Amodiaquine Modulates Aggregation and Disassembly of Amyloid-β and Tau and Attenuates Neuroinflammatory Responses and Aβ Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Evaluation of the Anti-Aggregation and Disaggregation Effects of AQ on Aβ42
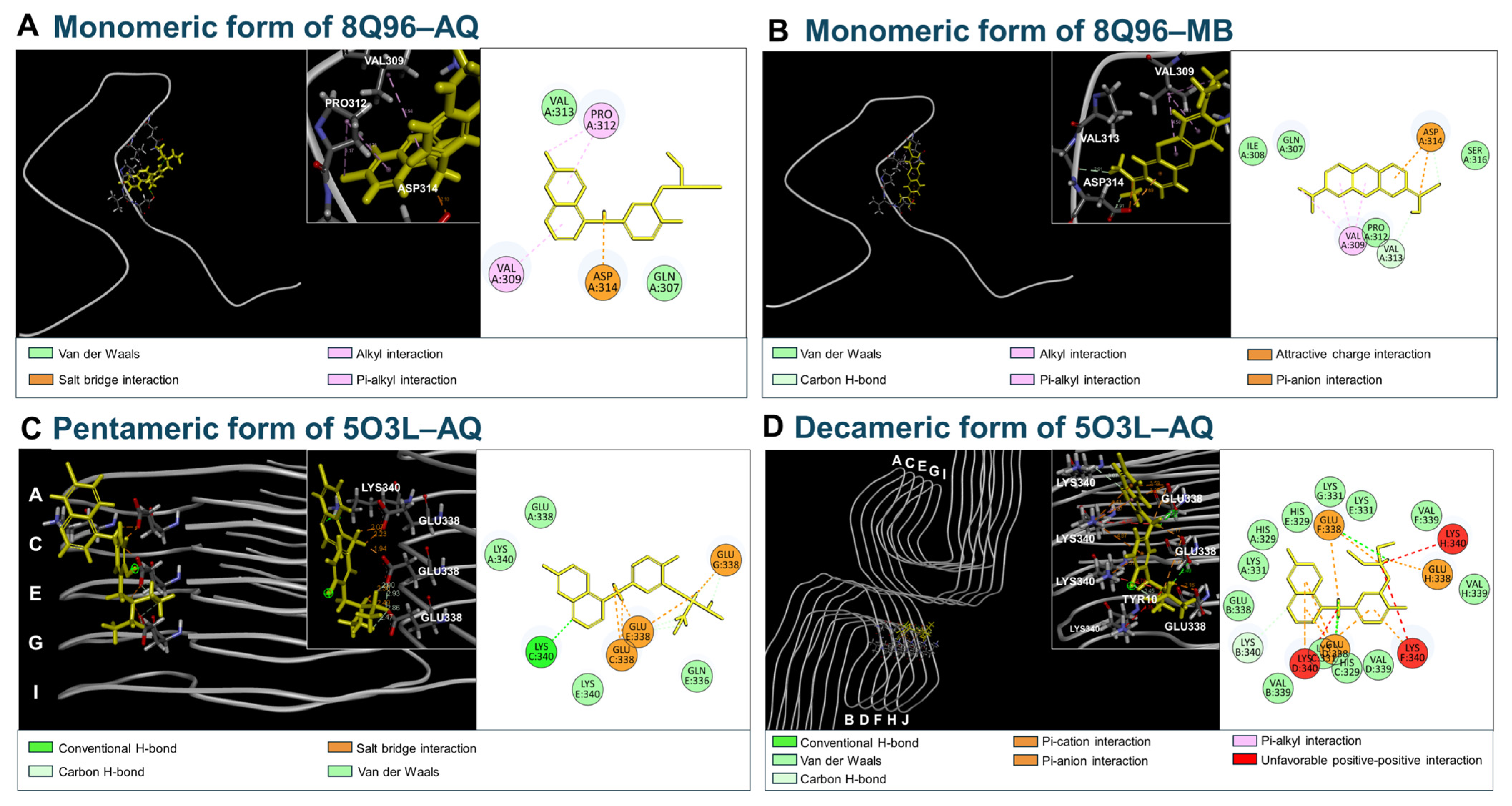
2.2. Molecular Docking Simulation of AQ with Aβ or Tau
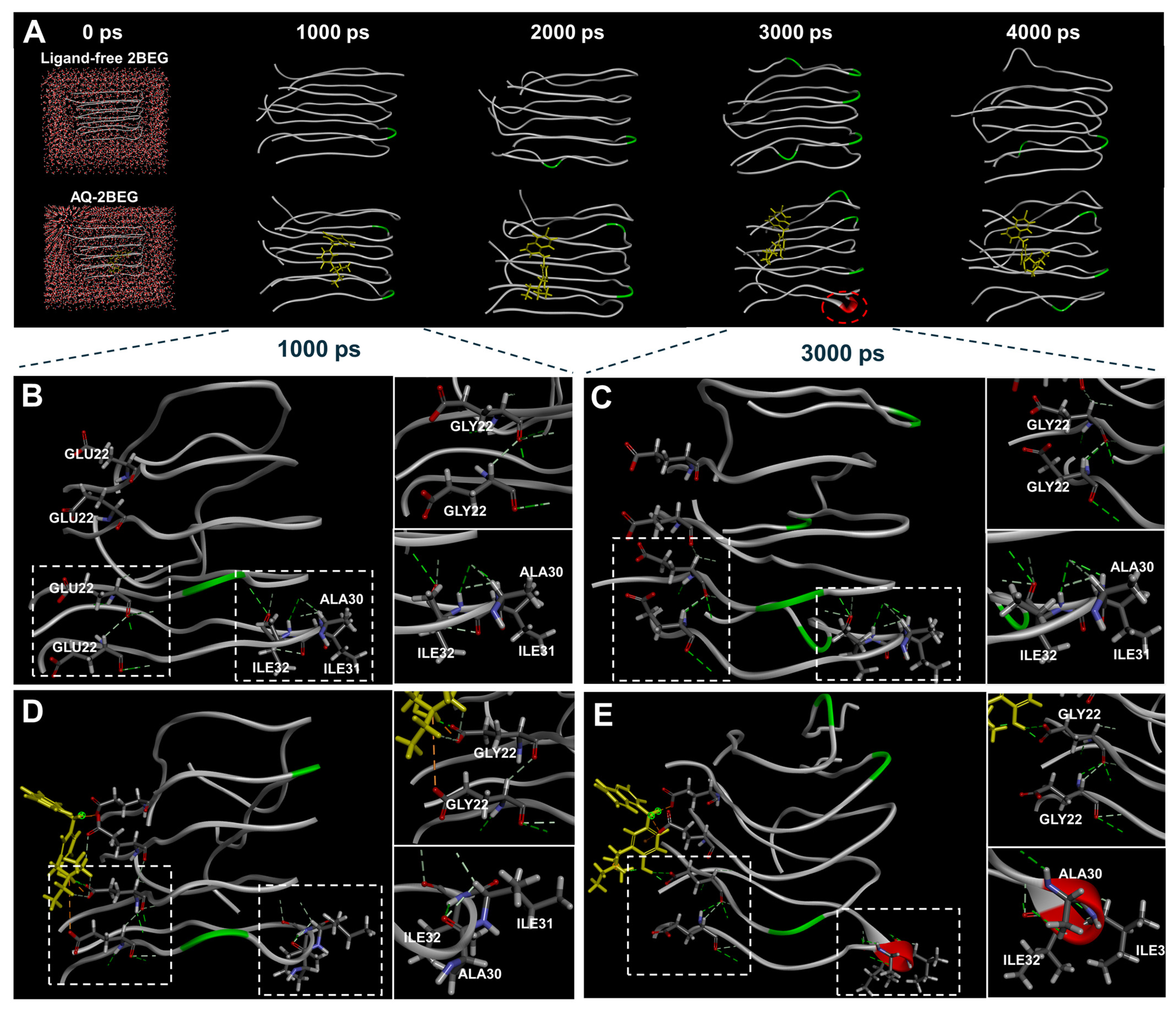
2.3. MD Simulations of AQ with Aβ or Tau
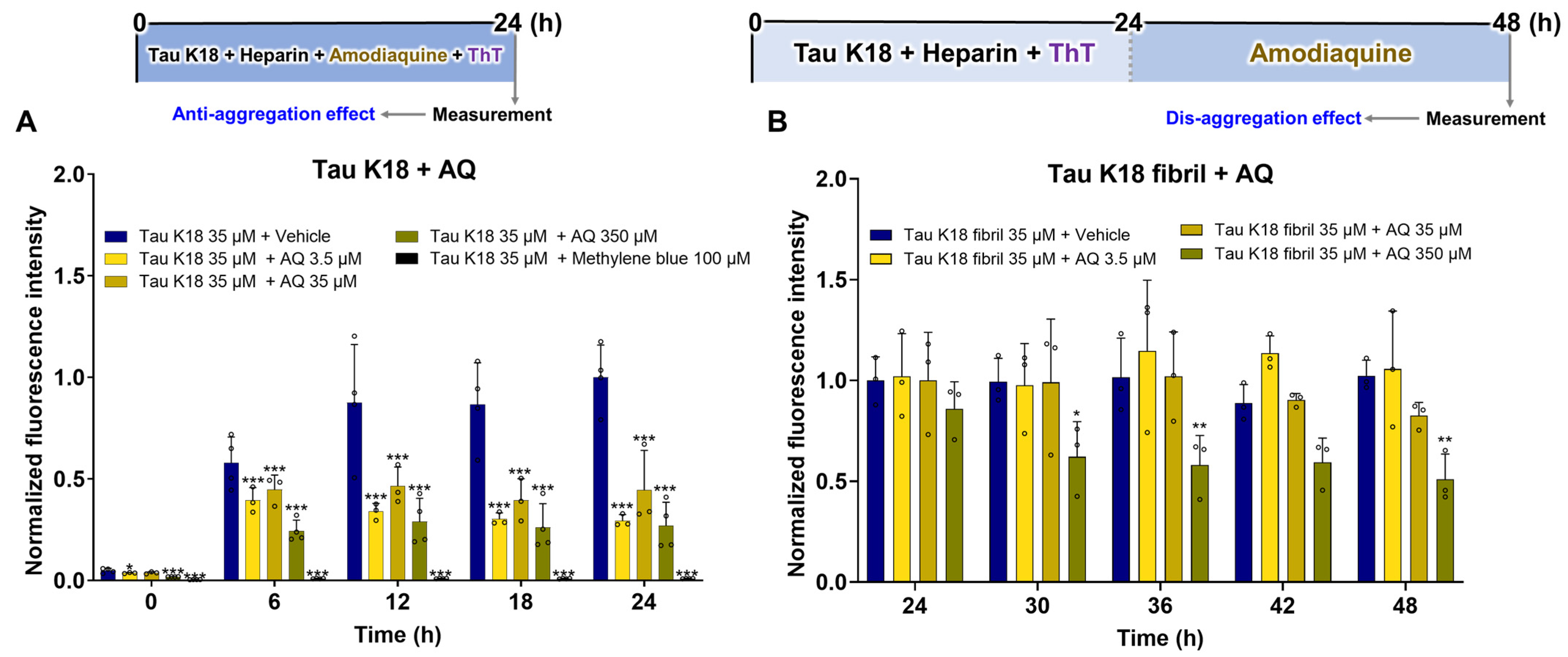
2.4. Evaluation of the Anti-Aggregation and Disaggregation Effects of AQ on Tau K18
2.5. Cell Culture Conditions of BV2 Cells and APP-H4 Cells
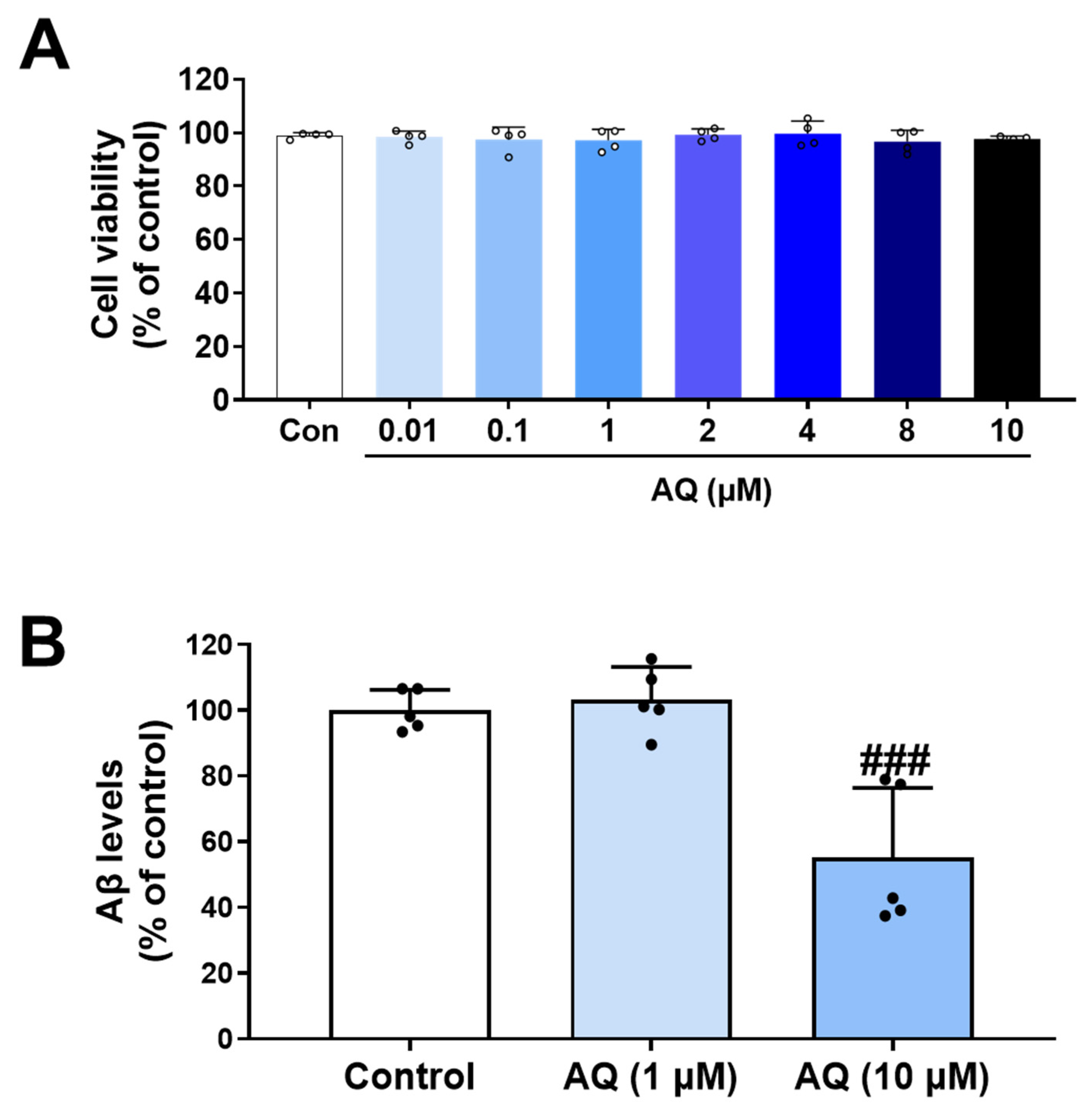
2.6. Cell Viability Assay in HT22 Cells, BV2 Cells and APP-H4 Cells
2.7. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR) Analysis of Inflammatory Cytokines in BV2 Cells
2.8. Enzyme-Linked Immunosorbent Assay (ELISA) in APP-H4 Cells
2.9. Prediction of Pharmacokinetic Characterization
2.10. Statistical Analysis
3. Results
3.1. Modulatory Effects of AQ on Aβ42
3.2. Molecular Docking of AQ to Aβ
3.3. Molecular Dynamics Simulation of AQ–Aβ Complex
3.4. Regulatory Effects of AQ on Tau K18
3.5. Molecular Docking of AQ to Tau
3.6. Molecular Dynamics Simulation of AQ–Tau Complex
3.7. Anti-Inflammatory Effect of AQ on Inflammatory Response Induced by Aβ42 and Tau K18
3.8. Inhibitory Effect of AQ on Production of Aβ
3.9. Prediction of Pharmacokinetics for AQ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-β |
| AD | Alzheimer’s disease |
| ADME | Absorption, distribution, metabolism, and excretion |
| APP | Amyloid precursor protein |
| AQ | Amodiaquine |
| BBB | Blood–brain barrier |
| Cdc25a | Cell division cycle 25A |
| CDK2 | Cyclin-dependent kinase 2 |
| CHARMm | Chemistry at HARvard Macromolecular Mechanics |
| Cq | Quantification cycle |
| DPBS | Dulbecco’s phosphate-buffered saline |
| E2F1 | E2F transcription factor 1 |
| EGCG | Epigallocatechin gallate |
| ELISA | Enzyme-linked immunosorbent assay |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| HIA | Human intestinal absorption |
| IL-6 | Interleukin-6 |
| ITC | Isothermal titration calorimetry |
| LSD | Least significant difference |
| MB | Methylene blue |
| MCM5 | Minichromosome maintenance complex component 5 |
| MD | Molecular dynamics |
