Base and Prime Editing for Inherited Retinal Diseases: Delivery Platforms, Safety, Efficacy, and Translational Perspectives
Abstract
1. Introduction
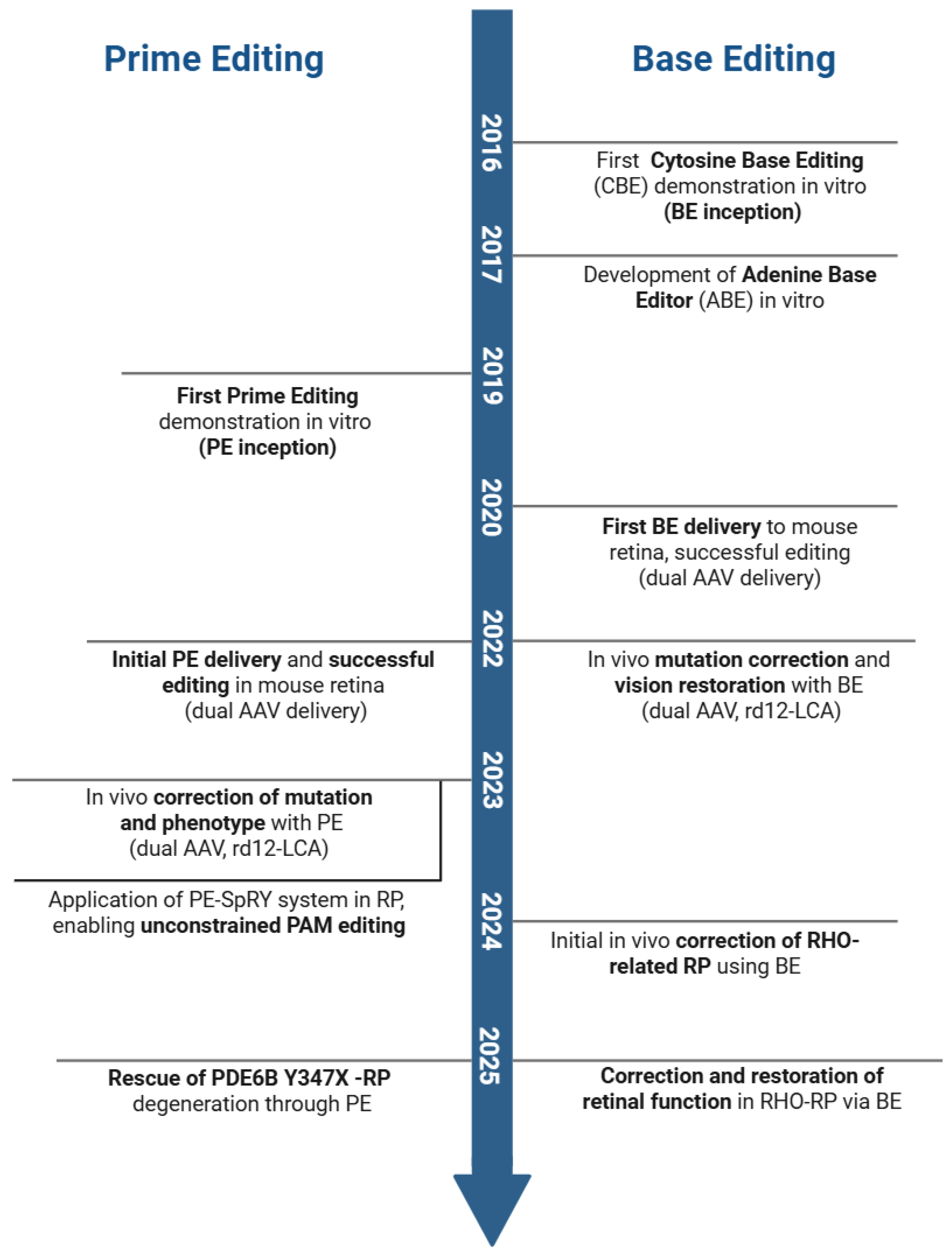
2. Technical Fundamentals and Optimization
2.1. Classification and Mechanisms of Base and Prime Editors
2.2. Optimization Strategies for Base Editors
2.3. Optimization Strategies for Prime Editors
2.4. Therapeutic Potential of Base and Prime Editors Targeting IRDs
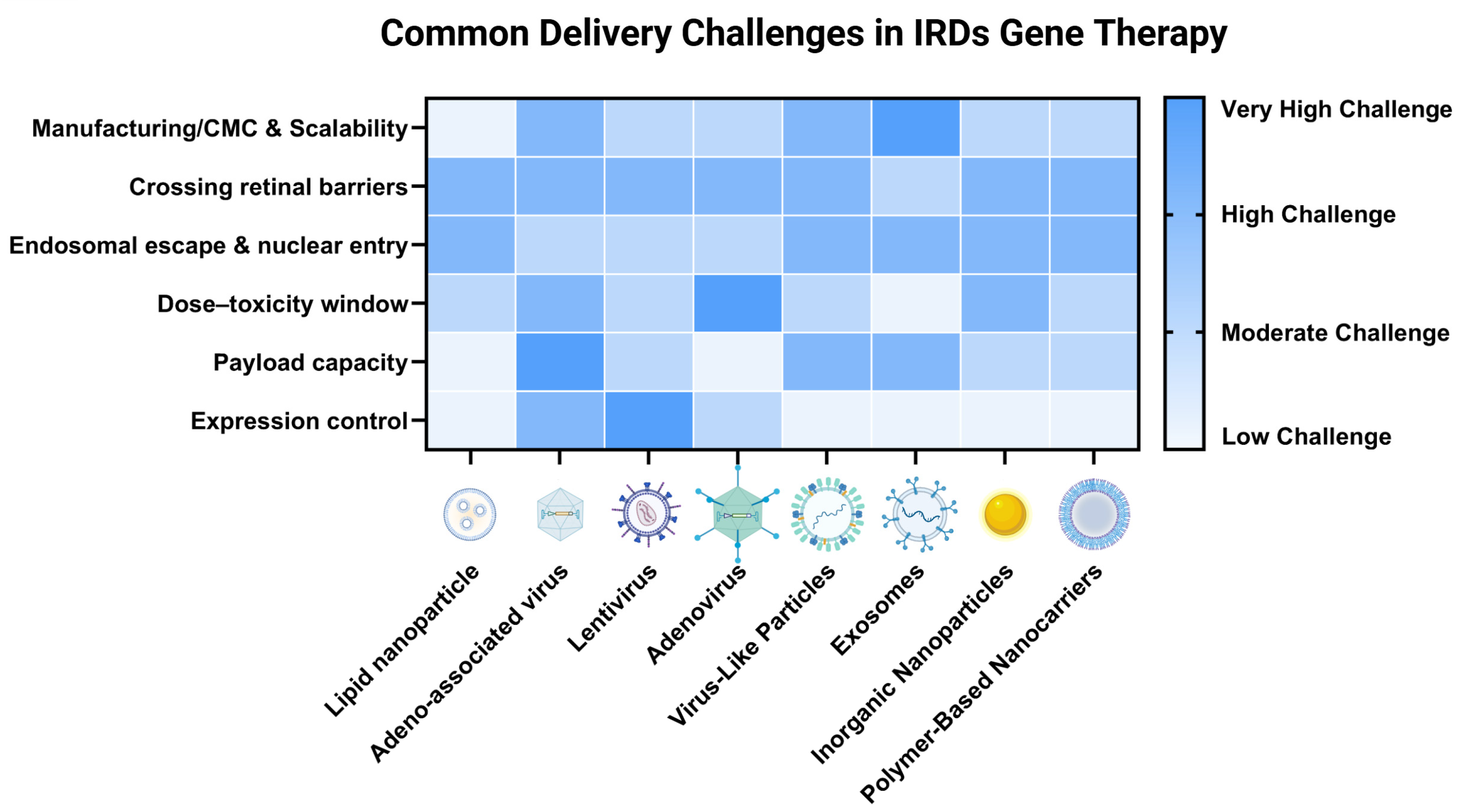
3. Delivery System Innovations
3.1. Viral Vector Delivery Methods
3.1.1. AAV
Dual-AAV
Single-AAV
3.1.2. LVs
3.1.3. AVs
3.2. Nonviral Vector Delivery Methods
3.2.1. Nonviral Nanoparticle Methods
LNPs
Inorganic Nanoparticles
VLPs
- mRNA-Packaging VLPs
- RNP-Packaged VLPs
- Advancing VLPs for IRDs Therapy Toward Clinical Translation
Other Potential Nanocarriers
3.3. Delivery Routes for Retinal Base and Prime Editing
3.3.1. Subretinal Injection
3.3.2. Intravitreal Injection
3.3.3. Suprachoroidal Injection
3.3.4. Comparative Considerations with Gene Augmentation
4. Preclinical Efficacy and Safety
4.1. Proof-of-Concept Studies in Preclinical Mouse Models
4.1.1. Base Editing
4.1.2. Prime Editing
4.2. Preclinical Models with Higher Clinical Relevance
4.3. Emerging Strategies and Key Considerations for Enhancing Efficacy and Safety
5. Preclinical-to-Clinical Translation
5.1. Functional Assessment and Clinical Endpoint Selection
5.2. Preclinical-to-Clinical Translation Barriers
Preclinical Model Limitations
6. Challenges and Future Opportunities
6.1. Common Challenges Across Gene Therapies for IRDs
6.2. Unique Translational Challenges of BE and PE
6.3. Preclinical-to-Clinical Translation Potential
6.4. The Future: AI-Driven Advancements in Base and Prime Editing for IRD Therapeutics
6.4.1. AI-Enhanced Editor Design
6.4.2. AI-Guided Evolution of AAV Capsids
6.4.3. AI-Powered Efficiency and Off-Target Prediction
6.4.4. AI-Driven Personalized Therapy
7. Conclusions
8. Method of Literature Search
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | adeno-associated virus |
| ABEs | adenine base editors |
| AI | artificial intelligence |
| AO | adaptive optics |
| ATRA | all-trans retinoic acid |
| AuNPs | gold nanoparticles |
| AYBEs | adenine transversion base editors |
| BCD | Bietti crystalline dystrophy |
| BCVA | Best-Corrected Visual Acuity |
| BE | base editing |
| CBEs | cytosine base editors |
| CGBEs | C-to-G base editors |
| CHM | choroideremia |
| CNNs | convolutional neural networks |
| CORD | cone-rod dystrophy |
| CPPs | cell-penetrating peptides |
| ddPCR | digital droplet PCR |
| DOPE | 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine |
| DOTAP | 1,2-dioleoyl-3-trimethylammonium-propane |
| DSBs | double-strand breaks |
| eCIS | extracellular contractile injection systems |
| ERG | electroretinography |
| FAF | fundus autofluorescence |
| FST | full-field stimulus testing |
| GMP | good manufacturing practice |
| gRNA | single guide RNA |
| HDR | homology directed repair |
| IRDs | inherited retinal diseases |
| ITRs | inverted terminal repeats |
| LCA | Leber congenital amaurosis |
| ILM | internal limiting membrane |
| LLVA | low-luminance visual acuity |
| LV | lentiviruses |
| mitoBEs | mitochondrial base editors |
| MLMT | multi-luminance mobility test |
| MLV | moloney murine leukemia virus |
| MP | microperimetry |
| MRDQ | Michigan retinal degeneration questionnaire |
| NES | nuclear export signals |
| NHPs | non-human primates |
| OCT | optical coherence tomography |
| OKR | optokinetic tracking response |
| OMRs | optomotor responses |
| ONL | outer nuclear layer |
| OS | outer segment |
| PAM | protospacer adjacent motif |
| PBS | primer binding site |
| PE | prime editing |
| PEG | polyethylene glycol |
| PLR | pupil light reflex |
| PLGA | poly lactic-co-glycolic acid |
| PRO | Patient-reported outcomes |
| PVC | photorhabdus virulence cassette |
| RP | retinitis pigmentosa |
| RPE | retinal pigment epithelial |
| RTT | reverse transcription template |
| SMOFs | surface-supported metal-organic frameworks |
| SNPs | silica nanoparticles |
| TALE | transcription activator-like effector |
| VEPs | visually evoked potentials |
| WES | whole-exome sequencing |
| XLRP | X-linked retinitis pigmentosa |
| XLRS | X-linked retinoschisis |
References
- RetNet: Diseases Table. Available online: https://retnet.org/disease (accessed on 14 October 2025).
- Ben-Yosef, T. Inherited Retinal Diseases. Int. J. Mol. Sci. 2022, 23, 13467. [Google Scholar] [CrossRef] [PubMed]
- Geiger, A.B.; Kennedy, J.G.; Staker, L.G.; Wensel, T.G.; Casson, R.J.; Thomas, P.Q. Shining light on CRISPR/Cas9 therapeutics for inherited retinal diseases. Prog. Retin. Eye Res. 2025, 107, 101376. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, N.; Li, J.; Zhao, M.; Huang, L. Stem cell therapy for inherited retinal diseases: A systematic review and meta-analysis. Stem Cell Res. Ther. 2023, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Wubben, T.J.; Zacks, D.N.; Besirli, C.G. Retinal neuroprotection: Current strategies and future directions. Curr. Opin. Ophthalmol. 2019, 30, 199–205. [Google Scholar] [CrossRef]
- Zhou, R.; Caspi, R.R. Ocular immune privilege. F1000 Biol. Rep. 2010, 2, 3. [Google Scholar] [CrossRef]
- The IRD Gene Therapy Pipeline. Retina Today. Available online: https://retinatoday.com/articles/2024-july-aug/the-ird-gene-therapy-pipeline (accessed on 14 October 2025).
