Research Progress on Nasal Delivery of siRNA Nanocarrier Systems for the Treatment of Neurodegenerative Diseases
Abstract
1. Introduction
2. Research Background of CNS Diseases and siRNA Therapy
2.1. Pathologic Mechanisms and Treatment Difficulties of CNS Diseases
2.2. The Mechanism of Action of siRNA in CNS Diseases
3. The Anatomical Basis and Advantages of the Nasal Cavity Delivery System
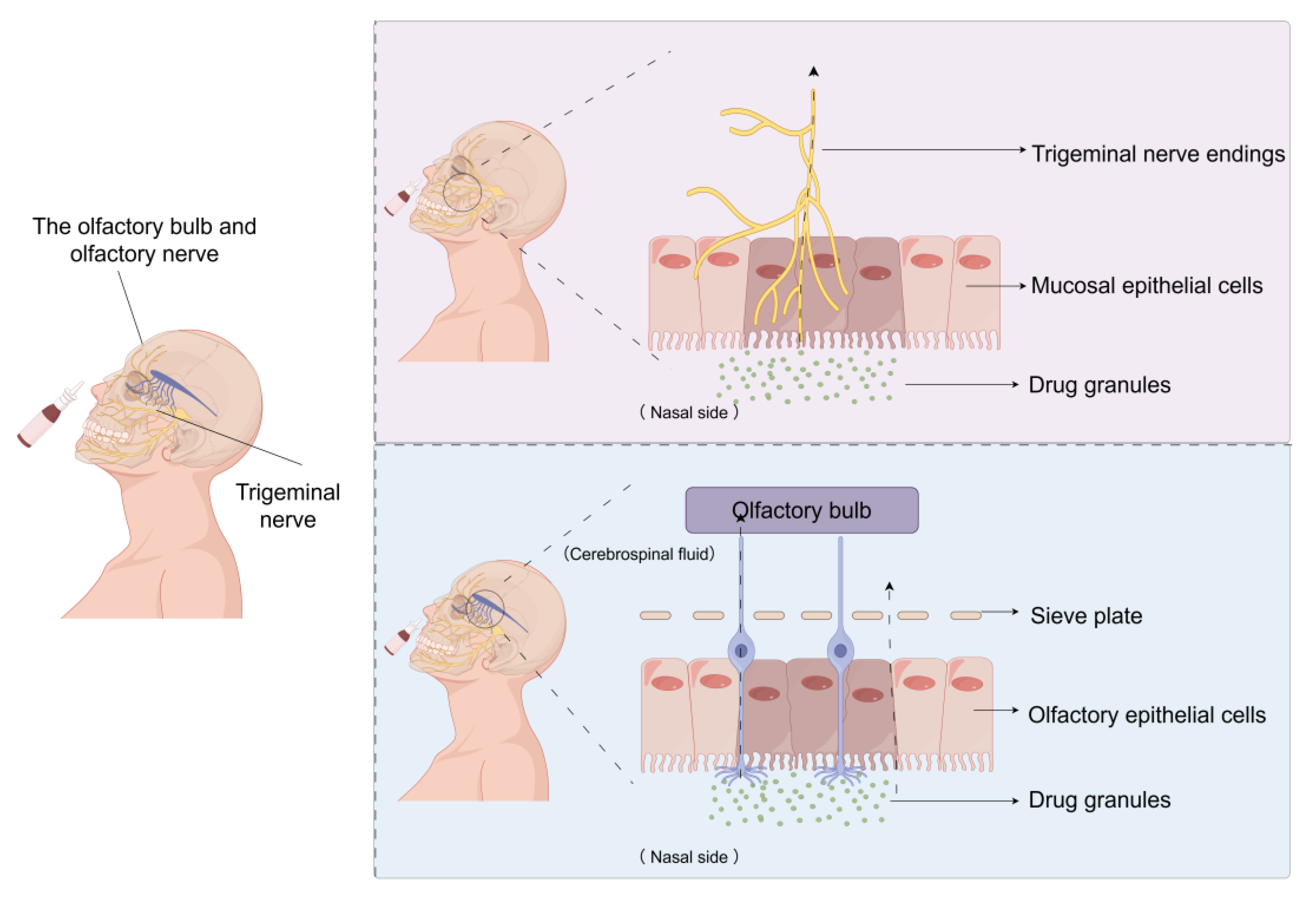
3.1. The Anatomical Connection Between the Nasal Cavity and the Brain
3.2. Advantages and Potential Limitations of Nasal Delivery
4. siRNA Nanocarrier Design Strategies
4.1. Basic Functions and Design Principles of Nanocarriers
4.2. The General Types of Nanocarriers
4.3. Dendrimers as Specialized Carriers
5. Delivery Mechanism of siRNA Nanocarriers for Nasal Delivery
5.1. The Pathway Through the Nasal Mucosa
5.2. Interaction Between Nanocarriers and Nasal Mucosa
5.3. siRNA Release and Intracellular Delivery Mechanism
6. Research Progress on In Vitro and In Vivo Delivery of siRNA Nanocarriers via Nasal Cavity
6.1. Delivery Efficiency and Gene Silencing Effect in Vitro Cell Models
6.2. Nasal Delivery Efficacy and Targeting in Animal Models
6.3. Challenges and Prospects of Preclinical Research
| siRNA Target/Strategy | Model (In Vitro/In Vivo) | Key Findings/Results | Reference |
|---|---|---|---|
| CD40 siRNA (polymeric nanocarriers) | Mouse immune-related models (in vitro/in vivo, macrophages and dendritic cells) | Downregulated CD40; inhibited macrophage and dendritic cell activation; enhanced anti-tumor effects | [112] |
| Bcl-2 pathway siRNA (HA-modified bola-type carriers) | Tumor-associated cell lines (in vitro/in vivo) | Improved transfection efficiency and silencing specificity; enhanced cell-specific delivery | [113] |
| Generic siRNA (liposomes) | Multiple in vitro cell lines | High biocompatibility; efficient membrane penetration; maintained siRNA activity | [34] |
| Generic siRNA (MOF nanoparticles) | Specific cell types (in vitro) | Overcame intracellular barriers; improved delivery efficiency | [40] |
| Generic siRNA (solid lipid–polymer hybrid nanoparticles, SLPHNs) | Various in vitro cell lines | Efficient siRNA delivery; maintained cell viability; reduced cytotoxicity | [46] |
| BACE1 siRNA (Rapa@DAK/siRNA nanoparticles) | Alzheimer’s disease transgenic mice (in vivo, nasal delivery) | Reduced BACE1 expression; decreased Aβ deposition; improved cognition | [58] |
| PAMAM dendrimers (generic siRNA delivery) | Mouse models (in vivo, nasal delivery) | Accurate brain delivery; favorable biocompatibility; no systemic toxicity | [83] |
| HMGB1 siRNA (PAMAM dendrimers) | Post-ischemic brain injury mice (in vivo, intranasal) | Target knockdown; robust neuroprotection | [82] |
| ApoE siRNA | Alzheimer’s disease mouse model (in vivo) | Reduced amyloid burden; improved neuronal survival | [119] |
| NMDAR siRNA (RVG-exosomes) | Orofacial neuropathic pain rats (in vivo) | Suppressed central sensitization; alleviated pain | [22] |
7. Challenges and Future Directions of Nasal Delivery siRNA Nanocarrier Systems
7.1. Technology and Biological Barriers
7.2. Strategies for Improving Delivery Efficiency and Targeting
7.3. Key Issues in Clinical Translation
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qu, Z.; Luo, J.; Li, Z.; Yang, R.; Zhao, J.; Chen, X.; Yu, S.; Shu, H. Advancements in strategies for overcoming the blood-brain barrier to deliver brain-targeted drugs. Front. Aging Neurosci. 2024, 16, 1353003. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, L.; Cao, F.; Wang, H.; Gong, P.; Ma, C.; Ren, L.; Lin, Y.; Lin, X. The barrier and interface mechanisms of the brain barrier, and brain drug delivery. Brain Res. Bull. 2022, 190, 69–83. [Google Scholar] [CrossRef]
- Akpinar Adscheid, S.; Türeli, A.E.; Günday-Türeli, N.; Schneider, M. Nanotechnological approaches for efficient N2B delivery: From small-molecule drugs to biopharmaceuticals. Beilstein J. Nanotechnol. 2024, 15, 1400–1414. [Google Scholar] [CrossRef]
- Kolls, B.J.; Muir, K.W.; Savitz, S.I.; Wechsler, L.R.; Pilitsis, J.G.; Rahimi, S.; Beckman, R.L.; Holmes, V.; Chen, P.R.; Albers, D.S.; et al. Experience with a hybrid recruitment approach of patient-facing web portal screening and subsequent phone and medical record review for a neurosurgical intervention trial for chronic ischemic stroke disability (PISCES III). Trials 2024, 25, 150. [Google Scholar] [CrossRef]
- Chaulagain, B.; Gothwal, A.; Lamptey, R.N.L.; Trivedi, R.; Mahanta, A.K.; Layek, B.; Singh, J. Experimental Models of In Vitro Blood–Brain Barrier for CNS Drug Delivery: An Evolutionary Perspective. Int. J. Mol. Sci. 2023, 24, 2710. [Google Scholar] [CrossRef]
