Toward Genotype-Informed Dosing of Voriconazole: Head-to-Head Simulations Across CYP2C19 Phenotypes Using Population Pharmacokinetic Models
Abstract
1. Introduction
2. Materials and Methods
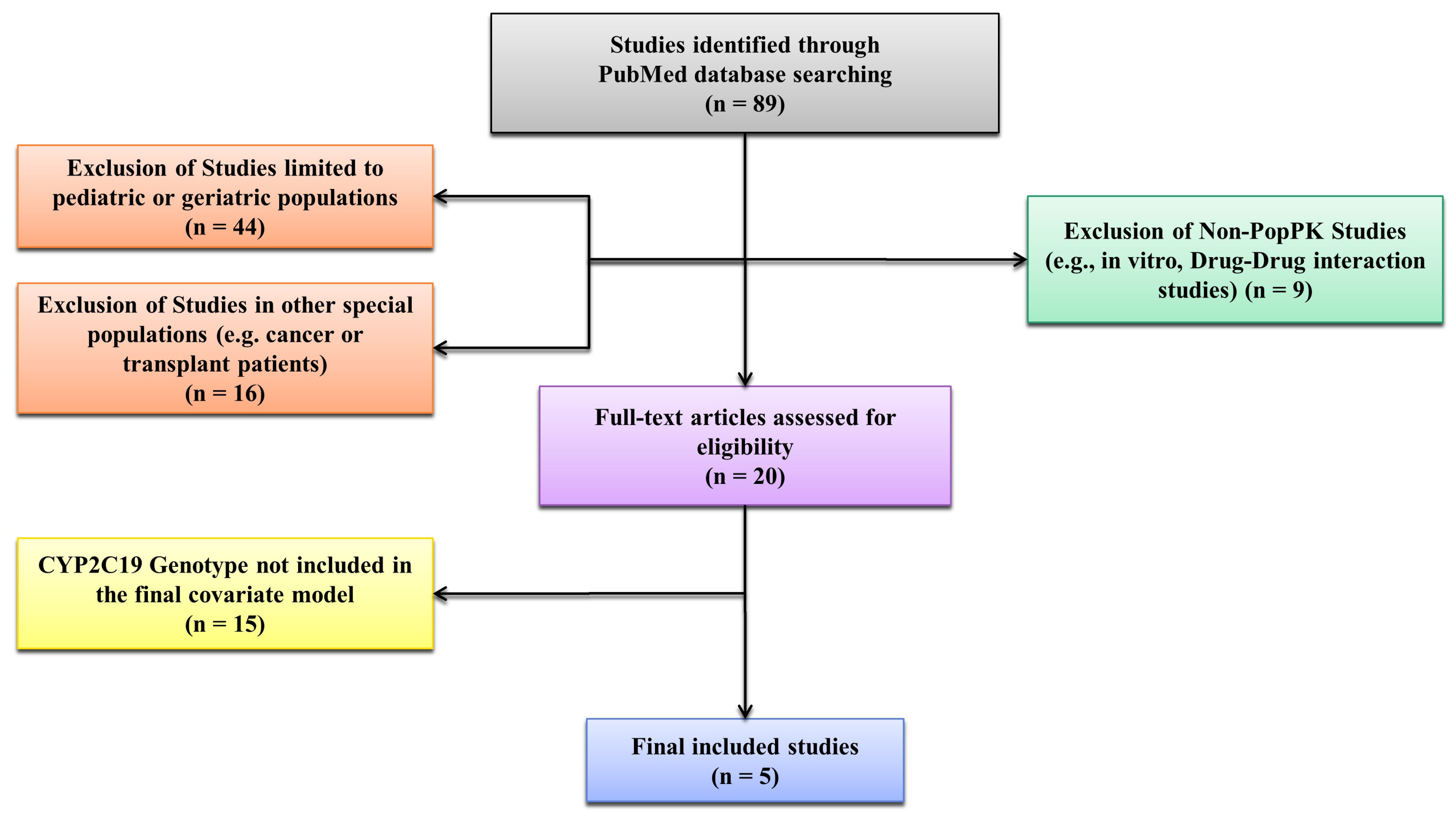
2.1. Literature Search and Model Selection
2.2. Virtual Cohort Generation and Covariates
2.3. Model Implementation and Simulations (Primary and Sensitivity Analyses)
2.4. Exposure Metrics and Genotype Stratification
- AUCτ,ss (area under the plasma concentration–time curve over τ at steady state);
- Ctrough,ss (minimum observed concentration within τ);
- Cavg,ss (average concentration, calculated as AUCτ,ss/τ);
- Cmax,ss (maximum observed concentration within τ);
- CLss/F (apparent clearance at steady state, calculated as Dose/AUCτ,ss); and
- Vss/F (apparent volume of distribution at steady state, derived as CLss/F × MRTss, where MRTss is mean residence time).