- A Patient Registry Study for Patients Treated with Voretigene Neparvovec in US. Available online: https://www.medthority.com/clinical-trials/luxturna/a-patient-registry-study-for-patients-treated-with-voretigene-neparvovec-in-us/ (accessed on 14 October 2025).
- Real World Data from the Treatment by Voretigene Neparvovec (Luxturna) of LCA2-RPE65 Patients Across 24 Countries—Euretina Brief. 10 October 2024. Available online: https://brief.euretina.org/clinical/real-world-data-from-the-treatment-by-voretigene-neparvovec-luxturna-of-lca2-rpe65-patients-across-24-countries (accessed on 14 October 2025).
- Fischer, M.D.; Maier, R.; Suhner, A.; Stiehl, D.; Fasser, C.; Leroy, B.P. PERCEIVE study report: Real-world safety and effectiveness of voretigene neparvovec. Investig. Ophthalmol. Vis. Sci. 2022, 63, 451. [Google Scholar]
- Corsi, K.; Chellat, F.; Yahia, L.; Fernandes, J.C. Mesenchymal stem cells, MG63 and HEK293 transfection using chitosan-DNA nanoparticles. Biomaterials 2003, 24, 1255–1264. [Google Scholar] [CrossRef]
- Feldhaus, B.; Weisschuh, N.; Nasser, F.; den Hollander, A.I.; Cremers, F.P.M.; Zrenner, E.; Kohl, S.; Zobor, D. CEP290 Mutation Spectrum and Delineation of the Associated Phenotype in a Large German Cohort: A Monocentric Study. Am. J. Ophthalmol. 2020, 211, 142–150. [Google Scholar] [CrossRef]
- Ewert, K.K.; Scodeller, P.; Simón-Gracia, L.; Steffes, V.M.; Wonder, E.A.; Teesalu, T.; Safinya, C.R. Cationic Liposomes as Vectors for Nucleic Acid and Hydrophobic Drug Therapeutics. Pharmaceutics 2021, 13, 1365. [Google Scholar] [CrossRef]
- Ghosh, R.; Tabrizi, S.J. Gene suppression approaches to neurodegeneration. Alzheimers Res. Ther. 2017, 9, 82. [Google Scholar] [CrossRef]
- Germain, N.D.; Chung, W.K.; Sarmiere, P.D. RNA interference (RNAi)-based therapeutics for treatment of rare neurologic diseases. Mol. Asp. Med. 2023, 91, 101148. [Google Scholar] [CrossRef] [PubMed]
- De Silva, S.R.; Moore, A.T. Optogenetic approaches to therapy for inherited retinal degenerations. J. Physiol. 2022, 600, 4623–4632. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.L.; Stefanidakis, M.; Wilson, C.J.; Baral, R.; Barrera, L.A.; Bounoutas, G.S.; Bumcrot, D.; Chao, H.; Ciulla, D.M.; DaSilva, J.A.; et al. Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat. Med. 2019, 25, 229–233. [Google Scholar] [CrossRef]
- Pierce, E.A.; Aleman, T.S.; Jayasundera, K.T.; Ashimatey, B.S.; Kim, K.; Rashid, A.; Jaskolka, M.C.; Myers, R.L.; Lam, B.L.; Bailey, S.T.; et al. Gene Editing for CEP290-Associated Retinal Degeneration. N. Engl. J. Med. 2024, 390, 1972–1984. [Google Scholar] [CrossRef]
- Chapman, J.R.; Taylor, M.R.G.; Boulton, S.J. Playing the end game: DNA double-strand break repair pathway choice. Mol. Cell 2012, 47, 497–510. [Google Scholar] [CrossRef]
- Cox, D.B.T.; Platt, R.J.; Zhang, F. Therapeutic genome editing: Prospects and challenges. Nat. Med. 2015, 21, 121–131. [Google Scholar] [CrossRef]
- Kosicki, M.; Tomberg, K.; Bradley, A. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 2018, 36, 765–771. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Z.; Zhang, Y.; Chen, M.; Sui, T.; Lai, L.; Li, Z. Large-Fragment Deletions Induced by Cas9 Cleavage while Not in the BEs System. Mol. Ther. Nucleic Acids 2020, 21, 523–526. [Google Scholar] [CrossRef]
- Leibowitz, M.L.; Papathanasiou, S.; Doerfler, P.A.; Blaine, L.J.; Sun, L.; Yao, Y.; Zhang, C.-Z.; Weiss, M.J.; Pellman, D. Chromothripsis as an on-target consequence of CRISPR-Cas9 genome editing. Nat. Genet. 2021, 53, 895–905. [Google Scholar] [CrossRef]
- Alanis-Lobato, G.; Zohren, J.; McCarthy, A.; Fogarty, N.M.E.; Kubikova, N.; Hardman, E.; Greco, M.; Wells, D.; Turner, J.M.A.; Niakan, K.K. Frequent loss of heterozygosity in CRISPR-Cas9-edited early human embryos. Proc. Natl. Acad. Sci. USA 2021, 118, e2004832117. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef] [PubMed]
- da Costa, B.L.; Levi, S.R.; Eulau, E.; Tsai, Y.-T.; Quinn, P.M.J. Prime Editing for Inherited Retinal Diseases. Front. Genome Ed. 2021, 3, 775330. [Google Scholar] [CrossRef] [PubMed]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; He, X.; Gao, X.D.; Li, F.; Ge, S.; Yang, Z.; Fan, X. Prime editing: Current advances and therapeutic opportunities in human diseases. Sci. Bull. 2023, 68, 3278–3291. [Google Scholar] [CrossRef]
- Abbaszadeh, A.; Shahlai, A. Artificial Intelligence for CRISPR Guide RNA Design: Explainable Models and Off-Target Safety. arXiv 2025, arXiv:2508.20130. [Google Scholar] [CrossRef]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Koblan, L.W.; Liu, D.R. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 2020, 38, 824–844. [Google Scholar] [CrossRef]
- Chen, P.J.; Liu, D.R. Prime editing for precise and highly versatile genome manipulation. Nat. Rev. Genet. 2023, 24, 161–177. [Google Scholar] [CrossRef]
- Nishida, K.; Arazoe, T.; Yachie, N.; Banno, S.; Kakimoto, M.; Tabata, M.; Mochizuki, M.; Miyabe, A.; Araki, M.; Hara, K.Y.; et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 2016, 353, aaf8729. [Google Scholar] [CrossRef]
- Zhao, D.; Li, J.; Li, S.; Xin, X.; Hu, M.; Price, M.A.; Rosser, S.J.; Bi, C.; Zhang, X. Glycosylase base editors enable C-to-A and C-to-G base changes. Nat. Biotechnol. 2021, 39, 35–40. [Google Scholar] [CrossRef]
- Kurt, I.C.; Zhou, R.; Iyer, S.; Garcia, S.P.; Miller, B.R.; Langner, L.M.; Grünewald, J.; Joung, J.K. CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells. Nat. Biotechnol. 2021, 39, 41–46. [Google Scholar] [CrossRef]
- Li, C.; Zhang, R.; Meng, X.; Chen, S.; Zong, Y.; Lu, C.; Qiu, J.-L.; Chen, Y.-H.; Li, J.; Gao, C. Targeted, random mutagenesis of plant genes with dual cytosine and adenine base editors. Nat. Biotechnol. 2020, 38, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Huang, X.; Wang, X.; Gou, S.; Liang, Y.; Chen, F.; Li, N.; Ouyang, Z.; Zhang, Q.; Ge, W.; et al. ACBE, a new base editor for simultaneous C-to-T and A-to-G substitutions in mammalian systems. BMC Biol. 2020, 18, 131. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xie, J.; Zhang, Q.; Wang, X.; Gou, S.; Lin, L.; Chen, T.; Ge, W.; Zhuang, Z.; Lian, M.; et al. AGBE: A dual deaminase-mediated base editor by fusing CGBE with ABE for creating a saturated mutant population with multiple editing patterns. Nucleic Acids Res. 2022, 50, 5384–5399. [Google Scholar] [CrossRef]
- Cox, D.B.T.; Gootenberg, J.S.; Abudayyeh, O.O.; Franklin, B.; Kellner, M.J.; Joung, J.; Zhang, F. RNA editing with CRISPR-Cas13. Science 2017, 358, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Mok, B.Y.; de Moraes, M.H.; Zeng, J.; Bosch, D.E.; Kotrys, A.V.; Raguram, A.; Hsu, F.; Radey, M.C.; Peterson, S.B.; Mootha, V.K.; et al. A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature 2020, 583, 631–637. [Google Scholar] [CrossRef]
- Cho, S.-I.; Lee, S.; Mok, Y.G.; Lim, K.; Lee, J.; Lee, J.M.; Chung, E.; Kim, J.-S. Targeted A-to-G base editing in human mitochondrial DNA with programmable deaminases. Cell 2022, 185, 1764–1776.e12. [Google Scholar] [CrossRef]
- Newby, G.A.; Liu, D.R. In vivo somatic cell base editing and prime editing. Mol. Ther. 2021, 29, 3107–3124. [Google Scholar] [CrossRef]
- Doman, J.L.; Raguram, A.; Newby, G.A.; Liu, D.R. Evaluation and minimization of Cas9-independent off-target DNA editing by cytosine base editors. Nat. Biotechnol. 2020, 38, 620–628. [Google Scholar] [CrossRef]
- Rees, H.A.; Liu, D.R. Base editing: Precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 2018, 19, 770–788. [Google Scholar] [CrossRef]
- Grünewald, J.; Zhou, R.; Iyer, S.; Lareau, C.A.; Garcia, S.P.; Aryee, M.J.; Joung, J.K. CRISPR DNA base editors with reduced RNA off-target and self-editing activities. Nat. Biotechnol. 2019, 37, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Koblan, L.W.; Doman, J.L.; Wilson, C.; Levy, J.M.; Tay, T.; Newby, G.A.; Maianti, J.P.; Raguram, A.; Liu, D.R. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat. Biotechnol. 2018, 36, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, C.; Xu, W.; Yuan, S.; Song, J.; Li, L.; Zhao, J.; Yang, J. Developing high-efficiency base editors by combining optimized synergistic core components with new types of nuclear localization signal peptide. Crop J. 2020, 8, 408–417. [Google Scholar] [CrossRef]
- Hu, J.; Guo, M.; Gao, Q.; Jia, H.; He, M.; Wang, Z.; Guo, L.; Liu, G.; Gao, Q.; Zhao, K.T. QBEmax is a sequence-permuted and internally protected base editor. Nat. Biotechnol. 2025, 1–7. [Google Scholar] [CrossRef]
- Yu, G.; Kim, H.K.; Park, J.; Kwak, H.; Cheong, Y.; Kim, D.; Kim, J.; Kim, J.; Kim, H.H. Prediction of efficiencies for diverse prime editing systems in multiple cell types. Cell 2023, 186, 2256–2272.e23. [Google Scholar] [CrossRef]
- Kantor, A.; McClements, M.E.; MacLaren, R.E. CRISPR-Cas9 DNA Base-Editing and Prime-Editing. Int. J. Mol. Sci. 2020, 21, 6240. [Google Scholar] [CrossRef]
- Chen, P.J.; Hussmann, J.A.; Yan, J.; Knipping, F.; Ravisankar, P.; Chen, P.-F.; Chen, C.; Nelson, J.W.; Newby, G.A.; Sahin, M.; et al. Enhanced prime editing systems by manipulating cellular determinants of editing outcomes. Cell 2021, 184, 5635–5652.e29. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Gao, X.D.; Podracky, C.J.; Nelson, A.T.; Koblan, L.W.; Raguram, A.; Levy, J.M.; Mercer, J.A.M.; Liu, D.R. Programmable deletion, replacement, integration and inversion of large DNA sequences with twin prime editing. Nat. Biotechnol. 2022, 40, 731–740. [Google Scholar] [CrossRef]
- Adikusuma, F.; Lushington, C.; Arudkumar, J.; Godahewa, G.I.; Chey, Y.C.J.; Gierus, L.; Piltz, S.; Geiger, A.; Jain, Y.; Reti, D.; et al. Optimized nickase- and nuclease-based prime editing in human and mouse cells. Nucleic Acids Res. 2021, 49, 10785–10795. [Google Scholar] [CrossRef]
- Doman, J.L.; Pandey, S.; Neugebauer, M.E.; An, M.; Davis, J.R.; Randolph, P.B.; McElroy, A.; Gao, X.D.; Raguram, A.; Richter, M.F.; et al. Phage-assisted evolution and protein engineering yield compact, efficient prime editors. Cell 2023, 186, 3983–4002.e26. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Sharp, P.A.; Langer, R. Engineered prime editors with minimal genomic errors. Nature 2025, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Wang, S.; Cai, Y.; Li, Z.; Li, K.M.; Wei, J.; Sun, C.; Zhu, H.; Chen, K.; Gao, C. Circular RNA-mediated inverse prime editing in human cells. Nat. Commun. 2025, 16, 5057. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Gao, X.D.; Krasnow, N.A.; McElroy, A.; Tao, Y.A.; Duby, J.E.; Steinbeck, B.J.; McCreary, J.; Pierce, S.E.; Tolar, J.; et al. Efficient site-specific integration of large genes in mammalian cells via continuously evolved recombinases and prime editing. Nat. Biomed. Eng. 2025, 9, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Hew, B.E.; Gupta, S.; Sato, R.; Waller, D.F.; Stoytchev, I.; Short, J.E.; Sharek, L.; Tran, C.T.; Badran, A.H.; Owens, J.B. Directed evolution of hyperactive integrases for site specific insertion of transgenes. Nucleic Acids Res. 2024, 52, e64. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Zhao, T.; Zhao, M.; Su, M.; Chen, Y.; Huang, Z.; Wang, Y.; Zhong, C.; Hu, Z.; et al. SunTag-PE: A modular prime editing system enables versatile and efficient genome editing. Commun. Biol. 2025, 8, 452. [Google Scholar] [CrossRef]
