Advancements in Targeted Quantum Dots Structures for Enhanced Cancer Treatment
Abstract
1. Introduction
2. Fundamentals of Quantum Dots
2.1. Types of Quantum Dots
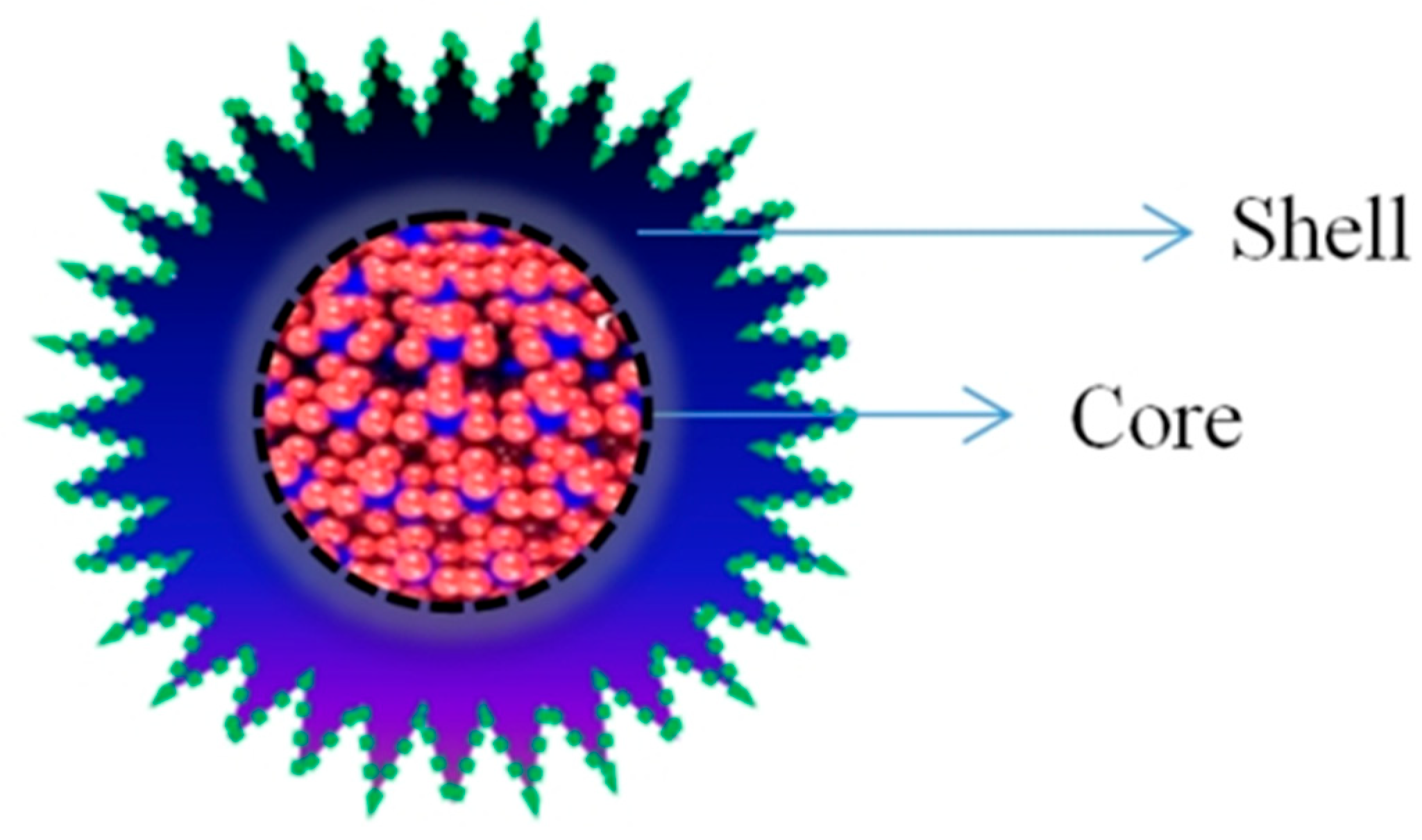
2.2. Heterostructure of QDs for Biomedical Application
| Type of Structure and Heterostructure | Material Used | Reference |
|---|---|---|
| Fish Scale-Derived Carbon Dots (FS-CDs) | fish scale | [35] |
| Phosphorus-doped CQDs (P-CQDs) | yeast cell walls | [36] |
| Fluorescent QD | based hydrogels | [37] |
| CuQDs | CuInS2 | [38] |
| Multifunctional microspheres (MFM) | Fluorescent source (CdSe/ZnS quantum dots), silica nanoparticles | [39] |
| CdSe:ZnS QDs | CdSe core—ZnS shells | [40] |
| Fluorescent carbon quantum dots (CQDs)—FS-CDs | Aegle marmelos fruit extract | [41] |
2.3. Quantum Dot-Based Formulation for Biomedical Applications
3. Quantum Dot-Based Cancer Care Drugs
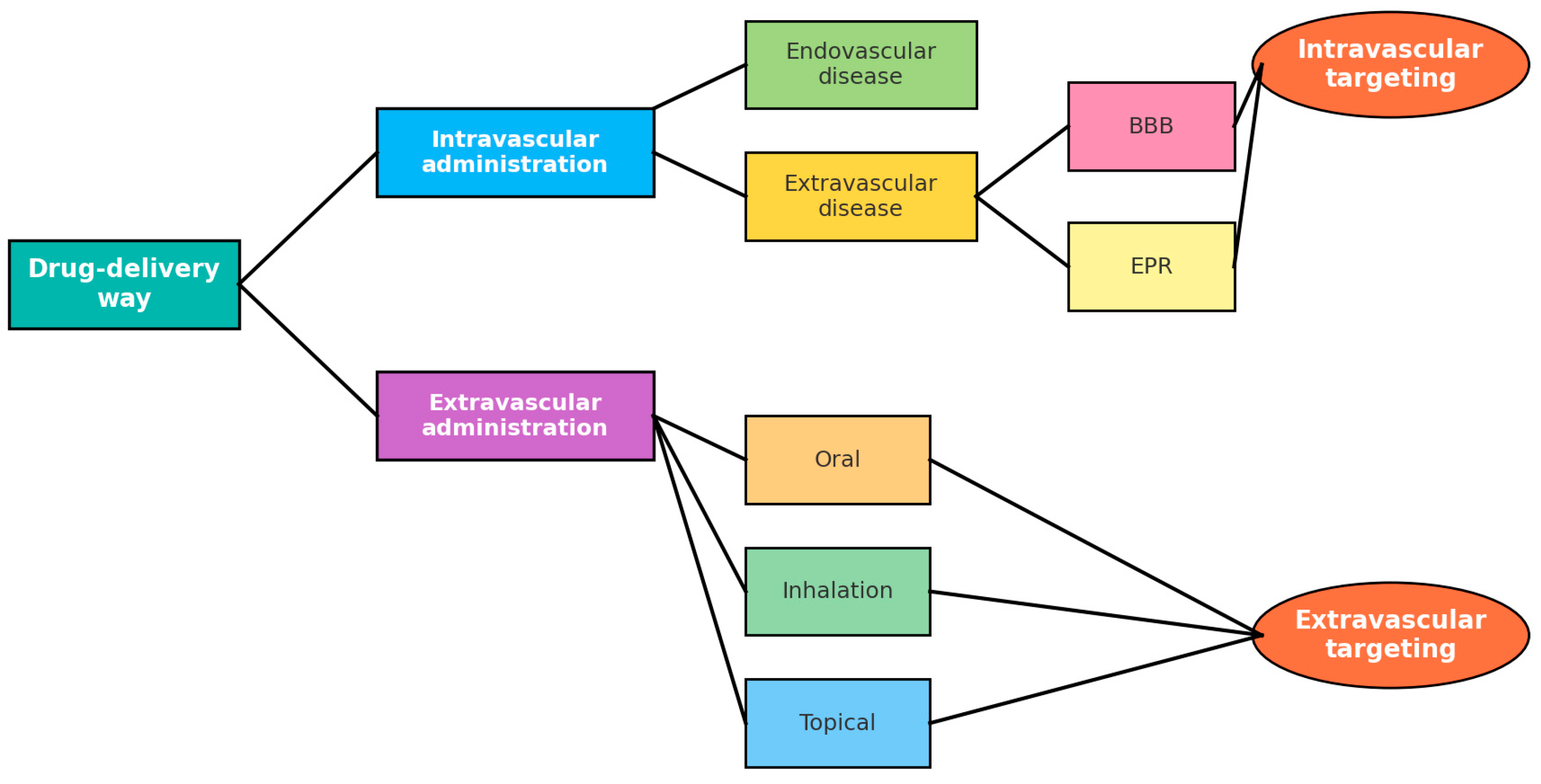
3.1. Cancer Environment and Location
3.2. Routes of Administration
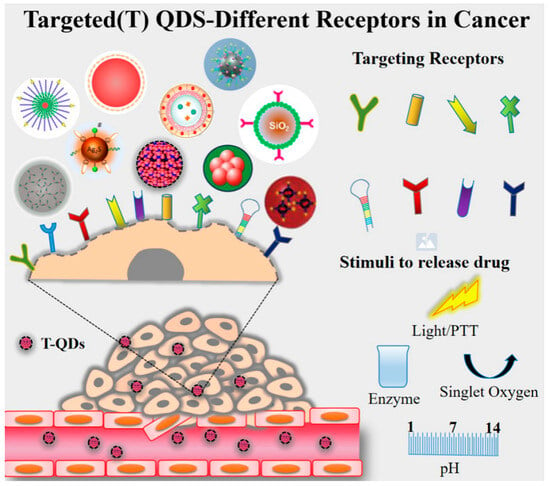
3.3. Targeting Strategies
4. Improve Targeting Ability and Therapeutic Properties of QD Heterostructure
4.1. Folate Receptors (FR)
4.2. Transferrin Receptor (Tfr)
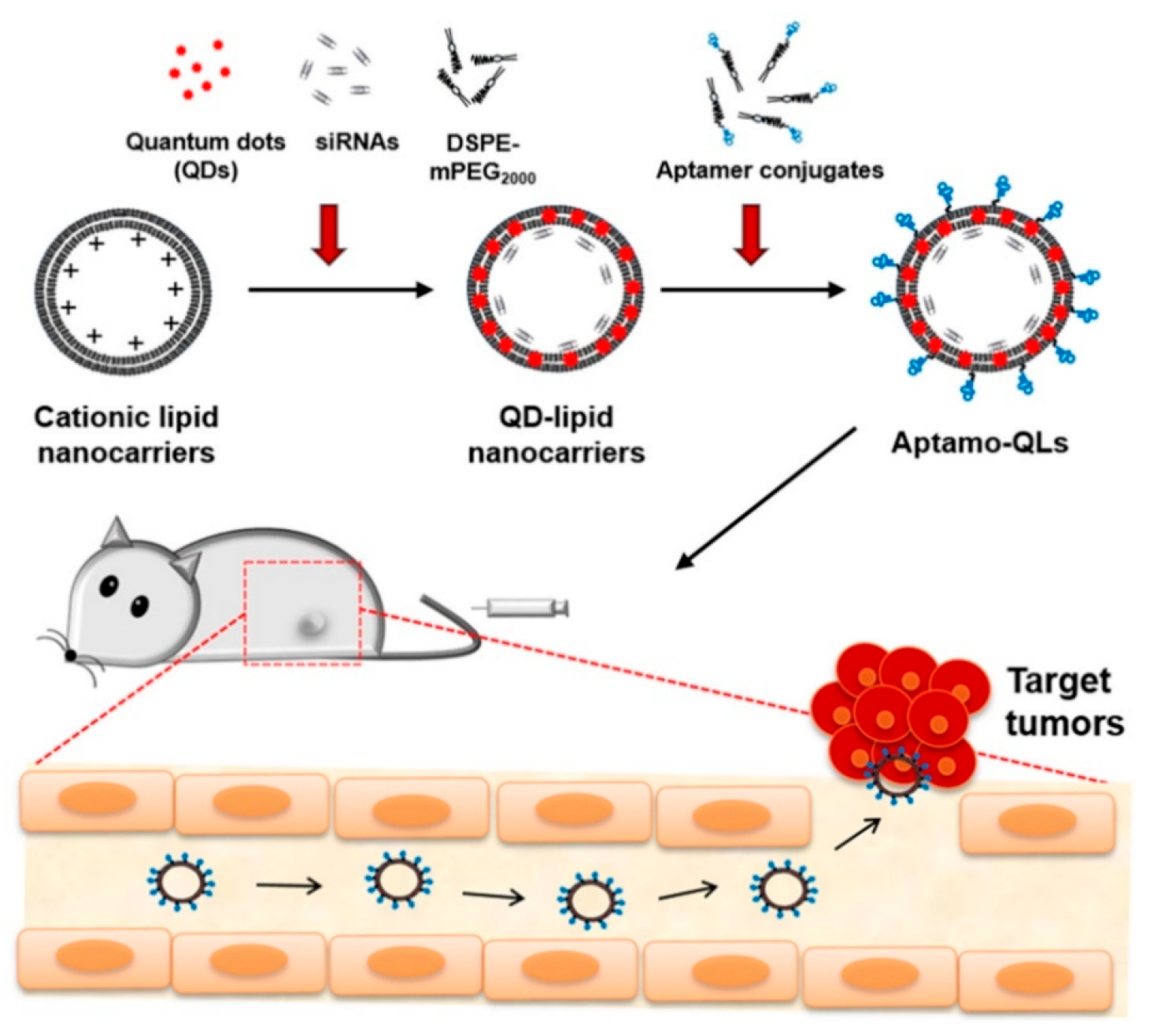
