New Kinetic Investigations to Better Understand the Mechanism of Polymorphic Transformations of Pharmaceutical Materials Induced by Milling
Abstract
1. Introduction
2. Materials and Methods
2.1. Milling
2.2. Laboratory X-Ray Diffraction
2.3. Synchrotron Experiments
2.4. DSC Experiments
2.5. Materials
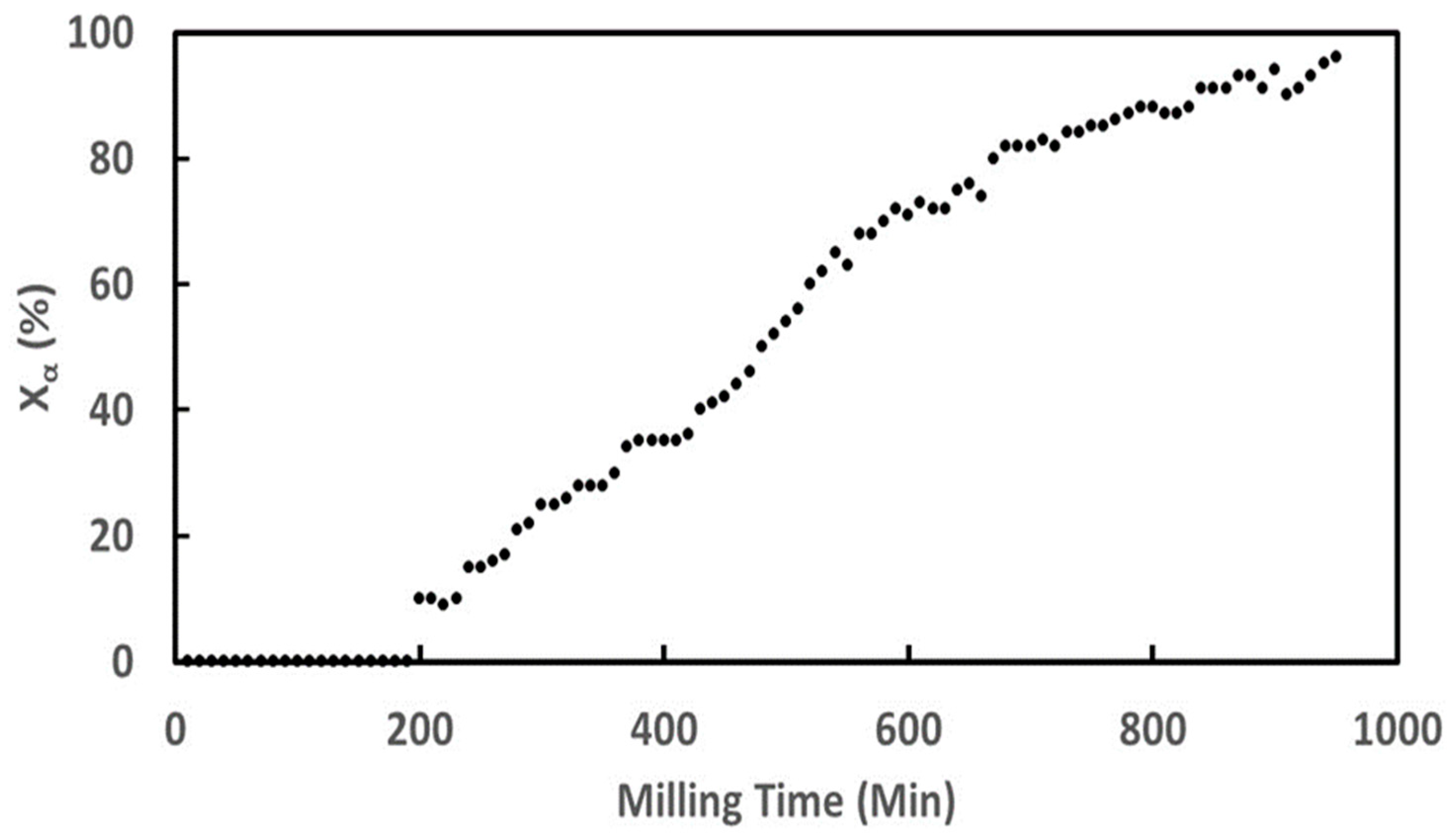
3. Results
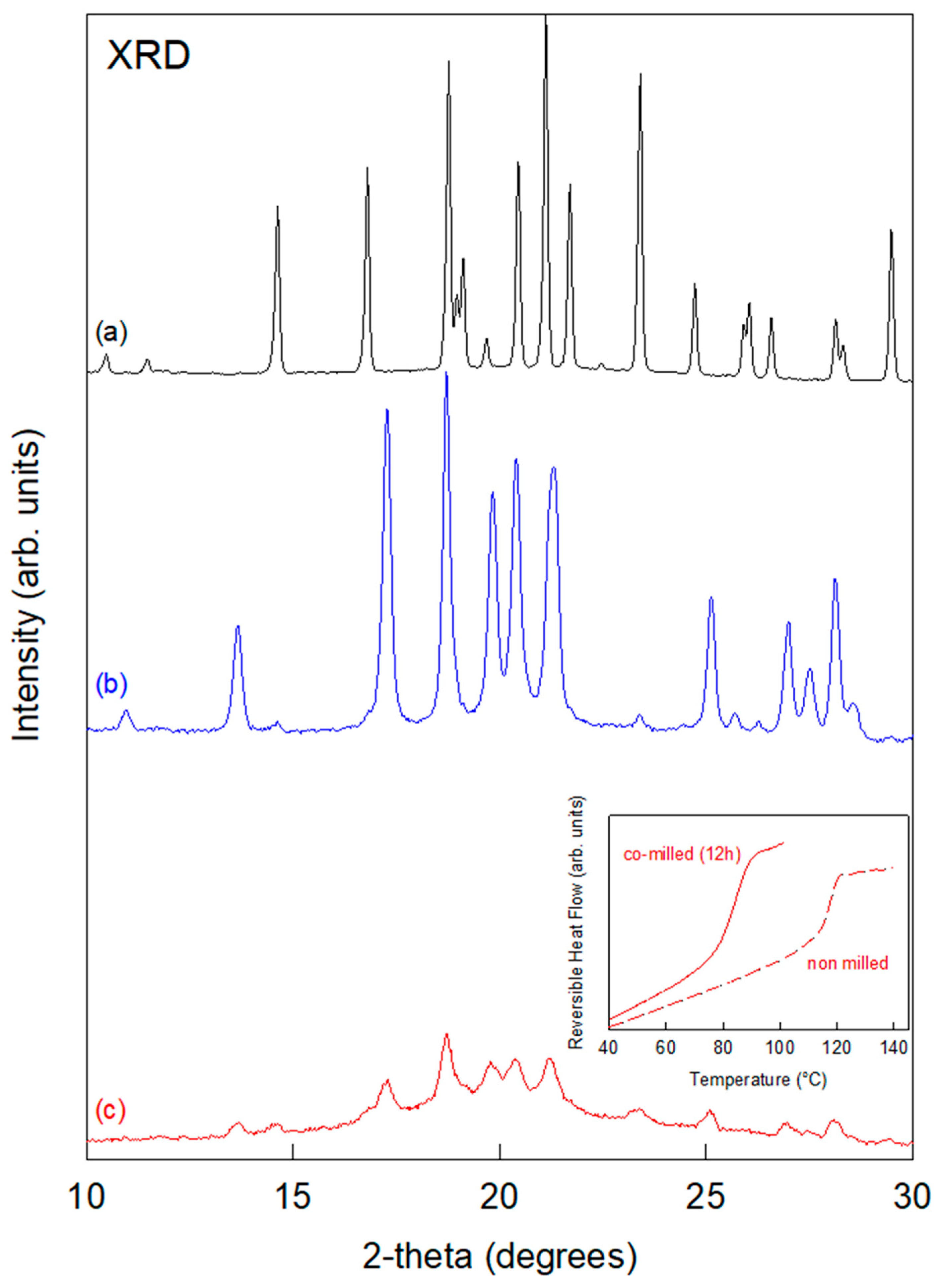
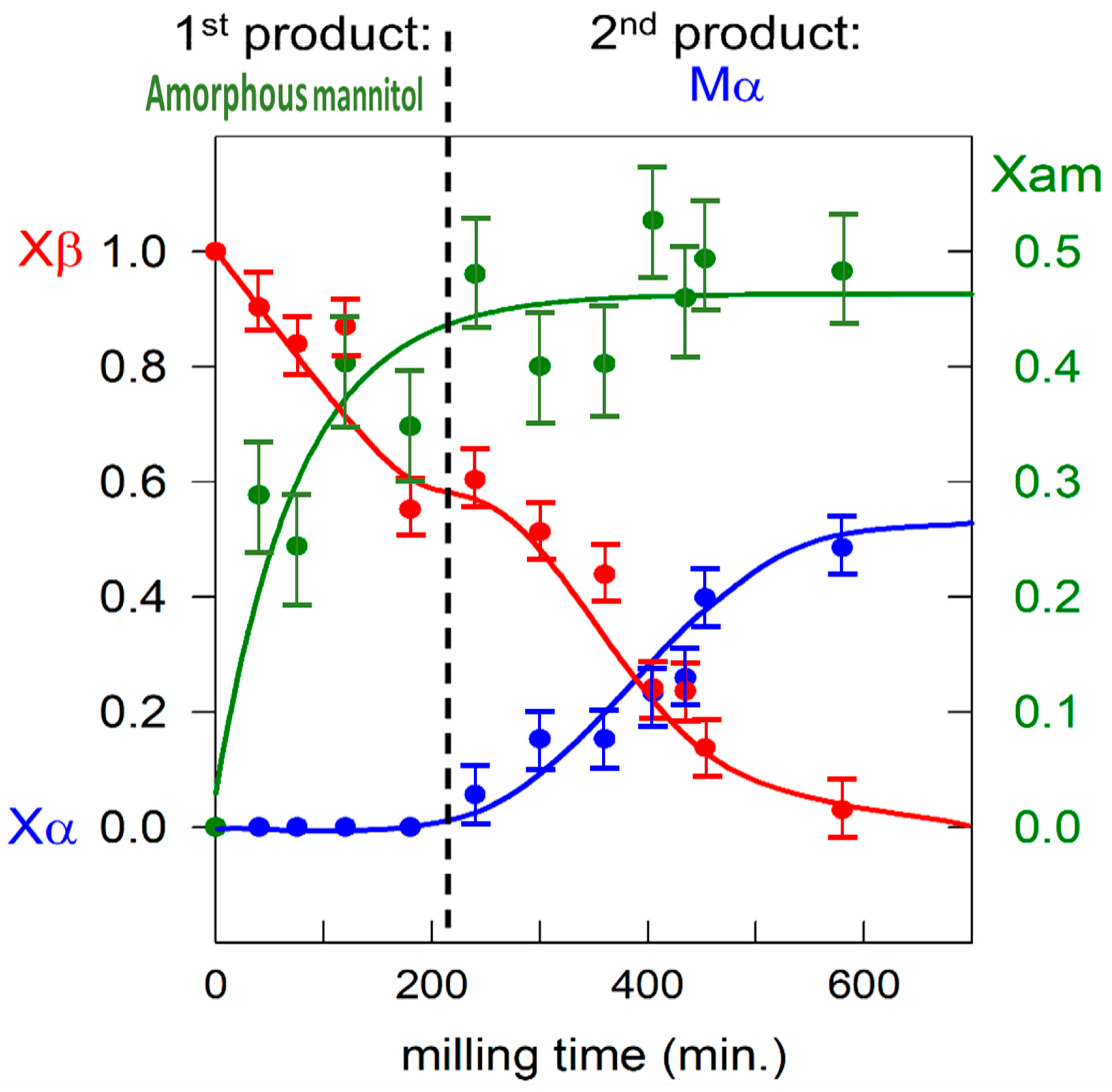
3.1. Mannitol
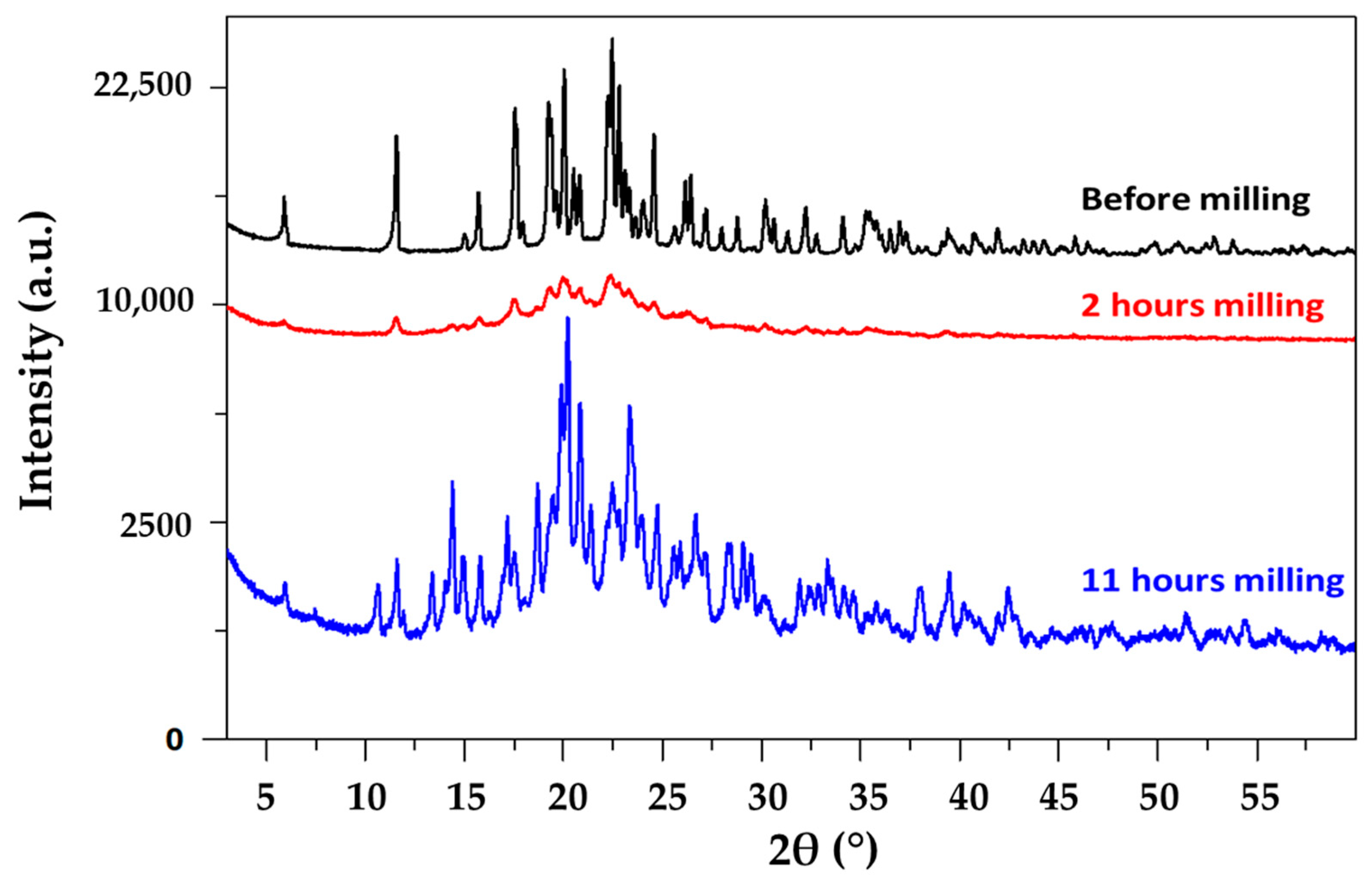
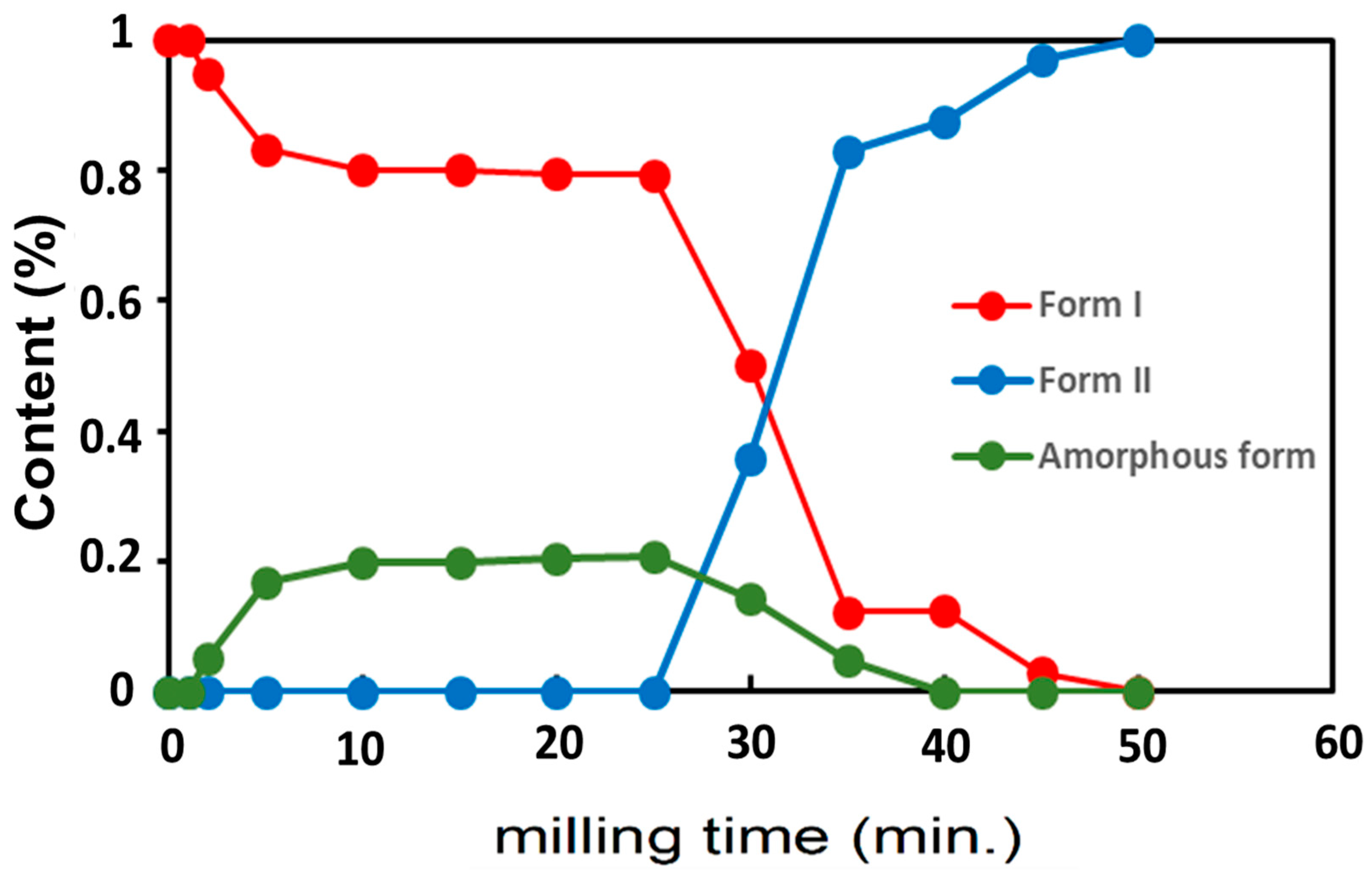
3.2. Famotidine
3.3. Sulfamerazine
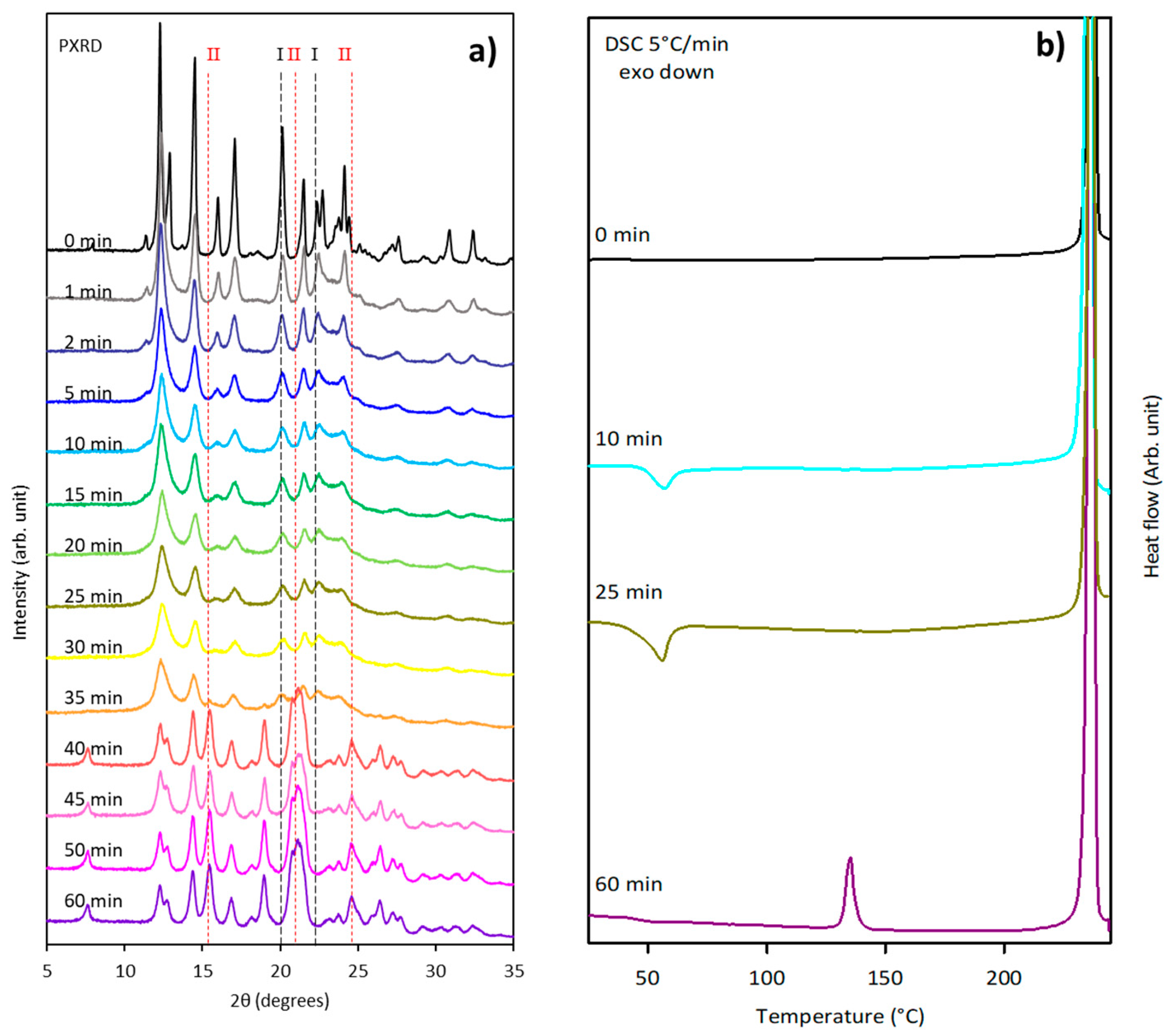
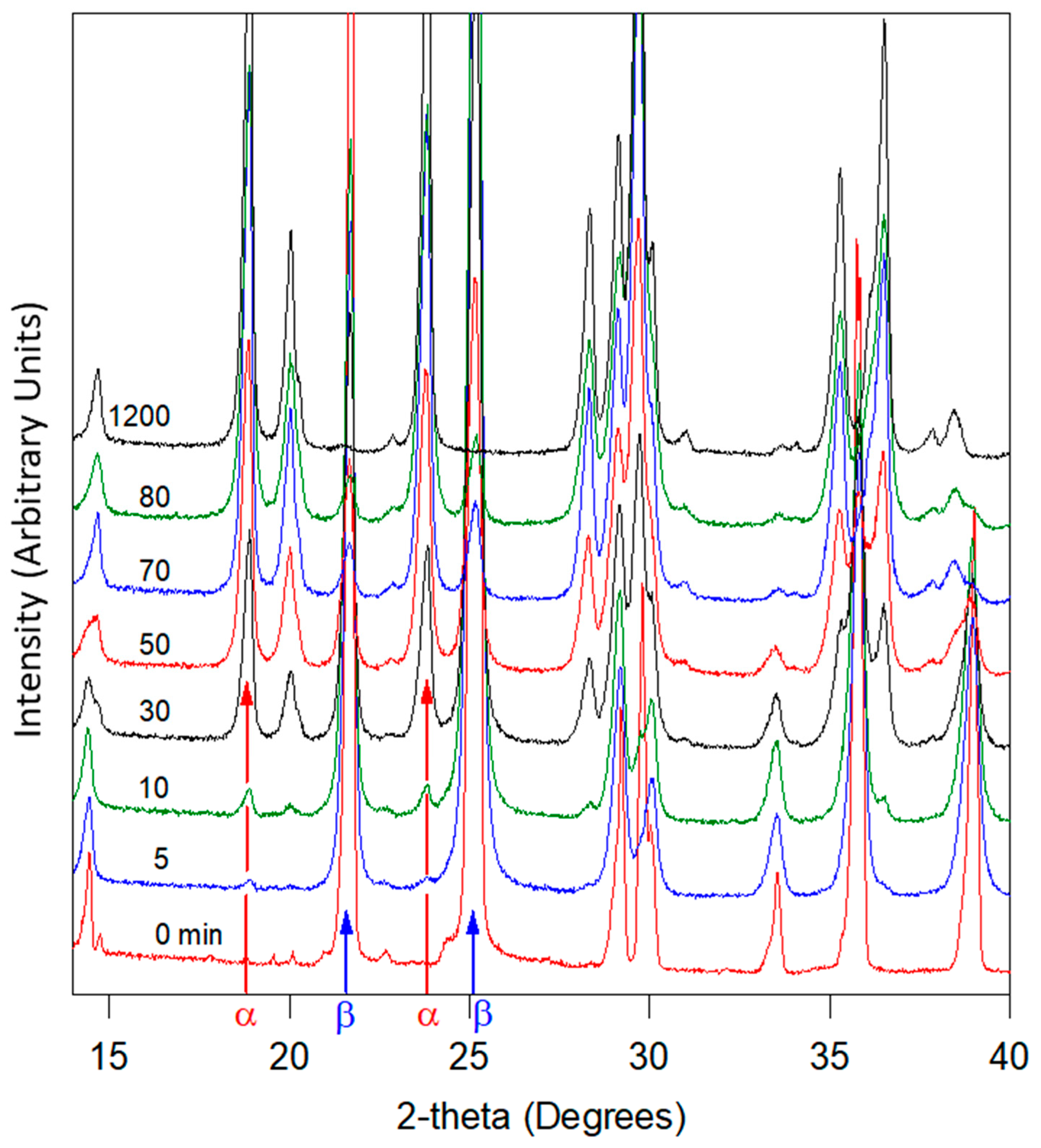
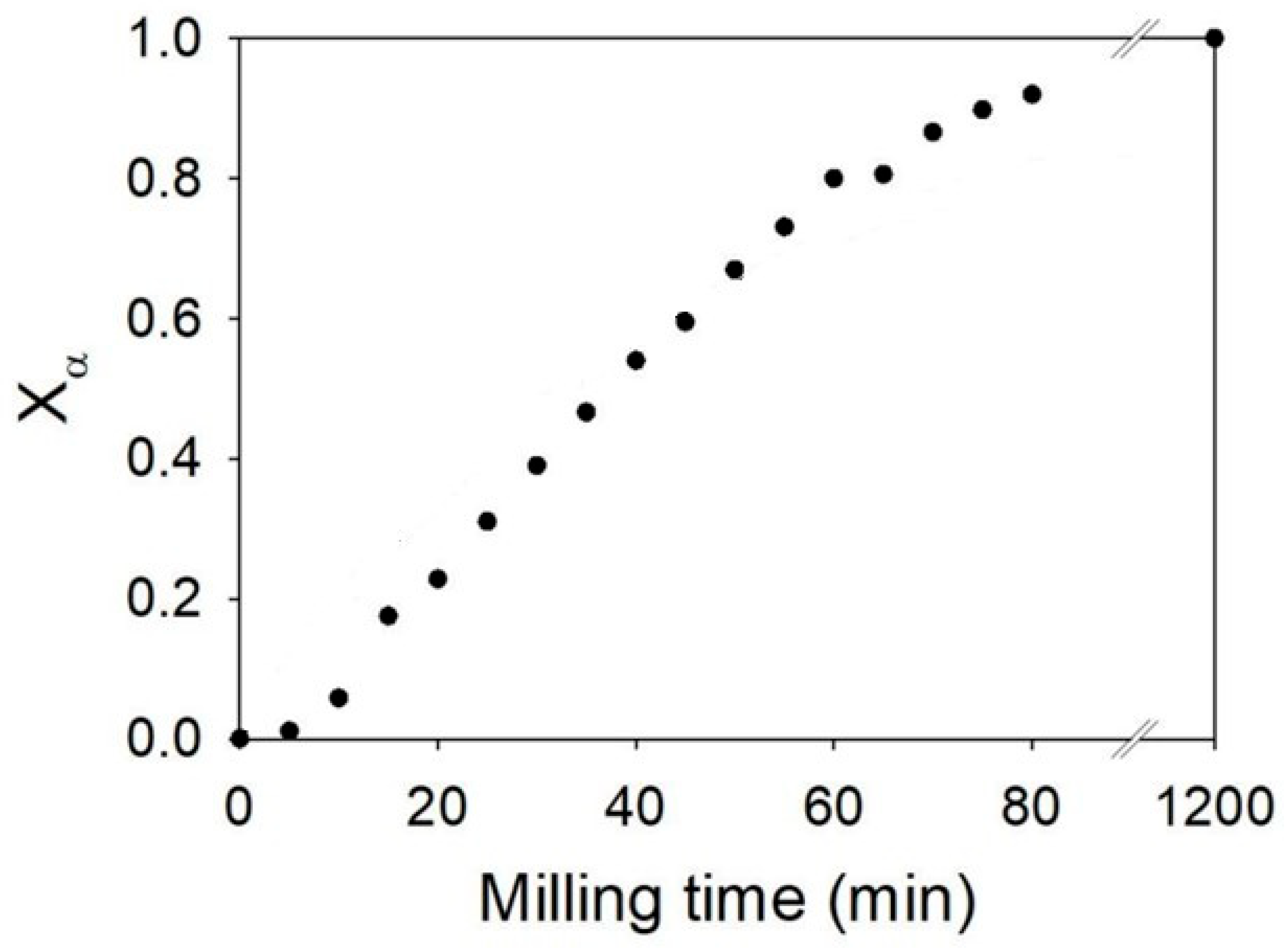
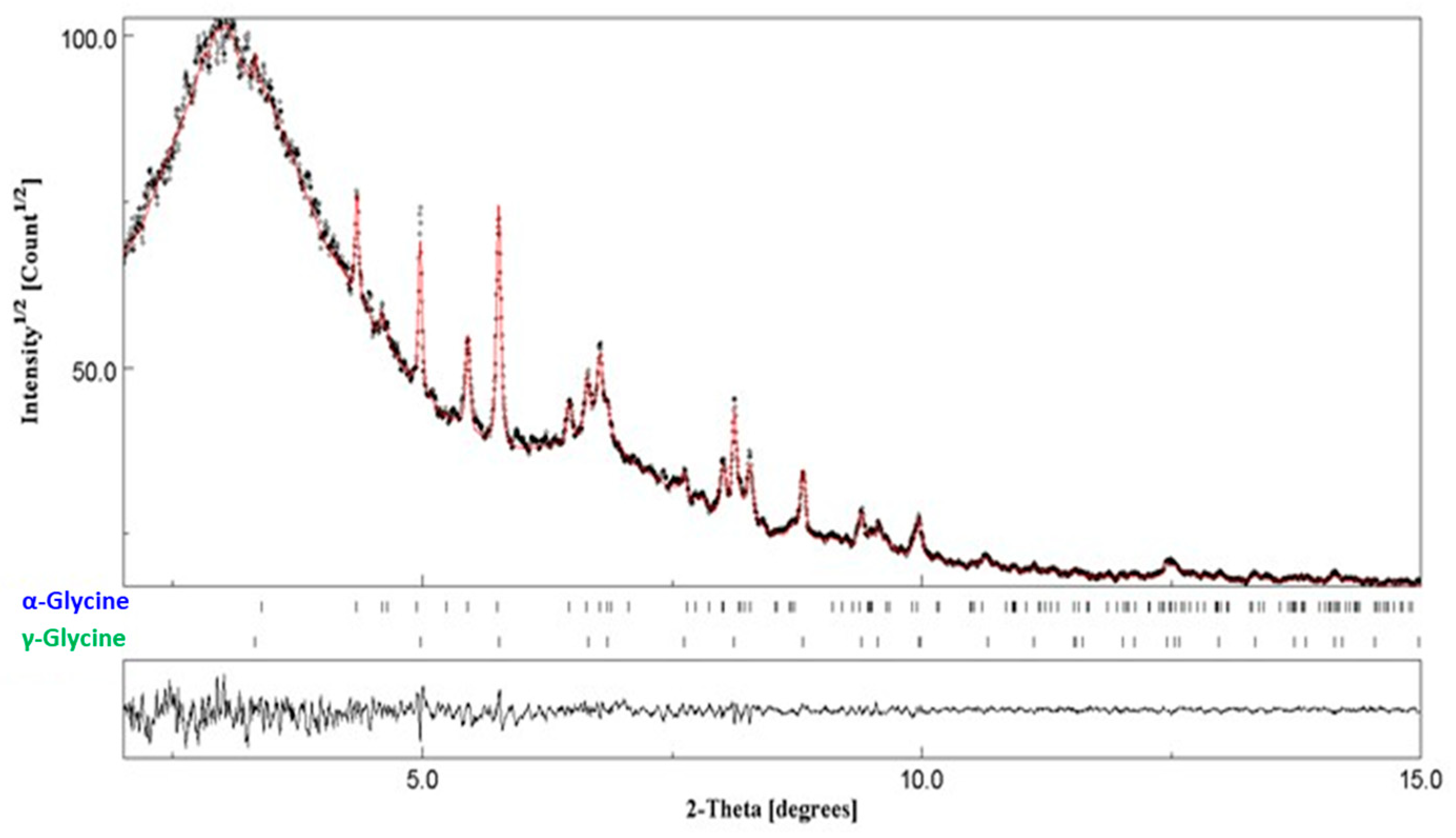
3.4. Glycine
3.4.1. Ex Situ Experiments
3.4.2. In Situ Experiments
4. Discussion
- -
- The existence of an induction time followed by a rapid transformation from the initial form to final form.
- -
- The transformation of part of the material (permanently or temporarily) into an amorphous material (except for glycine, for which any amorphization could be detected). It should be noted that the observation of transient amorphization could sometimes only be carried out indirectly.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Price, R.; Young, P.M. On the physical transformations of processed pharmaceutical solids. Micron 2005, 36, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Thakral, S.; Govindarajan, R.; Suryanarayanan, R. Processing-Induced Phase Transformations and Their Implications on Pharmaceutical Product Quality. In Polymorphism in the Pharmaceutical Industry: Solid Form and Drug Development; Wiley: Hoboken, NJ, USA, 2018; pp. 329–380. [Google Scholar]