| NFTs | Neurofibrillary tangles |
| NR | Newton-Raphson |
| PCNA | Proliferating cell nuclear antigen |
| PET | Positron emission tomography |
| PHFs | Paired helical filaments |
| PPB | Plasma protein binding |
| PS | Picosecond |
| RMS | Root mean square |
| RMSD | Root mean square deviation |
| SD | Standard deviations |
| SPR | Surface plasmon resonance |
| ThT | Thioflavin T |
| TNF-α | Tumor necrosis factor-alpha |
| WST-1 | Water-soluble tetrazolium-1 |
References
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chetelat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef]
- Bateman, R.J.; Xiong, C.; Benzinger, T.L.; Fagan, A.M.; Goate, A.; Fox, N.C.; Marcus, D.S.; Cairns, N.J.; Xie, X.; Blazey, T.M.; et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 2012, 367, 795–804. [Google Scholar] [CrossRef]
- Jia, J.; Ning, Y.; Chen, M.; Wang, S.; Yang, H.; Li, F.; Ding, J.; Li, Y.; Zhao, B.; Lyu, J.; et al. Biomarker Changes during 20 Years Preceding Alzheimer’s Disease. N. Engl. J. Med. 2024, 390, 712–722. [Google Scholar] [CrossRef]
- Malafaia, D.; Albuquerque, H.M.T.; Silva, A.M.S. Amyloid-beta and tau aggregation dual-inhibitors: A synthetic and structure-activity relationship focused review. Eur. J. Med. Chem. 2021, 214, 113209. [Google Scholar] [CrossRef]
- Thal, D.R.; Fandrich, M. Protein aggregation in Alzheimer’s disease: Abeta and tau and their potential roles in the pathogenesis of AD. Acta Neuropathol. 2015, 129, 163–165. [Google Scholar] [CrossRef]
- Ittner, L.M.; Gotz, J. Amyloid-beta and tau—A toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 2011, 12, 65–72. [Google Scholar] [CrossRef]
- Kim, S.; Hyun, D.G.; Nam, Y.; Shin, S.J.; Im, D.; Kim, H.S.; Leem, S.H.; Park, H.H.; Kim, B.H.; Park, Y.H.; et al. Genipin and pyrogallol: Two natural small molecules targeting the modulation of disordered proteins in Alzheimer’s disease. Biomed. Pharmacother. 2023, 168, 115770. [Google Scholar] [CrossRef]
- Nam, Y.; Shin, S.J.; Kumar, V.; Won, J.; Kim, S.; Moon, M. Dual modulation of amyloid beta and tau aggregation and dissociation in Alzheimer’s disease: A comprehensive review of the characteristics and therapeutic strategies. Transl. Neurodegener. 2025, 14, 15. [Google Scholar] [CrossRef]
