Integrating Graphene Oxide and Mesenchymal Stem Cells in 3D-Printed Systems for Drug Delivery and Tissue Regeneration
Abstract
1. Introduction
2. Methodology
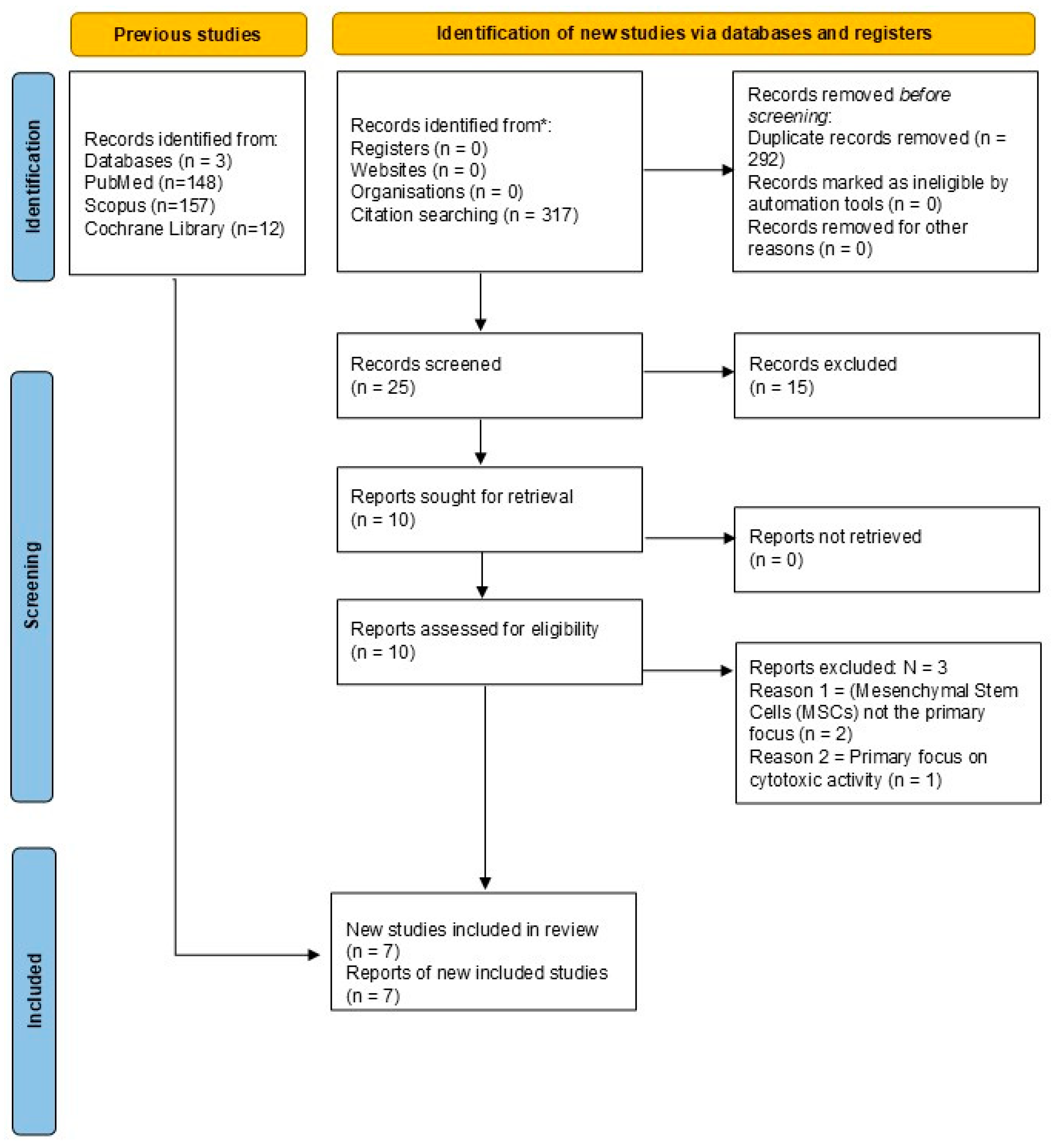
2.1. Databases and Search Strategy
- MEDLINE-PubMed: ((“Mesenchymal Stem Cells” [Mesh] OR “Mesenchymal Stem Cells” [tiab]) AND (“Graphene Oxide” [Mesh] OR “Graphene Oxide” [tiab] OR “GO” [tiab]) AND (“Regenerative Medicine” [Mesh] OR “Regenerative Medicine” [tiab] OR “Tissue Engineering” [tiab])) AND (2018:2025 [dp]);
- SCOPUS: TITLE-ABS-KEY (“Mesenchymal Stem Cells”) AND TITLE-ABS-KEY (“Graphene Oxide” OR GO) AND TITLE-ABS-KEY (“Regenerative Medicine” OR “Tissue Engineering”);
- Cochrane Library: (Mesenchymal Stem Cells): ti,ab,kw AND (Graphene Oxide OR GO): ti,ab,kw AND (Regenerative Medicine OR Tissue Engineering): ti,ab,kw.
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Collection
3. Literature Review
3.1. Properties of Mesenchymal Stem Cells (MSCs)
3.1.1. Differentiation
3.1.2. Immunomodulation
3.1.3. Angiogenesis
3.1.4. Secretome
3.1.5. Tissue Regeneration and Repair
3.1.6. Interaction with Stem Cells and Niches
3.1.7. Biological Functions and Cell Signaling of MSCs
3.1.8. Growth Factors, Cytokines, and Biologically Active Molecules
Growth Factors
- Platelet-derived growth factor (PDGF) stimulates cell proliferation and migration, playing a key role in wound healing and tissue regeneration [32].
- Vascular endothelial growth factor (VEGF) promotes angiogenesis—the formation of new blood vessels—which is essential for tissue repair and regeneration [33].
- Transforming growth factor beta (TGF-β) regulates cell proliferation, differentiation, and extracellular matrix production. It plays a crucial role in fibrogenesis and modulation of inflammatory processes [34].
- Epidermal growth factor (EGF) stimulates cell proliferation and differentiation and participates in tissue repair and wound healing [35].
- Insulin-like growth factor 1 (IGF-1) supports cell survival, growth, and differentiation and contributes to bone and cartilage regeneration [36].
- Fibroblast growth factor (FGF) is involved in tissue regeneration and angiogenesis by promoting cell proliferation and differentiation [37].
- Bone morphogenetic protein 2 (BMP-2) plays a fundamental role in cell differentiation and tissue remodeling. Produced by chondrocytes, it promotes cartilage and bone formation and has emerged as a promising agent for cartilage repair and chondrogenesis. However, its anabolic activity may also lead to aggrecan degradation and cartilage alterations, reflecting the complex nature of its cellular effects. Studies have shown that intra-articular administration of BMP-2 can induce osteophyte formation, indicating both regenerative and adverse effects [38]. BMP-2 interacts with matrix-degrading enzymes, influencing extracellular matrix homeostasis. A comprehensive understanding of BMP-2-mediated intracellular signaling is essential to harness its therapeutic potential while minimizing adverse outcomes [39].
- Bone morphogenetic protein 4 (BMP-4), a member of the TGF-β superfamily, was initially identified in bone extracts. It shows therapeutic potential in treating long bone fractures, osteoarthritis, rheumatoid arthritis, and cartilage regeneration. In vitro, BMP-4 supports the self-renewal of mouse embryonic stem cells (ESCs) when combined with leukemia inhibitory factor (LIF). Additionally, it has been used as a serum substitute for the expansion of human ES and iPS cells. Nevertheless, its application requires precise dosing and ethical considerations, highlighting the need for careful clinical use [40].