- Juban, A.; Briançon, S.; Puel, F. Processing-induced-transformations (PITs) during direct compression: Impact of tablet composition and compression load on phase transition of caffeine. Int. J. Pharm. 2016, 501, 253–264. [Google Scholar] [CrossRef]
- Boldyreva, E.V.; Shakhtshneider, T.P.; Ahsbahs, H.; Sowa, H.; Uchtmann, H. Effect of High Pressure on the Polymorphs of Paracetamol. J. Therm. Anal. Calorim. 2002, 68, 437–452. [Google Scholar] [CrossRef]
- Tsukushi, I.; Yamamuro, O.; Matsuo, T. Solid state amorphization of organic molecular crystals using a vibrating mill. Solid State Commun. 1995, 94, 1013–1018. [Google Scholar] [CrossRef]
- Willart, J.F.; Descamps, M. Solid State Amorphization of Pharmaceuticals. Mol. Pharm. 2008, 5, 905–920. [Google Scholar] [CrossRef]
- Descamps, M.; Willart, J.F. Perspectives on the amorphisation/milling relationship in pharmaceutical materials. Adv. Drug Deliv. Rev. 2016, 100, 51–66. [Google Scholar] [CrossRef]
- De Gusseme, A.; Neves, C.; Willart, J.F.; Rameau, A.; Descamps, M. Ordering and disordering of molecular solids upon mechanical milling: The case of fananserine. J. Pharm. Sci. 2008, 97, 5000–5012. [Google Scholar] [CrossRef] [PubMed]
- Guerain, M.; Willart, J.-F. Polymorphic transformations of pharmaceutical materials induced by mechanical milling: A review. Pharmaceutics 2025, 17, 946. [Google Scholar] [CrossRef]
- Dupont, A.; Guerain, M.; Danède, F.; Paccou, L.; Guinet, Y.; Hédoux, A.; Willart, J.-F. Kinetics and mechanism of polymorphic transformation of sorbitol under mechanical milling. Int. J. Pharm. 2020, 590, 119902. [Google Scholar] [CrossRef]