- Willems, S.; Muller, M.; Ohrndorf, J.; Heering, J.; Proschak, E.; Merk, D. Scaffold Hopping from Amodiaquine to Novel Nurr1 Agonist Chemotypes via Microscale Analogue Libraries. ChemMedChem 2022, 17, e202200026. [Google Scholar] [CrossRef]
- Albuquerque, H.M.T.; Nunes da Silva, R.; Pereira, M.; Maia, A.; Guieu, S.; Soares, A.R.; Santos, C.M.M.; Vieira, S.I.; Silva, A.M.S. Steroid-Quinoline Hybrids for Disruption and Reversion of Protein Aggregation Processes. ACS Med. Chem. Lett. 2022, 13, 443–448. [Google Scholar] [CrossRef]
- Bowroju, S.K.; Mainali, N.; Ayyadevara, S.; Penthala, N.R.; Krishnamachari, S.; Kakraba, S.; Shmookler Reis, R.J.; Crooks, P.A. Design and Synthesis of Novel Hybrid 8-Hydroxy Quinoline-Indole Derivatives as Inhibitors of Abeta Self-Aggregation and Metal Chelation-Induced Abeta Aggregation. Molecules 2020, 25, 3610. [Google Scholar] [CrossRef]
- Navarrete, L.P.; Guzman, L.; San Martin, A.; Astudillo-Saavedra, L.; Maccioni, R.B. Molecules of the quinoline family block tau self-aggregation: Implications toward a therapeutic approach for Alzheimer’s disease. J. Alzheimers Dis. 2012, 29, 79–88. [Google Scholar] [CrossRef]
- Manzoor, S.; Gabr, M.T.; Nafie, M.S.; Raza, M.K.; Khan, A.; Nayeem, S.M.; Arafa, R.K.; Hoda, N. Discovery of Quinolinone Hybrids as Dual Inhibitors of Acetylcholinesterase and Abeta Aggregation for Alzheimer’s Disease Therapy. ACS Chem. Neurosci. 2024, 15, 539–559. [Google Scholar] [CrossRef]
- Matosevic, A.; Opsenica, D.M.; Bartolic, M.; Marakovic, N.; Stoilkovic, A.; Komatovic, K.; Zandona, A.; Zunec, S.; Bosak, A. Derivatives of Amodiaquine as Potent Human Cholinesterases Inhibitors: Implication for Treatment of Alzheimer’s Disease. Molecules 2024, 29, 5357. [Google Scholar] [CrossRef]
- Majid, N.; Siddiqi, M.K.; Malik, S.; Khan, R.H. Revelation of anti-aggregating and disaggregating characteristic of amodiaquine via inhibition of human lysozyme fibrillation: Therapeutic alternative for treating amyloidopathies. J. Mol. Liq. 2023, 385, 122438. [Google Scholar] [CrossRef]
- Moon, M.; Jung, E.S.; Jeon, S.G.; Cha, M.Y.; Jang, Y.; Kim, W.; Lopes, C.; Mook-Jung, I.; Kim, K.S. Nurr1 (NR4A2) regulates Alzheimer’s disease-related pathogenesis and cognitive function in the 5XFAD mouse model. Aging Cell 2019, 18, e12866. [Google Scholar] [CrossRef]
