Three-Dimensional Printing in Paediatrics: Innovative Technology for Manufacturing Patient-Centred Drug Delivery Systems
Abstract
1. Introduction
2. Shortcomings of Medicinal Products for Paediatric Use
3. Principles and Advances in HME and FDM
3.1. Technology
3.2. APIs
3.3. Dose and Dose Flexibility
3.4. Excipients
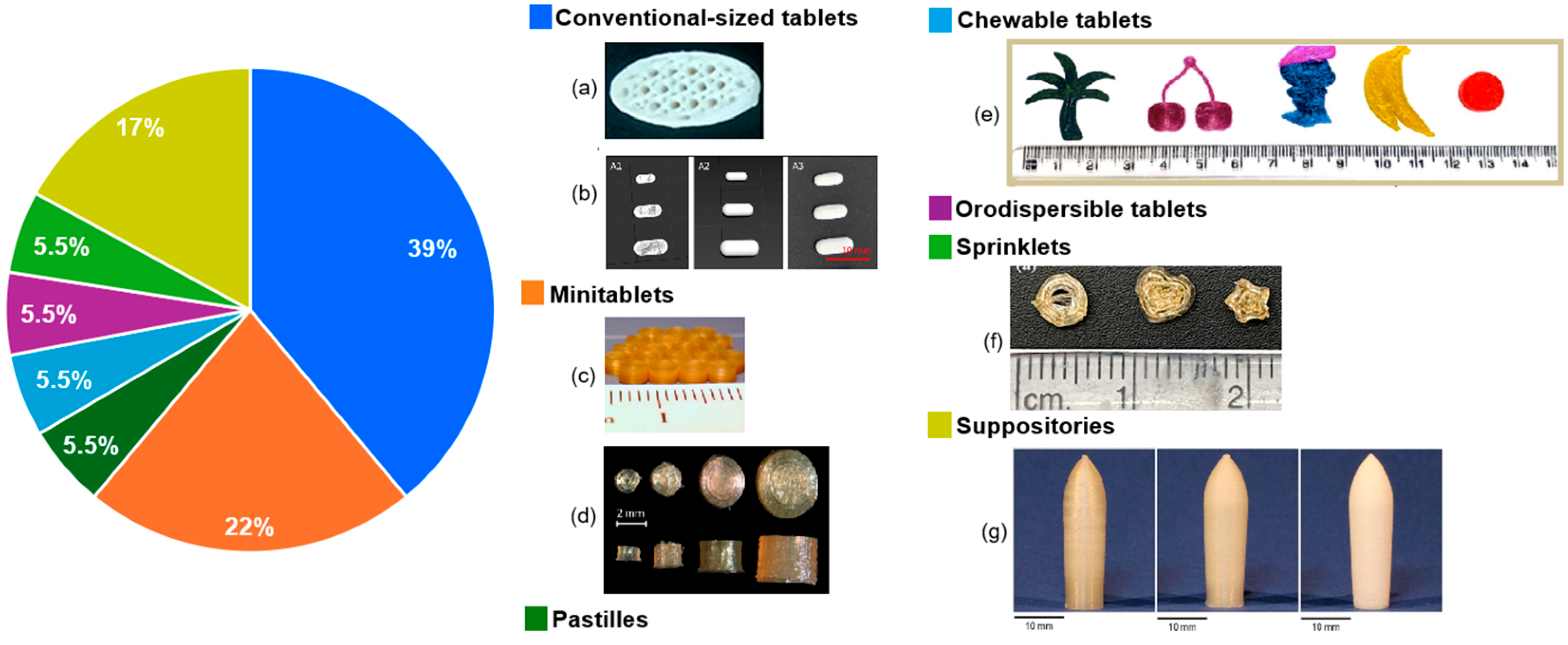
3.5. Dosage Forms and Acceptability
4. Principles and Advances in DPE
4.1. Technology
4.2. APIs and Doses
4.3. Excipients
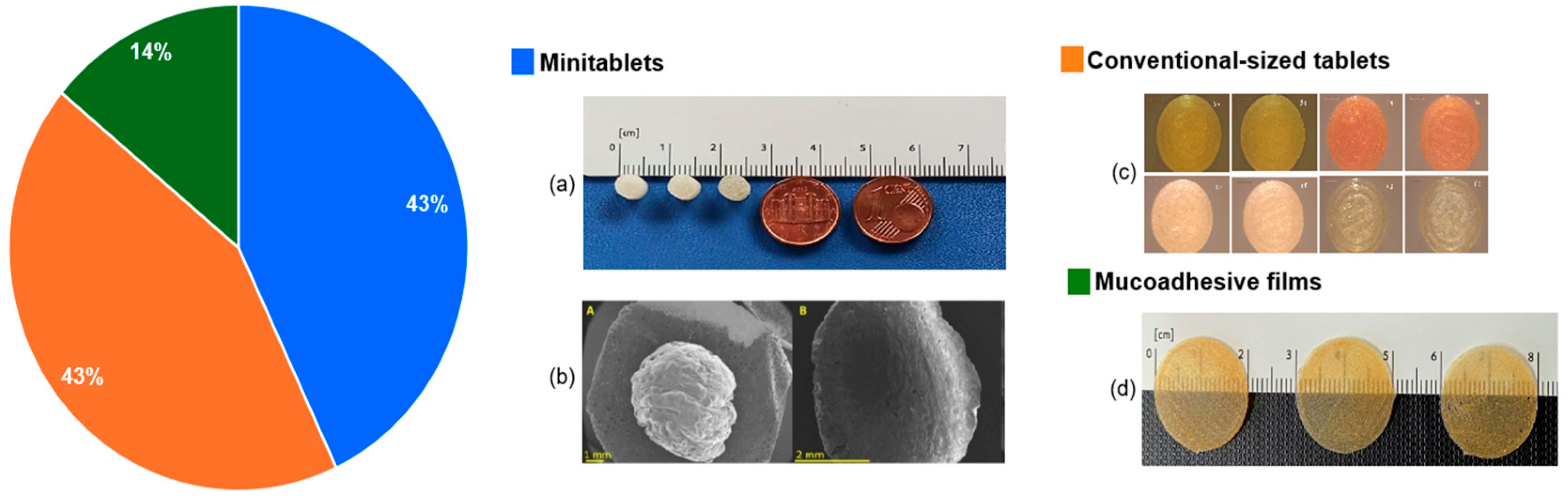
4.4. Dosage Forms and Acceptability
5. Principles and Advances in SSE
5.1. Technology
5.2. APIs and Doses
5.3. Excipients
5.4. Dosage Forms and Acceptability

| API Name, %, Dose/Unit | Excipients | Design | Size/Volume | Observations | Ref. | |
|---|---|---|---|---|---|---|
| Semisolid-Forming Excipients | Others | |||||
| Tablets | ||||||
| Fenofibrate, 41.9/45/53.2 mg/unit | Maisine CC/Captex 355 EP/NF/Capmul MCM EP/Soybean oil 32.5% | Croscarmellose sodium 5%—disintegrant Kolliphor EL, Tween 85 35%—solubilizers | Cylindrical | 7.9 or 8.5 mm diameter | - The lipid-based formulations’ redispersion was successful - Low drug loads restricted the possibility of printing tablets with a high API dose | [92] |
| 1% or 2%, Clopidogrel bisulphate, 2–10 mg/unit | Curablend 94.6%/94.8% | Polysorbate 80 2%—plasticiser Citric acid 1.2–2.4%—pH regulator | Round | Various sizes, n.s. | - Tablets had rapid drug release - disintegration was under 10 min - The clopidogrel–excipient mixture demonstrated chemical stability | [112] |
| Mini-tablets | ||||||
| Sildenafil, 4 mg/unit or Furosemide, 2 or 10 mg/unit | Gelucire 48/16 | Polysorbate 80—plasticiser | Cylindrical | 5 mm diameter | - The quality requirements of the European Pharmacopoeia were assessed, and it was decided that the uniformity of mass can guarantee the mini-tablets’ quality | [93] |
| Levothyroxine sodium, 0.061% | SH-E30 (HPMC) 5% | Round | 4.8 to 7 mm diameter | - SSE 3D printing was evaluated as a possible replacement for manual subdivision, and it was deemed appropriate with a maximum weight loss of 3% and a high dose accuracy - 3D printed tablets were found to be stable for at least three months | [94] | |
| Hydrocortisone | Gelucire 44/14, Precirol ATO 5, 70%/30%, 60%/40% or 50%/50% | Cylindrical | 1.5 × 6 mm | - Micro-extrusion was carried out, with a nozzle of 1.5 mm - Precirol ATO 5 had a double role: semi-solid forming excipient and taste-masking agent - The API dissolving rates were adjusted by varying the ratio of the two excipients, resulting in sustained release mini-tablets | [113] | |
| Chewable dosage forms | ||||||
| Lamotrigine, 10–20 mg, <1 mg/unit | HPMC 100/200/300 mg Gelatine 0.5/0.75/1/1.5% | Reduced syrup 4.29 g—sweetener Water 8.71 g | Star, trapezoidal, triangular, circular, donut, heart, etc. | About 10 mm | - For the formulations with low HPMC or gelatine content, the viscosity was reduced, and printing was challenging | [95] |
| Amlodipine besylate, 1.5–5 mg/unit | Glycerine 10% | SSG 7%—solubility enhancer CMC Na 1%—thickening agent Sucralose 0.1%—sweetener Lemon essence 0.05%—flavouring agent | Cartoons (flower, heart, bear, etc.) | 0.4 to 0.6 mm diameter | - Taste-masking was efficient - The dosage forms were found to be stable for at least four months | [96] |
| Propranolol hydrochloride, 0.455%, 1–5 mg/unit | Gelatine 12% Carrageenan 0.65% | CMS Na 6% Flavouring, colourants | Capsule, diamond, flower, bear | 20.3 mm diameter | - CMS-Na and carrageenan improved the gel ink’s viscosity and thixotropy - Immediate release was achieved - Taste-masking was accomplished | [97] |
| Propranolol, 1%/Spironolactone, 1%/ Prednisolone, 1% | CuraBlend | Cylindrical | Less than 20 mm diameter | - Uniformity of 500 mg showed less mass variation than lower weights, but all of the tablets complied with Ph. Eur. standards - Over the course of nine months, stability showed almost no fluctuation - Prednisolone tablets had the slowest rate of drug release, followed by propranolol and spironolactone - Transferring the liquid form of these tablets through a nasogastric tube was successful | [99] | |
| Propranolol, 1%, 3 mg, 4 mg, or 5 mg/unit | CuraBlend | Round or oval-shaped | Less than 20 mm diameter | - CMS-Na and carrageenan improved the gel ink’s viscosity and thixotropy - Immediate release was achieved - Taste-masking was accomplished - Adding the API to the semi-solid CuraBlend mix reduced viscosity and boosted fluidity - The SSE technique was automated, and three specific tablet weights were targeted | [100] | |
| Ranitidine hydrochloride, 1.004 g, 28 or 32 mg/unit | Xanthan gum 0.075 g Gelatine 2.4 g Carrageenan 0.6 g | Corn starch 0–1.5 g Strawberry essence 0.15 g Sweetener 1 g Purified water | Heart, bear, disc | 15 to 20.8 mm diameter | - Starch-free formulations produced a fast release of ranitidine, whereas integrating corn starch produced a slower release | [102] |
| Isoleucine, 14.4%, 50 to 200 mg/unit | Pectin Maltodextrin | Flavouring agents Colourants Water | Cylindrical | 10 mm diameter and smaller | - Children’s preference was assessed, and the most-liked formulation was the orange colour and taste - Clinical study in which the isoleucine mean blood levels were within the desired range - Tablets were stable for at least one month | [103] |
| Ibuprofen, 19.6 mg/g or Paracetamol, 22.9 mg/g | Chocolate, Corn syrup (1:1 w/w) | - | Star, cartoon characters | - Immediate release profile for both APIs (hydrophilic paracetamol and lipophilic ibuprofen) | [104] | |
| Paracetamol | Chocolate | - | Square | 8 × 8 × 5 mm to 22 × 22 × 17 mm | - 3D printed tablets were compared to mold-cast dosage forms - In line with FDA’s CDI values stated for commercial chewable tablets, both versions of dosage forms had a CDI that varied from 0.15 to 0.28 nm - 3D printed tablets passed all the quality requirements evaluations and allowed dose flexibility | [106] |
| F1: Omeprazole,1%, 7 mg/unit F2: Omeprazole, 22.5% pellets, 11 mg/unit | F1&F2: Carrageenan 2%, Xanthan gum 0.5%, F1: Glycerol 15% F2: Gelatine 8% | F1&F2: Sweetener, essence, lemon juice, water F1: CMC 3%—thickening agent, sodium bicarbonate 2.5%—to adjust the pH | Disc, lemon slice, heart | - | - The SSE 3D printing technique and the fluid bed pellet coating were combined - 3D printed hydrogels loaded with gastro-resistant omeprazole pellets (F2) were compared to hydrogels containing dissolved omeprazole (F1) - By using pellets, the bad taste of the API was masked - Only F2 was found to be gastro-resistant | [107] |
| Ibuprofen, 28%, 12–76 mg/unit Paracetamol, 40%, 6–77 mg/unit | Embedding medium: Glycerol EP: Gelatine (25:30) and water Paste: 2.98% Locust bean gum solution | Food dye | Lego-like brick | 20 mm diameter | - In this study both drugs had a similar release, although paracetamol is generally known to dissolve faster, the reason would be the delayed disintegration of the locust bean gum solution - Sweet flavour of the printed dosage form was identified | [105] |
| Metformin, 250 mg/unit | Gelatine 20% | Starch 5% | Rectangular | 23.8 mm/24.5 mm diameter, 4.3 mm/6.1 mm height | - A visually pleasing design was achieved - A rapid onset of the API’s action was observed, which can have an advantageous control for post-prandial glycemia in children compared to conventional dosage forms - In vivo studies should be further conducted to demonstrate the printed dosage form’s applicability | [115] |
