Insights into Photo Degradation and Stabilization Strategies of Antibody–Drug Conjugates with Camptothecin Payloads
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Light Exposure Studies
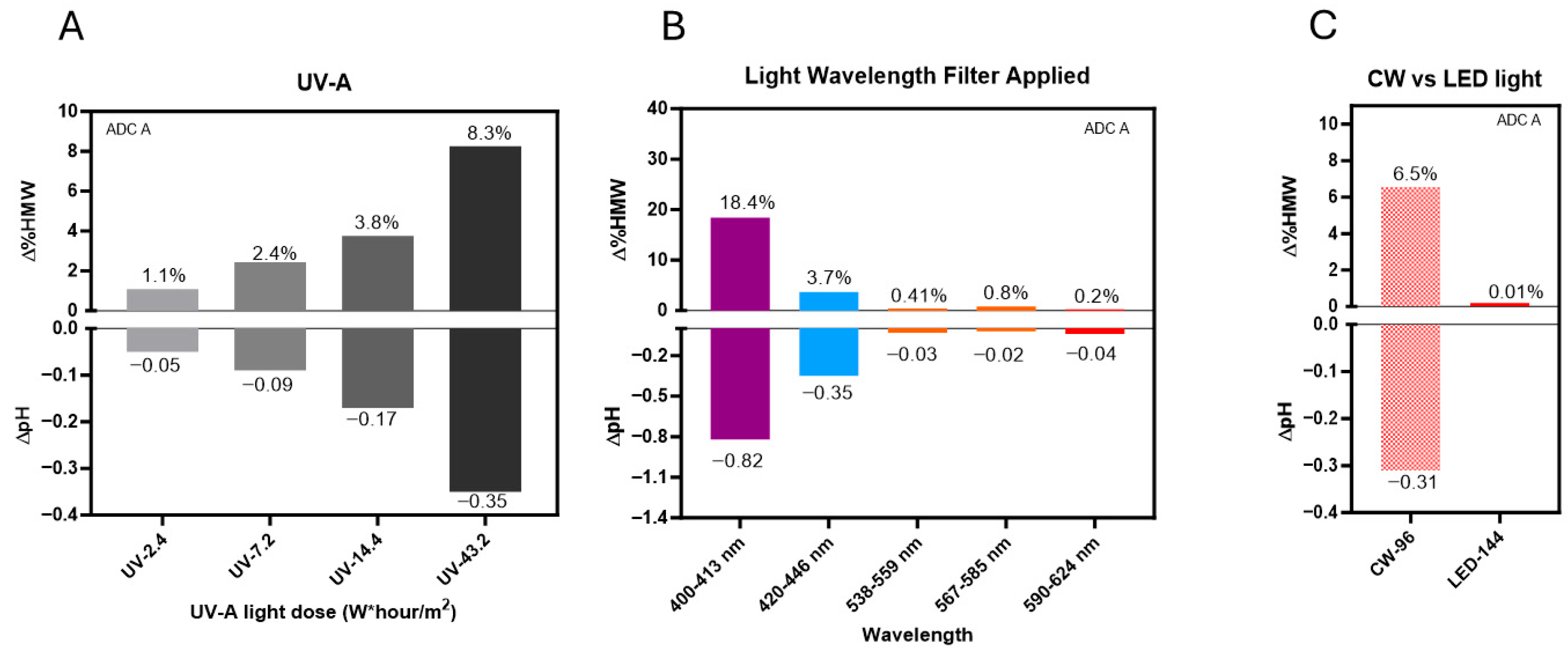
2.3. Light Wavelength Study
2.4. Size-Exclusion Ultra-Performance Liquid Chromatography (SE-UPLC)
2.5. Papain Digestion Reaction
2.6. Peptide Mapping by LC-MS/MS
2.7. Histidine Analysis LC-MS
3. Results
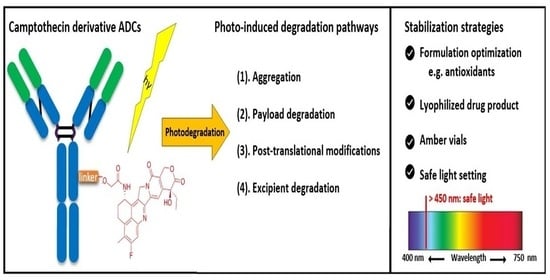
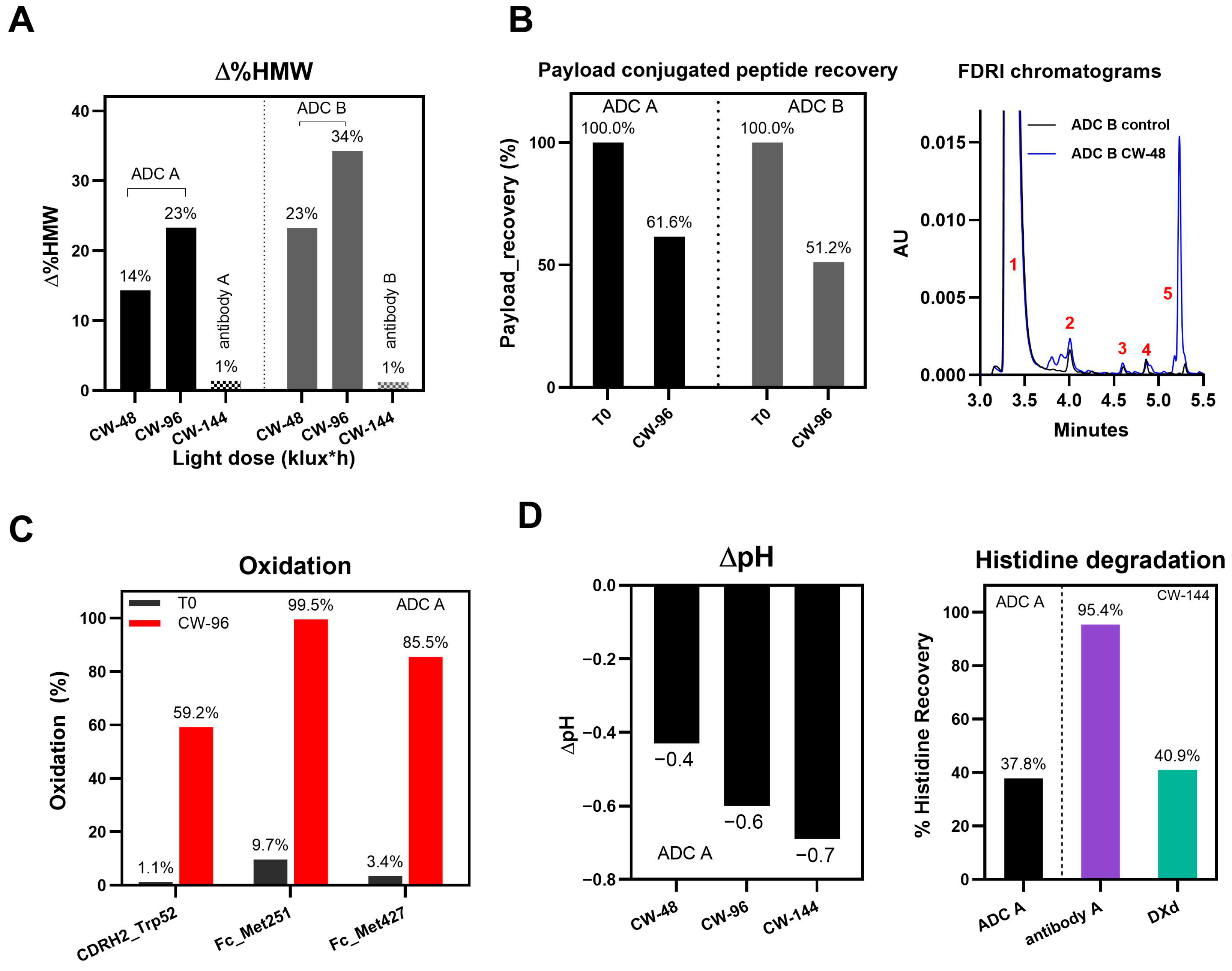
3.1. Photo Degradation Pathways of DXd ADCs
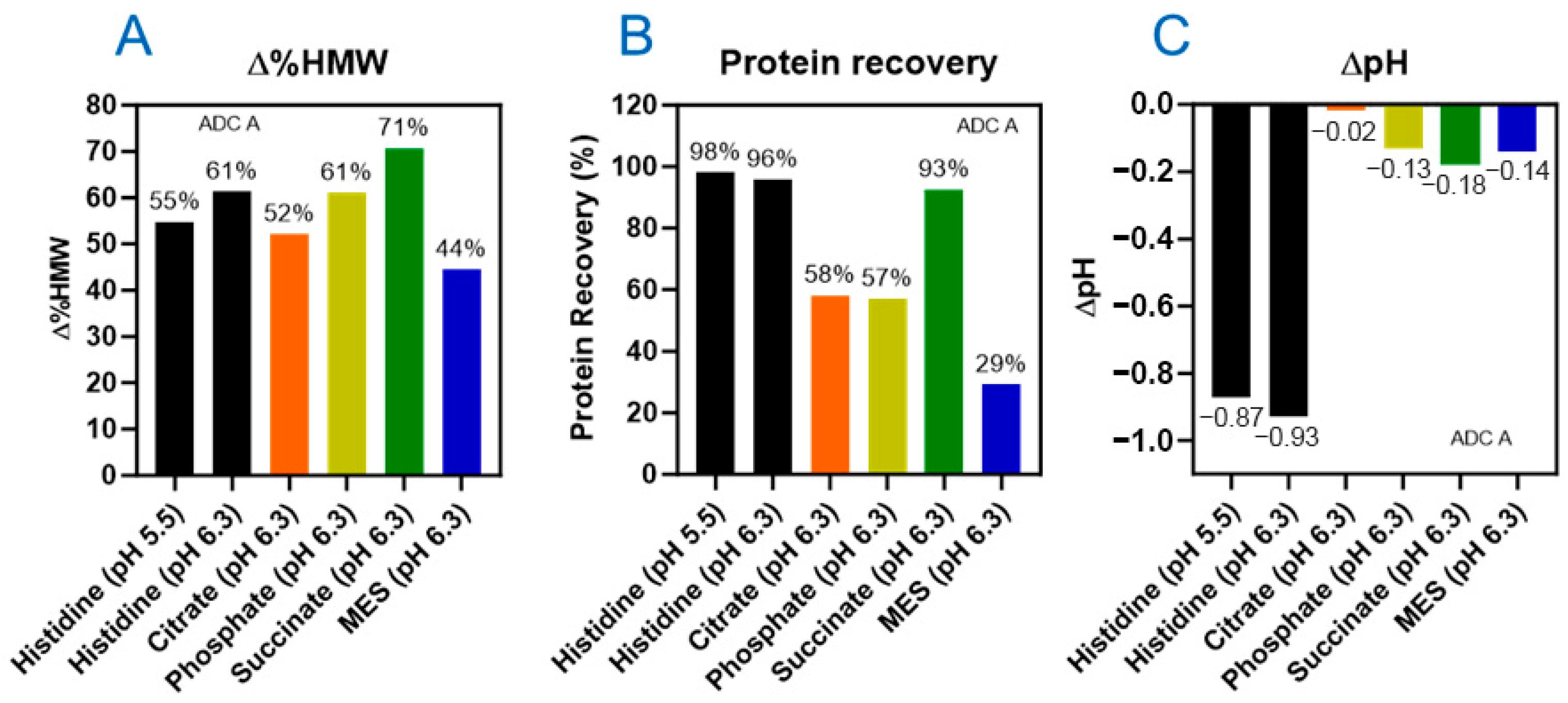
3.2. Histidine Buffer Safeguards DXd ADCs Against Light-Induced Damage
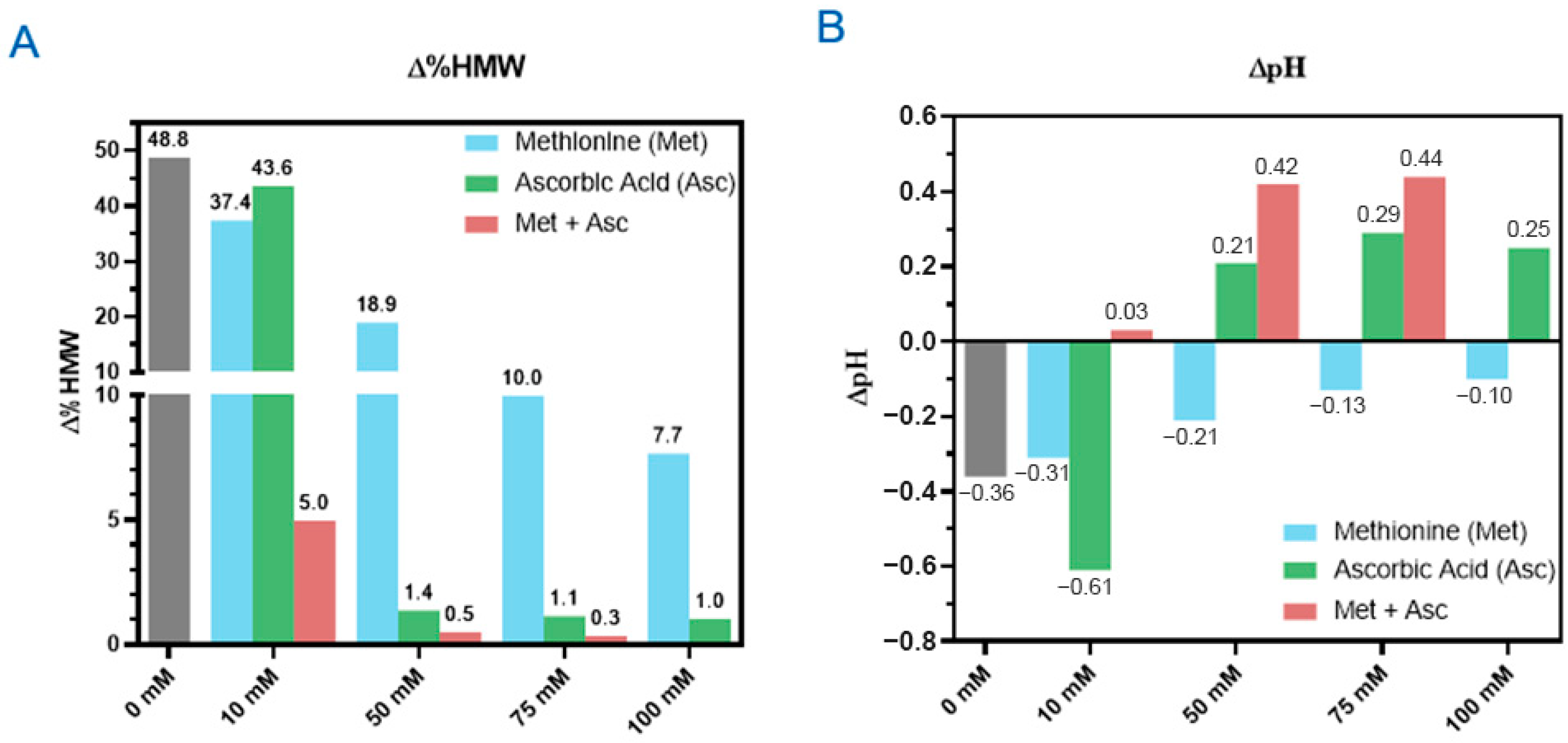
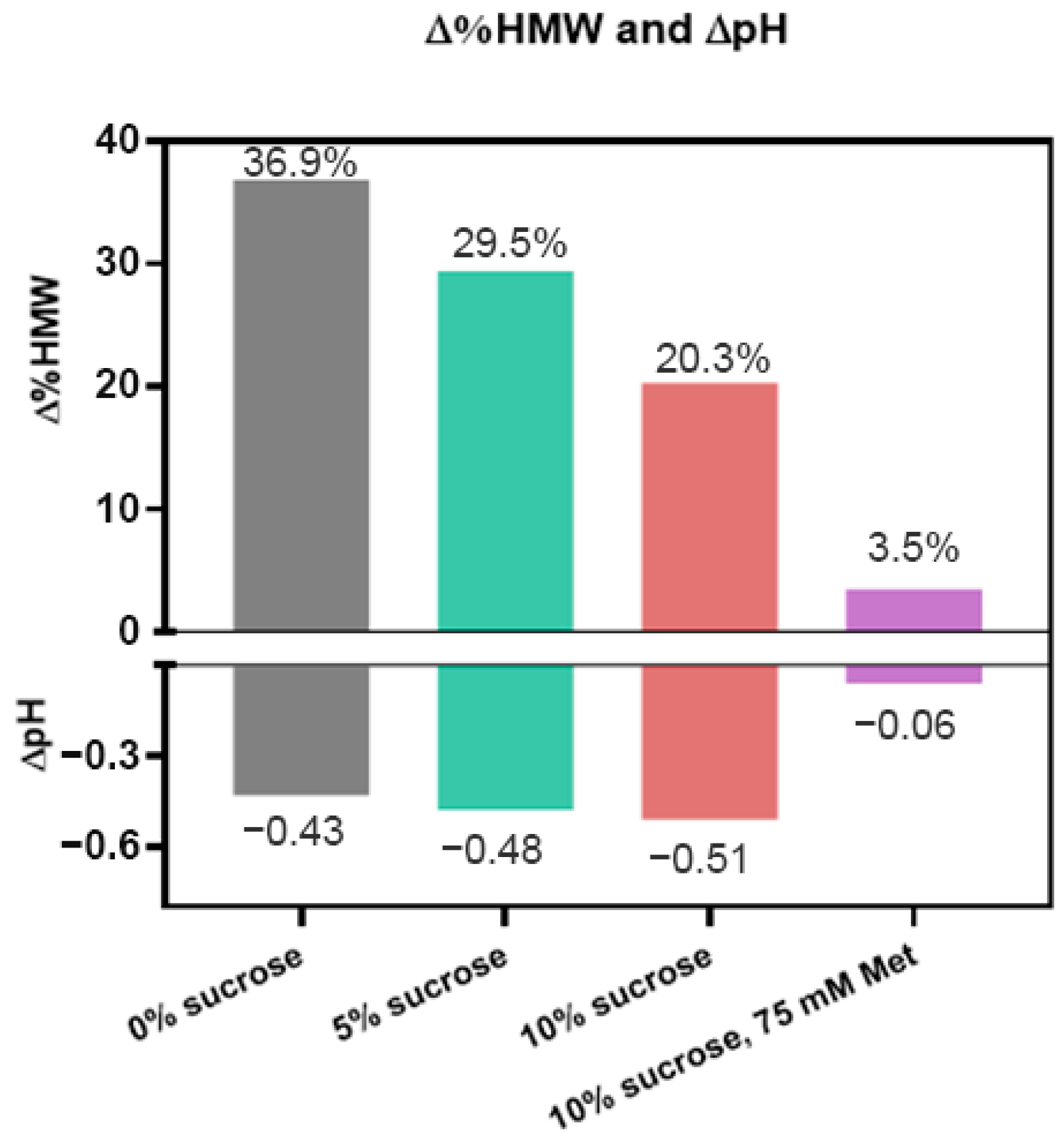
3.3. Three Excipients Significantly Enhance Photostability of DXd ADCs
3.4. DXd ADCs Are More Sensitive to Short-Wavelength Light
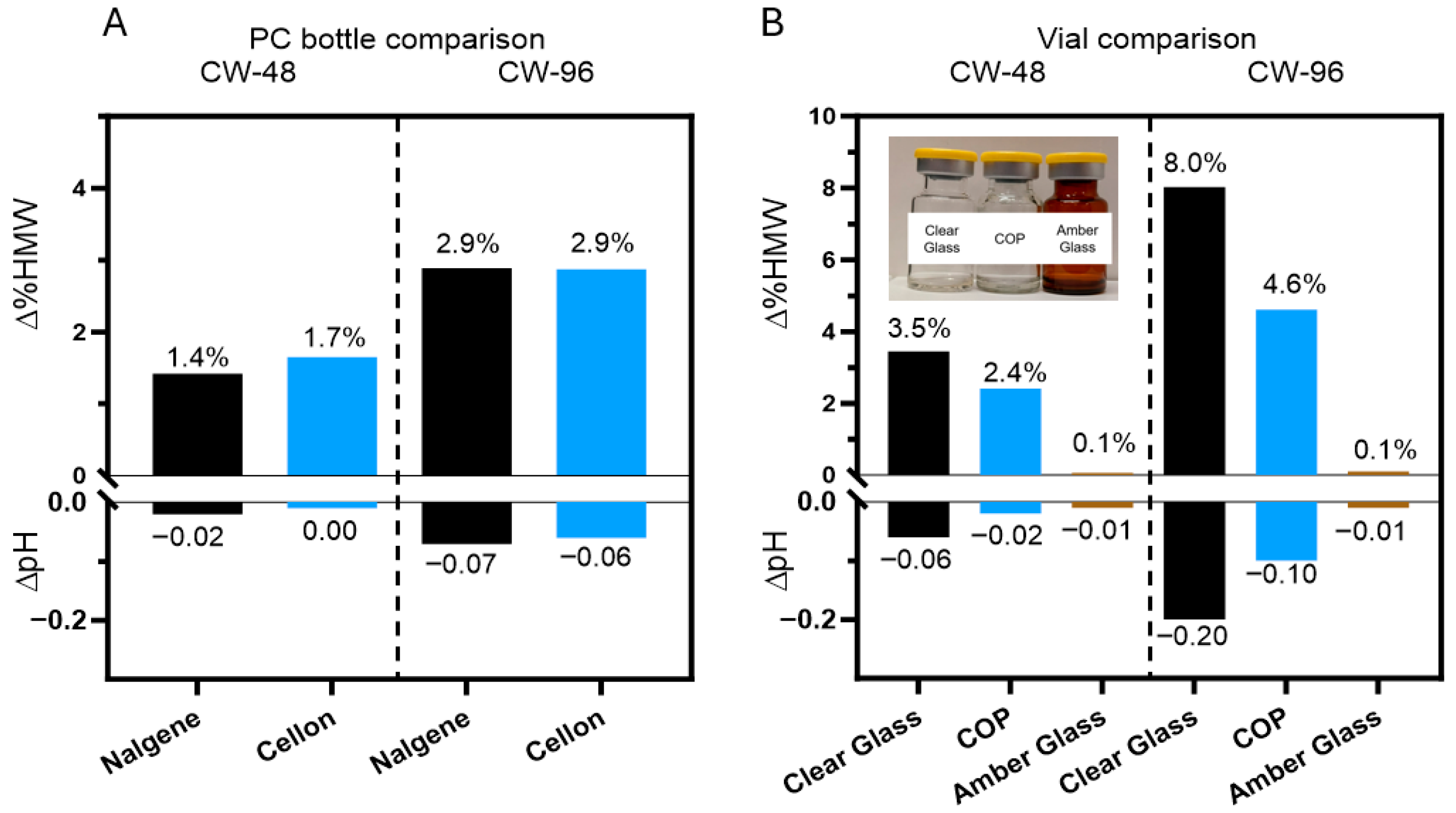
3.5. Containers Can Further Mitigate Photo Degradation of DXd ADCs
4. Discussion
4.1. Photo-Induced Degradation Pathways of DXd ADCs
4.2. Stabilization of DXd ADCs Through Formulation Development
4.3. Other Photostabilization Strategies for DXd-ADC Development
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Antibody–drug conjugates |
| CPT | Camptothecin |
| DAR | Drug-to-antibody ratio |
| DXd | Deruxtecan |
| FDRI | Free drug-related impurities |
| HMW | High molecular weight |
| PTM | Post-translational modifications |
References
- Conilh, L.; Sadilkova, L.; Viricel, W.; Dumontet, C. Payload diversification: A key step in the development of antibody-drug conjugates. J. Hematol. Oncol. 2023, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Pandiella, A.; Vargas-Castrillon, E.; Diaz-Rodriguez, E.; Iglesias-Hernangomez, T.; Martinez Cano, C.; Fernandez-Cuesta, I.; Winkow, E.; Perello, M.F. Trastuzumab deruxtecan in breast cancer. Crit. Rev. Oncol. Hematol. 2024, 198, 104355. [Google Scholar] [CrossRef]