2.5. Pharmacodynamic Targets and Surrogate
3. Results
3.1. Simulation Overview
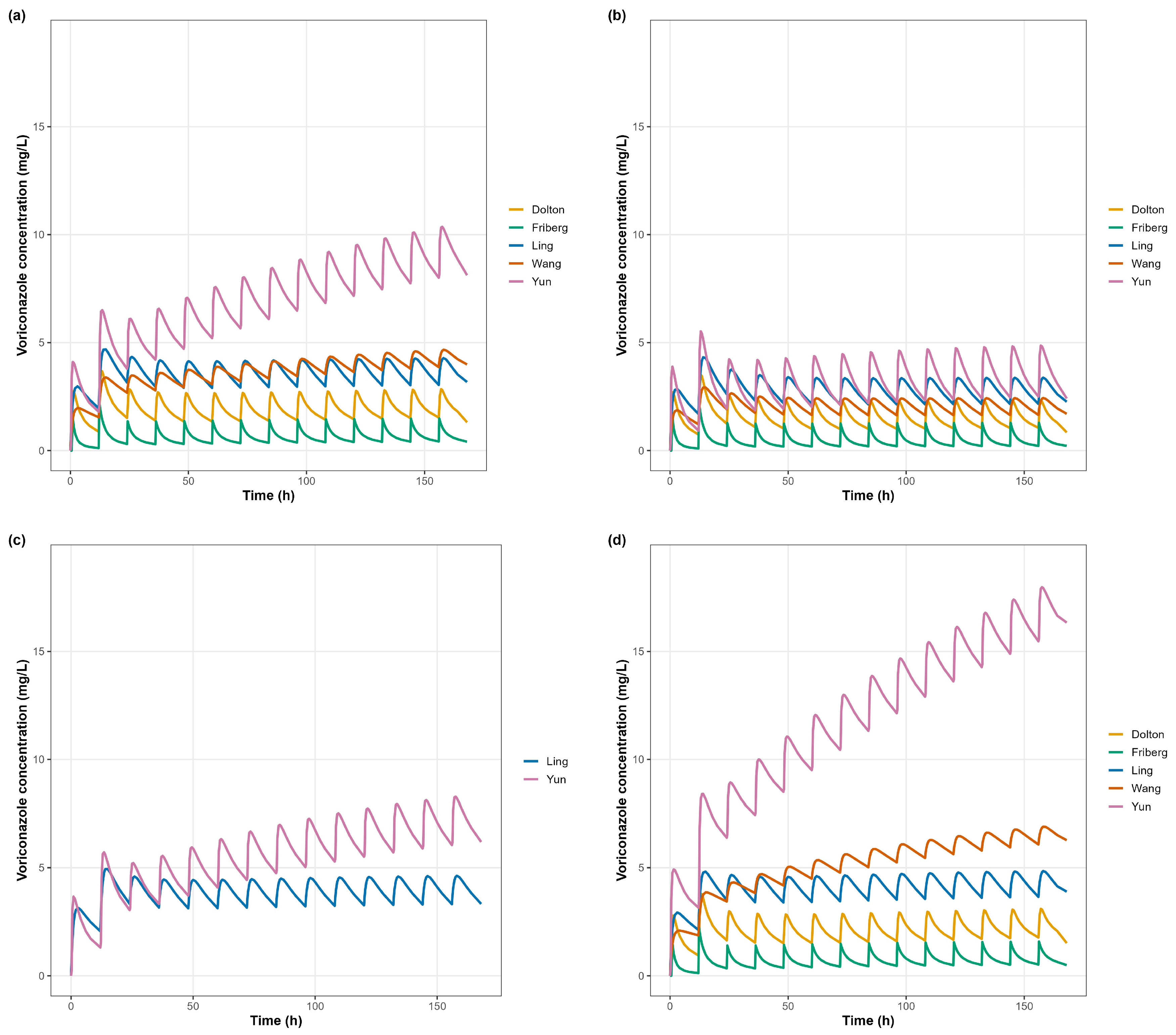
3.2. Concentration–Time Profiles (Primary Analysis)
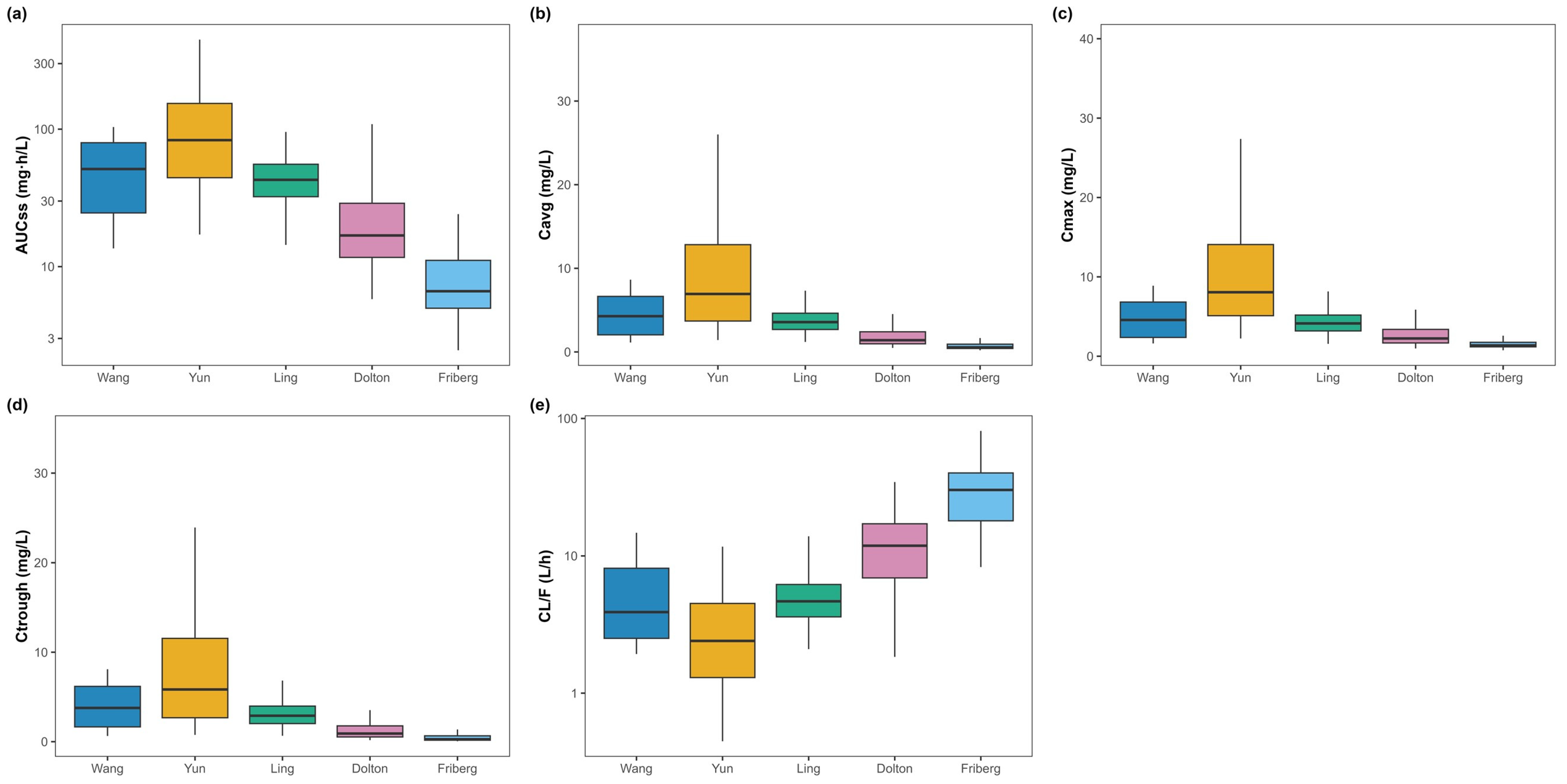
3.3. Primary Exposure Parameters
- Yun predicted the highest exposure and lowest clearance (AUCτ,ss 110.47 ± 85.79 mg·h/L; Cavg,ss 9.21 ± 7.15 mg/L; Ctrough,ss 7.96 ± 6.77 mg/L; CLss/F 3.19 ± 2.40 L/h).