- Dudognon, E.; Danède, F.; Guerain, M. Milling-Induced Phase Transformations, Underlying Mechanisms, and Resulting Physical States in an Enantiotropic System: The Case of Bezafibrate. Cryst. Growth Des. 2022, 22, 363–378. [Google Scholar] [CrossRef]
- Martinetto, P.; Bordet, P.; Descamps, M.; Dudognon, E.; Pagnoux, W.; Willart, J.-F. Structural Transformations of d-Mannitol Induced by in Situ Milling Using Real Time Powder Synchrotron Radiation Diffraction. Cryst. Growth Des. 2017, 17, 6111–6122. [Google Scholar] [CrossRef]
- Kaneniwa, N.; Otsuka, M. Effect of Grinding on the Transformations of Polymorphs of Chloramphenicol Palmitate. Chem. Pharm. Bull. 1985, 33, 1660–1668. [Google Scholar] [CrossRef]
- Dupont, A.; Guerain, M.; Danède, F.; Willart, J.F. Evidence of transient amorphization during the polymorphic transformation of sorbitol induced by milling. Int. J. Pharm. 2022, 623, 121929. [Google Scholar] [CrossRef]
- Matsuoka, M.; Hirata, J.; Yoshizawa, S. Kinetics of solid-state polymorphic transition of glycine in mechano-chemical processing. Chem. Eng. Res. Des. 2010, 88, 1169–1173. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Cheng, W.-T.; Wang, S.-L. Thermodynamic and kinetic characterization of polymorphic transformation of famotidine during grinding. Int. J. Pharm. 2006, 318, 86–91. [Google Scholar] [CrossRef]
- Macfhionnghaile, P.; Hu, Y.; Gniado, K.; Curran, S.; Mcardle, P.; Erxleben, A. Effects of Ball-Milling and Cryomilling on Sulfamerazine Polymorphs: A Quantitative Study. J. Pharm. Sci. 2014, 103, 1766–1778. [Google Scholar] [CrossRef] [PubMed]