- Cockrell, G.M.; Wolfe, M.S.; Wolfe, J.L.; Schoneich, C. Photoinduced aggregation of a model antibody-drug conjugate. Mol. Pharm. 2015, 12, 1784–1797. [Google Scholar] [CrossRef]
- Shah, D.D.; Zhang, J.; Maity, H.; Mallela, K.M.G. Effect of photo-degradation on the structure, stability, aggregation, and function of an igg1 monoclonal antibody. Int. J. Pharm. 2018, 547, 438–449. [Google Scholar] [CrossRef]
- Du, C.; Barnett, G.; Borwankar, A.; Lewandowski, A.; Singh, N.; Ghose, S.; Borys, M.; Li, Z.J. Protection of therapeutic antibodies from visible light induced degradation: Use safe light in manufacturing and storage. Eur. J. Pharm. Biopharm. 2018, 127, 37–43. [Google Scholar] [CrossRef]
- Lei, M.; Quan, C.; Wang, Y.J.; Kao, Y.-H.; Schöneich, C. Light-induced covalent buffer adducts to histidine in a model protein. Pharm. Res. 2018, 35, 67. [Google Scholar] [CrossRef]
- Zhang, Z.; Chow, S.Y.; De Guzman, R.; Joh, N.H.; Joubert, M.K.; Richardson, J.; Shah, B.; Wikstrom, M.; Zhou, Z.S.; Wypych, J. A mass spectrometric characterization of light-induced modifications in therapeutic proteins. J. Pharm. Sci. 2022, 111, 1556–1564. [Google Scholar] [CrossRef]
- Xu, C.F.; Chen, Y.; Yi, L.; Brantley, T.; Stanley, B.; Sosic, Z.; Zang, L. Discovery and characterization of histidine oxidation initiated cross-links in an igg1 monoclonal antibody. Anal. Chem. 2017, 89, 7915–7923. [Google Scholar] [CrossRef]
- Luis, L.M.; Hu, Y.; Zamiri, C.; Sreedhara, A. Determination of the acceptable ambient light exposure during drug product manufacturing for long-term stability of monoclonal antibodies. PDA J. Pharm. Sci. Technol. 2018, 72, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B. Immunogenicity of therapeutic proteins. Part 3: Impact of manufacturing changes. Biotechnol. Adv. 2007, 25, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Perez Medina Martinez, V.; Robles, M.C.; Juarez-Bayardo, L.C.; Espinosa-de la Garza, C.E.; Meneses, A.; Perez, N.O. Photodegradation of rituximab and critical evaluation of its sensibility to electromagnetic radiation. AAPS PharmSciTech 2022, 23, 271. [Google Scholar] [CrossRef] [PubMed]