- Friberg predicted the lowest exposure and highest clearance (AUCτ,ss 8.34 ± 4.58 mg·h/L; Cavg,ss 0.70 ± 0.38 mg/L; Ctrough,ss 0.42 ± 0.33 mg/L; CLss/F 30.94 ± 14.56 L/h).
- Wang and Ling yielded intermediate exposure (AUCτ,ss 51.91 ± 28.55 and 44.61 ± 15.75 mg·h/L; CLss/F 5.50 ± 3.20 and 5.12 ± 1.98 L/h, respectively).
- Dolton produced lower exposure with mid-to-higher clearance (AUCτ,ss 24.61 ± 20.63 mg·h/L; CLss/F 12.50 ± 6.89 L/h).
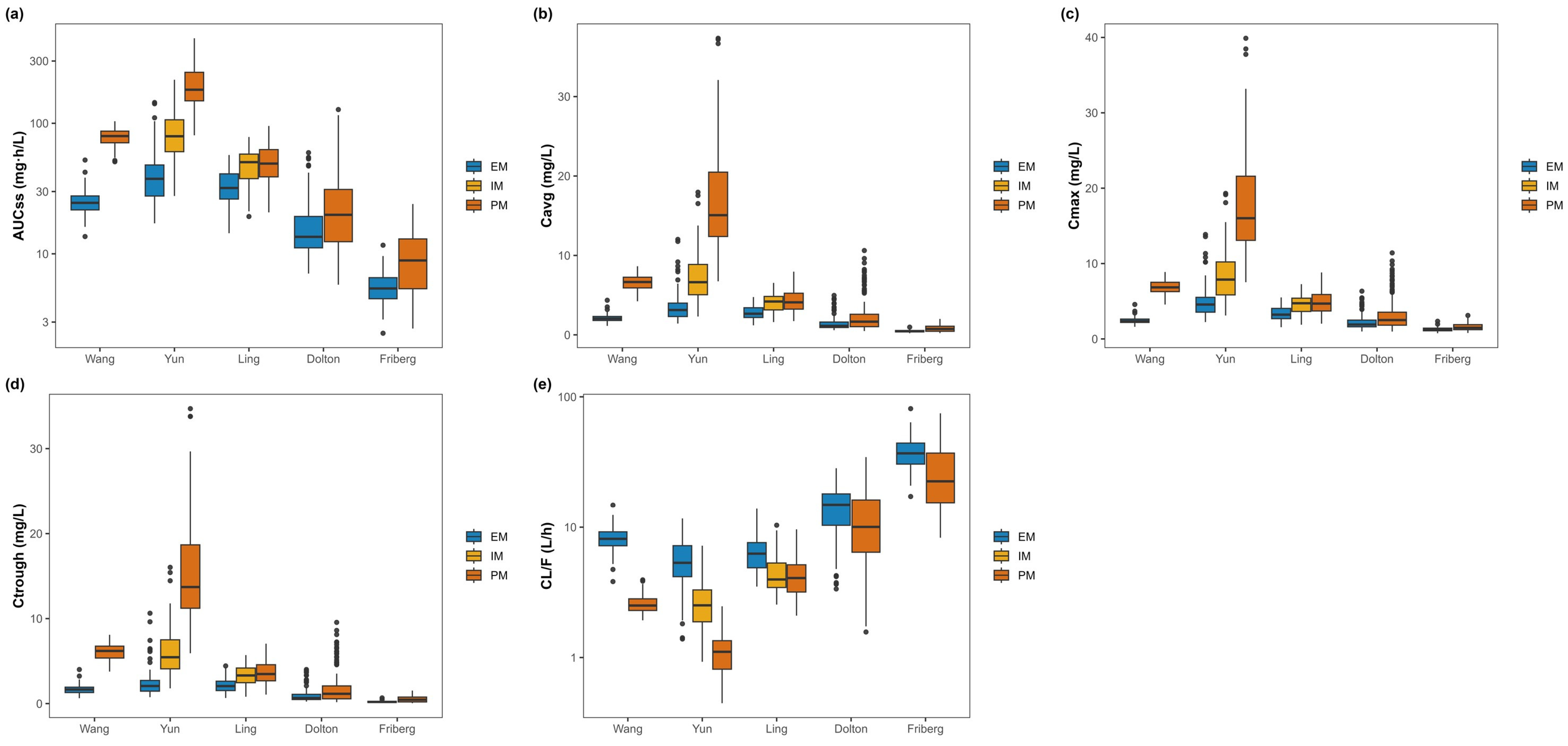
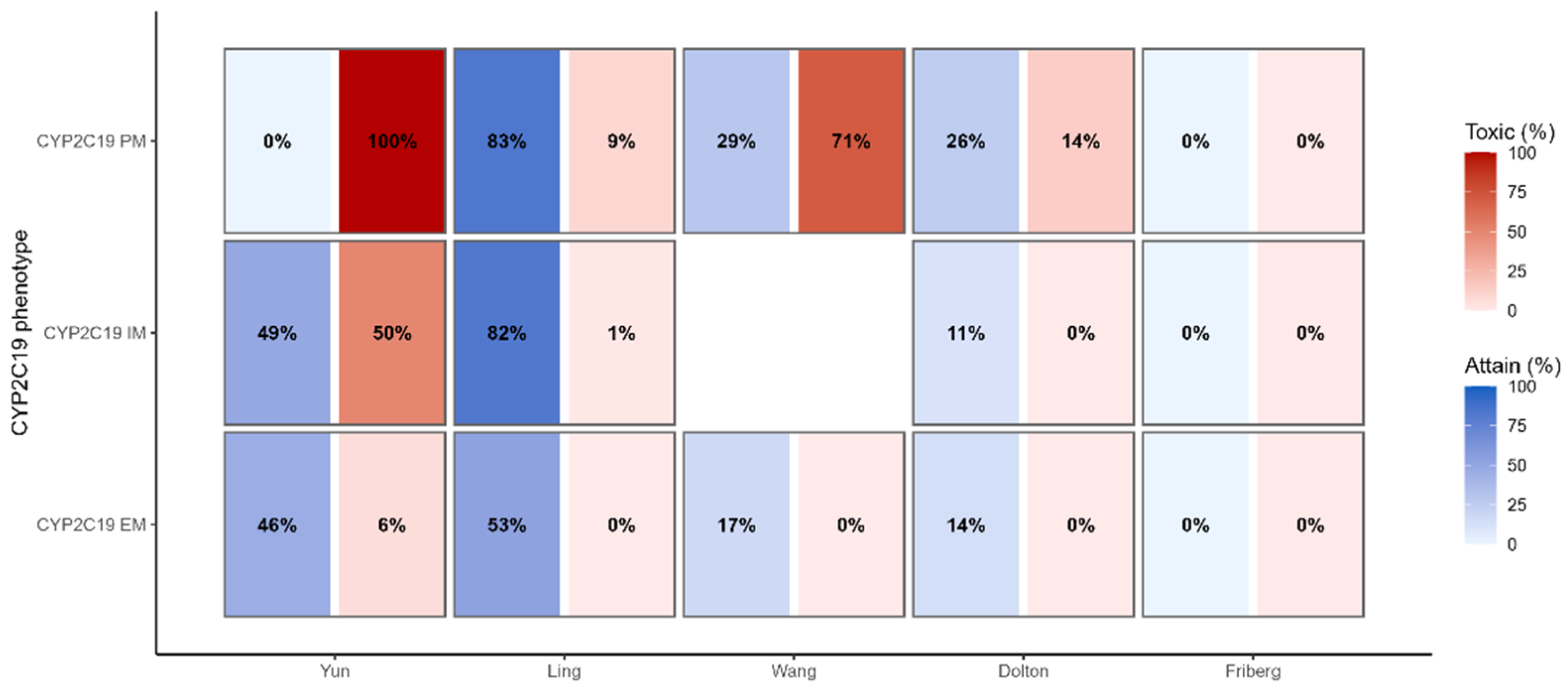
3.4. Genotype-Stratified Exposure (Primary Analysis)
- Yun showed a clear stepwise increase in exposure from EM → IM → PM with a reciprocal decline in CLss/F (AUCτ,ss: 42.64 ± 23.12, 86.52 ± 37.24, 202.25 ± 81.08 