- Lutterotti, L. Total pattern fitting for the combined size–strain–stress–texture determination in thin film diffraction. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2010, 268, 334–340. [Google Scholar] [CrossRef]
- Madsen, I.C.; Scarlett, N.V.Y.; Kern, A. Description and survey of methodologies for the determination of amorphous content via X-ray powder diffraction. Z. Für Krist. 2011, 226, 944–955. [Google Scholar] [CrossRef]
- Fitch, A.; Dejoie, C.; Covacci, E.; Confalonieri, G.; Grendal, O.; Claustre, L.; Guillou, P.; Kieffer, J.; de Nolf, W.; Petitdemange, S.; et al. ID22—The high-resolution powder-diffraction beamline at ESRF. J. Synchrotron Radiat. 2023, 30, 1003–1012. [Google Scholar] [CrossRef]
- Yu, L.; Mishra, D.S.; Rigsbee, D.R. Determination of the Glass Properties of D-Mannitol Using Sorbitol as an Impurity. J. Pharm. Sci. 1998, 87, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Caron, V.; Willart, J.-F.; Lefort, R.; Derollez, P.; Danède, F.; Descamps, M. Solid state amorphization kinetic of alpha lactose upon mechanical milling. Carbohydr. Res. 2011, 346, 2622–2628. [Google Scholar] [CrossRef] [PubMed]
- Caron, V.; Willart, J.F.; Danède, F.; Descamps, M. The implication of the glass transition in the formation of trehalose/mannitol molecular alloys by ball milling. Solid State Commun. 2007, 144, 288–292. [Google Scholar] [CrossRef]
- Caron, V. Mécanosynthèse et Vitrification à l’état Solide d’alliages Moléculaires. Ph.D. Thesis, Université des Sciences et Technologie de Lille, Villeneuve-d′Ascq, France, 2006. [Google Scholar]
- Willart, J.-F.; Carpentier, L.; Danède, F.; Descamps, M. Solid-State Vitrification of Crystalline Griseofulvin by Mechanical Milling. J. Pharm. Sci. 2012, 101, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Dupont, A. Cinétiques de Transformations de Produits Pharmaceutiques Sous Broyage. Ph.D. Thesis, Université de Lille, Lille, France, 2022. [Google Scholar]