- Kaukonen, M.; McClements, M.E.; MacLaren, R.E. CRISPR DNA Base Editing Strategies for Treating Retinitis Pigmentosa Caused by Mutations in Rhodopsin. Genes 2022, 13, 1327. [Google Scholar] [CrossRef]
- Ruan, G.-X.; Barry, E.; Yu, D.; Lukason, M.; Cheng, S.H.; Scaria, A. CRISPR/Cas9-Mediated Genome Editing as a Therapeutic Approach for Leber Congenital Amaurosis 10. Mol. Ther. 2017, 25, 331–341. [Google Scholar] [CrossRef]
- Nelson, C.E.; Hakim, C.H.; Ousterout, D.G.; Thakore, P.I.; Moreb, E.A.; Castellanos Rivera, R.M.; Madhavan, S.; Pan, X.; Ran, F.A.; Yan, W.X.; et al. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 2016, 351, 403–407. [Google Scholar] [CrossRef]
- Su, J.; She, K.; Song, L.; Jin, X.; Li, R.; Zhao, Q.; Xiao, J.; Chen, D.; Cheng, H.; Lu, F.; et al. In vivo base editing rescues photoreceptors in a mouse model of retinitis pigmentosa. Mol. Ther. Nucleic Acids 2023, 31, 596–609. [Google Scholar] [CrossRef]
- Wu, Y.; Wan, X.; Zhao, D.; Chen, X.; Wang, Y.; Tang, X.; Li, J.; Li, S.; Sun, X.; Bi, C.; et al. AAV-mediated base-editing therapy ameliorates the disease phenotypes in a mouse model of retinitis pigmentosa. Nat. Commun. 2023, 14, 4923. [Google Scholar] [CrossRef]
- Richter, M.F.; Zhao, K.T.; Eton, E.; Lapinaite, A.; Newby, G.A.; Thuronyi, B.W.; Wilson, C.; Koblan, L.W.; Zeng, J.; Bauer, D.E.; et al. Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat. Biotechnol. 2020, 38, 883–891. [Google Scholar] [CrossRef]
- Jo, D.H.; Jang, H.-K.; Cho, C.S.; Han, J.H.; Ryu, G.; Jung, Y.; Bae, S.; Kim, J.H. Visual function restoration in a mouse model of Leber congenital amaurosis via therapeutic base editing. Mol. Ther. Nucleic Acids 2023, 31, 16–27. [Google Scholar] [CrossRef]
- Choi, E.H.; Suh, S.; Foik, A.T.; Leinonen, H.; Newby, G.A.; Gao, X.D.; Banskota, S.; Hoang, T.; Du, S.W.; Dong, Z.; et al. In vivo base editing rescues cone photoreceptors in a mouse model of early-onset inherited retinal degeneration. Nat. Commun. 2022, 13, 1830. [Google Scholar] [CrossRef] [PubMed]
- Fry, L.E.; Major, L.; Salman, A.; McDermott, L.A.; Yang, J.; King, A.J.; McClements, M.E.; MacLaren, R.E. Comparison of CRISPR-Cas13b RNA base editing approaches for USH2A-associated inherited retinal degeneration. Commun. Biol. 2025, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Cideciyan, A.V.; Hood, D.C.; Huang, Y.; Banin, E.; Li, Z.Y.; Stone, E.M.; Milam, A.H.; Jacobson, S.G. Disease sequence from mutant rhodopsin allele to rod and cone photoreceptor degeneration in man. Proc. Natl. Acad. Sci. USA 1998, 95, 7103–7108. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; He, X.; Ma, L.; Gao, X.D.; Liu, P.; Shi, H.; Chai, P.; Ge, S.; Jia, R.; Liu, D.R.; et al. In vivo prime editing rescues photoreceptor degeneration in nonsense mutant retinitis pigmentosa. Nat. Commun. 2025, 16, 2394. [Google Scholar] [CrossRef]
- Prime Medicine Presents First In Vivo Proof-of-Concept Prime Editing Data Demonstrating Ability of Prime Editors to Treat Ophthalmological Diseases—Prime Medicine, Inc. Available online: https://investors.primemedicine.com/news-releases/news-release-details/prime-medicine-presents-first-vivo-proof-concept-prime-editing/ (accessed on 16 October 2025).
- Levy, J.M.; Yeh, W.-H.; Pendse, N.; Davis, J.R.; Hennessey, E.; Butcher, R.; Koblan, L.W.; Comander, J.; Liu, Q.; Liu, D.R. Cytosine and adenine base editing of the brain, liver, retina, heart and skeletal muscle of mice via adeno-associated viruses. Nat. Biomed. Eng. 2020, 4, 97–110. [Google Scholar] [CrossRef]
- Du, S.W.; Newby, G.A.; Salom, D.; Gao, F.; Menezes, C.R.; Suh, S.; Choi, E.H.; Chen, P.Z.; Liu, D.R.; Palczewski, K. In vivo photoreceptor base editing ameliorates rhodopsin-E150K autosomal-recessive retinitis pigmentosa in mice. Proc. Natl. Acad. Sci. USA 2024, 121, e2416827121. [Google Scholar] [CrossRef]
- Hu, S.; Chen, Y.; Zhou, Y.; Cao, T.; Liu, S.; Ding, C.; Xie, D.; Liang, P.; Huang, L.; Liu, H.; et al. In vivo adenine base editing ameliorates Rho-associated autosomal dominant retinitis pigmentosa. J. Genet. Genom. 2025, 52, 887–900. [Google Scholar] [CrossRef]
- Zhi, S.; Chen, Y.; Wu, G.; Wen, J.; Wu, J.; Liu, Q.; Li, Y.; Kang, R.; Hu, S.; Wang, J.; et al. Dual-AAV delivering split prime editor system for in vivo genome editing. Mol. Ther. 2022, 30, 283–294. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, W.; Zhang, S.; Feng, Y.; Xu, W.; Qi, J.; Zhang, Q.; Xu, C.; Liu, S.; Zhang, J.; et al. Vision rescue via unconstrained in vivo prime editing in degenerating neural retinas. J. Exp. Med. 2023, 220, e20220776. [Google Scholar] [CrossRef]
- She, K.; Liu, Y.; Zhao, Q.; Jin, X.; Yang, Y.; Su, J.; Li, R.; Song, L.; Xiao, J.; Yao, S.; et al. Dual-AAV split prime editor corrects the mutation and phenotype in mice with inherited retinal degeneration. Signal Transduct. Target. Ther. 2023, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, F.; Gao, G. CRISPR-Based Therapeutic Genome Editing: Strategies and In Vivo Delivery by AAV Vectors. Cell 2020, 181, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Vavassori, V.; Canarutto, D.; Jacob, A.; Castiello, M.C.; Javed, A.O.; Genovese, P. Gene Editing of Hematopoietic Stem Cells: Hopes and Hurdles Toward Clinical Translation. Front. Genome Ed. 2021, 3, 618378. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Staring, J.; Raaben, M.; Brummelkamp, T.R. Viral escape from endosomes and host detection at a glance. J. Cell Sci. 2018, 131, jcs216259. [Google Scholar] [CrossRef]
- Li, A.; Mitsunobu, H.; Yoshioka, S.; Suzuki, T.; Kondo, A.; Nishida, K. Cytosine base editing systems with minimized off-target effect and molecular size. Nat. Commun. 2022, 13, 4531. [Google Scholar] [CrossRef]
- Chen, F.; Alphonse, M.; Liu, Q. Strategies for nonviral nanoparticle-based delivery of CRISPR/Cas9 therapeutics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1609. [Google Scholar] [CrossRef]
- Wu, X.; Kriz, A.J.; Sharp, P.A. Target specificity of the CRISPR-Cas9 system. Quant. Biol. 2014, 2, 59–70. [Google Scholar] [CrossRef]
- Yin, H.; Song, C.-Q.; Suresh, S.; Wu, Q.; Walsh, S.; Rhym, L.H.; Mintzer, E.; Bolukbasi, M.F.; Zhu, L.J.; Kauffman, K.; et al. Structure-guided chemical modification of guide RNA enables potent non-viral in vivo genome editing. Nat. Biotechnol. 2017, 35, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Potter, J.; Kumar, S.; Zou, Y.; Quintanilla, R.; Sridharan, M.; Carte, J.; Chen, W.; Roark, N.; Ranganathan, S.; et al. Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection. J. Biotechnol. 2015, 208, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wagner, E.; Lächelt, U. Non-viral delivery of the CRISPR/Cas system: DNA versus RNA versus RNP. Biomater. Sci. 2022, 10, 1166–1192. [Google Scholar] [CrossRef] [PubMed]
- Ginn, S.L.; Amaya, A.K.; Alexander, I.E.; Edelstein, M.; Abedi, M.R. Gene therapy clinical trials worldwide to 2017: An update. J. Gene Med. 2018, 20, e3015. [Google Scholar] [CrossRef]
- Jony, M.J.; Joshi, A.; Dash, A.; Shukla, S. Non-Viral Delivery Systems to Transport Nucleic Acids for Inherited Retinal Disorders. Pharmaceuticals 2025, 18, 87. [Google Scholar] [CrossRef]
- Ramsay, E.; Lajunen, T.; Bhattacharya, M.; Reinisalo, M.; Rilla, K.; Kidron, H.; Terasaki, T.; Urtti, A. Selective drug delivery to the retinal cells: Biological barriers and avenues. J. Control. Release 2023, 361, 1–19. [Google Scholar] [CrossRef]
- Raguram, A.; Banskota, S.; Liu, D.R. Therapeutic in vivo delivery of gene editing agents. Cell 2022, 185, 2806–2827. [Google Scholar] [CrossRef]
- Gouveia, M.G.; Wesseler, J.P.; Ramaekers, J.; Weder, C.; Scholten, P.B.V.; Bruns, N. Polymersome-based protein drug delivery—Quo vadis? Chem. Soc. Rev. 2023, 52, 728–778. [Google Scholar] [CrossRef]
- Wagner, D.L.; Peter, L.; Schmueck-Henneresse, M. Cas9-directed immune tolerance in humans-a model to evaluate regulatory T cells in gene therapy? Gene Ther. 2021, 28, 549–559. [Google Scholar] [CrossRef]
- Drouin, L.M.; Agbandje-McKenna, M. Adeno-associated virus structural biology as a tool in vector development. Future Virol. 2013, 8, 1183–1199. [Google Scholar] [CrossRef]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L.; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, H.; Colosi, P. Effect of genome size on AAV vector packaging. Mol. Ther. 2010, 18, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Deverman, B.E.; Ravina, B.M.; Bankiewicz, K.S.; Paul, S.M.; Sah, D.W.Y. Gene therapy for neurological disorders: Progress and prospects. Nat. Rev. Drug Discov. 2018, 17, 641–659. [Google Scholar] [CrossRef]
- Goertsen, D.; Flytzanis, N.C.; Goeden, N.; Chuapoco, M.R.; Cummins, A.; Chen, Y.; Fan, Y.; Zhang, Q.; Sharma, J.; Duan, Y.; et al. AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nat. Neurosci. 2022, 25, 106–115. [Google Scholar] [CrossRef]
- Byrne, L.C.; Day, T.P.; Visel, M.; Strazzeri, J.A.; Fortuny, C.; Dalkara, D.; Merigan, W.H.; Schaffer, D.V.; Flannery, J.G. In vivo-directed evolution of adeno-associated virus in the primate retina. JCI Insight 2020, 5, e135112. [Google Scholar] [CrossRef]
- Dalkara, D.; Byrne, L.C.; Klimczak, R.R.; Visel, M.; Yin, L.; Merigan, W.H.; Flannery, J.G.; Schaffer, D.V. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 2013, 5, 189ra76. [Google Scholar] [CrossRef]
- Deverman, B.E.; Pravdo, P.L.; Simpson, B.P.; Kumar, S.R.; Chan, K.Y.; Banerjee, A.; Wu, W.-L.; Yang, B.; Huber, N.; Pasca, S.P.; et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 2016, 34, 204–209. [Google Scholar] [CrossRef]
- Planul, A.; Dalkara, D. Vectors and Gene Delivery to the Retina. Annu. Rev. Vis. Sci. 2017, 3, 121–140. [Google Scholar] [CrossRef]
- Maguire, A.M.; Simonelli, F.; Pierce, E.A.; Pugh, E.N.; Mingozzi, F.; Bennicelli, J.; Banfi, S.; Marshall, K.A.; Testa, F.; Surace, E.M.; et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008, 358, 2240–2248. [Google Scholar] [CrossRef]
- Palfi, A.; Chadderton, N.; Millington-Ward, S.; Post, I.; Humphries, P.; Kenna, P.F.; Farrar, G.J. AAV-PHP.eB transduces both the inner and outer retina with high efficacy in mice. Mol. Ther. Methods Clin. Dev. 2022, 25, 236–249. [Google Scholar] [CrossRef]