- Bone morphogenetic protein 7 (BMP-7) is another key member of the TGF-β family and is crucial for MSC differentiation. It is known to induce osteogenesis and promote bone tissue formation. It also contributes to maintaining MSC pluripotency and is involved in adipogenesis. During embryonic development, BMP-7 plays a central role in tissue and organ formation. Beyond its classical functions, BMP-7 modulates inflammatory responses and participates in tissue repair, making it a candidate for therapeutic strategies. However, its effects depend heavily on the cellular microenvironment and culture conditions [41].
- FGF-2 and VEGF: Fibroblast growth factor 2 (FGF-2) and vascular endothelial growth factor (VEGF) are both involved in regulating MSC differentiation. FGF-2, also known as basic FGF (bFGF), promotes proliferation and can guide MSCs toward specific lineages. VEGF is primarily associated with angiogenesis but also plays a role in MSC fate determination. Its expression varies depending on external stimuli and pathological conditions. The synergistic interaction of FGF-2, VEGF, and environmental factors critically influence MSC behavior in various tissues [42].
Cytokines
- Interleukin-6 (IL-6) is a pleiotropic cytokine that regulates inflammation and modulates immune response. It can act as either pro-inflammatory or anti-inflammatory, depending on the context [43].
- Interleukin-10 (IL-10) is an anti-inflammatory cytokine that plays a central role in immune regulation, supporting the balance between inflammation and regeneration [44].
- Tumor necrosis factor alpha (TNF-α) is a key regulator of inflammation with both pro-inflammatory and regenerative properties, depending on the tissue context [45].
Biologically Active Molecules
- Prostaglandins are lipid-derived signaling molecules synthesized from arachidonic acid which are involved in inflammation modulation and injury response [46].
- Matrix metalloproteinases (MMPs) is a family of enzymes that degrade extracellular matrix components, facilitating tissue remodeling and wound healing [47].
- Soluble cytokines include chemokines, which recruit inflammatory cells to injury sites and orchestrate local immune responses [48].
- Exosomes are nano-sized extracellular vesicles secreted by MSCs that contain proteins, lipids, and RNAs. They mediate intercellular communication and influence immune and inflammatory responses [49].
- Wnt-3, a canonical Wnt signaling pathway activator, is highly expressed in the dorsal midline region and is essential for spinal cord development. During vertebrate embryogenesis, it regulates self-renewal, proliferation, differentiation, and cell motility. Its activation governs cell fate decisions and plays a pivotal role in directing MSC differentiation into specific lineages [50].
- Wnt-4 is one of the most studied ligands of the Wnt family, which comprises 19 genes involved in stem cell regulation, development, and oncogenesis. Wnt-4 plays a key role in organogenesis, including sex determination and mammary gland development. Its dysregulation is linked to reduced bone mineral density, premature skeletal aging, endometriosis, and gynecological cancers. Wnt-4 can activate both β-catenin-dependent and independent pathways, acting as either an activator or suppressor, depending on the context. This functional duality highlights the need for further investigation into its role in MSC differentiation [51].
- Transforming growth factor beta (TGF-β) is ubiquitously expressed and essential for normal development and tissue homeostasis. There are three isoforms—TGF-β1, TGF-β2, and TGF-β3—which signal via binding to TGFBR2, triggering phosphorylation of TGFBR1. This activates SMAD2/3, which complexes with SMAD4 and translocates to the nucleus to regulate gene expression. This canonical signaling cascade is fundamental to controlling proliferation, differentiation, migration, and apoptosis. TGF-β plays an essential role in embryonic development and in maintaining adult tissue equilibrium [52].
- β-catenin is the central mediator of the canonical Wnt/β-catenin signaling pathway, and its cytosolic concentration is tightly regulated. In the absence of Wnt stimulation, cytosolic β-catenin is actively phosphorylated by a destruction complex comprising adenomatous polyposis coli (APC), Axin, glycogen synthase kinase 3β (GSK3β), casein kinase 1α (CK1α), and protein phosphatase 2A (PP2A). Within this complex, APC and Axin function as scaffolding proteins, facilitating β-catenin phosphorylation at Ser45 by CK1α and at Ser33, Ser37, and Thr41 by GSK3β. These phosphorylation events trigger ubiquitination and proteasomal degradation of β-catenin. Consequently, under basal conditions, β-catenin levels are maintained at a minimum. Upon Wnt stimulation, this phosphorylation cascade is disrupted, preventing β-catenin degradation. As a result, β-catenin accumulates in the cytosol and subsequently translocates into the nucleus, where it interacts with transcription factors to activate the expression of target genes involved in cell proliferation, differentiation, and survival. This tightly regulated mechanism underscores the pivotal role of β-catenin in mesenchymal stem cell (MSC) fate determination, highlighting its importance in orchestrating molecular pathways central to stem cell biology [53].
Wnt/β-Catenin Pathway
3.2. Graphene Oxide (GO)-Based Nanomaterials
3.2.1. Properties of Graphene Oxide
3.2.2. Advantages of Poly(L-Lactic Acid) (PLLA)
3.2.3. PLLA/GO Combination
Enhanced Scaffolds
Scaffold Fabrication Methodologies for GO/PLLA Composites
Drug Delivery
Cellular Stimulation
3.3. Applications for Regenerative Therapy
3.3.1. Bone Regeneration
3.3.2. Soft Tissue Regeneration
3.3.3. Combination Therapies
3.4. Properties and Production of Graphene Oxide
3.5. Cytotoxic Activity of Graphene Oxide-Based Nanomaterials
3.5.1. Factors Influencing Cytotoxicity
Particle Size and Shape
Degree of Oxidation and Functionalization
Dose and Concentration
Exposure Time
Cell Type
Cytotoxic Mechanisms
Studies and Examples
4. Results and Discussion
4.1. Molecular Mechanisms and Signaling Pathways of Graphene Oxide-Mediated MSC Modulation in 3D Systems
4.2. Impact of 3D Printing Parameters and Scaffold Design on Graphene Oxide–MSC Interactions
4.2.1. Specific 3D Printing Methods and Their Implications for GO–MSC Interactions
4.2.2. Material Combinations with Graphene Oxide in 3D Printing
4.2.3. Architectural Design Parameters and Their Influence on GO–MSC Interactions
5. Gaps in the Literature and Future Directions
5.1. Gaps in the Literature
5.2. Future Directions
Emerging Alternatives and Comparative Perspectives in Scaffold Design
5.3. Safety and Biocompatibility of Graphene Oxide in 3D Systems: Current Insights and Risk Mitigation
Key Factors Influencing Graphene Oxide Biocompatibility and Toxicity In Vivo
5.4. Translational Challenges and Regulatory Landscape for Graphene Oxide-Based Scaffolds
5.4.1. Emerging Regulatory Frameworks for Nanomaterials in Healthcare
5.4.2. Commercial Challenges and Market Adoption
5.4.3. Preclinical and Clinical Trial Landscape
- In Vivo Efficacy Studies: These involve using relevant animal models (e.g., rodents or large animals like pigs or sheep for orthopedic applications) to evaluate the scaffold’s ability to promote tissue regeneration, integrate with host tissue, and restore function [5]. These studies must mimic the human disease state as closely as possible, with a particular emphasis on assessing not just tissue formation but its functional integration and long-term stability within the native biological environment.
- Long-Term Safety Studies: Beyond acute toxicity, studies assessing biodistribution, degradation, and potential long-term systemic or local toxicities over extended periods (e.g., from 6 months to 1 year or more) are critical, including the sustained viability and function of MSCs within the implanted constructs [102].
- Immunogenicity and Biocompatibility: Comprehensive evaluation of immune responses, inflammatory reactions, and foreign body responses to the implanted scaffold, clarifying both acute and chronic immune responses that might influence the long-term viability of the MSCs and the successful integration of the scaffold are required [81].
- Good Laboratory Practice (GLP): All preclinical studies intended for regulatory submission must adhere to GLP guidelines to ensure data quality, integrity, and reproducibility [50].
- Safety Data: The biggest hurdle is establishing long-term safety, especially given the concerns surrounding nanomaterials and potential persistence or degradation products, which directly impact long-term MSC viability in vivo.