- Moon, H.; Jeon, S.G.; Kim, J.I.; Kim, H.S.; Lee, S.; Kim, D.; Park, S.; Moon, M.; Chung, H. Pharmacological Stimulation of Nurr1 Promotes Cell Cycle Progression in Adult Hippocampal Neural Stem Cells. Int. J. Mol. Sci. 2019, 21, 4. [Google Scholar] [CrossRef]
- Ryan, T.M.; Caine, J.; Mertens, H.D.; Kirby, N.; Nigro, J.; Breheney, K.; Waddington, L.J.; Streltsov, V.A.; Curtain, C.; Masters, C.L.; et al. Ammonium hydroxide treatment of Abeta produces an aggregate free solution suitable for biophysical and cell culture characterization. PeerJ 2013, 1, e73. [Google Scholar] [CrossRef]
- Nam, Y.; Prajapati, R.; Kim, S.; Shin, S.J.; Cheong, D.Y.; Park, Y.H.; Park, H.H.; Lim, D.; Yoon, Y.; Lee, G.; et al. Dual regulatory effects of neferine on amyloid-beta and tau aggregation studied by in silico, in vitro, and lab-on-a-chip technology. Biomed. Pharmacother. 2024, 172, 116226. [Google Scholar] [CrossRef]
- Kim, S.; Nam, Y.; Shin, S.J.; Prajapati, R.; Shin, S.M.; Kim, M.J.; Soo Kim, H.; Leem, S.H.; Kim, T.J.; Park, Y.H.; et al. Dual modulators of aggregation and dissociation of amyloid beta and tau: In vitro, in vivo, and in silico studies of Uncaria rhynchophylla and its bioactive components. Biomed. Pharmacother. 2022, 156, 113865. [Google Scholar] [CrossRef]
- Drolle, E.; Hane, F.; Lee, B.; Leonenko, Z. Atomic force microscopy to study molecular mechanisms of amyloid fibril formation and toxicity in Alzheimer’s disease. Drug Metab. Rev. 2014, 46, 207–223. [Google Scholar] [CrossRef]
- Moores, B.; Drolle, E.; Attwood, S.J.; Simons, J.; Leonenko, Z. Effect of surfaces on amyloid fibril formation. PLoS ONE 2011, 6, e25954. [Google Scholar] [CrossRef] [PubMed]
- Floden, A.M.; Li, S.; Combs, C.K. Beta-amyloid-stimulated microglia induce neuron death via synergistic stimulation of tumor necrosis factor alpha and NMDA receptors. J. Neurosci. 2005, 25, 2566–2575. [Google Scholar] [CrossRef]
- Gaikwad, S.; Puangmalai, N.; Bittar, A.; Montalbano, M.; Garcia, S.; McAllen, S.; Bhatt, N.; Sonawane, M.; Sengupta, U.; Kayed, R. Tau oligomer induced HMGB1 release contributes to cellular senescence and neuropathology linked to Alzheimer’s disease and frontotemporal dementia. Cell Rep. 2021, 36, 109419. [Google Scholar] [CrossRef] [PubMed]