mg·h/L; Ctrough,ss: 2.49 ± 1.65, 6.05 ± 2.79, 15.34 ± 6.25 mg/L; CLss/F: 5.69 ± 2.27, 2.27 ± 1.15, 1.15 ± 0.45 L/h for EM, IM, PM).
- Ling exhibited the same directionality with a modest gradient (AUCτ,ss EM 33.70 ± 9.92, IM 48.35 ± 13.80, PM 51.78 ± 16.56 mg·h/L; CLss/F EM 6.49 ± 2.06, IM 4.56 ± 1.59, PM 4.29 ± 1.47 L/h).
- Wang: PM exceeded EM in exposure with lower clearance (AUCτ,ss 24.90 ± 5.73 vs. 78.92 ± 11.49 mg·h/L; Ctrough,ss 1.65 ± 0.53 vs. 6.05 ± 0.98 mg/L; CLss/F 8.42 ± 1.81 vs. 2.59 ± 0.41 L/h for EM vs. PM).
- Friberg and Dolton: separation between (IM + PM) and EM was limited but directionally consistent (e.g., Dolton AUCτ,ss 27.70 ± 23.12 vs. 18.42 ± 12.40 mg·h/L; Friberg 9.72 ± 4.95 vs. 5.59 ± 1.66 mg·h/L for IM + PM vs. EM).