- Efficacy in Humans: Demonstrating robust and reproducible efficacy in complex human physiological environments, which are often different from animal models, particularly regarding the long-term functional integration of engineered tissues and the sustained therapeutic effect of MSCs, is a significant challenge.
- Trial Design: Designing rigorous clinical trials with appropriate endpoints, patient cohorts, and control groups is complex and resource-intensive.
- Ethical Considerations: Especially for cell-based combination products, ethical considerations related to stem cell use are paramount.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| AKI | Acute kidney injury |
| AMSC | Adipose-derived mesenchymal stromal cell |
| APC | Adenomatous polyposis coli |
| BUN | Blood urea nitrogen |
| BMP-2 | Bone morphogenetic protein 2 |
| BMP-4 | Bone morphogenetic protein 4 |
| BMP-7 | Bone morphogenetic protein 7 |
| CK1α | Casein kinase 1 alpha |
| Cr | Creatinine |
| ESCs | Embryonic stem cells |
| MSCs | Mesenchymal stem cells |
| DMEM | Dulbecco’s Modified Eagle Medium |
| EGF | Epidermal growth factor |
| EMT | Epithelial–mesenchymal transition |
| ROS | Reactive oxygen species |
| FGF | Fibroblast growth factor |
| FGF-2 | Fibroblast growth factor 2 |
| FoxO1 | Forkhead box O1 |
| GO | Graphene oxide |
| GSK3β | Glycogen synthase kinase 3 beta |
| hUC-MSC | Human umbilical cord-derived mesenchymal stem cell |
| IGF-1 | Insulin-like growth factor 1 |
| IL-10 | Interleukin-10 |
| IL-6 | Interleukin-6 |
| JNK | c-Jun N-terminal kinase |
| ECM | Extracellular matrix |
| MnSOD | Manganese superoxide dismutase |
| MMP | Mitochondrial membrane potential |
| MMPs | Matrix metalloproteinases |
| MSC | Mesenchymal stem cell |
| PEG | Polyethylene glycol |
| PCL | Polycaprolactone |
| PDGF | Platelet-derived growth factor |
| PLLA | Poly(L-lactic acid) |
| PP2A | Protein Phosphatase 2A |
| SLA | Sandblasted, large-grit, acid-etched |
| SMC | Smooth muscle cells |
| TAZ | Transcriptional coactivator with PDZ-binding motif |
| TCF/LEF | T-cell factor/lymphoid enhancer-binding factor family transcription factor |
| TGF-β | Transforming growth factor beta |
| TNF-α | Tumor necrosis factor alpha |
| VEGF | Vascular endothelial growth factor |
| Wnt-3a | Wnt family protein, subtype 3a |
| WNT-4 | Wnt family protein, subtype 4 |
References
- Hu, C.; Li, L. Preconditioning Influences Mesenchymal Stem Cell Properties in Vitro and in Vivo. J. Cell. Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef]
- Luo, M.; He, N.; Xu, Q.; Wen, Z.; Wang, Z.; Zhao, J.; Liu, Y. Roles of Prostaglandins in Immunosuppression. Clin. Immunol. 2024, 265, 110298. [Google Scholar] [CrossRef]
- Wang, L.-T.; Liu, K.-J.; Sytwu, H.-K.; Yen, M.-L.; Yen, B.L. Advances in Mesenchymal Stem Cell Therapy for Immune and Inflammatory Diseases: Use of Cell-Free Products and Human Pluripotent Stem Cell-Derived Mesenchymal Stem Cells. Stem Cells Transl. Med. 2021, 10, 1288–1303. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; An, Y.; Zhu, T.; Tang, S.; Huang, X.; Li, S.; Fu, F.; Chen, J.; Xuan, K. Mesenchymal Stem Cells: Emerging Concepts and Recent Advances in Their Roles in Organismal Homeostasis and Therapy. Front. Cell. Infect. Microbiol. 2023, 13, 1131218. [Google Scholar] [CrossRef]
- Chaudhary, D.; Trivedi, R.N.; Kathuria, A.; Goswami, T.K.; Khandia, R. Munjal Vitro And In Vivo Immunomodulating Properties of Mesenchymal Stem Cells. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 59–68. [Google Scholar] [CrossRef]
- Jung, S.-C.; Park, S. New Sources, Differentiation, and Therapeutic Uses of Mesenchymal Stem Cells 2.0. Int. J. Mol. Sci. 2023, 24, 3938. [Google Scholar] [CrossRef] [PubMed]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular Matrix: A Dynamic Microenvironment for Stem Cell Niche. Biochim. Biophys. Acta 2014, 1840, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Novoseletskaya, E.S.; Evdokimov, P.V.; Efimenko, A.Y. Extracellular Matrix-Induced Signaling Pathways in Mesenchymal Stem/Stromal Cells. Cell Commun. Signal 2023, 21, 244. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D Bioprinting Technology for Tissue/Organ Regenerative Engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Cao, T.; Rodríguez-Lozano, F.J.; Luong-Van, E.K.; Rosa, V. Graphene for the Development of the Next-Generation of Biocomposites for Dental and Medical Applications. Dent. Mater. 2017, 33, 765–774. [Google Scholar] [CrossRef]
- Biru, E.I.; Necolau, M.I.; Zainea, A.; Iovu, H. Graphene Oxide-Protein-Based Scaffolds for Tissue Engineering: Recent Advances and Applications. Polymers 2022, 14, 1032. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Viprya, P.; Kumar, D.; Kowshik, S. Study of Different Properties of Graphene Oxide (GO) and Reduced Graphene Oxide (rGO). Eng. Proc. 2023, 59, 84. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef]
- Ricci, A.; Cataldi, A.; Zara, S.; Gallorini, M. Graphene-Oxide-Enriched Biomaterials: A Focus on Osteo and Chondroinductive Properties and Immunomodulation. Materials 2022, 15, 2229. [Google Scholar] [CrossRef]
- Nanda, S.S.; Papaefthymiou, G.C.; Yi, D.K. Functionalization of Graphene Oxide and Its Biomedical Applications. Crit. Rev. Solid State Mater. Sci. 2015, 40, 291–315. [Google Scholar] [CrossRef]
- Wang, F.; Saure, L.M.; Schütt, F.; Lorich, F.; Rasch, F.; Nia, A.S.; Feng, X.; Seekamp, A.; Klüter, T.; Naujokat, H.; et al. Graphene Oxide Framework Structures and Coatings: Impact on Cell Adhesion and Pre-Vascularization Processes for Bone Grafts. Int. J. Mol. Sci. 2022, 23, 3379. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; Li, Y.-C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-Based Materials for Tissue Engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.S.; Kim, S. Human Adipose Mesenchymal Stem Cells Modulate Inflammation and Angiogenesis through Exosomes. Sci. Rep. 2022, 12, 2776. [Google Scholar] [CrossRef]
- Sekuła-Stryjewska, M.; Noga, S.; Dźwigońska, M.; Adamczyk, E.; Karnas, E.; Jagiełło, J.; Szkaradek, A.; Chytrosz, P.; Boruczkowski, D.; Madeja, Z.; Kotarba, A.; Lipińska, L.; Zuba-Surma, E.K. Graphene-based materials enhance cardiomyogenic and angiogenic differentiation capacity of human mesenchymal stem cells in vitro—Focus on cardiac tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111614. [Google Scholar] [CrossRef] [PubMed]
- Klimczak, A. Mesenchymal Stem/Progenitor Cells and Their Derivates in Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 6652. [Google Scholar] [CrossRef] [PubMed]
- Bydlowski, S.P.; Debes, A.A.; Maselli, L.M.F.; Janz, F.L. Características biológicas das células-tronco mesenquimais. Rev. Bras. Hematol. E Hemoter. 2009, 31, 25–35. [Google Scholar] [CrossRef]