- Triarico, S.; Maurizi, P.; Mastrangelo, S.; Attinà, G.; Capozza, M.A.; Ruggiero, A. Improving the Brain Delivery of Chemotherapeutic Drugs in Childhood Brain Tumors. Cancers 2019, 11, 824. [Google Scholar] [CrossRef]
- Berman-Booty, L.D.; Klein, S.K.; Mazur, C.; Schroeder, J.; Korte, S.; Ludwig, F.T.; Romeike, A.; Bolon, B.; Grieves, J.L. Toxicologic Pathology Forum: Opinion on Interpretive Challenges for Procedure-Related Effects Associated with Direct Central Nervous System Delivery of Oligonucleotides to Rodents, Dogs, and Nonhuman Primates. Toxicol. Pathol. 2023, 51, 375–389. [Google Scholar] [CrossRef]
- Drath, I.; Richter, F.; Feja, M. Nose-to-Brain Drug Delivery: From Bench to Bedside. Transl. Neurodegener. 2025, 14, 23. [Google Scholar] [CrossRef]
- Qiu, Y.; Huang, S.; Peng, L.; Yang, L.; Zhang, G.; Liu, T.; Yan, F.; Peng, X. The Nasal–Brain Drug Delivery Route: Mechanisms and Applications to Central Nervous System Diseases. MedComm 2025, 6, e70213. [Google Scholar] [CrossRef]
- Xu, J.; Ma, C.; Hua, M.; Li, J.; Xiang, Z.; Wu, J. CNS and CNS diseases in relation to their immune system. Front. Immunol. 2022, 13, 1063928. [Google Scholar] [CrossRef]
- Di Francesco, V.; Chua, A.J.; Huang, D.; D’Souza, A.; Yang, A.; Bleier, B.S.; Amiji, M.M. RNA therapies for CNS diseases. Adv. Drug Deliv. Rev. 2024, 208, 115283. [Google Scholar] [CrossRef]
- Teutsch, S.; Berkhout, A.; Raynes-Greenow, C.; Zurynski, Y.; Britton, P.N.; Jones, C.A. APSU Neonatal HSV study advisory group. Characteristics of neonatal herpes simplex central nervous system disease in Australia (1997–2020). J. Clin. Virol. 2023, 165, 105526. [Google Scholar] [CrossRef]
- Vuotto, C.; Battistini, L.; Caltagirone, C.; Borsellino, G. Gut Microbiota and Disorders of the Central Nervous System. Neuroscientist 2020, 26, 487–502. [Google Scholar] [CrossRef]
- Tao, W.; Zhang, Y.; Wang, B.; Nie, S.; Fang, L.; Xiao, J.; Wu, Y. Advances in molecular mechanisms and therapeutic strategies for central nervous system diseases based on gut microbiota imbalance. J. Adv. Res. 2025, 69, 261–278. [Google Scholar] [CrossRef]
- Bale, R.; Doshi, G. Deciphering the role of siRNA in anxiety and depression. Eur. J. Pharmacol. 2024, 981, 176868. [Google Scholar] [CrossRef]
- Zabel, M.D.; Mollnow, L.; Bender, H. siRNA Therapeutics for Protein Misfolding Diseases of the Central Nervous System. Methods Mol. Biol. 2021, 2282, 377–394. [Google Scholar] [CrossRef]
- Almarghalani, D.A.; Boddu, S.H.S.; Ali, M.; Kondaka, A.; Ta, D.; Shah, R.A.; Shah, Z.A. Small interfering RNAs based therapies for intracerebral hemorrhage: Challenges and progress in drug delivery systems. Neural Regen. Res. 2022, 17, 1717–1725. [Google Scholar] [CrossRef]
- Holm, A.; Hansen, S.N.; Klitgaard, H.; Kauppinen, S. Clinical advances of RNA therapeutics for treatment of neurological and neuromuscular diseases. RNA Biol. 2022, 19, 594–608. [Google Scholar] [CrossRef]
- Eyford, B.A.; Singh, C.S.B.; Abraham, T.; Munro, L.; Choi, K.B.; Hill, T.; Hildebrandt, R.; Welch, I.; Vitalis, T.Z.; Gabathuler, R.; et al. A Nanomule Peptide Carrier Delivers siRNA Across the Intact Blood-Brain Barrier to Attenuate Ischemic Stroke. Front. Mol. Biosci. 2021, 8, 611367. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, S.; Qin, F.; Fu, A.; Fu, C. Application progress of RVG peptides to facilitate the delivery of therapeutic agents into the central nervous system. RSC Adv. 2021, 11, 8505–8515. [Google Scholar] [CrossRef]
- Aundhia, C.; Parmar, G.; Talele, C.; Trivedi, R.; Kumari, M.; Chudasama, J. Impact and Significance of Viral Vectors for siRNA Delivery in the Treatment of Alzheimer’s Disease. Curr. Pharm. Biotechnol. 2025; ahead of print. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, Y.L.; Song, Q.X.; Yi, Y.T.; Feng, Y.H.; Li, Y.K.; Zhou, C.; Li, C.J.; Liu, F.; Shen, J.F. Engineered RVG-exosomes-mediated delivery of siRNAs targeting NMDAR alleviates orofacial neuropathic pain by suppressing central sensitivity. J. Pain. 2025, 33, 105457. [Google Scholar] [CrossRef]
- Patel, D.; Thakkar, H. Formulation considerations for improving intranasal delivery of CNS acting therapeutics. Ther. Deliv. 2022, 13, 371–381. [Google Scholar] [CrossRef]
- Sapru, M.K.; Yates, J.W.; Hogan, S.; Jiang, L.; Halter, J.; Bohn, M.C. Silencing of human alpha-synuclein in vitro and in rat brain using lentiviral-mediated RNAi. Exp. Neurol. 2006, 198, 382–390. [Google Scholar] [CrossRef]