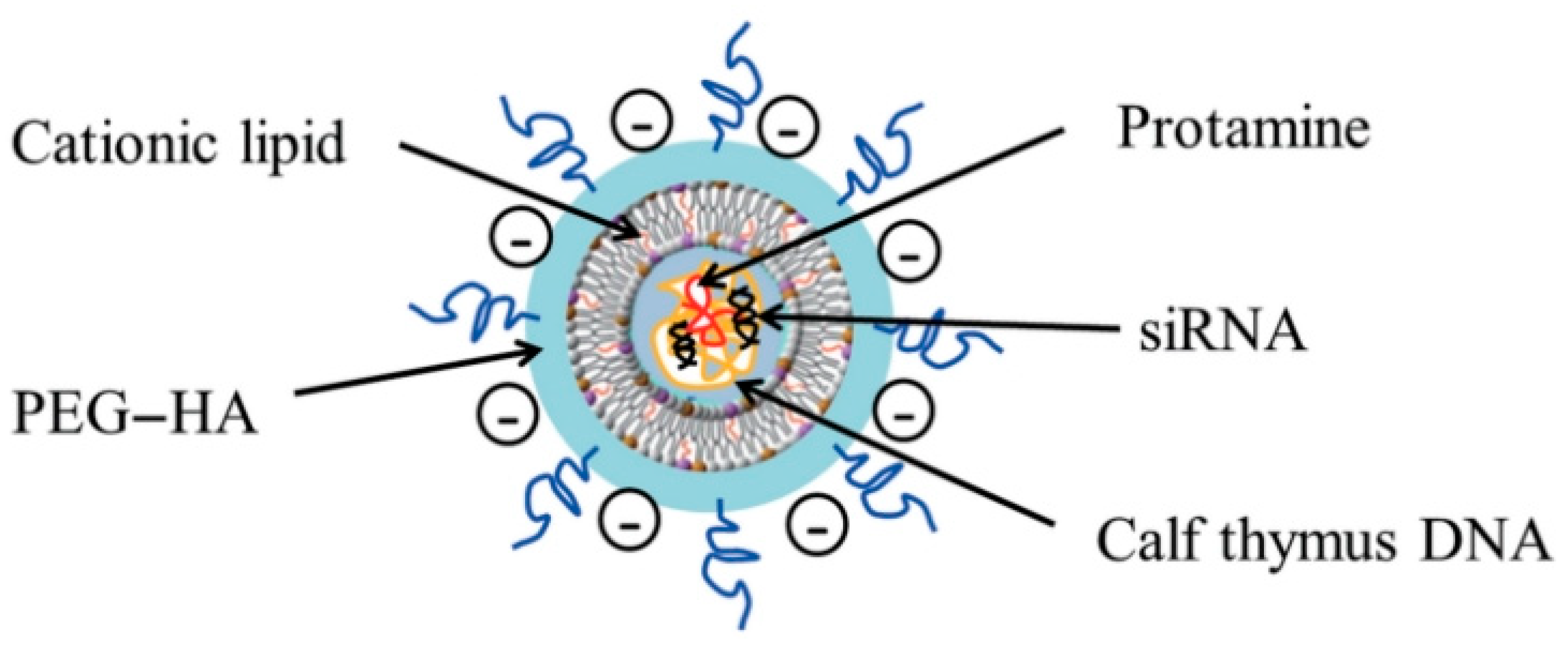
4.3. Aptamers (DNA, siRNA)
4.4. αvβ3 Integrin
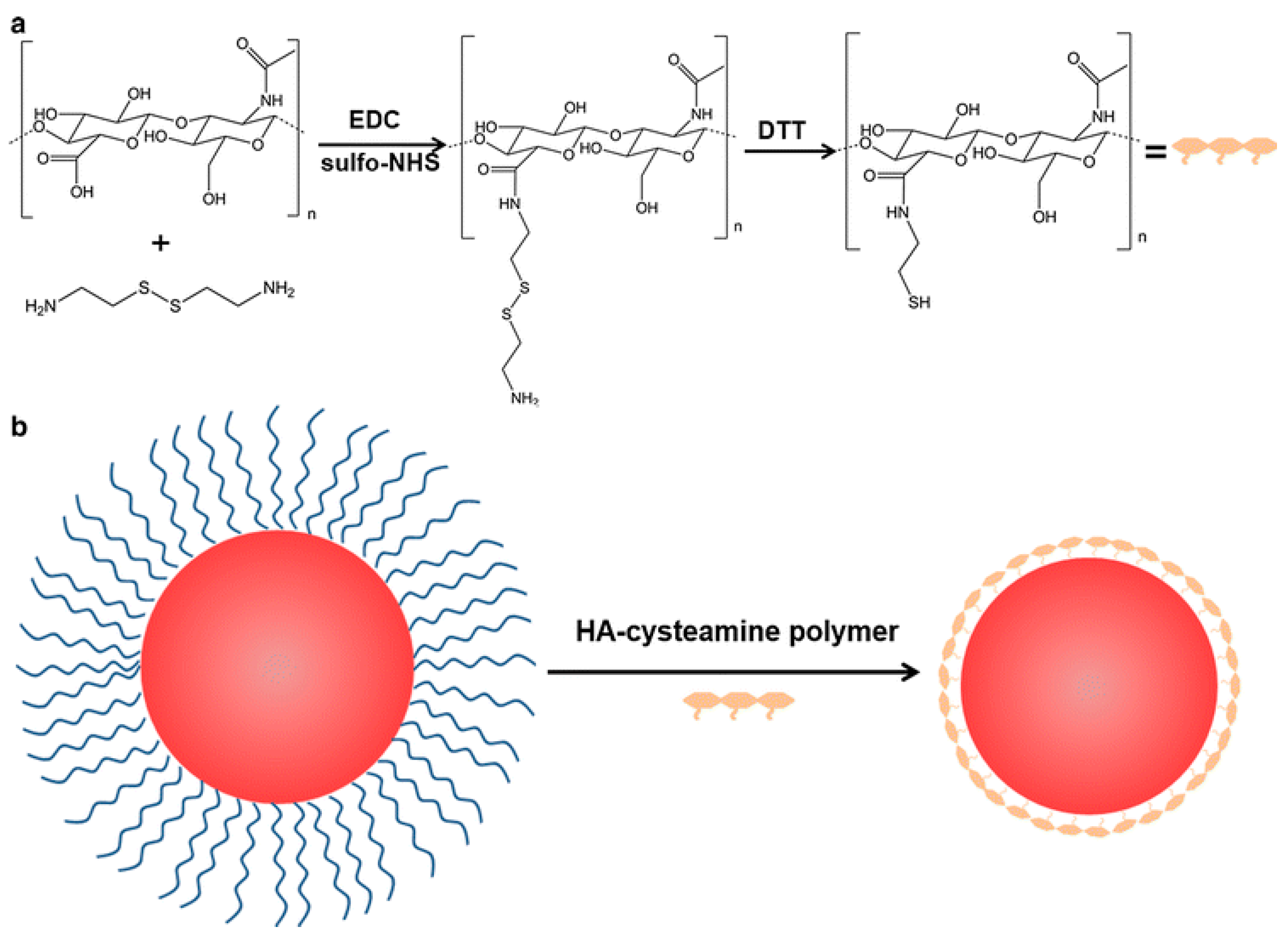
4.5. Hyaluronic Acid (HA)
4.6. Antibody (Ab)
4.7. Anti EGFR
4.8. Peptide
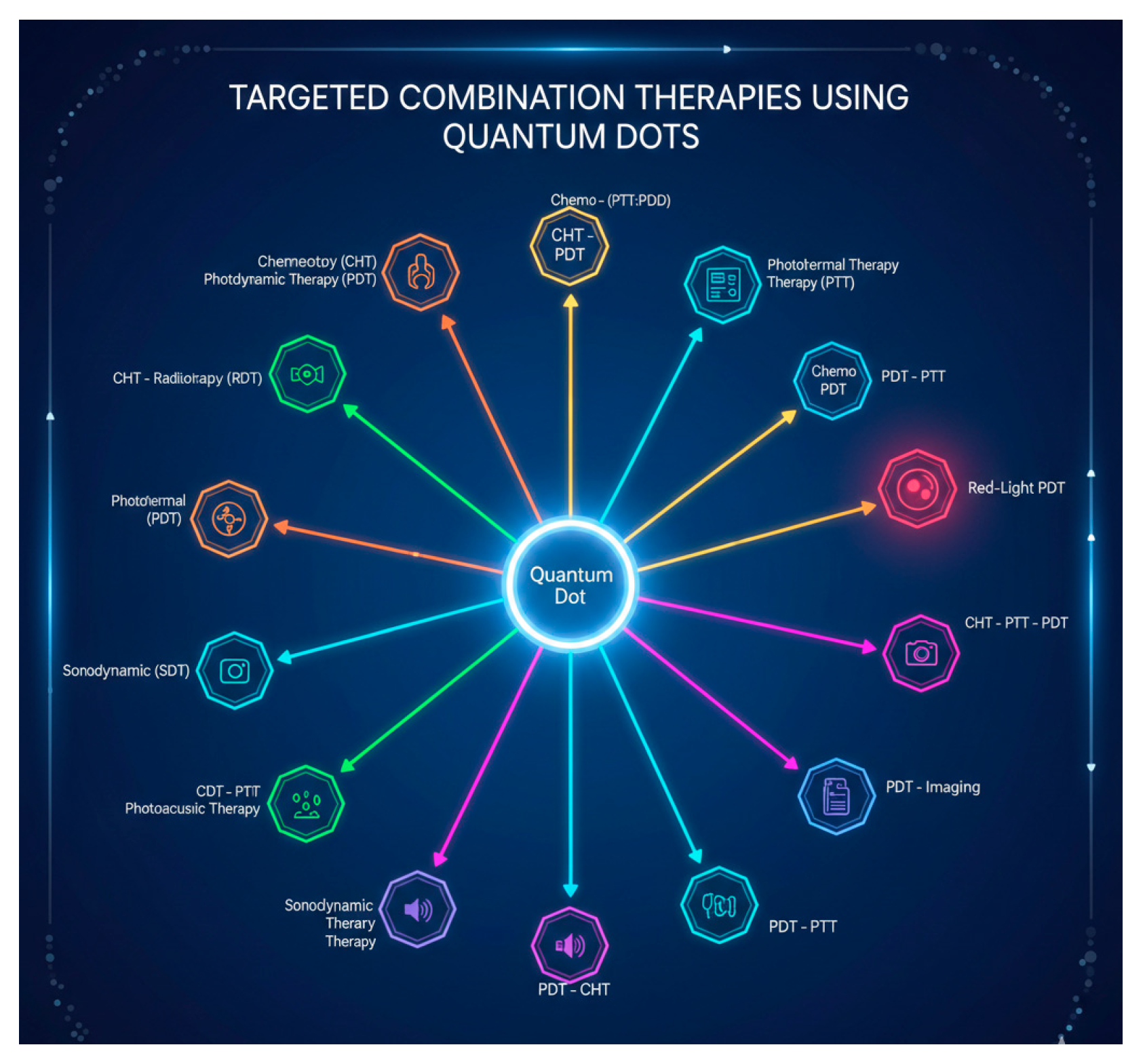
5. Targeted Combination Therapies Using Different QDs
5.1. Chemotherapy (CHT)-Photodynamic Therapy (PDT)
5.2. CHT-Radiotherapy (RDT)
5.3. Photothermal Therapy-(PTT)-PDT
5.4. Red Light PDT
5.5. Chemo-(PTT:PDT)
5.6. PDT-PTT
5.7. PDT-CHT
5.8. CDT-PDT
5.9. Multimodal PDT-PTT, Photoacoustic
5.10. Sonodyanamic (SDT)
5.11. PDT-Imaging
5.12. CHT-PTT:PDT
6. Quantum Dots in Targeted Imaging and Theranostic
6.1. Targeted Imaging and Therapy
6.2. Imaging with PTT
6.3. QDs-Hydrogels, Nanocomposites, and Layer-by-Layer System
7. Conclusions and Future Outlook
- Safer and sustainable QD formulations: Development of non-toxic, environmentally compliant QDs with high quantum yield and stability.
- Standardization of biological studies: Establishing uniform protocols for assessing in vivo pharmacokinetics, toxicity, and therapeutic efficacy.
- Integration with emerging technologies: Combining QDs with CRISPR, AI-based imaging, and nanotheranostics to enhance personalized medicine.