- Lam, X.M.; Yang, J.Y.; Cleland, J.L. Antioxidants for prevention of methionine oxidation in recombinant monoclonal antibody her2. J. Pharm. Sci. 1997, 86, 1250–1255. [Google Scholar] [CrossRef]
- Wang, W.; Vlasak, J.; Li, Y.; Pristatsky, P.; Fang, Y.; Pittman, T.; Roman, J.; Wang, Y.; Prueksaritanont, T.; Ionescu, R. Impact of methionine oxidation in human igg1 fc on serum half-life of monoclonal antibodies. Mol. Immunol. 2011, 48, 860–866. [Google Scholar] [CrossRef]
- Bertolotti-Ciarlet, A.; Wang, W.; Lownes, R.; Pristatsky, P.; Fang, Y.; McKelvey, T.; Li, Y.; Li, Y.; Drummond, J.; Prueksaritanont, T.; et al. Impact of methionine oxidation on the binding of human igg1 to fc rn and fc gamma receptors. Mol. Immunol. 2009, 46, 1878–1882. [Google Scholar] [CrossRef]
- Pan, H.; Chen, K.; Chu, L.; Kinderman, F.; Apostol, I.; Huang, G. Methionine oxidation in human igg2 fc decreases binding affinities to protein a and fcrn. Protein Sci. 2009, 18, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Walton, W.J.; Zhang, S.J.; Wilson, J.J.; Harvey, B.N.; Clemens, M.; Gu, Y. Impact of monoclonal antibody aggregates on effector function characterization. Antibodies 2025, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Lown, J.W.; Hsiao-Hsiung, C. Studies on the effects of the antitumor agent camptothecin and derivatives on deoxyribonucleic acid: Mechanism of the scission of deoxyribonucleic acid by photoactivated camptothecin. Biochem. Pharmacol. 1980, 29, 905–915. [Google Scholar] [CrossRef]
- Dodds, H.M.; Craik, D.J.; Rivory, L.P. Photodegradation of irinotecan (cpt-11) in aqueous solutions: Identification of fluorescent products and influence of solution composition. J. Pharm. Sci. 1997, 86, 1410–1416. [Google Scholar] [CrossRef]