- Ralph, G.S.; Radcliffe, P.A.; Day, D.M.; Carthy, J.M.; Leroux, M.A.; Lee, D.C.P.; Wong, L.F.; Bilsland, L.G.; Greensmith, L.; Kingsman, S.M.; et al. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat. Med. 2005, 11, 429–433. [Google Scholar] [CrossRef]
- Chung, S.; Peters, J.M.; Detyniecki, K.; Tatum, W.; Rabinowicz, A.L.; Carrazana, E. The nose has it: Opportunities and challenges for intranasal drug administration for neurologic conditions including seizure clusters. Epilepsy Behav. Rep. 2022, 21, 100581. [Google Scholar] [CrossRef]
- Jimenez-Mateos, E.M.; Engel, T.; Merino-Serrais, P.; McKiernan, R.C.; Tanaka, K.; Mouri, G.; Sano, T.; O’Tuathaigh, C.; Waddington, J.L.; Prenter, S.; et al. Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat. Med. 2012, 18, 1087–1094. [Google Scholar] [CrossRef]
- Rigaut, C.; Deruyver, L.; Niesen, M.; Vander Ghinst, M.; Goole, J.; Lambert, P.; Haut, B. What Are the Key Anatomical Features for the Success of Nose-to-Brain Delivery? A Study of Powder Deposition in 3D-Printed Nasal Casts. Pharmaceutics 2023, 15, 2661. [Google Scholar] [CrossRef]
- Kaikousidis, C.; Papakyriakopoulou, P.; Dokoumetzidis, A.; Valsami, G. Donepezil Brain and Blood Pharmacokinetic Modeling after Nasal Film and Oral Solution Administration in Mice. Pharmaceutics 2023, 15, 1409. [Google Scholar] [CrossRef]
- Yarragudi, S.B.; Kumar, H.; Jain, R.; Tawhai, M.; Rizwan, S. Olfactory Targeting of Microparticles Through Inhalation and Bi-directional Airflow: Effect of Particle Size and Nasal Anatomy. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 258–270. [Google Scholar] [CrossRef]
- Maaz, A.; Blagbrough, I.S.; De Bank, P.A. A Cell-Based Nasal Model for Screening the Deposition, Biocompatibility, and Transport of Aerosolized PLGA Nanoparticles. Mol. Pharm. 2024, 21, 1108–1124. [Google Scholar] [CrossRef]
- Botan, M.V.G.; da Silva, J.B.; Bruschi, M.L. Technological Strategies Applied to Pharmaceutical Systems for Intranasal Administration of Drugs Intended for Neurological Treatments: A Review. AAPS PharmSciTech 2024, 25, 258. [Google Scholar] [CrossRef]
- Salimi, M.; Nazari, M.; Shahsavar, P.; Dehghan, S.; Javan, M.; Mirnajafi-Zadeh, J.; Raoufy, M.R. Olfactory bulb stimulation mitigates Alzheimer’s-like disease progression. CNS Neurosci. Ther. 2024, 30, e70056. [Google Scholar] [CrossRef]
- Lee, D.; Shen, A.M.; Garbuzenko, O.B.; Minko, T. Liposomal Formulations of Anti-Alzheimer Drugs and siRNA for Nose-to-Brain Delivery: Design, Safety and Efficacy In Vitro. AAPS J. 2024, 26, 99. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, R.; Wang, Y.; Zhang, X.; Yang, Y.; Zhao, B.; Yang, L. A simple self-assembly nanomicelle based on brain tumor-targeting peptide-mediated siRNA delivery for glioma immunotherapy via intranasal administration. Acta Biomater. 2023, 155, 521–537. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Wu, S.; Zhang, R.; Zhou, B.; Zhang, X.; Tang, L.; Tian, Y.; Men, K.; Yang, L. Enhanced nose-to-brain delivery of siRNA using hyaluronan-enveloped nanomicelles for glioma therapy. J. Control Release 2022, 342, 66–80. [Google Scholar] [CrossRef]
- Supramaniam, A.; Tayyar, Y.; Clarke, D.T.W.; Kelly, G.; Acharya, D.; Morris, K.V.; McMillan, N.A.J.; Idris, A. Prophylactic intranasal administration of lipid nanoparticle formulated siRNAs reduce SARS-CoV-2 and RSV lung infection. J. Microbiol. Immunol. Infect. 2023, 56, 516–525. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, K.; Wang, D.; Yao, S.; Lu, L.; Wang, H.; Song, J.; Zhou, J.; Fan, X.; Wang, Y.; et al. Core-shell lipoplexes inducing active macropinocytosis promote intranasal delivery of c-Myc siRNA for treatment of glioblastoma. Acta Biomater. 2022, 138, 478–490. [Google Scholar] [CrossRef]
- Abudurexiti, M.; Xue, J.; Li, X.; Zhang, X.; Qiu, Y.; Xiong, S.; Liu, G.; Yuan, S.; Tang, R. Curcumin/TGF-β1 siRNA loaded solid lipid nanoparticles alleviate cerebral injury after intracerebral hemorrhage by transnasal brain targeting. Colloids Surf. B Biointerfaces 2024, 237, 113857. [Google Scholar] [CrossRef]
- Esmaeilpour, D.; Ghomi, M.; Zare, E.N.; Sillanpää, M. Nanotechnology-Enhanced siRNA Delivery: Revolutionizing Cancer Therapy. ACS Appl. Bio Mater. 2025, 8, 4549–4579. [Google Scholar] [CrossRef]
- Sristi; Gupta, G.; Abourehab, M.A.S.; Sahebkar, A.; Kesharwani, P. Recent advances in PD-L1 siRNA nanocarriers for cancer therapy. Int. J. Biol. Macromol. 2025, 311 Pt 3, 143994. [Google Scholar] [CrossRef]
- Morales-Becerril, A.; Aranda-Lara, L.; Isaac-Olivé, K.; Ocampo-García, B.E.; Morales-Ávila, E. Nanocarriers for delivery of siRNA as gene silencing mediator. EXCLI J. 2022, 21, 1028–1052. [Google Scholar] [CrossRef]
