Advances in 3D-Printed Drug Delivery and Screening Platforms for Bone Disease Therapy
Abstract
1. Introduction
2. Methods
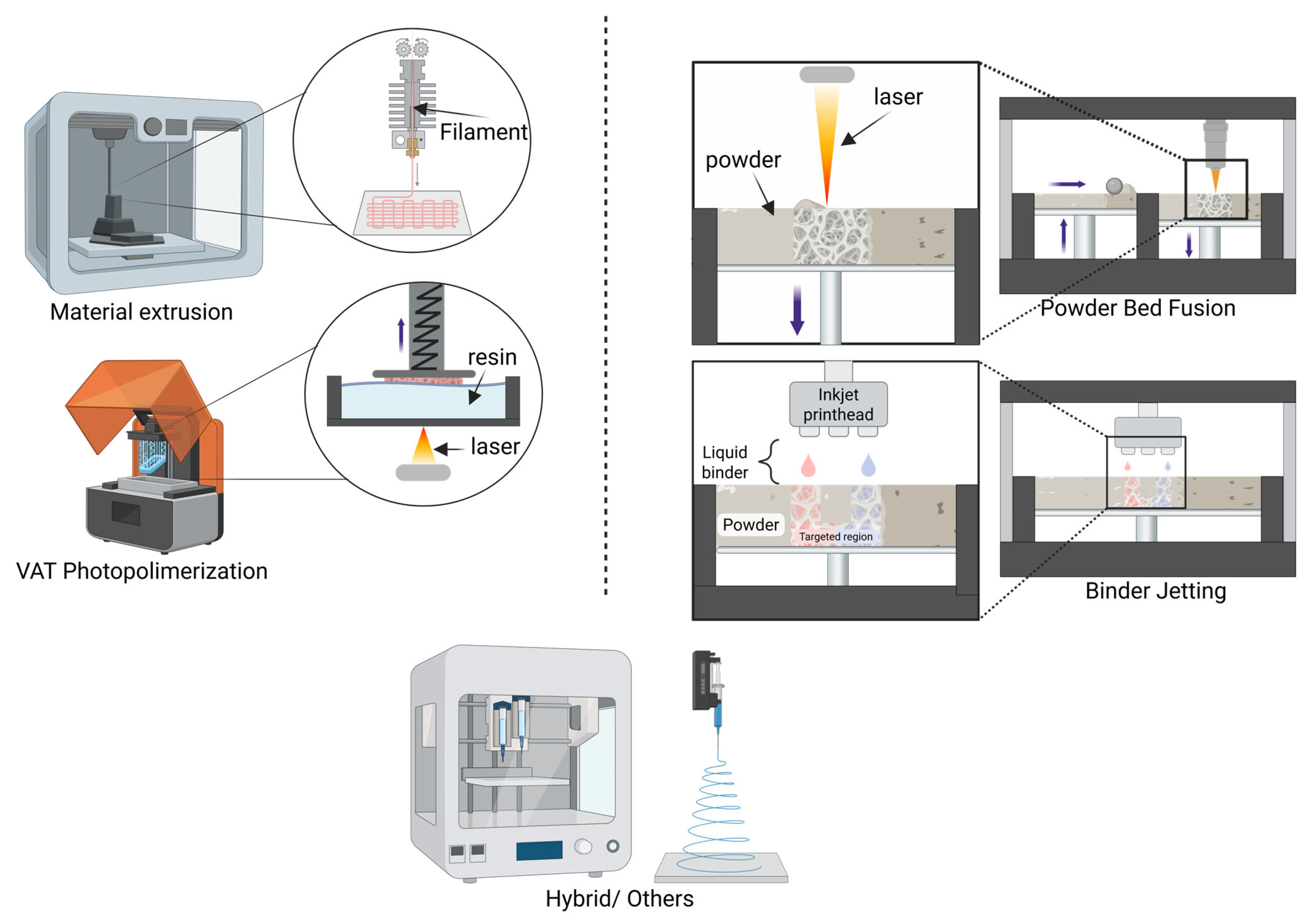
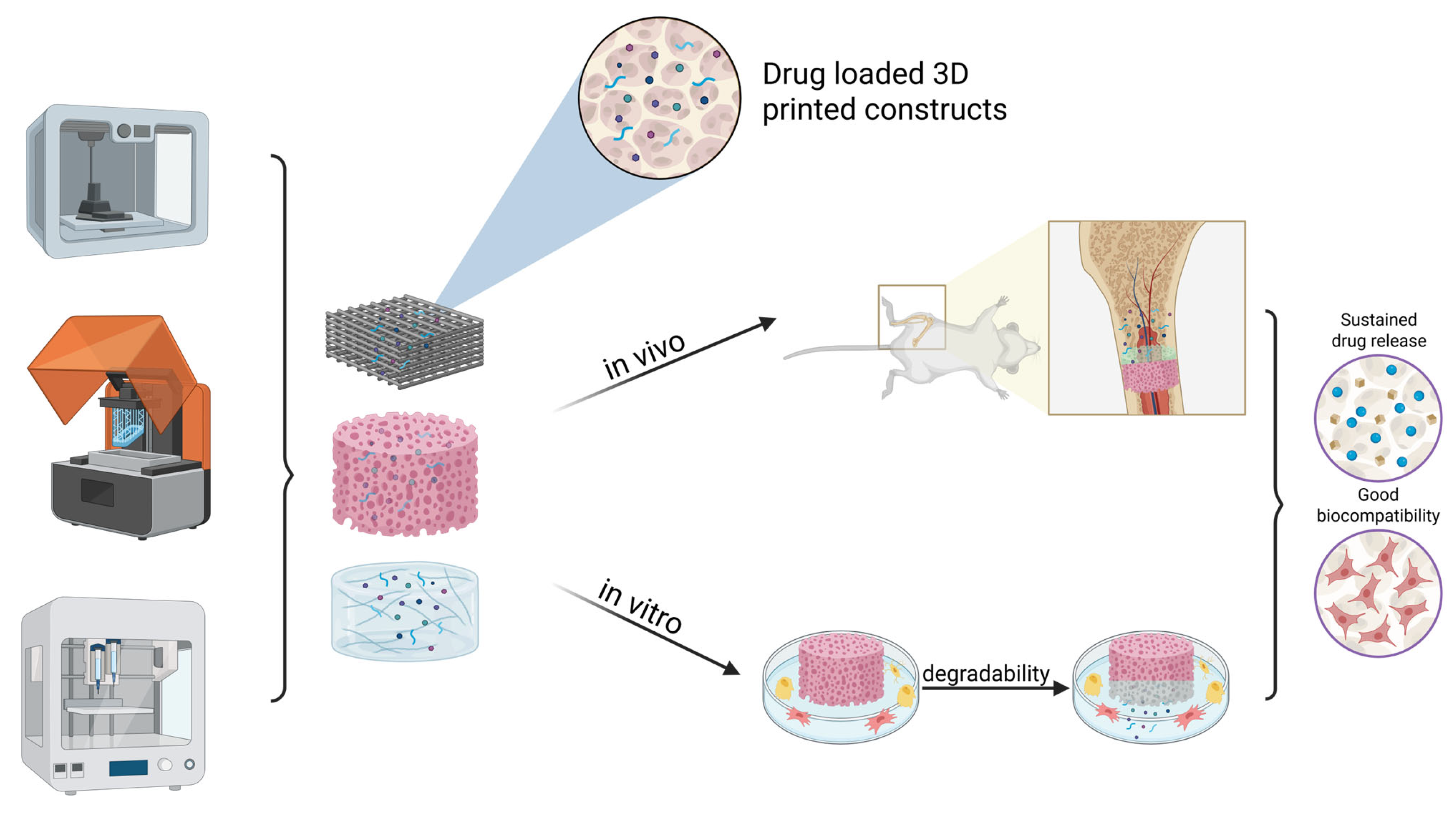
3. 3D-Printed Drug Delivery Systems
3.1. Antibiotics
3.1.1. Vancomycin
3.1.2. Ciprofloxacin (CFX)
3.1.3. Gentamicin
3.1.4. Tetracyclines
3.1.5. Other Antibiotics
3.2. Anticancer
3.2.1. Cisplatin
3.2.2. Doxorubicin
3.2.3. Zoledronate
3.3. Anti-Inflammatory and Other Drugs
4. 3D-Printed Drug Screening Models
4.1. 3D-Printed Drug Screening Models for Osteoporosis/Bone Defects
4.2. 3D-Printed Drug Screening Models for Osteoarthritis
4.3. Others
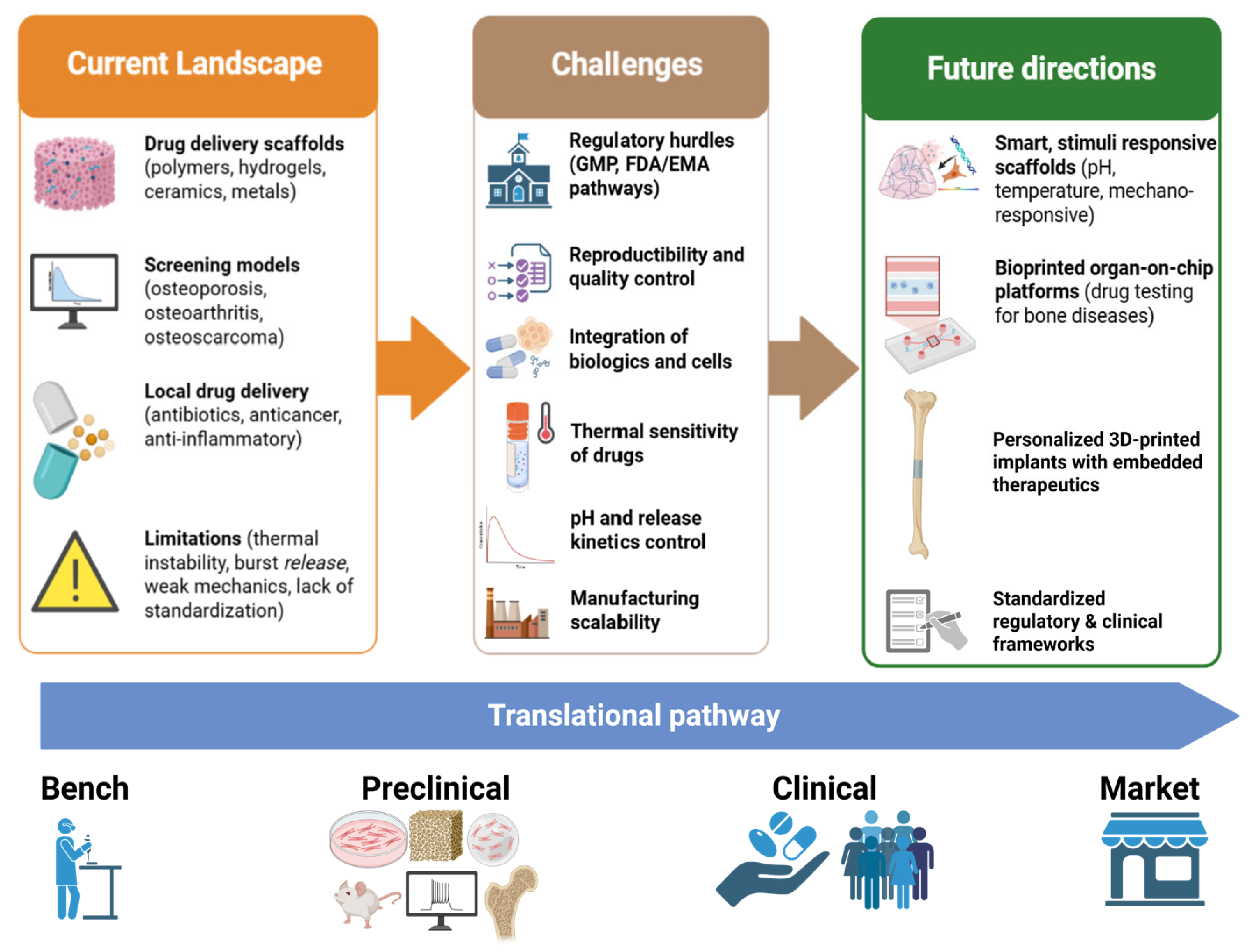
5. Future Perspectives and Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Chen, X.; Han, X.; Hong, X.; Li, X.; Zhang, H.; Li, M.; Wang, Z.; Zheng, A. A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future. Pharmaceutics 2023, 15, 416. [Google Scholar] [CrossRef]
- Lakkala, P.; Munnangi, S.R.; Bandari, S.; Repka, M. Additive manufacturing technologies with emphasis on stereolithography 3D printing in pharmaceutical and medical applications: A review. Int. J. Pharm. X 2023, 5, 100159. [Google Scholar] [CrossRef]
- Sarmah, P.; Gupta, K. Recent Advancements in Fabrication of Metal Matrix Composites: A Systematic Review. Materials 2024, 17, 4635. [Google Scholar] [CrossRef]
- Tuli, N.T.; Khatun, S.; Rashid, A.B. Unlocking the future of precision manufacturing: A comprehensive exploration of 3D printing with fiber-reinforced composites in aerospace, automotive, medical, and consumer industries. Heliyon 2024, 10, e27328. [Google Scholar] [CrossRef] [PubMed]
- Stanojević, G.; Medarević, D.; Adamov, I.; Pešić, N.; Kovačević, J.; Ibrić, S. Tailoring Atomoxetine Release Rate from DLP 3D-Printed Tablets Using Artificial Neural Networks: Influence of Tablet Thickness and Drug Loading. Molecules 2021, 26, 111. [Google Scholar] [CrossRef] [PubMed]
- Md. Shoaib, A.; Ayesha, A.; Iftikhar, A.; Sheikh, S.-u.-N. Pharmaceutical Product Development Exploiting 3D Printing Technology: Conventional to Novel Drug Delivery System. Curr. Pharm. Des. 2018, 24, 5029–5038. [Google Scholar] [CrossRef]
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 49. [Google Scholar] [CrossRef]
- Vanderburgh, J.; Sterling, J.A.; Guelcher, S.A. 3D Printing of Tissue Engineered Constructs for In Vitro Modeling of Disease Progression and Drug Screening. Ann. Biomed. Eng. 2017, 45, 164–179. [Google Scholar] [CrossRef]
- Marei, I.; Abu Samaan, T.; Al-Quradaghi, M.A.; Farah, A.A.; Mahmud, S.H.; Ding, H.; Triggle, C.R. 3D Tissue-Engineered Vascular Drug Screening Platforms: Promise and Considerations. Front. Cardiovasc. Med. 2022, 9, 847554. [Google Scholar] [CrossRef]
- Steinberg, E.; Friedman, R.; Goldstein, Y.; Friedman, N.; Beharier, O.; Demma, J.A.; Zamir, G.; Hubert, A.; Benny, O. A fully 3D-printed versatile tumor-on-a-chip allows multi-drug screening and correlation with clinical outcomes for personalized medicine. Commun. Biol. 2023, 6, 1157. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Soker, S.; Skardal, A. 3D bioprinting for high-throughput screening: Drug screening, disease modeling, and precision medicine applications. Appl. Phys. Rev. 2019, 6, 011302. [Google Scholar] [CrossRef]
- Thiruchandran, G.; Dean, O.; Alim, D.; Crawford, A.; Salim, O. Three-dimensional printing in orthopaedic surgery: A review of current and future applications. J. Orthop. 2025, 59, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Bădilă, A.E.; Rădulescu, D.M.; Niculescu, A.G.; Grumezescu, A.M.; Rădulescu, M.; Rădulescu, A.R. Recent Advances in the Treatment of Bone Metastases and Primary Bone Tumors: An Up-to-Date Review. Cancers 2021, 13, 4229. [Google Scholar] [CrossRef] [PubMed]
- Emami, A.; Namdari, H.; Parvizpour, F.; Arabpour, Z. Challenges in osteoarthritis treatment. Tissue Cell 2023, 80, 101992. [Google Scholar] [CrossRef]
- Patel, D.; Wairkar, S. Bone regeneration in osteoporosis: Opportunities and challenges. Drug Deliv. Transl. Res. 2023, 13, 419–432. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, J.W. Mimicking microbone tissue by 3-dimensional printing. Organoid 2024, 4, e4. [Google Scholar] [CrossRef]
- Kortam, S.; Lu, Z.; Zreiqat, H. Recent advances in drug delivery systems for osteosarcoma therapy and bone regeneration. Commun. Mater. 2024, 5, 168. [Google Scholar] [CrossRef]
- Nair, A.; Varghese, B.A.; Gopi, S.; Jacob, J. 11—Smart drug delivery systems of natural products for inflammation: From fundamentals to the clinic. In Inflammation and Natural Products; Gopi, S., Amalraj, A., Kunnumakkara, A., Thomas, S., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 217–238. [Google Scholar] [CrossRef]
- Codrea, C.I.; Fruth, V. Three-Dimensionally Printed Scaffolds and Drug Delivery Systems in Treatment of Osteoporosis. Biomimetics 2025, 10, 429. [Google Scholar] [CrossRef]
- Motiei, M.; Kashanian, S.; Lucia, L.A.; Khazaei, M. Intrinsic parameters for the synthesis and tuned properties of amphiphilic chitosan drug delivery nanocarriers. J. Control. Release 2017, 260, 213–225. [Google Scholar] [CrossRef]
- Mirchandani, Y.; Patravale, V.B.; S, B. Solid lipid nanoparticles for hydrophilic drugs. J. Control. Release 2021, 335, 457–464. [Google Scholar] [CrossRef]
- Borandeh, S.; van Bochove, B.; Teotia, A.; Seppälä, J. Polymeric drug delivery systems by additive manufacturing. Adv. Drug Deliv. Rev. 2021, 173, 349–373. [Google Scholar] [CrossRef] [PubMed]
- Farjaminejad, S.; Farjaminejad, R.; Hasani, M.; Shojaei, S. Innovative Drug Delivery Systems in Bone Regeneration: Benefits and Applications in Tissue Engineering. J. Bionic Eng. 2025, 22, 2286–2307. [Google Scholar] [CrossRef]
- Qiu, E.; Liu, F. PLGA-based drug delivery systems in treating bone tumors. Front. Bioeng. Biotechnol. 2023, 11, 1199343. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-Based Nanoparticles for Drug/Gene Delivery: An Overview of the Production Techniques and Difficulties Encountered in Their Industrial Development. ACS Mater. Au 2023, 3, 600–619. [Google Scholar] [CrossRef]
- Grini, M.I.; Benbayer, C.; Saidi-Besbes, S.; Elaissari, A. Advances in mesoporous silica nanoparticles as carriers for drug delivery and other biomedical applications. Microporous Mesoporous Mater. 2025, 391, 113603. [Google Scholar] [CrossRef]
- Qiu, C.; Wu, Y.; Guo, Q.; Shi, Q.; Zhang, J.; Meng, Y.; Xia, F.; Wang, J. Preparation and application of calcium phosphate nanocarriers in drug delivery. Mater. Today Bio 2022, 17, 100501. [Google Scholar] [CrossRef]
- Bai, L.; Tao, G.; Feng, M.; Xie, Y.; Cai, S.; Peng, S.; Xiao, J. Hydrogel Drug Delivery Systems for Bone Regeneration. Pharmaceutics 2023, 15, 1334. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiong, Y.; Zhao, Y. Beyond Drug Delivery: Metal–Organic Framework-Derived Nanosystems for Bone Regeneration under Complicated Pathological Microenvironments. Acc. Mater. Res. 2024, 5, 1532–1543. [Google Scholar] [CrossRef]
- Borges, R.; Pelosine, A.M.; de Souza, A.C.S.; Machado, J.; Justo, G.Z.; Gamarra, L.F.; Marchi, J. Bioactive Glasses as Carriers of Cancer-Targeted Drugs: Challenges and Opportunities in Bone Cancer Treatment. Materials 2022, 15, 9082. [Google Scholar] [CrossRef]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Wang, H.; Tian, L.; Goldstein, A.; Liu, J.; Lo, H.-C.; Sheng, K.; Welte, T.; Wong, S.T.C.; Gugala, Z.; Stossi, F.; et al. Bone-in-culture array as a platform to model early-stage bone metastases and discover anti-metastasis therapies. Nat. Commun. 2017, 8, 15045. [Google Scholar] [CrossRef]
- Paek, K.; Kim, S.; Tak, S.; Kim, M.K.; Park, J.; Chung, S.; Park, T.H.; Kim, J.A. A high-throughput biomimetic bone-on-a-chip platform with artificial intelligence-assisted image analysis for osteoporosis drug testing. Bioeng. Transl. Med. 2023, 8, e10313. [Google Scholar] [CrossRef]
- Fois, M.G.; van Griensven, M.; Giselbrecht, S.; Habibović, P.; Truckenmüller, R.K.; Tahmasebi Birgani, Z.N. Mini-bones: Miniaturized bone in vitro models. Trends Biotechnol. 2024, 42, 910–928. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Li, X.; Lai, C.; Li, L.; Wu, H.; Wang, Y.; Shi, X. Fabrication of a hydroxyapatite-PDMS microfluidic chip for bone-related cell culture and drug screening. Bioact. Mater. 2021, 6, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Natoli, D.; Levin, M.; Tsygan, L.; Liu, L. Chapter 33—Development, Optimization, and Scale-Up of Process Parameters: Tablet Compression. In Developing Solid Oral Dosage Forms, 2nd ed.; Qiu, Y., Chen, Y., Zhang, G.G.Z., Yu, L., Mantri, R.V., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 917–951. [Google Scholar] [CrossRef]
- Shanmugam, S. Granulation techniques and technologies: Recent progresses. Bioimpacts 2015, 5, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.C. Chapter 27—Coating of pharmaceutical dosage forms. In Remington, 23rd ed.; Adejare, A., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 551–564. [Google Scholar] [CrossRef]
- Jadhav, A.; Jadhav, V.S. A review on 3D printing: An additive manufacturing technology. Mater. Today Proc. 2022, 62, 2094–2099. [Google Scholar] [CrossRef]
- Gail Kim, R.; Abisado, M.; Villaverde, J.; Sampedro, G. A Survey of Image-Based Fault Monitoring in Additive Manufacturing: Recent Developments and Future Directions. Sensors 2023, 23, 6821. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Bottini, L.; Boschetto, A.; Veniali, F.; Gaudenzi, P. 7—Additive manufacturing for CubeSat structure fabrication. In Next Generation CubeSats and SmallSats; Branz, F., Cappelletti, C., Ricco, A.J., Hines, J.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 153–180. [Google Scholar] [CrossRef]
- Chartrain, N.A.; Gilchrist, K.H.; Ho, V.B.; Klarmann, G.J. 3D bioprinting for the repair of articular cartilage and osteochondral tissue. Bioprinting 2022, 28, e00239. [Google Scholar] [CrossRef]
- Lan, W.; Huang, X.; Huang, D.; Wei, X.; Chen, W. Progress in 3D printing for bone tissue engineering: A review. J. Mater. Sci. 2022, 57, 12685–12709. [Google Scholar] [CrossRef]
- Sabahi, N.; Roohani, I.; Wang, C.H.; Li, X. Material extrusion 3D printing of bioactive smart scaffolds for bone tissue engineering. Addit. Manuf. 2025, 98, 104636. [Google Scholar] [CrossRef]
- Nugroho, W.T.; Dong, Y.; Pramanik, A.; Chithirai Pon Selvan, M.; Zhang, Z.; Ramakrishna, S. Additive manufacturing of re-entrant structures: Well-tailored structures, unique properties, modelling approaches and real applications. Addit. Manuf. 2023, 78, 103829. [Google Scholar] [CrossRef]
- Tymrak, B.M.; Kreiger, M.; Pearce, J. Mechanical Properties of Components Fabricated with Open-Source 3-D Printers Under Realistic Environmental Conditions. Mater. Des. 2014, 58, 242–246. [Google Scholar] [CrossRef]
- Lefay, C.; Guillaneuf, Y. Recyclable/degradable materials via the insertion of labile/cleavable bonds using a comonomer approach. Prog. Polym. Sci. 2023, 147, 101764. [Google Scholar] [CrossRef]
- Islam, A.; Rahman, M.Z. 13.18—Recent advances in additive manufacturing techniques: An in-depth review. In Comprehensive Materials Processing, 2nd ed.; Hashmi, S., Ed.; Elsevier: Oxford, UK, 2024; pp. 352–378. [Google Scholar] [CrossRef]
- Khanna, N.; Zadafiya, K.; Patel, T.; Kaynak, Y.; Rahman Rashid, R.A.; Vafadar, A. Review on machining of additively manufactured nickel and titanium alloys. J. Mater. Res. Technol. 2021, 15, 3192–3221. [Google Scholar] [CrossRef]
- Lai, H.; Peng, X.; Li, L.; Zhu, D.; Xiao, P. Novel monomers for photopolymer networks. Prog. Polym. Sci. 2022, 128, 101529. [Google Scholar] [CrossRef]