3.5. Secondary Exposure Parameters
3.6. Therapeutic Target Attainment
3.7. Sensitivity Analyses (Model-Specific Cohorts)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schulz, J.; Thomas, A.; Saleh, A.; Mikus, G.; Kloft, C.; Michelet, R. Towards the Elucidation of the Pharmacokinetics of Voriconazole: A Quantitative Characterization of Its Metabolism. Pharmaceutics 2022, 14, 477. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, N.; Kreuter, R.; Blank, A.; Weiss, J.; Burhenne, J.; Haefeli, W.E.; Mikus, G. Autoinhibitory properties of the parent but not of the N-oxide metabolite contribute to infusion rate-dependent voriconazole pharmacokinetics. Br. J. Clin. Pharmacol. 2017, 83, 1954–1965. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhang, C.; Qin, Z.; Wang, D.; Yang, J.; Zhang, X. Impact of CYP2C19 Phenotype and Drug-Drug Interactions on Voriconazole Concentration in Pediatric Patients. Antimicrob. Agents Chemother. 2021, 65, e0020721. [Google Scholar] [CrossRef] [PubMed]
- Hamadeh, I.S.; Klinker, K.P.; Borgert, S.J.; Richards, A.I.; Li, W.; Mangal, N.; Hiemenz, J.W.; Schmidt, S.; Langaee, T.Y.; Peloquin, C.A.; et al. Impact of the CYP2C19 genotype on voriconazole exposure in adults with invasive fungal infections. Pharmacogenet. Genom. 2017, 27, 190–196. [Google Scholar] [CrossRef]
- Jiang, Z.; Wei, Y.; Huang, W.; Li, B.; Zhou, S.; Liao, L.; Li, T.; Liang, T.; Yu, X.; Li, X.; et al. Population pharmacokinetics of voriconazole and initial dosage optimization in patients with talaromycosis. Front. Pharmacol. 2022, 13, 982981. [Google Scholar] [CrossRef]
- Pascual, A.; Calandra, T.; Bolay, S.; Buclin, T.; Bille, J.; Marchetti, O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 2008, 46, 201–211. [Google Scholar] [CrossRef]
- Takesue, Y.; Hanai, Y.; Oda, K.; Hamada, Y.; Ueda, T.; Mayumi, T.; Matsumoto, K.; Fujii, S.; Takahashi, Y.; Miyazaki, Y.; et al. Clinical Practice Guideline for the Therapeutic Drug Monitoring of Voriconazole in Non-Asian and Asian Adult Patients: Consensus Review by the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. Clin. Ther. 2022, 44, 1604–1623. [Google Scholar] [CrossRef]
- Hu, L.; Huang, J.; Li, Y.; He, G. Clinical application of voriconazole in pediatric patients: A systematic review. Ital. J. Pediatr. 2024, 50, 113. [Google Scholar] [CrossRef]
- Jin, H.; Wang, T.; Falcione, B.A.; Olsen, K.M.; Chen, K.; Tang, H.; Hui, J.; Zhai, S. Trough concentration of voriconazole and its relationship with efficacy and safety: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2016, 71, 1772–1785. [Google Scholar] [CrossRef]
- Dolton, M.J.; Mikus, G.; Weiss, J.; Ray, J.E.; McLachlan, A.J. Understanding variability with voriconazole using a population pharmacokinetic approach: Implications for optimal dosing. J. Antimicrob. Chemother. 2014, 69, 1633–1641. [Google Scholar] [CrossRef]
- Friberg, L.E.; Ravva, P.; Karlsson, M.O.; Liu, P. Integrated population pharmacokinetic analysis of voriconazole in children, adolescents, and adults. Antimicrob. Agents Chemother. 2012, 56, 3032–3042. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, S.; Sun, J.; Cai, J.; Cheng, X.; Dong, H.; Wang, X.; Xing, J.; Dong, W.; Yao, H.; et al. Identification of factors influencing the pharmacokinetics of voriconazole and the optimization of dosage regimens based on Monte Carlo simulation in patients with invasive fungal infections. J. Antimicrob. Chemother. 2014, 69, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Rhee, S.J.; Park, W.B.; Yu, K.S.; Jang, I.J.; Lee, S. A Personalized CYP2C19 Phenotype-Guided Dosing Regimen of Voriconazole Using a Population Pharmacokinetic Analysis. J. Clin. Med. 2019, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Yang, X.; Dong, L.; Jiang, Y.; Zou, S.; Hu, N. Influence of C-reactive protein on the pharmacokinetics of voriconazole in relation to the CYP2C19 genotype: A population pharmacokinetics analysis. Front. Pharmacol. 2024, 15, 1455721. [Google Scholar] [CrossRef]