- Pérez-Carrión, M.D.; Posadas, I.; Ceña, V. Nanoparticles and siRNA: A New Era in Therapeutics? Pharmacol. Res. 2024, 201, 107102. [Google Scholar] [CrossRef]
- Zohora, F.T.; Pathmanathan, R.; Chowdhury, E.H. Application of Strontium Chloride Hexahydrate to Synthesize Strontium-Substituted Carbonate Apatite as a pH-Sensitive, Biologically Safe, and Highly Efficient siRNA Nanocarrier. ACS Appl. Bio Mater. 2025, 8, 348–367. [Google Scholar] [CrossRef]
- Chang, Y.T.; Huang, T.H.; Alalaiwe, A.; Hwang, E.; Fang, J.Y. Small interfering RNA-based nanotherapeutics for treating skin-related diseases. Expert. Opin. Drug Deliv. 2023, 20, 757–772. [Google Scholar] [CrossRef]
- de Araujo, M.M.; Borgheti-Cardoso, L.N.; Praça, F.G.; Marcato, P.D.; Bentley, M.V.L.B. Solid Lipid-Polymer Hybrid Nanoplatform for Topical Delivery of siRNA: In Vitro Biological Activity and Permeation Studies. J. Funct. Biomater. 2023, 14, 374. [Google Scholar] [CrossRef]
- Xiong, L.; Chen, S.; Li, S.; He, D.; Wang, Y.; Zhang, Q.; He, Z.; Li, M.; He, Q. ATP-responsive tumor targeted lipid nanoparticle for enhanced siRNA delivery and improved treatment efficacy in melanoma. J. Control Release 2025, 382, 113622. [Google Scholar] [CrossRef]
- Karlsson, J.; Tzeng, S.Y.; Hemmati, S.; Luly, K.M.; Choi, O.; Rui, Y.; Wilson, D.R.; Kozielski, K.L.; Quiñones-Hinojosa, A.; Green, J.J. Photocrosslinked Bioreducible Polymeric Nanoparticles for Enhanced Systemic siRNA Delivery as Cancer Therapy. Adv. Funct. Mater. 2021, 31, 2009768. [Google Scholar] [CrossRef]
- Hassani Besheli, N.; Verbakel, J.; Hosseini, M.; Andrée, L.; Joosten, B.; Walboomers, X.F.; Cambi, A.; Yang, F.; Leeuwenburgh, S.C.G. Cellular Uptake of Modified Mesoporous Bioactive Glass Nanoparticles for Effective Intracellular Delivery of Therapeutic Agents. Int. J. Nanomedicine 2023, 18, 1599–1612. [Google Scholar] [CrossRef]
- Ray, R.; Ghosh, S.; Maity, A.; Jana, N.R. Arginine Surface Density of Nanoparticles Controls Nonendocytic Cell Uptake and Autophagy Induction. ACS Appl. Mater. Interfaces 2024, 16, 5451–5461. [Google Scholar] [CrossRef]
- Oberländer, J.; Champanhac, C.; da Costa Marques, R.; Landfester, K.; Mailänder, V. Temperature, concentration, and surface modification influence the cellular uptake and the protein corona of polystyrene nanoparticles. Acta Biomater. 2022, 148, 271–278. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Shaw, S.; Arora, H.; Seth, P.; Jana, N.R. Nuclear Transport of the Molecular Drug via Nanocarrier-Based Nonendocytic Cellular Uptake. ACS Appl. Mater. Interfaces 2023, 15, 39176–39185. [Google Scholar] [CrossRef]
- Huang, L.; Mao, X.; Li, J.; Li, Q.; Shen, J.; Liu, M.; Fan, C.; Tian, Y. Nanoparticle Spikes Enhance Cellular Uptake via Regulating Myosin IIA Recruitment. ACS Nano 2023, 17, 9155–9166. [Google Scholar] [CrossRef]
- Huang, X.; Guo, H.; Wang, L.; Zhang, Z.; Zhang, W. Biomimetic cell membrane-coated nanocarriers for targeted siRNA delivery in cancer therapy. Drug Discov. Today 2023, 28, 103514. [Google Scholar] [CrossRef]
- Charbe, N.B.; Amnerkar, N.D.; Ramesh, B.; Tambuwala, M.M.; Bakshi, H.A.; Aljabali, A.A.A.; Khadse, S.C.; Satheeshkumar, R.; Satija, S.; Metha, M.; et al. Small interfering RNA for cancer treatment: Overcoming hurdles in delivery. Acta Pharm. Sin B 2020, 10, 2075–2109. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tu, B.; Sun, Y.; Liao, L.; Lu, X.; Liu, E.; Huang, Y. Nanobody-based drug delivery systems for cancer therapy. J. Control Release 2025, 381, 113562. [Google Scholar] [CrossRef]
- D’Angelo, N.A.; Noronha, M.A.; Câmara, M.C.C.; Kurnik, I.S.; Feng, C.; Araujo, V.H.S.; Santos, J.H.P.M.; Feitosa, V.; Molino, J.V.D.; Rangel-Yagui, C.O.; et al. Doxorubicin nanoformulations on therapy against cancer: An overview from the last 10 years. Biomater. Adv. 2022, 133, 112623. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, W.; Xia, X.; Lei, T.; Yang, Z.; Jia, W.; Zhou, Y.; Cheng, G.; Gao, H. Intranasal Delivery of BACE1 siRNA and Rapamycin by Dual Targets Modified Nanoparticles for Alzheimer’s Disease Therapy. Small 2022, 18, e2203182. [Google Scholar] [CrossRef]
- Paroha, S.; Verma, J.; Dubey, R.D.; Dewangan, R.P.; Molugulu, N.; Bapat, R.A.; Sahoo, P.K.; Kesharwani, P. Recent advances and prospects in gemcitabine drug delivery systems. Int. J. Pharm. 2021, 592, 120043. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, D.; Lian, B.; Shi, K.; Chen, P.; Li, Y.; Lin, W.; Ding, L.; Long, Q.; Wang, Y.; et al. Cargo-selective and adaptive delivery of nucleic acid therapeutics by bola-amphiphilic dendrimers. Proc. Natl. Acad. Sci. USA 2023, 120, e2220787120. [Google Scholar] [CrossRef]