- Sondag, C.M.; Dhawan, G.; Combs, C.K. Beta amyloid oligomers and fibrils stimulate differential activation of primary microglia. J. Neuroinflamm. 2009, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Gay, M.; Carato, P.; Coevoet, M.; Renault, N.; Larchanche, P.E.; Barczyk, A.; Yous, S.; Buee, L.; Sergeant, N.; Melnyk, P. New phenylaniline derivatives as modulators of amyloid protein precursor metabolism. Bioorg Med. Chem. 2018, 26, 2151–2164. [Google Scholar] [CrossRef]
- Colombo, A.; Bastone, A.; Ploia, C.; Sclip, A.; Salmona, M.; Forloni, G.; Borsello, T. JNK regulates APP cleavage and degradation in a model of Alzheimer’s disease. Neurobiol. Dis. 2009, 33, 518–525. [Google Scholar] [CrossRef]
- Heidari, R.; Babaei, H.; Eghbal, M.A. Amodiaquine-induced toxicity in isolated rat hepatocytes and the cytoprotective effects of taurine and/or N-acetyl cysteine. Res. Pharm. Sci. 2014, 9, 97–105. [Google Scholar]
- Adjei, G.O.; Goka, B.Q.; Rodrigues, O.P.; Hoegberg, L.C.; Alifrangis, M.; Kurtzhals, J. Amodiaquine-associated adverse effects after inadvertent overdose and after a standard therapeutic dose. Ghana. Med. J. 2009, 43, 135–138. [Google Scholar] [CrossRef]
- Convertino, M.; Vitalis, A.; Caflisch, A. Disordered binding of small molecules to Abeta(12-28). J. Biol. Chem. 2011, 286, 41578–41588. [Google Scholar] [CrossRef]
- Ryan, T.M.; Friedhuber, A.; Lind, M.; Howlett, G.J.; Masters, C.; Roberts, B.R. Small amphipathic molecules modulate secondary structure and amyloid fibril-forming kinetics of Alzheimer disease peptide Abeta1–42. J. Biol. Chem. 2012, 287, 16947–16954. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Khan, J.M.; Malik, A.; Rehman, M.T.; Husain, F.M.; AlAjmi, M.F.; Hamdan Ali Alghamdi, O.; Khan, A. Quinoline yellow dye stimulates whey protein fibrillation via electrostatic and hydrophobic interaction: A biophysical study. J. Dairy Sci. 2021, 104, 5141–5151. [Google Scholar] [CrossRef]
- Khan, M.S.; Rehman, M.T.; Bhat, S.A.; Tabrez, S.; Hussain, A.; Husain, F.M.; AlAjmi, M.F.; Alamery, S.F.; Sumbul, S. Food additive dye (quinoline yellow) promotes unfolding and aggregation of myoglobin: A spectroscopic and molecular docking analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 214, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Xue, C.; Wang, H.; Guo, Z. Distinguishing the Effect on the Rate and Yield of Abeta42 Aggregation by Green Tea Polyphenol EGCG. ACS Omega 2020, 5, 21497–21505. [Google Scholar] [CrossRef] [PubMed]