| Amoxicillin, 200 mg/unit | Corn starch and glycerol | Sweetener, plasticiser and flavouring agent: acacia honey, 30% | Bear-shaped | 12.90 × 16.94 × 5.44 mm (width × length × height) after drying | - Use of naturally derived components with the safety of the formulation for children in mind - A sensory analysis of four different types of placebo gels was conducted, and most of the participants chose the formulation with the highest amount of honey (30%) - PermeaPad® barrier was used as a model membrane for the prediction of API oral absorption | [117] |
| Isoniazid, 5% | Gelatine, carrageenan gum | Sweeteners: maltitol and xylitol, flavouring | Cylindrical | 5.6 mm height and ~12 mm diameter | - Low printing speeds resulted in warping - An evaluation of taste-masking with an electronic tongue was conducted and the findings denoted a balanced taste of the printed dosage forms | [118] |
| Sulfamethoxazole 13.7% single-layer and 27.4 bilayer, Trimethoprim 2.7% single-layer and 5.4% bilayer, 100/20 mg single layer and 200/40 mg bilayer | Gelatine and pregelatinized starch | Water, osmotic filler: mannitol, sweeteners: sucralose, maltitol syrup 80/55, colouring and flavouring agent | Cylindrical | 14 × 8 × 5 mm (length × width × height) | - Single-layer tablets yielded a potential bioequivalence to commercial products with the same APIs - Bilayer technique is promising for taste-masking the bitter API; however, the printing process needs refining, and the long-term stability has to be assessed in the future - The formulation is clinically feasible due to its enhanced palatability when compared to conventional oral suspensions, demonstrated by patient adherence testing | [121] |
| Ondansetron, 4 mg/unit | Pectin, gelatine | Thickener: PVP, plasticiser: glycerol, pH modifier: citric acid, conserving agent: potassium sorbate, sweeteners, flavouring, colouring | Cylindrical | 10 mm diameter and 4.5 mm height | - It was demonstrated that the ondansetron content and model size had a linear relationship | [101] |
| Dexamethasone, 2 mg and 12 mg/unit | Gelatine, carrageenan, Xanthan gum Pectin | Water, sweeteners: sorbitol solution and saccharin sodium, bitter-blocker: GABA, citric acid, polysorbate 80, raspberry flavouring, colourant Sweeteners: sucrose, maltose, maltodextrin, colourants, various flavours | Cylindrical | Varying diameters and heights | - The tablets were specifically designed for nausea in paediatric oncology patients - GABA was an effective taste-masker in combination with this API | [119] |
| Amino acids: Isoleucine 20%, 22.5%, 40%; Valine 17.5%, 20%, 40%; Citrulline 30%, various doses | Cylindrical | ~1 cm to 6 mm diameter | - A clinical study was conducted - The chewable dosage forms’ plasma levels of amino acids were akin to conventional formulations’ levels - Shape and texture of chewable dosage forms were well-liked by patients | [122] | ||
| Orodispersible dosage forms | ||||||
| Hydrochlorothiazide, 40.40%, 10 mg/unit | PVP 8.10% Lactose monohydrate 18.20%—binders | Ac-Di-Sol 30.30%—disintegrant Banana flavouring essence 3% | Cylindrical tablets | 5 mm diameter | - Molding and SSE 3DP were compared - All 3D printed dosage forms complied with the Ph. Eur. standards - DSC demonstrated that 3D printing resulted in a more compact and stable structure | [108] |
| Hydrochlorothiazide, 40.40%, 10.32 mg/unit | PVP K30 8.1% Lactose monohydrate 18.2%—binders | Ac-Di-Sol 30.30%—disintegrant Banana flavouring essence 3% | Cylindrical tablets | About 5 mm diameter | - Different printing surfaces were tested (steel, glass, polypropylene, blue tape, and methacrylate) out of which polypropylene and glass were the most fitting - The disintegration time was modified by changing the infill percentage - The weight of the dosage forms was constant | [109] |
| Levetiracetam, 27.6 g | Water PVA-PEG (Kollicoat IR) 31 g-hydrophyllic matrix | - | Cylindrical tablets | 10 mm diameter | - A limitation of this study was the size of the tablets, which is not appropriate for younger paediatric patients (neonates, infants) | [89] |
| Carbamazepine, 0.46 mg/unit | Water Lactose monohydrate 50%—diluent Kollidon VA 30 5%—binder | SSG 40%—superdisintegrant Sucrose 5%—sweetener | Round tablets | 3 mm diameter | - The taste of the printed dosage forms was assessed, and it was determined that the bitter taste of the API is indistinguishable - Advantages such as rapid breakdown and fractioned dose are attributed to the resulting dosage forms | [110] |
| Clorpromazine hydrochloride, 2.5% | Pullulan 48.8–50% | Sucrose, maltose, isomalt, glucose, fructose 48.8–50% | Round films | 20 mm × 30 mm × 0.8 mm | - Oromucosal films were successfully developed - Five sweeteners were evaluated by a human taste panel, and sucralose was the favourite - E-tongue sensor test results were in agreement with those delivered by human panellists | [116] |
| Warfarin, 3.9 to 7.4 mg/unit | PVA or HPC 20% | Round films | 25–200 mm3 | - The PVA films were extremely curved and stiff after drying, making them unsuitable for use as orodispersible films - The neutral surface pH of both the drug-loaded and unloaded films suggests that they can be used inside the oral cavity without causing discomfort | [111] | |
| Dexamethasone, 1%/3%, 0.25 to 5 mg/unit | HPMCAS | Diluent: D-Mannitol, D-Sorbitol, plasticizer and suspension stabiliser: PEG, superdisintegrant: CMC, flavour enhancers: citric acid and rebaudioside A | Cylindrical | Various sizes | - GRAS excipients were exclusively used - Ink homogeneity was determined by taking multiple samples from different places in the syringe | [120] |
6. Other 3D Printing Techniques
7. Practical Implications and Future Directions
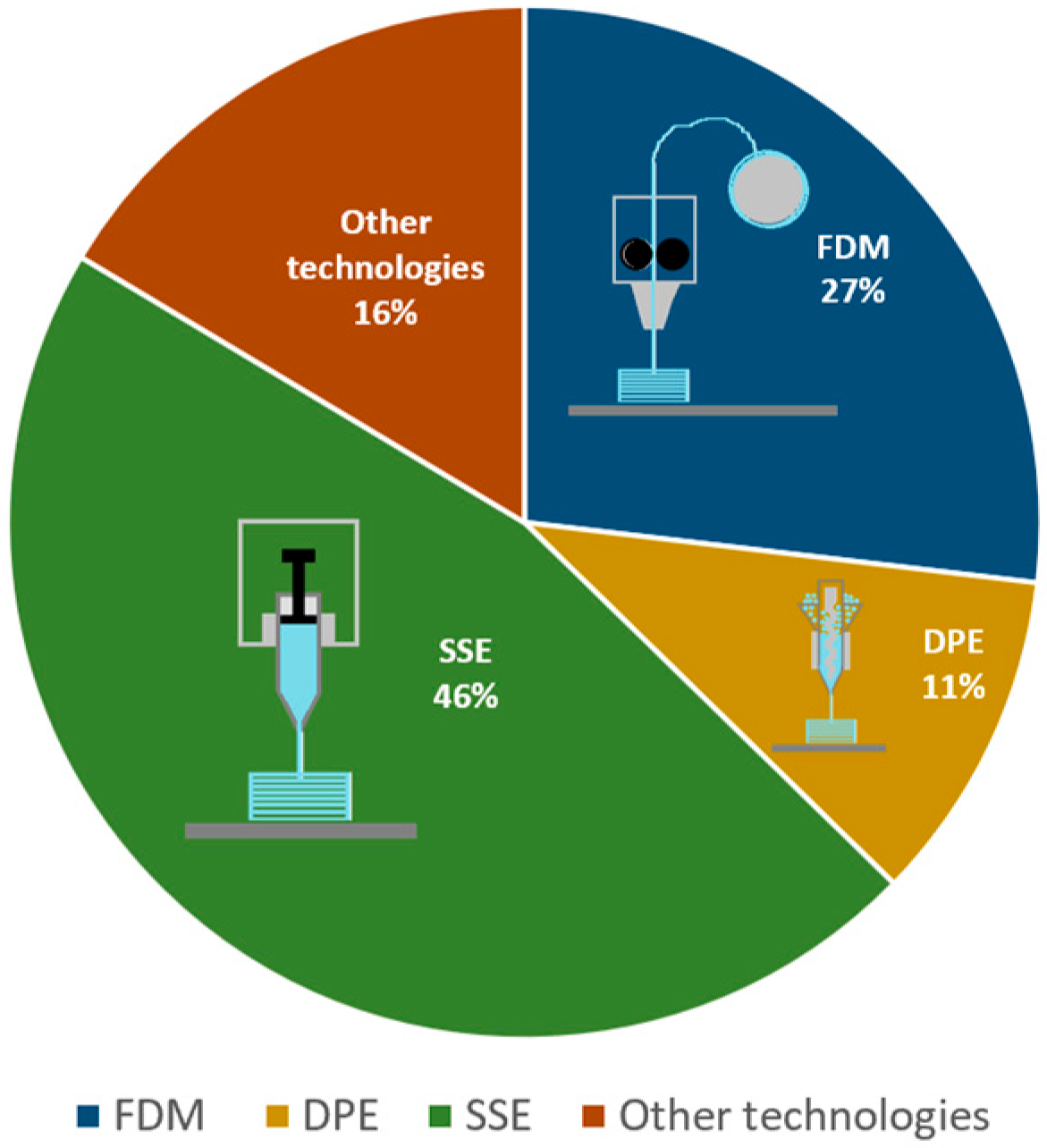
- Evolving 3D printing technologies. As shown in the sections above, FDM, DPE, and SSE were the methods of choice for paediatric drug 3DP. Several review papers offer detailed descriptions of these technologies, with an overview of their background, process stages and equipment, process control, material-related information, and even an inventory of 3DP techniques tested on particular APIs [149]. However, their working principles and unique characteristics influence their suitability for prepare medicines for children. The table below gathers some important points to consider regarding the three technologies, which could guide the choice of a method when the preparation of 3DP paediatric drugs is considered.
- improved excipient development and selection. Excipients with specific properties are requested for each 3DP technology, and as discussed before, the performance of well-known conventional material is still to be understood in conjunction with different APIs and new 3DP technologies. However, the development of new excipients, tailored to undergo the stages of 3DP, with good safety profiles for all categories of paediatric patients, is crucial.