- Zawadzki, S.; Martín-Serrano, Á.; Okła, E.; Kędzierska, M.; Garcia-Gallego, S.; López, P.O.; de la Mata, F.J.; Michlewska, S.; Makowski, T.; Ionov, M.; et al. Synthesis and biophysical evaluation of carbosilane dendrimers as therapeutic siRNA carriers. Sci. Rep. 2024, 14, 1615. [Google Scholar] [CrossRef] [PubMed]
- Mirhadi, E.; Mashreghi, M.; Faal Maleki, M.; Alavizadeh, S.H.; Arabi, L.; Badiee, A.; Jaafari, M.R. Redox-sensitive nanoscale drug delivery systems for cancer treatment. Int. J. Pharm. 2020, 589, 119882. [Google Scholar] [CrossRef]
- Pan, P.; Svirskis, D.; Rees, S.W.P.; Barker, D.; Waterhouse, G.I.N.; Wu, Z. Photosensitive drug delivery systems for cancer therapy: Mechanisms and applications. J. Control Release 2021, 338, 446–461. [Google Scholar] [CrossRef]
- Liang, F.; Cui, S.; Yang, J.; He, Z.; Zhu, L. Nanoplatform-Enabled Genetic Interventions for Central Nervous System Disorders: Advances in Delivery Strategies and Therapeutic Potential. Adv. Genet. 2025, 6, e00010. [Google Scholar] [CrossRef]
- Young, H.; He, Y.; Joo, B.; Ferguson, S.; Demko, A.; Butterfield, S.K.; Lowe, J.; Mjema, N.F.; Sheth, V.; Whitehead, L.; et al. Toward the Scalable, Rapid, Reproducible, and Cost-Effective Synthesis of Personalized Nanomedicines at the Point of Care. Nano Lett. 2024, 24, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Tan, P.; Fu, S.; Tian, X.; Zhang, H.; Ma, X.; Gu, Z.; Luo, K. Preparation and application of pH-responsive drug delivery systems. J. Control Release 2022, 348, 206–238. [Google Scholar] [CrossRef]
- Romero-Ben, E.; Goswami, U.; Soto-Cruz, J.; Mansoori-Kermani, A.; Mishra, D.; Martin-Saldaña, S.; Muñoz-Ugartemendia, J.; Sosnik, A.; Calderón, M.; Beloqui, A.; et al. Polymer-based nanocarriers to transport therapeutic biomacromolecules across the blood-brain barrier. Acta Biomater. 2025, 196, 17–49. [Google Scholar] [CrossRef] [PubMed]
- Markowicz-Piasecka, M.; Darłak, P.; Markiewicz, A.; Sikora, J.; Kumar Adla, S.; Bagina, S.; Huttunen, K.M. Current approaches to facilitate improved drug delivery to the central nervous system. Eur. J. Pharm. Biopharm. 2022, 181, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Butola, M.; Nainwal, N. Non-Invasive Techniques of Nose to Brain Delivery Using Nanoparticulate Carriers: Hopes and Hurdles. AAPS PharmSciTech 2024, 25, 256. [Google Scholar] [CrossRef]
- Li, X.; Luo, Y.; Huang, Z.; Wang, Y.; Wu, J.; Zhou, S. Multifunctional Liposomes Remodeling Tumor Immune Microenvironment for Tumor Chemoimmunotherapy. Small Methods 2023, 7, e2201327. [Google Scholar] [CrossRef]
- Sufianov, A.; Beilerli, A.; Kudriashov, V.; Ilyasova, T.; Wenjie, B.; Beylerli, O. Advances in transdermal siRNAs delivery: A review of current research progress. Noncoding RNA Res. 2023, 8, 392–400. [Google Scholar] [CrossRef]
- Waghule, T.; Saha, R.N.; Alexander, A.; Singhvi, G. Tailoring the multi-functional properties of phospholipids for simple to complex self-assemblies. J. Control Release 2022, 349, 460–474. [Google Scholar] [CrossRef]
- Harshita; Harish, V.; Upendra, S.L.; Mohd, S.; Singh, S.K.; Agrawal, P.; Vishwas, S.; Dua, K. Next-Gen Cancer Treatment: Nanotechnology-Driven siRNA Delivery Solutions. Assay Drug Dev. Technol. 2025, 23, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Kalaimani, K.; Balachandran, S.; Boopathy, L.K.; Roy, A.; Jayachandran, B.; Sankaranarayanan, S.; Arumugam, M.K. Recent advancements in small interfering RNA based therapeutic approach on breast cancer. Eur. J. Pharmacol. 2024, 981, 176877. [Google Scholar] [CrossRef]
- Nandy, U.; Azad Mandal, A.K.; Pranav. Nanomaterial-Mediated Nucleic Acid Delivery for Pancreatic Cancer Therapeutics. ACS Appl. Bio Mater. 2025, 8, 6676–6700. [Google Scholar] [CrossRef]
- Mignani, S.; Shi, X.; Karpus, A.; Majoral, J.P. Non-Invasive Intranasal Administration Route Directly to the Brain Using Dendrimer Nanoplatforms: An Opportunity to Develop New CNS Drugs. Eur. J. Med. Chem. 2021, 209, 112905. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Muñoz, A.; Correa-Basurto, J.; Bello, M. Binding of Folate-G4-PAMAM dendrimer conjugate with indomethacin via ligand diffusion MD simulations. J. Biomol. Struct. Dyn. 2022, 40, 4739–4749. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Guo, D.D.; Zhu, Y. Research progress in polyamide-amine dendrimer functionalized ionic separation media. Se Pu 2025, 43, 726–733. (In Chinese) [Google Scholar] [CrossRef]