- Kellish, P.C.; Marsic, D.; Crosson, S.M.; Choudhury, S.; Scalabrino, M.L.; Strang, C.E.; Hill, J.; McCullough, K.T.; Peterson, J.J.; Fajardo, D.; et al. Intravitreal injection of a rationally designed AAV capsid library in non-human primate identifies variants with enhanced retinal transduction and neutralizing antibody evasion. Mol. Ther. 2023, 31, 3441–3456. [Google Scholar] [CrossRef]
- Hung, S.S.C.; Chrysostomou, V.; Li, F.; Lim, J.K.H.; Wang, J.-H.; Powell, J.E.; Tu, L.; Daniszewski, M.; Lo, C.; Wong, R.C.; et al. AAV-Mediated CRISPR/Cas Gene Editing of Retinal Cells In Vivo. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3470–3476. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Muhuri, M.; Li, S.; Qin, W.; Xu, G.; Luo, L.; Li, J.; Letizia, A.J.; Wang, S.K.; Chan, Y.K.; et al. Circumventing cellular immunity by miR142-mediated regulation sufficiently supports rAAV-delivered OVA expression without activating humoral immunity. JCI Insight 2019, 5, e99052. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Lee, C.M.; Hurley, A.E.; Jarrett, K.E.; De Giorgi, M.; Lu, W.; Balderrama, K.S.; Doerfler, A.M.; Deshmukh, H.; Ray, A.; et al. A Self-Deleting AAV-CRISPR System for In Vivo Genome Editing. Mol. Ther. Methods Clin. Dev. 2019, 12, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.D.; Aschenbrenner, S.; Grosse, S.; Rapti, K.; Domenger, C.; Fakhiri, J.; Mastel, M.; Börner, K.; Eils, R.; Grimm, D.; et al. Cell-specific CRISPR-Cas9 activation by microRNA-dependent expression of anti-CRISPR proteins. Nucleic Acids Res. 2019, 47, e75. [Google Scholar] [CrossRef]
- An, M.; Davis, J.R.; Levy, J.M.; Serack, F.E.; Harvey, J.W.; Brauer, P.P.; Pirtle, C.P.; Berríos, K.N.; Newby, G.A.; Yeh, W.-H.; et al. In vivo base editing extends lifespan of a humanized mouse model of prion disease. Nat. Med. 2025, 31, 1319–1328. [Google Scholar] [CrossRef]
- Chu, D.; Sullivan, C.C.; Weitzman, M.D.; Du, L.; Wolf, P.L.; Jamieson, S.W.; Thistlethwaite, P.A. Direct comparison of efficiency and stability of gene transfer into the mammalian heart using adeno-associated virus versus adenovirus vectors. J. Thorac. Cardiovasc. Surg. 2003, 126, 671–679. [Google Scholar] [CrossRef]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune Responses to Viral Gene Therapy Vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef]
- Charlesworth, C.T.; Deshpande, P.S.; Dever, D.P.; Camarena, J.; Lemgart, V.T.; Cromer, M.K.; Vakulskas, C.A.; Collingwood, M.A.; Zhang, L.; Bode, N.M.; et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat. Med. 2019, 25, 249–254. [Google Scholar] [CrossRef]
- Hanlon, K.S.; Kleinstiver, B.P.; Garcia, S.P.; Zaborowski, M.P.; Volak, A.; Spirig, S.E.; Muller, A.; Sousa, A.A.; Tsai, S.Q.; Bengtsson, N.E.; et al. High levels of AAV vector integration into CRISPR-induced DNA breaks. Nat. Commun. 2019, 10, 4439. [Google Scholar] [CrossRef]
- Farkas, M.H.; Au, E.D.; Carroll, L.; Owen, L.A. Random integration of AAV plasmid into the mouse genome following sub-retinal delivery. Investig. Ophthalmol. Vis. Sci. 2022, 63, 73–A0046. [Google Scholar]
- Greig, J.A.; Martins, K.M.; Breton, C.; Lamontagne, R.J.; Zhu, Y.; He, Z.; White, J.; Zhu, J.-X.; Chichester, J.A.; Zheng, Q.; et al. Integrated vector genomes may contribute to long-term expression in primate liver after AAV administration. Nat. Biotechnol. 2024, 42, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Monteys, A.M.; Hundley, A.A.; Ranum, P.T.; Tecedor, L.; Muehlmatt, A.; Lim, E.; Lukashev, D.; Sivasankaran, R.; Davidson, B.L. Regulated control of gene therapies by drug-induced splicing. Nature 2021, 596, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, Q.; Cheng, Y.; Wang, X.; Deng, Z.; Zhou, F.; Sun, Y. Design and Engineering of Light-Induced Base Editors Facilitating Genome Editing with Enhanced Fidelity. Adv. Sci. 2024, 11, e2305311. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Y.; Wei, Y.; Zheng, S.; Gou, S.; Chen, T.; Yang, Y.; Lan, T.; Chen, M.; Liao, Y.; et al. Eliminating predictable DNA off-target effects of cytosine base editor by using dual guiders including sgRNA and TALE. Mol. Ther. 2022, 30, 2443–2451. [Google Scholar] [CrossRef]
- Tornabene, P.; Trapani, I. Can Adeno-Associated Viral Vectors Deliver Effectively Large Genes? Hum. Gene Ther. 2020, 31, 47–56. [Google Scholar] [CrossRef]
- Lennon, C.W.; Belfort, M. Inteins. Curr. Biol. 2017, 27, R204–R206. [Google Scholar] [CrossRef]
- Aranko, A.S.; Wlodawer, A.; Iwaï, H. Nature’s recipe for splitting inteins. Protein Eng. Des. Sel. 2014, 27, 263–271. [Google Scholar] [CrossRef]
- Xu, Z.; Yue, Y.; Lai, Y.; Ye, C.; Qiu, J.; Pintel, D.J.; Duan, D. Trans-splicing adeno-associated viral vector-mediated gene therapy is limited by the accumulation of spliced mRNA but not by dual vector coinfection efficiency. Hum. Gene Ther. 2004, 15, 896–905. [Google Scholar] [CrossRef]
- Duan, D.; Yue, Y.; Engelhardt, J.F. Expanding AAV packaging capacity with trans-splicing or overlapping vectors: A quantitative comparison. Mol. Ther. 2001, 4, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Liang, S.-Q.; Liu, B.; Liu, P.; Kwan, S.-Y.; Wolfe, S.A.; Xue, W. A flexible split prime editor using truncated reverse transcriptase improves dual-AAV delivery in mouse liver. Mol. Ther. 2022, 30, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Böck, D.; Rothgangl, T.; Villiger, L.; Schmidheini, L.; Matsushita, M.; Mathis, N.; Ioannidi, E.; Rimann, N.; Grisch-Chan, H.M.; Kreutzer, S.; et al. In vivo prime editing of a metabolic liver disease in mice. Sci. Transl. Med. 2022, 14, eabl9238. [Google Scholar] [CrossRef] [PubMed]
- Tornabene, P.; Trapani, I.; Minopoli, R.; Centrulo, M.; Lupo, M.; de Simone, S.; Tiberi, P.; Dell’Aquila, F.; Marrocco, E.; Iodice, C.; et al. Intein-mediated protein trans-splicing expands adeno-associated virus transfer capacity in the retina. Sci. Transl. Med. 2019, 11, eaav4523. [Google Scholar] [CrossRef]
- Nelson, J.W.; Randolph, P.B.; Shen, S.P.; Everette, K.A.; Chen, P.J.; Anzalone, A.V.; An, M.; Newby, G.A.; Chen, J.C.; Hsu, A.; et al. Engineered pegRNAs improve prime editing efficiency. Nat. Biotechnol. 2022, 40, 402–410. [Google Scholar] [CrossRef]
- Davis, J.R.; Banskota, S.; Levy, J.M.; Newby, G.A.; Wang, X.; Anzalone, A.V.; Nelson, A.T.; Chen, P.J.; Hennes, A.D.; An, M.; et al. Efficient prime editing in mouse brain, liver and heart with dual AAVs. Nat. Biotechnol. 2024, 42, 253–264. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, S.; Jia, Y.; Shan, H.; Chen, M.; Song, Y.; Lai, L.; Li, Z. Efficient and high-fidelity base editor with expanded PAM compatibility for cytidine dinucleotide. Sci. China Life Sci. 2021, 64, 1355–1367. [Google Scholar] [CrossRef]
- Kim, E.; Koo, T.; Park, S.W.; Kim, D.; Kim, K.; Cho, H.-Y.; Song, D.W.; Lee, K.J.; Jung, M.H.; Kim, S.; et al. In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni. Nat. Commun. 2017, 8, 14500. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, S.; Zhang, C.; Gao, N.; Li, M.; Wang, D.; Wang, D.; Liu, D.; Liu, H.; Ong, S.-G.; et al. A compact Cas9 ortholog from Staphylococcus Auricularis (SauriCas9) expands the DNA targeting scope. PLoS Biol. 2020, 18, e3000686. [Google Scholar] [CrossRef]
- Davis, J.R.; Wang, X.; Witte, I.P.; Huang, T.P.; Levy, J.M.; Raguram, A.; Banskota, S.; Seidah, N.G.; Musunuru, K.; Liu, D.R. Efficient in vivo base editing via single adeno-associated viruses with size-optimized genomes encoding compact adenine base editors. Nat. Biomed. Eng. 2022, 6, 1272–1283. [Google Scholar] [CrossRef]
- Zhang, H.; Bamidele, N.; Liu, P.; Ojelabi, O.; Gao, X.D.; Rodriguez, T.; Cheng, H.; Kelly, K.; Watts, J.K.; Xie, J.; et al. Adenine Base Editing In Vivo with a Single Adeno-Associated Virus Vector. GEN Biotechnol. 2022, 1, 285–299. [Google Scholar] [CrossRef]
- Yi, Z.; Zhang, X.; Wei, X.; Li, J.; Ren, J.; Zhang, X.; Zhang, Y.; Tang, H.; Chang, X.; Yu, Y.; et al. Programmable DNA pyrimidine base editing via engineered uracil-DNA glycosylase. Nat. Commun. 2024, 15, 6397. [Google Scholar] [CrossRef]
- Shams, A.; Higgins, S.A.; Fellmann, C.; Laughlin, T.G.; Oakes, B.L.; Lew, R.; Kim, S.; Lukarska, M.; Arnold, M.; Staahl, B.T.; et al. Comprehensive deletion landscape of CRISPR-Cas9 identifies minimal RNA-guided DNA-binding modules. Nat. Commun. 2021, 12, 5664. [Google Scholar] [CrossRef]
- Kabadi, A.M.; Ousterout, D.G.; Hilton, I.B.; Gersbach, C.A. Multiplex CRISPR/Cas9-based genome engineering from a single lentiviral vector. Nucleic Acids Res. 2014, 42, e147. [Google Scholar] [CrossRef]
- Meunier, A.; Pohl, M. Lentiviral vectors for gene transfer into the spinal cord glial cells. Gene Ther. 2009, 16, 476–482. [Google Scholar] [CrossRef]
- Joglekar, A.V.; Sandoval, S. Pseudotyped Lentiviral Vectors: One Vector, Many Guises. Hum. Gene Ther. Methods 2017, 28, 291–301. [Google Scholar] [CrossRef]
- Kymäläinen, H.; Appelt, J.U.; Giordano, F.A.; Davies, A.F.; Ogilvie, C.M.; Ahmed, S.G.; Laufs, S.; Schmidt, M.; Bode, J.; Yáñez-Muñoz, R.J.; et al. Long-term episomal transgene expression from mitotically stable integration-deficient lentiviral vectors. Hum. Gene Ther. 2014, 25, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Yang, S.; Hu, X.; Yin, D.; Dai, Y.; Qian, X.; Wang, D.; Pan, X.; Hong, J.; Sun, X.; et al. Lentiviral delivery of co-packaged Cas9 mRNA and a Vegfa-targeting guide RNA prevents wet age-related macular degeneration in mice. Nat. Biomed. Eng. 2021, 5, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.G.; Dang, Y.; Abraham, S.; Ma, H.; Zhang, J.; Guo, H.; Cai, Y.; Mikkelsen, J.G.; Wu, H.; Shankar, P.; et al. Lentivirus pre-packed with Cas9 protein for safer gene editing. Gene Ther. 2016, 23, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Grüter, O.; Kostic, C.; Crippa, S.V.; Perez, M.-T.R.; Zografos, L.; Schorderet, D.F.; Munier, F.L.; Arsenijevic, Y. Lentiviral vector-mediated gene transfer in adult mouse photoreceptors is impaired by the presence of a physical barrier. Gene Ther. 2005, 12, 942–947. [Google Scholar] [CrossRef]
- Suh, S.; Choi, E.H.; Leinonen, H.; Foik, A.T.; Newby, G.A.; Yeh, W.-H.; Dong, Z.; Kiser, P.D.; Lyon, D.C.; Liu, D.R.; et al. Restoration of visual function in adult mice with an inherited retinal disease via adenine base editing. Nat. Biomed. Eng. 2021, 5, 169–178. [Google Scholar] [CrossRef]
- Milone, M.C.; O’Doherty, U. Clinical use of lentiviral vectors. Leukemia 2018, 32, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zheng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.; Zhang, X.-Y.; Reiser, J. Altering the tropism of lentiviral vectors through pseudotyping. Curr. Gene Ther. 2005, 5, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Mou, H.; Li, S.; Li, Y.; Hough, S.; Tran, K.; Li, J.; Yin, H.; Anderson, D.G.; Sontheimer, E.J.; et al. Adenovirus-Mediated Somatic Genome Editing of Pten by CRISPR/Cas9 in Mouse Liver in Spite of Cas9-Specific Immune Responses. Hum. Gene Ther. 2015, 26, 432–442. [Google Scholar] [CrossRef]