- easy design and formulation. Up to this point, formulation and design strategies have aimed at obtaining multiple dosage forms in various shapes and sizes with specific API release times to match the needs of children of different ages. The inclusion of nanosystems into the printed products has also been mentioned as a novel delivery strategy [141]. Future studies could tackle controlled release, varied nanosystem addition, and improvements in size, texture, palatability, and ease of administration. A thorough knowledge of children’s preferences and needs regarding taste, texture, colour, and administration skills would contribute to the development of 3DP drugs; therefore, more studies are required to collect patient feedback.
- regulatory framework. The approaches mentioned above cannot succeed without clear regulation concerning the 3DP drug production and clinical studies. The FDA initiated a discussion about point-of-care manufacturing. The published paper emphasises that point-of-care manufacturing requires solid quality systems and adaptable, risk-based Good Manufacturing Practice (GMP) frameworks designed for small-batch, bespoke production [160]. These ideas promote the safe, decentralised production of patient-specific medications with real-time quality monitoring using 3D printing of pharmaceuticals. EMA proposes recruiting specialised expertise, modernising regulations, tackling point-of-care manufacturing issues, and promoting flexibility in GMP application [161].
- clinical translation. Although FDM is the most studied academic approach with many formulation studies and geometry/acceptability trials, continuous in-line quality control for routine clinical use, commercialisation of standardised API-loaded filaments, and GMP-compliant implementations are still lacking. DPE is still limited by variable powder flow, non-uniform API distribution, and a lack of validated quality control methods. Prior to clinical implementation, feed systems, stability, and GMP/point-of-care conditions must be certified. Regarding SSE, some clinical studies were already performed on paediatric 3DP drugs developed and manufactured in hospital pharmacies with a GMP-certified 3D printer. They demonstrated, on the one hand, the need for this therapeutic alternative, but also the feasibility of point-of-care manufacturing of small batches of 3D-printed medicines and their acceptability [103,121,122]. Customised dosing was based on routinely monitored amino acid blood levels and guided the 3D-printed formulations. The focus was on comparing adherence and acceptability versus conventionally compounded medicines [122]. In addition to that, 3D printing is slowly but surely integrating itself as a safer and more effective way of preparing dosage forms when compared to compounding in community pharmacies. One study prepared capsules with minoxidil with a 3D printer by automatic filling, effectively reducing the time needed for production. The stability of the pharma-inks and capsules was also tested and found to be adequate [153]. This demonstrates SSE’s advanced technological maturity. Scaling-up, standardised regulatory procedures, and dependable automated QC are the remaining challenges, nonetheless.
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| HME | Hot melt extrusion |
| FDM | Fused deposition modelling |
| DPE | Direct powder extrusion |
| SSE | Semi-solid extrusion |
| SLS | Selective laser sintering |
| BJ | Binder-jet |
| WHO | World Health Organization |
| APIs | Active pharmaceutical ingredients |
| ODTs | Orodispersible tablets |
| PAM | Pressure-assisted microsyringe |
| ODFs | Orodispersible films |
| Tm | Melting temperature |
| Tg | Glass transition temperature |
| STEP | Safety and Toxicity of Excipients for Paediatrics |
| GRAS | Generally Recognised as Safe |
| HPC | Hydroxypropylcellulose |
| HPMC | Hydroxypropylmetylcellulose |
| HPMC-AS | Hypromellose acetate succinate |
| BCS | Biopharmaceutical Classification System |
| PVP | Polyvinylpyrrolidone |
| PVA | Poly(vinyl) alcohol |
| PEO | Polyethylene oxide |
| PEG | Polyethylene glycol |
| TPGS | D-α-Tocopheryl PEG 1000 succinate |
| DPH | Diphenhydramine hydrochloride |
| FTIR | Fourier Transform Infrared Spectroscopy |
| CMS-Na | Sodium carboxymethyl starch |
| GABA | Gamma-aminobutyric acid |
| SSG | Sodium starch glycolate |
| FDA | Food and Drug Administration |
| CDI | Chewing Difficulty Index |
| DoD | Drop on demand |
| CIJ | Continuous inkjet |
| MW | Molecular weight |
| PBPK | Physiologically based pharmacokinetic |
| CJ-3DP | Colour-jet 3D printing |
| AIDS | Acquired Immune Deficiency Syndrome |
| NIR | Near-infrared |
| TRL | Technology Readiness Level |
| GMP | Good Manufacturing Practice |
References
- Lopez, F.L.; Ernest, T.B.; Tuleu, C.; Gul, M.O. Formulation approaches to pediatric oral drug delivery: Benefits and limitations of current platforms. Expert. Opin. Drug Deliv. 2015, 12, 1727–1740. [Google Scholar] [CrossRef]
- Sam, T.; Ernest, T.B.; Walsh, J.; Williams, J.L. A benefit/risk approach towards selecting appropriate pharmaceutical dosage forms—An application for paediatric dosage form selection. Int. J. Pharm. 2012, 435, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Matsui, D. Current Issues in Pediatric Medication Adherence. Pediatr. Drugs 2007, 9, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.; Cram, A.; Woertz, K.; Breitkreutz, J.; Winzenburg, G.; Turner, R.; Tuleu, C. Playing hide and seek with poorly tasting paediatric medicines: Do not forget the excipients. Adv. Drug Deliv. Rev. 2014, 73, 14–33. [Google Scholar] [CrossRef]
- Belayneh, A.; Tadese, E.; Molla, F. Safety and biopharmaceutical challenges of excipients in off-label pediatric formulations. Int. J. Gen. Med. 2020, 13, 1051–1066. [Google Scholar] [CrossRef]
- Zajicek, A.; Fossler, M.J.; Barrett, J.S.; Worthington, J.H.; Ternik, R.; Charkoftaki, G.; Lum, S.; Breitkreutz, J.; Baltezor, M.; Macheras, P.; et al. A report from the pediatric formulations task force: Perspectives on the state of child-friendly oral dosage forms. AAPS J. 2013, 15, 1072–1081. [Google Scholar] [CrossRef]
- Dumpa, N.; Butreddy, A.; Wang, H.; Komanduri, N.; Bandari, S.; Repka, M.A. 3D printing in personalized drug delivery: An overview of hot-melt extrusion-based fused deposition modeling. Int. J. Pharm. 2021, 600, 120501. [Google Scholar] [CrossRef]
- Madla, C.M.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing Technologies, Implementation and Regulation: An Overview. In 3D Printing of Pharmaceuticals; Basit, A.W., Gaisford, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 21–40. ISBN 978-3-319-90755-0. [Google Scholar] [CrossRef]
- Ayyoubi, S.; Ruijgrok, L.; van der Kuy, H.; ten Ham, R.; Thielen, F. What Does Pharmaceutical 3D Printing Cost? A Framework and Case Study with Hydrocortisone for Adrenal Insufficiency. Pharmacoecon. Open 2025, 9, 207–215. [Google Scholar] [CrossRef]
- McCloskey, A.P.; Bracken, L.; Vasey, N.; Ehtezazi, T. 3D printing—An alternative strategy for pediatric medicines. Expert. Rev. Clin. Pharmacol. 2023, 16, 613–616. [Google Scholar] [CrossRef]
- Ahmed, M.; Tomlin, S.; Tuleu, C.; Garfield, S. Real-World Evidence of 3D Printing of Personalised Paediatric Medicines and Evaluating Its Potential in Children with Cancer: A Scoping Review. Pharmaceutics 2024, 16, 1212. [Google Scholar] [CrossRef] [PubMed]
- Preis, M.; Öblom, H. 3D-Printed Drugs for Children-Are We Ready Yet? AAPS PharmSciTech 2017, 18, 303–308. [Google Scholar] [CrossRef]
- Lafeber, I.; Ruijgrok, E.J.; Guchelaar, H.J.; Schimmel, K.J.M. 3D Printing of Pediatric Medication: The End of Bad Tasting Oral Liquids?-A Scoping Review. Pharmaceutics 2022, 14, 416. [Google Scholar] [CrossRef]