- Sheykhloo, H.; Milani, M.; Najafi, F.; Bani, F.; Zarebkohan, A. Conjugation of Gentamicin to Polyamidoamine Dendrimers Improved Anti-bacterial Properties against Pseudomonas aeruginosa. Adv. Pharm. Bull. 2021, 11, 675–683. [Google Scholar] [CrossRef]
- Tamaki, M.; Kojima, C. pH-Switchable LCST/UCST-type thermosensitive behaviors of phenylalanine-modified zwitterionic dendrimers. RSC Adv. 2020, 10, 10452–10460. [Google Scholar] [CrossRef] [PubMed]
- Kojima, C.; Fu, Y.; Tamaki, M. Control of Stimuli Sensitivity in pH-Switchable LCST/UCST-Type Thermosensitive Dendrimers by Changing the Dendrimer Structure. Polymers 2022, 14, 2426. [Google Scholar] [CrossRef]
- Kim, I.D.; Shin, J.H.; Kim, S.W.; Choi, S.; Ahn, J.; Han, P.L.; Park, J.S.; Lee, H.K.; Lee, J.K. Intranasal Delivery of HMGB1 siRNA Confers Target Gene Knockdown and Robust Neuroprotection in the Post-Ischemic Brain. Mol. Ther. 2012, 20, 829–839. [Google Scholar] [CrossRef]
- Katare, Y.K.; Daya, R.P.; Sookram Gray, C.; Luckham, R.E.; Bhandari, J.; Chauhan, A.S.; Mishra, R.K. Brain Targeting of a Water-Insoluble Antipsychotic Drug Haloperidol via the Intranasal Route Using PAMAM Dendrimer. Mol. Pharm. 2015, 12, 3380–3388. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Manavi, M.S.; Nazari, A.; Momayezi, A.; Faghihkhorasani, F.; Rasool Riyadh Abdulwahid, A.H.; Rezaei-Tazangi, F.; Kavei, M.; Rezaei, R.; Mobarak, H.; et al. Nano-scale delivery systems for siRNA delivery in cancer therapy: New era of gene therapy empowered by nanotechnology. Environ. Res. 2023, 239 Pt 2, 117263. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Addison, M.L.; Dear, J.W.; Webb, D.J. Small interfering RNA: Discovery, pharmacology and clinical development-An introductory review. Br. J. Pharmacol. 2023, 180, 2697–2720. [Google Scholar] [CrossRef]
- Mestre, H.; Mori, Y.; Nedergaard, M. The Brain’s Glymphatic System: Current Controversies. Trends Neurosci. 2020, 43, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, C.; Huang, Y.; Ma, Y.; Song, Q.; Chen, H.; Jiang, G.; Gao, X. Intranasal Drug Delivery: The Interaction between Nanoparticles and the Nose-to-Brain Pathway. Adv. Drug Deliv. Rev. 2024, 207, 115196. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Shastri, D.H.; Shah, J.; Nair, A.B.; Jacob, S. Nasal Delivery to the Brain: Harnessing Nanoparticles for Effective Drug Transport. Pharmaceutics 2024, 16, 481. [Google Scholar] [CrossRef] [PubMed]
- Elkomy, M.H.; Zaki, R.M.; Alsaidan, O.A.; Elmowafy, M.; Zafar, A.; Shalaby, K.; Abdelgawad, M.A.; Abo El-Ela, F.I.; Rateb, M.E.; Naguib, I.A.; et al. Intranasal Nanotransferosomal Gel for Quercetin Brain Targeting: I. Optimization, Characterization, Brain Localization, and Cytotoxic Studies. Pharmaceutics 2023, 15, 1805. [Google Scholar] [CrossRef]
- Sanchez-Castillo, L.V.; Guareschi, F.; Tsekoura, E.; Patterlini, V.; Delledonne, A.; Ferraboschi, I.; Sissa, C.; Suman, J.; Sonvico, F.; Narain, R. Formulation of siRNA nanoparticles, transfection and enhanced adhesion -penetration in nasal mucosal tissue. J. Control Release 2025, 383, 113790. [Google Scholar] [CrossRef]
- Tam, A.; Kulkarni, J.; An, K.; Li, L.; Dorscheid, D.R.; Singhera, G.K.; Bernatchez, P.; Reid, G.; Chan, K.; Witzigmann, D.; et al. Lipid nanoparticle formulations for optimal RNA-based topical delivery to murine airways. Eur. J. Pharm. Sci. 2022, 176, 106234. [Google Scholar] [CrossRef]
- Pho, T.; Champion, J.A. Surface Engineering of Protein Nanoparticles Modulates Transport, Adsorption, and Uptake in Mucus. ACS Appl. Mater. Interfaces 2022, 14, 51697–51710. [Google Scholar] [CrossRef]
- Gao, X.; Xiong, Y.; Chen, H.; Gao, X.; Dai, J.; Zhang, Y.; Zou, W.; Gao, Y.; Jiang, Z.; Han, B. Mucus adhesion vs. mucus penetration? Screening nanomaterials for nasal inhalation by MD simulation. J. Control Release 2023, 353, 366–379. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Junior, E.R.; Silva, J.M.; Salomão, M.A.; de Almeida Oliveira, N.C.; de Freitas, C.S.; Ferreira, N.N.; Moreno, N.S.; Rodero, C.F.; Graziani, D.; Zucolotto, V.; et al. Optimized mucus adhesion and penetration of lipid-polymer nanoparticles enables effective nose-to-brain delivery of perillyl alcohol for glioblastoma therapy. Drug Deliv. Transl. Res. 2025; ahead of print. [Google Scholar] [CrossRef]
- Deshmukh, V.; Narwade, M.; Gajbhiye, K.R. Intranasal Delivery of Paclitaxel-Loaded Ligand Conjugated Polymeric Nanoparticles for Targeted Brain Delivery. AAPS PharmSciTech 2025, 26, 49. [Google Scholar] [CrossRef]