- Addressing conflicting results: Systematic comparative studies across different QD types, sizes, and functionalizations to resolve inconsistencies in biodistribution, clearance, and cellular interactions.
- Scalable manufacturing: Advancing reproducible and cost-effective synthesis methods to facilitate commercialization for both biomedical and electronic applications.
Funding
Data Availability Statement
Conflicts of Interest
References
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Alivisatos, A.P. Semiconductor Clusters, Nanocrystals, and Quantum Dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Nie, S. Chemical analysis and cellular imaging with quantum dots. Analyst 2004, 129, 672–677. [Google Scholar] [CrossRef]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef]
- Kadian, S.; Shukla, S.; Yadav, A.K.; Arya, B.; Sethi, S.; Chaudhary, V.; Narayan, R. Recent Advancements in Graphene Quantum Dot-Based Bioimaging and Drug Delivery Systems. MedComm 2025, 6, e70320. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef]
- Yong, K.-T.; Law, W.-C.; Hu, R.; Ye, L.; Liu, L.; Swihart, M.T.; Prasad, P.N. Nanotoxicity assessment of quantum dots: From cellular to primate studies. Chem. Soc. Rev. 2013, 42, 1236–1250. [Google Scholar] [CrossRef]
- Rzigalinski, B.A.; Strobl, J.S. Cadmium-containing nanoparticles: Perspectives on pharmacology and toxicology of quantum dots. Toxicol. Appl. Pharmacol. 2009, 238, 280–288. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Li, J.; Tian, Y.; Kang, Y.; Ren, G.; Liu, W.; Wang, H.; Wang, B.; Yan, L.; et al. Targeted Delivery of Doxorubicin Using Transferrin-Conjugated Carbon Dots for Cancer Therapy. ACS Appl. Bio Mater. 2021, 4, 7280–7289. [Google Scholar] [CrossRef]
- Yazdian, F. 13-Aptamer-functionalized quantum dots for targeted cancer therapy. In Aptamers Engineered Nanocarriers for Cancer Therapy; Kesharwani, P., Ed.; Woodhead Publishing: Sawston, UK, 2023; pp. 295–315. [Google Scholar]
- Choi, H.S.; Liu, W.; Liu, F.; Nasr, K.; Misra, P.; Bawendi, M.G.; Frangioni, J.V. Design considerations for tumour-targeted nanoparticles. Nat. Nanotechnol. 2010, 5, 42–47. [Google Scholar] [CrossRef]
- Shen, T.; Li, B.; Zheng, K.; Pullerits, T.; Cao, G.; Tian, J. Surface Engineering of Quantum Dots for Remarkably High Detectivity Photodetectors. J. Phys. Chem. Lett. 2018, 9, 3285–3294. [Google Scholar] [CrossRef]
- Liu, L.; Miao, Q.; Liang, G. Quantum Dots as Multifunctional Materials for Tumor Imaging and Therapy. Materials 2013, 6, 483–499. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Fontana, F.; Tapeinos, C.; Shahbazi, M.-A.; Han, H.; Santos, H.A. Nanoparticles-based phototherapy systems for cancer treatment: Current status and clinical potential. Bioact. Mater. 2023, 23, 471–507. [Google Scholar] [CrossRef]
- Rosenthal, S.J.; Chang, J.C.; Kovtun, O.; McBride, J.R.; Tomlinson, I.D. Biocompatible quantum dots for biological applications. Chem. Biol. 2011, 18, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhao, F.; Wang, J.; Zu, Y.; Gu, Z.; Zhao, Y. A Safe-by-Design Strategy towards Safer Nanomaterials in Nanomedicines. Adv. Mater. 2019, 31, 1805391. [Google Scholar] [CrossRef] [PubMed]
- Al-Jamal, W.T.; Kostarelos, K. Liposome-nanoparticle hybrids for multimodal diagnostic and therapeutic applications. Nanomedicine 2007, 2, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Zayed, D.G.; AbdElhamid, A.S.; Freag, M.S.; Elzoghby, A.O. Hybrid Quantum dot-based Theranostic Nanomedicines for tumor-targeted Drug Delivery and Cancer Imaging. Nanomedicine 2019, 14, 225–228. [Google Scholar] [CrossRef]
- Guo, W.; Song, X.; Liu, J.; Liu, W.; Chu, X.; Lei, Z. Quantum Dots as a Potential Multifunctional Material for the Enhancement of Clinical Diagnosis Strategies and Cancer Treatments. Nanomaterials 2024, 14, 1088. [Google Scholar] [CrossRef]
- Talapin, D.V.; Lee, J.-S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of Colloidal Nanocrystals for Electronic and Optoelectronic Applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef]
- Efros, A.; Efros, A. Interband Light Absorption in Semiconductor Spheres. Sov. Phys. Semicond. 1982, 16, 772–775. [Google Scholar]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef]
- Ding, R.; Chen, Y.; Wang, Q.; Wu, Z.; Zhang, X.; Li, B.; Lin, L. Recent advances in quantum dots-based biosensors for antibiotics detection. J. Pharm. Anal. 2022, 12, 355–364. [Google Scholar] [CrossRef]
- Daby, T.P.M.; Modi, U.; Yadav, A.K.; Bhatia, D.; Solanki, R. Bioimaging and therapeutic applications of multifunctional carbon quantum dots: Recent progress and challenges. Next Nanotechnol. 2025, 8, 100158. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, F.; Dai, Y.; Wang, M.; Liu, J. Safe-by-design strategies towards bismuth-based nanomaterials in tumor diagnosis and therapy. Nano Today 2025, 62, 102714. [Google Scholar] [CrossRef]
- Alhussaini, M.S.; Alyahya, A.A.I.; Al-Ghanayem, A.A. Recent progress in quantum dots for antimicrobial therapy and bioimaging: A comprehensive review (2018 to mid-2025). Dye. Pigment. 2026, 245, 113294. [Google Scholar] [CrossRef]
- Li, M.; Huang, Y.; Shen, C.; Wang, Y.; Lin, Y.; Wang, Z.; Chen, N.; Luo, Y. Application of quantum dots in cancer diagnosis and treatment: Advances and perspectives. Nano Res. 2025, 18, 94907163. [Google Scholar] [CrossRef]
- Mondal, S.; Das, S.; Sharma, B.; Nayak, R.; Rahman, M.Z. Recent progress of carbon-based quantum dots and nanotubes for cancer targeting and drug delivery applications. J. Drug Deliv. Sci. Technol. 2025, 108, 106896. [Google Scholar] [CrossRef]
- Pareek, A.; Kumar, D.; Pareek, A.; Gupta, M.M. Advancing Cancer Therapy with Quantum Dots and Other Nanostructures: A Review of Drug Delivery Innovations, Applications, and Challenges. Cancers 2025, 17, 878. [Google Scholar] [CrossRef]
- Chuang, C.-H.M.; Brown, P.R.; Bulović, V.; Bawendi, M.G. Improved performance and stability in quantum dot solar cells through band alignment engineering. Nat. Mater. 2014, 13, 796–801. [Google Scholar] [CrossRef]
- Ruzycka-Ayoush, M.; Kowalik, P.; Kowalczyk, A.; Bujak, P.; Nowicka, A.M.; Wojewodzka, M.; Kruszewski, M.; Grudzinski, I.P. Quantum dots as targeted doxorubicin drug delivery nanosystems in human lung cancer cells. Cancer Nanotechnol. 2021, 12, 8. [Google Scholar] [CrossRef]
- Davodabadi, F.; Mirinejad, S.; Fathi-Karkan, S.; Majidpour, M.; Ajalli, N.; Sheervalilou, R.; Sargazi, S.; Rozmus, D.; Rahdar, A.; Diez-Pascual, A.M. Aptamer-functionalized quantum dots as theranostic nanotools against cancer and bacterial infections: A comprehensive overview of recent trends. Biotechnol. Prog. 2023, 39, e3366. [Google Scholar] [CrossRef]
- Dirheimer, L.; Pons, T.; Marchal, F.; Bezdetnaya, L. Quantum Dots Mediated Imaging and Phototherapy in Cancer Spheroid Models: State of the Art and Perspectives. Pharmaceutics 2022, 14, 2136. [Google Scholar] [CrossRef]
- Tai, W.; Mahato, R.; Cheng, K. The role of HER2 in cancer therapy and targeted drug delivery. J. Control. Release 2010, 146, 264–275. [Google Scholar] [CrossRef]
- Ruan, J.; Song, H.; Qian, Q.; Li, C.; Wang, K.; Bao, C.; Cui, D. HER2 monoclonal antibody conjugated RNase-A-associated CdTe quantum dots for targeted imaging and therapy of gastric cancer. Biomaterials 2012, 33, 7093–7102. [Google Scholar] [CrossRef]
- Srinivas, V.; Molangiri, A.; Mallepogu, A.; Kona, S.R.; Ibrahim, A.; Duttaroy, A.K.; Basak, S. Maternal n-3 PUFA deficiency alters uterine artery remodeling and placental epigenome in the mice. J. Nutr. Biochem. 2021, 96, 108784. [Google Scholar] [CrossRef]
- Anselmo-Lima, W.T.; Romano, F.R.; Tamashiro, E.; Roithmann, R.; Dinarte, V.R.P.; Piltcher, O.B.; Miyake, M.M.; Fornazieri, M.A.; Nakanishi, M.; Bezerra, T.F.P.; et al. Brazilian guideline for the use of immunobiologicals in chronic rhinosinusitis with nasal polyps—2024 update. Braz. J. Otorhinolaryngol. 2024, 90, 101394. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Agathokleous, E. Smoke-water commonly induces hormetic dose responses in plants. Sci. Total Environ. 2021, 765, 142776. [Google Scholar] [CrossRef]
- Ziaee, N.; Farhadian, N.; Abnous, K.; Matin, M.M.; Khoshnood, A.; Yaghoobi, E. Dual targeting of Mg/N doped-carbon quantum dots with folic and hyaluronic acid for targeted drug delivery and cell imaging. Biomed. Pharmacother. 2023, 164, 114971. [Google Scholar] [CrossRef]
- Devi, S.; Kumar, M.; Tiwari, A.; Tiwari, V.; Kaushik, D.; Verma, R.; Bhatt, S.; Sahoo, B.M.; Bhattacharya, T.; Alshehri, S.; et al. Quantum Dots: An Emerging Approach for Cancer Therapy. Front. Mater. 2022, 8, 798440. [Google Scholar] [CrossRef]
- Lee, C.M.; Jang, D.; Cheong, S.J.; Kim, E.M.; Jeong, M.H.; Kim, S.H.; Kim, D.W.; Lim, S.T.; Sohn, M.H.; Jeong, H.J. Surface engineering of quantum dots for in vivo imaging. Nanotechnology 2010, 21, 285102. [Google Scholar] [CrossRef]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum dots in biomedical applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef]
- García de Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor quantum dots: Technological progress and future challenges. Science 2021, 373, eaaz8541. [Google Scholar] [CrossRef]
