J. Nanomed. 2024, 19, 12999–13027. [Google Scholar] [CrossRef]
- Elhabal, S.F.; Abdelmonem, R.; El Nashar, R.M.; Elrefai, M.F.M.; Hamdan, A.M.E.; Safwat, N.A.; Shoela, M.S.; Hassan, F.E.; Rizk, A.; Kabil, S.L.; et al. Enhanced Antibacterial Activity of Clindamycin Using Molecularly Imprinted Polymer Nanoparticles Loaded with Polyurethane Nanofibrous Scaffolds for the Treatment of Acne Vulgaris. Pharmaceutics 2024, 16, 947. [Google Scholar] [CrossRef]
- Ewedah, T.M.; Abdalla, A.; Hagag, R.S.; Elhabal, S.F.; Teaima, M.H.; El-Nabarawi, M.A.; Schlatter, G.; Shoueir, K.R. Enhancing Cellular Affinity for Skin Disorders: Electrospun Polyurethane/Collagen Nanofiber Mats Coated with Phytoceramides. Int. J. Pharm. 2024, 663, 124541. [Google Scholar] [CrossRef]
- Fathy Elhabal, S.; El-Nabarawi, M.A.; Abdelaal, N.; Elrefai, M.F.M.; Ghaffar, S.A.; Khalifa, M.M.; Mohie, P.M.; Waggas, D.S.; Hamdan, A.M.E.; Alshawwa, S.Z.; et al. Development of Canagliflozin Nanocrystals Sublingual Tablets in the Presence of Sodium Caprate Permeability Enhancer: Formulation Optimization, Characterization, in-Vitro, in Silico, and in-Vivo Study. Drug Deliv. 2023, 30, 2241665. [Google Scholar] [CrossRef]
- Mahu, S.C.; Florin Spac, A.; Ciobanu, C.; Hancianu, M.; Agoroaei, L.; Butnaru, E. Quantitative Determination of Bisoprolol Fumarate by HPLC I. Method Validation. Pharmacia 2021, 68, 69–77. [Google Scholar]
- Feng, Y.; Chen, H.; Chen, S.; Zhang, K.; Yun, D.; Liu, D.; Zeng, J.; Yang, C.; Xie, Q. Disulfiram-Loaded PLGA Nanoparticles Modified with a Phenyl Borate Chitosan Conjugate Enhance Hepatic Carcinoma Treatment. Int. J. Pharm. 2025, 671, 125293. [Google Scholar] [CrossRef]
- Elhabal, S.F.; Al-Zuhairy, S.A.-K.S.; Elrefai, M.F.M.; El-Nabarawi, M.A.; Hababeh, S.; Attalla, K.Z.; Shoela, M.S.; Nelson, J.; Fady, M.; Elzohairy, N.A.; et al. Chitosan-Based Intelligent Microneedles for Delivery of Amphotericin B Loaded Oleosomes: Antifungal Ocular Patch Targeting for Effective Against Fungal Keratitis Using Rabbit Model via TLR4/NLRP3 Pathway. Int. J. Nanomed. 2025, 20, 5949–5981. [Google Scholar] [CrossRef]
- Larreina Vicente, N.; Srinivas, M.; Tagit, O. Perfluorocarbon-Loaded Poly(Lactide-Co-Glycolide) Nanoparticles from Core to Crust: Multifaceted Impact of Surfactant on Particle Ultrastructure, Stiffness, and Cell Uptake. ACS Appl. Polym. Mater. 2025, 7, 2864–2878. [Google Scholar] [CrossRef]
- Güven, U.M.; Berkman, M.S.; Şenel, B.; Yazan, Y. Development and in Vitro/in Vivo Evaluation of Thermo-Sensitive in Situ Gelling Systems for Ocular Allergy. Braz. J. Pharm. Sci. 2019, 55, e17511. [Google Scholar] [CrossRef]
- Huo, P.; Han, X.; Zhang, W.; Zhang, J.; Kumar, P.; Liu, B. Electrospun Nanofibers of Polycaprolactone/Collagen as a Sustained-Release Drug Delivery System for Artemisinin. Pharmaceutics 2021, 13, 1228. [Google Scholar] [CrossRef] [PubMed]
- Elhabal, S.F.; Faheem, A.M.; Hababeh, S.; Nelson, J.; Elzohairy, N.A.; Ibrahim, Y.F.; Ewedah, T.M.; Mousa, I.S.; Allam, K.M.; Hamdan, A.M.E. Augmented Marshmallow Extract Lipid Nanoparticles with Clove Oil Embedded in Collagen Sponge for Ultimate Antimicrobial Healing of Diabetic Mouth Ulcer. Pharmaceutics 2025, 17, 611. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, Y.; Zhang, L.; Wang, Q.; Zhang, D. Stability of Nanosuspensions in Drug Delivery. J. Control. Release 2013, 172, 1126–1141. [Google Scholar] [CrossRef]