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef]
- Caesar, I.; Jonson, M.; Nilsson, K.P.; Thor, S.; Hammarstrom, P. Curcumin promotes A-beta fibrillation and reduces neurotoxicity in transgenic Drosophila. PLoS ONE 2012, 7, e31424. [Google Scholar] [CrossRef]
- Bieschke, J.; Russ, J.; Friedrich, R.P.; Ehrnhoefer, D.E.; Wobst, H.; Neugebauer, K.; Wanker, E.E. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Proc. Natl. Acad. Sci. USA 2010, 107, 7710–7715. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.M.; Roberts, B.R.; McColl, G.; Hare, D.J.; Doble, P.A.; Li, Q.X.; Lind, M.; Roberts, A.M.; Mertens, H.D.; Kirby, N.; et al. Stabilization of nontoxic Abeta-oligomers: Insights into the mechanism of action of hydroxyquinolines in Alzheimer’s disease. J. Neurosci. 2015, 35, 2871–2884. [Google Scholar] [CrossRef]
- Furumoto, S.; Tago, T.; Harada, R.; Kudo, Y.; Okamura, N. 18F-Labeled 2-Arylquinoline Derivatives for Tau Imaging: Chemical, Radiochemical, Biological and Clinical Features. Curr. Alzheimer Res. 2017, 14, 178–185. [Google Scholar] [CrossRef]
- Okamura, N.; Furumoto, S.; Harada, R.; Tago, T.; Yoshikawa, T.; Fodero-Tavoletti, M.; Mulligan, R.S.; Villemagne, V.L.; Akatsu, H.; Yamamoto, T.; et al. Novel 18F-labeled arylquinoline derivatives for noninvasive imaging of tau pathology in Alzheimer disease. J. Nucl. Med. 2013, 54, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Elbatrawy, A.A.; Hyeon, S.J.; Yue, N.; Osman, E.E.A.; Choi, S.H.; Lim, S.; Kim, Y.K.; Ryu, H.; Cui, M.; Nam, G. “Turn-On” Quinoline-Based Fluorescent Probe for Selective Imaging of Tau Aggregates in Alzheimer’s Disease: Rational Design, Synthesis, and Molecular Docking. ACS Sens. 2021, 6, 2281–2289. [Google Scholar] [CrossRef] [PubMed]




| Peptide/Protein | PDB Code | Simulation Models | Amino Acid Sequences |
|---|---|---|---|
| Aβ | 1Z0Q | Monomeric form of 1Z0Q | 1DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA42 |
| 2BEG | Monomeric form of 2BEG | 17LVFFAEDVGSNKGAIIGLMVGGVVIA42 | |
| Pentameric form of 2BEG | |||