- Geutskens, S.B.; van der Eb, M.M.; Plomp, A.C.; Jonges, L.E.; Cramer, S.J.; Ensink, N.G.; Kuppen, P.J.; Hoeben, R.C. Recombinant adenoviral vectors have adjuvant activity and stimulate T cell responses against tumor cells. Gene Ther. 2000, 7, 1410–1416. [Google Scholar] [CrossRef]
- Muruve, D.A. The innate immune response to adenovirus vectors. Hum. Gene Ther. 2004, 15, 1157–1166. [Google Scholar] [CrossRef]
- Imperiale, M.J.; Kochanek, S. Adenovirus vectors: Biology, design, and production. Curr. Top. Microbiol. Immunol. 2004, 273, 335–357. [Google Scholar] [CrossRef]
- Romano, G.; Michell, P.; Pacilio, C.; Giordano, A. Latest developments in gene transfer technology: Achievements, perspectives, and controversies over therapeutic applications. Stem Cells 2000, 18, 19–39. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Trapani, I.; Puppo, A.; Auricchio, A. Vector platforms for gene therapy of inherited retinopathies. Prog. Retin. Eye Res. 2014, 43, 108–128. [Google Scholar] [CrossRef]
- Kazemian, P.; Yu, S.-Y.; Thomson, S.B.; Birkenshaw, A.; Leavitt, B.R.; Ross, C.J.D. Lipid-Nanoparticle-Based Delivery of CRISPR/Cas9 Genome-Editing Components. Mol. Pharm. 2022, 19, 1669–1686. [Google Scholar] [CrossRef]
- van der Aa, M.A.E.M.; Mastrobattista, E.; Oosting, R.S.; Hennink, W.E.; Koning, G.A.; Crommelin, D.J.A. The nuclear pore complex: The gateway to successful nonviral gene delivery. Pharm. Res. 2006, 23, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Antas, P.; Carvalho, C.; Cabral-Teixeira, J.; de Lemos, L.; Seabra, M.C. Toward low-cost gene therapy: mRNA-based therapeutics for treatment of inherited retinal diseases. Trends Mol. Med. 2024, 30, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Roldão, A.; Mellado, M.C.M.; Castilho, L.R.; Carrondo, M.J.T.; Alves, P.M. Virus-like particles in vaccine development. Expert. Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef]
- Pozzi, D.; Caracciolo, G. Looking Back, Moving Forward: Lipid Nanoparticles as a Promising Frontier in Gene Delivery. ACS Pharmacol. Transl. Sci. 2023, 6, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hu, Z.; Song, F.; Xu, Y.; Han, X. Lipid nanoparticles: Composition, formulation, and application. Mol. Ther. Methods Clin. Dev. 2025, 33, 101463. [Google Scholar] [CrossRef]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Wittrup, A.; Ai, A.; Liu, X.; Hamar, P.; Trifonova, R.; Charisse, K.; Manoharan, M.; Kirchhausen, T.; Lieberman, J. Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat. Biotechnol. 2015, 33, 870–876. [Google Scholar] [CrossRef]
- Maier, M.A.; Jayaraman, M.; Matsuda, S.; Liu, J.; Barros, S.; Querbes, W.; Tam, Y.K.; Ansell, S.M.; Kumar, V.; Qin, J.; et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 2013, 21, 1570–1578. [Google Scholar] [CrossRef]
- Jang, H.-K.; Jo, D.H.; Lee, S.-N.; Cho, C.S.; Jeong, Y.K.; Jung, Y.; Yu, J.; Kim, J.H.; Woo, J.-S.; Bae, S. High-purity production and precise editing of DNA base editing ribonucleoproteins. Sci. Adv. 2021, 7, eabg2661. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Sahu, B.; Gao, S.; Schur, R.M.; Vaidya, A.M.; Maeda, A.; Palczewski, K.; Lu, Z.-R. Targeted Multifunctional Lipid ECO Plasmid DNA Nanoparticles as Efficient Non-viral Gene Therapy for Leber’s Congenital Amaurosis. Mol. Ther. Nucleic Acids 2017, 7, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Kim, J.; Eygeris, Y.; Jozic, A.; Sahay, G. Illuminating endosomal escape of polymorphic lipid nanoparticles that boost mRNA delivery. Biomater. Sci. 2021, 9, 4289–4300. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Barrera, M.; Gautam, M.; Lokras, A.; Vlasova, K.; Foged, C.; Sahay, G. Lipid Nanoparticle-Enabled Intracellular Delivery of Prime Editors. AAPS J. 2023, 25, 65. [Google Scholar] [CrossRef]
- Herrera-Barrera, M.; Ryals, R.C.; Gautam, M.; Jozic, A.; Landry, M.; Korzun, T.; Gupta, M.; Acosta, C.; Stoddard, J.; Reynaga, R.; et al. Peptide-guided lipid nanoparticles deliver mRNA to the neural retina of rodents and nonhuman primates. Sci. Adv. 2023, 9, eadd4623. [Google Scholar] [CrossRef]
- Narsineni, L.; Chen, D.-W.; Foldvari, M. BDNF gene delivery to the retina by cell adhesion peptide-conjugated gemini nanoplexes in vivo. J. Control. Release 2023, 359, 244–256. [Google Scholar] [CrossRef]
- Rajala, A.; Wang, Y.; Zhu, Y.; Ranjo-Bishop, M.; Ma, J.-X.; Mao, C.; Rajala, R.V.S. Nanoparticle-assisted targeted delivery of eye-specific genes to eyes significantly improves the vision of blind mice in vivo. Nano Lett. 2014, 14, 5257–5263. [Google Scholar] [CrossRef]
- Bisht, R.; Mandal, A.; Jaiswal, J.K.; Rupenthal, I.D. Nanocarrier mediated retinal drug delivery: Overcoming ocular barriers to treat posterior eye diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1473. [Google Scholar] [CrossRef]
- Wang, Y.; Shahi, P.K.; Xie, R.; Zhang, H.; Abdeen, A.A.; Yodsanit, N.; Ma, Z.; Saha, K.; Pattnaik, B.R.; Gong, S. A pH-responsive silica-metal-organic framework hybrid nanoparticle for the delivery of hydrophilic drugs, nucleic acids, and CRISPR-Cas9 genome-editing machineries. J. Control. Release 2020, 324, 194–203. [Google Scholar] [CrossRef]
- Wang, Y.; Shahi, P.K.; Wang, X.; Xie, R.; Zhao, Y.; Wu, M.; Roge, S.; Pattnaik, B.R.; Gong, S. In vivo targeted delivery of nucleic acids and CRISPR genome editors enabled by GSH-responsive silica nanoparticles. J. Control. Release 2021, 336, 296–309. [Google Scholar] [CrossRef]
- Sioson, V.A.; Kim, M.; Joo, J. Challenges in delivery systems for CRISPR-based genome editing and opportunities of nanomedicine. Biomed. Eng. Lett. 2021, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Kabra, M.; Shahi, P.K.; Wang, Y.; Sinha, D.; Spillane, A.; Newby, G.A.; Saxena, S.; Tong, Y.; Chang, Y.; Abdeen, A.A.; et al. Nonviral base editing of KCNJ13 mutation preserves vision in a model of inherited retinal channelopathy. J. Clin. Investig. 2023, 133, e171356. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-J.; Yang, P.; Ban, Q.; Yang, Y.-P.; Wang, M.-L.; Chien, C.-S.; Chen, S.-J.; Sun, N.; Zhu, Y.; Liu, H.; et al. Dual Supramolecular Nanoparticle Vectors Enable CRISPR/Cas9-Mediated Knockin of Retinoschisin 1 Gene-A Potential Nonviral Therapeutic Solution for X-Linked Juvenile Retinoschisis. Adv. Sci. 2020, 7, 1903432. [Google Scholar] [CrossRef] [PubMed]
- Banskota, S.; Raguram, A.; Suh, S.; Du, S.W.; Davis, J.R.; Choi, E.H.; Wang, X.; Nielsen, S.C.; Newby, G.A.; Randolph, P.B.; et al. Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell 2022, 185, 250–265.e16. [Google Scholar] [CrossRef]
- Lyu, P.; Lu, Z.; Cho, S.-I.; Yadav, M.; Yoo, K.W.; Atala, A.; Kim, J.-S.; Lu, B. Adenine Base Editor Ribonucleoproteins Delivered by Lentivirus-like Particles Show High On-Target Base Editing and Undetectable RNA Off-Target Activities. CRISPR J. 2021, 4, 69–81. [Google Scholar] [CrossRef]
- Haldrup, J.; Andersen, S.; Labial, A.R.L.; Wolff, J.H.; Frandsen, F.P.; Skov, T.W.; Rovsing, A.B.; Nielsen, I.; Jakobsen, T.S.; Askou, A.L.; et al. Engineered lentivirus-derived nanoparticles (LVNPs) for delivery of CRISPR/Cas ribonucleoprotein complexes supporting base editing, prime editing and in vivo gene modification. Nucleic Acids Res. 2023, 51, 10059–10074. [Google Scholar] [CrossRef]
- Galla, M.; Will, E.; Kraunus, J.; Chen, L.; Baum, C. Retroviral pseudotransduction for targeted cell manipulation. Mol. Cell 2004, 16, 309–315. [Google Scholar] [CrossRef]
- Prel, A.; Caval, V.; Gayon, R.; Ravassard, P.; Duthoit, C.; Payen, E.; Maouche-Chretien, L.; Creneguy, A.; Nguyen, T.H.; Martin, N.; et al. Highly efficient in vitro and in vivo delivery of functional RNAs using new versatile MS2-chimeric retrovirus-like particles. Mol. Ther. Methods Clin. Dev. 2015, 2, 15039. [Google Scholar] [CrossRef]
- Knopp, Y.; Geis, F.K.; Heckl, D.; Horn, S.; Neumann, T.; Kuehle, J.; Meyer, J.; Fehse, B.; Baum, C.; Morgan, M.; et al. Transient Retrovirus-Based CRISPR/Cas9 All-in-One Particles for Efficient, Targeted Gene Knockout. Mol. Ther. Nucleic Acids 2018, 13, 256–274. [Google Scholar] [CrossRef]
- Lu, B.; Javidi-Parsijani, P.; Makani, V.; Mehraein-Ghomi, F.; Sarhan, W.M.; Sun, D.; Yoo, K.W.; Atala, Z.P.; Lyu, P.; Atala, A. Delivering SaCas9 mRNA by lentivirus-like bionanoparticles for transient expression and efficient genome editing. Nucleic Acids Res. 2019, 47, e44. [Google Scholar] [CrossRef]
- Wang, W.; Bartholomae, C.C.; Arens, A.; Gabriel, R.; Glimm, H.; von Kalle, C.; Schmidt, M. Genome Wide Characterization of Insertion Profiles of Integration Deficient Lentiviral Vectors Using Two-Sided Non-Restrictive and Standard LAM-PCR. Mol. Ther. 2010, 18, S135. [Google Scholar] [CrossRef]
- Segel, M.; Lash, B.; Song, J.; Ladha, A.; Liu, C.C.; Jin, X.; Mekhedov, S.L.; Macrae, R.K.; Koonin, E.V.; Zhang, F. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science 2021, 373, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Tiroille, V.; Krug, A.; Bokobza, E.; Kahi, M.; Bulcaen, M.; Ensinck, M.M.; Geurts, M.H.; Hendriks, D.; Vermeulen, F.; Larbret, F.; et al. Nanoblades allow high-level genome editing in murine and human organoids. Mol. Ther. Nucleic Acids 2023, 33, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Halsey, R.J.; Tanzer, F.L.; Meyers, A.; Pillay, S.; Lynch, A.; Shephard, E.; Williamson, A.-L.; Rybicki, E.P. Chimaeric HIV-1 subtype C Gag molecules with large in-frame C-terminal polypeptide fusions form virus-like particles. Virus Res. 2008, 133, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Gee, P.; Lung, M.S.Y.; Okuzaki, Y.; Sasakawa, N.; Iguchi, T.; Makita, Y.; Hozumi, H.; Miura, Y.; Yang, L.F.; Iwasaki, M.; et al. Extracellular nanovesicles for packaging of CRISPR-Cas9 protein and sgRNA to induce therapeutic exon skipping. Nat. Commun. 2020, 11, 1334. [Google Scholar] [CrossRef]
- Indikova, I.; Indik, S. Highly efficient ‘hit-and-run’ genome editing with unconcentrated lentivectors carrying Vpr.Prot.Cas9 protein produced from RRE-containing transcripts. Nucleic Acids Res. 2020, 48, 8178–8187. [Google Scholar] [CrossRef]
- Lyu, P.; Javidi-Parsijani, P.; Atala, A.; Lu, B. Delivering Cas9/sgRNA ribonucleoprotein (RNP) by lentiviral capsid-based bionanoparticles for efficient ‘hit-and-run’ genome editing. Nucleic Acids Res. 2019, 47, e99. [Google Scholar] [CrossRef]
- An, M.; Raguram, A.; Du, S.W.; Banskota, S.; Davis, J.R.; Newby, G.A.; Chen, P.Z.; Palczewski, K.; Liu, D.R. Engineered virus-like particles for transient delivery of prime editor ribonucleoprotein complexes in vivo. Nat. Biotechnol. 2024, 42, 1526–1537. [Google Scholar] [CrossRef]