- Xing, F.; Xu, J.; Yu, P.; Zhou, Y.; Zhe, M.; Luo, R.; Liu, M.; Xiang, Z.; Duan, X.; Ritz, U. Recent advances in biofabrication strategies based on bioprinting for vascularized tissue repair and regeneration. Mater. Des. 2023, 229, 111885. [Google Scholar] [CrossRef]
- Huh, J.T.; Yoo, J.J.; Atala, A.; Lee, S.J. Chapter 74—Three-dimensional bioprinting for tissue engineering. In Principles of Tissue Engineering, 5th ed.; Lanza, R., Langer, R., Vacanti, J.P., Atala, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1391–1415. [Google Scholar] [CrossRef]
- Budharaju, H.; Suresh, S.; Sekar, M.P.; De Vega, B.; Sethuraman, S.; Sundaramurthi, D.; Kalaskar, D.M. Ceramic materials for 3D printing of biomimetic bone scaffolds—Current state-of-the-art & future perspectives. Mater. Des. 2023, 231, 112064. [Google Scholar] [CrossRef]
- Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Advincula, R.C. Mechanical characterization of 3D-printed polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Sun, Y.; Juncos Bombin, A.D.; Boyd, P.; Dunne, N.; McCarthy, H.O. Application of 3D printing & 3D bioprinting for promoting cutaneous wound regeneration. Bioprinting 2022, 28, e00230. [Google Scholar] [CrossRef]
- Yu, H.Z. Chapter 6—Beyond metals and alloys: Additive friction stir deposition of metal matrix composites. In Additive Friction Stir Deposition; Yu, H.Z., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 203–232. [Google Scholar] [CrossRef]
- Joharji, L.; Mishra, R.B.; Alam, F.; Tytov, S.; Al-Modaf, F.; El-Atab, N. 4D printing: A detailed review of materials, techniques, and applications. Microelectron. Eng. 2022, 265, 111874. [Google Scholar] [CrossRef]
- Mohammadi, H.; Ahmad, Z.; Petrů, M.; Mazlan, S.A.; Faizal Johari, M.A.; Hatami, H.; Rahimian Koloor, S.S. An insight from nature: Honeycomb pattern in advanced structural design for impact energy absorption. J. Mater. Res. Technol. 2023, 22, 2862–2887. [Google Scholar] [CrossRef]
- Shahriar, M.A.; Kobir, M.H.; Rahman, S.; Rahman, M.Z.; Saha, B. 13.03—Overview of additive manufacturing and applications of 3D printed composites. In Comprehensive Materials Processing, 2nd ed.; Hashmi, S., Ed.; Elsevier: Oxford, UK, 2024; pp. 58–76. [Google Scholar] [CrossRef]
- Tufail, A.; Schmidt, F.; Maqbool, M. 15—Three-dimensional printing of hydroxyapatite. In Handbook of Ionic Substituted Hydroxyapatites; Khan, A.S., Chaudhry, A.A., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 355–381. [Google Scholar] [CrossRef]
- Sinha, P.; Mukhopadhyay, T. Programmable multi-physical mechanics of mechanical metamaterials. Mater. Sci. Eng. R Rep. 2023, 155, 100745. [Google Scholar] [CrossRef]
- Hansen, C.J.; Peterson, A.M.; Park, J.H. Chapter 23—3D printing. In Handbook of Thermoset Plastics, 4th ed.; Dodiuk, H., Ed.; William Andrew Publishing: Boston, MA, USA, 2022; pp. 1021–1043. [Google Scholar] [CrossRef]
- V, K.; Kumar, B.N.; Kumar, S.S.; M, V. Magnesium role in additive manufacturing of biomedical implants—Challenges and opportunities. Addit. Manuf. 2022, 55, 102802. [Google Scholar] [CrossRef]
- Karunakaran, R.; Ortgies, S.; Tamayol, A.; Bobaru, F.; Sealy, M.P. Additive manufacturing of magnesium alloys. Bioact. Mater. 2020, 5, 44–54. [Google Scholar] [CrossRef]
- Elliott, A.M.; Cramer, C.L.; Nandwana, P.; Chmielus, M.; Mostafaei, A. Binder Jet-Metals. In Encyclopedia of Materials: Metals and Alloys; Caballero, F.G., Ed.; Elsevier: Oxford, UK, 2022; pp. 120–133. [Google Scholar] [CrossRef]
- Szymczyk-Ziółkowska, P.; Łabowska, M.B.; Detyna, J.; Michalak, I.; Gruber, P. A review of fabrication polymer scaffolds for biomedical applications using additive manufacturing techniques. Biocybern. Biomed. Eng. 2020, 40, 624–638. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.; Shi, J. Additive manufacturing of zirconia ceramics: A state-of-the-art review. J. Mater. Res. Technol. 2020, 9, 9029–9048. [Google Scholar] [CrossRef]
- Peng, B.; Xu, H.; Song, F.; Wen, P.; Tian, Y.; Zheng, Y. Additive manufacturing of porous magnesium alloys for biodegradable orthopedic implants: Process, design, and modification. J. Mater. Sci. Technol. 2024, 182, 79–110. [Google Scholar] [CrossRef]
- Pontes, A.J. Chapter 7—Designing for additive manufacturing. In Design and Manufacturing of Plastics Products; Pouzada, A.S., Ed.; William Andrew Publishing: Norwich, NY, USA, 2021; pp. 249–292. [Google Scholar] [CrossRef]
- Lee, J.Y.; An, J.; Chua, C.K. Fundamentals and applications of 3D printing for novel materials. Appl. Mater. Today 2017, 7, 120–133. [Google Scholar] [CrossRef]
- Lv, X.; Ye, F.; Cheng, L.; Fan, S.; Liu, Y. Binder jetting of ceramics: Powders, binders, printing parameters, equipment, and post-treatment. Ceram. Int. 2019, 45, 12609–12624. [Google Scholar] [CrossRef]
- Dinh, P.; Hutchinson, B.K.; Zalavras, C.; Stevanovic, M.V. Reconstruction of osteomyelitis defects. Semin. Plast. Surg. 2009, 23, 108–118. [Google Scholar] [CrossRef]
- Nandi, S.K.; Mukherjee, P.; Roy, S.; Kundu, B.; De, D.K.; Basu, D. Local antibiotic delivery systems for the treatment of osteomyelitis–A review. Mater. Sci. Eng. C 2009, 29, 2478–2485. [Google Scholar] [CrossRef]
- Hasan, R.; Schaner, K.; Schroeder, M.; Wohlers, A.; Shreffler, J.; Schaper, C.; Subramanian, H.; Brooks, A. Extended release combination antibiotic therapy from a bone void filling putty for treatment of osteomyelitis. Pharmaceutics 2019, 11, 592. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, R.P.; Ninomiya, M.J.; El-Atawneh, I.M.; Knapp, E.K.; de Mesy Bentley, K.L.; Dunman, P.M.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Calcium phosphate spacers for the local delivery of sitafloxacin and rifampin to treat orthopedic infections: Efficacy and proof of concept in a mouse model of single-stage revision of device-associated osteomyelitis. Pharmaceutics 2019, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 2006, 42 (Suppl. S1), S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Vorpagel, E.R.; Laskin, J. Experimental and theoretical studies of the structures and interactions of vancomycin antibiotics with cell wall analogues. J. Am. Chem. Soc. 2008, 130, 13013–13022. [Google Scholar] [CrossRef]
- Yamaga, C.; Bostick, D.L.; Tabak, Y.P.; Liu-Ferrara, A.; Morel, D.; Yu, K. 210. Vancomycin Infusion Frequency and Intensity: Analysis of Real-World Data Generated from Automated Infusion Devices. Open Forum Infect. Dis. 2020, 7, S108. [Google Scholar] [CrossRef]
- Lietman, P.S.; Schaad, U.B.; McCracken Jr, G.H.; Nelson, J.D. Clinical pharmacology and efficacy of vancomycin in pediatric patients. J. Pediatr. 1980, 96, 119–126. [Google Scholar] [CrossRef]
- Wang, H.; Huang, L.; Wang, J.; Ni, Y.; Zhu, Z.; Gao, P.; Hu, Y.; Zhang, L.; Yang, J.; Fang, L. Population pharmacokinetic study of vancomycin in Chinese pediatric patients with hematological malignancies. Pharmacother. J. Hum. Human. Pharmacol. Drug Ther. 2020, 40, 1201–1209. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Panagopoulos, G.N.; Kokkalis, Z.T.; Koulouvaris, P.; Megaloikonomos, P.D.; Igoumenou, V.; Mantas, G.; Moulakakis, K.G.; Sfyroeras, G.S.; Lazaris, A. Vascular injury in orthopedic trauma. Orthopedics 2016, 39, 249–259. [Google Scholar] [CrossRef]
- Amon, M.; Laschke, M.W.; Harder, Y.; Vollmar, B.; Menger, M.D. Impact of severity of local soft-tissue trauma on long-term manifestation of microcirculatory and microlymphatic dysfunctions. J. Trauma. Acute Care Surg. 2006, 61, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Fang, T.; Wang, Y.; Dong, J. The controlled release of vancomycin in gelatin/β-TCP composite scaffolds. J. Biomed. Mater. Res. Part A 2012, 100, 2295–2301. [Google Scholar] [CrossRef] [PubMed]
- Anvari Kohestani, A.; Pishbin, F. 3D printing of bone scaffolds based on alginate/gelatin hydrogel ink containing bioactive glass 45S5 and ZIF-8 nanoparticles with sustained drug-release capability. Adv. Eng. Mater. 2023, 25, 2300563. [Google Scholar] [CrossRef]
- Choi, C.E.; Chakraborty, A.; Adzija, H.; Shamiya, Y.; Hijazi, K.; Coyle, A.; Rizkalla, A.; Holdsworth, D.W.; Paul, A. Metal organic framework-incorporated three-dimensional (3D) bio-printable hydrogels to facilitate bone repair: Preparation and in vitro bioactivity analysis. Gels 2023, 9, 923. [Google Scholar] [CrossRef]
- Lin, M.; Stehle, Y.; Chen, L.; Yang, M.; Zeng, K.; Wang, C.; Zhang, R.; Zhang, H.; Yang, J.; Hu, D.; et al. A 3D-printed chitosan-based pH-responsive dual functional scaffold for osteomyelitis: Synergistic antibacterial and osteogenic treatment. Carbohydr. Polym. 2025, 366, 123866. [Google Scholar] [CrossRef]
- Lu, L.; Yuan, S.; Wang, J.; Shen, Y.; Deng, S.; Xie, L.; Yang, Q. The formation mechanism of hydrogels. Curr. Stem Cell Res. Ther. 2018, 13, 490–496. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Q.; Ren, B.; Muskhelishvili, L.; Davis, K.; Wynne, R.; Rua, D.; Cao, X. Subacute pulmonary toxicity of glutaraldehyde aerosols in a human in vitro airway tissue model. Int. J. Mol. Sci. 2022, 23, 12118. [Google Scholar] [CrossRef]
- Zeiger, E.; Gollapudi, B.; Spencer, P. Genetic toxicity and carcinogenicity studies of glutaraldehyde—A review. Mutat. Res./Rev. Mutat. Res. 2005, 589, 136–151. [Google Scholar] [CrossRef]
- Hafezi, F.; Scoutaris, N.; Douroumis, D.; Boateng, J. 3D printed chitosan dressing crosslinked with genipin for potential healing of chronic wounds. Int. J. Pharm. 2019, 560, 406–415. [Google Scholar] [CrossRef]
- López-González, I.; Hernández-Heredia, A.B.; Rodríguez-López, M.I.; Auñón-Calles, D.; Boudifa, M.; Gabaldón, J.A.; Meseguer-Olmo, L. Evaluation of the In Vitro Antimicrobial Efficacy against Staphylococcus aureus and epidermidis of a Novel 3D-Printed Degradable Drug Delivery System Based on Polycaprolactone/Chitosan/Vancomycin—Preclinical Study. Pharmaceutics 2023, 15, 1763. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, X.; Du, H.; Ha, Y.; Xu, Y.; Ao, R.; He, C. Bifunctional Hydrogel-Integrated 3D Printed Scaffold for Repairing Infected Bone Defects. ACS Biomater. Sci. Eng. 2023, 9, 4583–4596. [Google Scholar] [CrossRef]
- Iglesias-Mejuto, A.; Magariños, B.; Ferreira-Gonçalves, T.; Starbird-Pérez, R.; Álvarez-Lorenzo, C.; Reis, C.P.; Ardao, I.; García-González, C.A. Vancomycin-loaded methylcellulose aerogel scaffolds for advanced bone tissue engineering. Carbohydr. Polym. 2024, 324, 121536. [Google Scholar] [CrossRef]
- Gao, X.; Xu, Z.; Li, S.; Cheng, L.; Xu, D.; Li, L.; Chen, L.; Xu, Y.; Liu, Z.; Liu, Y. Chitosan-vancomycin hydrogel incorporated bone repair scaffold based on staggered orthogonal structure: A viable dually controlled drug delivery system. RSC Adv. 2023, 13, 3759–3765. [Google Scholar] [CrossRef]
- Huang, H.; Wu, Z.; Yang, Z.; Fan, X.; Bai, S.; Luo, J.; Chen, M.; Xie, X. In vitroapplication of drug-loaded hydrogel combined with 3D-printed porous scaffolds. Biomed. Mater. 2022, 17, 065019. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, W.; Yang, W.; Bi, J.; Li, H.; Gao, X.; Zhang, B.; Shi, G.; Li, K.; Wei, Z.; et al. Vancomycin-encapsulated hydrogel loaded microarc-oxidized 3D-printed porous Ti6Al4V implant for infected bone defects: Reconstruction, anti-infection, and osseointegration. Bioact. Mater. 2024, 42, 18–31. [Google Scholar] [CrossRef]
- Zhang, B.; Pei, X.; Zhou, C.; Fan, Y.; Jiang, Q.; Ronca, A.; D’Amora, U.; Chen, Y.; Li, H.; Sun, Y. The biomimetic design and 3D printing of customized mechanical properties porous Ti6Al4V scaffold for load-bearing bone reconstruction. Mater. Des. 2018, 152, 30–39. [Google Scholar] [CrossRef]
- Zhang, G.-F.; Liu, X.; Zhang, S.; Pan, B.; Liu, M.-L. Ciprofloxacin derivatives and their antibacterial activities. Eur. J. Med. Chem. 2018, 146, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Brouqui, P.; Rousseau, M.C.; Stein, A.; Drancourt, M.; Raoult, D. Treatment of Pseudomonas aeruginosa-infected orthopedic prostheses with ceftazidime-ciprofloxacin antibiotic combination. Antimicrob. Agents Chemother. 1995, 39, 2423–2425. [Google Scholar] [CrossRef] [PubMed]