- Racaniello, G.F.; Silvestri, T.; Pistone, M.; D’Amico, V.; Arduino, I.; Denora, N.; Lopedota, A.A. Innovative Pharmaceutical Techniques for Paediatric Dosage Forms: A Systematic Review on 3D Printing, Prilling/Vibration and Microfluidic Platform. J. Pharm. Sci. 2024, 113, 1726–1748. [Google Scholar] [CrossRef]
- Ianno, V.; Vurpillot, S.; Prillieux, S.; Espeau, P. Pediatric Formulations Developed by Extrusion-Based 3D Printing: From Past Discoveries to Future Prospects. Pharmaceutics 2024, 16, 441. [Google Scholar] [CrossRef]
- Quodbach, J.; Bogdahn, M.; Breitkreutz, J.; Chamberlain, R.; Eggenreich, K.; Elia, A.G.; Gottschalk, N.; Gunkel-Grabole, G.; Hoffmann, L.; Kapote, D.; et al. Quality of FDM 3D Printed Medicines for Pediatrics: Considerations for Formulation Development, Filament Extrusion, Printing Process and Printer Design. Ther. Innov. Regul. Sci. 2022, 56, 910–928. [Google Scholar] [CrossRef]
- Tong, H.; Zhang, J.; Ma, J.; Zhang, J. Perspectives on 3D printed personalized medicines for pediatrics. Int. J. Pharm. 2024, 653, 123867. [Google Scholar] [CrossRef] [PubMed]
- Tegegne, A.M.; Ayenew, K.D.; Selam, M.N. Review on Recent Advance of 3DP-Based Pediatric Drug Formulations. Biomed. Res. Int. 2024, 2024, 4875984. [Google Scholar] [CrossRef]
- Lu, H.; Rosenbaum, S. Developmental pharmacokinetics in pediatric populations. J. Pediatr. Pharmacol. Ther. 2014, 19, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Zhao, L.; Shen, L. Medication adherence and pharmaceutical design strategies for pediatric patients: An overview. Drug Discov. Today 2023, 28, 103766. [Google Scholar] [CrossRef] [PubMed]
- Zahn, J.; Hoerning, A.; Trollmann, R.; Rascher, W.; Neubert, A. Manipulation of medicinal products for oral administration to paediatric patients at a german university hospital: An observational study. Pharmaceutics 2020, 12, 583. [Google Scholar] [CrossRef]
- Binson, G.; Sanchez, C.; Waton, K.; Chanat, A.; Maio, M.D.; Beuzit, K.; Dupuis, A. Accuracy of dose administered to children using off-labelled or unlicensed oral dosage forms. Pharmaceutics 2021, 13, 1014. [Google Scholar] [CrossRef]
- Siafaka, P.; Ipekci, E.; Caglar, E.Ş.; Ustundag Okur, N.; Buyukkayhan, D. Current status of pediatric formulations for chronic and acute children’ diseases: Applications and future perspectives. Medeni. Med. J. 2021, 36, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, H.K.; Marriott, J.F. Formulations for children: Problems and solutions. Br. J. Clin. Pharmacol. 2015, 79, 405–418. [Google Scholar] [CrossRef]
- Ruiz, F.; Nunn, A.; Gill, A.; Clapham, D.; Fotaki, N.; Salunke, S.; Cram, A.; O’Brien, F. A review of paediatric injectable drug delivery to inform the study of product acceptability—An introduction. Eur. J. Pharm. Biopharm. 2023, 188, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Charro, M.B.; Guy, R.H. Effective use of transdermal drug delivery in children. Adv. Drug Deliv. Rev. 2014, 73, 63–82. [Google Scholar] [CrossRef]
- Mistry, P.; Batchelor, H. Evidence of acceptability of oral paediatric medicines: A review. J. Pharm. Pharmacol. 2017, 69, 361–376. [Google Scholar] [CrossRef]
- Gaikwad, S.S.; Morales, J.O.; Lande, N.B.; Catalán-Figueroa, J.; Laddha, U.D.; Kshirsagar, S.J. Exploring paediatric oral suspension development: Challenges, requirements, and formulation advancements. Int. J. Pharm. 2024, 657, 124169. [Google Scholar] [CrossRef]
- Michele, T.M.; Knorr, B.; Vadas, E.B.; Reiss, T.F. Safety of chewable tablets for children. J. Asthma 2002, 39, 391–403. [Google Scholar] [CrossRef]
- Rai, A.; Rawat, S.S.; Rathi, R.; Raina, D.; Odeku, O.A.; Singh, I. Mucoadhesive Drug Delivery Systems for Pediatric and Geriatric Patients. Fabad J. Pharm. Sci. 2023, 48, 561–584. [Google Scholar] [CrossRef]
- Tai, J.; Han, M.; Lee, D.; Park, I.H.; Lee, S.H.; Kim, T.H. Different Methods and Formulations of Drugs and Vaccines for Nasal Administration. Pharmaceutics 2022, 14, 1073. [Google Scholar] [CrossRef] [PubMed]
- Palmer, E.A. How Safe are Ocular Drugs in Pediatrics? Ophthalmology 1986, 93, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Magdy, M.; Elmowafy, E.; Elassal, M.; Ishak, R.A.H. Localized drug delivery to the middle ear: Recent advances and perspectives for the treatment of middle and inner ear diseases. J. Drug Deliv. Sci. Technol. 2022, 69, 103149. [Google Scholar] [CrossRef]
- Kwok, P.C.L.; Chan, H.K. Delivery of inhalation drugs to children for asthma and other respiratory diseases. Adv. Drug Deliv. Rev. 2014, 73, 83–88. [Google Scholar] [CrossRef]
- Hanning, S.M.; Walker, E.; Sutcliffe, E.; Tuleu, C. The rectal route of medicine administration for children: Let’s get to the bottom of it! Eur. J. Pharm. Biopharm. 2020, 157, 25–27. [Google Scholar] [CrossRef]
- Pires, L.R.; Vinayakumar, K.B.; Turos, M.; Miguel, V.; Gaspar, J. A perspective on microneedle-based drug delivery and diagnostics in paediatrics. J. Pers. Med. 2019, 9, 49. [Google Scholar] [CrossRef]
- Tambe, S.; Jain, D.; Agarwal, Y.; Amin, P. Hot-melt extrusion: Highlighting recent advances in pharmaceutical applications. J. Drug Deliv. Sci. Technol. 2021, 63, 102452. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.M.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef]
- Fanous, M.; Gold, S.; Hirsch, S.; Ogorka, J.; Imanidis, G. Development of immediate release (IR) 3D-printed oral dosage forms with focus on industrial relevance. Eur. J. Pharm. Sci. 2020, 155, 105558. [Google Scholar] [CrossRef]
- Yang, T.L.; Stogiannari, M.; Janeczko, S.; Khoshan, M.; Lin, Y.; Isreb, A.; Habashy, R.; Giebułtowicz, J.; Peak, M.; Alhnan, M.A. Towards point-of-care manufacturing and analysis of immediate-release 3D printed hydrocortisone tablets for the treatment of congenital adrenal hyperplasia. Int. J. Pharm. 2023, 642, 123072. [Google Scholar] [CrossRef]
- Gorkem Buyukgoz, G.; Kossor, C.G.; Ji, S.; Guvendiren, M.; Davé, R.N. Dose Titration of Solid Dosage Forms via FDM 3D-Printed Mini-Tablets. Pharmaceutics 2022, 14, 2305. [Google Scholar] [CrossRef] [PubMed]
- Krause, J.; Müller, L.; Sarwinska, D.; Seidlitz, A.; Sznitowska, M.; Weitschies, W. 3D printing of mini tablets for pediatric use. Pharmaceuticals 2021, 14, 143. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Fullbrook, D.H.G.; Vilain, L.; Derrar, Y.; Nandi, U.; Grau, C.; Morales, A.; Hooper, G.; Hiezl, Z.; Douroumis, D. Personalised tasted masked chewable 3d printed fruit-chews for paediatric patients. Pharmaceutics 2021, 13, 1301. [Google Scholar] [CrossRef]
- Patel, H.; Raje, V.; Maczko, P.; Patel, K. Application of 3D printing technology for the development of dose adjustable geriatric and pediatric formulation of celecoxib. Int. J. Pharm. 2024, 655, 123941. [Google Scholar] [CrossRef] [PubMed]
- Kocabas, L.I.; Ayyoubi, S.; Tajqurishi, M.; Quodbach, J.; Vermonden, T.; Kok, R.J. 3D-printed prednisolone phosphate suppositories with tunable dose and rapid release for the treatment of inflammatory bowel disease. Int. J. Pharm. 2024, 649, 123639. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Model List of Essential Medicines for Children 9th List (2023). Available online: https://iris.who.int/bitstream/handle/10665/371091/WHO-MHP-HPS-EML-2023.03-eng.pdf?sequence=1 (accessed on 7 January 2025).