- Yang, N.; Sun, Q.; Wang, Y.; Mei, D.; Wang, X.; Zhang, J.; Liu, D.; Huo, R.; Tian, Y.; Su, Y.; et al. Endosomal disruption by co-encapsulating gentamicin in lipid nanoparticles for efficient siRNA delivery and cancer therapy. Asian J. Pharm. Sci. 2025, 20, 101011. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, H.; Du Rietz, H.; Johansson, J.M.; Eriksson, H.C.; Zedan, W.; Huang, L.; Wallin, J.; Wittrup, A. Single-cell quantification and dose-response of cytosolic siRNA delivery. Nat. Commun. 2023, 14, 1075. [Google Scholar] [CrossRef]
- Li, L.; He, A.; Zhao, H.; Tian, C.; Liu, S.; Stuart, M.A.C.; Wang, J.; Liu, W. Rational design and structure-activity relationship of random copolymers for enhanced siRNA delivery. J. Colloid. Interface Sci. 2025, 690, 137273. [Google Scholar] [CrossRef]
- Teng, Z.; Yang, J.; Chen, X.; Liu, Y. Intranasal Morphology Transformation Nanomedicines for Long-Term Intervention of Allergic Rhinitis. ACS Nano 2023, 17, 25322–25334. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, J.; Zhou, J.E.; Wang, J.; Wang, Z.; Luo, S.; Wang, Y.; Zhu, S.; Yang, F.; Tang, J.; et al. Monitoring the in vivo siRNA release from lipid nanoparticles based on the fluorescence resonance energy transfer principle. Asian J. Pharm. Sci. 2023, 18, 100769. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Li, J.; Ruan, H.; Xia, Q.; Hou, X.; Wang, Z.; Guo, T.; Zhu, C.; Feng, N.; Zhang, Y. Microneedle-mediated nose-to-brain drug delivery for improved Alzheimer’s disease treatment. J. Control Release 2024, 366, 712–731. [Google Scholar] [CrossRef]
- Arnold, A.E.; Czupiel, P.; Shoichet, M. Engineered polymeric nanoparticles to guide the cellular internalization and trafficking of small interfering ribonucleic acids. J. Control Release 2017, 259, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Hao, J.; Fang, X.; Sha, X. Factors influencing the transfection efficiency and cellular uptake mechanisms of Pluronic P123-modified polypropyleneimine/pDNA polyplexes in multidrug resistant breast cancer cells. Colloids Surf. B Biointerfaces 2016, 140, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Zhao, Y.; Miao, L.; Satterlee, A.; Haynes, M.; Luo, C.; Musetti, S.; Huang, L. Dual Functional LipoMET Mediates Envelope-type Nanoparticles to Combinational Oncogene Silencing and Tumor Growth Inhibition. Mol. Ther. 2017, 25, 1567–1579. [Google Scholar] [CrossRef]
- Maher, R.; Moreno-Borrallo, A.; Jindal, D.; Mai, B.T.; Ruiz-Hernandez, E.; Harkin, A. Intranasal Polymeric and Lipid-Based Nanocarriers for CNS Drug Delivery. Pharmaceutics 2023, 15, 746. [Google Scholar] [CrossRef]
- Lanier, O.L.; D’Andrea, A.P.; Shodeinde, A.; Peppas, N.A. siRNA Delivery from Cationic Nanocarriers Prepared by Diffusion-assisted Loading in the Presence and Absence of Electrostatic Interactions. J. Appl. Polym. Sci. 2024, 141, e55029. [Google Scholar] [CrossRef]
- Costa, C.; Oliveira, I.S.; Silva, J.P.N.; Silva, S.G.; Botelho, C.; do Vale, M.L.C.; Real Oliveira, M.E.C.D.; Gomes, A.C.; Marques, E.F. Effective cytocompatible nanovectors based on serine-derived gemini surfactants and monoolein for small interfering RNA delivery. J. Colloid. Interface Sci. 2021, 584, 34–44. [Google Scholar] [CrossRef]
- Guo, S.; Cázarez-Márquez, F.; Jiao, H.; Foppen, E.; Korpel, N.L.; Grootemaat, A.E.; Liv, N.; Gao, Y.; van der Wel, N.; Zhou, B.; et al. Specific Silencing of Microglial Gene Expression in the Rat Brain by Nanoparticle-Based Small Interfering RNA Delivery. ACS Appl. Mater. Interfaces 2022, 14, 5066–5079. [Google Scholar] [CrossRef]
- Wang, J.; Mao, K.; Cong, X.; Tan, H.; Wu, C.; Hu, Z.; Yang, Y.G.; Sun, T. Nanoparticle delivery of CD40 siRNA suppresses alloimmune responses by inhibiting activation and differentiation of DCs and macrophages. Sci. Adv. 2022, 8, eabq3699. [Google Scholar] [CrossRef]
- Jia, N.; Ma, J.; Gao, Y.; Hu, H.; Chen, D.; Zhao, X.; Yuan, Y.; Qiao, M. HA-Modified R8-Based Bola-Amphiphile Nanocomplexes for Effective Improvement of siRNA Delivery Efficiency. ACS Biomater. Sci. Eng. 2020, 6, 2084–2093. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Sun, W.; Xie, J.; Zhang, Z.; Luo, J.; Han, X.; Xiong, Y.; Yang, Y.; Zhang, Y. RNA nanotherapeutics for hepatocellular carcinoma treatment. Theranostics 2025, 15, 965–992. [Google Scholar] [CrossRef]
- Mo, Y.; Keszei, A.F.A.; Kothari, S.; Liu, H.; Pan, A.; Kim, P.; Bu, J.; Kamanzi, A.; Dai, D.L.; Mazhab-Jafari, M.T.; et al. Lipid-siRNA Organization Modulates the Intracellular Dynamics of Lipid Nanoparticles. J. Am. Chem. Soc. 2025, 147, 10430–10445. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Huang, K.; Wu, Y.; Lin, Z.; Yin, L. Hypoxia-reinforced antitumor RNA interference mediated by micelleplexes with programmed disintegration. Acta Biomater. 2022, 148, 194–205. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, F.; Liu, Y.; Zheng, M.; Wang, Y.; Zhang, D.; Anraku, Y.; Zou, Y.; Li, J.; Wu, H.; et al. Blood-brain barrier-penetrating siRNA nanomedicine for Alzheimer’s disease therapy. Sci. Adv. 2020, 6, eabc7031. [Google Scholar] [CrossRef]