- LeBel, M. Ciprofloxacin: Chemistry, mechanism of action, resistance, antimicrobial spectrum, pharmacokinetics, clinical trials, and adverse reactions. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1988, 8, 3–30. [Google Scholar] [CrossRef]
- Wald, E.R. Risk factors for osteomyelitis. Am. J. Med. 1985, 78, 206–212. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, A.; Li, X.; Ma, Z.; Zhang, D. Efficacy Evaluation of Ciprofloxacin-Loaded Poly (Trimethylene Carbonate) Implants in the Treatment of Chronic Osteomyelitis. Front. Bioeng. Biotechnol. 2022, 10, 864041. [Google Scholar] [CrossRef]
- Baggio, D.; Ananda-Rajah, M.R. Fluoroquinolone antibiotics and adverse events. Aust. Prescr. 2021, 44, 161–164. [Google Scholar] [CrossRef]
- Sterne, G.M.; Richardson, M.L.; Warren, B.H. Imaging findings in two cases of fluoroquinolone-induced Achilles tendinopathy. Radiol. Case Rep. 2006, 1, 87–91. [Google Scholar] [CrossRef]
- Cámara-Torres, M.; Duarte, S.; Sinha, R.; Egizabal, A.; Álvarez, N.; Bastianini, M.; Sisani, M.; Scopece, P.; Scatto, M.; Bonetto, A.; et al. 3D additive manufactured composite scaffolds with antibiotic-loaded lamellar fillers for bone infection prevention and tissue regeneration. Bioact. Mater. 2021, 6, 1073–1082. [Google Scholar] [CrossRef]
- Moroni, L.; De Wijn, J.; Van Blitterswijk, C. Three-dimensional fiber-deposited PEOT/PBT copolymer scaffolds for tissue engineering: Influence of porosity, molecular network mesh size, and swelling in aqueous media on dynamic mechanical properties. J. Biomed. Mater. Res. Part. A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2005, 75, 957–965. [Google Scholar] [CrossRef]
- Zheng, H.; Zuo, B. Functional silk fibroin hydrogels: Preparation, properties and applications. J. Mater. Chem. B 2021, 9, 1238–1258. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.; Pinho, A.R.; Gomes, M.C.; Demidov, Y.; Krakor, E.; Grume, D.; Herb, M.; Le, K.; Mano, J.; Mathur, S. Fabrication of antibacterial, osteo-inductor 3D printed aerogel-based scaffolds by incorporation of drug laden hollow mesoporous silica microparticles into the self-assembled silk fibroin biopolymer. Macromol. Biosci. 2022, 22, 2100442. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, M.; Averianov, I.; Gofman, I.; Shevchenko, N.; Rubinstein, A.; Egorova, T.; Trulioff, A.; Nashchekina, Y.; Kudryavtsev, I.; Demyanova, E. Drug loaded 3D-printed poly (ε-caprolactone) scaffolds for local antibacterial or anti-inflammatory treatment in bone regeneration. Polymers 2023, 15, 3957. [Google Scholar] [CrossRef]
- Timofticiuc, I.A.; Călinescu, O.; Iftime, A.; Dragosloveanu, S.; Caruntu, A.; Scheau, A.E.; Badarau, I.A.; Didilescu, A.C.; Caruntu, C.; Scheau, C. Biomaterials Adapted to Vat Photopolymerization in 3D Printing: Characteristics and Medical Applications. J. Funct. Biomater. 2023, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, S.M.; Quinn, D.J.; Ingram, R.J.; Gilmore, B.F.; Donnelly, R.F.; Taggart, C.C.; Scott, C.J. Gentamicin-loaded nanoparticles show improved antimicrobial effects towards Pseudomonas aeruginosa infection. Int. J. Nanomed. 2012, 7, 4053–4063. [Google Scholar] [CrossRef]
- Witkowski, J.; Wnukiewicz, W.; Reichert, P. Polymers as Carriers of Gentamicin in Traumatology and Orthopedic Surgery—Current State of Knowledge. Polim. Med. 2016, 46, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Hodiamont, C.J.; van den Broek, A.K.; de Vroom, S.L.; Prins, J.M.; Mathôt, R.A.A.; van Hest, R.M. Clinical Pharmacokinetics of Gentamicin in Various Patient Populations and Consequences for Optimal Dosing for Gram-Negative Infections: An Updated Review. Clin. Pharmacokinet. 2022, 61, 1075–1094. [Google Scholar] [CrossRef] [PubMed]
- Neut, D.; van de Belt, H.; Stokroos, I.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Biomaterial-associated infection of gentamicin-loaded PMMA beads in orthopaedic revision surgery. J. Antimicrob. Chemother. 2001, 47, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Tappa, K.; Jammalamadaka, U.; Weisman, J.A.; Ballard, D.H.; Wolford, D.D.; Pascual-Garrido, C.; Wolford, L.M.; Woodard, P.K.; Mills, D.K. 3D Printing Custom Bioactive and Absorbable Surgical Screws, Pins, and Bone Plates for Localized Drug Delivery. J. Funct. Biomater. 2019, 10, 17. [Google Scholar] [CrossRef]
- Weisman, J.A.; Nicholson, J.C.; Tappa, K.; Jammalamadaka, U.; Wilson, C.G.; Mills, D.K. Antibiotic and chemotherapeutic enhanced three-dimensional printer filaments and constructs for biomedical applications. Int. J. Nanomed. 2015, 10, 357–370. [Google Scholar] [CrossRef]
- Mills, D.K.; Jammalamadaka, U.; Tappa, K.; Weisman, J. Studies on the cytocompatibility, mechanical and antimicrobial properties of 3D printed poly(methyl methacrylate) beads. Bioact. Mater. 2018, 3, 157–166. [Google Scholar] [CrossRef]
- Szoradi, G.T.; Feier, A.M.; Zuh, S.G.; Russu, O.M.; Pop, T.S. Polymethyl Methacrylate Bone Cement Polymerization Induced Thermal Necrosis at the Cement–Bone Interface: A Narrative Review. Appl. Sci. 2024, 14, 11651. [Google Scholar] [CrossRef]
- Gundapaneni, D.; Goswami, T. Thermal isotherms in PMMA and cell necrosis during total hip arthroplasty. J. Appl. Biomater. Funct. Mater. 2014, 12, 193–202. [Google Scholar] [CrossRef]
- Boner, V.; Kuhn, P.; Mendel, T.; Gisep, A. Temperature evaluation during PMMA screw augmentation in osteoporotic bone—An in vitro study about the risk of thermal necrosis in human femoral heads. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 90, 842–848. [Google Scholar] [CrossRef]
- Niedźwiedź, M.J.; Gil, M.; Gargol, M.; Podkościelny, W.M.; Mergo, P. Determination of the optimal extrusion temperature of the PMMA optical fibers. Photonics Lett. Pol. 2019, 11, 7–9. [Google Scholar]
- Frunzaverde, D.; Cojocaru, V.; Ciubotariu, C.-R.; Miclosina, C.-O.; Ardeljan, D.D.; Ignat, E.F.; Marginean, G. The influence of the printing temperature and the filament color on the dimensional accuracy, tensile strength, and friction performance of FFF-printed PLA specimens. Polymers 2022, 14, 1978. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, R.; Mangiafico, A.; Basile, L.; Ruozi, B.; Furneri, P.M. Amphiphilic ion pairs of tobramycin with lipoamino acids. Eur. J. Med. Chem. 2011, 46, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Maane, M.; Xiu, F.; Bellstedt, P.; Kullak-Ublick, G.A.; Visentin, M. Characterization of ligand-induced thermal stability of the human organic cation transporter 2 (OCT2). Front. Pharmacol. 2023, 14, 1154213. [Google Scholar] [CrossRef]
- Biscaia, S.; Branquinho, M.V.; Alvites, R.D.; Fonseca, R.; Sousa, A.C.; Pedrosa, S.S.; Caseiro, A.R.; Guedes, F.; Patrício, T.; Viana, T. 3D printed poly (ε-caprolactone)/hydroxyapatite scaffolds for bone tissue engineering: A comparative study on a composite preparation by melt blending or solvent casting techniques and the influence of bioceramic content on scaffold properties. Int. J. Mol. Sci. 2022, 23, 2318. [Google Scholar] [CrossRef]
- Agrawal, A.; Hussain, C.M. 3D-Printed Hydrogel for Diverse Applications: A Review. Gels 2023, 9, 960. [Google Scholar] [CrossRef]
- Yu, X.; Wang, P.; Gao, J.; Fu, Y.; Wang, Q.; Chen, J.; Chen, S.; Ding, J. Wet 3D printing of biodegradable porous scaffolds to enable room-temperature deposition modeling of polymeric solutions for regeneration of articular cartilage. Biofabrication 2024, 16, 035007. [Google Scholar] [CrossRef]
- Cochis, A.; Bonetti, L.; Sorrentino, R.; Contessi Negrini, N.; Grassi, F.; Leigheb, M.; Rimondini, L.; Farè, S. 3D Printing of Thermo-Responsive Methylcellulose Hydrogels for Cell-Sheet Engineering. Materials 2018, 11, 579. [Google Scholar] [CrossRef]
- Montero, J.; Becerro, A.; Pardal-Peláez, B.; Quispe-López, N.; Blanco, J.F.; Gómez-Polo, C. Main 3D Manufacturing Techniques for Customized Bone Substitutes. A Systematic Review. Materials 2021, 14, 2524. [Google Scholar] [CrossRef]
- Nguyen, F.; Starosta, A.L.; Arenz, S.; Sohmen, D.; Dönhöfer, A.; Wilson, D.N. Tetracycline antibiotics and resistance mechanisms. Biol. Chem. 2014, 395, 559–575. [Google Scholar] [CrossRef]
- Martin, V.; Bettencourt, A.F.; Santos, C.; Fernandes, M.H.; Gomes, P.S. Unveiling the osteogenic potential of tetracyclines: A comparative study in human mesenchymal stem cells. Cells 2023, 12, 2244. [Google Scholar] [CrossRef]
- Pasternak, B.; Aspenberg, P. Metalloproteinases and their inhibitors—Diagnostic and therapeutic opportunities in orthopedics. Acta Orthop. 2009, 80, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Ahangar, P.; Li, J.; Nkindi, L.S.; Mohammadrezaee, Z.; Cooke, M.E.; Martineau, P.A.; Weber, M.H.; Saade, E.; Nateghi, N.; Rosenzweig, D.H. A nanoporous 3D-printed scaffold for local antibiotic delivery. Micromachines 2023, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Souza, L.R.d.; Litina, C.; Markaki, A.E.; Al-Tabbaa, A. Feasibility of using 3D printed polyvinyl alcohol (PVA) for creating self-healing vascular tunnels in cement system. Materials 2019, 12, 3872. [Google Scholar] [CrossRef] [PubMed]
- Karyappa, R.; Nagaraju, N.; Yamagishi, K.; Koh, X.Q.; Zhu, Q.; Hashimoto, M. 3D printing of polyvinyl alcohol hydrogels enabled by aqueous two-phase system. Mater. Horiz. 2024, 11, 2701–2717. [Google Scholar] [CrossRef]
- Duran, C.; Subbian, V.; Giovanetti, M.T.; Simkins, J.R.; Beyette Jr, F.R. Experimental desktop 3D printing using dual extrusion and water-soluble polyvinyl alcohol. Rapid Prototyp. J. 2015, 21, 528–534. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, G.; Junka, R.; Chang, N.; Anwar, A.; Wang, H.; Yu, X. Fabrication of polylactic acid (PLA)-based porous scaffold through the combination of traditional bio-fabrication and 3D printing technology for bone regeneration. Colloids Surf. B Biointerfaces 2021, 197, 111420. [Google Scholar] [CrossRef]
- Dussault, A.; Pitaru, A.A.; Weber, M.H.; Haglund, L.; Rosenzweig, D.H.; Villemure, I. Optimizing design parameters of PLA 3D-printed scaffolds for bone defect repair. Surgeries 2022, 3, 162–174. [Google Scholar] [CrossRef]
- Buj-Corral, I.; Bagheri, A.; Petit-Rojo, O. 3D printing of porous scaffolds with controlled porosity and pore size values. Materials 2018, 11, 1532. [Google Scholar] [CrossRef]
- Huber, F.; Vollmer, D.; Vinke, J.; Riedel, B.; Zankovic, S.; Schmal, H.; Seidenstuecker, M. Influence of 3D printing parameters on the mechanical stability of PCL scaffolds and the proliferation behavior of bone cells. Materials 2022, 15, 2091. [Google Scholar] [CrossRef]
- Temple, J.P.; Hutton, D.L.; Hung, B.P.; Huri, P.Y.; Cook, C.A.; Kondragunta, R.; Jia, X.; Grayson, W.L. Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J. Biomed. Mater. Res. Part A 2014, 102, 4317–4325. [Google Scholar] [CrossRef]
- Chamundeswari, V.N.; Siang, L.Y.; Chuah, Y.J.; Tan, J.S.; Wang, D.-A.; Loo, S.C.J. Sustained releasing sponge-like 3D scaffolds for bone tissue engineering applications. Biomed. Mater. 2017, 13, 015019. [Google Scholar] [CrossRef]
- Ahad, N.A.; Rozali, F.Z.; Rosli, N.H.; Hanif, N.I.H.; Parimin, N. Oil and water absorption behavior of TPU/natural fibers composites. Solid. State Phenom. 2018, 280, 374–381. [Google Scholar] [CrossRef]
- Desai, S.M.; Sonawane, R.Y.; More, A.P. Thermoplastic polyurethane for three-dimensional printing applications: A review. Polym. Adv. Technol. 2023, 34, 2061–2082. [Google Scholar] [CrossRef]