- Mazziotta, C.; Lanzillotti, C.; Iaquinta, M.R.; Taraballi, F.; Torreggiani, E.; Rotondo, J.C.; Otòn-Gonzalez, L.; Mazzoni, E.; Frontini, F.; Bononi, I. MicroRNAs Modulate Signaling Pathways in Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 2362. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Platelet-Derived Growth Factor (PDGF) Therapy in Myocardial Infarction: Challenges and Opportunities. Int. J. Cardiol. 2021, 341, 74–75. [Google Scholar] [CrossRef]
- Beheshtizadeh, N.; Gharibshahian, M.; Bayati, M.; Maleki, R.; Strachan, H.; Doughty, S.; Tayebi, L. Vascular Endothelial Growth Factor (VEGF) Delivery Approaches in Regenerative Medicine. Biomed. Pharmacother. 2023, 166, 115301. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β Signaling in Health, Disease, and Therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef]
- Jagiełło, J.; Sekuła-Stryjewska, M.; Noga, S.; Adamczyk, E.; Dźwigońska, M.; Kurcz, M.; Kurp, K.; Winkowska-Struzik, M.; Karnas, E.; Boruczkowski, D.; et al. Impact of Graphene-Based Surfaces on the Basic Biological Properties of Human Umbilical Cord Mesenchymal Stem Cells: Implications for Ex Vivo Cell Expansion Aimed at Tissue Repair. Int. J. Mol. Sci. 2019, 20, 4561. [Google Scholar] [CrossRef]
- Miller, B.S.; Rogol, A.D.; Rosenfeld, R.G. The History of the Insulin-Like Growth Factor System. Horm. Res. Paediatr. 2022, 95, 619–630. [Google Scholar] [CrossRef]
- Goldfarb, M. Fibroblast Growth Factor Homologous Factors: Canonical and Non-Canonical Mechanisms of Action. J. Physiol. 2024, 602, 4097–4110. [Google Scholar] [CrossRef] [PubMed]
- Gillman, C.E.; Jayasuriya, A.C. FDA-Approved Bone Grafts and Bone Graft Substitute Devices in Bone Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112466. [Google Scholar] [CrossRef]
- Aykul, S.; Maust, J.; Martinez-Hackert, E. BMP-4 Extraction from Extracellular Matrix and Analysis of Heparin-Binding Properties. Mol. Biotechnol. 2022, 64, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, X.; Wang, S.; Cao, X.; Yuan, Z.; Getachew, T.; Mwacharo, J.M.; Haile, A.; Sun, W. BMP7 Functions to Regulate Proliferation of Dermal Papilla Cells in Hu Sheep. Genes 2022, 13, 201. [Google Scholar] [CrossRef] [PubMed]
- Pei, F.; Ma, L.; Jing, J.; Feng, J.; Yuan, Y.; Guo, T.; Han, X.; Ho, T.-V.; Lei, J.; He, J. Sensory Nerve Niche Regulates Mesenchymal Stem Cell Homeostasis via FGF/mTOR/Autophagy Axis. Nat. Commun. 2023, 14, 344. [Google Scholar] [CrossRef]
- Kistner, T.M.; Pedersen, B.K.; Lieberman, D.E. Interleukin 6 as an Energy Allocator in Muscle Tissue. Nat. Metab. 2022, 4, 170–179. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, G.; Gao, T.; Huang, T.; Zou, M.; Zou, Y.; Duan, S. Epigenetic Changes Associated with Interleukin-10. Front. Immunol. 2020, 11, 1105. [Google Scholar] [CrossRef] [PubMed]
- Kurtulus, I.; Basim, S.; Ozdenkaya, Y. Can Serum Tumor Necrosis Factor-Alpha Predict Peritoneal Adhesions Prior to Secondary Laparoscopic Procedures? J. Visc. Surg. 2023, 160, 261–268. [Google Scholar] [CrossRef]
- Wautier, J.-L.; Wautier, M.-P. Pro- and Anti-Inflammatory Prostaglandins and Cytokines in Humans: A mini review. Int. J. Mol. Sci. 2023, 24, 9647. [Google Scholar] [CrossRef]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Melgrati, S.; Sozzani, S.; Thelen, M. Editorial: Insights in Cytokines and Soluble Mediators in Immunity: 2022. Front. Immunol. 2023, 14, 1194553. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef]
- Gao, K.; Zhang, T.; Wang, F.; Lv, C. Therapeutic Potential of Wnt-3a in Neurological Recovery after Spinal Cord Injury. Eur. Neurol. 2019, 81, 197–204. [Google Scholar] [CrossRef]
- Vander Ark, A.; Cao, J.; Li, X. TGF-β Receptors: In and beyond TGF-β Signaling. Cell. Signal 2018, 52, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z.; Ji, H. Direct Targeting of β-catenin in the Wnt Signaling Pathway: Current Progress and Perspectives. Med. Res. Rev. 2021, 41, 2109–2129. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Lim, K.-T. Graphene Oxide-Based Stimuli-Responsive Platforms for Biomedical Applications. Molecules 2021, 26, 2797. [Google Scholar] [CrossRef]
- Xu, D.; Wang, C.; Wu, J.; Fu, Y.; Li, S.; Hou, W.; Lin, L.; Li, P.; Yu, D.; Zhao, W. Effects of Low-Concentration Graphene Oxide Quantum Dots on Improving the Proliferation and Differentiation Ability of Bone Marrow Mesenchymal Stem Cells through the Wnt/β-Catenin Signaling Pathway. ACS Omega 2022, 7, 13546–13556. [Google Scholar] [CrossRef]
- Ray, S.; Adelnia, H.; Ta, H.T. Collagen and the Effect of Poly-L-Lactic Acid Based Materials on Its Synthesis. Biomater. Sci 2021, 9, 5714–5731. [Google Scholar] [CrossRef]
- Mazzuco, R.; Evangelista, C.; Gobbato, D.O.; Almeida, L.M. Clinical and Histological Comparative Outcomes after Injections of POLY-L-LACTIC Acid and Calcium Hydroxyapatite in Arms: A Split Side Study. J. Cosmet. Dermatol. 2022, 21, 6727–6733. [Google Scholar] [CrossRef]
- Ao, Y.-J.; Yi, Y.; Wu, G.-H. Application of PLLA (Poly-L-Lactic Acid) for Rejuvenation and Reproduction of Facial Cutaneous Tissue in Aesthetics: A Review. Medicine 2024, 103, 37506. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Yang, F.; Shuai, Y.; Peng, S.; Chen, S.; Deng, Y.; Feng, P. Silicon Dioxide Nanoparticles Decorated on Graphene Oxide Nanosheets and Their Application in Poly(l-Lactic Acid) Scaffold. J. Adv. Res 2023, 48, 175–190. [Google Scholar] [CrossRef]
- Díaz, E.; Iglesias, N.; Ribeiro, S.; Lanceros-Méndez, S. Cytocompatible Scaffolds of Poly(L-Lactide)/Reduced Graphene Oxide for Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2021, 32, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Fu, Z.; Liu, X.; He, H.; Li, Q.; Fan, S.; Zhao, L. Adaptação Das Estruturas e Propriedades de Misturas Biodegradáveis de Poli(Ácido Láctico)/Poli(Butileno Adipato-Co-Ácido Tereftálico) Por Meio de Óxido de Grafeno Reativo. Int. J. Biol. Macromol. 2025, 302, 140455. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alhadlaq, H.A. Reduced Graphene Oxide Mitigates Cadmium-Induced Cytotoxicity and Oxidative Stress in HepG2 Cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 143, 111515. [Google Scholar] [CrossRef]
- Nichols, F.; Chen, S. Graphene Oxide Quantum Dot-Based Functional Nanomaterials for Effective Antimicrobial Applications. Chem. Rec. 2020, 20, 1505–1515. [Google Scholar] [CrossRef]
- Wroblewski, O.M.; Vega-Soto, E.E.; Nguyen, M.H.; Cederna, P.S.; Larkin, L.M. Impact of Human Epidermal Growth Factor on Tissue-Engineered Skeletal Muscle Structure and Function. Tissue Eng. Part A 2021, 27, 1151–1159. [Google Scholar] [CrossRef]
- Maleki, M.; Zarezadeh, R.; Nouri, M.; Sadigh, A.R.; Pouremamali, F.; Asemi, Z.; Kafil, H.S.; Alemi, F.; Yousefi, B. Graphene Oxide: A Promising Material for Regenerative Medicine and Tissue Engineering. Biomol. Concepts 2020, 11, 182–200. [Google Scholar] [CrossRef] [PubMed]