- Mahalingaiah, P.K.; Ciurlionis, R.; Durbin, K.R.; Yeager, R.L.; Philip, B.K.; Bawa, B.; Mantena, S.R.; Enright, B.P.; Liguori, M.J.; Van Vleet, T.R. Potential mechanisms of target-independent uptake and toxicity of antibody-drug conjugates. Pharmacol. Ther. 2019, 200, 110–125. [Google Scholar] [CrossRef]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaia, N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef]
- Paul, R.; Graff-Meyer, A.; Stahlberg, H.; Lauer, M.E.; Rufer, A.C.; Beck, H.; Briguet, A.; Schnaible, V.; Buckel, T.; Boeckle, S. Structure and function of purified monoclonal antibody dimers induced by different stress conditions. Pharm. Res. 2012, 29, 2047–2059. [Google Scholar] [CrossRef] [PubMed]
- Bessa, J.; Boeckle, S.; Beck, H.; Buckel, T.; Schlicht, S.; Ebeling, M.; Kiialainen, A.; Koulov, A.; Boll, B.; Weiser, T.; et al. The immunogenicity of antibody aggregates in a novel transgenic mouse model. Pharm. Res. 2015, 32, 2344–2359. [Google Scholar] [CrossRef]
- Li, Y.; Gu, C.; Gruenhagen, J.; Yehl, P.; Chetwyn, N.P.; Medley, C.D. An enzymatic deconjugation method for the analysis of small molecule active drugs on antibody-drug conjugates. MAbs 2016, 8, 698–705. [Google Scholar] [CrossRef]
- Liu, D.; Ren, D.; Huang, H.; Dankberg, J.; Rosenfeld, R.; Cocco, M.J.; Li, L.; Brems, D.N.; Remmele, R.L., Jr. Structure and stability changes of human igg1 fc as a consequence of methionine oxidation. Biochemistry 2008, 47, 5088–5100. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef]
- Ji, J.A.; Zhang, B.; Cheng, W.; Wang, Y.J. Methionine, tryptophan, and histidine oxidation in a model protein, pth: Mechanisms and stabilization. J. Pharm. Sci. 2009, 98, 4485–4500. [Google Scholar] [CrossRef] [PubMed]
- Ziomkowska, B. Deactivation rate of camptothecin determined by factor analysis of steady-state fluorescence and absorption spectra. Opt. Appicata 2006, 36, 137–146. [Google Scholar]