- Wei, C.; Solanki, N.G.; Vasoya, J.M.; Shah, A.V.; Serajuddin, A.T. Development of 3D printed tablets by fused deposition modeling using polyvinyl alcohol as polymeric matrix for rapid drug release. J. Pharm. Sci. 2020, 109, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Ng, N.; Haq, R.A.; Marwah, O.; Ho, F.; Adzila, S. Optimization of polyvinyl alcohol (PVA) support parameters for fused deposition modelling (FDM) by using design of experiments (DOE). Mater. Today Proc. 2022, 57, 1226–1234. [Google Scholar] [CrossRef]
- Poole, K. Efflux pumps as antimicrobial resistance mechanisms. Ann. Med. 2007, 39, 162–176. [Google Scholar] [CrossRef]
- Barza, M.; Brown, R.B.; Shanks, C.; Gamble, C.; Weinstein, L. Relation between lipophilicity and pharmacological behavior of minocycline, doxycycline, tetracycline, and oxytetracycline in dogs. Antimicrob. Agents Chemother. 1975, 8, 713–720. [Google Scholar] [CrossRef]
- El-Habashy, S.E.; El-Kamel, A.H.; Essawy, M.M.; Abdelfattah, E.A.; Eltaher, H.M. 3D printed bioinspired scaffolds integrating doxycycline nanoparticles: Customizable implants for in vivo osteoregeneration. Int. J. Pharm. 2021, 607, 121002. [Google Scholar] [CrossRef]
- Zeltz, C.; Gullberg, D. The integrin–collagen connection–a glue for tissue repair? J. Cell Sci. 2016, 129, 653–664. [Google Scholar] [CrossRef]
- Somaiah, C.; Kumar, A.; Mawrie, D.; Sharma, A.; Patil, S.D.; Bhattacharyya, J.; Swaminathan, R.; Jaganathan, B.G. Collagen promotes higher adhesion, survival and proliferation of mesenchymal stem cells. PLoS ONE 2015, 10, e0145068. [Google Scholar] [CrossRef]
- Martin, V.; Ribeiro, I.A.; Alves, M.M.; Gonçalves, L.; Claudio, R.A.; Grenho, L.; Fernandes, M.H.; Gomes, P.; Santos, C.F.; Bettencourt, A.F. Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 15–26. [Google Scholar] [CrossRef]
- Hedström, S.Å. General and local antibiotic treatment of chronic osteomyelitis. Scand. J. Infect. Dis. 1969, 1, 175–180. [Google Scholar] [CrossRef]
- Wu, W.; Ye, C.; Zheng, Q.; Wu, G.; Cheng, Z. A therapeutic delivery system for chronic osteomyelitis via a multi-drug implant based on three-dimensional printing technology. J. Biomater. Appl. 2016, 31, 250–260. [Google Scholar] [CrossRef]
- Bansal, R.; Sharma, D.; Singh, R. Tuberculosis and its treatment: An overview. Mini Rev. Med. Chem. 2018, 18, 58–71. [Google Scholar] [CrossRef]
- Gong, D.; Ma, Y.; Yang, X.; Xie, W.; Shao, L.; Zhen, P. Study on cytotoxicity of three-dimensional printed β-tricalcium phosphate loaded poly (lactide-co-glycolide) anti-tuberculosis drug sustained release microspheres and its effect on osteogenic differentiation of bone marrow mesenchymal stem cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2018, 32, 1131–1136. [Google Scholar] [CrossRef]
- Bai, J.; Wang, H.; Gao, W.; Liang, F.; Wang, Z.; Zhou, Y.; Lan, X.; Chen, X.; Cai, N.; Huang, W.; et al. Melt electrohydrodynamic 3D printed poly (ε-caprolactone)/polyethylene glycol/roxithromycin scaffold as a potential anti-infective implant in bone repair. Int. J. Pharm. 2020, 576, 118941. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Yoon, S.J.; Moon, Y.J.; Chun, H.J.; Yang, D.H. Doxorubicin· hydrochloride/cisplatin-loaded hydrogel/nanosized (2-hydroxypropyl)-beta-cyclodextrin local drug-delivery system for osteosarcoma treatment in vivo. Nanomaterials 2019, 9, 1652. [Google Scholar] [CrossRef]
- Chen, C.; Dong, J.; Chen, H.; Wang, X.; Mei, J.; Wang, L.; Xian, C.J. Preparation of adriamycin gelatin microsphere-loaded decellularized periosteum that is cytotoxic to human osteosarcoma cells. J. Cell. Physiol. 2019, 234, 10771–10781. [Google Scholar] [CrossRef]
- Jing, Z.; Ni, R.; Wang, J.; Lin, X.; Fan, D.; Wei, Q.; Zhang, T.; Zheng, Y.; Cai, H.; Liu, Z. Practical strategy to construct anti-osteosarcoma bone substitutes by loading cisplatin into 3D-printed titanium alloy implants using a thermosensitive hydrogel. Bioact. Mater. 2021, 6, 4542–4557. [Google Scholar] [CrossRef]
- Raj, P.; Lal, B.; Gadewar, M.; Singh, A.; Prashanth, G. Cisplatin and nano-particle formulations of cisplatin for cancer therapy: A Review. J. Pharm. Res. Int. 2022, 34, 34–49. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Xu, W.; Shao, L.H.; Shen, H.Q.; Liu, H.W. Enhanced osseointegration and antimicrobial properties of 3D-Printed porous titanium alloys with copper-strontium doped calcium silicate coatings. J. Biomater. Appl. 2025, 39, 607–619. [Google Scholar] [CrossRef]
- Marchandet, L.; Lallier, M.; Charrier, C.; Baud’huin, M.; Ory, B.; Lamoureux, F. Mechanisms of resistance to conventional therapies for osteosarcoma. Cancers 2021, 13, 683. [Google Scholar] [CrossRef]
- Zhu, C.; He, M.; Sun, D.; Huang, Y.; Huang, L.; Du, M.; Wang, J.; Wang, J.; Li, Z.; Hu, B.; et al. 3D-Printed Multifunctional Polyetheretherketone Bone Scaffold for Multimodal Treatment of Osteosarcoma and Osteomyelitis. ACS Appl. Mater. Interfaces 2021, 13, 47327–47340. [Google Scholar] [CrossRef]
- Ahangar, P.; Akoury, E.; Ramirez Garcia Luna, A.S.; Nour, A.; Weber, M.H.; Rosenzweig, D.H. Nanoporous 3D-printed scaffolds for local doxorubicin delivery in bone metastases secondary to prostate cancer. Materials 2018, 11, 1485. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, J.; Wu, Q.; Luo, G.; Zhao, M.; Zhong, G.; Zheng, Y.; Meng, X.; Cheng, S.; Zhang, Y. Multifunctional 3D-printed scaffolds eradiate orthotopic osteosarcoma and promote osteogenesis via microwave thermo-chemotherapy combined with immunotherapy. Biomaterials 2023, 301, 122236. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, S.; Zhu, M.; Zhu, Y.; Zhang, Y.; Liu, Z.; Zhang, C. 3D-printed magnetic Fe(3)O(4)/MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermia. J. Mater. Chem. B 2014, 2, 7583–7595. [Google Scholar] [CrossRef]
- Jaiswal, C.; Dey, S.; Prasad, J.; Gupta, R.; Agarwala, M.; Mandal, B.B. 3D bioprinted microfluidic based osteosarcoma-on-a chip model as a physiomimetic pre-clinical drug testing platform for anti-cancer drugs. Biomaterials 2025, 320, 123267. [Google Scholar] [CrossRef]
- Liu, X.; Hu, H.; Ma, J.; Wang, B. Mineralized cellulose nanofibers reinforced bioactive hydrogel remodels the osteogenic and angiogenic microenvironment for enhancing bone regeneration. Carbohydr. Polym. 2025, 357, 123480. [Google Scholar] [CrossRef]
- Wang, L.; Dai, Z.; Bi, J.; Chen, Y.; Wang, Z.; Sun, Z.; Ji, Z.; Wang, H.; Zhang, Y.; Wang, L.; et al. Polydopamine-functionalized calcium-deficient hydroxyapatite 3D-printed scaffold with sustained doxorubicin release for synergistic chemo-photothermal therapy of osteosarcoma and accelerated bone regeneration. Mater. Today Bio 2024, 29, 101253. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, W.; Wang, B.; Zhu, H.; Zhang, B.; Feng, M. Delivery of hydrophilic drug doxorubicin hydrochloride-targeted liver using apoAI as carrier. J. Drug Target. 2013, 21, 367–374. [Google Scholar] [CrossRef]
- Park, C.; Lee, J.H.; Jin, G.; Ngo, H.V.; Park, J.-B.; Tran, T.T.; Tran, P.H.; Lee, B.-J. Release kinetics of hydroxypropyl methylcellulose governing drug release and hydrodynamic changes of matrix tablet. Curr. Drug Deliv. 2022, 19, 520–533. [Google Scholar] [CrossRef]
- Heshmatnezhad, F.; Nazar, A.R.S.; Aghaei, H.; Varshosaz, J. Production of doxorubicin-loaded PCL nanoparticles through a flow-focusing microfluidic device: Encapsulation efficacy and drug release. Soft Matter 2021, 17, 10675–10682. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tu, S.; Steffen, D.; Xiong, M. Iron complexation to histone deacetylase inhibitors SAHA and LAQ824 in PEGylated liposomes can considerably improve pharmacokinetics in rats. J. Pharm. Pharm. Sci. 2014, 17, 583–602. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; He, D.; Wang, S.; Ding, P.; Wang, J.; Ju, J. Preparation, characterization, and pharmacokinetics study of a novel genistein-loaded mixed micelles system. Drug Dev. Ind. Pharm. 2018, 44, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Van Oss, C.; Giese, R. The hydrophilicity and hydrophobicity of clay minerals. Clays Clay Miner. 1995, 43, 474–477. [Google Scholar] [CrossRef]
- Huang, W.Y.; Hibino, T.; Suye, S.-i.; Fujita, S. Electrospun collagen core/poly-l-lactic acid shell nanofibers for prolonged release of hydrophilic drug. RSC Adv. 2021, 11, 5703–5711. [Google Scholar] [CrossRef]
- Yeo, Y.; Park, K. Control of encapsulation efficiency and initial burst in polymeric microparticle systems. Arch. Pharmacal Res. 2004, 27, 1–12. [Google Scholar] [CrossRef]
- Kovshova, T.; Osipova, N.; Alekseeva, A.; Malinovskaya, J.; Belov, A.; Budko, A.; Pavlova, G.; Maksimenko, O.; Nagpal, S.; Braner, S. Exploring the interplay between drug release and targeting of lipid-like polymer nanoparticles loaded with doxorubicin. Molecules 2021, 26, 831. [Google Scholar] [CrossRef]
- Terpos, E. Bisphosphonate anticancer activity in multiple myeloma. Anti-Cancer Agents Med. Chem. Anti-Cancer Agents 2012, 12, 123–128. [Google Scholar] [CrossRef]
- Burkinshaw, R.; Thorpe, H.; Coleman, R. Bisphosphonates and prevention of metastases: The AZURE study. Breast Cancer Online 2007, 10, e21. [Google Scholar] [CrossRef]
- Di, W.; Shuai, Y.; Bo, W.; Wei, T.; Jinpeng, H.; Qian, G.; Deng, Y. A bifunctional zoledronate sustained-release system in scaffold: Tumor therapy and bone repair. Colloids Surf. B Biointerfaces 2023, 222, 113064. [Google Scholar] [CrossRef]
- Ran, Z.; Wang, Y.; Li, J.; Xu, W.; Tan, J.; Cao, B.; Luo, D.; Ding, Y.; Wu, J.; Wang, L.; et al. 3D-printed biodegradable magnesium alloy scaffolds with zoledronic acid-loaded ceramic composite coating promote osteoporotic bone defect repair. Int. J. Bioprint 2023, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Traore, Y.L.; Walker, L.; Yang, S.; Ho, E.A. Fused deposition modeling three-dimensional printing of flexible polyurethane intravaginal rings with controlled tunable release profiles for multiple active drugs. Drug Deliv. Transl. Res. 2022, 12, 906–924. [Google Scholar] [CrossRef] [PubMed]
- Albu, P.; Budiul, M.; Mateescu, M.; Chiriac, V.; Vlase, G.; Vlase, T. Studies regarding the induced thermal degradation, kinetic analysis and possible interactions with various excipients of an osseointegration agent: Zoledronic acid. J. Therm. Anal. Calorim. 2017, 130, 403–411. [Google Scholar] [CrossRef]
- Pang, R.; Lai, M.K.; Ismail, K.I.; Yap, T.C. Characterization of the Dimensional Precision, Physical Bonding, and Tensile Performance of 3D-Printed PLA Parts with Different Printing Temperature. J. Manuf. Mater. Process. 2024, 8, 56. [Google Scholar] [CrossRef]
- Liu, Q.; Qiu, L.; Wang, Y.; Lv, G.; Liu, G.; Wang, S.; Lin, J. Solvent effect on molecular structure, IR spectra, thermodynamic properties and chemical stability of zoledronic acid: DFT study. J. Mol. Model. 2016, 22, 84. [Google Scholar] [CrossRef]
- Johnson, D.B.; Lopez, M.J.; Kelley, B. Dexamethasone. In StatPearls [Internet]; StatPearls Publishing: Orlando, FL, USA, 2023. [Google Scholar]
- Zhang, X.; Geven, M.A.; Wang, X.; Qin, L.; Grijpma, D.W.; Peijs, T.; Eglin, D.; Guillaume, O.; Gautrot, J.E. A drug eluting poly(trimethylene carbonate)/poly(lactic acid)-reinforced nanocomposite for the functional delivery of osteogenic molecules. Int. J. Nanomed. 2018, 13, 5701–5718. [Google Scholar] [CrossRef]
- Chen, C.H.; Dash, B.S.; Ting, W.C.; Chen, J.P. Bone Tissue Engineering with Adipose-Derived Stem Cells in Polycaprolactone/Graphene Oxide/Dexamethasone 3D-Printed Scaffolds. ACS Biomater. Sci. Eng. 2024, 10, 6425–6440. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, S.; Xie, C.; Liang, Q.; Xu, D.; Chen, W.; Xiao, X. The fabrication of multifunctional sodium alginate scaffold incorporating ibuprofen-loaded modified PLLA microspheres based on cryogenic 3D printing. J. Biomater. Sci. Polym. Ed. 2022, 33, 1269–1288. [Google Scholar] [CrossRef]