- Wang, H.; Dumpa, N.; Bandari, S.; Durig, T.; Repka, M.A. Fabrication of Taste-Masked Donut-Shaped Tablets Via Fused Filament Fabrication 3D Printing Paired with Hot-Melt Extrusion Techniques. AAPS PharmSciTech 2020, 21, 243. [Google Scholar] [CrossRef]
- Roulon, S.; Soulairol, I.; Lavastre, V.; Payre, N.; Cazes, M.; Delbreilh, L.; Alié, J. Production of reproducible filament batches for the fabrication of 3d printed oral forms. Pharmaceutics 2021, 13, 472. [Google Scholar] [CrossRef]
- Parulski, C.; Bya, L.A.; Goebel, J.; Servais, A.C.; Lechanteur, A.; Evrard, B. Development of 3D printed mini-waffle shapes containing hydrocortisone for children’s personalized medicine. Int. J. Pharm. 2023, 642, 123131. [Google Scholar] [CrossRef]
- Palekar, S.; Kumar, P.; Mishra, S.M.; Kipping, T.; Patel, K. Application of 3D printing technology and quality by design approach for development of age-appropriate pediatric formulation of baclofen. Int. J. Pharm. 2019, 556, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3D Printed “Starmix” Drug Loaded Dosage Forms for Paediatric Applications. Pharm. Res. 2018, 35, 34. [Google Scholar] [CrossRef]
- Pawar, A.; Karanwad, T.; Banerjee, S. 3D printed tinidazole tablets coupled with melt-extrusion techniques for formulating child friendly medicines. Eur. J. Pharm. Biopharm. 2024, 203, 114471. [Google Scholar] [CrossRef]
- Łyszczarz, E.; Brniak, W.; Szafraniec-Szczęsny, J.; Majka, T.M.; Majda, D.; Zych, M.; Pielichowski, K.; Jachowicz, R. The impact of the preparation method on the properties of orodispersible films with aripiprazole: Electrospinning vs. casting and 3D printing methods. Pharmaceutics 2021, 13, 1122. [Google Scholar] [CrossRef] [PubMed]
- Monteil, M.; Sanchez-Ballester, N.M.; Aubert, A.; Gimello, O.; Begu, S.; Soulairol, I. HME coupled with FDM 3D printing of a customized oral solid form to treat pediatric epilepsy. Int. J. Pharm. 2025, 673, 125345. [Google Scholar] [CrossRef]
- Ferreira, M.; Lopes, C.M.; Gonçalves, H.; Pinto, J.F.; Catita, J. Personalised Esomeprazole and Ondansetron 3D Printing Formulations in Hospital Paediatric Environment: I-Pre-Formulation Studies. Appl. Sci. 2022, 12, 10585. [Google Scholar] [CrossRef]
- Persaud, S.; Eid, S.; Swiderski, N.; Serris, I.; Cho, H. Preparations of rectal suppositories containing artesunate. Pharmaceutics 2020, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Liu, D.; Xie, H.; Sun, Y.; Fang, Y.; Du, L.; Jin, Y. 3D-printed cannabidiol hollow suppositories for treatment of epilepsy. Int. J. Pharm. 2025, 670, 125141. [Google Scholar] [CrossRef]
- Ahirwar, K.; Shukla, R. Preformulation Studies: A Versatile Tool in Formulation Design. In Drug Formulation Design; Intech Open: London, UK, 2023; ISBN 978-1-83768-471-7. [Google Scholar] [CrossRef]
- EMA Guideline on Pharmaceutical Development of Medicines for Paediatric Use. Available online: https://www.tga.gov.au/sites/default/files/2024-07/Guidelineonpharmaceuticaldevelopmentofmedicinesforpaediatric%20use%20.pdf (accessed on 6 October 2025).
- Kristensen, H.G. WHO guideline development of paediatric medicines: Points to consider in pharmaceutical development. Int. J. Pharm. 2012, 435, 134–135. [Google Scholar] [CrossRef]
- STEP Database. Available online: https://step-db.ucl.ac.uk/eupfi/appDirectLink.do?appFlag=login (accessed on 13 October 2025).
- Salunke, S.; Giacoia, G.; Tuleu, C. The STEP (safety and toxicity of excipients for paediatrics) database. Part 1-A need assessment study. Int. J. Pharm. 2012, 435, 101–111. [Google Scholar] [CrossRef]
- Solanki, N.G.; Tahsin, M.; Shah, A.V.; Serajuddin, A.T.M. Formulation of 3D Printed Tablet for Rapid Drug Release by Fused Deposition Modeling: Screening Polymers for Drug Release, Drug-Polymer Miscibility and Printability. J. Pharm. Sci. 2018, 107, 390–401. [Google Scholar] [CrossRef]
- Couți, N.; Porfire, A.; Iovanov, R.; Crișan, A.G.; Iurian, S.; Casian, T.; Tomuță, I. Polyvinyl Alcohol, a Versatile Excipient for Pharmaceutical 3D Printing. Polymers 2024, 16, 517. [Google Scholar] [CrossRef]
- Parteck® MXP Technical Information. Available online: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/203/518/parteck-mxp-techinfo-141464-mk.pdf (accessed on 26 March 2024).
- Bracken, L.; Habashy, R.; McDonough, E.; Wilson, F.; Shakeshaft, J.; Ohia, U.; Garcia-Sorribes, T.; Isreb, A.; Alhnan, M.A.; Peak, M. Creating Acceptable Tablets 3D (CAT 3D): A Feasibility Study to Evaluate the Acceptability of 3D Printed Tablets in Children and Young People. Pharmaceutics 2022, 14, 516. [Google Scholar] [CrossRef] [PubMed]
- Eudragit E PO. Available online: https://www.pharmaexcipients.com/product/eudragit-e-po/?attachment_id=201953&download_file=2ife21drt18ev (accessed on 1 April 2024).
- Alhijjaj, M.; Belton, P.; Qi, S. An investigation into the use of polymer blends to improve the printability of and regulate drug release from pharmaceutical solid dispersions prepared via fused deposition modeling (FDM) 3D printing. Eur. J. Pharm. Biopharm. 2016, 108, 111–125. [Google Scholar] [CrossRef]
- Gelucire 48/16. Available online: https://www.pharmaexcipients.com/wp-content/uploads/2020/03/Gelucire-48-16_solubility-and-bioavailability-enhancer-from-Gattefosse.pdf (accessed on 1 April 2024).
- Januskaite, P.; Xu, X.; Ranmal, S.R.; Gaisford, S.; Basit, A.W.; Tuleu, C.; Goyanes, A. I spy with my little eye: A paediatric visual preferences survey of 3d printed tablets. Pharmaceutics 2020, 12, 1100. [Google Scholar] [CrossRef]
- Kozarewicz, P. Regulatory perspectives on acceptability testing of dosage forms in children. Int. J. Pharm. 2014, 469, 245–248. [Google Scholar] [CrossRef]
- Mitra, B.; Thool, P.; Meruva, S.; Aycinena, J.A.; Li, J.; Patel, J.; Patel, K.; Agarwal, A.; Karki, S.; Bowen, W. Decoding the small size challenges of mini-tablets for enhanced dose flexibility and micro-dosing. Int. J. Pharm. 2020, 574, 118905. [Google Scholar] [CrossRef] [PubMed]
- Klingmann, V.; Spomer, N.; Lerch, C.; Stoltenberg, I.; Frömke, C.; Bosse, H.M.; Breitkreutz, J.; Meissner, T. Favorable acceptance of mini-tablets compared with syrup: A randomized controlled trial in infants and preschool children. J. Pediatr. 2013, 163, 1728–1732.e1. [Google Scholar] [CrossRef]
- Lura, A.; Tardy, G.; Kleinebudde, P.; Breitkreutz, J. Tableting of mini-tablets in comparison with conventionally sized tablets: A comparison of tableting properties and tablet dimensions. Int. J. Pharm. X 2020, 2, 100061. [Google Scholar] [CrossRef]
- Kukkar, V.; Anand, V.; Kataria, M.; Gera, M.; Choudhury, P.K. Mixing and formulation of low dose drugs: Underlying problems and solutions. Thai J. Pharm. Sci. 2008, 32, 43–58. [Google Scholar] [CrossRef]
- Cilurzo, F.; Musazzi, U.M.; Franzé, S.; Selmin, F.; Minghetti, P. Orodispersible dosage forms: Biopharmaceutical improvements and regulatory requirements. Drug Discov. Today 2018, 23, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Spector, A.C.; Reed, D.R.; Coldwell, S.E. The Bad Taste of Medicines: Overview of Basic Research on Bitter Taste. Clin. Ther. 2013, 35, 1225. [Google Scholar] [CrossRef] [PubMed]
- Jannin, V.; Lemagnen, G.; Gueroult, P.; Larrouture, D.; Tuleu, C. Rectal route in the 21st Century to treat children. Adv. Drug Deliv. Rev. 2014, 73, 34–49. [Google Scholar] [CrossRef]
- Aguilar-de-Leyva, Á.; Casas, M.; Ferrero, C.; Linares, V.; Caraballo, I. 3D Printing Direct Powder Extrusion in the Production of Drug Delivery Systems: State of the Art and Future Perspectives. Pharmaceutics 2024, 16, 437. [Google Scholar] [CrossRef]
- Pistone, M.; Racaniello, G.F.; Rizzi, R.; Iacobazzi, R.M.; Arduino, I.; Lopalco, A.; Lopedota, A.A.; Denora, N. Direct cyclodextrin based powder extrusion 3D printing of budesonide loaded mini-tablets for the treatment of eosinophilic colitis in paediatric patients. Int. J. Pharm. 2023, 632, 122592. [Google Scholar] [CrossRef] [PubMed]
- Boniatti, J.; Januskaite, P.; da Fonseca, L.B.; Viçosa, A.L.; Amendoeira, F.C.; Tuleu, C.; Basit, A.W.; Goyanes, A.; Ré, M.I. Direct powder extrusion 3d printing of praziquantel to overcome neglected disease formulation challenges in paediatric populations. Pharmaceutics 2021, 13, 1114. [Google Scholar] [CrossRef]