- Rodrigues, A.; Alves, P.M.; Coroadinha, A.; Rodrigues, A.; Alves, P.M.; Coroadinha, A. Production of Retroviral and Lentiviral Gene Therapy Vectors: Challenges in the Manufacturing of Lipid Enveloped Virus. In Viral Gene Therapy; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- van der Loo, J.C.M.; Wright, J.F. Progress and challenges in viral vector manufacturing. Hum. Mol. Genet. 2016, 25, R42–R52. [Google Scholar] [CrossRef]
- Park, E.Y.; Minkner, R. A systematic approach for scalable purification of virus-like particles. Protein Expr. Purif. 2025, 228, 106664. [Google Scholar] [CrossRef]
- Li, M.; Liu, Z.; Wang, D.; Ye, J.; Shi, Z.; Pan, C.; Zhang, Q.; Ju, R.; Zheng, Y.; Liu, Y. Intraocular mRNA delivery with endogenous MmPEG10-based virus-like particles. Exp. Eye Res. 2024, 243, 109899. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, N.; Zhao, X.; Su, X.; Liu, Z. Recent advancements in polymer science for retinal diseases: New frontiers in drug delivery systems. APL Bioeng. 2025, 9, 020902. [Google Scholar] [CrossRef] [PubMed]
- Eblimit, A.; Makia, M.S.; Strayve, D.; Crane, R.; Conley, S.M.; Sinha, T.; Acharya, G.; Al-Ubaidi, M.R.; Naash, M.I. Co-Injection of Sulfotyrosine Facilitates Retinal Uptake of Hyaluronic Acid Nanospheres Following Intravitreal Injection. Pharmaceutics 2021, 13, 1510. [Google Scholar] [CrossRef] [PubMed]
- Suresh, B.; Ramakrishna, S.; Kim, H. Cell-Penetrating Peptide-Mediated Delivery of Cas9 Protein and Guide RNA for Genome Editing. Methods Mol. Biol. 2017, 1507, 81–94. [Google Scholar] [CrossRef]
- Farkhani, S.M.; Valizadeh, A.; Karami, H.; Mohammadi, S.; Sohrabi, N.; Badrzadeh, F. Cell penetrating peptides: Efficient vectors for delivery of nanoparticles, nanocarriers, therapeutic and diagnostic molecules. Peptides 2014, 57, 78–94. [Google Scholar] [CrossRef]
- Wei, G.; Wang, Y.; Huang, X.; Hou, H.; Zhou, S. Peptide-Based Nanocarriers for Cancer Therapy. Small Methods 2018, 2, 1700358. [Google Scholar] [CrossRef]
- Aslan, C.; Zolbanin, N.M.; Faraji, F.; Jafari, R. Exosomes for CRISPR-Cas9 Delivery: The Cutting Edge in Genome Editing. Mol. Biotechnol. 2024, 66, 3092–3116. [Google Scholar] [CrossRef]
- Wallen, M.; Aqil, F.; Spencer, W.; Gupta, R.C. Exosomes as an Emerging Plasmid Delivery Vehicle for Gene Therapy. Pharmaceutics 2023, 15, 1832. [Google Scholar] [CrossRef]
- Iqbal, Z.; Rehman, K.; Mahmood, A.; Shabbir, M.; Liang, Y.; Duan, L.; Zeng, H. Exosome for mRNA delivery: Strategies and therapeutic applications. J. Nanobiotechnol. 2024, 22, 395. [Google Scholar] [CrossRef]
- Yang, Q.; Li, S.; Ou, H.; Zhang, Y.; Zhu, G.; Li, S.; Lei, L. Exosome-based delivery strategies for tumor therapy: An update on modification, loading, and clinical application. J. Nanobiotechnol. 2024, 22, 41. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.B.; Kim, H.J.; Kang, S.-W.; Yoo, T.-H. Exosome-Based Drug Delivery: Translation from Bench to Clinic. Pharmaceutics 2023, 15, 2042. [Google Scholar] [CrossRef] [PubMed]
- Kreitz, J.; Friedrich, M.J.; Guru, A.; Lash, B.; Saito, M.; Macrae, R.K.; Zhang, F. Programmable protein delivery with a bacterial contractile injection system. Nature 2023, 616, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Kreitz, J.; Yang, V.; Friedrich, M.J.; Pham, J.; Macrae, R.K.; Zhang, F. Targeted delivery of diverse biomolecules with engineered bacterial nanosyringes. Nat. Biotechnol. 2025, 1–5. [Google Scholar] [CrossRef]
- Muller, A.; Sullivan, J.; Schwarzer, W.; Wang, M.; Park-Windhol, C.; Hasler, P.W.; Janeschitz-Kriegl, L.; Duman, M.; Klingler, B.; Matsell, J.; et al. High-efficiency base editing in the retina in primates and human tissues. Nat. Med. 2025, 31, 490–501. [Google Scholar] [CrossRef]
- Gautam, M.; Jozic, A.; Su, G.L.-N.; Herrera-Barrera, M.; Curtis, A.; Arrizabalaga, S.; Tschetter, W.; Ryals, R.C.; Sahay, G. Lipid nanoparticles with PEG-variant surface modifications mediate genome editing in the mouse retina. Nat. Commun. 2023, 14, 6468. [Google Scholar] [CrossRef]
- Vagni, P.; Perlini, L.E.; Chenais, N.A.L.; Marchetti, T.; Parrini, M.; Contestabile, A.; Cancedda, L.; Ghezzi, D. Gene Editing Preserves Visual Functions in a Mouse Model of Retinal Degeneration. Front. Neurosci. 2019, 13, 945. [Google Scholar] [CrossRef]
- Sisk, R.A.; Berger, T.A.; Williams, E.R.; Riemann, C.D. Intraoperative bleb behavior in subretinal gene augmentation therapy for inherited retinal diseases. Retina 2023, 43, 1763–1772. [Google Scholar] [CrossRef]
- Daruich, A.; Rateaux, M.; Batté, E.; de Vergnes, N.; Valleix, S.; Robert, M.P.; Gignac, D.B. 12-month outcomes after voretigene neparvovec gene therapy in paediatric patients with RPE65-mediated inherited retinal dystrophy. Br. J. Ophthalmol. 2025, 109, 281–285. [Google Scholar] [CrossRef]
- Wang, T.; Yu, T.; Liu, Q.; Sung, T.-C.; Higuchi, A. Lipid nanoparticle technology-mediated therapeutic gene manipulation in the eyes. Mol. Ther. Nucleic Acids 2024, 35, 102236. [Google Scholar] [CrossRef]
- Egger, D.; Heger, K.A.; Bolz, M.; Brinkmann, M.P.; Krepler, K.; Vecsei-Marlovits, P.V.; Wedrich, A.; Waldstein, S.M. Intravitreal therapy-success stories and challenges. Wien. Med. Wochenschr. 2025, 175, 162–174. [Google Scholar] [CrossRef]
- Fung, S.; Syed, Y.Y. Suprachoroidal Space Triamcinolone Acetonide: A Review in Uveitic Macular Edema. Drugs 2022, 82, 1403–1410. [Google Scholar] [CrossRef]
- Pavlásek, J.; Mašánová, C.; Bielik, P.; Murgaš, K. Voltammetrically Determined Differences in Changes Evoked by KC1 Microinjections on Catecholamine Levels in the Reticular Formation and Corpus Striatum of the Rat. Physiol. Res. 1992, 41, 191–199. [Google Scholar]
- Scruggs, B.A.; Berger, A.; Knudsen, T.; Kopp, F.N.; Hill, M.; Trncic, E.; Anderson, K.; Iezzi, R.; Marmorstein, A.D. Retinal gene therapy using epiretinal AAV-containing fibrin hydrogel implants. Sci. Adv. 2025, 11, eadv7922. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, H.; Jia, H.; Wang, H.; Huang, Z.; Tang, Y.; Wang, Z.; Hu, J.; Zhao, X.; Li, T.; et al. The clinical safety landscape for ocular AAV gene therapies: A systematic review and meta-analysis. iScience 2025, 28, 112265. [Google Scholar] [CrossRef] [PubMed]
- Moffit, J.S.; Alatsis, K.R.; Blanset, D.L.; Buss, N.; Rana, P. Nonclinical strategies and considerations to enable the redosing of gene therapies. Mol. Ther. Methods Clin. Dev. 2025, 33, 101520. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, S.; Davis, A.E.; Lo, C.-H.; Wang, Q.; Li, T.; Ning, K.; Zhang, Q.; Zhao, J.; Wang, S.; et al. Efficient Rescue of Retinal Degeneration in Pde6a Mice by Engineered Base Editing and Prime Editing. Adv. Sci. 2024, 11, e2405628. [Google Scholar] [CrossRef]
- Jang, H.; Jo, D.H.; Cho, C.S.; Shin, J.H.; Seo, J.H.; Yu, G.; Gopalappa, R.; Kim, D.; Cho, S.-R.; Kim, J.H.; et al. Application of prime editing to the correction of mutations and phenotypes in adult mice with liver and eye diseases. Nat. Biomed. Eng. 2022, 6, 181–194. [Google Scholar] [CrossRef]
- Kumar, S.; Fry, L.E.; Wang, J.H.; Martin, K.R.; Hewitt, A.W.; Chen, F.K.; Liu, G.S. RNA-targeting strategies as a platform for ocular gene therapy. Prog. Retin. Eye Res. 2023, 92, 101110. [Google Scholar] [CrossRef]
- Jo, D.H.; Bae, S.; Kim, H.H.; Kim, J.-S.; Kim, J.H. In vivo application of base and prime editing to treat inherited retinal diseases. Prog. Retin. Eye Res. 2023, 94, 101132. [Google Scholar] [CrossRef]
- Stefanidakis, M.; Maeder, M.; Bounoutas, G.; Yudkoff, C.; Chao, H.; Haskett, S.; Nguyen, D.; Samuelsson, S.J.; Giannoukos, G.; Ciulla, D.; et al. Efficient in vivo editing of CEP290 IVS26 by EDIT-101 as a novel therapeutic for treatment of Leber Congenital Amaurosis 10. Investig. Ophthalmol. Vis. Sci. 2018, 59, 385. [Google Scholar]
- Pniakowska, Z.; Dzieża, N.; Kustosik, N.; Przybylak, A.; Jurowski, P. Genetic Therapies for Retinitis Pigmentosa: Current Breakthroughs and Future Directions. J. Clin. Med. 2025, 14, 5661. [Google Scholar] [CrossRef] [PubMed]
- Mundisugih, J.; Kumar, S.; Kizana, E. Adeno-associated virus-mediated gene therapy for cardiac tachyarrhythmia: A systematic review and meta-analysis. Heart Rhythm. 2024, 21, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.D.; Ochakovski, G.A.; Beier, B.; Seitz, I.P.; Vaheb, Y.; Kortuem, C.; Reichel, F.F.L.; Kuehlewein, L.; Kahle, N.A.; Peters, T.; et al. Efficacy and Safety of Retinal Gene Therapy Using Adeno-Associated Virus Vector for Patients with Choroideremia: A Randomized Clinical Trial. JAMA Ophthalmol. 2019, 137, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Yu, S.; Li, H.; Chen, S.; Luo, J.; Wang, H.; Guan, Y.; Zhang, H.; Yin, S.; et al. Gene replacement therapy in Bietti crystalline corneoretinal dystrophy: An open-label, single-arm, exploratory trial. Signal Transduct. Target. Ther. 2024, 9, 95. [Google Scholar] [CrossRef]
- Pechnikova, N.A.; Poimenidou, M.; Iliadis, I.; Zafeiriou-Chatziefraimidou, M.; Iaremenko, A.V.; Yaremenko, T.V.; Domvri, K.; Yaremenko, A.V. Pre-Clinical and Clinical Advances in Gene Therapy of X-Linked Retinitis Pigmentosa: Hope on the Horizon. J. Clin. Med. 2025, 14, 898. [Google Scholar] [CrossRef]
- Gehrke, M.; Diedrichs-Möhring, M.; Bogedein, J.; Büning, H.; Michalakis, S.; Wildner, G. Immunogenicity of Novel AAV Capsids for Retinal Gene Therapy. Cells 2022, 11, 1881. [Google Scholar] [CrossRef]
- Mansouri, V. X-Linked Retinitis Pigmentosa Gene Therapy: Preclinical Aspects. Ophthalmol. Ther. 2023, 12, 7–34. [Google Scholar] [CrossRef]
- Banou, L.; Sarrafpour, S.; Teng, C.C.; Liu, J. Ocular Gene Therapy: An Overview of Viral Vectors, Immune Responses, and Future Directions. Yale J. Biol. Med. 2024, 97, 491–503. [Google Scholar] [CrossRef]
- Georgiou, M.; Fujinami, K.; Michaelides, M. Inherited retinal diseases: Therapeutics, clinical trials and end points—A review. Clin. Exper. Ophthalmol. 2021, 49, 270–288. [Google Scholar] [CrossRef]
- Merle, D.A.; Hertens, L.; Dimopoulos, S.; Kohl, S.; Van Haute, M.; De Baere, E.; De Bruyne, M.; Janssens, B.; Rüther, K.; Huchzermeyer, C.; et al. Short-Term Outcomes of Pediatric Patients with Mild Autosomal Recessive RPE65—Associated Retinal Dystrophy Treated with Voretigene Neparvovec. Trans. Vis. Sci. Technol. 2025, 14, 8. [Google Scholar] [CrossRef]
- Maguire, A.M.; Russell, S.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Drack, A.V.; Simonelli, F.; Leroy, B.P.; Reape, K.Z.; High, K.A.; et al. Durability of Voretigene Neparvovec for Biallelic RPE65-Mediated Inherited Retinal Disease. Ophthalmology 2021, 128, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, X.; Cui, S.; Wang, G.; Liu, Y.; Qu, G.; Jiang, L.; Liu, Y.; Li, X. Safety and Vision Outcomes Following Gene Therapy for Bietti Crystalline Dystrophy: A Nonrandomized Clinical Trial. JAMA Ophthalmol. 2025, 143, 126–133. [Google Scholar] [CrossRef] [PubMed]