- Wei, H.; Chen, W.; Chen, S.; Zhang, T.; Xiao, X. 3D printing of MOF-reinforced methacrylated gelatin scaffolds for bone regeneration. J. Biomater. Sci. Polym. Ed. 2024, 35, 443–462. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Y.; Pan, J.; Wang, H.; Li, H.; Xu, X.; Fu, X.; Shi, R.; Luo, Z.; Li, Y.; et al. A hybrid 3D-printed aspirin-laden liposome composite scaffold for bone tissue engineering. J. Mater. Chem. B 2019, 7, 619–629. [Google Scholar] [CrossRef]
- Vu, A.A.; Bose, S. Vitamin D3 release from traditionally and additively manufactured tricalcium phosphate bone tissue engineering scaffolds. Ann. Biomed. Eng. 2020, 48, 1025–1033. [Google Scholar] [CrossRef]
- Bose, S.; Chaudhari, V.S.; Kushram, P. 3D printed scaffolds with quercetin and vitamin D3 nanocarriers: In vitro cellular evaluation. J. Biomed. Mater. Res. Part A 2024, 112, 2110–2123. [Google Scholar] [CrossRef]
- Deng, N.; Sun, J.; Li, Y.; Chen, L.; Chen, C.; Wu, Y.; Wang, Z.; Li, L. Experimental study of rhBMP-2 chitosan nano-sustained release carrier-loaded PLGA/nHA scaffolds to construct mandibular tissue-engineered bone. Arch. Oral. Biol. 2019, 102, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Su, Y.; Cao, R.; Wang, K.; Yang, P. Enhanced osteogenic activity and bone repair ability of PLGA/MBG scaffolds doped with ZIF-8 nanoparticles loaded with BMP-2. Int. J. Nanomed. 2023, 18, 5055–5072. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.H.; Lin, Y.H.; Shie, M.Y.; Lin, C.P. Effects of bone morphogenic protein-2 loaded on the 3D-printed MesoCS scaffolds. J. Formos. Med. Assoc. 2018, 117, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wei, Y.; Xuan, C.; Liu, L.; Lai, C.; Chai, M.; Zhang, Z.; Wang, L.; Shi, X. A Biomimetic Biphasic Osteochondral Scaffold with Layer-Specific Release of Stem Cell Differentiation Inducers for the Reconstruction of Osteochondral Defects. Adv. Healthc. Mater. 2020, 9, e2000076. [Google Scholar] [CrossRef]
- Cheng, W.X.; Liu, Y.Z.; Meng, X.B.; Zheng, Z.T.; Li, L.L.; Ke, L.Q.; Li, L.; Huang, C.S.; Zhu, G.Y.; Pan, H.D.; et al. PLGA/β-TCP composite scaffold incorporating cucurbitacin B promotes bone regeneration by inducing angiogenesis. J. Orthop. Transl. 2021, 31, 41–51. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Jo, Y.; Bose, S. In vivo and In vitro properties evaluation of curcumin loaded MgO doped 3D printed TCP scaffolds. J. Mater. Chem. B 2023, 11, 4725–4739. [Google Scholar] [CrossRef]
- Bose, S.; Banerjee, D.; Vu, A.A. Ginger and Garlic Extracts Enhance Osteogenesis in 3D Printed Calcium Phosphate Bone Scaffolds with Bimodal Pore Distribution. ACS Appl. Mater. Interfaces 2022, 14, 12964–12975. [Google Scholar] [CrossRef]
- Bose, S.; White, B.; Chaudhari, V.S. Gingerol encapsulated lipid nanoparticles on 3D-printed scaffolds for orthopedic and dental applications. Biomater. Adv. 2025, 172, 214194. [Google Scholar] [CrossRef]
- Hock, F.J.; Pugsley, M.K. Drug Discovery and Evaluation: Safety and Pharmacokinetic Assays; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Singh, V.K.; Seed, T.M. How necessary are animal models for modern drug discovery? Expert. Opin. Drug Discov. 2021, 16, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Brown, P.C.; Chow, E.C.; Ewart, L.; Ferguson, S.S.; Fitzpatrick, S.; Freedman, B.S.; Guo, G.L.; Hedrich, W.; Heyward, S. 3D cell culture models: Drug pharmacokinetics, safety assessment, and regulatory consideration. Clin. Transl. Sci. 2021, 14, 1659–1680. [Google Scholar] [CrossRef] [PubMed]
- Foglizzo, V.; Cocco, E.; Marchiò, S. Advanced cellular models for preclinical drug testing: From 2D cultures to organ-on-a-chip technology. Cancers 2022, 14, 3692. [Google Scholar] [CrossRef] [PubMed]
- Badr-Eldin, S.M.; Aldawsari, H.M.; Kotta, S.; Deb, P.K.; Venugopala, K.N. Three-dimensional in vitro cell culture models for efficient drug discovery: Progress so far and future prospects. Pharmaceuticals 2022, 15, 926. [Google Scholar] [CrossRef]
- Li, S.; Yang, K.; Chen, X.; Zhu, X.; Zhou, H.; Li, P.; Chen, Y.; Jiang, Y.; Li, T.; Qin, X. Simultaneous 2D and 3D cell culture array for multicellular geometry, drug discovery and tumor microenvironment reconstruction. Biofabrication 2021, 13, 045013. [Google Scholar] [CrossRef]
- Xu, Y.; Pachnikova, G.; Wang, H.; Wu, Y.; Przybilla, D.; Schäfer, R.; Chen, Z.; Zhu, S.; Keilholz, U. IC50: An unsuitable measure for large-sized prostate cancer spheroids in drug sensitivity evaluation. Bosn. J. Basic. Med. Sci. 2022, 22, 580. [Google Scholar] [CrossRef]
- Abbas, Z.N.; Al-Saffar, A.Z.; Jasim, S.M.; Sulaiman, G.M. Comparative analysis between 2D and 3D colorectal cancer culture models for insights into cellular morphological and transcriptomic variations. Sci. Rep. 2023, 13, 18380. [Google Scholar] [CrossRef]
- Jota Baptista, C.V.; Faustino-Rocha, A.I.; Oliveira, P.A. Animal models in pharmacology: A brief history awarding the nobel prizes for physiology or medicine. Pharmacology 2021, 106, 356–368. [Google Scholar] [CrossRef]
- Admane, P.; Mane, S.; Vinchurkar, K. 3D Printing Technology in the Pharmaceutical Industry and Its Application in Drug Delivery in the Context of Personalized Medication. In Personalized Medicine-New Perspectives; IntechOpen: London, UK, 2024. [Google Scholar]
- Mihaylova, A.; Shopova, D.; Parahuleva, N.; Yaneva, A.; Bakova, D. (3D) bioprinting—Next dimension of the pharmaceutical sector. Pharmaceuticals 2024, 17, 797. [Google Scholar] [CrossRef] [PubMed]
- Bisht, B.; Hope, A.; Mukherjee, A.; Paul, M.K. Advances in the fabrication of scaffold and 3D printing of biomimetic bone graft. Ann. Biomed. Eng. 2021, 49, 1128–1150. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, D.; Demir, A.; Bermingham, M.; Dargusch, M. Challenges and opportunities in the selective laser melting of biodegradable metals for load-bearing bone scaffold applications. Metall. Mater. Trans. A 2020, 51, 3311–3334. [Google Scholar] [CrossRef]
- Entezari, A.; Roohani-Esfahani, S.-I.; Zhang, Z.; Zreiqat, H.; Dunstan, C.R.; Li, Q. Fracture behaviors of ceramic tissue scaffolds for load bearing applications. Sci. Rep. 2016, 6, 28816. [Google Scholar] [CrossRef]
- Li, X.; Fan, L.; Li, J. Extrusion-based 3D printing of high internal phase emulsions stabilized by co-assembled β-cyclodextrin and chitosan. Food Hydrocoll. 2023, 134, 108036. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Fan, L.; Li, J. Fabrication of fat-reduced water-in-oil emulsion and the application in 3D printing. Food Res. Int. 2023, 172, 113118. [Google Scholar] [CrossRef]
- Ghosh, S.; Yadav, A.; Rani, S.; Takkar, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. 3D Printed Hierarchical Porous Poly(ε-caprolactone) Scaffolds from Pickering High Internal Phase Emulsion Templating. Langmuir 2023, 39, 1927–1946. [Google Scholar] [CrossRef]
- Zhang, J.; Griesbach, J.; Ganeyev, M.; Zehnder, A.K.; Zeng, P.; Schädli, G.N.; Leeuw, A.; Lai, Y.; Rubert, M.; Müller, R. Long-term mechanical loading is required for the formation of 3D bioprinted functional osteocyte bone organoids. Biofabrication 2022, 14, 035018. [Google Scholar] [CrossRef]
- Breathwaite, E.; Weaver, J.; Odanga, J.; Dela Pena-Ponce, M.; Lee, J.B. 3D Bioprinted Osteogenic Tissue Models for In Vitro Drug Screening. Molecules 2020, 25, 3442. [Google Scholar] [CrossRef]
- Ayan, B.; Heo, D.; Zhang, Z.; Dey, M.; Povilianskas, A.; Drapaca, C.; Ozbolat, I. Aspiration-assisted bioprinting for precise positioning of biologics. Sci. Adv. 2020, 6, eaaw5111. [Google Scholar] [CrossRef]
- Khoshnood, N.; Zamanian, A. A comprehensive review on scaffold-free bioinks for bioprinting. Bioprinting 2020, 19, e00088. [Google Scholar] [CrossRef]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, I.N.; Olivos III, D.J.; Brinker, A.; Alvarez, M.B.; Smith, L.J.; Chu, T.-M.G.; Kacena, M.A.; Wagner, D.R. Scaffold-free bioprinting of mesenchymal stem cells using the Regenova printer: Spheroid characterization and osteogenic differentiation. Bioprinting 2019, 15, e00050. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, C.C.; da Cunha, J.B.; de Souza, V.M.; de Andrade, K.C.; Moraes, Â.M.; Coimbra, I.B. Amniotic fluid MSCs for scaffold-free cartilage repair: Spheroid fusion and chondrogenic microtissue development. Future Sci. OA 2025, 11, 2476922. [Google Scholar] [CrossRef]
- Apostu, D.; Lucaciu, O.; Mester, A.; Oltean-Dan, D.; Baciut, M.; Baciut, G.; Bran, S.; Onisor, F.; Piciu, A.; Pasca, R.D. Systemic drugs with impact on osteoarthritis. Drug Metab. Rev. 2019, 51, 498–523. [Google Scholar] [CrossRef]
- Oliveira, M.B.; Neto, A.I.; Correia, C.R.; Rial-Hermida, M.I.; Alvarez-Lorenzo, C.; Mano, J.F. Superhydrophobic chips for cell spheroids high-throughput generation and drug screening. ACS Appl. Mater. Interfaces 2014, 6, 9488–9495. [Google Scholar] [CrossRef]
- Yan, Y.; Song, L.; Madinya, J.; Ma, T.; Li, Y. Derivation of cortical spheroids from human induced pluripotent stem cells in a suspension bioreactor. Tissue Eng. Part A 2018, 24, 418–431. [Google Scholar] [CrossRef]
- Han, W.; El Botty, R.; Montaudon, E.; Malaquin, L.; Deschaseaux, F.; Espagnolle, N.; Marangoni, E.; Cottu, P.; Zalcman, G.; Parrini, M.C.; et al. In vitro bone metastasis dwelling in a 3D bioengineered niche. Biomaterials 2021, 269, 120624. [Google Scholar] [CrossRef]
- Ji, X.; Bei, H.P.; Zhong, G.; Shao, H.; He, X.; Qian, X.; Zhang, Y.; Zhao, X. Premetastatic Niche Mimicking Bone-On-A-Chip: A Microfluidic Platform to Study Bone Metastasis in Cancer Patients. Small 2023, 19, e2207606. [Google Scholar] [CrossRef]
- Lu, Z.; Miao, X.; Zhang, C.; Sun, B.; Skardal, A.; Atala, A.; Ai, S.; Gong, J.; Hao, Y.; Zhao, J.; et al. An osteosarcoma-on-a-chip model for studying osteosarcoma matrix-cell interactions and drug responses. Bioact. Mater. 2024, 34, 1–16. [Google Scholar] [CrossRef]
- Pellegrini, E.; Desando, G.; Petretta, M.; Cellamare, A.; Cristalli, C.; Pasello, M.; Manara, M.C.; Grigolo, B.; Scotlandi, K. A 3D collagen-based bioprinted model to study osteosarcoma invasiveness and drug response. Polymers 2022, 14, 4070. [Google Scholar] [CrossRef]
- Adhami, M.; Dastidar, A.G.; Anjani, Q.K.; Detamornrat, U.; Tarrés, Q.; Delgado-Aguilar, M.; Acheson, J.G.; Manda, K.; Clarke, S.A.; Moreno-Castellanos, N.; et al. 3D-printing of dipyridamole/thermoplastic polyurethane materials for bone regeneration. Drug Deliv. Transl. Res. 2025, 15, 2467–2482. [Google Scholar] [CrossRef]
- Bharathi, R.; Harini, G.; Sankaranarayanan, A.; Shanmugavadivu, A.; Vairamani, M.; Selvamurugan, N. Nuciferine-loaded chitosan hydrogel-integrated 3D-printed polylactic acid scaffolds for bone tissue engineering: A combinatorial approach. Int. J. Biol. Macromol. 2023, 253, 127492. [Google Scholar] [CrossRef]
- Farto-Vaamonde, X.; Auriemma, G.; Aquino, R.P.; Concheiro, A.; Alvarez-Lorenzo, C. Post-manufacture loading of filaments and 3D printed PLA scaffolds with prednisolone and dexamethasone for tissue regeneration applications. Eur. J. Pharm. Biopharm. 2019, 141, 100–110. [Google Scholar] [CrossRef]
- Oryan, A.; Hassanajili, S.; Sahvieh, S. Zoledronate loaded polylactic acid/polycaprolactone/hydroxyapatite scaffold accelerates regeneration and led to enhance structural performance and functional ability of the radial bone defect in rat. Iran. J. Vet. Res. 2023, 24, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Remy, M.T.; Upara, C.; Ding, Q.J.; Miszuk, J.M.; Sun, H.; Hong, L. MicroRNA-200c release from Gelatin-Coated 3D-Printed PCL scaffolds enhances bone regeneration. ACS Biomater. Sci. Eng. 2024, 10, 2337–2350. [Google Scholar] [CrossRef] [PubMed]
- Riester, O.; Laufer, S.; Deigner, H.-P. Direct 3D printed biocompatible microfluidics: Assessment of human mesenchymal stem cell differentiation and cytotoxic drug screening in a dynamic culture system. J. Nanobiotechnol. 2022, 20, 540. [Google Scholar] [CrossRef] [PubMed]