- Perlovich, G.L.; Hansen, L.K.; Bauer-Brandl, A. The Polymorphism of Glycine. Thermochemical and structural aspects. J. Therm. Anal. Calorim. 2001, 66, 699–715. [Google Scholar] [CrossRef]
- Dujardin, N.; Willart, J.F.; Dudognon, E.; Danède, F.; Descamps, M. Mechanism of Solid State Amorphization of Glucose upon Milling. J. Phys. Chem. B 2013, 117, 1437–1443. [Google Scholar] [CrossRef]
- Descamps, M.; Willart, J.F.; Dudognon, E.; Caron, V. Transformation of pharmaceutical compounds upon milling and comilling: The role of Tg. J. Pharm. Sci. 2007, 96, 1398–1407. [Google Scholar] [CrossRef]
- Hu, Y.; Erxleben, A.; Hodnett, B.K.; Li, B.; McArdle, P.; Rasmuson, Å.C.; Ryder, A.G. Solid-State Transformations of Sulfathiazole Polymorphs: The Effects of Milling and Humidity. Cryst. Growth Des. 2013, 13, 3404–3413. [Google Scholar] [CrossRef]
- Desprez, S. Transformation de Phases Induites par Broyage Dans un Composé Moléculaire: L’indométhacine. Ph.D. Thesis, Université de Lille, Lille, France, 2004. [Google Scholar]
- Bauer-Brandl, A. Polymorphic transitions of cimetidine during manufacture of solid dosage forms. Int. J. Pharm. 1996, 140, 195–206. [Google Scholar] [CrossRef]
- Chieng, N.; Zujovic, Z.; Bowmaker, G.; Rades, T.; Saville, D. Effect of milling conditions on the solid-state conversion of ranitidine hydrochloride form 1. Int. J. Pharm. 2006, 327, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Amaro, M.I.; Simon, A.; Cabral, L.M.; de Sousa, V.P.; Healy, A.M. Rivastigmine hydrogen tartrate polymorphs: Solid-state characterisation of transition and polymorphic conversion via milling. Solid State Sci. 2015, 49, 29–36. [Google Scholar] [CrossRef]