- Singh, D.; Thapa, S.; Singh, K.R.B.; Verma, R.; Singh, R.P.; Singh, J. Cadmium selenide quantum dots and its biomedical applications. Mater. Lett. X 2023, 18, 100200. [Google Scholar] [CrossRef]
- Sengupta, S.; Pal, S.; Pal, A.; Maity, S.; Sarkar, K.; Das, M. A review on synthesis, toxicity profile and biomedical applications of graphene quantum dots (GQDs). Inorganica Chim. Acta 2023, 557, 121677. [Google Scholar] [CrossRef]
- Yang, H.-L.; Bai, L.-F.; Geng, Z.-R.; Chen, H.; Xu, L.-T.; Xie, Y.-C.; Wang, D.-J.; Gu, H.-W.; Wang, X.-M. Carbon quantum dots: Preparation, optical properties, and biomedical applications. Mater. Today Adv. 2023, 18, 100376. [Google Scholar] [CrossRef]
- Singh, K.R.B.; Nayak, V.; Sabui, P.; Mallick, S.; Singh, J.; Singh, R.P. Bioinspired quantum dots: Promising nanosystems for biomedical application. Nano-Struct. Nano-Objects 2022, 32, 100921. [Google Scholar] [CrossRef]
- Salvi, A.; Kharbanda, S.; Thakur, P.; Shandilya, M.; Thakur, A. Biomedical application of carbon quantum dots: A review. Carbon Trends 2024, 17, 100407. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Pourmoslemi, S.; Khan, A.; Riahi, Z.; Rhim, J.-W. Sulfur quantum dots as sustainable materials for biomedical applications: Current trends and future perspectives. Colloids Surf. B Biointerfaces 2024, 237, 113863. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, Z.; Wei, J.; Dai, H.; Chen, Y.; Liu, S.; Duan, Z.; Xie, F.; Zhang, W.; Guo, R. Quantum dots-hydrogel composites for biomedical applications. Chin. Chem. Lett. 2022, 33, 1245–1253. [Google Scholar] [CrossRef]
- He, X.; Ma, N. An overview of recent advances in quantum dots for biomedical applications. Colloids Surf. B Biointerfaces 2014, 124, 118–131. [Google Scholar] [CrossRef]
- Panja, A.; Patra, P. A review on Quantum Dots (QDs) and their biomedical applications. 4Open 2023, 6, 1. [Google Scholar] [CrossRef]
- Le, N.; Kim, K. Current Advances in the Biomedical Applications of Quantum Dots: Promises and Challenges. Int. J. Mol. Sci. 2023, 24, 12682. [Google Scholar] [CrossRef]
- Yong, K.T.; Wang, Y.; Roy, I.; Rui, H.; Swihart, M.T.; Law, W.C.; Kwak, S.K.; Ye, L.; Liu, J.; Mahajan, S.D.; et al. Preparation of quantum dot/drug nanoparticle formulations for traceable targeted delivery and therapy. Theranostics 2012, 2, 681–694. [Google Scholar] [CrossRef]
- Jin, T.; Tiwari, D.K.; Tanaka, S.; Inouye, Y.; Yoshizawa, K.; Watanabe, T.M. Antibody-protein A conjugated quantum dots for multiplexed imaging of surface receptors in living cells. Mol. Biosyst. 2010, 6, 2325–2331. [Google Scholar] [CrossRef]
- Osypiw, A.R.C.; Lee, S.; Jung, S.-M.; Leoni, S.; Smowton, P.M.; Hou, B.; Kim, J.M.; Amaratunga, G.A.J. Solution-processed colloidal quantum dots for light emission. Mater. Adv. 2022, 3, 6773–6790. [Google Scholar] [CrossRef]
- Muñoz, R.; Santos, E.M.; Galan-Vidal, C.A.; Miranda, J.M.; Lopez-Santamarina, A.; Rodriguez, J.A. Ternary Quantum Dots in Chemical Analysis. Synthesis and Detection Mechanisms. Molecules 2021, 26, 2764. [Google Scholar] [CrossRef]
- Abbas, Z.; Rehman, S. An Overview of Cancer Treatment Modalities. In Neoplasm; Shahzad, H.N., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Levy, M.; Chowdhury, P.P.; Nagpal, P. Quantum dot therapeutics: A new class of radical therapies. J. Biol. Eng. 2019, 13, 48. [Google Scholar] [CrossRef]
- Liang, Z.; Khawar, M.B.; Liang, J.; Sun, H. Bio-Conjugated Quantum Dots for Cancer Research: Detection and Imaging. Front. Oncol. 2021, 11, 749970. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, Z.; Gao, S.; Goh, B.L.; Samsudin, I.B.; Lwe, K.W.; Wu, Y.; Wu, C.; Su, X. Recent advances in non-toxic quantum dots and their biomedical applications. Prog. Nat. Sci. Mater. Int. 2019, 29, 628–640. [Google Scholar] [CrossRef]
- Nabil, M.; Megahed, F. Quantum Dot Nanomaterials: Preparation, Characterization, Advanced Bio-Imaging and Therapeutic Applications. J. Fluoresc. 2024, 34, 2467–2484. [Google Scholar] [CrossRef]
- Channa, A.I.; Tong, X.; Xu, J.-Y.; Liu, Y.; Wang, C.; Sial, M.N.; Yu, P.; Ji, H.; Niu, X.; Wang, Z.M. Tailored near-infrared-emitting colloidal heterostructured quantum dots with enhanced visible light absorption for high performance photoelectrochemical cells. J. Mater. Chem. A 2019, 7, 10225–10230. [Google Scholar] [CrossRef]
- Ju, F.; Atyah, M.M.; Horstmann, N.; Gul, S.; Vago, R.; Bruns, C.J.; Zhao, Y.; Dong, Q.Z.; Ren, N. Characteristics of the cancer stem cell niche and therapeutic strategies. Stem Cell Res. Ther. 2022, 13, 233. [Google Scholar] [CrossRef]
- Dragu, D.L.; Necula, L.G.; Bleotu, C.; Diaconu, C.C.; Chivu-Economescu, M. Therapies targeting cancer stem cells: Current trends and future challenges. World J. Stem Cells 2015, 7, 1185–1201. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Hamidu, A.; Pitt, W.G.; Husseini, G.A. Recent Breakthroughs in Using Quantum Dots for Cancer Imaging and Drug Delivery Purposes. Nanomaterials 2023, 13, 2566. [Google Scholar] [CrossRef]
- Rhyner, M.N.; Smith, A.M.; Gao, X.; Mao, H.; Yang, L.; Nie, S. Quantum dots and multifunctional nanoparticles: New contrast agents for tumor imaging. Nanomedicine 2006, 1, 209–217. [Google Scholar] [CrossRef]
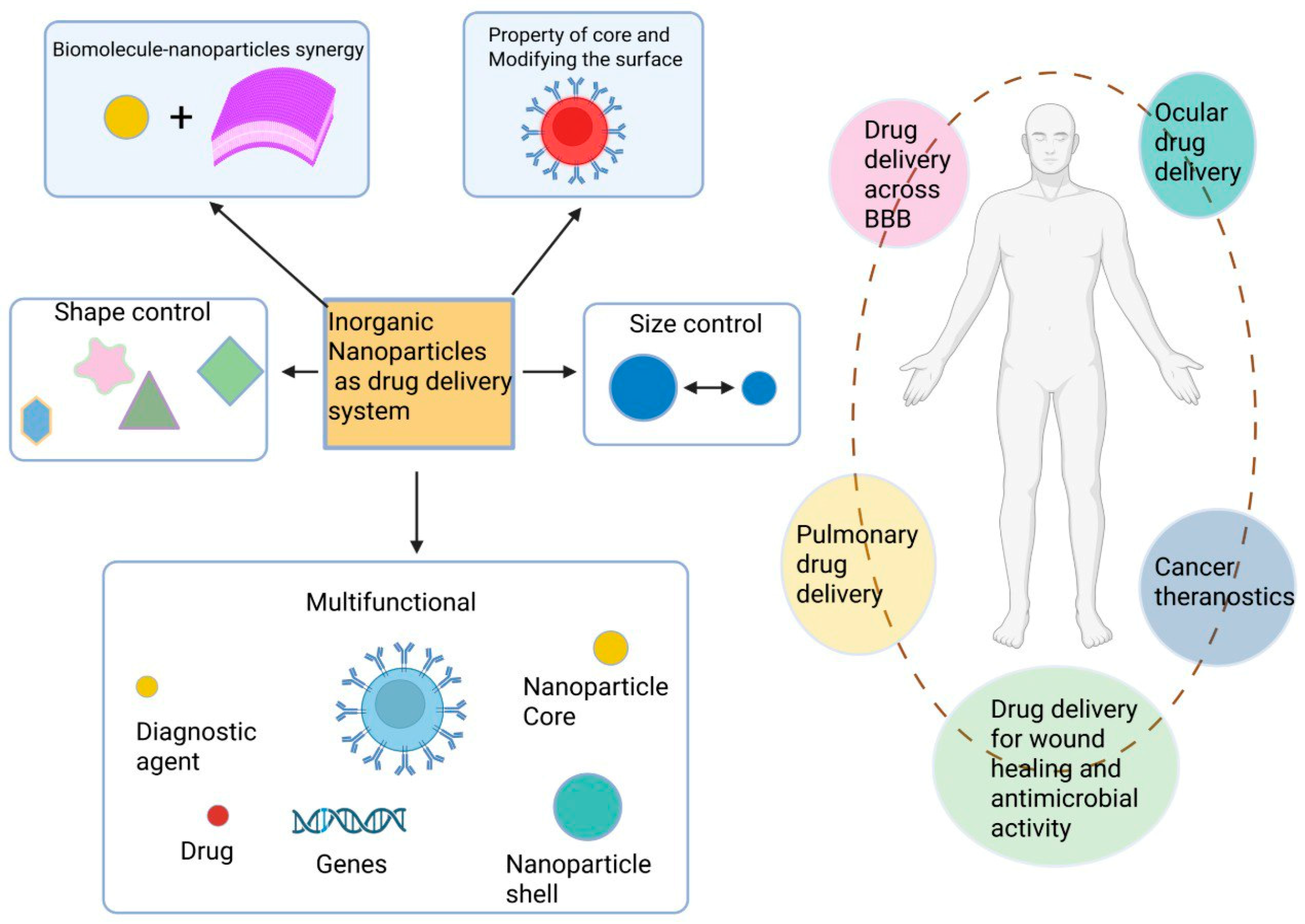
- Asad, S.; Jacobsen, A.-C.; Teleki, A. Inorganic nanoparticles for oral drug delivery: Opportunities, barriers, and future perspectives. Curr. Opin. Chem. Eng. 2022, 38, 100869. [Google Scholar] [CrossRef]
- McCright, J.; Naiknavare, R.; Yarmovsky, J.; Maisel, K. Targeting Lymphatics for Nanoparticle Drug Delivery. Front. Pharmacol. 2022, 13, 887402. [Google Scholar] [CrossRef]
- Schudel, A.; Francis, D.M.; Thomas, S.N. Material design for lymph node drug delivery. Nat. Rev. Mater. 2019, 4, 415–428. [Google Scholar] [CrossRef]
- Praphawatvet, T.; Peters, J.I.; Williams, R.O., 3rd. Inhaled nanoparticles-An updated review. Int. J. Pharm. 2020, 587, 119671. [Google Scholar] [CrossRef]
- Unnikrishnan, G.; Joy, A.; Megha, M.; Kolanthai, E.; Senthilkumar, M. Exploration of inorganic nanoparticles for revolutionary drug delivery applications: A critical review. Discov. Nano 2023, 18, 157. [Google Scholar] [CrossRef]
- Liu, M.; Du, H.; Zhang, W.; Zhai, G. Internal stimuli-responsive nanocarriers for drug delivery: Design strategies and applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1267–1280. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-organic framework functionalization and design strategies for advanced electrochemical energy storage devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef]
- Mohkam, M.; Sadraeian, M.; Lauto, A.; Gholami, A.; Nabavizadeh, S.H.; Esmaeilzadeh, H.; Alyasin, S. Exploring the potential and safety of quantum dots in allergy diagnostics. Microsyst. Nanoeng. 2023, 9, 145. [Google Scholar] [CrossRef]
- Suwatthanarak, T.; Tanaka, M.; Minamide, T.; Harvie, A.J.; Tamang, A.; Critchley, K.; Evans, S.D.; Okochi, M. Screening and characterisation of CdTe/CdS quantum dot-binding peptides for material surface functionalisation. RSC Adv. 2020, 10, 8218–8223. [Google Scholar] [CrossRef]
- Rahim, M.A.; Jan, N.; Khan, S.; Shah, H.; Madni, A.; Khan, A.; Jabar, A.; Khan, S.; Elhissi, A.; Hussain, Z.; et al. Recent Advancements in Stimuli Responsive Drug Delivery Platforms for Active and Passive Cancer Targeting. Cancers 2021, 13, 670. [Google Scholar] [CrossRef]
- Sun, Y.; Davis, E. Nanoplatforms for Targeted Stimuli-Responsive Drug Delivery: A Review of Platform Materials and Stimuli-Responsive Release and Targeting Mechanisms. Nanomaterials 2021, 11, 746. [Google Scholar] [CrossRef]
- Aboulkheyr Es, H.; Montazeri, L.; Aref, A.R.; Vosough, M.; Baharvand, H. Personalized Cancer Medicine: An Organoid Approach. Trends Biotechnol. 2018, 36, 358–371. [Google Scholar] [CrossRef]
- Wang, Q.; Chao, Y.M. Multifunctional quantum dots and liposome complexes in drug delivery. J. Biomed. Res. 2018, 32, 91–106. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Younis, M.A.; Alsharidah, M.; Al Rugaie, O.; Tawfeek, H.M. Biomedical Applications of Quantum Dots: Overview, Challenges, and Clinical Potential. Int. J. Nanomed. 2022, 17, 1951–1970. [Google Scholar] [CrossRef]
- Ryvolova, M.; Chomoucka, J.; Janu, L.; Drbohlavova, J.; Adam, V.; Hubalek, J.; Kizek, R. Biotin-modified glutathione as a functionalized coating for bioconjugation of CdTe-based quantum dots. Electrophoresis 2011, 32, 1619–1622. [Google Scholar] [CrossRef]
- Sahu, A.; Kumar, D. Core-shell quantum dots: A review on classification, materials, application, and theoretical modeling. J. Alloys Compd. 2022, 924, 166508. [Google Scholar] [CrossRef]
- Belli, C.; Trapani, D.; Viale, G.; D’Amico, P.; Duso, B.A.; Della Vigna, P.; Orsi, F.; Curigliano, G. Targeting the microenvironment in solid tumors. Cancer Treat. Rev. 2018, 65, 22–32. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.R.; Campbell, P.J.; Futreal, P.A. The cancer genome. Nature 2009, 458, 719–724. [Google Scholar] [CrossRef]
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.Y.; Leem, S.H.; Lee, J.H.; Kim, H.S. Dual Relationship Between Stromal Cells and Immune Cells in the Tumor Microenvironment. Front. Immunol. 2022, 13, 864739. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y.; et al. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 204. [Google Scholar] [CrossRef]