- Lipreri, M.V.; Totaro, M.T.; Raimondi, I.; Cortini, M.; Baldini, N.; Avnet, S. A customizable micropatterned platform for osteosarcoma spheroid generation, imaging, and drug screening. Biomater. Adv. 2025, 177, 214419. [Google Scholar] [CrossRef]
- Fan, S.; Tan, Y.; Yuan, X.; Liu, C.; Wu, X.; Dai, T.; Ni, S.; Wang, J.; Weng, Y.; Zhao, H. Regulation of the immune microenvironment by pioglitazone-loaded polylactic glycolic acid nanosphere composite scaffolds to promote vascularization and bone regeneration. J. Tissue Eng. 2024, 15, 20417314241231452. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Chen, J.; Gong, W.; Tu, X. The Osteocyte with SB216763-Activated Canonical Wnt Signaling Constructs a Multifunctional 4D Intelligent Osteogenic Module. Biomolecules 2024, 14, 354. [Google Scholar] [CrossRef]
- Zha, Y.; Li, Y.; Lin, T.; Chen, J.; Zhang, S.; Wang, J. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics 2021, 11, 397–409. [Google Scholar] [CrossRef]
- Tan, Y.; Fan, S.; Wu, X.; Liu, M.; Dai, T.; Liu, C.; Ni, S.; Wang, J.; Yuan, X.; Zhao, H. Fabrication of a three-dimensional printed gelatin/sodium alginate/nano-attapulgite composite polymer scaffold loaded with leonurine hydrochloride and its effects on osteogenesis and vascularization. Int. J. Biol. Macromol. 2023, 249, 126028. [Google Scholar] [CrossRef] [PubMed]
- Noory, P.; Farmani, A.R.; Ai, J.; Bahrami, N.; Bayat, M.; Ebrahimi-Barough, S.; Farzin, A.; Shojaie, S.; Hajmoradi, H.; Mohamadnia, A.; et al. Enhancing in vitro osteogenic differentiation of mesenchymal stem cells via sustained dexamethasone delivery in 3D-Printed hybrid scaffolds based on polycaprolactone-nanohydroxyapatite/alginate-gelatin for bone regeneration. J. Biol. Eng. 2025, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yang, J.; Liu, L.; Hu, Z.; Lin, R.; Tang, L.; Yu, M.; Chen, Z.; Gao, C.; Zhang, M.; et al. An electrostatic encapsulation strategy to motivate 3D-printed polyelectrolyte scaffolds for repair of osteoporotic bone defects. Bioact. Mater. 2025, 46, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, R.; Delgado, J.; Delgado, L.M.; Pérez, R.A. Silica 3D printed scaffolds as pH stimuli-responsive drug release platform. Mater. Today Bio 2024, 28, 101187. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, W.; Wu, S.; Kuss, M.; Jiang, X.; Untrauer, J.B.; Reid, S.P.; Duan, B. 3D printed composite scaffolds with dual small molecule delivery for mandibular bone regeneration. Biofabrication 2020, 12, 035020. [Google Scholar] [CrossRef]
- Wu, Y.; Woodbine, L.; Carr, A.M.; Pillai, A.R.; Nokhodchi, A.; Maniruzzaman, M. 3D Printed Calcium Phosphate Cement (CPC) Scaffolds for Anti-Cancer Drug Delivery. Pharmaceutics 2020, 12, 1077. [Google Scholar] [CrossRef]
- Xu, T.; Gao, S.; Yang, N.; Zhao, Q.; Zhang, Y.; Li, T.; Liu, Z.; Han, B. A personalized biomimetic dual-drug delivery system via controlled release of PTH(1-34) and simvastatin for in situ osteoporotic bone regeneration. Front. Bioeng. Biotechnol. 2024, 12, 1355019. [Google Scholar] [CrossRef]
- Wen, Y.T.; Dai, N.T.; Hsu, S.H. Biodegradable water-based polyurethane scaffolds with a sequential release function for cell-free cartilage tissue engineering. Acta Biomater. 2019, 88, 301–313. [Google Scholar] [CrossRef]
- Romero-Torrecilla, J.A.; de Anleo, M.E.-G.; Martínez-Ohárriz, C.; Ripalda-Cemboráin, P.; López-Martínez, T.; Abizanda, G.; Valdés-Fernández, J.; Prandota, J.; Muiños-López, E.; Garbayo, E. 3D-printed polycaprolactone scaffolds functionalized with poly (lactic-co-glycolic) acid microparticles enhance bone regeneration through tunable drug release. Acta Biomater. 2025, 198, 219–233. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Li, H.; Xu, X.; Fu, X.; Pan, J.; Wang, H.; Fuh, J.Y.H.; Bai, Y.; Wei, S. An effective dual-factor modified 3D-printed PCL scaffold for bone defect repair. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2167–2179. [Google Scholar] [CrossRef]
- Nichols, D.A.; Sondh, I.S.; Little, S.R.; Zunino, P.; Gottardi, R. Design and validation of an osteochondral bioreactor for the screening of treatments for osteoarthritis. Biomed. Microdevices 2018, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Sun, M.; Chen, B.; Saiding, Q.; Zhang, J.; Song, H.; Deng, L.; Wang, P.; Gong, W.; Cui, W. Lotus seedpod-inspired internal vascularized 3D printed scaffold for bone tissue repair. Bioact. Mater. 2021, 6, 1639–1652. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Meng, K.; Li, Z.; Lan, S.; Ren, Z.; Fu, X.; Li, C.; Sun, T.; Xie, D.; Zhang, Z.; et al. An Integrated Microcurrent Delivery System Facilitates Human Parathyroid Hormone Delivery for Enhancing Osteoanabolic Effect. Small Methods 2025, 9, e2401144. [Google Scholar] [CrossRef] [PubMed]
- Pektas, H.K.; Demidov, Y.; Ahvan, A.; Abie, N.; Georgieva, V.S.; Chen, S.; Farè, S.; Brachvogel, B.; Mathur, S.; Maleki, H. MXene-Integrated Silk Fibroin-Based Self-Assembly-Driven 3D-Printed Theragenerative Scaffolds for Remotely Photothermal Anti-Osteosarcoma Ablation and Bone Regeneration. ACS Mater. Au 2023, 3, 711–726. [Google Scholar] [CrossRef]
- Bubpamala, T.; Promoppatum, P.; Pholpabu, P. Drug-Releasing Tannic Acid-Mediated Adhesive PEG Hydrogel for Porous Titanium Implants. ACS Omega 2024, 9, 887–895. [Google Scholar] [CrossRef]
- Kwon, B.J.; Seon, G.M.; Lee, M.H.; Koo, M.A.; Kim, M.S.; Kim, D.; Han, J.J.; Kim, D.; Kim, J.; Park, J.C. Locally delivered ethyl-2,5-dihydroxybenzoate using 3D printed bone implant for promotion of bone regeneration in a osteoporotic animal model. Eur. Cell Mater. 2018, 35, 1–12. [Google Scholar] [CrossRef]
- Fan, D.; Zhang, C.; Wang, H.; Wei, Q.; Cai, H.; Wei, F.; Bian, Z.; Liu, W.; Wang, X.; Liu, Z. Fabrication of a composite 3D-printed titanium alloy combined with controlled in situ drug release to prevent osteosarcoma recurrence. Mater. Today Bio 2023, 20, 100683. [Google Scholar] [CrossRef]
- Jing, Z.; Yuan, W.; Wang, J.; Ni, R.; Qin, Y.; Mao, Z.; Wei, F.; Song, C.; Zheng, Y.; Cai, H.; et al. Simvastatin/hydrogel-loaded 3D-printed titanium alloy scaffolds suppress osteosarcoma via TF/NOX2-associated ferroptosis while repairing bone defects. Bioact. Mater. 2024, 33, 223–241. [Google Scholar] [CrossRef]
- Bose, S.; Sarkar, N.; Majumdar, U. Micelle encapsulated curcumin and piperine-laden 3D printed calcium phosphate scaffolds enhance in vitro biological properties. Colloids Surf. B Biointerfaces 2023, 231, 113563. [Google Scholar] [CrossRef]
- Vu, A.A.; Kushram, P.; Bose, S. Effects of Vitamin A (Retinol) Release from Calcium Phosphate Matrices and Porous 3D Printed Scaffolds on Bone Cell Proliferation and Maturation. ACS Appl. Bio Mater. 2022, 5, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Onyiriuka, A. Mineral and bone disorders secondary to chronic kidney disease (renal osteodystrophy). Sri Lanka J. Diabetes Endocrinol. Metab. 2015, 5, 91. [Google Scholar] [CrossRef]
- Evaggelou, E.; Lambrou, G.I. Acid-base disorders and the impact on metabolic bone disease in hemodialysis patients. J. Res. Pract. Musculoskelet. Syst. 2021, 5, 65–70. [Google Scholar] [CrossRef]
- Prasad, N.; Hamosh, A.; Sponseller, P. Orthopaedic Manifestations of Inborn Errors of Metabolism. J. Bone Jt. Surg. Rev. 2021, 9, e20. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, G.; Zimran, A.; Bembi, B.; Kanis, J.; Reginster, J.-Y.; Rizzoli, R.; Cooper, C.; Brandi, M.L. Gaucher disease and bone manifestations. Calcif. Tissue Int. 2014, 95, 477–494. [Google Scholar] [CrossRef]
- Rosenbloom, B.E.; Weinreb, N.J. Gaucher disease: A comprehensive review. Crit. Rev. Oncog. 2013, 18, 163–175. [Google Scholar] [CrossRef]
- Mucci, J.M.; Rozenfeld, P. Pathogenesis of bone alterations in Gaucher disease: The role of immune system. J. Immunol. Res. 2015, 2015, 192761. [Google Scholar] [CrossRef]
- Soloveva, A.; Yatsyk, G.; Ponomarev, R.; Lukina, K.; Kostina, I.; Mamonov, V.; Lukina, E. Reversible and irreversible radiological signs of bone involvement in type I Gaucher disease. Russ. J. Hematol. Transfusiol. 2019, 64, 49–59. [Google Scholar] [CrossRef]
- Wenstrup, R.; Roca-Espiau, M.; Weinreb, N.; Bembi, B. Skeletal aspects of Gaucher disease: A review. Br. J. Radiol. 2002, 75, A2–A12. [Google Scholar] [CrossRef]
- Barranger, J.; O’Rourke, E. Lessons learned from the development of enzyme therapy for Gaucher disease. J. Inherit. Metab. Dis. 2001, 24, 89–96. [Google Scholar] [CrossRef]
- Amato, D.; Patterson, M.A. Combined miglustat and enzyme replacement therapy in two patients with type 1 Gaucher disease: Two case reports. J. Med. Case Rep. 2018, 12, 19. [Google Scholar] [CrossRef]
- Banerjee, D.; Ivanova, M.M.; Celik, N.; Kim, M.H.; Derman, I.D.; Limgala, R.P.; Ozbolat, I.T.; Goker-Alpan, O. Biofabrication of anin-vitrobone model for Gaucher disease. Biofabrication 2023, 15, 045023. [Google Scholar] [CrossRef] [PubMed]
- Barneveld, R.A.; Tegelaers, F.P.; Ginns, E.I.; Visser, P.; Laanen, E.A.; Brady, R.O.; Galjaard, H.; Barranger, J.A.; Reuser, A.J.; Tager, J.M. Monoclonal antibodies against human β-glucocerebrosidase. Eur. J. Biochem. 1983, 134, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, M.M.; Dao, J.; Kasaci, N.; Friedman, A.; Noll, L.; Goker-Alpan, O. Wnt signaling pathway inhibitors, sclerostin and DKK-1, correlate with pain and bone pathology in patients with Gaucher disease. Front. Endocrinol. 2022, 13, 1029130. [Google Scholar] [CrossRef] [PubMed]
- Duong, L.H.; Chen, P.-C. A novel solvent bonding method for creating a 3D, nonplanar, and hybrid PLA/PMMA microfluidic chip. In Proceedings of the 2018 IEEE 13th Annual International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Singapore, 1–4 April 2018; pp. 177–180. [Google Scholar]
- Lynh, H.D.; Pin-Chuan, C. Novel solvent bonding method for creation of a three-dimensional, non-planar, hybrid PLA/PMMA microfluidic chip. Sens. Actuators A Phys. 2018, 280, 350–358. [Google Scholar] [CrossRef]
- Reif, R.; Wang, M.; Joshi, S.; A’Amar, O.; Bigio, I.J. Optical method for real-time monitoring of drug concentrations facilitates the development of novel methods for drug delivery to brain tissue. J. Biomed. Opt. 2007, 12, 034036. [Google Scholar] [CrossRef]
- Di Prima, M.; Coburn, J.; Hwang, D.; Kelly, J.; Khairuzzaman, A.; Ricles, L. Additively manufactured medical products—The FDA perspective. 3D Print. Med. 2016, 2, 1. [Google Scholar] [CrossRef]
- Beitler, B.G.; Abraham, P.F.; Glennon, A.R.; Tommasini, S.M.; Lattanza, L.L.; Morris, J.M.; Wiznia, D.H. Interpretation of regulatory factors for 3D printing at hospitals and medical centers, or at the point of care. 3D Print. Med. 2022, 8, 7. [Google Scholar] [CrossRef]
- Food and Drug Administration, USA, Highlights of Prescribing Information—Spritam. 2015. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207958s000lbl.pdf (accessed on 15 October 2025).
- Velagaleti, R.; Burns, P.K.; Gill, M.; Prothro, J. Impact of current good manufacturing practices and emission regulations and guidances on the discharge of pharmaceutical chemicals into the environment from manufacturing, use, and disposal. Environ. Health Perspect. 2002, 110, 213–220. [Google Scholar] [CrossRef]
- Parramon-Teixido, C.J.; Rodríguez-Pombo, L.; Basit, A.W.; Worsley, A.; Cañete-Ramírez, C.; Alvarez-Lorenzo, C.; Cabañas-Poy, M.J.; Goyanes, A. A framework for conducting clinical trials involving 3D printing of medicines at the point-of-care. Drug Deliv. Transl. Res. 2025, 15, 3078–3097. [Google Scholar] [CrossRef]
- European Medicines Agency. Quality Innovation Group. 2024. Available online: https://www.ema.europa.eu/en/committees/working-parties-other-groups/chmp-working-parties-other-groups/quality-innovation-group (accessed on 15 October 2025).