- Racaniello, G.F.; Pistone, M.; Meazzini, C.; Lopedota, A.; Arduino, I.; Rizzi, R.; Lopalco, A.; Musazzi, U.M.; Cilurzo, F.; Denora, N. 3D printed mucoadhesive orodispersible films manufactured by direct powder extrusion for personalized clobetasol propionate based paediatric therapies. Int. J. Pharm. 2023, 643, 123214. [Google Scholar] [CrossRef]
- Tabriz, A.G.; Nandi, U.; Scoutaris, N.; Sanfo, K.; Alexander, B.; Gong, Y.; Hui, H.W.; Kumar, S.; Douroumis, D. Personalised paediatric chewable Ibuprofen tablets fabricated using 3D micro-extrusion printing technology. Int. J. Pharm. 2022, 626, 122135. [Google Scholar] [CrossRef]
- Mora-Castaño, G.; Rodríguez-Pombo, L.; Carou-Senra, P.; Januskaite, P.; Rial, C.; Bendicho-Lavilla, C.; Couce, M.L.; Millán-Jiménez, M.; Caraballo, I.; Basit, A.W.; et al. Optimising 3D printed medications for rare diseases: In-line mass uniformity testing in direct powder extrusion 3D printing. Int. J. Pharm. 2025, 668, 124964. [Google Scholar] [CrossRef] [PubMed]
- Totaro, M.; Racaniello, G.F.; Lopalco, A.; Lopedota, A.A.; Denora, N. Development of 3D-Printed Captopril Mini-Tablets with customized release profiles for paediatric hypertension therapy. Int. J. Pharm. 2025, 678, 125685. [Google Scholar] [CrossRef]
- Malebari, A.M.; Kara, A.; Khayyat, A.N.; Mohammad, K.A.; Serrano, D.R. Development of Advanced 3D-Printed Solid Dosage Pediatric Formulations for HIV Treatment. Pharmaceuticals 2022, 15, 435. [Google Scholar] [CrossRef]
- Ozon, E.A.; Sarbu, I.; Popovici, V.; Mitu, M.A.; Musuc, A.M.; Karampelas, O.; Velescu, B.S. Three-Dimensional Printing Technologies in Oral Films Manufacturing—A Minireview. Processes 2023, 11, 2628. [Google Scholar] [CrossRef]
- Neagu, O.M.; Ghitea, T.; Marian, E.; Vlase, L.; Vlase, A.M.; Ciavoi, G.; Fehér, P.; Pallag, A.; Bácskay, I.; Nemes, D.; et al. Formulation and Characterization of Mucoadhesive Polymeric Films Containing Extracts of Taraxaci Folium and Matricariae Flos. Molecules 2023, 28, 4002. [Google Scholar] [CrossRef] [PubMed]
- El Aita, I.; Rahman, J.; Breitkreutz, J.; Quodbach, J. 3D-Printing with precise layer-wise dose adjustments for paediatric use via pressure-assisted microsyringe printing. Eur. J. Pharm. Biopharm. 2020, 157, 59–65. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Han, X.; Hong, X.; Li, X.; Zhang, H.; Li, M.; Wang, Z.; Zheng, A. A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future. Pharmaceutics 2023, 15, 416. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Januskaite, P.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A. Semi-solid extrusion 3D printing in drug delivery and biomedicine: Personalised solutions for healthcare challenges. J. Control. Release 2021, 332, 367–389. [Google Scholar] [CrossRef]
- Johannesson, J.; Khan, J.; Hubert, M.; Teleki, A.; Bergström, C.A.S. 3D-printing of solid lipid tablets from emulsion gels. Int. J. Pharm. 2021, 597, 120304. [Google Scholar] [CrossRef]
- Lafeber, I.; Tichem, J.M.; Ouwerkerk, N.; van Unen, A.D.; van Uitert, J.J.D.; Bijleveld-Olierook, H.C.M.; Kweekel, D.M.; Zaal, W.M.; Le Brun, P.P.H.; Guchelaar, H.J.; et al. 3D printed furosemide and sildenafil tablets: Innovative production and quality control. Int. J. Pharm. 2021, 603, 120694. [Google Scholar] [CrossRef]
- Liu, L.; Fu, K.; Hong, S.; Wang, Z.; Mo, M.; Li, S.; Yu, Y.; Chen, J.; Chen, J.; Zeng, W.; et al. Improving the quality and clinical efficacy of subdivided levothyroxine sodium tablets by 3D printing technology. J. Drug Deliv. Sci. Technol. 2023, 89, 105008. [Google Scholar] [CrossRef]
- Tagami, T.; Ito, E.; Kida, R.; Hirose, K.; Noda, T.; Ozeki, T. 3D printing of gummy drug formulations composed of gelatin and an HPMC-based hydrogel for pediatric use. Int. J. Pharm. 2021, 594, 120118. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Kang, D.; Liu, B.; Zhang, H.; Wang, Z.; Gao, X.; Zheng, A. Feasibility of developing hospital preparation by semisolid extrusion 3D printing: Personalized amlodipine besylate chewable tablets. Pharm. Dev. Technol. 2022, 27, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Liang, E.; Wang, Z.; Li, X.; Wang, S.; Han, X.; Chen, D.; Zheng, A. 3D Printing Technology Based on Versatile Gelatin-Carrageenan Gel System for Drug Formulations. Pharmaceutics 2023, 15, 1218. [Google Scholar] [CrossRef]
- Zhu, C.; Tian, Y.; Zhang, E.; Gao, X.; Zhang, H.; Liu, N.; Han, X.; Sun, Y.; Wang, Z.; Zheng, A. Semisolid Extrusion 3D Printing of Propranolol Hydrochloride Gummy Chewable Tablets: An Innovative Approach to Prepare Personalized Medicine for Pediatrics. AAPS PharmSciTech 2022, 23, 166. [Google Scholar] [CrossRef]
- Bahman, M.; Sandler Topelius, N.; Viitala, T. Semi-solid extruded tablets for personalized pediatric use: Development, Quality control and In-Vitro Assessment of Enteral Tube Administration. Eur. J. Pharm. Sci. 2025, 211, 107122. [Google Scholar] [CrossRef]
- Roostar, K.; Meos, A.; Laidmäe, I.; Aruväli, J.; Räikkönen, H.; Peltonen, L.; Airaksinen, S.; Topelius, N.S.; Heinämäki, J.; Paaver, U. Towards a Customized Oral Drug Therapy for Pediatric Applications: Chewable Propranolol Gel Tablets Printed by an Automated Extrusion-Based Material Deposition Method. Pharmaceutics 2025, 17, 881. [Google Scholar] [CrossRef]
- Veselý, M.; Záruba, D.; Elbl, J. Development of 3D-Printed Chewable Gummy Tablets with Adjustable Ondansetron Content for the Treatment of Pediatric Patients. Pharmaceutics 2025, 17, 458. [Google Scholar] [CrossRef]
- Herrada-Manchón, H.; Rodríguez-González, D.; Alejandro Fernández, M.; Suñé-Pou, M.; Pérez-Lozano, P.; García-Montoya, E.; Aguilar, E. 3D printed gummies: Personalized drug dosage in a safe and appealing way. Int. J. Pharm. 2020, 587, 119687. [Google Scholar] [CrossRef]
- Goyanes, A.; Madla, C.M.; Umerji, A.; Duran Piñeiro, G.; Giraldez Montero, J.M.; Lamas Diaz, M.J.; Gonzalez Barcia, M.; Taherali, F.; Sánchez-Pintos, P.; Couce, M.L.; et al. Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: First single-centre, prospective, crossover study in patients. Int. J. Pharm. 2019, 567, 118497. [Google Scholar] [CrossRef]
- Karavasili, C.; Gkaragkounis, A.; Moschakis, T.; Ritzoulis, C.; Fatouros, D.G. Pediatric-friendly chocolate-based dosage forms for the oral administration of both hydrophilic and lipophilic drugs fabricated with extrusion-based 3D printing. Eur. J. Pharm. Sci. 2020, 147, 105291. [Google Scholar] [CrossRef]
- Rycerz, K.; Stepien, K.A.; Czapiewska, M.; Arafat, B.T.; Habashy, R.; Isreb, A.; Peak, M.; Alhnan, M.A. Embedded 3D Printing of Novel Bespoke Soft Dosage Form Concept for Pediatrics. Pharmaceutics 2019, 11, 630. [Google Scholar] [CrossRef] [PubMed]
- Chachlioutaki, K.; Karavasili, C.; Mavrokefalou, E.E.; Gioumouxouzis, C.I.; Ritzoulis, C.; Fatouros, D.G. Quality control evaluation of paediatric chocolate-based dosage forms: 3D printing vs mold-casting method. Int. J. Pharm. 2022, 624, 121991. [Google Scholar] [CrossRef]
- Rouaz-El Hajoui, K.; Herrada-Manchón, H.; Rodríguez-González, D.; Fernández, M.A.; Aguilar, E.; Suñé-Pou, M.; Nardi-Ricart, A.; Pérez-Lozano, P.; García-Montoya, E. Pellets and gummies: Seeking a 3D printed gastro-resistant omeprazole dosage for paediatric administration. Int. J. Pharm. 2023, 643, 123289. [Google Scholar] [CrossRef]
- Suárez-González, J.; Magariños-Triviño, M.; Díaz-Torres, E.; Cáceres-Pérez, A.R.; Santoveña-Estévez, A.; Fariña, J.B. Individualized orodispersible pediatric dosage forms obtained by molding and semi-solid extrusion by 3D printing: A comparative study for hydrochlorothiazide. J. Drug Deliv. Sci. Technol. 2021, 66, 102884. [Google Scholar] [CrossRef]
- Eduardo, D.T.; Ana, S.E.; José, B.F. A micro-extrusion 3D printing platform for fabrication of orodispersible printlets for pediatric use. Int. J. Pharm. 2021, 605, 120854. [Google Scholar] [CrossRef]
- Hu, J.; Fitaihi, R.; Abukhamees, S.; Abdelhakim, H.E. Formulation and Characterisation of Carbamazepine Orodispersible 3D-Printed Mini-Tablets for Paediatric Use. Pharmaceutics 2023, 15, 250. [Google Scholar] [CrossRef]
- Sjöholm, E.; Sandler, N. Additive manufacturing of personalized orodispersible warfarin films. Int. J. Pharm. 2019, 564, 117–123. [Google Scholar] [CrossRef]
- Shokraneh, F.; Filppula, A.M.; Tornio, A.; Aruväli, J.; Paaver, U.; Topelius, N.S. Automated extrusion-based dispensing: Personalized dosing and quality control of clopidogrel tablets for pediatric care. Eur. J. Pharm. Sci. 2025, 204, 106967. [Google Scholar] [CrossRef]