- Liao, D.; Johnson, R.S. Hypoxia: A key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007, 26, 281–290. [Google Scholar] [CrossRef]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef] [PubMed]
- Castro-Giner, F.; Aceto, N. Tracking cancer progression: From circulating tumor cells to metastasis. Genome Med. 2020, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shao, X.; Zhang, Y.; Zhu, M.; Wang, F.X.C.; Mu, J.; Li, J.; Yao, H.; Chen, K. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023, 12, 11149–11165. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, H.; Liu, G.; Wu, J.; Yuan, Y.; Shang, A. Tumor Microenvironment: Lactic Acid Promotes Tumor Development. J. Immunol. Res. 2022, 2022, 3119375. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Y.; Gillies, R.J. Tumor pH and its measurement. J. Nucl. Med. 2010, 51, 1167–1170. [Google Scholar] [CrossRef]
- Justus, C.R.; Dong, L.; Yang, L.V. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front. Physiol. 2013, 4, 354. [Google Scholar] [CrossRef]
- Salavati, H.; Debbaut, C.; Pullens, P.; Ceelen, W. Interstitial fluid pressure as an emerging biomarker in solid tumors. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188792. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Yu, A.R.; Lee, J.J.; Lee, Y.J.; Lim, S.M.; Kim, J.S. Measurement of Tumor Pressure and Strategies of Imaging Tumor Pressure for Radioimmunotherapy. Nucl. Med. Mol. Imaging 2019, 53, 235–241. [Google Scholar] [CrossRef]
- Sever, R.; Brugge, J.S. Signal transduction in cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a006098. [Google Scholar] [CrossRef]
- Mroz, E.A.; Rocco, J.W. The challenges of tumor genetic diversity. Cancer 2017, 123, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Nigam, M.; Kunjwal, S.S.; Sergey, P.; Mishra, A.P.; Sharifi-Rad, J. Cancer Stem Cells: From an Insight into the Basics to Recent Advances and Therapeutic Targeting. Stem Cells Int. 2022, 2022, 9653244. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, V. Exposure to Inorganic Nanoparticles: Routes of Entry, Immune Response, Biodistribution and In Vitro/In Vivo Toxicity Evaluation. Toxics 2017, 5, 29. [Google Scholar] [CrossRef]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef]
- Zeng, S.; Baillargeat, D.; Ho, H.-P.; Yong, K.-T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef]
- Yong, K.-T.; Qian, J.; Roy, I.; Lee, H.H.; Bergey, E.J.; Tramposch, K.M.; He, S.; Swihart, M.T.; Maitra, A.; Prasad, P.N. Quantum Rod Bioconjugates as Targeted Probes for Confocal and Two-Photon Fluorescence Imaging of Cancer Cells. Nano Lett. 2007, 7, 761–765. [Google Scholar] [CrossRef]
- Kurosawa, Y.; Nirengi, S.; Homma, T.; Esaki, K.; Ohta, M.; Clark, J.F.; Hamaoka, T. A single-dose of oral nattokinase potentiates thrombolysis and anti-coagulation profiles. Sci. Rep. 2015, 5, 11601. [Google Scholar] [CrossRef]
- Di, Y.; Wang, P.; Li, C.; Xu, S.; Tian, Q.; Wu, T.; Tian, Y.; Gao, L. Design, Bioanalytical, and Biomedical Applications of Aptamer-Based Hydrogels. Front. Med. 2020, 7, 456. [Google Scholar] [CrossRef]
- Lin, B.; Xiao, F.; Jiang, J.; Zhao, Z.; Zhou, X. Engineered aptamers for molecular imaging. Chem. Sci. 2023, 14, 14039–14061. [Google Scholar] [CrossRef]
- Weaver, C.; DeRosier, M.E. Commentary on Scaling-Up Evidence-Based Interventions in Public Systems. Prev. Sci. 2019, 20, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Lee, Y.-H.; Chou, C.-L.; Chang, Y.-S.; Liu, W.-C.; Chiu, H.-W. Oxidative stress and potential effects of metal nanoparticles: A review of biocompatibility and toxicity concerns. Environ. Pollut. 2024, 346, 123617. [Google Scholar] [CrossRef]
- Chen, K.; Chen, X. Design and development of molecular imaging probes. Curr. Top. Med. Chem. 2010, 10, 1227–1236. [Google Scholar] [CrossRef]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- Dighe, S.; Jog, S.; Momin, M.; Sawarkar, S.; Omri, A. Intranasal Drug Delivery by Nanotechnology: Advances in and Challenges for Alzheimer’s Disease Management. Pharmaceutics 2024, 16, 58. [Google Scholar] [CrossRef]
- Gupta, M.; Agrawal, U.; Vyas, S.P. Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert. Opin. Drug Deliv. 2012, 9, 783–804. [Google Scholar] [CrossRef]
- Attama, A.; Ogbonna, J.D.N.; Uchechi, O. Nanoparticles for Dermal and Transdermal Drug Delivery. In Application of Nanotechnology in Drug Delivery; Sezer, A.D., Ed.; IntechOpen: London, UK, 2014. [Google Scholar]
- Chen, Y.; Feng, X. Gold nanoparticles for skin drug delivery. Int. J. Pharm. 2022, 625, 122122. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G.; Vierl, U. Nanotechnology and the transdermal route: A state of the art review and critical appraisal. J. Control. Release 2010, 141, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Labouta, H.I.; Schneider, M. Interaction of inorganic nanoparticles with the skin barrier: Current status and critical review. Nanomedicine 2013, 9, 39–54. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Luo, Z.; Dai, L.; Guo, X.; Cai, K. Design of nanocarriers based on complex biological barriers in vivo for tumor therapy. Nano Today 2017, 15, 56–90. [Google Scholar] [CrossRef]
- Tee, J.K.; Yip, L.X.; Tan, E.S.; Santitewagun, S.; Prasath, A.; Ke, P.C.; Ho, H.K.; Leong, D.T. Nanoparticles’ interactions with vasculature in diseases. Chem. Soc. Rev. 2019, 48, 5381–5407. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Liu, Y.; Liu, X.; Su, K.; Shi, K. Influencing factors and strategies of enhancing nanoparticles into tumors in vivo. Acta Pharm. Sin. B 2021, 11, 2265–2285. [Google Scholar] [CrossRef]
- Lee, H.; Hoang, B.; Fonge, H.; Reilly, R.M.; Allen, C. In vivo distribution of polymeric nanoparticles at the whole-body, tumor, and cellular levels. Pharm. Res. 2010, 27, 2343–2355. [Google Scholar] [CrossRef] [PubMed]
- Soo Choi, H.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef]
- Poon, W.; Zhang, Y.-N.; Ouyang, B.; Kingston, B.R.; Wu, J.L.Y.; Wilhelm, S.; Chan, W.C.W. Elimination Pathways of Nanoparticles. ACS Nano 2019, 13, 5785–5798. [Google Scholar] [CrossRef] [PubMed]
- Riviere, J.E. Pharmacokinetics of nanomaterials: An overview of carbon nanotubes, fullerenes and quantum dots. WIREs Nanomed. Nanobiotechnol. 2009, 1, 26–34. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle Uptake: The Phagocyte Problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Xiao, Q.; Zoulikha, M.; Qiu, M.; Teng, C.; Lin, C.; Li, X.; Sallam, M.A.; Xu, Q.; He, W. The effects of protein corona on in vivo fate of nanocarriers. Adv. Drug Deliv. Rev. 2022, 186, 114356. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef]
- Rabanel, J.M.; Aoun, V.; Elkin, I.; Mokhtar, M.; Hildgen, P. Drug-loaded nanocarriers: Passive targeting and crossing of biological barriers. Curr. Med. Chem. 2012, 19, 3070–3102. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in nanomaterial-based targeted drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef]
- Bhandari, S.; Mondal, D.; Nataraj, S.K.; Balakrishna, R.G. Biomolecule-derived quantum dots for sustainable optoelectronics. Nanoscale Adv. 2019, 1, 913–936. [Google Scholar] [CrossRef]
- Drbohlavova, J.; Adam, V.; Kizek, R.; Hubalek, J. Quantum Dots—Characterization, Preparation and Usage in Biological Systems. Int. J. Mol. Sci. 2009, 10, 656–673. [Google Scholar] [CrossRef] [PubMed]
- Pinaud, F.; Michalet, X.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Iyer, G.; Weiss, S. Advances in fluorescence imaging with quantum dot bio-probes. Biomaterials 2006, 27, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- López-Rios de Castro, R.; Ziolek, R.M.; Ulmschneider, M.B.; Lorenz, C.D. Therapeutic Peptides Are Preferentially Solubilized in Specific Microenvironments within PEG–PLGA Polymer Nanoparticles. Nano Lett. 2024, 24, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Marinelli, L.; Ciulla, M.; Ritsema, J.A.S.; van Nostrum, C.F.; Cacciatore, I.; Dimmito, M.P.; Palmerio, F.; Orlando, G.; Robuffo, I.; Grande, R.; et al. Preparation, Characterization, and Biological Evaluation of a Hydrophilic Peptide Loaded on PEG-PLGA Nanoparticles. Pharmaceutics 2022, 14, 1821. [Google Scholar] [CrossRef]
- Watcharadulyarat, N.; Rattanatayarom, M.; Ruangsawasdi, N.; Patikarnmonthon, N. PEG–PLGA nanoparticles for encapsulating ciprofloxacin. Sci. Rep. 2023, 13, 266. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Trasande, L.; Kannan, K. Occurrence of Polyethylene Terephthalate and Polycarbonate Microplastics in Infant and Adult Feces. Environ. Sci. Technol. Lett. 2021, 8, 989–994. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Cvetković, I.; Hong, D.; Wan, X.; Zhang, W.; Abraham, T.; Malsam, J. Polyester polyols and polyurethanes from ricinoleic acid. J. Appl. Polym. Sci. 2008, 108, 1184–1190. [Google Scholar] [CrossRef]
- Alibolandi, M.; Abnous, K.; Sadeghi, F.; Hosseinkhani, H.; Ramezani, M.; Hadizadeh, F. Folate receptor-targeted multimodal polymersomes for delivery of quantum dots and doxorubicin to breast adenocarcinoma: In vitro and in vivo evaluation. Int. J. Pharm. 2016, 500, 162–178. [Google Scholar] [CrossRef]