- Alexander, A.E.; Wake, N.; Chepelev, L.; Brantner, P.; Ryan, J.; Wang, K.C. A guideline for 3D printing terminology in biomedical research utilizing ISO/ASTM standards. 3D Print. Med. 2021, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Török, G.; Gombocz, P.; Bognár, E.; Nagy, P.; Dinya, E.; Kispélyi, B.; Hermann, P. Effects of disinfection and sterilization on the dimensional changes and mechanical properties of 3D printed surgical guides for implant therapy—Pilot study. BMC Oral. Health 2020, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Rynio, P.; Galant, K.; Wójcik, Ł.; Grygorcewicz, B.; Kazimierczak, A.; Falkowski, A.; Gutowski, P.; Dołęgowska, B.; Kawa, M. Effects of Sterilization Methods on Different 3D Printable Materials for Templates of Physician-Modified Aortic Stent Grafts Used in Vascular Surgery-A Preliminary Study. Int. J. Mol. Sci. 2022, 23, 3539. [Google Scholar] [CrossRef] [PubMed]
- Pérez Davila, S.; González Rodríguez, L.; Chiussi, S.; Serra, J.; González, P. How to Sterilize Polylactic Acid Based Medical Devices? Polymers 2021, 13, 2115. [Google Scholar] [CrossRef]
- Abdella, S.; Youssef, S.H.; Afinjuomo, F.; Song, Y.; Fouladian, P.; Upton, R.; Garg, S. 3D Printing of Thermo-Sensitive Drugs. Pharmaceutics 2021, 13, 1524. [Google Scholar] [CrossRef]
- Peng, H.; Han, B.; Tong, T.; Jin, X.; Peng, Y.; Guo, M.; Li, B.; Ding, J.; Kong, Q.; Wang, Q. 3D printing processes in precise drug delivery for personalized medicine. Biofabrication 2024, 16, 032001. [Google Scholar] [CrossRef]
- Dumpa, N.; Butreddy, A.; Wang, H.; Komanduri, N.; Bandari, S.; Repka, M.A. 3D printing in personalized drug delivery: An overview of hot-melt extrusion-based fused deposition modeling. Int. J. Pharm. 2021, 600, 120501. [Google Scholar] [CrossRef]
- Rengarajan, V.; Clyde, A.; Pontsler, J.; Valiente, J.; Peel, A.; Huang, Y. Assessing Leachable Cytotoxicity of 3D-Printed Polymers and Facile Detoxification Methods. 3D Print. Addit. Manuf. 2023, 10, 1110–1121. [Google Scholar] [CrossRef]
- Gharehdaghi, N.; Nokhbatolfoghahaei, H.; Khojasteh, A. 4D printing of smart scaffolds for bone regeneration: A systematic review. Biomed. Mater. 2024, 20, 012003. [Google Scholar] [CrossRef]
- Serrano, D.R.; Luciano, F.C.; Anaya, B.J.; Ongoren, B.; Kara, A.; Molina, G.; Ramirez, B.I.; Sánchez-Guirales, S.A.; Simon, J.A.; Tomietto, G.; et al. Artificial Intelligence (AI) Applications in Drug Discovery and Drug Delivery: Revolutionizing Personalized Medicine. Pharmaceutics 2024, 16, 1328. [Google Scholar] [CrossRef]
- Pettersson, A.B.V.; Ballardini, R.M.; Mimler, M.; Li, P.; Salmi, M.; Minssen, T.; Gibson, I.; Mäkitie, A. Core Legal Challenges for Medical 3D Printing in the EU. Healthcare 2024, 12, 1114. [Google Scholar] [CrossRef]
- Annaratone, L.; De Palma, G.; Bonizzi, G.; Sapino, A.; Botti, G.; Berrino, E.; Mannelli, C.; Arcella, P.; Di Martino, S.; Steffan, A.; et al. Basic principles of biobanking: From biological samples to precision medicine for patients. Virchows Arch. 2021, 479, 233–246. [Google Scholar] [CrossRef]

| Broad 3D Printing Technology | Specific 3D Printing Technology | Drugs | Scaffold Material | 3D-Printer Commercial Name | Diseases Targeted + Delivery/Screening | Biological and Mechanical Properties | Release Profile | In Vitro | In Vivo | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Material Extrusion | FDM (Fused Deposition Modeling) | Dipyridamole (DIP) | Thermoplastic polyurethane (TPU) | Flashforge Creator Pro (Flashforge, China) | -Bone defects -Drug delivery system | -Shore hardness–80A -Moderate flexibility -Resistance to deformation -Non-degradable, good biocompatibility -Osseointegration -High cell viability and proliferation at DIP 10% w/w -High opacity -Compression modulus of 10 MPa | -Slow, sustained over 30 days -10% DIP, the release was ~90% (2888 µg), vs. 5% DIP, where the release was ~3% (58 µg) -No burst | MC3T3-E1 (murine pre-osteoblasts) | Not tested | [237] |
| Tetracycline | TPU + PVA porous filaments (PORO-LAY LAY-FOMM 60; LAY-FELT) | Flashforge Creator Pro (Flashforge, China) | -Bone defects -Drug delivery system | -High porosity -Release and absorption properties -High cell viability | Not specified | Staphylococcus aureus (cat# 470179-208), Pseudomonas fragi (cat# 470179-090); human ACL fibroblasts (from three patients) | Not tested | [134] | ||
| Nuciferine (NF) | PLA/CS-NF porous scaffold (PLA with chitosan-nuciferine) | Ultimaker 3 | -Bone defects -Drug delivery system | -Low molecular weight -75–85% deacetylation -Average pore size: 100–325 µm -Osteoblast differentiation | -Burst day 1, then sustained to 21 days; -68.5–71.8% cumulative by day 21 (dose-dependent 60–100 μM) | Mouse MSCs (C3H10T1/2) | Not tested | [238] | ||
| Prednisolone; Dexamethasone | PLA (Polylactic Acid) | Regemat3D (Spain) | -Bone defects -Drug delivery system | -Osteoinductivity | -Loaded filament scaffolds: <10% in first 2 weeks (sustained) -Post-print loaded: ~50% burst in first 6 h | Murine fibroblasts (CCL-163, ATCC); human bone marrow MSCs (hMSC; ATCC PCS-500-012) | Not tested | [239] | ||
| Minocycline | PLA scaffold coated with type-I collagen + citrate-hydroxyapatite nanoparticles (HA NPs) | BQ Hephestos i3 (Prusa-style) | -Osteomyelitis -Drug delivery system | -Compressive strength: ≈13 MPa -High Young’s modulus values -Low plasticity -Cell adhesion | -Burst within first hour, then gradual 4–24 h | Staphylococcus aureus; human bone-marrow-derived stromal cells (hMSCs) | Not tested | [153] | ||
| Gentamicin; Tobramycin; Nitrofurantoin | PMMA bone cement (Orthoset 3) or PLA pellets, silicone-oil coated | MakerBot Replicator (first gen) | -Osteomyelitis -Drug delivery system | Not mentioned | Not specified | Escherichia coli and Staphylococcus aureus | Not tested | [118] | ||
| Zoledronate | PLA/PCL + HA NPs (indirect: printed + freeze-dried) | ZMorph (indirect printing + freeze-drying) | -Bone defect -Drug delivery system | -Pore diameter ≈ 141.01 ± 48.25 µm -Wall thickness ≈ 27.51 ± 2.94 µm -Porosity ≈ 69–71% -Compressive modulus ≈ 0.6339 ± 0.03995 MPa -Weight loss ≈ 2 ± 2.5% at 35 days; -Slow degradation -Structural stability | Not specified | Not tested | Forty adult males (Wistar rats) | [240] | ||
| No drug (Plasmid DNA encoding microRNA-200c) | PCL scaffold coated with collagen/gelatin sponges | Regemat 3D + Facilan PCL 100 | -Bone defect -Drug delivery system | -Pore size 450 µm; -Porosity 55.75%; -Gelatin coating thickness ≈ 17.52 µm; -Basic pH gelatin slowed the release and improved regeneration | -Collagen/acidic gelatin: rapid over 72 h -Basic gelatin: slowed, sustained over 72 h -PCL + polymer coats mimic sponge controls -Burst vs. sustained, depending on polymer | Human preosteoblasts, embryonic palatal mesenchymal (HEPM; ATCC) | 12-week-old male Sprague–Dawley rats (N per group = 3–7) | [241] | ||
| Any drug | PLA/PMMA/PC microfluidic chips | Ultimaker 3 | -Osteosarcoma model; -Drug-screening platform | Not mentioned | No specific drug tested | SaOS-2 osteoblasts; human MSCs (hMSCs) | Not mentioned | [242] | ||
| Doxorubicin | PLA | Creality Ender 3 Pro | -Osteosarcoma model -Drug-screening platform | -The surface microstructure allowed the optimal development of spheroids and their immobilization | Not specified | Osteosarcoma lines 143B, MG63 (ATCC) | Not tested | [243] | ||
| 3D bioprinting | Icariin; Purmorphamine; PD98059; U0126 | Scaffold-free-spheroids (bone marrow-derived mesenchymal stem cells (BM-MSC)) | Regenova® (Cyfuse) | -Osteoporosis model -Drug-screening platform | -Icariin and Purmorphamine are osteoinductive drugs -Osteogenesis promoters -PD98059; U0126 are osteogenesis inhibitors | Not specified | Bone marrow MSCs at passage 4 (LifeNet Health) | Not tested | [224] | |
| Vancomycin | Methylcellulose + nanohydroxyapatite (aerogel ink) | Cellink BIO X (BIOX) | -Osteomyelitis -Drug delivery system | -Bone repair and antimicrobial activity -Optimal porosity -Textural stability after 7 months | -Burst first 8 h, then sustained to 72 h | Mouse embryo fibroblasts (NIH/3T3); MC3T3-E1 pre-osteoblasts | Artemia salina eggs; fresh fertilized hens’ eggs; tests in triplicate | [94] | ||
| Pioglitazone (PIO) | nano-attapulgite/PVA/gelatin | Not specified | -Bone defect -Drug delivery system | -High viscosity -Osteogenic differentiation -PIO clear absorbance: 238 nm -Minimal cytotoxicity | -PLGA nanospheres reduced burst; sustained to day 9 (with PNs), total ~32.5% ± 1.5% on day 9 -Without PNs ~41% ± 2.5% at day 5 | RAW 264.7 (mouse macrophage); mouse BMSCs (CRL-12424, ATCC); HUVECs | Sprague–Dawley rats, 3-month-old (N = 30) | [244] | ||
| SB216763 | PCL | Bioprinter DOME-PCI3D | -Osteoporosis model -Drug-screening platform | -Induces angiogenesis -Osteogenic differentiation (Wnt signaling) -High cell survival rate (91% after 7 days) | Not specified | HUVECs; murine ST2 stromal cell line | Not tested | [245] | ||
| Growth factor (VEGF) | PCL modified with amine groups (CP05) | 3D printer (Allcct, China) | -Bone defect -Drug-screening platform | -PCL molecular weight: 50 kDa -Printing temperature: 180 °C -PCL scaffold was optimal for the porous structure of the bone, in which new vessels (due to VEGF) started to form | -Controllable release (qualitative) | Rat BMSCs (rBMSCs) | Male Sprague–Dawley rats (N = 40) | [246] | ||
| Leonurine hydrochloride | Gelatin/alginate + nano-attapulgite | RegenHU 3D Discovery (microvalve) | -Bone defects with vascular impairment -Drug delivery system | -Good biocompatibility -Bone mineralization -Increased blood vessel formation -Tube formation and cellular migration (key elements for angiogenesis) | -Explosive within 72 h, then stabilized -~18% over 17 days (nanostructured scaffold) | BMSCs; HUVECs | Male Wistar rats, 8–10 weeks (N = 50). | [247] | ||
| Dexamethasone (DEX); Amphotericin B; Gentamicin | PCL + nano-HA + alginate/gelatin hydrogel | Two-nozzle pneumatic printer | -Osteomyelitis -Drug delivery system | -PCL-nHA high compressive modulus: 46.37 ± 2.58 MPa -PCL-nHA low compressive strength 4.51 ± 0.47 MPa -Dexamethasone microparticles (MPs-DEX) -spherical: 0.24–5.58 µm -Negative zeta potential of the drug microparticles | -Free MPs-DEX: 55.8% day-1 burst, ~90.4% by day 30. -MPs-DEX entrapped: ~7.0% day-1 then sustained to ~55.8% by day 30 | Human endometrial MSCs (hEnMSCs). | Not tested | [248] | ||
| Doxycycline (in DX/HAp/PCL NPs) | Gelatin + PVA + hyaluronic acid; integrated with DX/HAp/PCL NPs | Robota (dual extrusion) | -Osteomyelitis -Drug delivery system | -Osteoconduction; -Bioresorption and immune tolerance; -Pore sizes (90.4 ± 3.9 and 196.6 ± 38.8 µm); | -Wet-state scaffolds: 100% by 15 days -NPs alone: slower (to 24–28 days) -Biphasic 24-day profiles with method-dependent differences | Not tested | New Zealand white rabbits (N = 30) | [150] | ||
| Growth factor (BMP-2) | PCL + mesoporous calcium silicate NPs | BioScaffolder 3.1 (GeSiM) | -Orofacial bone repair -Drug delivery system | -BMP-2 did not affect the mechanical integrity of the PCL scaffold -Porosity: 65% -Pore sizes: 542 ± 13 µm -Good cell viability and adhesion | -Burst day 1 -Sustained, day 14 amount ~4× day 1 | Human Wharton’s Jelly MSCs (WJMSCs) | Not tested | [200] | ||
| BMP-2 (in ZIF-8 NPs) | PLGA + MBG + ZIF-8 | EnvisionTEC FDM bioprinter | -Bone defects -Drug delivery system | -Compressive strength: ≈2.9 MPa -Increased mechanical strength due to the addition of ZIF-8 -Osteoinductive activity | -Slow; -~40% by 72 h, ~57% by 174 h, continuing thereafter | MC3T3-E1 | Sprague–Dawley rats (N = 12). | [199] | ||
| Salvianolic acid B | Alginate/ε-polylysine | BioScaffolder 3.1 (GeSiM) | -Bone defects -Drug delivery system | -Angiogenesis promoter -Suppressed osteoclast activity -+146% bone mass | -~60% cumulative by day 80 | C3H10 cells | Female Sprague–Dawley rats, 10 weeks (N = 32) | [249] | ||
| Ibuprofen (IBU) | GelMA + ZIF-8 nanoparticles | Not specified | -Bone defects -Drug delivery system | Not mentioned | Not specified | MC3T3-E1 | Not tested | [194] | ||
| Model protein (Cytochrome C) | Sol–gel Ca-doped silica (TEOS/CaCl2 inks) | Cellink BIO X (mechanical extrusion) | -Bone regeneration -Drug delivery system | -Calcium increased the pore size (from 1.66 nm to 9.10 nm) -Brittle behavior at 120 N -Adsorption of Cytochrome C of 84% after 5 days -pH-controlled release -By increasing the calcium concentration, larger drug molecules can be released | -pH-dependent -No release at pH 7.5 -Initial burst (24 h) at pH ≤ 5.5, then fast to 72 h -Continued up to 28 d (21 days at pH 5.5). | Rat MSCs (rMSCs) | Not tested | [250] | ||
| Resveratrol (RVS); Strontium ranelate (SrRn) | PCL/hydrogel | Not stated | -Craniomaxillofacial bone defects -Drug delivery system | -Mandibular bone formation after 8 weeks in rats’ mandibular defects -Enhanced angiogenesis -Osteoclast inhibition | SrRn: >70% day 1 burst, then sustained ≥21 days -RVS: <30% over 21 days -Scaffolds are structurally stable over 21 days. | Mouse MSCs; human osteoclasts (from PB monocytes); HUVECs | Sprague–Dawley rats: bone defect only (N = 5), scaffolds only (N = 6), scaffolds + RVS + SrRn (N = 6) | [251] | ||
| 5-Fluorouracil | Calcium phosphate cement (CPC) | Bioprinter + Innotere | -Osteosarcoma -Drug delivery system | -Poor solubility of the anticancer drug -Shape fidelity and increased ultimate tensile strength | -Complete release within 2 h for all coated scaffolds | HeLa; HEK293T cell lines | Not tested | [252] | ||
| Parathormone (1-34)(PTH1-34); Simvastatin | GelMA + PLA | Sunp Biomaker Pro (Sunp Biotech) | -Osteoporosis -Drug delivery system | -Mechanical strength similar to natural bone -Stable biodegradation -Porosity rates were around 50% -Compressive modulus of GelMA-PLA: ≈40 MPa -Water absorption of GelMA-PLA: ≈255% | -Simvastatin: 6.29% initial, then gradual to day 19 -PTH1-34 minimal initial, cumulative peak ~day 40 (PLGA microspheres mediated) | MC3T3-E1. | 12-week-old female SD rats, sham (N = 3) and OVX (N = 21) | [253] | ||
| Water-based extrusion printing | (general drug-releasing) | PCL HIPE Pickering emulsions (PCL + PVA dispersed phase) | Not specified | -Osteosarcoma model -Drug screening platform | -Good cellular adhesion -Optimal pore morphology that resembles the natural bone | Not specified | MG63 osteoblasts | Not tested | [222] | |
| SDF-1; Y27632 | PU (Polyurethane) dispersion + collagen | Not specified | -Osteoarthritis -Drug delivery system | -Promotion of cartilage proliferation -Cell free scaffold -Stromal cell-derived factor attracted mesenchymal stem cells from the surroundings, which helped in cartilage development | -Y27632 burst at 12 h increases with loading (12–30%) -Then, ~0.10–0.19%/h -Scaffold releases reach effective ~10 µM by 48–62 h | Human bone marrow MSCs (hMSCs) | Adult male New Zealand white rabbits; independent triplicate runs | [254] | ||