- Bellet, P.; Gasparotto, M.; Pressi, S.; Fortunato, A.; Scapin, G.; Mba, M.; Menna, E.; Filippini, F. Graphene-Based Scaffolds for Regenerative Medicine. Nanomaterials 2021, 11, 404. [Google Scholar] [CrossRef]
- Faria, G.S.; Lima, A.M.; Brandão, L.P.; da Costa, A.P.; Nardecchia, S.; Ribeiro, A.A.; Pinheiro, W.A. Produção e caracterização de óxido de grafeno e óxido de grafeno reduzido com diferentes tempos de oxidação. Matéria 2018, 22, e11918. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Yu, Y.; Wang, L.; Zhao, M. Umbilical Cord-derived Mesenchymal Stem Cell Secretome Promotes Skin Regeneration and Rejuvenation: From Mechanism to Therapeutics. Cell Prolif. 2024, 57, 13586. [Google Scholar] [CrossRef]
- Ikram, R.; Shamsuddin, S.A.A.; Mohamed Jan, B.; Abdul Qadir, M.; Kenanakis, G.; Stylianakis, M.M.; Anastasiadis, S.H. Impact of Graphene Derivatives as Artificial Extracellular Matrices on Mesenchymal Stem Cells. Molecules 2022, 27, 379. [Google Scholar] [CrossRef]
- Moo, J.G.S.; Khezri, B.; Webster, R.D.; Pumera, M. Graphene Oxides Prepared by Hummers’, Hofmann’s, and Staudenmaier’s Methods: Dramatic Influences on Heavy-Metal-Ion Adsorption. ChemPhysChem 2014, 15, 2922–2929. [Google Scholar] [CrossRef]
- Chua, R.; Cai, Y.; Lim, P.Q.; Kumar, S.; Satish, R.; Manalastas, W.; Ren, H.; Verma, V.; Meng, S.; Morris, S.A.; et al. Hydrogen-Bonding Interactions in Hybrid Aqueous/Nonaqueous Electrolytes Enable Low-Cost and Long-Lifespan Sodium-Ion Storage. ACS Appl. Mater. Interfaces 2020, 12, 22862–22872. [Google Scholar] [CrossRef]
- Flores-Rojas, G.G.; Gómez-Lazaro, B.; López-Saucedo, F.; Vera-Graziano, R.; Bucio, E.; Mendizábal, E. Electrospun Scaffolds for Tissue Engineering: A Review. Macromol 2023, 3, 524–553. [Google Scholar] [CrossRef]
- Gong, X.; Tang, C.Y.; Wong, C.T.; Lu, W.W.; Zhang, Y.; Lam, W.M.; Wu, S.; Liu, J. Fabrication of Poly(Lactic Acid) Scaffolds by a Modified Solvent Casting/Porogen Leaching Method. e-Polymers 2010, 10. [Google Scholar] [CrossRef]
- Saravanan, S.; Chawla, A.; Vairamani, M.; Sastry, T.P.; Subramanian, K.S.; Selvamurugan, N. Scaffolds Containing Chitosan, Gelatin and Graphene Oxide for Bone Tissue Regeneration in Vitro and in Vivo. Int. J. Biol. Macromol. 2017, 104, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Tupone, M.G.; Panella, G.; d’Angelo, M.; Castelli, V.; Caioni, G.; Catanesi, M.; Benedetti, E.; Cimini, A. An Update on Graphene-Based Nanomaterials for Neural Growth and Central Nervous System Regeneration. Int. J. Mol. Sci. 2021, 22, 13047. [Google Scholar] [CrossRef]
- Banerjee, D.; Singh, Y.P.; Datta, P.; Ozbolat, V.; O’Donnell, A.; Yeo, M.; Ozbolat, I.T. Strategies for 3D Bioprinting of Spheroids: A Comprehensive Review. Biomaterials 2022, 291, 121881. [Google Scholar] [CrossRef]
- Ni, F.; Chen, Y.; Wang, Z.; Zhang, X.; Gao, F.; Shao, Z.; Wang, H. Graphene Derivative Based Hydrogels in Biomedical Applications. J. Tissue Eng. 2024, 15, 20417314241282131. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I. Thermomechanical Properties of Polylactic Acid-Graphene Composites: A State-of-the-Art Review for Biomedical Applications. Materials 2017, 10, 748. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Montazeri, F. Cell Origin and Microenvironment: The Players of Differentiation Capacity in Human Mesenchymal Stem Cells. Tissue Cell 2025, 93, 102709. [Google Scholar] [CrossRef]
- López Tenorio, D.; Valencia, C.H.; Valencia, C.; Zuluaga, F.; Valencia, M.E.; Mina, J.H.; Grande Tovar, C.D. Evaluation of the Biocompatibility of CS-Graphene Oxide Compounds In Vivo. Int. J. Mol. Sci. 2019, 20, 1572. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Choi, H.E.; Kim, K.S. Graphene-Based Nanomaterials as Drug Delivery Carriers. In Multifaceted Biomedical Applications of Graphene; Han, D.-W., Hong, S.W., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2022; Volume 1351, pp. 109–124. ISBN 978-981-16-4922-6. [Google Scholar]
- Li, J.; Ding, J.; Liu, T.; Liu, J.F.; Yan, L.; Chen, X. Poly(Lactic Acid) Controlled Drug Delivery. In Industrial Applications of Poly(lactic acid); Di Lorenzo, M.L., Androsch, R., Eds.; Advances in Polymer Science; Springer International Publishing: Cham, Switzerland, 2017; Volume 282, pp. 109–138. ISBN 978-3-319-75458-1. [Google Scholar]
- Frigerio, G.; Motta, S.; Siani, P.; Donadoni, E.; Di Valentin, C. Unveiling the Drug Delivery Mechanism of Graphene Oxide Dots at the Atomic Scale. J. Control. Release 2025, 379, 344–362. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Kim, S.; Min, D.-H. Biosensors Based on Graphene Oxide and Its Biomedical Application. Adv. Drug Deliv. Rev. 2016, 105, 275–287. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical Functionalization of Graphene and Its Applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, Biodegradation and Excretion of Polylactic Acid (PLA) in Medical Implants and Theranostic Systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of Drug Release from Delivery Systems Based on Hydroxypropyl Methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Sontakke, A.D.; Tiwari, S.; Purkait, M.K. A Comprehensive Review on Graphene Oxide-Based Nanocarriers: Synthesis, Functionalization and Biomedical Applications. FlatChem 2023, 38, 100484. [Google Scholar] [CrossRef]
- Vuppaladadium, S.S.R.; Agarwal, T.; Kulanthaivel, S.; Mohanty, B.; Barik, C.S.; Maiti, T.K.; Pal, S.; Pal, K.; Banerjee, I. Silanization Improves Biocompatibility of Graphene Oxide. Mater. Sci. Eng. C 2020, 110, 110647. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Luo, Q.; Ju, Y.; Song, G. A Mini Review Focused on the Recent Applications of Graphene Oxide in Stem Cell Growth and Differentiation. Nanomaterials 2018, 8, 736. [Google Scholar] [CrossRef]
- Ding, D.-C.; Shyu, W.-C.; Lin, S.-Z. Mesenchymal Stem Cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Q.; Xu, T.; Zhang, H.; Zhang, M.; Lu, L.; Hao, Y.; Fuh, J.; Zhao, X. Photocrosslinkable Nanocomposite Ink for Printing Strong, Biodegradable and Bioactive Bone Graft. Biomaterials 2020, 263, 120378. [Google Scholar] [CrossRef] [PubMed]
- Pulingam, T.; Thong, K.L.; Appaturi, J.N.; Lai, C.W.; Leo, B.F. Mechanistic Actions and Contributing Factors Affecting the Antibacterial Property and Cytotoxicity of Graphene Oxide. Chemosphere 2021, 281, 130739. [Google Scholar] [CrossRef] [PubMed]
- Kregielewski, K.; Fraczek, W.; Grodzik, M. Graphene Oxide Enhanced Cisplatin Cytotoxic Effect in Glioblastoma and Cervical Cancer. Molecules 2023, 28, 6253. [Google Scholar] [CrossRef] [PubMed]
- Cerverò-Varona, A.; Canciello, A.; Peserico, A.; Haidar Montes, A.A.; Citeroni, M.R.; Mauro, A.; Russo, V.; Moffa, S.; Pilato, S.; Di Giacomo, S.; et al. Graphene Oxide Accelerates TGFβ-Mediated Epithelial-Mesenchymal Transition and Stimulates pro-Inflammatory Immune Response in Amniotic Epithelial Cells. Mater. Today Bio 2023, 22, 100758. [Google Scholar] [CrossRef]