- Stapf, M.; Pömpner, N.; Teichgräber, U.; Hilger, I. Heterogeneous response of different tumor cell lines to methotrexate-coupled nanoparticles in presence of hyperthermia. Int. J. Nanomed. 2016, 11, 485–500. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Shi, Y.; Jiang, L.; Wang, L.; Rashid, H.U.; Yuan, M.; Liu, X. Advances in liposomes loaded with photoresponse materials for cancer therapy. Biomed. Pharmacother. 2024, 174, 116586. [Google Scholar] [CrossRef]
- López-Gutiérrez, N.; Romero-González, R.; Martínez Vidal, J.L.; Frenich, A.G. Determination of polyphenols in grape-based nutraceutical products using high resolution mass spectrometry. LWT-Food Sci. Technol. 2016, 71, 249–259. [Google Scholar] [CrossRef]
- Yang, C.; Ding, N.; Xu, Y.; Qu, X.; Zhang, J.; Zhao, C.; Hong, L.; Lu, Y.; Xiang, G. Folate receptor-targeted quantum dot liposomes as fluorescence probes. J. Drug Target. 2009, 17, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; Jeong, H.Y.; Kang, S.J.; Jeong, I.H.; Choi, M.J.; You, Y.M.; Im, C.S.; Song, I.H.; Lee, T.S.; Lee, J.S.; et al. Anti-EGF Receptor Aptamer-Guided Co-Delivery of Anti-Cancer siRNAs and Quantum Dots for Theranostics of Triple-Negative Breast Cancer. Theranostics 2019, 9, 837–852. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Wilson, R.L.; Cubeddu, L.X. Quantum Dot Research in Breast Cancer: Challenges and Prospects. Materials 2024, 17, 2152. [Google Scholar] [CrossRef]
- Monteiro, C.A.P.; Oliveira, A.; Silva, R.C.; Lima, R.R.M.; Souto, F.O.; Baratti, M.O.; Carvalho, H.F.; Santos, B.S.; Cabral Filho, P.E.; Fontes, A. Evaluating internalization and recycling of folate receptors in breast cancer cells using quantum dots. J. Photochem. Photobiol. B 2020, 209, 111918. [Google Scholar] [CrossRef]
- Zhang, D.; Meegoda, J.N.; da Silva, B.M.G.; Hu, L. Impact of de-ionized water on changes in porosity and permeability of shales mineralogy due to clay-swelling. Sci. Rep. 2021, 11, 20049. [Google Scholar] [CrossRef]
- Kunachowicz, D.; Kłosowska, K.; Sobczak, N.; Kepinska, M. Applicability of Quantum Dots in Breast Cancer Diagnostic and Therapeutic Modalities—A State-of-the-Art Review. Nanomaterials 2024, 14, 1424. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Wang, H.; Sun, H.; Wei, H.; Xi, P.; Nie, S.; Ren, Q. Biocompatible hyaluronic acid polymer-coated quantum dots for CD44+ cancer cell-targeted imaging. J. Nanoparticle Res. 2014, 16, 2621. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Yu, Y.; Yu, R.N.; Wan, M.; Zhang, R.Y.; Zhao, Y.D. Tracking the down-regulation of folate receptor-α in cancer cells through target specific delivery of quantum dots coupled with antisense oligonucleotide and targeted peptide. Small 2013, 9, 4183–4193. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L.; Ming, X.; Nakagawa, O. The chemistry and biology of oligonucleotide conjugates. Acc. Chem. Res. 2012, 45, 1067–1076. [Google Scholar] [CrossRef]
- Ran, R.; Liu, Y.; Gao, H.; Kuang, Q.; Zhang, Q.; Tang, J.; Fu, H.; Zhang, Z.; He, Q. PEGylated Hyaluronic Acid-Modified Liposomal Delivery System with Anti-γ-Glutamylcyclotransferase siRNA for Drug-Resistant MCF-7 Breast Cancer Therapy. J. Pharm. Sci. 2015, 104, 476–484. [Google Scholar] [CrossRef]
- Wang, F.B.; Rong, Y.; Fang, M.; Yuan, J.P.; Peng, C.W.; Liu, S.P.; Li, Y. Recognition and capture of metastatic hepatocellular carcinoma cells using aptamer-conjugated quantum dots and magnetic particles. Biomaterials 2013, 34, 3816–3827. [Google Scholar] [CrossRef] [PubMed]
- Ladju, R.B.; Pascut, D.; Massi, M.N.; Tiribelli, C.; Sukowati, C.H.C. Aptamer: A potential oligonucleotide nanomedicine in the diagnosis and treatment of hepatocellular carcinoma. Oncotarget 2018, 9, 2951–2961. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Asghar, W.; Demirci, U.; Wan, Y. Nanostructured substrates for isolation of circulating tumor cells. Nano Today 2013, 8, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Saharkhiz, S.; Nasri, N.; Dini, G.; Yousefnia, S. Development of a new smart theranostic anti-PSMA-aptamer conjugated cationic-lipid coated mesoporous silica platform for targeted delivery of paclitaxel and CdSe/ZnS quantum dots to LNCaP cell line. J. Drug Deliv. Sci. Technol. 2023, 88, 104964. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- Lammertink, B.H.A.; Deckers, R.; Derieppe, M.; De Cock, I.; Lentacker, I.; Storm, G.; Moonen, C.T.W.; Bos, C. Dynamic Fluorescence Microscopy of Cellular Uptake of Intercalating Model Drugs by Ultrasound-Activated Microbubbles. Mol. Imaging Biol. 2017, 19, 683–693. [Google Scholar] [CrossRef]
- Hou, J.; Zhao, Y.; Sun, L.; Zou, X. Enzyme/GSH/pH-responsive hyaluronic acid grafted porous silica nanocarriers bearing Ag2S QDs for fluorescence imaging and combined therapy. Carbohydr. Polym. 2023, 305, 120547. [Google Scholar] [CrossRef]
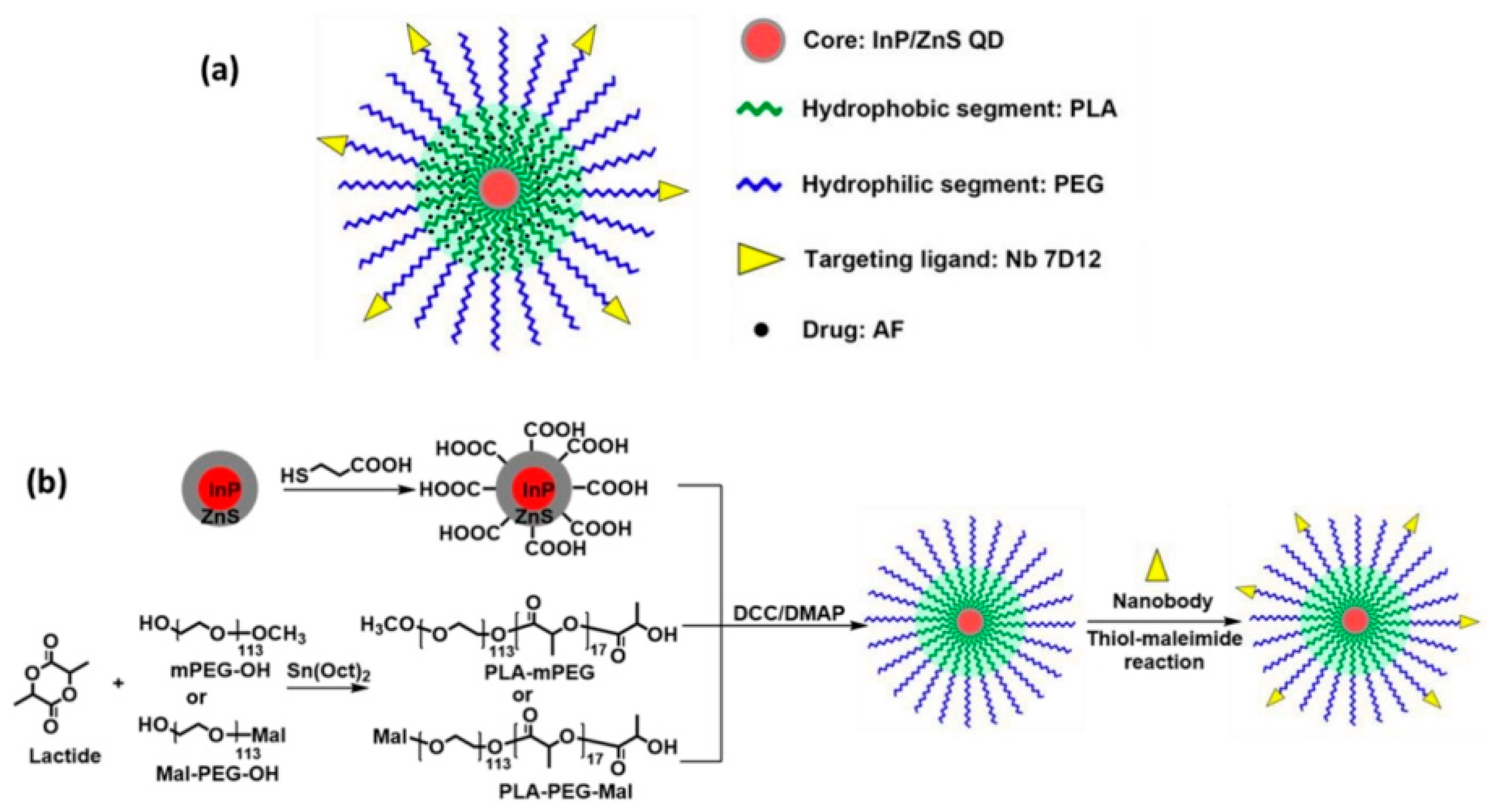
- Wang, Y.; Wang, Y.; Chen, G.; Li, Y.; Xu, W.; Gong, S. Quantum-Dot-Based Theranostic Micelles Conjugated with an Anti-EGFR Nanobody for Triple-Negative Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 30297–30305. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, J.; Dong, C.; Li, Y.; Yu, Q.; Wang, X.; Chen, Z.; Li, J.; Yang, Y.; Wang, H. Polyvalent Aptamer-Functionalized NIR-II Quantum Dots for Targeted Theranostics in High PD-L1-Expressing Tumors. ACS Appl. Mater. Interfaces 2024, 16, 21571–21581. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, G.; Zhang, Y.; Chen, G.; Li, F.; Dai, H.; Wang, Q. Ag2S Quantum Dot: A Bright and Biocompatible Fluorescent Nanoprobe in the Second Near-Infrared Window. ACS Nano 2012, 6, 3695–3702. [Google Scholar] [CrossRef]
- Sun, P.; Li, K.; Liu, X.; Wang, J.; Qiu, X.; Wei, W.; Zhao, J. Peptide-mediated Aqueous Synthesis of NIR-II Emitting Ag2S Quantum Dots for Rapid Photocatalytic Bacteria Disinfection. Angew. Chem. Int. Ed. 2023, 62, e202300085. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.; Gong, C.; Zhang, J.; Wang, X.; Wang, X.; Gu, Y.; Guo, G.; Chen, L.; Luo, F.; Zhao, X.; et al. Polymeric matrix for drug delivery: Honokiol-loaded PCL-PEG-PCL nanoparticles in PEG-PCL-PEG thermosensitive hydrogel. J. Biomed. Mater. Res. A 2010, 93, 219–226. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, J.; Sun, C. Ditelluride-Bridged PEG-PCL Copolymer as Folic Acid-Targeted and Redox-Responsive Nanoparticles for Enhanced Cancer Therapy. Front. Chem. 2020, 8, 156. [Google Scholar] [CrossRef]
- Hou, Z.; Zhou, W.; Guo, X.; Zhong, R.; Wang, A.; Li, J.; Cen, Y.; You, C.; Tan, H.; Tian, M. Poly(ε-Caprolactone)-Methoxypolyethylene Glycol (PCL-MPEG)-Based Micelles for Drug-Delivery: The Effect of PCL Chain Length on Blood Components, Phagocytosis, and Biodistribution. Int. J. Nanomed. 2022, 17, 1613–1632. [Google Scholar] [CrossRef]
- Jin, P.; Ma, D.; Gao, Y.; Wang, L.; Gao, Z.; Zhang, Y.; Liu, M.; Xu, J.; Wang, J. Determination of Cisplatin Cross-Linked Hyaluronic Acid (CPHA) Hydrogel and DNA Using the Fluorescent Response from Mercaptopropionic Acid (MPA) Capped Cadmium Telluride Quantum Dots (CdTe QDs). Anal. Lett. 2021, 54, 2411–2422. [Google Scholar] [CrossRef]
- Tan, W.B.; Jiang, S.; Zhang, Y. Quantum-dot based nanoparticles for targeted silencing of HER2/neu gene via RNA interference. Biomaterials 2007, 28, 1565–1571. [Google Scholar] [CrossRef]
- Mulder, W.J.M.; Castermans, K.; van Beijnum, J.R.; oude Egbrink, M.G.A.; Chin, P.T.K.; Fayad, Z.A.; Löwik, C.W.G.M.; Kaijzel, E.L.; Que, I.; Storm, G.; et al. Molecular imaging of tumor angiogenesis using αvβ3-integrin targeted multimodal quantum dots. Angiogenesis 2009, 12, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; He, J.; Zhang, Y.; Fan, L.; Zhao, Y.; Xu, T.; Nie, Z.; Li, X.; Huang, Z.; Lu, B.; et al. Synthesis and in vitro evaluation of a hyaluronic acid–quantum dots–melphalan conjugate. Carbohydr. Polym. 2015, 121, 132–139. [Google Scholar] [CrossRef]
- Tao, J.; Feng, S.; Liu, B.; Pan, J.; Li, C.; Zheng, Y. Hyaluronic acid conjugated nitrogen-doped graphene quantum dots for identification of human breast cancer cells. Biomed. Mater. 2021, 16, 055001. [Google Scholar] [CrossRef] [PubMed]
- Kaveh Zenjanab, M.; Abdolahinia, E.D.; Alizadeh, E.; Hamishehkar, H.; Shahbazi, R.; Ranjbar-Navazi, Z.; Jahanban-Esfahlan, R.; Fathi, M.; Mohammadi, S.A. Hyaluronic Acid-Targeted Niosomes for Effective Breast Cancer Chemostarvation Therapy. ACS Omega 2024, 9, 10875–10885. [Google Scholar] [CrossRef] [PubMed]
- Rakovich, T.Y.; Mahfoud, O.K.; Mohamed, B.M.; Prina-Mello, A.; Crosbie-Staunton, K.; Van Den Broeck, T.; De Kimpe, L.; Sukhanova, A.; Baty, D.; Rakovich, A.; et al. Highly sensitive single domain antibody-quantum dot conjugates for detection of HER2 biomarker in lung and breast cancer cells. ACS Nano 2014, 8, 5682–5695. [Google Scholar] [CrossRef]
- Pietilä, M.; Lehenkari, P.; Kuvaja, P.; Kaakinen, M.; Kaul, S.C.; Wadhwa, R.; Uemura, T. Mortalin antibody-conjugated quantum dot transfer from human mesenchymal stromal cells to breast cancer cells requires cell–cell interaction. Exp. Cell Res. 2013, 319, 2770–2780. [Google Scholar] [CrossRef]
- Zdobnova, T.A.; Stremovskiy, O.A.; Lebedenko, E.N.; Deyev, S.M. Self-assembling complexes of quantum dots and scFv antibodies for cancer cell targeting and imaging. PLoS ONE 2012, 7, e48248. [Google Scholar] [CrossRef]