- Makhlouf, A.; Elnawawy, T. Hair Regrowth Boosting via Minoxidil Cubosomes: Formulation Development, in Vivo Hair Regrowth Evaluation, Histopathological Examination and Confocal Laser Microscopy Imaging. Int. J. Pharm. 2023, 634, 122665. [Google Scholar] [CrossRef]
- Csidey, M.; Csorba, A.; Kormányos, K.; Náray, A.; Kéki-Kovács, K.; Németh, O.; Knézy, K.; Bausz, M.; Szigeti, A.; Szabó, D.; et al. Examination of the Corneal Endothelium in Patients with Congenital Aniridia with a PAX6 Mutation Using In Vivo Confocal Laser Scanning Microscopy. Cornea 2025, 44, 324–331. [Google Scholar] [CrossRef]
- Sarhan, A.; Rokne, J.; Alhajj, R. Glaucoma detection using image processing techniques: A literature review. Comput. Med. Imaging Graph. 2019, 78, 101657. [Google Scholar] [CrossRef]
- Badran, M.M.; Alomrani, A.H.; Almomen, A.; Bin Jardan, Y.A.; Abou El Ela, A.E.S. Novel Metoprolol-Loaded Chitosan-Coated Deformable Liposomes in Thermosensitive In Situ Gels for the Management of Glaucoma: A Repurposing Approach. Gels 2022, 8, 635. [Google Scholar] [CrossRef]
- Phulke, S.; Kaushik, S.; Kaur, S.; Pandav, S.S. Steroid-Induced Glaucoma: An Avoidable Irreversible Blindness. J. Curr. Glaucoma Pract. 2017, 11, 67–72. [Google Scholar]
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, e11686. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, C.; Chang, Y.; Su, M.; Yu, R.; Huang, Z.; Guo, Y.-W.; Jin, X. Design, Synthesis, and Evaluation of Novel ROCK Inhibitors for Glaucoma Treatment: Insights into In Vitro and In Vivo Efficacy and Safety. J. Med. Chem. 2025, 68, 10008–10030. [Google Scholar] [CrossRef]
- Laengle, U.W.; Court, M.; Markstein, R.; Germann, P.G.; Nogues, V.; Roman, D. Effects of Anti-Glaucoma Drugs Timolol and GLC756, a Novel Mixed Dopamine D2 Receptor Agonist and D1 Receptor Antagonist, on Endotoxin-Induced-Uveitis and -Arthritis in Rats. Exp. Toxicol. Pathol. 2005, 57, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Schuman, J.S. Effects of Systemic β-Blocker Therapy on the Efficacy and Safety of Topical Brimonidine and Timolol. Ophthalmology 2000, 107, 1171–1177. [Google Scholar] [CrossRef]
- Gallego, B.I.; Salazar, J.J.; De Hoz, R.; Rojas, B.; Ramírez, A.I.; Salinas-Navarro, M.; Ortín-Martínez, A.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Perez, M.P.; et al. IOP Induces Upregulation of GFAP and MHC-II and Microglia Reactivity in Mice Retina Contralateral to Experimental Glaucoma. J. Neuroinflamm. 2012, 9, 92. [Google Scholar]
- Fernández-Albarral, J.A.; Ramírez, A.I.; de Hoz, R.; Matamoros, J.A.; Salobrar-García, E.; Elvira-Hurtado, L.; López-Cuenca, I.; Sánchez-Puebla, L.; Salazar, J.J.; Ramírez, J.M. Glaucoma: From Pathogenic Mechanisms to Retinal Glial Cell Response to Damage. Front. Cell. Neurosci. 2024, 18, 1354569. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Albarral, J.A.; de Hoz, R.; Matamoros, J.A.; Chen, L.; López-Cuenca, I.; Salobrar-García, E.; Sánchez-Puebla, L.; Ramírez, J.M.; Triviño, A.; Salazar, J.J.; et al. Retinal Changes in Astrocytes and Müller Glia in a Mouse Model of Laser-Induced Glaucoma: A Time-Course Study. Biomedicines 2022, 10, 939. [Google Scholar] [CrossRef]