| 2NAO | Hexameric form of 2NAO | 1DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA42 | |
| Tau | 8Q96 | Monomeric form of 8Q96 | 274KVQIINKKLDLSNVQSKCGSKDNIKHVSGGGSCQIVYKPVDLSKVTSKCGSLGNIH329 |
| 5O3L | Pentameric form of 5O3L | 306VQIVYKPVDLSKVTSKCGSLGNIHHKPGGGQVEVKSEKLDFKDRVQSKIGSLDNITHVPGGGNKKIETHKLTF378 | |
| Decameric form of 5O3L |
| Ligands | Binding Energy | Interaction Residues | ||||
|---|---|---|---|---|---|---|
| −CDOCKER Interaction Energy | Ligand Strain Energy # | −CDOCKER Energy | H-Bond Interaction | Hydrophobic Interaction | Other | |
| Monomeric form of 1Z0Q | ||||||
| AQ | 26.5208 | 21.7129 | 4.8079 | Ala21 and Glu22 (Carbon H-bond) | Val18 (Alkyl), His14, Val18 and Ala21 (π-alkyl) | Glu22 (Attractive charge), Val24 and Ser26 (Van der Waals) |
| Morin | 16.0720 | 4.8304 | 11.2416 | Ala21, Val24 and Ser26 (H-bond) | Ala21 (π-alkyl) | His14, Val18 and Glu22 (Van der Waals) |
| Monomeric form of 2BEG | ||||||
| AQ | 30.9364 | 23.6421 | 7.2942 | Met35 (Carbon H-bond) | Ile32 (Alkyl), Leu34 (π-alkyl) | Asp23, Ala21, Glu22, Gly33 and Val36 (Van der Waals) |
| Morin | 22.5316 | 4.9058 | 17.6258 | Ala21 (H-bond), Asp23 (H-bond and π-donor H bond) | Ala21 and Val36 (π-alkyl) | Phe19, Glu22, Val24, Gly25 and Leu34 (Van der Waals) |
| Pentameric form of 2BEG | ||||||
| AQ | 73.4199 | 28.2477 | 45.1722 | Glu22D (H-bond and Carbon H-bond), Glu22E (Carbon H-bond) | - | Glu22BCD (Salt bridge), Glu22CE (Attractive charge), Glu22BC (π -anion), Phe20CDE and Val24BCD (Van der Waals) |
| Hexameric form of 2NAO | ||||||
| AQ | 47.1806 | 23.8639 | 23.3167 | Gly9F and Glu11E (H-bond), His13E (π-donor H bond) | Phe4F (π-alkyl), His6F (π-π T-shaped), Try10F and His6F (π-π stacked) | Glu11E (Attractive charge), Tyr10F and His6 (π-cation), His6E, SER8F, GLY9E, Glu11D, Val12DEF, Try10ED and His13F (Van der Waals) |
| Ligands | Binding Energy | Interaction Residues | ||||
|---|---|---|---|---|---|---|
| −CDOCKER Interaction Energy | Ligand Strain Energy # | −CDOCKER Energy | H-Bond Interaction | Hydrophobic Interaction | Other | |
| Monomeric form of 8Q96 | ||||||
| AQ | 26.3056 | 23.2522 | 3.0534 | - | Pro312 (Alkyl), Pro312 and Val309 (π-alkyl) | Val313 and Gln307 (Van der Waals) |
| Methylene blue | 23.3931 | 15.6787 | 7.7144 | Val313 and Asp314 (Carbon H-bond) | Val309 (Alkyl and π-alkyl) | Asp314 (Attractive charge and π-anion), Ile308, Gln307 and Ser316 (Van der Waals) |