- Andes, D.; Marchillo, K.; Stamstad, T.; Conklin, R. In vivo pharmacokinetics and pharmacodynamics of a new triazole, voriconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 2003, 47, 3165–3169. [Google Scholar] [CrossRef]
- Veringa, A.; Bruggemann, R.J.; Span, L.F.R.; Biemond, B.J.; de Boer, M.G.J.; van den Heuvel, E.R.; Klein, S.K.; Kraemer, D.; Minnema, M.C.; Prakken, N.H.J.; et al. Therapeutic drug monitoring-guided treatment versus standard dosing of voriconazole for invasive aspergillosis in haematological patients: A multicentre, prospective, cluster randomised, crossover clinical trial. Int. J. Antimicrob. Agents 2023, 61, 106711. [Google Scholar] [CrossRef]
- Leveque, D.; Nivoix, Y.; Jehl, F.; Herbrecht, R. Clinical pharmacokinetics of voriconazole. Int. J. Antimicrob. Agents 2006, 27, 274–284. [Google Scholar] [CrossRef]
- Moriyama, B.; Obeng, A.O.; Barbarino, J.; Penzak, S.R.; Henning, S.A.; Scott, S.A.; Agundez, J.; Wingard, J.R.; McLeod, H.L.; Klein, T.E.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP2C19 and Voriconazole Therapy. Clin. Pharmacol. Ther. 2017, 102, 45–51. [Google Scholar] [CrossRef]
- Wang, J.; Shen, Y.; Wu, Z.; Ge, W. Population Pharmacokinetics of Voriconazole and Dose Optimization in Elderly Chinese Patients. J. Clin. Pharmacol. 2024, 64, 253–263. [Google Scholar] [CrossRef]
- Zhong, X.; Tong, X.; Ju, Y.; Du, X.; Li, Y. Interpersonal Factors in the Pharmacokinetics and Pharmacodynamics of Voriconazole: Are CYP2C19 Genotypes Enough for Us to Make a Clinical Decision? Curr. Drug Metab. 2018, 19, 1152–1158. [Google Scholar] [CrossRef]
- Shi, C.; Xiao, Y.; Mao, Y.; Wu, J.; Lin, N. Voriconazole: A Review of Population Pharmacokinetic Analyses. Clin. Pharmacokinet. 2019, 58, 687–703. [Google Scholar] [CrossRef]
- Bolcato, L.; Khouri, C.; Veringa, A.; Alffenaar, J.W.C.; Yamada, T.; Naito, T.; Lamoureux, F.; Fonrose, X.; Stanke-Labesque, F.; Gautier-Veyret, E. Combined Impact of Inflammation and Pharmacogenomic Variants on Voriconazole Trough Concentrations: A Meta-Analysis of Individual Data. J. Clin. Med. 2021, 10, 2089. [Google Scholar] [CrossRef]
| Study | N (Male/Female) | Subject Characteristics (n) | Age | Body Weight | CYP2C19 Phenotype | Samples | Data | Structural Model | Pharmacokinetic Parameters | Model Variability | Covariates | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Per Subject | Total | |||||||||||
| Friberg et al. [11] | 173 (101/72) | Immunocompromised children (112), adolescents (26), and healthy adults (35) | 12.9 (2–55) | 38.7 (10.8–97) | EM (98) IM (66) UM (4) PM (5) | 19.3 | 3336 | Rich data from five PK studies | 2-Compartment model with first-order oral absorption, with a lag time, and mixed linear and Michaelis–Menten elimination | Ka = 100 h−1 (fixed) [adults] Ka = 1.19 h−1 (children) Ka = 1.19 × (1 − 0.615 × ADO) h−1 (pediatrics) F = 0.642 Lag time = 0.949 h (adults) Lag time = 0.12 h (pediatrics) V1 = 79.0 × (WT/70) L V2 = 103 × (WT/70) L Q = 15.5 × (WT/70)0.75 L/h (adults) Q = 15.5 × (WT/70)0.75 × (1 + 0.637) L/h (pediatrics) CL = 6.16 × (WT/70)0.75 L/h Vmax,1 = 114 × (WT/70)0.75 mg/h Vmax,inh = 0.82 (adults/adolescents) Vmax,inh = 0.75 (children) T50 = 2.41 h Vmax = Vmax,1 × {1 − Vmax,inh × (T − 1)/[(T − 1) + (T50 − 1)]} mg/h Km = 1.15 mg/L | BSV Ka = 89.8% (pediatrics) BSV logit (F) = 0.78 (adults) BSV logit (F) = 2.3 (pediatrics) BSV V1 = 14% BSV V2 = 77% BSV Q = 42.4% BSV CL = 44% (adults) BSV CL = 75% (pediatrics) BSV Vmax,1 = 79% (adults) BSV Vmax,1 = 24% (children) BSV Vmax,1 = 28% (adolescents) BSV Km = 136% Proportional REE = 37% to 59% | V1: WT V2: WT Q: WT CL: WT Vmax,1: WT Vmax,inh: Study population (children or adolescents) Ka: Study population (adolescents only, ADO = 1) Lag time: Study population (adults vs. pediatrics) |