- Enrique, R.A.; Bellon, P. Nonequilibrium fluctuations, effective temperature, and effective interactions driven by irradiation of alloys. Phys. Rev. B 2004, 70, 224106. [Google Scholar] [CrossRef]
- Aji, D.P.B.; Khouri, J.; Johari, G.P. Non-exponential relaxation, fictive temperatures, and dispersive kinetics in the liquid-glass-liquid transition range of acetaminophen, sulfathiazole, and their mixtures. J. Chem. Phys. 2014, 141, 174507. [Google Scholar] [CrossRef] [PubMed]
- Madan, T.; Kakkar, A. Preparation and Characterization of Ranitidine-HC1 Crystals. Drug Dev. Ind. Pharm. 1994, 20, 1571–1588. [Google Scholar] [CrossRef]
- Mahlin, D.; Bergström, C.A.S. Early drug development predictions of glass-forming ability and physical stability of drugs. Eur. J. Pharm. Sci. 2013, 49, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Hsu, C.-H.; Ke, W.-T. Solid-state transformation of different gabapentin polymorphs upon milling and co-milling. Int. J. Pharm. 2010, 396, 83–90. [Google Scholar] [CrossRef]
- Otsuka, M.; Otsuka, K.; Kaneniwa, N. Relation Between Polymorphic Transformation Pathway During Grinding and the Physicochemical Properties of Bulk Powders for Pharmaceutical Preparations. Drug Dev. Ind. Pharm. 1994, 20, 1649–1660. [Google Scholar] [CrossRef]
- Luisi, B.S.; Medek, A.; Liu, Z.; Mudunuri, P.; Moulton, B. Milling-Induced Disorder of Pharmaceuticals: One-Phase or Two-Phase System? J. Pharm. Sci. 2012, 101, 1475–1485. [Google Scholar] [CrossRef]