- von Krusenstiern, L.; Liu, J.; Liao, E.; Gow, J.A.; Chen, G.; Ong, T.; Lotery, A.J.; Jalil, A.; Lam, B.L.; MacLaren, R.E. Changes in Retinal Sensitivity Associated with Cotoretigene Toliparvovec in X-Linked Retinitis Pigmentosa with RPGR Gene Variations. JAMA Ophthalmol. 2023, 141, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.L.; Pennesi, M.E.; Kay, C.N.; Panda, S.; Gow, J.A.; Zhao, G.; MacLaren, R.E.; XIRIUS Study Group. Assessment of Visual Function with Cotoretigene Toliparvovec in X-Linked Retinitis Pigmentosa in the Randomized XIRIUS Phase 2/3 Study. Ophthalmology 2024, 131, 1083–1093. [Google Scholar] [CrossRef]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65 -mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Jolly, J.K.; Grigg, J.R.; McKendrick, A.M.; Fujinami, K.; Cideciyan, A.V.; Thompson, D.A.; Matsumoto, C.; Asaoka, R.; Johnson, C.; Dul, M.W.; et al. ISCEV and IPS guideline for the full-field stimulus test (FST). Doc. Ophthalmol. 2024, 148, 3–14. [Google Scholar] [CrossRef]
- Tuohy, G.P.; Megaw, R. A Systematic Review and Meta-Analyses of Interventional Clinical Trial Studies for Gene Therapies for the Inherited Retinal Degenerations (IRDs). Biomolecules 2021, 11, 760. [Google Scholar] [CrossRef]
- Yamamoto, M.; Matsuyama, T.; Maeda, T.; Takagi, S.; Motozawa, N.; Sakai, D.; Hirami, Y.; Maeda, A.; Kurimoto, Y.; Takahashi, M.; et al. Detailed Evaluation of Chromatic Pupillometry and Full-Field Stimulus Testing to Assess Ultralow Vision in Retinitis Pigmentosa. Ophthalmol. Sci. 2023, 3, 100328. [Google Scholar] [CrossRef]
- Rukmini, A.V.; Milea, D.; Gooley, J.J. Chromatic Pupillometry Methods for Assessing Photoreceptor Health in Retinal and Optic Nerve Diseases. Front. Neurol. 2019, 10, 76. [Google Scholar] [CrossRef]
- Brar, A.S.; Parameswarappa, D.C.; Takkar, B.; Narayanan, R.; Jalali, S.; Mandal, S.; Fujinami, K.; Padhy, S.K. Gene Therapy for Inherited Retinal Diseases: From Laboratory Bench to Patient Bedside and Beyond. Ophthalmol. Ther. 2024, 13, 21–50. [Google Scholar] [CrossRef] [PubMed]
- Sobh, M.; Lagali, P.S.; Ghiasi, M.; Montroy, J.; Dollin, M.; Hurley, B.; Leonard, B.C.; Dimopoulos, I.; Lafreniere, M.; Fergusson, D.A.; et al. Safety and Efficacy of Adeno-Associated Viral Gene Therapy in Patients with Retinal Degeneration: A Systematic Review and Meta-Analysis. Trans. Vis. Sci. Technol. 2023, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Cehajic-Kapetanovic, J.; Xue, K.; de la Camara, C.M.-F.; Nanda, A.; Davies, A.; Wood, L.J.; Salvetti, A.P.; Fischer, M.D.; Aylward, J.W.; Barnard, A.R.; et al. Retinal gene therapy in X-linked retinitis pigmentosa caused by mutations in RPGR: Results at 6 months in a first in human clinical trial. Nat. Med. 2020, 26, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Igoe, J.M.; Lam, B.L.; Gregori, N.Z. Update on Clinical Trial Endpoints in Gene Therapy Trials for Inherited Retinal Diseases. J. Clin. Med. 2024, 13, 5512. [Google Scholar] [CrossRef]
- Audo, I.; Patalano, F.; Naujoks, C.; Spera, C.; Fischer, M.D.; Green, J.; Kay, C.; Durham, T.; Williamson, N.; Bradley, H.; et al. Development of Novel Patient-Reported Outcome (PRO) and Observer-Reported Outcome (ObsRO) Instruments in Retinitis Pigmentosa (RP) and Leber Congenital Amaurosis (LCA): ViSIO-PRO and ViSIO-ObsRO. Ophthalmol. Ther. 2023, 12, 2069–2085. [Google Scholar] [CrossRef]
- Jayasundera, K.T.; Abuzaitoun, R.O.; Popova, L.; Abalem, M.F.; Andrews, C.A.; Lacy, G.D.; Fresco, D.M.; Musch, D.C. Construct Validity of Inherited Retinal Disease-Specific Patient-Reported Outcome Measures. Am. J. Ophthalmol. 2023, 248, 116–126. [Google Scholar] [CrossRef]
- Gouveia, N.; Karuntu, J.; Almushattat, H.; Silva, R.; Boon, C.; Marques, J.P. Retinitis Pigmentosa GTPase regulator-Associated Retinal Degeneration: Integrating Patient-Reported Outcomes, Genetic, and Structural Biomarkers. Ophthalmol. Sci. 2026, 6, 100915. [Google Scholar] [CrossRef]
- Bucher, K.; Rodríguez-Bocanegra, E.; Dauletbekov, D.; Fischer, M.D. Immune responses to retinal gene therapy using adeno-associated viral vectors—Implications for treatment success and safety. Prog. Retin. Eye Res. 2021, 83, 100915. [Google Scholar] [CrossRef]
- Testa, F.; Bacci, G.; Falsini, B.; Iarossi, G.; Melillo, P.; Mucciolo, D.P.; Murro, V.; Salvetti, A.P.; Sodi, A.; Staurenghi, G.; et al. Voretigene neparvovec for inherited retinal dystrophy due to RPE65 mutations: A scoping review of eligibility and treatment challenges from clinical trials to real practice. Eye 2024, 38, 2504–2515. [Google Scholar] [CrossRef]
- Marie, M.; Churet, L.; Gautron, A.-S.; Farjo, R.; Mizuyoshi, K.; Stevenson, V.; Khabou, H.; Léveillard, T.; Sahel, J.-A.; Lorget, F. Preclinical safety and biodistribution of SPVN06, a novel gene- and mutation-independent gene therapy for rod-cone dystrophies. Gene Ther. 2025, 1–14. [Google Scholar] [CrossRef]
- Zhao, Q.; Wei, L.; Chen, Y. From bench to bedside: Developing CRISPR/Cas-based therapy for ocular diseases. Pharmacol. Res. 2025, 213, 107638. [Google Scholar] [CrossRef] [PubMed]
- Daich Varela, M.; Cabral De Guimaraes, T.A.; Georgiou, M.; Michaelides, M. Leber congenital amaurosis/early-onset severe retinal dystrophy: Current management and clinical trials. Br. J. Ophthalmol. 2022, 106, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Chen, S.; Li, W.; Zhang, J.; Qu, B.; Qiao, J.; Meng, X.; Yu, S.; Liu, X.; Xu, B.; et al. Unravelling CYP4V2: Clinical features, genetic insights, pathogenic mechanisms and therapeutic strategies in Bietti crystalline corneoretinal dystrophy. Prog. Retin. Eye Res. 2025, 107, 101377. [Google Scholar] [CrossRef] [PubMed]
- Pierce, E.A.; Ashimatey, B.S.; Jayasundera, T.; Hoyng, C.; Lam, B.L.; Lorenz, B.; Kim, K.; Rashid, A.; Myers, R.; Pennesi, M.E. Twelve-month Natural History Study of Centrosomal Protein 290 (CEP290)-associated Inherited Retinal Degeneration. Ophthalmol. Sci. 2024, 4, 100483. [Google Scholar] [CrossRef]
- Kharisova, C.B.; Kitaeva, K.V.; Solovyeva, V.V.; Sufianov, A.A.; Sufianova, G.Z.; Akhmetshin, R.F.; Bulgar, S.N.; Rizvanov, A.A. Looking to the Future of Viral Vectors in Ocular Gene Therapy: Clinical Review. Biomedicines 2025, 13, 365. [Google Scholar] [CrossRef]
- Kashiwakura, Y.; Baatartsogt, N.; Yamazaki, S.; Nagao, A.; Amano, K.; Suzuki, N.; Matsushita, T.; Sawada, A.; Higasa, S.; Yamasaki, N.; et al. The seroprevalence of neutralizing antibodies against the adeno-associated virus capsids in Japanese hemophiliacs. Mol. Ther. Methods Clin. Dev. 2022, 27, 404–414. [Google Scholar] [CrossRef]
- Padhy, S.K.; Takkar, B.; Narayanan, R.; Venkatesh, P.; Jalali, S. Voretigene Neparvovec and Gene Therapy for Leber’s Congenital Amaurosis: Review of Evidence to Date. Appl. Clin. Genet. 2020, 13, 179–208. [Google Scholar] [CrossRef]
- Shamshad, A.; Kang, C.; Jenny, L.A.; Persad-Paisley, E.M.; Tsang, S.H. Translatability barriers between preclinical and clinical trials of AAV gene therapy in inherited retinal diseases. Vis. Res. 2023, 210, 108258. [Google Scholar] [CrossRef]
- Fischer, M.D.; McClements, M.E.; Martinez-Fernandez de la Camara, C.; Bellingrath, J.-S.; Dauletbekov, D.; Ramsden, S.C.; Hickey, D.G.; Barnard, A.R.; MacLaren, R.E. Codon-Optimized RPGR Improves Stability and Efficacy of AAV8 Gene Therapy in Two Mouse Models of X-Linked Retinitis Pigmentosa. Mol. Ther. 2017, 25, 1854–1865. [Google Scholar] [CrossRef]
- Shi, L.F.; Hall, A.J.; Thompson, D.A. Full-field stimulus threshold testing: A scoping review of current practice. Eye 2024, 38, 33–53. [Google Scholar] [CrossRef]
- Roth-Cline, M.; Nelson, R.M. Parental Permission and Child Assent in Research on Children. Yale J. Biol. Med. 2013, 86, 291–301. [Google Scholar] [PubMed]
- Song, C.; Dufour, V.L.; Cideciyan, A.V.; Ye, G.-J.; Swider, M.; Newmark, J.A.; Timmers, A.M.; Robinson, P.M.; Knop, D.R.; Chulay, J.D.; et al. Dose Range Finding Studies with Two RPGR Transgenes in a Canine Model of X-Linked Retinitis Pigmentosa Treated with Subretinal Gene Therapy. Hum. Gene Ther. 2020, 31, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Alipanahi, R.; Safari, L.; Khanteymoori, A. DTMP-prime: A deep transformer-based model for predicting prime editing efficiency and PegRNA activity. Mol. Ther. Nucleic Acids 2024, 35, 102370. [Google Scholar] [CrossRef] [PubMed]
- Mathis, N.; Marquart, K.F.; Allam, A.; Krauthammer, M.; Schwank, G. Systematic pegRNA design with PRIDICT2.0 and ePRIDICT for efficient prime editing. Nat. Protoc. 2025, 1–21. [Google Scholar] [CrossRef]
- Park, J.; Yu, G.; Seo, S.-Y.; Yang, J.; Kim, H.H. SynDesign: Web-based prime editing guide RNA design and evaluation tool for saturation genome editing. Nucleic Acids Res. 2024, 52, W121–W125. [Google Scholar] [CrossRef]
- Zhang, W.; Petri, K.; Ma, J.; Lee, H.; Tsai, C.-L.; Joung, J.K.; Yeh, J.-R.J. Enhancing CRISPR prime editing by reducing misfolded pegRNA interactions. eLife 2024, 12, RP90948. [Google Scholar] [CrossRef]
- Huang, J.; Lin, Q.; Fei, H.; He, Z.; Xu, H.; Li, Y.; Qu, K.; Han, P.; Gao, Q.; Li, B.; et al. Discovery of deaminase functions by structure-based protein clustering. Cell 2023, 186, 3182–3195.e14. [Google Scholar] [CrossRef]
- Ogden, P.J.; Kelsic, E.D.; Sinai, S.; Church, G.M. Comprehensive AAV capsid fitness landscape reveals a viral gene and enables machine-guided design. Science 2019, 366, 1139–1143. [Google Scholar] [CrossRef]
- Marques, A.D.; Kummer, M.; Kondratov, O.; Banerjee, A.; Moskalenko, O.; Zolotukhin, S. Applying machine learning to predict viral assembly for adeno-associated virus capsid libraries. Mol. Ther. Methods Clin. Dev. 2021, 20, 276–286. [Google Scholar] [CrossRef]
- Bryant, D.H.; Bashir, A.; Sinai, S.; Jain, N.K.; Ogden, P.J.; Riley, P.F.; Church, G.M.; Colwell, L.J.; Kelsic, E.D. Deep diversification of an AAV capsid protein by machine learning. Nat. Biotechnol. 2021, 39, 691–696. [Google Scholar] [CrossRef]
- Ding, X.; Chen, X.; Sullivan, E.E.; Shay, T.F.; Gradinaru, V. Fast, accurate ranking of engineered proteins by target-binding propensity using structure modeling. Mol. Ther. 2024, 32, 1687–1700. [Google Scholar] [CrossRef]
- Eid, F.-E.; Chen, A.T.; Chan, K.Y.; Huang, Q.; Zheng, Q.; Tobey, I.G.; Pacouret, S.; Brauer, P.P.; Keyes, C.; Powell, M.; et al. Systematic multi-trait AAV capsid engineering for efficient gene delivery. Nat. Commun. 2024, 15, 6602. [Google Scholar] [CrossRef]
- Liu, F.; Huang, S.; Hu, J.; Chen, X.; Song, Z.; Dong, J.; Liu, Y.; Huang, X.; Wang, S.; Wang, X.; et al. Design of prime-editing guide RNAs with deep transfer learning. Nat. Mach. Intell. 2023, 5, 1261–1274. [Google Scholar] [CrossRef]