| Wang et al. [12] | 151 (104/47) | Invasive fungal infection patients (n = 151) | 59 (18–99) | 59.1 (35.0–80.0) | EM (64) IM (65) PM (19) UM (3) | 4 | 406 | Sparse sampling | 1-Compartment model with first-order absorption and elimination | Ka = 1.1 h−1 (fixed) F = 0.895 V = 200 × [1 − 0.0098 × (AGE − 61)] L CL = 6.95 × [1 − 0.012 × (AGE − 61)] × (1 − 0.37 × PM) × [1 − 0.0016 × (ALP − 104)] L/h | BSV F = 18.9% BSV V = 25.4% BSV CL = 28.7% Proportional REE = 10.8% Additive REE = 0.016 mg/L | V: AGE CL: AGE, CYP2C19 genotype (PM), ALP |
| Dolton et al. [10] | 240 (152/88) | Healthy adults (63) and adult patients with fungal infection or at risk for fungal infections (177) | 34 (18–88) | 69 (39–115) | EM&RM (56) IM&PM (38) UM (146) | 14 | 3352 | Rich data from five PK studies and sparse data from a TDM study | 2-Compartment model with first-order oral absorption and Michaelis–Menten elimination | Ka = 0.53 h−1 Lag_time = 0.162 h F = 0.942 L V1 = 27.1 L V2 = 127 L Q = 35.1 L/h Km = 3.33 mg/L Vmax = 43.9 × (1 − 0.412 × CYP2C19) × (1 − 0.429 × RIT) × (1 + 1.07 × SJW) × (1 + 2.03 × POR) × (1 + 0.366 × POP) × (1 + 0.564 × MET) × (1 + 0.557 × DEX) × (1 + 1.11 × HV) | BSV Ka = 41.6% BSV F = 36.7% BSV V1 = 83.4% BSV V2 = 38.1% BSV Vmax = 26.8% BSV Km = 64.5% Prop REE = 33.8% Add REE = 0.005 mg/L | Vmax: CYP2C19 genotype, short-term ritonavir, St John’s wort, phenytoin, rifampicin, glucocorticoids (prednisone, prednisolone, methylprednisolone, dexamethasone), study population (healthy volunteers) |
| Yun et al. [13] | 193 (164/29) | Healthy Subjects (93) Patients (100) | 34 (18–80) | 66.0 (40.8–88.5) | EM (75), IM (70), PM (48) | 9.5 | 1828 | Rich data from a study | 3-compartment model with a first-order oral absorption, lag time, and elimination along with an inhibition compartment model | Ka = 1.23 h−1 F1 = 0.876 ALAG1 = 0.237 h CL = 45.3 L/h V2 = 35.7 L V3 = 58.9 L Q2 = 10.9 L/h V4 = 25.4 L Q3 = 54.6 L/h RCLF = 0.162 KIC = 0.002 µM−1 CL0 = 45.3 × (WT/70)^0.595 × (1 − 0.186·IM − 0.746·PM) × (1 − 0.75·LIVER) INH = RCLF + (1 − RCLF) × (1 − (Cinh/(IC50 + Cinh))) CL = CL0 × INH | BSV CL = 21.4% BSV V2 = 40.2% BSV V3 = 20.6% BSV Q2 = 28.8% BSV KA = 87.8% BSV F1 = 84.4% BSV RCLF = 54.4% Add REE (healthy) 0.208 mg/L Add REE (patient) 0.799 mg/L | CL: CYP2C19 phenotype, WT, liver dysfunction (grade 3) RCLF:CYP2C19 phenotype V3: WT Q2: WT |
| Ling et al. [14] | 167 (119/48) | Patients with invasive fungal infections | 68.87 ± 14.87 (71.16–97) | 64.48 ± 12.24 (65.37–100) | EM (66), IM (72) PM (29) | 1.4 | 232 | Rich data from a study | 1-Compartment model with first-order absorption and elimination | Ka = 1.10 h−1 (fixed) F = 0.965 V = 134 × (WT/65)(2.21) L CL = 3.83 L/h × 0.794(IM) × 0.635(PM) × CRP(−0.153); apply to EM/IM only × (ALB/34.8)(0.664) × (AGE/71)(−0.582) × 1.41(Female) | BSV CL = 38.9% BSV V = 45.2% Prop REE = 14.7% Add REE = 0.58 µg/mL | CL:CYP2C19 phenotype (IM, PM) ALB AGE Gender WT V: WT |
| Model | CYP2C19 Phenotype | AUCτ,ss (mg·h/L) | Cavg,ss (mg/L) | Cmax,ss (mg/L) | Ctrough,ss (mg/L) | CLss/F (L/h) | Vss/F (L) |
|---|---|---|---|---|---|---|---|
| Friberg | Total | 8.34 ± 4.58 | 0.70 ± 0.38 | 1.48 ± 0.44 | 0.42 ± 0.33 | 30.94 ± 14.56 | 134.43 ± 50.62 |
| EM | 5.59 ± 1.66 | 0.47 ± 0.14 | 1.28 ± 0.29 | 0.22 ± 0.11 | 39.05 ± 11.88 | 162.85 ± 40.97 | |
| IM + PM | 9.72 ± 4.95 | 0.81 ± 0.41 | 1.58 ± 0.47 | 0.52 ± 0.36 | 26.89 ± 14.10 | 120.22 ± 49.05 | |