- Halim, A.; Liu, L.; Ariyanti, A.D.; Ju, Y.; Luo, Q.; Song, G. Low-Dose Suspended Graphene Oxide Nanosheets Induce Antioxidant Response and Osteogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells via JNK-Dependent FoxO1 Activation. J. Mater. Chem. B 2019, 7, 5998–6009. [Google Scholar] [CrossRef]
- Park, R.; Yoon, J.W.; Lee, J.-H.; Hong, S.W.; Kim, J.H. Phenotypic Change of Mesenchymal Stem Cells into Smooth Muscle Cells Regulated by Dynamic Cell-Surface Interactions on Patterned Arrays of Ultrathin Graphene Oxide Substrates. J. Nanobiotechnol. 2022, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Liu, L.; Ariyanti, A.D.; Ju, Y.; Luo, Q.; Song, G. Low-Dose Suspended Graphene Oxide Nanosheets Induce Antioxidant Response and Osteogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells via JNK-Dependent FoxO1 Activation. J. Mater. Chem. B 2019, 7, 5998–6009. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.-S.; Kung, M.-L.; Chen, F.-C.; Ke, Y.-C.; Shen, C.-C.; Yang, Y.-C.; Tang, C.-M.; Yeh, C.-A.; Hsieh, H.-H.; Hsu, S.-h. Nanogold-Carried Graphene Oxide: Anti-Inflammation and Increased Differentiation Capacity of Mesenchymal Stem Cells. Nanomaterials 2021, 11, 2046. [Google Scholar] [CrossRef]
- Karimzadeh, P.; Foroutan, T.; Nafar, M.; Kalavati, S. Impact of Nanographene Oxide on Cisplatin Induced Acute Kidney Injury Managed by Stem Cells Therapy. Iran. J. Kidney Dis. 2023, 17, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, C.; Li, Y.; Li, Y.; Chen, G.; He, Y.; Yi, C.; Wang, C.; Yu, D. Dose-dependent Cytotoxicity Induced by Pristine Graphene Oxide Nanosheets for Potential Bone Tissue Regeneration. J. Biomed. Mater. Res. 2020, 108, 614–624. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, H.; Fang, Y.; Cao, Y.; Huang, J.; Zhang, M.; Dai, J.; Shi, X.; Zhang, Z. Enhanced Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells on Graphene Oxide-Incorporated Electrospun Poly(Lactic-Co-Glycolic Acid) Nanofibrous Mats. ACS Appl. Mater. Interfaces 2015, 7, 6331–6339. [Google Scholar] [CrossRef]
- Kenry; Lee, W.C.; Loh, K.P.; Lim, C.T. When Stem Cells Meet Graphene: Opportunities and Challenges in Regenerative Medicine. Biomaterials 2018, 155, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Lim, C.H.; Kenry; Su, C.; Loh, K.P.; Lim, C.T. Cell-Assembled Graphene Biocomposite for Enhanced Chondrogenic Differentiation. Small 2015, 11, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.; Kim, S.-H.; Kim, J.; Lee, J.-R.; Jeong, G.-J.; Yoon, J.-K.; Kang, S.; Bhang, S.H.; Yoon, H.H.; Lee, J.-C.; et al. Graphene Oxide Reinforced Hydrogels for Osteogenic Differentiation of Human Adipose-Derived Stem Cells. RSC Adv. 2017, 7, 20779–20788. [Google Scholar] [CrossRef]
- Park, J.; Kim, I.Y.; Patel, M.; Moon, H.J.; Hwang, S.; Jeong, B. 2D and 3D Hybrid Systems for Enhancement of Chondrogenic Differentiation of Tonsil-Derived Mesenchymal Stem Cells. Adv. Funct. Mater. 2015, 25, 2573–2582. [Google Scholar] [CrossRef]
- Chien, S.-Y.; Tsai, C.-H.; Liu, S.-C.; Huang, C.-C.; Lin, T.-H.; Yang, Y.-Z.; Tang, C.-H. Noggin Inhibits IL-1β and BMP-2 Expression, and Attenuates Cartilage Degeneration and Subchondral Bone Destruction in Experimental Osteoarthritis. Cells 2020, 9, 927. [Google Scholar] [CrossRef]
- Hart, D.A. What Molecular Recognition Systems Do Mesenchymal Stem Cells/Medicinal Signaling Cells (MSC) Use to Facilitate Cell-Cell and Cell Matrix Interactions? A Review of Evidence and Options. Int. J. Mol. Sci 2021, 22, 8637. [Google Scholar] [CrossRef]
- Kong, Y.; Ao, J.; Chen, Q.; Su, W.; Zhao, Y.; Fei, Y.; Ma, J.; Ji, M.; Mi, L. Evaluating Differentiation Status of Mesenchymal Stem Cells by Label-Free Microscopy System and Machine Learning. Cells 2023, 12, 1524. [Google Scholar] [CrossRef]
- Durairaj, S.; Sridhar, D.; Ströhle, G.; Li, H.; Chen, A. Bactericidal Effect and Cytotoxicity of Graphene Oxide/Silver Nanocomposites. ACS Appl. Mater. Interfaces 2024, 16, 18300–18310. [Google Scholar] [CrossRef]
- Raslan, A.; Saenz Del Burgo, L.; Ciriza, J.; Pedraz, J.L. Graphene Oxide and Reduced Graphene Oxide-Based Scaffolds in Regenerative Medicine. Int. J. Pharm. 2020, 580, 119226. [Google Scholar] [CrossRef] [PubMed]




| Category | Molecule | Function | References |
|---|---|---|---|
| Growth Factors | Platelet-Derived Growth Factor (PDGF) | Stimulates cell proliferation and migration, key in wound healing and tissue regeneration | [33] |
| Vascular Endothelial Growth Factor (VEGF) | Promotes angiogenesis, essential for tissue repair and regeneration | [34] | |
| Transforming Growth Factor Beta (TGF-β) | Regulates cell proliferation, differentiation, and extracellular matrix production | [35] | |
| Epidermal Growth Factor (EGF) | Stimulates cell proliferation and differentiation, participates in tissue repair | ||
| Insulin-Like Growth Factor 1 (IGF-1) | Supports cell survival, growth, and differentiation, contributes to bone and cartilage regeneration | [37] | |
| Fibroblast Growth Factor (FGF) | Involved in tissue regeneration and angiogenesis by promoting cell proliferation and differentiation | [38] | |
| Bone Morphogenetic Protein 2 (BMP-2) | Promotes cartilage and bone formation, involved in osteogenesis and bone homeostasis | [2,3] | |
| Bone Morphogenetic Protein 4 (BMP-4) | Involved in bone and cartilage development, muscle development, and fracture repair | [4,5] | |
| Bone Morphogenetic Protein 7 (BMP-7) | Induces osteoblast differentiation, involved in bone homeostasis and kidney development | [6,7] | |
| Cytokines | Interleukin-6 (IL-6) | Regulates inflammation and modulates the immune response | [43] |
| Interleukin-10 (IL-10) | Anti-inflammatory cytokine, central in immune regulation | [44] | |
| Tumor Necrosis Factor Alpha (TNF-α) | Key regulator of inflammation, with both pro-inflammatory and regenerative properties | [45] | |
| Biologically Active Molecules | Prostaglandins | Lipid-derived signaling molecules involved in inflammation modulation and injury response | [46] |
| Matrix Metalloproteinases (MMPs) | Enzymes that degrade extracellular matrix components, facelifted tissue remodeling and wound healing | [55] | |
| Soluble Cytokines | Including chemokines, which recruit inflammatory cells to injury sites and orchestrate local immune responses | [48] | |
| Exosomes | Nano-sized extracellular vesicles secreted by MSCs, mediating intercellular communication and influencing immune and inflammatory responses | [49] | |
| Signaling Pathways | Wnt-3 | Regulates cell fate decisions, involved in mesenchymal stem cell differentiation | [8,9] |
| Wnt-4 | Plays a role in organogenesis, sex determination, and mammary gland development | [10,11] | |
| β-Catenin | Central mediator of the canonical Wnt signaling pathway, regulates gene expression | [19,27] | |
| Wnt/β-Catenin Pathway | Integrates signals from various pathways, regulates stem cell pluripotency and cell fate decisions | [29,30,56] |