- Wu, Y.; Xue, P.; Kang, Y.; Hui, K.M. Highly specific and ultrasensitive graphene-enhanced electrochemical detection of low-abundance tumor cells using silica nanoparticles coated with antibody-conjugated quantum dots. Anal. Chem. 2013, 85, 3166–3173. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, J.Y.; Nehrbass, U.; Song, R.; Choi, Y. Detection of melanoma using antibody-conjugated quantum dots in a coculture model for high-throughput screening system. Analyst 2012, 137, 1440–1445. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Qasim, M.; Kim, J.H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.A.P.; de Carvalho, S.M.; Mansur, H.S. Bioengineered quantum dot/chitosan-tripeptide nanoconjugates for targeting the receptors of cancer cells. Int. J. Biol. Macromol. 2016, 82, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Phung, L.T.; Kitwetcharoen, H.; Chamnipa, N.; Boonchot, N.; Thanonkeo, S.; Tippayawat, P.; Klanrit, P.; Yamada, M.; Thanonkeo, P. Changes in the chemical compositions and biological properties of kombucha beverages made from black teas and pineapple peels and cores. Sci. Rep. 2023, 13, 7859. [Google Scholar] [CrossRef]
- Solhi, L.; Guccini, V.; Heise, K.; Solala, I.; Niinivaara, E.; Xu, W.; Mihhels, K.; Kröger, M.; Meng, Z.; Wohlert, J.; et al. Understanding Nanocellulose–Water Interactions: Turning a Detriment into an Asset. Chem. Rev. 2023, 123, 1925–2015. [Google Scholar] [CrossRef]
- Wang, Y.; Boulic, M.; Phipps, R.; Plagmann, M.; Cunningham, C.; Guyot, G. Field performance of a solar air heater used for space heating and ventilation–A case study in New Zealand primary schools. J. Build. Eng. 2023, 76, 106802. [Google Scholar] [CrossRef]
- Gunti, S.; Notkins, A.L. Polyreactive Antibodies: Function and Quantification. J. Infect. Dis. 2015, 212 (Suppl. S1), S42–S46. [Google Scholar] [CrossRef]
- Ahirwar, S.; Mallick, S.; Bahadur, D. Photodynamic therapy using graphene quantum dot derivatives. J. Solid State Chem. 2020, 282, 121107. [Google Scholar] [CrossRef]
- Murali, G.; Kwon, B.; Kang, H.; Modigunta, J.K.R.; Park, S.; Lee, S.; Lee, H.; Park, Y.H.; Kim, J.; Park, S.Y.; et al. Hematoporphyrin Photosensitizer-Linked Carbon Quantum Dots for Photodynamic Therapy of Cancer Cells. ACS Appl. Nano Mater. 2022, 5, 4376–4385. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Charron, G.; Stuchinskaya, T.; Edwards, D.R.; Russell, D.A.; Nann, T. Insights into the Mechanism of Quantum Dot-Sensitized Singlet Oxygen Production for Photodynamic Therapy. J. Phys. Chem. C 2012, 116, 9334–9342. [Google Scholar] [CrossRef]
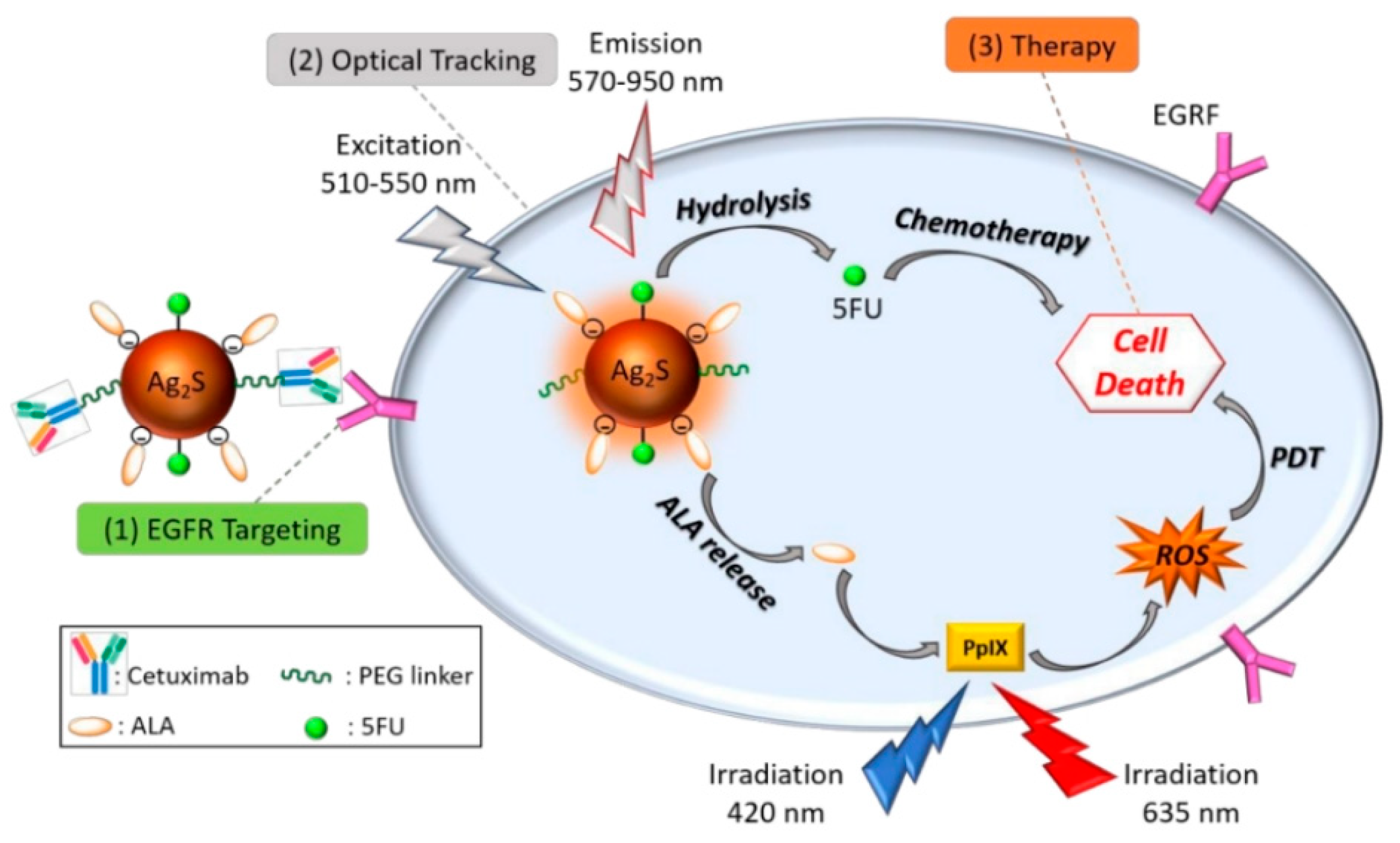
- Hashemkhani, M.; Demirci, G.; Bayir, A.; Muti, A.; Sennaroglu, A.; Mohammad Hadi, L.; Yaghini, E.; Loizidou, M.; MacRobert, A.J.; Yagci Acar, H. Cetuximab-Ag2S quantum dots for fluorescence imaging and highly effective combination of ALA-based photodynamic/chemo-therapy of colorectal cancer cells. Nanoscale 2021, 13, 14879–14899. [Google Scholar] [CrossRef]
- Abrishami, A.; Bahrami, A.R.; Nekooei, S.; Sh Saljooghi, A.; Matin, M.M. Hybridized quantum dot, silica, and gold nanoparticles for targeted chemo-radiotherapy in colorectal cancer theranostics. Commun. Biol. 2024, 7, 393. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Norwitz, E.R.; Shaw, J. Contemporary management of fibroids in pregnancy. Rev. Obstet. Gynecol. 2010, 3, 20–27. [Google Scholar] [PubMed]
- Guo, S.; Song, Z.; Ji, D.-K.; Reina, G.; Fauny, J.-D.; Nishina, Y.; Ménard-Moyon, C.; Bianco, A. Combined Photothermal and Photodynamic Therapy for Cancer Treatment Using a Multifunctional Graphene Oxide. Pharmaceutics 2022, 14, 1365. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef]
- Matveeva, O.; Nechipurenko, Y.; Lagutkin, D.; Yegorov, Y.E.; Kzhyshkowska, J. SARS-CoV-2 infection of phagocytic immune cells and COVID-19 pathology: Antibody-dependent as well as independent cell entry. Front. Immunol. 2022, 13, 1050478. [Google Scholar] [CrossRef]
- Sultana, N.; Pathak, R.; Samanta, S.; Sen Sarma, N. A comprehensive analysis of photothermal therapy (PTT) and photodynamic therapy (PDT) for the treatment of cancer. Process Biochem. 2025, 148, 17–31. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, H.; Yang, Z.; Zhong, X.; Chen, Y.; Dai, W.; Zhang, X. Aptamer-Conjugated Graphene Quantum Dots/Porphyrin Derivative Theranostic Agent for Intracellular Cancer-Related MicroRNA Detection and Fluorescence-Guided Photothermal/Photodynamic Synergetic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 159–166. [Google Scholar] [CrossRef]
- Wang, R.; Shen, J.; Ma, Y.; Qin, X.; Qin, X.; Yang, F.; Ostrikov, K.; Zhang, Q.; He, J.; Zhong, X. Cancer-targeting carbon quantum dots synthesized by plasma electrochemical method for red-light-activated photodynamic therapy. Plasma Process. Polym. 2024, 21, 2300174. [Google Scholar] [CrossRef]
- Yadav, P.K.; Chandra, S.; Kumar, V.; Kumar, D.; Hasan, S.H. Carbon Quantum Dots: Synthesis, Structure, Properties, and Catalytic Applications for Organic Synthesis. Catalysts 2023, 13, 422. [Google Scholar] [CrossRef]
- Wen, D.; Zhang, X.; Ding, L.; Wen, H.; Liu, W.; Zhang, C.; Wang, B.; Li, L.; Diao, H. Folic acid functionalized aggregation-induced emission nanoparticles for tumor cell targeted imaging and photodynamic therapy. RSC Adv. 2022, 12, 4484–4489. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef]
- Singh, S.; Chakraborty, A.; Singh, V.; Molla, A.; Hussain, S.; Singh, M.K.; Das, P. DNA mediated assembly of quantum dot–protoporphyrin IX FRET probes and the effect of FRET efficiency on ROS generation. Phys. Chem. Chem. Phys. 2015, 17, 5973–5981. [Google Scholar] [CrossRef]
- Yi, J.; Liu, L.; Gao, W.; Zeng, J.; Chen, Y.; Pang, E.; Lan, M.; Yu, C. Advances and perspectives in phototherapy-based combination therapy for cancer treatment. J. Mater. Chem. B 2024, 12, 6285–6304. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Yang, Y.; Yu, Y.; Zhang, Y.; Zhu, D.; Yu, X.; Ouyang, X.; Xie, Z.; Zhao, Y.; et al. Recent Advances in Nanomaterials-Based Chemo-Photothermal Combination Therapy for Improving Cancer Treatment. Front. Bioeng. Biotechnol. 2019, 7, 293. [Google Scholar] [CrossRef]
- Miao, Y.; Wang, X.; Sun, J.; Yan, Z. Recent advances in the biomedical applications of black phosphorus quantum dots. Nanoscale Adv. 2021, 3, 1532–1550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.; Zhou, N.; Yuan, P.; Su, Y.; Shao, M.; Chi, C.; Pan, F. Near-infrared light triggered photo-therapy, in combination with chemotherapy using magnetofluorescent carbon quantum dots for effective cancer treating. Carbon 2017, 118, 752–764. [Google Scholar] [CrossRef]
- Nam, J.; Son, S.; Ochyl, L.J.; Kuai, R.; Schwendeman, A.; Moon, J.J. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat. Commun. 2018, 9, 1074. [Google Scholar] [CrossRef]
- Casserly, C.M.; Turner, J.N.; O’Sullivan, J.J.; Bruen, M.; Bullock, C.; Atkinson, S.; Kelly-Quinn, M. Impact of low-head dams on bedload transport rates in coarse-bedded streams. Sci. Total Environ. 2020, 716, 136908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wen, L.; Huang, R.; Wang, H.; Hu, X.; Xing, D. Mitochondrial specific photodynamic therapy by rare-earth nanoparticles mediated near-infrared graphene quantum dots. Biomaterials 2018, 153, 14–26. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, S.; Jia, Q.; Huang, L.; Lan, M.; Wang, P.; Zhang, W. Lysosome-targetable carbon dots for highly efficient photothermal/photodynamic synergistic cancer therapy and photoacoustic/two-photon excited fluorescence imaging. Chem. Eng. J. 2020, 388, 124212. [Google Scholar] [CrossRef]
- Chen, P.-H.; Hu, Z.; An, E.; Okeke, I.; Zheng, S.; Luo, X.; Gong, A.; Jaime-Figueroa, S.; Crews, C.M. Modulation of Phosphoprotein Activity by Phosphorylation Targeting Chimeras (PhosTACs). ACS Chem. Biol. 2021, 16, 2808–2815. [Google Scholar] [CrossRef]
- Liu, F.; Lin, J.; Luo, Y.; Xie, D.; Bian, J.; Liu, X.; Yue, J. Sialic acid-targeting multi-functionalized silicon quantum dots for synergistic photodynamic and photothermal cancer therapy. Biomater. Sci. 2023, 11, 4009–4021. [Google Scholar] [CrossRef]
- Zhou, M.; Ni, Q.W.; Yang, S.Y.; Qu, C.Y.; Zhao, P.C.; Zhang, J.C.; Xu, L.M. Effects of integrin-targeted photodynamic therapy on pancreatic carcinoma cell. World J. Gastroenterol. 2013, 19, 6559–6567. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A. The flaws and human harms of animal experimentation. Camb. Q. Healthc. Ethics 2015, 24, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Luo, Y.; Tsai, P.; Wang, J.; Chen, X. Metal ions doped carbon quantum dots: Synthesis, physicochemical properties, and their applications. TrAC Trends Anal. Chem. 2018, 103, 87–101. [Google Scholar] [CrossRef]
- Samimi, S.; Ardestani, M.S.; Dorkoosh, F.A. Preparation of carbon quantum dots- quinic acid for drug delivery of gemcitabine to breast cancer cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102287. [Google Scholar] [CrossRef]
- Lu, F.; Li, Z.; Kang, Y.; Su, Z.; Yu, R.; Zhang, S. Black phosphorus quantum dots encapsulated in anionic waterborne polyurethane nanoparticles for enhancing stability and reactive oxygen species generation for cancer PDT/PTT therapy. J. Mater. Chem. B 2020, 8, 10650–10661. [Google Scholar] [CrossRef]
- Einafshar, E.; Ghorbani, A. Advances in Black Phosphorus Quantum Dots for Cancer Research: Synthesis, Characterization, and Applications. Top. Curr. Chem. 2024, 382, 25. [Google Scholar] [CrossRef]