- Palanivel, V.; Gupta, V.; Chitranshi, N.; Tietz, O.; Wall, R.V.; Blades, R.; Thananthirige, K.P.M.; Salkar, A.; Shen, C.; Mirzaei, M.; et al. Neuropeptide Y Receptor Activation Preserves Inner Retinal Integrity through PI3K/Akt Signaling in a Glaucoma Mouse Model. PNAS Nexus 2024, 3, pgae299. [Google Scholar] [CrossRef] [PubMed]
- Girkin, C.A.; Strickland, R.G.; Somerville, M.M.; Anne Garner, M.; Grossman, G.H.; Blake, A.; Kumar, N.; Ianov, L.; Fazio, M.A.; Clark, M.E.; et al. Acute Ocular Hypertension in the Living Human Eye: Model Description and Initial Cellular Responses to Elevated Intraocular Pressure. Vision. Res. 2024, 223, 108465. [Google Scholar] [CrossRef]
- Calvo, P.; Thomas, C.; Alonso, M.J.; Vila-Jato, J.L.; Robinson, J.R.; Alonso, M.J. Study of the Mechanism of Interaction of Poly(E-Caprolactone) Nanocapsules with the Cornea by Confocal Laser Scanning Microscopy. Int. J. Pharm. 1994, 103, 283–291. [Google Scholar] [CrossRef]
- Elhabal, S.F.; Ashour, H.A.; Mohamed Elrefai, M.F.; Teaima, M.H.; Elzohairy, N.A.; Kholeif, N.A.; El-Nabarawi, M. Innovative Transdermal Delivery of Microneedle Patch for Dual Drugs Febuxostat and Lornoxicam: In Vitro and in Vivo Efficacy for Treating Gouty Arthritis. J. Drug Deliv. Sci. Technol. 2025, 110, 107053. [Google Scholar] [CrossRef]
- Madani, F.; Esnaashari, S.S.; Mujokoro, B.; Dorkoosh, F.; Khosravani, M.; Adabi, M. Investigation of Effective Parameters on Size of Paclitaxel Loaded PLGA Nanoparticles. Adv. Pharm. Bull. 2018, 8, 77–84. [Google Scholar] [CrossRef]
- Budhian, A.; Siegel, S.J.; Winey, K.I. Production of Haloperidol-Loaded PLGA Nanoparticles for Extended Controlled Drug Release of Haloperidol. J. Microencapsul. 2005, 22, 773–785. [Google Scholar] [CrossRef]
- Spaeth, G.L. European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition. Br. J. Ophthalmol. 2021, 105 (Suppl. S1), 1–169. [Google Scholar] [CrossRef]
- Sukmawati, A.; Utami, W.; Yuliani, R.; Da’I, M.; Nafarin, A. Effect of Tween 80 on Nanoparticle Preparation of Modified Chitosan for Targeted Delivery of Combination Doxorubicin and Curcumin Analogue. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Malé, Maldives, 6–8 March 2018; Institute of Physics Publishing: Bristol, UK, 2018; Volume 311. [Google Scholar]
- Elhabal, S.F.; Faheem, A.M.; Hababeh, S.; Nelson, J.; Elzohairy, N.A.; AbdelGhany Morsy, S.A.; Ewedah, T.M.; Mousa, I.S.; Fouad, M.A.; Hamdan, A.M.E. Dissolving Microneedles Containing Lactoferrin Nanosuspension for Enhancement of Antimicrobial and Anti-Inflammatory Effects in the Treatment of Dry Eye Disease. Pharmaceutics 2025, 17, 653. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Chauhan, M.K. Optimization and Characterization of Brimonidine Tartrate Nanoparticles-Loaded In Situ Gel for the Treatment of Glaucoma. Curr. Eye Res. 2021, 46, 1703–1716. [Google Scholar] [CrossRef]
- Sah, A.K.; Suresh, P.K.; Verma, V.K. PLGA Nanoparticles for Ocular Delivery of Loteprednol Etabonate: A Corneal Penetration Study. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- ELhabal, S.F.; El-Nabarawi, M.A.; Hassanin, S.O.; Hassan, F.E.; Abbas, S.S.; Gebril, S.M.; Albash, R. Transdermal Fluocinolone Acetonide Loaded Decorated Hyalurosomes Cellulose Acetate/Polycaprolactone Nanofibers Mitigated Freund’s Adjuvant-Induced Rheumatoid Arthritis in Rats. J. Pharm. Investig. 2024, 55, 113–132. [Google Scholar] [CrossRef]