- Qi, P.; Volkin, D.B.; Zhao, H.; Nedved, M.L.; Hughes, R.; Bass, R.; Yi, S.C.; Panek, M.E.; Wang, D.; Dalmonte, P.; et al. Characterization of the photodegradation of a human igg1 monoclonal antibody formulated as a high-concentration liquid dosage form. J. Pharm. Sci. 2009, 98, 3117–3130. [Google Scholar] [CrossRef]
- Sreedhara, A.; Yin, J.; Joyce, M.; Lau, K.; Wecksler, A.T.; Deperalta, G.; Yi, L.; John Wang, Y.; Kabakoff, B.; Kishore, R.S. Effect of ambient light on igg1 monoclonal antibodies during drug product processing and development. Eur. J. Pharm. Biopharm. 2016, 100, 38–46. [Google Scholar] [CrossRef]
- Hernandez-Jimenez, J.; Salmeron-Garcia, A.; Cabeza, J.; Velez, C.; Capitan-Vallvey, L.F.; Navas, N. The effects of light-accelerated degradation on the aggregation of marketed therapeutic monoclonal antibodies evaluated by size-exclusion chromatography with diode array detection. J. Pharm. Sci. 2016, 105, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Schoneich, C. Photo-degradation of therapeutic proteins: Mechanistic aspects. Pharm. Res. 2020, 37, 45. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Chapter nine—Ascorbic acid as antioxidant. In Vitamins and Hormones; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2023; Volume 121, pp. 247–270. [Google Scholar]
- Peng, M.; Liu, Y.; Zhang, H.; Cui, Y.; Zhai, G.; Chen, C. Photostability study of doxorubicin aqueous solution enhanced by inclusion interaction between doxorubicin and hydroxypropyl-β-cyclodextrin. Chin. J. Chem. 2010, 28, 1291–1295. [Google Scholar] [CrossRef]
- Syed, Y.Y. Sacituzumab govitecan: First approval. Drugs 2020, 80, 1019–1025. [Google Scholar] [CrossRef]
- Matsukawa, R.; Yamane, M.; Kanai, M. Histidine photooxygenation chemistry: Mechanistic evidence and elucidation. Chem. Rec. 2023, 23, e202300198. [Google Scholar] [CrossRef] [PubMed]