- Linol, J.; Morelli, T.; Petit, M.N.; Coquerel, G. Inversion of the relative stability between two polymorphic forms of (±) modafinil under dry high-energy milling: Comparisons with results obtained under wet high-energy milling. Cryst. Growth Des. 2007, 7, 1608–1611. [Google Scholar] [CrossRef]
- Otsuka, M.; Kaneniwa, N. Effect of seed crystals on solid-state transformation of polymorphs of chloramphenicol palmitate during grinding1. J. Pharm. Sci. 1986, 75, 506–511. [Google Scholar] [CrossRef]
- Thayyil, M.S. Fragility of Cimetidine Drug Probed by Broadband Dielectric Spectroscopy. Transl. Med. 2014, 4, 138. [Google Scholar]
- Kaneniwa, N.; Ichikawa, J.-I.; Matsumoto, T. Preparation of Phenylbutazone Polymorphs and Their Transformation in Solution. Chem. Pharm. Bull. 1988, 36, 1063–1073. [Google Scholar] [CrossRef]
- Fukuoka, E.; Makita, M.; Yamamura, S. Glassy State of Pharmaceuticals. III: Thermal Properties and Stability of Glassy Pharmaceuticals and Their Binary Glass Systems. Chem. Pharm. Bull. 1989, 37, 1047–1050. [Google Scholar] [CrossRef]
- Kohno, Y.; Hiyoshi, R.I.; Yamaguchi, Y.; Matsumoto, S.; Koseki, A.; Takahashi, O.; Yamasaki, K.; Ueda, K. Molecular Dynamics Studies of the Structural Change in 1,3-Diamino-2,4,6-trinitrobenzene (DATB) in the Crystalline State under High Pressure. J. Phys. Chem. A 2009, 113, 2551–2560. [Google Scholar] [CrossRef]
- Taddei, P.; Torreggiani, A.; Fini, G. Vibrational study of polymorphism of tetralin derivative for treatment of cardiovascular diseases. Biopolymers 2002, 67, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Pirttimäki, J.; Laine, E.; Ketolainen, J.; Paronen, P. Effects of grinding and compression on crystal structure of anhydrous caffeine. Int. J. Pharm. 1993, 95, 93–99. [Google Scholar] [CrossRef]