- Gebreel, R.M.; Edris, N.A.; Elmofty, H.M.; Tadros, M.I.; El-Nabarawi, M.A.; Hassan, D.H. Development and Characterization of PLGA Nanoparticle-Laden Hydrogels for Sustained Ocular Delivery of Norfloxacin in the Treatment of Pseudomonas Keratitis: An Experimental Study. Drug Des. Dev. Ther. 2021, 15, 399–418. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhu, X.; Liu, D.; Gong, Y.; Sun, J.; Dong, C. Continuous Delivery of Propranolol from Liposomes-in-Microspheres Significantly Inhibits Infantile Hemangioma Growth. Int. J. Nanomed. 2017, 12, 6923–6936. [Google Scholar] [CrossRef]
- Younis, M.K.; Elakkad, Y.E.; Fakhr Eldeen, R.R.; Ali, I.H.; Khalil, I.A. Propranolol-Loaded Trehalosome as Antiproliferative Agent for Treating Skin Cancer: Optimization, Cytotoxicity, and In Silico Studies. Pharmaceutics 2023, 15, 2033. [Google Scholar] [CrossRef]
- Ger, T.Y.; Yang, C.J.; Ghosh, S.; Lai, J.Y. Biofunctionalization of Nanoceria with Sperminated Hyaluronan Enhances Drug Delivery Performance for Corneal Alkali Burn Therapy. Chem. Eng. J. 2023, 476, 146864. [Google Scholar] [CrossRef]
- . Mahar, R.; Chakraborty, A.; Nainwal, N.; Bahuguna, R.; Sajwan, M.; Jakhmola, V. Application of PLGA as a Biodegradable and Biocompatible Polymer for Pulmonary Delivery of Drugs. AAPS PharmSciTech 2023, 24, 39. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Z.; Wu, D.; Hao, B.; Liu, Y.; Wang, X.; Pu, W.; Yi, Y.; Shang, R.; Wang, S. The Effect of Polymer Blends on the In Vitro Release/Degradation and Pharmacokinetics of Moxidectin-Loaded PLGA Microspheres. Int. J. Mol. Sci. 2023, 24, 14729. [Google Scholar] [CrossRef]
- Guo, W.; Quan, P.; Fang, L.; Cun, D.; Yang, M. Sustained Release Donepezil Loaded PLGA Microspheres for Injection: Preparation, in Vitro and in Vivo Study. Asian J. Pharm. Sci. 2015, 10, 405–414. [Google Scholar] [CrossRef]
- Lagreca, E.; Onesto, V.; Di Natale, C.; La Manna, S.; Netti, P.A.; Vecchione, R. Recent Advances in the Formulation of PLGA Microparticles for Controlled Drug Delivery. Prog. Biomater. 2020, 9, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Egea, M.A.; Cano, A.; Espina, M.; Calpena, A.C.; Ettcheto, M.; Camins, A.; Souto, E.B.; Silva, A.M.; García, M.L. PEGylated PLGA Nanospheres Optimized by Design of Experiments for Ocular Administration of Dexibuprofen-in Vitro, Ex Vivo and in Vivo Characterization. Colloids Surf. B Biointerfaces 2016, 145, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G. Sparfloxacin-loaded PLGA nanoparticles for sustained ocular drug delivery. Nanomedicine 2010, 6, 324–333. [Google Scholar] [CrossRef] [PubMed]




| Factors (Independent Variables) | Design Levels | ||
|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | |
| X1: Bisoprolol (mg) | 12.5 | 25 | 37.5 |
| X2: PLGA (mg) | 22.5 | 25 | 27.5 |
| X3: Tween 80 (mg) | 60 | 80 | 100 |
| Responses (Dependent variables) | Goal | ||
| Y1: P.S (nm) | Minimize | ||
| Y2: P.D.I (nm) | Minimize | ||
| Y3: Z.P (mV) | Maximize | ||
| Y4: E.E (%) | Maximize | ||
| Factors | Responses | ||||||
|---|---|---|---|---|---|---|---|