- Agon, V.V.; Bubb, W.A.; Wright, A.; Hawkins, C.L.; Davies, M.J. Sensitizer-mediated photooxidation of histidine residues: Evidence for the formation of reactive side-chain peroxides. Free Radic. Biol. Med. 2006, 40, 698–710, Erratum in Free Radic. Biol. Med. 2006, 40, 2242. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yamniuk, A.; Dai, J.; Chen, S.; Stetsko, P.; Ditto, N.; Zhang, Y. Investigation of a degradant in a biologics formulation buffer containing l-histidine. Pharm. Res. 2015, 32, 2625–2635. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, Y.; Shintani, K.; Hayashihara-Kakuhou, K.; Zukawa, T.; Morita, Y.; Nakazawa, T.; Yoshida, T.; Ohkubo, T.; Uchiyama, S. Effect of uvc irradiation on the oxidation of histidine in monoclonal antibodies. Sci. Rep. 2020, 10, 6333. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Z.; Cheetham, J.; Ren, D.; Zhou, Z.S. Discovery and characterization of a photo-oxidative histidine-histidine cross-link in igg1 antibody utilizing 18O-labeling and mass spectrometry. Anal. Chem. 2014, 86, 4940–4948. [Google Scholar] [CrossRef]
- Weber, J.; Buske, J.; Mader, K.; Garidel, P.; Diederichs, T. Oxidation of polysorbates—An underestimated degradation pathway? Int. J. Pharm. X 2023, 6, 100202. [Google Scholar] [CrossRef]
- Kerwin, B.A. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: Structure and degradation pathways. J. Pharm. Sci. 2008, 97, 2924–2935. [Google Scholar] [CrossRef]
- Donbrow, M.; Azaz, E.; Pillersdorf, A. Autoxidation of polysorbates. J. Pharm. Sci. 1978, 67, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Gopalrathnam, G.; Sharma, A.N.; Dodd, S.W.; Huang, L. Impact of stainless steel exposure on the oxidation of polysorbate 80 in histidine placebo and active monoclonal antibody formulation. PDA J. Pharm. Sci. Technol. 2018, 72, 163–175. [Google Scholar] [CrossRef]