- Ferguson, C.M.; Hildebrand, S.; Godinho, B.M.D.C.; Buchwald, J.; Echeverria, D.; Coles, A.; Grigorenko, A.; Vangjeli, L.; Sousa, J.; McHugh, N.; et al. Silencing Apoe with divalent-siRNAs improves amyloid burden and activates immune response pathways in Alzheimer’s disease. Alzheimers Dement. 2024, 20, 2632–2652. [Google Scholar] [CrossRef]
- Lan, H.; Zhang, W.; Jin, K.; Liu, Y.; Wang, Z. Modulating barriers of tumor microenvironment through nanocarrier systems for improved cancer immunotherapy: A review of current status and future perspective. Drug Deliv. 2020, 27, 1248–1262. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.U.; Busignies, V.; Coelho Silva Ribeiro, M.; Almeida Lage, N.; Tchoreloff, P.; Escriou, V.; Charrueau, C. Fate of Tableted Freeze-Dried siRNA Lipoplexes in Gastrointestinal Environment. Pharmaceutics 2021, 13, 1807. [Google Scholar] [CrossRef]
- Iqubal, M.K.; Kaur, H.; Md, S.; Alhakamy, N.A.; Iqubal, A.; Ali, J.; Baboota, S. A technical note on emerging combination approach involved in the onconanotherapeutics. Drug Deliv. 2022, 29, 3197–3212. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yu, J.; Luo, Q.; Bai, Y.; Gao, Q.; Yu, R. siHIF-1α-loaded micellar nanoparticles inhibit M1 macrophage activation to ameliorate chronic rhinosinusitis. Free Radic. Biol. Med. 2025, 237, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, M.C.P.; Kont, A.; Aburto, M.R.; Cryan, J.F.; O’Driscoll, C.M. Advances in the Design of (Nano)Formulations for Delivery of Antisense Oligonucleotides and Small Interfering RNA: Focus on the Central Nervous System. Mol. Pharm. 2021, 18, 1491–1506. [Google Scholar] [CrossRef]
- Guo, T.; Dong, F.; Yin, J.; Wang, X.; Min, P.; Zhang, J.; Cheng, H.; Zhang, J. A novel ultrasound-responsive cluster bomb system for efficient siRNA delivery in brain. Ultrason. Sonochem 2025, 120, 107446. [Google Scholar] [CrossRef]
- Nandi, A.; Halder, R.; Patra, D.; Hussain, A.; Roy, S.; Das Sarma, J.; Shunmugam, R. Unique Polyester-Based Biodegradable Multiarm Nano-Carrier for Cancer Cell Specific Mitochondrial Delivery of Chemotherapeutics. Biomacromolecules 2025, 26, 5182–5194. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Qi, X. Advances and Challenges of Stimuli-Responsive Nucleic Acids Delivery System in Gene Therapy. Pharmaceutics 2023, 15, 1450. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Kuo, W.T. Redox/pH-Responsive 2-in-1 Chimeric Nanoparticles for the Co-Delivery of Doxorubicin and siRNA. Polymers 2021, 13, 4362. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, X.; Zhu, Y.; Song, X.; Li, Q.; Zhang, S. A pH-Responsive Nanoplatform Based on Fluorescent Conjugated Polymer Dots for Imaging-Guided Multitherapeutics Delivery and Combination Cancer Therapy. ACS Biomater. Sci. Eng. 2022, 8, 161–169. [Google Scholar] [CrossRef]
- Sainz-Ramos, M.; Gallego, I.; Villate-Beitia, I.; Zarate, J.; Maldonado, I.; Puras, G.; Pedraz, J.L. How Far Are Non-Viral Vectors to Come of Age and Reach Clinical Translation in Gene Therapy? Int. J. Mol. Sci. 2021, 22, 7545. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Wu, W.; Liu, Y.; Li, W.; Chen, X.; Yu, S.; Gong, G.; Shu, H. Research Progress on Nasal Delivery of siRNA Nanocarrier Systems for the Treatment of Neurodegenerative Diseases. Pharmaceutics 2025, 17, 1407. https://doi.org/10.3390/pharmaceutics17111407
Huang Q, Wu W, Liu Y, Li W, Chen X, Yu S, Gong G, Shu H. Research Progress on Nasal Delivery of siRNA Nanocarrier Systems for the Treatment of Neurodegenerative Diseases. Pharmaceutics. 2025; 17(11):1407. https://doi.org/10.3390/pharmaceutics17111407
Chicago/Turabian StyleHuang, Qingqing, Wei Wu, Yinghai Liu, Weiqing Li, Xin Chen, Sixun Yu, Gu Gong, and Haifeng Shu. 2025. "Research Progress on Nasal Delivery of siRNA Nanocarrier Systems for the Treatment of Neurodegenerative Diseases" Pharmaceutics 17, no. 11: 1407. https://doi.org/10.3390/pharmaceutics17111407
APA StyleHuang, Q., Wu, W., Liu, Y., Li, W., Chen, X., Yu, S., Gong, G., & Shu, H. (2025). Research Progress on Nasal Delivery of siRNA Nanocarrier Systems for the Treatment of Neurodegenerative Diseases. Pharmaceutics, 17(11), 1407. https://doi.org/10.3390/pharmaceutics17111407