- Protopapa, C.; Siamidi, A.; Kolipaka, S.S.; Junqueira, L.A.; Douroumis, D.; Vlachou, M. In Vitro Profile of Hydrocortisone Release from Three-Dimensionally Printed Paediatric Mini-Tablets. Pharmaceutics 2024, 16, 385. [Google Scholar] [CrossRef]
- Bernhardt, M.B.; Shokraneh, F.; Hrizanovska, L.; Lahtinen, J.; Brasher, C.A.; Sandler, N. Automated 3D Printing-Based Non-Sterile Compounding Technology for Pediatric Corticosteroid Dosage Forms in a Health System Pharmacy Setting. Pharmaceutics 2025, 17, 762. [Google Scholar] [CrossRef]
- Santamaría, K.J.; Anaya, B.J.; Lalatsa, A.; González-Barranco, P.; Cantú-Cárdenas, L.; Serrano, D.R. Engineering 3D Printed Gummies Loaded with Metformin for Paediatric Use. Gels 2024, 10, 620. [Google Scholar] [CrossRef]
- Chachlioutaki, K.; Li, X.; Koltsakidis, S.; Abdelhakim, H.E.; Bouropoulos, N.; Tzetzis, D.; Karavasili, C.; Fatouros, D.G. How sugar types and fabrication methods affect palatability in paediatric-friendly oromucosal pullulan films of chlorpromazine hydrochloride. Carbohydr. Polym. 2025, 348, 122802. [Google Scholar] [CrossRef]
- Imbriano, A.; Fratini, C.; Bondi, G.; D’Abbrunzo, I.; Bertoni, S.; Tiboni, M.; Abruzzo, A.; Hasa, D.; Pagano, C.; Casettari, L. 3D-printed chewable gummy tablets: A new tool for oral amoxicillin administration in paediatric population. Int. J. Pharm. 2025, 677, 125645. [Google Scholar] [CrossRef]
- Moreira, A.d.O.E.; Neta, L.M.S.A.; Pietroluongo, M.; Matos, A.P.d.S.; Correa, B.B.; Ortiz, B.H.; Guimarães, A.d.S.; Nele, M.; Santos, C.M.; Fai, A.E.C.; et al. Three-Dimensional-Printed Isoniazid Chewable Gels for On-Demand Latent Tuberculosis Treatment in Children. Pharmaceutics 2025, 17, 658. [Google Scholar] [CrossRef]
- Dadkhah, A.; Gutowski, T.; Wansing, E.M.; von Hugo, A.; Woessmann, W.; Winkler, B.; Franke, G.; Baehr, M.; Langebrake, C. Development of 3D printed dexamethasone chewable tablets for prophylaxis of chemotherapy-induced nausea and vomiting in children. Int. J. Pharm. X 2025, 10, 100380. [Google Scholar] [CrossRef]
- Paccione, N.; Guarnizo-Herrero, V.; Navarro-Alvarez, A.; Scaini, D.; Larrarte, E.; Pedraz, J.L. Development of personalized dexamethasone orodispersible solid oral dosage forms by semisolid extrusion 3D printing. Int. J. Pharm. 2025, 683, 126090. [Google Scholar] [CrossRef]
- Stoops, M.; Do, B.; Ramos, S.; Tan, B.X.; Sheng Chua, N.Y.; Mazet, R.; Guiblin, N.; Michelet, A.; Flynn, S.; Abbou, S.; et al. Clinical implementation of a paediatric 3D-printed combination of Sulfamethoxazole and Trimethoprim. Int. J. Pharm. 2025, 676, 125581. [Google Scholar] [CrossRef]
- Rodríguez-Pombo, L.; de Castro-López, M.J.; Sánchez-Pintos, P.; Giraldez-Montero, J.M.; Januskaite, P.; Duran-Piñeiro, G.; Dolores Bóveda, M.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A.; et al. Paediatric clinical study of 3D printed personalised medicines for rare metabolic disorders. Int. J. Pharm. 2024, 657, 124140. [Google Scholar] [CrossRef]
- Strand, D.S.; Kim, D.; Peura, D.A. 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver 2017, 11, 27–37. [Google Scholar] [CrossRef]
- CAPTEX® MEDIUM CHAIN TRIGLYCERIDES. Available online: https://www.abiteccorp.com/en/product-repository/captex-medium-chain-triglycerides/ (accessed on 16 September 2024).
- Maisine® CC. Available online: https://www.pharmaexcipients.com/wp-content/uploads/2020/03/Maisine-CC_gattefosse-pharmaceutical-oil-for-solubility-and-bioavailability-enhancement.pdf (accessed on 16 September 2024).
- Gelucire® 44/14. Available online: https://www.gattefosse.com/pharmaceuticals/product-finder/gelucire-4414 (accessed on 23 January 2025).
- CAPMUL® MONO AND DIGLYCERIDES. Available online: https://www.abiteccorp.com/en/product-repository/capmul-mono-and-diglycerides/ (accessed on 16 September 2024).
- Krämer, J.; Gajendran, J.; Guillot, A.; Barakat, A. Chewable Oral Drug Products. In In Vitro Drug Release Testing of Special Dosage Forms; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 27–53. ISBN 9781118675748. [Google Scholar] [CrossRef]
- Parhi, R. A review of three-dimensional printing for pharmaceutical applications: Quality control, risk assessment and future perspectives. J. Drug Deliv. Sci. Technol. 2021, 64, 102571. [Google Scholar] [CrossRef]
- Hirshfield, L.; Giridhar, A.; Taylor, L.S.; Harris, M.T.; Reklaitis, G.V. Dropwise additive manufacturing of pharmaceutical products for solvent-based dosage forms. J. Pharm. Sci. 2014, 103, 496–506. [Google Scholar] [CrossRef]
- Konta, A.A.; García-Piña, M.; Serrano, D.R. Personalised 3D printed medicines: Which techniques and polymers are more successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef]
- Sundarkumar, V.; Wang, W.; Nagy, Z.; Reklaitis, G. Manufacturing pharmaceutical mini-tablets for pediatric patients using drop-on-demand printing. Int. J. Pharm. 2023, 644, 123355. [Google Scholar] [CrossRef]
- Cui, M.; Pan, H.; Fang, D.; Sun, H.; Qiao, S.; Pan, W. Exploration and evaluation of dynamic dose-control platform for pediatric medicine based on Drop-on-Powder 3D printing technology. Int. J. Pharm. 2021, 596, 120201. [Google Scholar] [CrossRef]
- Sundarkumar, V.; Wang, W.; Mills, M.; Oh, S.W.; Nagy, Z.; Reklaitis, G. Developing a Modular Continuous Drug Product Manufacturing System with Real Time Quality Assurance for Producing Pharmaceutical Mini-Tablets. J. Pharm. Sci. 2024, 113, 937–947. [Google Scholar] [CrossRef]
- Li, Z.R.; Wang, C.Y.; Lin, W.W.; Chen, Y.T.; Liu, X.Q.; Jiao, Z. Handling Delayed or Missed Dose of Antiseizure Medications: A Model-Informed Individual Remedial Dosing. Neurology 2023, 100, E921–E931. [Google Scholar] [CrossRef]
- Li, X.; Liang, E.; Hong, X.; Han, X.; Li, C.; Wang, Y.; Wang, Z.; Zheng, A. In vitro and in vivo bioequivalence study of 3d-printed instant-dissolving levetiracetam tablets and subsequent personalized dosing for chinese children based on physiological pharmacokinetic modeling. Pharmaceutics 2022, 14, 20. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, X.; Hong, X.; Han, X.; Li, J.; Duan, S.; Wu, J.; Wang, Z.; Zheng, A. 3D printing personalized orally disintegrating tablets with complex structures for the treatment of special populations. Int. J. Pharm. 2025, 673, 125371. [Google Scholar] [CrossRef]
- Alhnan, M.A.; Okwuosa, T.C.; Sadia, M.; Wan, K.W.; Ahmed, W.; Arafat, B. Emergence of 3D Printed Dosage Forms: Opportunities and Challenges. Pharm. Res. 2016, 33, 1817–1832. [Google Scholar] [CrossRef]
- Wang, Z.; Han, X.; Chen, R.; Li, J.; Gao, J.; Zhang, H.; Liu, N.; Gao, X.; Zheng, A. Innovative color jet 3D printing of levetiracetam personalized paediatric preparations. Asian J. Pharm. Sci. 2021, 16, 374–386. [Google Scholar] [CrossRef]
- Iwanaga, S.; Arai, K.; Nakamura, M. Inkjet bioprinting. In Essentials of 3D Biofabrication and Translation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 61–79. [Google Scholar] [CrossRef]
- Kondiah, P.P.D.; Rants, T.A.; Mdanda, S. An Oral 3D Printed PLGA-Tocopherol PEG Succinate. Biomedicines 2022, 10, 1470. [Google Scholar] [CrossRef]
- Charoo, N.A.; Barakh Ali, S.F.; Mohamed, E.M.; Kuttolamadom, M.A.; Ozkan, T.; Khan, M.A.; Rahman, Z. Selective laser sintering 3D printing–an overview of the technology and pharmaceutical applications. Drug Dev. Ind. Pharm. 2020, 46, 869–877. [Google Scholar] [CrossRef]
- Gueche, Y.A.; Sanchez-Ballester, N.M.; Cailleaux, S.; Bataille, B.; Soulairol, I. Selective laser sintering (Sls), a new chapter in the production of solid oral forms (sofs) by 3d printing. Pharmaceutics 2021, 13, 1212. [Google Scholar] [CrossRef]
- Tarekegn, K.; Lonsako, S.; Hyattsville, M.D.; Ghidey, F. Role of Continuity of Care in HIV/AIDS Treatment and Care Program in Ethiopia. Open Forum Infect. Dis. 2015, 2, 408. [Google Scholar] [CrossRef]
- Kayalar, C.; Rahman, Z.; Mohamed, E.M.; Dharani, S.; Khuroo, T.; Helal, N.; Kuttolamadom, M.A.; Khan, M.A. Preparation and Characterization of 3D-Printed Dose-Flexible Printlets of Tenofovir Disoproxil Fumarate. AAPS PharmSciTech 2023, 24, 171. [Google Scholar] [CrossRef]
- Pansare, S.J.; Kayalar, C.; Shaikh, R.; Dongala, B.P.; Thota, S.K.; Kuttolamadom, M.A.; Rahman, Z.; Khan, M.A. Design of SLS 3D-printed pediatric combination printlets of lamivudine and tenofovir disoproxil fumarate by understanding the impact of formulation and process variables on flow, spectral, thermal and performance characteristics. Int. J. Pharm. 2025, 682, 125942. [Google Scholar] [CrossRef]