- Lei, M.; Quan, C.; Wang, J.Y.; Kao, Y.H.; Schoneich, C. Light-induced histidine adducts to an igg1 molecule via oxidized histidine residue and the potential impact of polysorbate-20 concentration. Pharm. Res. 2021, 38, 491–501. [Google Scholar] [CrossRef]
- Baptista, M.S.; Cadet, J.; Greer, A.; Thomas, A.H. Photosensitization reactions of biomolecules: Definition, targets and mechanisms. Photochem. Photobiol. 2021, 97, 1456–1483. [Google Scholar] [CrossRef]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type i and type ii photosensitized oxidation reactions: Guidelines and mechanistic pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef]
- Escudero, D. Revising intramolecular photoinduced electron transfer (pet) from first-principles. Acc. Chem. Res. 2016, 49, 1816–1824. [Google Scholar]
- Liu, J.; Wei, Y.; Ma, L.; Jin, X.; Sun, S.; Liu, M.; Du, J.; Xu, X.; Tian, H.; Ma, X. Photo-induced energy transfer polymerization. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Van den Ende, W.; Valluru, R. Sucrose, sucrosyl oligosaccharides, and oxidative stress: Scavenging and salvaging? J. Exp. Bot. 2009, 60, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Weiss, S.J.; Levine, R.L. Methionine oxidation and reduction in proteins. Biochim. Biophys. Acta 2014, 1840, 901–905. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M.; Tu, Y.J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Kanoh, M.; Okazaki, M.; Maeda, R.; Mori, S.; Akimoto, S.; Inagaki, M.; Nishi, H. Photoprotective effects of selected amino acids on naproxen photodegradation in aqueous media. Pharmaceuticals 2020, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Detoni, C.B.; Souto, G.D.; da Silva, A.L.; Pohlmann, A.R.; Guterres, S.S. Photostability and skin penetration of different e-resveratrol-loaded supramolecular structures. Photochem. Photobiol. 