| Pentameric form of 5O3L | ||||||
| AQ | 57.4125 | 29.4652 | 27.9473 | Lys340C (H-bond), Glu338EG (Carbon H-bond) | - | Glu338A, Lys340AE and Gln336E (Van der Waals), Glu338CEG (Salt bridge) |
| Decameric form of 5O3L | ||||||
| AQ | 45.2739 | 33.1491 | 12.1302 | Glu338DF (H-bond), Lys340B (Carbon H-bond) | Lys340D (π-alkyl) | His329ACE, Lys331ACEG, Glu338B and ValBDFH (Van der Waals), Glu338FH (Salt bridge), Glu338DFH (Attractive charge), Lys340DF (π-cation), Glu338D (π-anion) |
| Pharmacokinetic Properties | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound name | logPo/w a | PPB b | Caco2 c | HIA d | BBB e | CYP _2C19 _inhibition f | CYP 2D6 _inhibition g | Total Clearance h |
| AQ | 4.95 | 95.9 | 41.78 | 94.9 | 5.76 | Non | Inhibitor | 1.265 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.; Kim, S.; Kim, N.-H.; Shin, S.J.; Kumar, V.; Son, J.G.; Lee, M.; Kim, C.-g.; Lim, E.-K.; Chung, H.; et al. Amodiaquine Modulates Aggregation and Disassembly of Amyloid-β and Tau and Attenuates Neuroinflammatory Responses and Aβ Production. Pharmaceutics 2025, 17, 1417. https://doi.org/10.3390/pharmaceutics17111417
Jang S, Kim S, Kim N-H, Shin SJ, Kumar V, Son JG, Lee M, Kim C-g, Lim E-K, Chung H, et al. Amodiaquine Modulates Aggregation and Disassembly of Amyloid-β and Tau and Attenuates Neuroinflammatory Responses and Aβ Production. Pharmaceutics. 2025; 17(11):1417. https://doi.org/10.3390/pharmaceutics17111417
Chicago/Turabian StyleJang, Sinae, Sujin Kim, Na-Hyun Kim, Soo Jung Shin, Vijay Kumar, Jeong Gyu Son, Minseok Lee, Choon-gil Kim, Eun-Kyung Lim, Hyunju Chung, and et al. 2025. "Amodiaquine Modulates Aggregation and Disassembly of Amyloid-β and Tau and Attenuates Neuroinflammatory Responses and Aβ Production" Pharmaceutics 17, no. 11: 1417. https://doi.org/10.3390/pharmaceutics17111417
APA StyleJang, S., Kim, S., Kim, N.-H., Shin, S. J., Kumar, V., Son, J. G., Lee, M., Kim, C.-g., Lim, E.-K., Chung, H., Koh, Y. H., Nam, Y., & Moon, M. (2025). Amodiaquine Modulates Aggregation and Disassembly of Amyloid-β and Tau and Attenuates Neuroinflammatory Responses and Aβ Production. Pharmaceutics, 17(11), 1417. https://doi.org/10.3390/pharmaceutics17111417