| Fabrication Method | Basic Principle | Advantages of GO/PLLA Composites | Disadvantages or Limitations of GO/PLLA Composites | References |
|---|---|---|---|---|
| 3D Printing (Extrusion and FDM) | Deposits material layer by layer | - High geometric precision and control over internal architecture (porosity and geometry) - Possibility of incorporating cells and biomolecules (bioprinting) - Good reproducibility | - Limited resolution at the nanometer scale (for extremely small pores) - Material viscosity can be challenging - Potential mechanical or thermal stress on cells (bioprinting) | [50] |
| 3D Printing (SLA and DLP) | Light-induced polymerization of photoinitiator in resin | - High resolution and fine details, allowing for complex structures - Compatible with certain photoinitiator-functionalized GOs - Less mechanical stress for cells (if used in bioprinting) | - Restriction to photopolymerizable materials - Requires removal of residual photoinitiators - High equipment and material costs | [63] |
| Electrospinning | Submicron and nanometer fibers formed by electric field | - Produces membranes with high surface area and interconnected porosity - Mimics natural ECM structure - Allows fiber orientation - Suitable for incorporating nanomaterials like GO | - Difficulty in controlling 3D porosity and thickness (usually forms as 2D or 2.5D) - Low mechanical strength in some configurations - Can be challenging for large-volume production | [64] |
| Particulate Leaching | Material encapsulated with removable particles | - Simple and low cost - Allows high porosity and interconnected pores - Control over pore size by porogen size | - Difficulty in achieving uniform porosity and complex architecture - Porogen residues can be toxic - Less control over the final scaffold shape | [65] |
| Freeze-Drying | Freezing followed by solvent sublimation | - Produces highly porous scaffolds with interconnected pores - Suitable for heat-sensitive materials (GO/PLLA) - Simple and low-cost | - Limited control over pore size and geometry (irregularity) - Often low mechanical properties - Difficult controlling pore orientation | [66] |
| Solvent Casting and Evaporation | Solvent evaporation from a polymer solution | - Simple and low-cost for films or thin layers - Good for GO/PLLA composites | - Difficulty in creating complex 3D structures and interconnected pores - Solvent residues can be toxic to cells | [9] |
| Self-Assembly | Spontaneous organization of components into structures | - Allows nanoscale structures with high precision and order - Mimics natural biological processes - Can form porous hydrogels | - Difficult to control macro-structure and scaffold scale - High cost of precursors - Complex process for large-scale fabrication | [67] |
| Spheroid and Microsphere Formation | Cell or polymeric agglomeration into micro-structures | - Creates modular units for tissue engineering - Can be used for delivering cells or growth factors - Potential for in situ vascularization upon implantation | - Limited control over spheroid internal morphology - Challenges in mechanical stability when used as isolated scaffolds - Difficulty in fusing into larger, complex structures | [68] |
| Gelling and In Situ Hydrogel Formation | Transformation of solution into gel at the site of interest (or in vitro) | - Allows gentle encapsulation of cells (bioprinting) and biomolecules - High similarity to natural ECM in terms of water content - Injectable for minimally invasive applications | - Low mechanical strength for tissues requiring support - Degradation rate is difficult to control precisely - Some gelling initiators may be cytotoxic for GO/PLLA | [69] |
| Melt Processing | Processing polymers above their melting temperatures | - Wide variety of techniques (injection molding and extrusion) - High production capacity and cost-effectiveness - Improves GO dispersion in some cases | - High temperatures can degrade GO (functionalities) or biomolecules - Difficulty in creating interconnected porosity without secondary porogens - Less suitable for cell incorporation | [70] |
| Drug Release Characteristics | Specific Advantages of GO/PLLA Composites | References |
|---|---|---|
| High Loading Capacity and Versatility | - GO has a large surface area and functional groups that allow a high loading capacity of hydrophilic and hydrophobic drugs via π-π stacking interactions, hydrogen bonding, and electrostatic adsorption | [71] |
| Specificity and Targeting | - GO can be functionalized to target the composite to specific locations (cells and tissues), minimizing systemic effects - Systems can be responsive to stimuli (pH, enzymes, and light), allowing for controlled and on-demand release | [44] |
| Controlled and Sustained Release | - The PLLA matrix offers sustained drug release, useful for long-term treatments and reduced dosing frequency - The release profile can be adjusted by the GO/PLLA ratio and material architecture | [50] |
| Biocompatibility and Biodegradability | - Both GO (in appropriate concentrations) and PLLA are biocompatible, reducing adverse reactions - PLLA’s biodegradability ensures safe disposal of the material after its function | [72] |
| Stability | GO/PLLA combination can protect the drug from degradation and optimize bioavailability | [53] |
| Drug Release Characteristics | Disadvantages and Specific Challenges of GO/PLLA Composites | References |
|---|---|---|
| Nonspecific Adsorption and Complexity | - Potential for nonspecific adsorption of proteins and other biomolecules on GO, affecting drug release or causing unwanted interactions - Functionalization for high specificity can increase manufacturing complexity and cost | [73] |
| Loading Limitations | - Loading capacity may be limited by suboptimal drug–polymer–GO interactions or solubility restrictions - GO aggregation can reduce the available surface area | [74] |
| Challenges in Controlled Release | - PLLA degradation rates can be too slow for some applications, necessitating modifications - Precise control over release kinetics can be challenging in complex biological environments - Initial “burst release” can occur and may be undesirable for certain treatments | [75] |
| Long-Term Biosafety Concerns | - Although biocompatible, the long-term safety and metabolic fate of GO, PLLA, and their degradation products in vivo still require further, more in-depth studies - PLLA degradation can generate acidic byproducts - GO cytotoxicity can be an issue at high concentrations or with certain cell types | [76] |
| Homogeneity and Reproducibility | - Difficulty in ensuring homogeneous GO dispersion within the PLLA matrix, which directly impacts the consistency of drug release | [77] |
| Factor | Description | Effect on Cytotoxicity | References |