| Run | A: Bisoprolol (mg) | B: PLGA (mg) | C: Tween 80 (mg) | PS (nm) | PDI | ZP (mv) | EE% |
| 1 | 37.5 | 25 | 60 | 503 0.32 | 0.49 0.12 | −29.3 0.31 | 80 ± 0.46 |
| 2 | 12.5 | 27.5 | 100 | 179 0.24 | 0.221 0.01 | −21.8 0.45 | 50 ± 0.98 |
| 3 | 12.5 | 27.5 | 60 | 140 0.18 | 0.22 0.02 | −21.8 0.32 | 55 ± 0.72 |
| 4 | 12.5 | 22.5 | 100 | 140 0.52 | 0.24 0.02 | −13.3 0.51 | 65 ± 0.76 |
| 5 | 25 | 25 | 100 | 384 0.71 | 0.96 0.05 | −18.6 0.14 | 45 ± 0.88 |
| 6 | 12.5 | 25 | 80 | 389 0.42 | 0.39 0.01 | −22.9 0.52 | 60 ± 0.35 |
| 7 | 37.5 | 25 | 100 | 636 0.31 | 0.69 0.03 | −12 0.60 | 85 ± 0.65 |
| 8 | 25 | 25 | 100 | 784 0.71 | 0.96 0.05 | −18.6 0.14 | 45 ± 0.35 |
| 9 | 25 | 22.5 | 60 | 105 0.35 | 0.411 0.14 | −18.7 0.41 | 75 ± 0.98 |
| 10 | 12.5 | 25 | 80 | 389 0.53 | 0.39 0.11 | −22.9 0.61 | 60 ± 0.34 |
| 11 | 37.5 | 27.5 | 100 | 195 0.62 | 0.127 0.04 | −30.5 0.80 | 40 ± 0.56 |
| 12 | 37.5 | 22.5 | 80 | 147 0.15 | 0.34 0.03 | −21.5 0.47 | 70 ± 0.82 |
| 13 | 25 | 27.5 | 80 | 484 0.50 | 0.96 0.04 | −18.6 0.38 | 45 ± 0.47 |
| 14 | 37.5 | 27.5 | 60 | 503 0.32 | 0.49 0.06 | −29.3 0.31 | 80 ± 0.37 |
| 15 | 12.5 | 22.5 | 80 | 140 ± 0.87 | 0.24 0.04 | −13.3 0.16 | 65 ± 0.72 |
| 16 | 25 | 27.5 | 80 | 484 0.53 | 0.96 0.04 | −18.6 0.38 | 45 ± 0.85 |
| Parameters | BSP-PLGA-NPs Freshly Prepared | BSP-PLGA-NPs After Three Months of Storage at 4 °C | BSP-PLGA-NPs After Three Months of Storage at 25 °C |
|---|---|---|---|
| PS (nm) | 105 ± 0.35 | 140 ± 0.73 | 164 ± 0.51 |
| PDI | 0.411 ± 0.14 | 0.435 ± 0.12 | 0.487 ± 0.11 |
| ZP (mV) | −18.7 ± 0.41 | −16 ± 0.021 | 14 ± 0.03 |
| EE (%) | 75 ± 0.98 | 71 ± 0.06 | 69 ± 0.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhabal, S.F.; Mahfouz, O.M.; Elrefai, M.F.M.; Teaima, M.H.; Abdalla, A.; El-Nabarawi, M. Sustained Intraocular Pressure Reduction Using Bisoprolol-Loaded PLGA Nanoparticles: A Promising Strategy for Enhanced Ocular Delivery with Reduced GFAP Expression Indicative of Lower Glial Activation. Pharmaceutics 2025, 17, 1418. https://doi.org/10.3390/pharmaceutics17111418
Elhabal SF, Mahfouz OM, Elrefai MFM, Teaima MH, Abdalla A, El-Nabarawi M. Sustained Intraocular Pressure Reduction Using Bisoprolol-Loaded PLGA Nanoparticles: A Promising Strategy for Enhanced Ocular Delivery with Reduced GFAP Expression Indicative of Lower Glial Activation. Pharmaceutics. 2025; 17(11):1418. https://doi.org/10.3390/pharmaceutics17111418
Chicago/Turabian StyleElhabal, Sammar Fathy, Omnia Mohamed Mahfouz, Mohamed Fathi Mohamed Elrefai, Mahmoud H. Teaima, Ahmed Abdalla, and Mohamed El-Nabarawi. 2025. "Sustained Intraocular Pressure Reduction Using Bisoprolol-Loaded PLGA Nanoparticles: A Promising Strategy for Enhanced Ocular Delivery with Reduced GFAP Expression Indicative of Lower Glial Activation" Pharmaceutics 17, no. 11: 1418. https://doi.org/10.3390/pharmaceutics17111418
APA StyleElhabal, S. F., Mahfouz, O. M., Elrefai, M. F. M., Teaima, M. H., Abdalla, A., & El-Nabarawi, M. (2025). Sustained Intraocular Pressure Reduction Using Bisoprolol-Loaded PLGA Nanoparticles: A Promising Strategy for Enhanced Ocular Delivery with Reduced GFAP Expression Indicative of Lower Glial Activation. Pharmaceutics, 17(11), 1418. https://doi.org/10.3390/pharmaceutics17111418