- Descamps, M.; Correia, N.T.; Derollez, P.; Danede, F.; Capet, F. Plastic and Glassy Crystal States of Caffeine. J. Phys. Chem. B 2005, 109, 16092–16098. [Google Scholar] [CrossRef] [PubMed]
- Descamps, M.; Decroix, A.A. Polymorphism and disorder in caffeine: Dielectric investigation of molecular mobilities. J. Mol. Struct. 2014, 1078, 165–173. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerain, M.; Dupont, A.; Danède, F.; Barkhatova, D.; Willart, J.-F. New Kinetic Investigations to Better Understand the Mechanism of Polymorphic Transformations of Pharmaceutical Materials Induced by Milling. Pharmaceutics 2025, 17, 1404. https://doi.org/10.3390/pharmaceutics17111404
Guerain M, Dupont A, Danède F, Barkhatova D, Willart J-F. New Kinetic Investigations to Better Understand the Mechanism of Polymorphic Transformations of Pharmaceutical Materials Induced by Milling. Pharmaceutics. 2025; 17(11):1404. https://doi.org/10.3390/pharmaceutics17111404
Chicago/Turabian StyleGuerain, Mathieu, Anthony Dupont, Florence Danède, Darina Barkhatova, and Jean-François Willart. 2025. "New Kinetic Investigations to Better Understand the Mechanism of Polymorphic Transformations of Pharmaceutical Materials Induced by Milling" Pharmaceutics 17, no. 11: 1404. https://doi.org/10.3390/pharmaceutics17111404
APA StyleGuerain, M., Dupont, A., Danède, F., Barkhatova, D., & Willart, J.-F. (2025). New Kinetic Investigations to Better Understand the Mechanism of Polymorphic Transformations of Pharmaceutical Materials Induced by Milling. Pharmaceutics, 17(11), 1404. https://doi.org/10.3390/pharmaceutics17111404