|---|---|---|---|
| Particle Size | Smaller particles present a larger surface area for interactions. | Increases membrane penetration and tissue infiltration, enhancing toxic effects. | [82] |
| Particle Shape | Spherical, lamellar, or elongated morphologies. | Different shapes affect endocytosis and membrane dynamics, potentially causing mechanical damage. | [16] |
| Degree of Oxidation | Presence of oxygen-containing functional groups such as epoxides and hydroxyls. | It can trigger oxidative stress and metabolic disturbances. | [59] |
| Functionalization | Chemical modification to reduce intrinsic toxicity. | Improves biocompatibility and stability in aqueous media. | [62] |
| Dose and Concentration | Higher concentrations are associated with increased toxicity. | Increases oxidative stress, inflammatory responses, and cell death. | [51] |
| Exposure Time | Duration of exposure to nanomaterials. | Prolonged exposures increase the likelihood of intracellular accumulation and cumulative damage. | [53] |
| Cell Type | Metabolic and cell cycle differences among cell types. | Influences susceptibility to nanomaterials and the extent of cytotoxic effects. | [71] |
| Cytotoxicity Mechanisms | Generation of reactive oxygen species (ROS), inflammation, cell membrane damage. | Oxidation of lipids, proteins, and nucleic acids, activation of inflammatory pathways. | [54] |
| Authors | Graphene-Based Nanomaterials (Exposure Conditions) | Effect on Mesenchymal Stem Cell | Related Mechanisms | References |
|---|---|---|---|---|
| Cerverò-Varona; Canciello; Peserico; Haidar Montes et al., 2023 | GO [20 µg/mL for 24 h] | Induced and accelerated the EMT | Intracellular activation of TGFβ1-SMAD2/3 signaling pathway | [87] |
| Xu; Wang; Wu; Fu et al., 2022 | GO [0.1, 1, 5, and 10 μg/mL for 24 h] | Induced and accelerated the EMT low-dose GO [0.1, 1 μg/mL] | Activated the Wnt/β-catenin signaling pathway | [47] |
| Park; Yoon; Lee; Hong et al., 2022 | GO [2 μg/mL for 24 h] | Spontaneous surface-induced differentiation of MSCs to SMCs | TGFβ1-induced differentiation | [88] |
| Halim; Liu; Ariyanti; Ju et al., 2019 | GO [0, 0.1, 1, 10, and 100 μg/mL for 0, 24, 48, and 72 h] | Antioxidant response and osteogenic differentiation of bone marrow-derived MSC | Activation and nuclear localization of FoxO1, depending on the JNK activity | [89] |
| Jagiełło; Sekuła-Stryjewska; Noga; Adamczyk et al., 2019 | GO [10–30 µg/cm2 and 3–10 µg/cm2 for 24, 48, and 72 h] * | Maintenance of the appropriate phenotype of hUC-MSC | Low expression of CD45 and high expression of the CD90 and CD105 antigens | [28] |
| Zhang; Wei; Li; Li et al., 2020 | GO [0.02, 0.1, and 0.5 mg/mL for 24 h] | Increased ROS generation and MMP loss | Upregulation of cleaved caspase-3, LC3-II/I, and beclin-1 and a downregulation of Bcl-2 and caspase-3 | [90] |
| Authors | GO or Scaffold Formulation | Model (In Vitro or In Vivo) | Key Findings on MSCs | Noted Limitations | References |
|---|---|---|---|---|---|
| Cerverò-Varona; Canciello; Peserico; Haidar Montes et al., 2023 | GO-coated glass surfaces | In vitro (amniotic epithelial cells) | Induced EMT (upregulation of Snail, Twist, ZEB, and vimentin; downregulation of CYTO8); activated TGFβ1–SMAD2/3; enhanced migration; increased pro-inflammatory cytokines. | Specific focus on AECs and not directly MSCs; findings in 2D culture. | [87] |
| Xu; Wang; Wu; Fu et al., 2022 | GO (0.1 and 1 μg/mL) | In vitro (MSCs) | Enhanced proliferation and osteogenic differentiation; activated Wnt/β-catenin (increased active β-catenin, p-GSK-3β); inhibited chondrogenic and adipogenic differentiation. | Specific GO concentrations; limited scope to osteogenic outcomes in 2D. | [47] |
| Park; Yoon; Lee; Hong et al., 2022 | GO nanosheets (0.1 μg/mL) | In vitro (MSCs) | Promoted MSC proliferation; maintained oxidative balance (upregulated MnSOD, catalase); activated JNK/FoxO1 pathway for osteogenesis. | Specific patterning (100 μm) might limit broader applicability; focus on MSCs. | [89] |
| Halim; Liu; Ariyanti; Ju et al., 2019 | GO-Au nanocomposites | In vitro (MSCs) and in vivo (anti-inflammatory model) | GO-Au(x2) showed highest antioxidant capacity, enhanced proliferation and spreading, and suppressed immune responses (monocyte-macrophage transition, platelet activity). | Limited to low GO doses; primary in vitro assessment. | [90] |
| Jagiełło; Sekuła-Stryjewska; Noga; Adamczyk et al., 2019 | Graphene-based nanostructures | In vivo (acute kidney injury (AKI) model) | Reduced serum Cr and BUN; increased cell proliferation (Ki-67+); reduced apoptosis and necrosis; decreased cyst formation and intratubular debris. | Primarily focused on cytotoxicity and viability and less on lineage-specific differentiation mechanisms. | [28] |
| Zhang; Wei; Li; Li et al., 2020 | Graphene-based nanostructures | In vivo (acute kidney injury (AKI) model) | Enhanced MSC efficacy in kidney regeneration is achieved by improving cell–cell–ECM interactions. | Focus on cytotoxicity and apoptosis mechanisms at high concentrations and not differentiation. | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grecca, I.S.G.; Miola, V.F.B.; Ferreira, J.C.; Vinholo, T.R.; da Silva, L.M.D.; Totti, P.G.F.; Gianini, S.H.S.; Souza, M.d.S.S.d.; Souza, J.d.S.S.d.; Araújo, A.C.; et al. Integrating Graphene Oxide and Mesenchymal Stem Cells in 3D-Printed Systems for Drug Delivery and Tissue Regeneration. Pharmaceutics 2025, 17, 1088. https://doi.org/10.3390/pharmaceutics17081088
Grecca ISG, Miola VFB, Ferreira JC, Vinholo TR, da Silva LMD, Totti PGF, Gianini SHS, Souza MdSSd, Souza JdSSd, Araújo AC, et al. Integrating Graphene Oxide and Mesenchymal Stem Cells in 3D-Printed Systems for Drug Delivery and Tissue Regeneration. Pharmaceutics. 2025; 17(8):1088. https://doi.org/10.3390/pharmaceutics17081088
Chicago/Turabian StyleGrecca, Igor Soares Gianini, Vitor Fernando Bordin Miola, Júlia Carolina Ferreira, Thiago Rissato Vinholo, Laira Mireli Dias da Silva, Paulo Gabriel Friedrich Totti, Silvia Helena Soares Gianini, Maricelma da Silva Soares de Souza, Juliana da Silva Soares de Souza, Adriano Cressoni Araújo, and et al. 2025. "Integrating Graphene Oxide and Mesenchymal Stem Cells in 3D-Printed Systems for Drug Delivery and Tissue Regeneration" Pharmaceutics 17, no. 8: 1088. https://doi.org/10.3390/pharmaceutics17081088
APA StyleGrecca, I. S. G., Miola, V. F. B., Ferreira, J. C., Vinholo, T. R., da Silva, L. M. D., Totti, P. G. F., Gianini, S. H. S., Souza, M. d. S. S. d., Souza, J. d. S. S. d., Araújo, A. C., Guiguer, E. L., Spilla, C. S. G., Bechara, M. D., Roque, D. D., Pereira, E. d. S. B. M., & Pomini, K. T. (2025). Integrating Graphene Oxide and Mesenchymal Stem Cells in 3D-Printed Systems for Drug Delivery and Tissue Regeneration. Pharmaceutics, 17(8), 1088. https://doi.org/10.3390/pharmaceutics17081088