2012, 88, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Johann, F.; Woll, S.; Gieseler, H. Evaluating the potential of cyclodextrins in reducing aggregation of antibody-drug conjugates with different payloads. J. Pharm. Sci. 2024, 113, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Celestino, M.T.; Magalhães, U.d.O.; Fraga, A.G.M.; Carmo, F.A.d.; Lione, V.; Castro, H.C.; Sousa, V.P.d.; Rodrigues, C.R.; Cabral, L.M. Rational use of antioxidants in solid oral pharmaceutical preparations. Braz. J. Pharm. Sci. 2012, 48, 405–415. [Google Scholar] [CrossRef]
| FDRI Peak | Structure * |
|---|---|
| 1 | 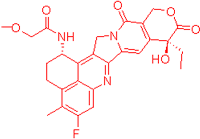 |
| 2 | 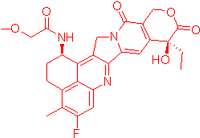 |
| 3 | 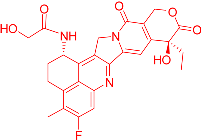 |
| 4 | 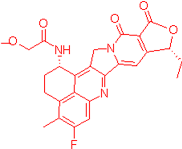 |
| 5 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, S.; Bulos, J.; Uroza, R.; Zhao, Y.; Pan, X.; Su, Y.; Qiu, H.; Olagunju, B.; Wang, W.; Liu, D.; et al. Insights into Photo Degradation and Stabilization Strategies of Antibody–Drug Conjugates with Camptothecin Payloads. Pharmaceutics 2025, 17, 1397. https://doi.org/10.3390/pharmaceutics17111397
Luo S, Bulos J, Uroza R, Zhao Y, Pan X, Su Y, Qiu H, Olagunju B, Wang W, Liu D, et al. Insights into Photo Degradation and Stabilization Strategies of Antibody–Drug Conjugates with Camptothecin Payloads. Pharmaceutics. 2025; 17(11):1397. https://doi.org/10.3390/pharmaceutics17111397
Chicago/Turabian StyleLuo, Shukun, Joshua Bulos, Ricky Uroza, Yimeng Zhao, Xiao Pan, Yue Su, Haibo Qiu, Babatunde Olagunju, Wenhua Wang, Dingjiang Liu, and et al. 2025. "Insights into Photo Degradation and Stabilization Strategies of Antibody–Drug Conjugates with Camptothecin Payloads" Pharmaceutics 17, no. 11: 1397. https://doi.org/10.3390/pharmaceutics17111397
APA StyleLuo, S., Bulos, J., Uroza, R., Zhao, Y., Pan, X., Su, Y., Qiu, H., Olagunju, B., Wang, W., Liu, D., & Shameem, M. (2025). Insights into Photo Degradation and Stabilization Strategies of Antibody–Drug Conjugates with Camptothecin Payloads. Pharmaceutics, 17(11), 1397. https://doi.org/10.3390/pharmaceutics17111397