- Wang, M.; Liang, Y.; Liu, Y.; Ren, G.; Zhang, Z.; Wu, S.; Shen, J. Ultrasmall black phosphorus quantum dots: Synthesis, characterization, and application in cancer treatment. Analyst 2018, 143, 5822–5833. [Google Scholar] [CrossRef]
- Yang, S.; Wang, X.; He, P.; Xu, A.; Wang, G.; Duan, J.; Shi, Y.; Ding, G. Graphene Quantum Dots with Pyrrole N and Pyridine N: Superior Reactive Oxygen Species Generation Efficiency for Metal-Free Sonodynamic Tumor Therapy. Small 2021, 17, 2004867. [Google Scholar] [CrossRef]
- Zhang, Y.; Pang, L.; Ma, C.; Tu, Q.; Zhang, R.; Saeed, E.; Mahmoud, A.E.; Wang, J. Small Molecule-Initiated Light-Activated Semiconducting Polymer Dots: An Integrated Nanoplatform for Targeted Photodynamic Therapy and Imaging of Cancer Cells. Anal. Chem. 2014, 86, 3092–3099. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Gedda, G.R.; Thakur, M.; Bhaisare, M.L.; Talib, A.; Khan, M.S.; Wu, S.-M.; Wu, H.-F. Theranostic carbon dots ‘clathrate-like’ nanostructures for targeted photo-chemotherapy and bioimaging of cancer. J. Ind. Eng. Chem. 2017, 56, 62–73. [Google Scholar] [CrossRef]
- Nasrin, A.; Hassan, M.; Gomes, V.G. Two-photon active nucleus-targeting carbon dots: Enhanced ROS generation and photodynamic therapy for oral cancer. Nanoscale 2020, 12, 20598–20603. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ai, S.; Lu, X.; Liu, S.; Guan, W. Nanotechnology-based strategies for gastric cancer imaging and treatment. RSC Adv. 2021, 11, 35392–35407. [Google Scholar] [CrossRef]
- Scheck, M.K.; Hofheinz, R.D.; Lorenzen, S. HER2-Positive Gastric Cancer and Antibody Treatment: State of the Art and Future Developments. Cancers 2024, 16, 1336. [Google Scholar] [CrossRef] [PubMed]



| Property | Traditional Organic Fluorophores | Quantum Dots (QDs) | References |
|---|---|---|---|
| Chemical properties | Poor chemical resistance | Chemically resilient; pH sensitivity determined by surface coatings | [43,53,54,55,56,57,58,59,60] |
| Dimensions | Molecular (<0.5 nm) | Colloidal (1.5–10 nm diameter) | [43,53,54] |
| Hydrodynamic radius | <0.6 nm a | 1.4–40 nm b (depends on coating and ligand) | [61,62] |
| Absorption spectra | Discrete bands, FWHM ≈ 35–100 nm cde | Strong and broad absorption | [56,57] |
| Emission spectra | Broad, red-tailed, asymmetric, FWHM ≈ 35–100 nm | Narrow, symmetric, FWHM ≈ 30–90 nm | [56,57,61,62] |
| Two-photon cross-section | 10–500 GM | 2000–47,700 GM f | [56,58] |
| Molar absorption coefficient | 103–105 cm−1 mol−1 L | 105–106 cm−1 mol−1 L | [43,53,54,55,57,58,59,60] |
| Quantum yield | 0.05–1.0 | >20% g (ligand/shell dependent) | [57,58,59,60] |
| Fluorescence lifetime | <5 ns, mono-exponential | >10 ns, multi-exponential | [55,61] |
| Solubility/Dispersibility | Determined by substitution pattern | Controlled via surface chemistry (ligands) | [53,56] |
| Thermal stability | Variable; depends on dye | High; shell/ligand dependent | [57,62] |
| Photostability | Poor; prone to photobleaching | Excellent; long observation time | [55,56,57] |
| Bioconjugation labels | Mostly monovalent | Multivalent scaffolds; diverse conjugation | [58,59] |
| Single-molecule analysis | Limited by bleaching | Effective; restricted by blinking | [57,61] |
| Spectral multiplexing | Possible but limited | Excellent; ≥5 distinct colors achievable | [56,57,58] |
| Multifunctionality | Difficult and rare | High potential for multifunctional integration | [57,58,59,60] |
| Toxicity | Depends on dye chemistry | Related to heavy-metal content (e.g., Cd, Pb) | [55,57,62] |
| Methods of Fabrication | Quantum Dots Engineered | Characteristics | Refs. |
|---|---|---|---|
| Electron beam lithography | QD nanostructures | Optical properties preserved after cross-linking | [66] |
| QD microarrays | Fluorescence Bioaffinity | [67] | |
| Reactive ion etching | Indium gallium nitride (InGaN) QDs | Strong and distinct photoluminescence signal | [68] |
| Sol-gel | Titanium dioxide (TiO2) QDs | Extensive surface area, photocatalytic properties | [47] |
| Zinc selenide (ZnSe) QDs encapsulated in Silicon dioxide (SiO2) | - | [48] | |
| Cadmium sulfide (CdS) and Ni-doped CdS | Highly crystalline | [38] | |
| Zinc oxide (ZnO)@polymer core/shell | Quantum yield above 50% | [39] | |
| Zinc oxide (ZnO) QDs | Strong photoluminescence efficiency | [30] | |
| Microemulsion (reverse micelle) | Zinc sulfide (ZnS) QDs | Nanocrystal with high purity, Photoluminescence peak observed at 365 nm Quantum confinement effect | [51] |
| Cadmium sulfide/Zinc sulfide (CdS/ZnS) semiconductor QDs | Excellent luminescence and photostability | [51] | |
| Cadmium selenide@Zinc sulfide (CdSe@ZnS) within monodisperse silica | Good monodispersity High luminescence | [63] | |
| Microemulsion (gas contacting technique) | Zinc selenide (ZnSe) QDs | Excellent photostability and size-influenced luminescence | [69] |
| Microemulsion method + ultrasonic waves (sono-microemulsion method) | Cadmium sulfide (CdS) | Restricted size distribution High-order crystalline arrangement and purity | [70] |
| Physical vapor deposition | Niobium pentoxide (Nb2O5) QDs | Quantum confinement effect | [21] |
| RF magnetron sputtering | Cadmium selenide (CdSe) QDs | Optical properties | [69] |
| Solvothermal | Zinc Oxide (ZO) QDs | Minuscule size High purity, superior crystallinity, and large surface area | [70] |
| Graphene QDs (GQDs) | Resilient stability, photoluminescence quantum yield of 11.4%, biocompatibility, mild toxicity | [21] | |
| Hydrothermal | Nitrogen- and sulfur-doped carbon QDs (N, S-doped CQDs) | Small Spherical Green emission | [20] |
| Fluorescence quantum yield (10.35%) | |||
| Nitrogen-doped carbon QDs (N-CQDs) | Low toxicity excellent photostability | [40] | |
| Silicon QDs | Excellent water dispersibility High photoluminescence Strong pH stability | [71] | |
| Tin oxide/Tin sulfide in reduced bovine serum albumin (SnO2/SnS2 @r-BSA2) | Specific selectivity Long term stability Enhanced reproducibility | [72] | |
| Nitrogen-doped Graphene QDs (N-GQDs) | High quanta yield Persistent fluorescence stability Enhanced sensitivity and specificity | [73,74] | |
| Molecular beam epitaxy | Indium arsenide gallium arsenide core/shell (InAs/GaAs) QDs | Strong photoluminescence intensity High structural properties | [75] |
| Quantum Dot of Compounds | Size Spectrum (Diameter in nm) | Range of Emission Spectrum (nm) |
|---|---|---|
| Cadmium sulfide (CdS) | 2.8–5.4 | 410–460 |
| Cadmium telluride (CdTe) | 3.1–9.1 | 520–750 |
| Cadmium selenide (CdSe) | 2–8 | 480–680 |
| CdTe/CdSe | 4–9.2 | 650–840 |
| Indium phosphide (InP) | 2.5–4.5 | 610–710 |
| Indium arsenide (InAs) | 3.2–6 | 860–1270 |
| Lead selenide (PbSe) | 3.2–4.1 | 1110–1310 |
| 1-Dodecanethiol silver sulfide (Dt)-Ag2S) | 5.4–10 | 1000–1300 |
| Surface Optimization Techniques | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| Ligand exchange | Feasibility of processing, Small dimensions of QD | Degradation of photophysical properties in QDs present in aqueous environment (i.e., reduced PLQY) QD core is suspected to be oxidation | [94,110,111,112] |
| Surface silanization | Enhances biocompatibility, High cross-linking in ligand molecules, Terminal groups enable further coating by exposing their reactive ends (e.g., thiol), Fine-tuning the QD response to light is enabled by controlling the thickness of the silica shell, Improves PLQY of QDs, Improves photochemical stability. | Large hydrodynamic size, Aggregation of QDs in aqueous solution | [113,114,115] |
| Amphiphilic ligands | Increased chemical stability, Increased colloidal stability, Excellent biocompatibility, and strong fluorescence signals with high stability. | Size enlargement, Surface defects | [112,113,116] |
| Microsphere coating | Improvement in the stability of QD, High fluorescence, Effectively conceals QD toxicity | The formation of a uniform microsphere is obstructed, Reduced PLQY, Encapsulating the QDs with high concentrations finds QD aggregation. | [114,117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shukla, N.; Cárdenas, C.Y.; Chanderiya, A.; Polozhentsev, O.E.; Das, R.; Vyas, S.; Mukhanova, E.; Soldatov, A.; Belbekhouche, S. Advancements in Targeted Quantum Dots Structures for Enhanced Cancer Treatment. Pharmaceutics 2025, 17, 1396. https://doi.org/10.3390/pharmaceutics17111396
Shukla N, Cárdenas CY, Chanderiya A, Polozhentsev OE, Das R, Vyas S, Mukhanova E, Soldatov A, Belbekhouche S. Advancements in Targeted Quantum Dots Structures for Enhanced Cancer Treatment. Pharmaceutics. 2025; 17(11):1396. https://doi.org/10.3390/pharmaceutics17111396
Chicago/Turabian StyleShukla, Nutan, Carol Y. Cárdenas, Aayushi Chanderiya, Oleg E. Polozhentsev, Ratnesh Das, Supriya Vyas, Elizaveta Mukhanova, Alexander Soldatov, and Sabrina Belbekhouche. 2025. "Advancements in Targeted Quantum Dots Structures for Enhanced Cancer Treatment" Pharmaceutics 17, no. 11: 1396. https://doi.org/10.3390/pharmaceutics17111396
APA StyleShukla, N., Cárdenas, C. Y., Chanderiya, A., Polozhentsev, O. E., Das, R., Vyas, S., Mukhanova, E., Soldatov, A., & Belbekhouche, S. (2025). Advancements in Targeted Quantum Dots Structures for Enhanced Cancer Treatment. Pharmaceutics, 17(11), 1396. https://doi.org/10.3390/pharmaceutics17111396