| Wang | Total | 51.91 ± 28.55 | 4.33 ± 2.38 | 4.66 ± 2.34 | 3.85 ± 2.34 | 5.50 ± 3.20 | 31.24 ± 17.22 |
| EM | 24.90 ± 5.73 | 2.07 ± 0.48 | 2.43 ± 0.42 | 1.65 ± 0.53 | 8.42 ± 1.81 | 47.18 ± 8.81 | |
| PM | 78.92 ± 11.49 | 6.58 ± 0.96 | 6.88 ± 0.90 | 6.05 ± 0.98 | 2.59 ± 0.41 | 15.30 ± 2.29 | |
| Dolton | Total | 24.61 ± 20.63 | 2.05 ± 1.72 | 2.85 ± 1.76 | 1.59 ± 1.59 | 12.50 ± 6.89 | 62.05 ± 30.48 |
| EM | 18.42 ± 12.40 | 1.53 ± 1.03 | 2.34 ± 1.10 | 1.04 ± 0.94 | 14.48 ± 6.31 | 70.94 ± 27.27 | |
| IM + PM | 27.70 ± 23.12 | 2.31 ± 1.93 | 3.10 ± 1.97 | 1.76 ± 1.79 | 11.51 ± 6.97 | 57.60 ± 30.89 | |
| Yun | Total | 110.47 ± 85.79 | 9.21 ± 7.15 | 10.39 ± 7.19 | 7.96 ± 6.77 | 3.19 ± 2.40 | 17.44 ± 12.22 |
| EM | 42.64 ± 23.12 | 3.55 ± 1.93 | 4.93 ± 2.18 | 2.49 ± 1.65 | 5.69 ± 2.27 | 29.99 ± 11.33 | |
| IM | 86.52 ± 37.24 | 7.21 ± 3.10 | 8.34 ± 3.30 | 6.05 ± 2.79 | 2.27 ± 1.15 | 15.57 ± 6.41 | |
| PM | 202.25 ± 81.08 | 16.85 ± 6.76 | 17.90 ± 7.01 | 15.34 ± 6.25 | 1.15 ± 0.45 | 6.77 ± 2.63 | |
| Ling | Total | 44.61 ± 15.75 | 3.72 ± 1.31 | 4.28 ± 1.37 | 3.08 ± 1.27 | 5.12 ± 1.98 | 28.80 ± 10.55 |
| EM | 33.70 ± 9.92 | 2.81 ± 0.83 | 3.37 ± 0.89 | 2.16 ± 0.80 | 6.49 ± 2.06 | 36.07 ± 10.81 | |
| IM | 48.35 ± 13.80 | 4.03 ± 1.15 | 4.63 ± 1.21 | 3.31 ± 1.15 | 4.56 ± 1.59 | 25.74 ± 8.49 | |
| PM | 51.78 ± 16.56 | 4.31 ± 1.38 | 4.84 ± 1.46 | 3.65 ± 1.29 | 4.29 ± 1.47 | 24.59 ± 8.15 |
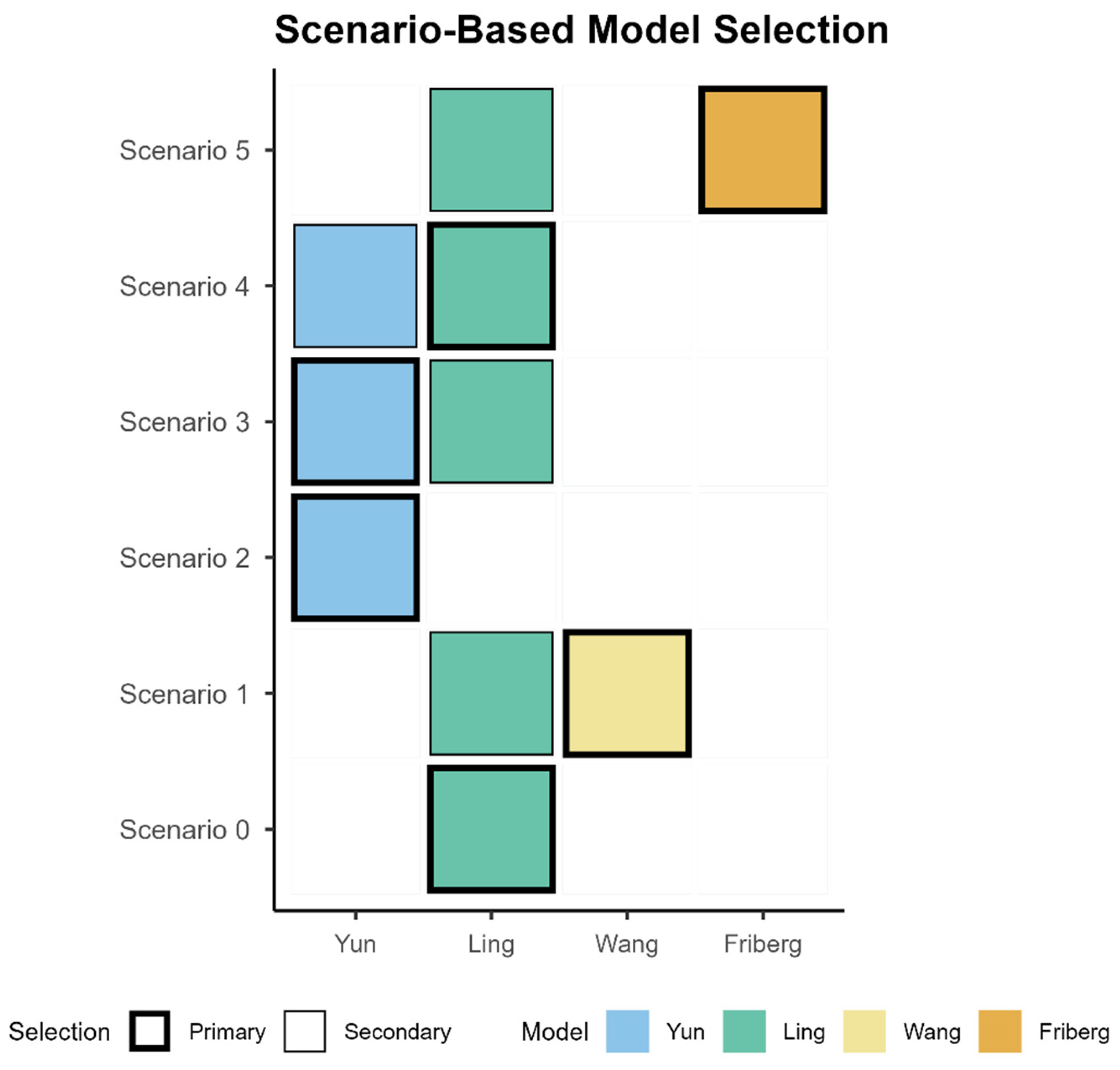
| Scenario | Description | Model | Prescribing |
|---|---|---|---|
| Scenario 0 | Adult standard start, safety-first | Primary: Ling | Start with standard dosing and early TDM |
| Scenario 1 | Limited genotype info (EM vs. PM only), need lower/upper-bound check | Primary: Wang Secondary: Ling | Define phenotype-specific assumptions for the therapeutic window and verify with early TDM; adjust if attainment or exceedance is observed. |
| Scenario 2 | Hepatotoxicity concern or rising liver enzymes (e.g., ALT/AST) under the standardized regimen; prioritization of upper-bound control. | Primary: Yun | Reduce dose or extend dosing interval to control the upper bound; intensify TDM to confirm trough ≤5.5 mg/L; Continue close LFT monitoring and re-titrate if needed. |
| Scenario 3 | Genotype confirmed | Primary: Yun Secondary: Ling | Conservative start or interval extension for IM/PM, uptitrate EM if below target—TDM-guided |
| Scenario 4 | Inflammation or covariate-anchored adjustment (CRP increased, low ALB, elderly) | Primary: Ling Secondary: Yun | Reduce dose or extend interval per covariates |
| Scenario 5 | Suspected underexposure | Primary: Friberg Secondary: Ling | If below target, uptitrate by 20–30% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Lee, N.; Rhee, S.-j.; Kim, Y. Toward Genotype-Informed Dosing of Voriconazole: Head-to-Head Simulations Across CYP2C19 Phenotypes Using Population Pharmacokinetic Models. Pharmaceutics 2025, 17, 1398. https://doi.org/10.3390/pharmaceutics17111398
Lee Y, Lee N, Rhee S-j, Kim Y. Toward Genotype-Informed Dosing of Voriconazole: Head-to-Head Simulations Across CYP2C19 Phenotypes Using Population Pharmacokinetic Models. Pharmaceutics. 2025; 17(11):1398. https://doi.org/10.3390/pharmaceutics17111398
Chicago/Turabian StyleLee, Yeobin, Nai Lee, Su-jin Rhee, and Yun Kim. 2025. "Toward Genotype-Informed Dosing of Voriconazole: Head-to-Head Simulations Across CYP2C19 Phenotypes Using Population Pharmacokinetic Models" Pharmaceutics 17, no. 11: 1398. https://doi.org/10.3390/pharmaceutics17111398
APA StyleLee, Y., Lee, N., Rhee, S.-j., & Kim, Y. (2025). Toward Genotype-Informed Dosing of Voriconazole: Head-to-Head Simulations Across CYP2C19 Phenotypes Using Population Pharmacokinetic Models. Pharmaceutics, 17(11), 1398. https://doi.org/10.3390/pharmaceutics17111398