| Melt-extrusion | Ciprofloxacin (CFX); Gentamicin | copolymer PEOT/PBT (poly(ethylene oxide terephthalate)/poly(butylene terephthalate)) | Not specified | -Osteomyelitis -Drug delivery system | -Interconnected pore network -High porosity -Load-bearing mechanics | -CFX/MgAl: sustained ~1 month -GTM/ZrP: burst <24 h, then slow -Kinetics: Stage I (modified Freundlich), Stage II diffusion-limited; incomplete total release due to polymer diffusion limits | Staphylococcus epidermidis; Pseudomonas aeruginosa; human MSCs (hMSCs) | Not tested | [106] | |
| Micro-extrusion | Ciprofloxacin | Methacrylated silk fibroin (SF-MA) + HMSC-MA silica capsules (polymer–silica aerogel) | Micro-extrusion system (name not specified) | -Osteomyelitis -Drug delivery system | -Large bacteriostatic rings -Osteoinductive -Osteogenic | -Burst first 4 h; ~35% by 72 h (most), up to ~50% with higher HMSC content due to high loading (95%) and mesopores | Staphylococcus aureus (ATCC 29213); Escherichia coli (K-12 DH5α); MC3T3-E1; human MSCs | Not tested | [109] | |
| Melt Electrowriting (MEW) + FDM (hybrid) | —(BMP-2 in PLGA MicroPs as cargo) | PCL mesh + PLGA microparticles (InductOs BMP-2 formulation) | MEW (outer) + FDM (core) | -Critical-sized bone defects -Drug delivery system | -PLGA allowed the controlled release of rhBMP-2 -Limited ectopic bone growth | -15-day study -Most rhBMP-2 released within 24 h -High-dose MPs ~73% vs. low-dose ~69%; -Amidation did not alter MPs’ kinetics | Human periosteum-derived MSCs (hPMSCs) | Female Sprague–Dawley rats, 8–12 weeks; groups ≥6 animals; randomized double-blind; doses: 0.2–1.2 µg rhBMP-2 | [255] | |
| Melt Electrohydrodynamic Printing | Roxithromycin | PCL/PEG | Custom system | -Osteomyelitis -Drug delivery system | -The addition of PEG increased the hydrophilicity of the scaffold -Increased wettability -Increased cellular proliferation | ->70% within 6 h (burst), then slow to 24 h -PEG increased early release (0–15% PEG: 72–88% at 6 h; 76–91% at 24 h) | Escherichia coli; Staphylococcus aureus; MG63 cells | Not tested | [158] | |
| Modified/Hybrid/Custom extrusion | Aspirin | PCL | Custom system | -Bone defect -Drug delivery system | Not mentioned | -Sustained release | Human MSCs (hMSCs) | Not tested | [256] | |
| Vat Photopolymerization | SLA (Stereolithography) | Cisplatin | DS-3000 biocompatible resin (DWS) | DWS 028J+, Italy | -Osteosarcoma model -Drug screening model | -Osteosarcoma cells were taken from a patient with residual cancerous cells (HBCx-66) -Pore sizes: 100–500 µm -Young’s modulus: ≈ 2.45 GPa | -Initial burst, then sustained >15 days -Release coupled to PLGA-PEG-PLGA hydrogel degradation within 3D-printed implant | Bone marrow MSCs; breast cancer PDX cells; osteosarcoma (143B, HOS, MG63) | 5-week-old female BALB/c nude mice; 5 groups, N = 5/group | [233] |
| Bioreactor to test candidate drugs | Somos WaterShed XC11122 resin; agarose porous medium; GelMA hydrogel scaffold | 3D Systems Viper si2 | -Osteoarthritis model -Drug screening platform | -Fluid velocity inside the bioreactor is tunable to test the drug’s elution -Hydraulic resistance | Not specified | Not tested | Not tested | [257] | ||
| DLP (Digital Light Processing) | Vancomycin | GelMA with Zn-MOF | CELLINK Lumen XTM DLP bioprinter | -Osteomyelitis -Drug delivery system | -Compressive modulus 52.14 ± 19.42 kPa -If ZIF-8 nanoparticles are added, the compressive modulus increases to 128.13 ± 19.46 kPa -Enhanced cell adhesion, migration, and growth | -Sustained over 48 h -GelMA ~93% and GelMA/ZIF-8 ~88% cumulative | Escherichia coli DH5α; human adipose-derived stem cells (hASCs) | Not tested | [86] | |
| Deferoxamine (DPO) liposomes | TCP (tricalcium phosphate) + GelMA | AUTOCERA-M (Beijing, China) | -Bone defect -Drug delivery system | -GelMA microspheres diameter: 200 ± 30 µm -Porosity 64 ± 5% -Polydispersity index 0.19 ± 0.03 -Bioceramic compressive strength: 2.2 ± 0.2 MPa -Suitable for non-load-bearing applications | -Pure DFO liposomes: 67.6% in 6 h -Scaffold-loaded DFO: ~36% at 6 h; ~69% by 7 days -Total in vitro 48 h for pure liposomes | Rat MC3T3-E1 | Rats divided into control, β-TCP, and TGL groups (N = 10 each; total N = 30). | [258] | ||
| High-res Photopolymerization | Human parathyroid hormone (1-34 PTH) | XS-BIO resin; silver-coated syringe tip & steel counter-electrodes (electrodes as metals) | Miro P213 (Xingsheng) | -Osteoporosis -Drug delivery system | -No fracture in the microchannels at compressive loads of 40 N -No deformation for 20 s at forces of 20 N | Not specified | HUVECs | Female Sprague–Dawley rats (N = 30); OVX (N = 25) and sham (N = 5) | [259] | |
| Photo-crosslinking-assisted printing | Sorafenib | Methacrylated silk fibroin (SF-MA) + cetyltrimethylammonium bromide (CTAB); Ti3C2Tₓ MXene | Photo-crosslinking SF-MA gel setup | -Osteosarcoma model -Drug screening platform | -Controlled pore sizes -MXene increased the photothermal conversion -Laser irradiation was used to increase the scaffolds’ temperature and to favor the release of sorafenib | -pH- and laser-responsive -At pH 7: 76% at 5 h; pH 4.3: 18% at 5 h -Acidic + laser: 35% at 5 h (vs 18% without) | MC3T3-E1; MG-63 | Not tested | [260] | |
| Powder Bed Fusion (PBF) | SLS (Selective Laser Sintering) | Zoledronate | PLLA + mesoporous silicon (SBA15NH2) | Home-made SLS system | -Osteosarcoma -Drug delivery system | -In the acidic pH, the release is approximately two times higher, a key aspect for localized treatment of osteosarcoma or osteoporosis | -Initial burst phase release -Fast first 24 h, then slow -Acidic pH: 16.7% (24 h) + 21.7% later -Neutral pH: 9.6% (24 h) + 11.7% later | MG-63 (osteosarcoma); RAW264.7 (mouse macrophage/osteoclast precursor) | Not tested | [184] |
| SLM (Selective Laser Melting) | Doxorubicin; indoleamine 2,3-dioxygenase inhibitor | Ti6Al4V + Zn(NO3)2·6H2O; Metal-organic-framework (MW) composite | Concept Laser M2 | -Osteosarcoma -Drug delivery system | -High biocompatibility -Corrosion resistance -Bone-like elastic modulus -Microwave thermal sensitivity | -Acidic pH 5.5 + MW: 61.6% at 24 h; acidic no MW: 39.5% -Neutral pH 7.4: 27.2% (no MW) or 33.9% (with MW) | Murine OS K7M2; rBMSCs | BALB/c mice (male, 4–5 weeks) and male SD rats (numbers not specified) | [168] | |
| Strontium ranelate | Tannic Acid (TA) coated with PEG hydrogel | TRUMPF TruPrint 1000 | -Osteoporosis model -Drug screening model | -The PEG coating increased the titanium crosslinkability -Optimal biodegradability -The release was diffusion-based, and it was slower than in other materials, given that the tannic acid scaffold is denser | -TA 20% → ~90% release in 2 days -TA 80% → ~50% release in 15 days | L929 mouse fibroblasts | Not tested | [261] | ||
| Ethyl-2,5-Dihydroxibenzoate | Titanium coated with PLGA | Winforsys Metasys250 | -Osteoporosis -Drug delivery system | -Inhibition of bone resorption -Promoter of bone formation -PLGA coating further stimulated the bone formation | Not specified | MC3T3-E1 mouse pre-osteoblasts | Female Sprague–Dawley rats (N = 20; 3 months old) | [262] | ||
| Zoledronic acid (ZA) | JDBM Mg-Nd-Zn-Zr alloy + ceramic coating | ZRapid Tech SLM150 | -Osteoporosis -Drug delivery systems | -Porosity: 80% -Pore-diameter: 600 µm -The ceramic coating reduced the biodegradability to ≈5 mg/day -Corrosion resistance | -Slow, controllable -~8% in first 3 days -~54% by 30 days (release driven by coating degradation) | Rat BMSCs (rBMSCs); mouse BMMs (bone-marrow macrophages) | Female Sprague–Dawley rats, 3-month-old (N = 60) | [185] | ||
| EBM (Electron Beam Melting) | Paclitaxel | Titanium + PEG | Q10plus, GE, SUA | -Osteosarcoma -Drug delivery system | -In the acidic environment, the osteosarcoma cells were successfully killed | -pH-responsive: ~11% at 72 h (pH 7.4) -~85% at 72 h (pH 6.5) -Stable at 7.4, fast intracellular release at 6.5 -The releases were controlled for over 1 month | 143B, HOS, MG63; SaOS2, U2OS | BALB/c nude mice (N = 30), 4 groups, n = 6/group | [263] | |
| Cisplatin | Ti6Al4V + PLGA-PEG-PLGA hydrogel | Arcam S12 (Molndal/Gothenburg, Sweden) | -Osteosarcoma -Drug delivery system | -Low elastic modulus -Low stiffness -Good osteoinduction -Large drug-loading due to the porous structure | -Initial burst then sustained >15 days -Release matched hydrogel degradation -Some hydrogel detachment observed | Osteosarcoma 143B, HOS, MG63 | BALB/c nude mice, 5 groups (N = 5/group) | [162] | ||
| Simvastatin | Porous Ti6Al4V (3DTi) + hydrogel | Arcam S12 (Molndal/Gothenburg, Sweden) | -Bone defect (osteosarcoma) -Drug delivery system -In vitro and in vivo (rabbit condyles) | -The tumor volume reduced by 59–77% without any other damage -The release was sustained for 18 days -Anti-osteosarcoma and osteogenic effects | -Continuous release up to 18 days aligned with hydrogel degradation | 143B (human OS); L929 (mouse fibroblasts) | Six-week-old female BALB/c nude mice; 3 groups (n = 6 each). | [264] | ||
| Binder Jetting | Binder Jet 3D Printing | Curcumin loaded with Magnesium | β-TCP (β-tricalcium phosphate) | ExOne (Irwin, PA) | -Osteosarcoma -Drug delivery system | -In vivo osseointegration; -In vitro chemoprevention; -High cell viability; | -30 days: pH 7.4 ~22% (TCP) vs. ~17% (Mg-TCP); pH 5.0 ~30% (TCP) vs. ~23% (Mg-TCP) -Weibull fits R2 > 0.9 | THP1-derived osteoclasts; hFOB; MG-63; S. aureus; P. aeruginosa | Sprague–Dawley rats (supplier Envigo, numbers not specified) | [203] |
| Gingerol; Allicin | β-TCP | ExOne | -Osteoporosis -Drug delivery system | -Porosity: 50% -Antimicrobial activity -Ginger promoted osteoblast proliferation by over 50% -Reduced osteoclast activity -Porous core with dense exterior with 10 MPa compressive strength -Bone healing after 10 weeks | -Allicin sustained ~2 days (35–45%); gingerol release higher with PEG 50:50 vs. 65:35 | Human fetal osteoblasts (hFOB); osteoclasts (THP1-derived) | Sprague–Dawley rats (N = 20) | [204] | ||
| Quercetin; Vitamin D3 (lipid NPs) | β-TCP | ExOne | -Osteosarcoma -Drug delivery system | -Compressive strength around 10 MPa; -Osteoblast proliferation -Osteoclast inhibition -Antimicrobial activity against Staphylococcus aureus and Pseudomonas aeruginosa | -Biphasic; method- and pH-dependent; up to ~60 days -Power-law kinetics (R2 > 0.99) -↑ Acidic pH release -Above 80% release rate at pH 7.4 -Controlled release for 60 days | hFOB; THP-1-derived osteoclasts; MG-63; P. aeruginosa; S. aureus | Not tested | [197] | ||
| Curcumin + Piperine (coated with Carvacrol) | β-TCP | ProMetal® (ExOne) | -Osteosarcoma -Drug delivery system | -Compressive strength of 12.3 MPa -Pore diameter: 400 µm | -Biphasic; method- and pH dependent; -Up to ~60 days Power-law kinetics (R2 > 0.99) -↑ Acidic pH release -In combination with piperine, the curcumin availability increases to over 2000% -94% antibacterial activity -Cumulative release of 80% for curcumin and 90% piperine -Controlled release for 60 days | hFOB (passage 6); MG-63 (passage 6); Staphylococcus aureus | Not tested | [265] | ||
| Gingerol | β-TCP | ExOne Innovent+ | -Osteosarcoma -Drug delivery system | -Antioxidant properties -Tumor necrosis factor suppression -No cytotoxicity | -In a very acidic environment (pH 5.0), there is an initial burst release of 16% compared to 12% in pH 7.4 | hFOB (ATCC CRL-11372); MG-63; Staphylococcus aureus | Not tested | [205] | ||
| Retinol (vitamin A) | β-TCP scaffold with PCL/PEG coating | ExOne powder-bed printer | -Bone defect -Drug delivery scaffold | -Bulk density: ≈1.0 g/cm−3 -Porosity: 50% -Compressive strength: 5.1 ± 1.1 MPa | -Full release by 1 day at pH 5 -~50% plateau by 7 days at pH 7.4; Weibull fits reported; | hFOB; THP-1-derived osteoclasts | Not tested | [266] | ||
| Vitamin D3 | β-TCP scaffold with PCL/PEG coating | ExOne | -Bone defect -Drug delivery scaffold | -Pore diameter: 350 µm -Porosity: 48.9 ± 2.54% -Compressive strength: 7.0 ± 0.66 MPa -Relative density: 32.2 ± 1.23% -Osteoblast proliferation was enhanced by 64% compared to traditional osteoinductive methods | -3D scaffolds > porogen scaffolds -After 60 days in PBS: 3D-printed model released ~47.5% vs. porogen ~37.5% | hFOB | Not tested | [196] | ||
| Sitafloxacin; Rifampin | α-TCP/HA; PLGA coating | Modified ZPrinter 450 (3D Systems) | -Osteomyelitis -Drug delivery system | -Reduced bacterial colonization -Improved mechanical robustness -The PLGA coating increased the Young’s modulus by three times, and the maximum stress by 4.4 times | ->95% in 12 h (burst). PLGA-coated: biphasic -Initial burst then ~zero-order for 2 weeks (dose-dependent) | Not tested | Female Balb/cJ mice (13–15 weeks) | [76] |
| Drug Class | Main 3D Printing Techniques | Release Profile | Biocompatibility/Biological Response | References |
|---|---|---|---|---|
| Antibiotics |
|
|
| [76,86,94,106,109,118,134,150,153,248] |
| Anti-inflammatory |
|
|
| [194,239,248,256] |
| Anticancer |
|
|
| [162,168,184,185,233,240,243,252,259,263] |
| Other drugs and growth factors |
|
|
| [196,197,199,200,204,205,249,259,261,265,266] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timofticiuc, I.-A.; Grigore, A.-G.; Tomescu, E.-T.; Vlaicu, T.-M.; Dragosloveanu, S.; Scheau, A.-E.; Caruntu, A.; Dragosloveanu, C.D.M.; Badarau, I.A.; Didilescu, A.C.; et al. Advances in 3D-Printed Drug Delivery and Screening Platforms for Bone Disease Therapy. Pharmaceutics 2025, 17, 1372. https://doi.org/10.3390/pharmaceutics17111372
Timofticiuc I-A, Grigore A-G, Tomescu E-T, Vlaicu T-M, Dragosloveanu S, Scheau A-E, Caruntu A, Dragosloveanu CDM, Badarau IA, Didilescu AC, et al. Advances in 3D-Printed Drug Delivery and Screening Platforms for Bone Disease Therapy. Pharmaceutics. 2025; 17(11):1372. https://doi.org/10.3390/pharmaceutics17111372
Chicago/Turabian StyleTimofticiuc, Iosif-Aliodor, Alex-Gabriel Grigore, Elena-Teodora Tomescu, Teona-Maria Vlaicu, Serban Dragosloveanu, Andreea-Elena Scheau, Ana Caruntu, Christiana Diana Maria Dragosloveanu, Ioana Anca Badarau, Andreea Cristiana Didilescu, and et al. 2025. "Advances in 3D-Printed Drug Delivery and Screening Platforms for Bone Disease Therapy" Pharmaceutics 17, no. 11: 1372. https://doi.org/10.3390/pharmaceutics17111372
APA StyleTimofticiuc, I.-A., Grigore, A.-G., Tomescu, E.-T., Vlaicu, T.-M., Dragosloveanu, S., Scheau, A.-E., Caruntu, A., Dragosloveanu, C. D. M., Badarau, I. A., Didilescu, A. C., Caruntu, C., & Scheau, C. (2025). Advances in 3D-Printed Drug Delivery and Screening Platforms for Bone Disease Therapy. Pharmaceutics, 17(11), 1372. https://doi.org/10.3390/pharmaceutics17111372