- Saraswat, P.; Ranjan, R. Unlocking the potential of CRISPR tools and databases for precision genome editing. Front. Plant Sci. 2025, 16, 1563711. [Google Scholar] [CrossRef] [PubMed]
- Sanvicente-García, M.; García-Valiente, A.; Jouide, S.; Jaraba-Wallace, J.; Bautista, E.; Escobosa, M.; Sánchez-Mejías, A.; Güell, M. CRISPR-Analytics (CRISPR-A): A platform for precise analytics and simulations for gene editing. PLoS Comput. Biol. 2023, 19, e1011137. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.R.; Snoek, J.; Rinn, J.L. Basset: Learning the regulatory code of the accessible genome with deep convolutional neural networks. Genome Res. 2016, 26, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Wu, L.; Li, S.; Zheng, J.; Li, N.; Xiao, X.; Zhang, H.; Fei, T.; Xie, L.; Zuo, Z.; et al. Deep learning models incorporating endogenous factors beyond DNA sequences improve the prediction accuracy of base editing outcomes. Cell Discov. 2024, 10, 20. [Google Scholar] [CrossRef]
- Pasupuleti, M.K. AI-Driven Bioinformatics: Transforming Genomics, Gene Editing, and Personalized Medicine. Int. J. Acad. Ind. Res. Innov. 2025, 5, 42–53. [Google Scholar] [CrossRef]
- Pushkaran, A.C.; Arabi, A.A. From understanding diseases to drug design: Can artificial intelligence bridge the gap? Artif. Intell. Rev. 2024, 57, 86. [Google Scholar] [CrossRef]

| Base Editing | Prime Editing | |
|---|---|---|
| Components | nCas9/dCas9-DNA deaminases (cytidine/adenosine deaminases) | nCas9–reverse transcriptase + pegRNA |
| Editing scope | Specific base conversions (C→T, T→C, A→G, G→A) | All 12 single-base substitutions; small insertions and deletions |
| Advantages | High editing efficiency (generally increased efficiency in non-dividing cells); simple design | Broad editable scope; high precision (minimal bystander editing); less constrained by PAM |
| Disadvantages | Narrow editing scope; fixed editing window with limited flexibility DNA/RNA off-targets; bystander editing | Lower editing efficiency in photoreceptors due to payload size; complex design; byproducts (unedited/partially edited); large payload, challenging delivery |
| AAV | LV | AdV | LNPs | Polymer-Based Nanocarriers | Inorganic Nanoparticles | Exosomes | VLPs | Electroporation | |
|---|---|---|---|---|---|---|---|---|---|
| Payload Capacity | ~4.7 kb | ~10 kb | ~30 kb | flexible | flexible | flexible | flexible | flexible | flexible |
| Cargo Type | DNA | DNA | DNA | RNA | RNA, RNP, DNA | RNP | RNP, RNA | RNA, RNP | RNP, RNA, DNA |
| Key Advantages | Non-integrating; | Efficient delivery to non-dividing cells | Non-integrating; large payload; strong expression | Biocompatibility; easy to engineer; Mature manufacturing and scale-up; scalability for GMP production | Customizable targeting | Customizable functionality | Low immunogenicity; barrier-penetrating ability | Natural viral properties (efficient intracellular delivery, endosomal escape, tissue targeting via envelope glycoproteins/ligands); transient expression window and low off-target risk; scalability for GMP production | High efficiency; strong temporal control; easy to use |
| Key Limitations | Pre-existing neutralizing antibodies common and redosing challenges; payload size limited; off-target risks | narrower cell targeting than AAV; integration risks | Strong immunogenicity and redosing challenges; Complex manufacturing | non-specific targeting, toxicity control; Poor stability | low transfection efficiency; toxicity control | Clearance issues (low biodegradability and accumulation risk); toxicity control; non-specific targeting; low transfection efficiency | Variable loading efficiency; purification and potency consistency challenges | Stability/consistency challenges; complex manufacturing | Impacts cell viability; not feasible in vivo |
| Study ID | Disease | Mutation | Editor | Delivery | Animal Models | Average Editing Efficiency | Structural/Functional Improvement | Safety |
|---|---|---|---|---|---|---|---|---|
| Muller et al., 2025 [211] | Stargardt disease | ABCA4 c.5882G > A | ABE8.5m | Dual AAV9-PHP.eB, subretinal injection (mice); dual AAV5, subretinal injection (non-human primates, NHPs) | Mouse: ABCA4hu1961E/ms1961G NHP: cynomolgus macaques | AAV5-v2-SABE1,NHPs: 75% of cones, 87% of RPE cells | Not Applicable | No significant off-target detected in human explants; editing confined to injected eye; bystander editing: A8 (silent edit) |
| Hu et al., 2025 [75] | adRP | RHO p.Q344ter, p.T17M | ABE7.10 ABE8e | Dual AAV8, subretinal injection | RHOQ344ter/+ and RHOT17M/+ knock-in mice | ABE8e, RhoQ344ter/+ mice, P15–P21: 15.29% at 2 months; 5.56% at P300 P29–P35: 3.18% at 2 months; 3.00% at P300 | P15–21: preserved ONL thickness and scotopic and photopic ERG responses P29–35: no significant improvement | Off-target efficiency (ABE8e, RhoQ344ter/+): P15–21, 6.32%, P29-35, 1.72% |
| Kabra et al., 2023 [178] | LCA16 | KCNJ13 c.158G > A | ABE8e | GSH-responsive silica nanocapsules (SNC), subretinal injection | KCNJ13W53X/+ knock-in mice; KCNJ13W53X/+ΔR mice (for functional readouts) | 16.8% on-target | ERG c-wave recovered; OCT shows preserved retinal structure | No significant off-target, indels low; bystanders rare and mostly silent/low-frequency missense; SNC did not upregulate CD8/Iba1 versus viral vectors |
| Wu et al., 2023 [65] | arRP | PDE6B c.1678 C > T | SpRY-ABE8e | Dual AAV5, subretinal injection | rd10 mice | cDNA: target-only 34.07% | ONL, rod OS length, scotopic/photopic ERG responses, cone structure partially preserved; water-maze success reached 100% | No significant off-target, bystander-only 1.27%; indels 0.72% histology unremarkable aside from injection artifacts (excluded) |
| Choi et al., 2022 [68] | LCA2 | RPE65 c.130C > T | NG-ABEmax | Single-vector lentivirus, subretinal injection. | rd12 mice; cone-dominant rd12Gnat1−/− mice (for functional readouts) | cDNA: 54%; precise correction (no bystanders) 27% | Scotopic /photopic ERG responses, cone function and numbers preserved to 6 months VEPs present but attenuated/delayed; single-neuron responses increased. | No significant off-target; cDNA bystanders mainly at A8 (21%) and A3 (8%); indels low histology unremarkable; |
| Suh et al., 2020 [146] | LCA2 | RPE65 c.130C > T | ABEmax | Single-vector lentivirus, subretinal injection. | rd12 mice | gDNA target-only: sgRNA-A5 15.95%; sgRNA-A6 5.22%; | Visual cycle (11-cis-retinal, all-trans-retinyl esters) partially reconstructed; function of the retina and entire visual pathway (ERG, OMR, VEP, V1 neurons) partially recovered | No significant off-target; indels low (A5 0.48%; A6 0.16%); bystanders mainly at T5 for A5; |
| Levy et al., 2020 [73] | Not a disease-correction study for the eye | None (test locus DNMT1 to quantify in vivo efficiency) | CBE3.9max ABEmax | Dual AAV-PHP.B/Anc80, subretinal injection | RHO-Cre; Ai9 mice | PHP.B + CBE: 48%in transduced rods; Anc80 + ABE: 37% in transduced rods | Not Applicable | ABE: very low indels in retinal cells. CBE: substantial indels in retina; base edit/indel ratio ~2:1 to 1:1; indels up to 34%. * no retina-specific genome-wide off-target profiling reported |
| Liu et al., 2024 [223] | arRP | PDE6A c.2009A > G | CBE4max-NG; PEmax | In vivo plasmid electroporation at P0 (subretinal + pulses); dual AAV2.NN, subretinal injection | PDE6A mice | CBE, electrop: 23.8% PE, electrop: 21.5% Dual AAV-PE: 9.4% | OS, ONL thickness, ERG, OKR improved; P14 injection effective but weaker than P0–P3 injection | CBE, electrop: bystander 13.6%, indels 5.5%; PE, electrop: bystander 0%, indels: 0.9%; Dual AAV-PE: bystander 0%, indels 0.4% No significant off-target (CBE, AAV-PE); Editing confined to injected eye |
| Du et al., 2024 [74] | arRP | RHO c.448G > A | SpCas9-ABEmax | Dual AAV2/8, subretinal injection | Homozygous RHO-E150K mice | P21 injection, cDNA: total 18.2%, precise 11.9% | P21 injection: no ERG rescue P15 injection: ERG improved, ONL preserved (in thickest treated regions); rhodopsin expression restored | No significant off-target; No detectable increase in bystander editing or indels at on-/off-target sites |
| Fu et al., 2025 [71] | arRP | PDE6B c.1041 C > A | PE2 (RTΔRnH, epegRNA) | Dual AAV2, subretinal injection; engineered VLPs | rd1 mice | AAV2, gDNA: 26.47% | AAV2: ONL, OS preserved; ERG, PLR improved; better performance in light–dark and visual cliff tests; benefits persisted to 8 weeks. (eVLPs-PE preserved ONL but was inferior to AAV-PE) | No significant off-target; editing confined to injected eye |
| She et al., 2023 [78] | LCA2 | RPE65 c.130C > T | PE3 | Dual AAV8, subretinal injection | rd12 mice | Precise editing (gDNA): 3.6% (low dose), 11.4% (high dose) | Retinal structure, ERG responses preserved better performance in visual cliff tests | No detectable off-target substitutions or indels above untreated background very high dose (3 × 1010 GC/eye) reduced ERG amplitudes |
| Qin et al., 2023 [77] | arRP | PDE6B c.1678 C > T | PESpRY: PE2 fused to SpRY | Dual AAV8, subretinal injection | rd10 mice | 76.34% in transduced cells of retinas 40.86% in total cells of retinas | Substantial rod/cone preservation; stronger rescue with P14 vs. P21 dosing; significant ERG recovery; improved OMR, avoidance tests, and water-maze; benefits persisted to at least P240 | No significant off-target editing above untreated background; indels rare (0.14%); histology unremarkable |
| Jang et al., 2022 [224] | LCA2 | RPE65 c.130C > T | PE2 | Dual AAV2, subretinal injection | rd12 mice | 7.7% (estimated 33% within transduced regions) | ERG, optomotor thresholds improved | No detectable off-target edits at 20 candidate sites, no detectable bystander, substitutions or indels at/near on-target |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, Y.; Li, J.; Li, X.; Li, T. Base and Prime Editing for Inherited Retinal Diseases: Delivery Platforms, Safety, Efficacy, and Translational Perspectives. Pharmaceutics 2025, 17, 1405. https://doi.org/10.3390/pharmaceutics17111405
Zhang H, Li Y, Li J, Li X, Li T. Base and Prime Editing for Inherited Retinal Diseases: Delivery Platforms, Safety, Efficacy, and Translational Perspectives. Pharmaceutics. 2025; 17(11):1405. https://doi.org/10.3390/pharmaceutics17111405
Chicago/Turabian StyleZhang, Haoliang, Yuxuan Li, Jiajie Li, Xiaosa Li, and Tong Li. 2025. "Base and Prime Editing for Inherited Retinal Diseases: Delivery Platforms, Safety, Efficacy, and Translational Perspectives" Pharmaceutics 17, no. 11: 1405. https://doi.org/10.3390/pharmaceutics17111405
APA StyleZhang, H., Li, Y., Li, J., Li, X., & Li, T. (2025). Base and Prime Editing for Inherited Retinal Diseases: Delivery Platforms, Safety, Efficacy, and Translational Perspectives. Pharmaceutics, 17(11), 1405. https://doi.org/10.3390/pharmaceutics17111405