- Funk, N.L.; Januskaite, P.; Beck, R.C.R.; Basit, A.W.; Goyanes, A. 3D printed dispersible efavirenz tablets: A strategy for nasogastric administration in children. Int. J. Pharm. 2024, 660, 124299. [Google Scholar] [CrossRef] [PubMed]
- Kayalar, C.; Pansare, S.J.; Sibhat, G.; Kuttolamadom, M.; Rahman, Z.; Khan, M.A. Development and Characterization of Printlets of Lamivudine for Pediatric Patients Using Selective Laser Sintering. Pharmaceuticals 2025, 18, 356. [Google Scholar] [CrossRef] [PubMed]
- Paccione, N.; Guarnizo-Herrero, V.; Ramalingam, M.; Larrarte, E.; Pedraz, J.L. Application of 3D printing on the design and development of pharmaceutical oral dosage forms. J. Control Release 2024, 373, 463–480. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Otero-Espinar, F.J.; Goyanes, Á. 3D printing of pharmaceutical products. In Additive Manufacturing; Elsevier: Amsterdam, The Netherlands, 2021; pp. 569–597. [Google Scholar] [CrossRef]
- Huanbutta, K.; Burapapadh, K.; Sriamornsak, P.; Sangnim, T. Practical Application of 3D Printing for Pharmaceuticals in Hospitals and Pharmacies. Pharmaceutics 2023, 15, 1877. [Google Scholar] [CrossRef]
- Goyanes, A.; Scarpa, M.; Kamlow, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Patient acceptability of 3D printed medicines. Int. J. Pharm. 2017, 530, 71–78. [Google Scholar] [CrossRef]
- Rodríguez-Maciñeiras, X.; Bendicho-Lavilla, C.; Rial, C.; Garba-Mohammed, K.; Worsley, A.; Díaz-Torres, E.; Orive-Martínez, C.; Orive-Mayor, Á.; Basit, A.W.; Alvarez-Lorenzo, C.; et al. Advancing medication compounding: Use of a pharmaceutical 3D printer to auto-fill minoxidil capsules for dispensing to patients in a community pharmacy. Int. J. Pharm. 2025, 671, 125251. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Xu, X.; Ong, J.J.; Teyeb, A.; Gaisford, S.; Campos-Álvarez, A.; Stulz, A.; Marcuta, C.; Kraschew, L.; Mohr, W.; et al. A case study on decentralized manufacturing of 3D printed medicines. Int. J. Pharm. X 2023, 5, 100184. [Google Scholar] [CrossRef] [PubMed]
- Trenfield, S.J.; Xu, X.; Goyanes, A.; Rowland, M.; Wilsdon, D.; Gaisford, S.; Basit, A.W. Releasing fast and slow: Non-destructive prediction of density and drug release from SLS 3D printed tablets using NIR spectroscopy. Int. J. Pharm. X 2023, 5, 100148. [Google Scholar] [CrossRef]
- Bendicho-Lavilla, C.; Rodríguez-Pombo, L.; Januskaite, P.; Rial, C.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A. Ensuring the quality of 3D printed medicines: Integrating a balance into a pharmaceutical printer for in-line uniformity of mass testing. J. Drug Deliv. Sci. Technol. 2024, 92, 105337. [Google Scholar] [CrossRef]
- Salunke, S.; Agrawal, A.; Walsh, J.; Nunn, A.; Hughes, K.; Kuehl, P.; Caivano, G.; Clapham, D.; Thompson, K.; Rumondor, A.; et al. Selecting appropriate excipients for paediatric dosage form − Paediatric excipients risk assessment (PERA) framework—Part 1. Eur. J. Pharm. Biopharm. 2024, 203, 114458. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Salunke, S.; Rumondor, A.; Thompson, K.; Caivano, G.; Walsh, J.; Enright, B.; Sherratt, P.; Hughes, K.; Clapham, D.; et al. Paediatric excipient risk assessment (PERA) tool and application for selecting appropriate excipients for paediatric dosage forms—Part 2. Eur. J. Pharm. Biopharm. 2024, 203, 114447. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, M.; Muñiz Castro, B.; Gavins, F.K.H.; Ong, J.J.; Gaisford, S.; Pérez, G.; Basit, A.W.; Cabalar, P.; Goyanes, A. M3DISEEN: A novel machine learning approach for predicting the 3D printability of medicines. Int. J. Pharm. 2020, 590, 119837. [Google Scholar] [CrossRef] [PubMed]
- FDA Distributed Manufacturing and Point-of-Care Manufacturing of Drugs. Available online: https://www.fda.gov/media/162157/download?attachment (accessed on 8 October 2025).
- DRAFT—EMA Regulatory Science Strategy to 2025. Available online: https://www.ema.europa.eu/system/files/documents/regulatory-procedural-guideline/ema_regulatory_science_to_2025_en.pdf (accessed on 8 October 2025).
| Route of Administration | Dosage Forms | Shortcoming | References |
|---|---|---|---|
| Oral | Solution and syrup | Excipient safety issues Taste, aftertaste, and smell issues Very small volumes used for younger children Measuring device needed Stability issues Misdosing issues when measuring the needed volumes | [20,27] |
| Suspensions and emulsions | Excipient safety issues (solvents, sugar, flavours, dyes) Risk of medication errors because of the need to redisperse the API Thermodynamic instability | [28] | |
| Capsule | Swallowing issues because of their size Risk of choking Taste and aftertaste issues Dosing issues, opening is often needed | [27] | |
| Dispersible tablets, powders, granules, pellets or sprinkles for reconstitution | Instructions can be complicated when reconstituted in solvents Risk of local injury when the used volume of liquid is not appropriate | [24] | |
| Tablets | Swallowing issues because of their size Risk of choking Taste and aftertaste issues Dosing issues, splitting is often needed | [23] | |
| Chewable tablets | Dose flexibility needed Splitting is not possible Controlled release can be technologically challenging Taste masking is difficult Bioavailability may be changed by retention time in the mouth. Potential overdose if used improperly as a candy Patients should be instructed carefully when administering | [1,29] | |
| ODTs | Dose flexibility needed Splitting is prohibited because of their fragility | [1] | |
| ODFs | Mouthfeel and taste issues Controlled release can be technologically challenging Uniformity of dose can be difficult to achieve Only small doses can be incorporated | [1] | |
| Mucoadhesive films | Pharmacokinetic and pharmacodynamic challenges Standard- derived methods for determining in vitro, in vivo, and ex vivo mucoadhesive properties need to be determined before formulation | [30] | |
| Nasal | Sprays, drops | Can cause damage of nasal mucosa The nasal cavity’s state impacts drug absorption | [31] |
| Ocular | Drops, ointments, gels, and inserts | Difficulty of administration Systemic side effect risk due to the fact that ocular dosing is not weight-adjustable Requirement for customised paediatric delivery systems to administer lower drug doses | [24,32] |
| Otic | Ear drops | Needs frequent use which can lead to poor patient compliance | [33] |
| Pulmonar | Aerosol devices | Devices intended for adults are adapted for children Very common off-label use Need for proper face-masks designed for children | [34] |
| Rectal | Suppositories | Portions of adult-use suppositories are used, leading to inaccurate doses, instability, improper shape for rectal insertion Undesirable route in children’s opinion | [24,35] |
| Injectable | Injectable solutions and suspensions | Excipient safety is imperative Higher costs Need for trained specialists for administration Acceptability challenges Serial dilution errors | [25] |
| Transdermal | Transdermal patches | Suitable only for some APIs Difficult to modify drug dose for premature neonates | [26] |
| Transdermal microneedles | Complicated to design proper structures to target the various paediatric age groups | [36] |
| API Name, Percentage, Dose/Unit | Excipients | Design | Size/Volume | Observations | Ref. | ||
|---|---|---|---|---|---|---|---|
| Filament-Forming Polymers | Plasticizers | Others | |||||
| Tablets | |||||||
| Caffeine, 5%, 10%, 20% | HPC SSL/Kollidon VA64/Kollicoat IR | Xylitol/PEG 4000 | PEG4000, maltodextrin, dibasic calcium phosphate—pore formers | Honeycomb | 8 × 16 × 4 mm | - Rapid release - Dissolution rate of drugs might be affected by just 10 °C variation in 3D printing temperature | [39] |
| Amiodarone hydrochloride, 20% | PEO (PolyoxN10) 40% | Glycerol 2% | D-sorbitol 37%—filler, colloidal anhydrous silica 1%—flow activator | Serpentine shape | - | - The influence of powder storage on batch reproducibility, water absorption was observed, which decreased powder flow and had a plasticising effect in the HME process - Content uniformity was proven | [48] |
| Placebo | Eudragit EPO 45% | Triethylcitrate 5% | Sodium stearyl fumarate—flow activator, TiO2 1%—colouring agent, talc 49%—filler | Convex tablets | 6 mm/8 mm/10 mm diameter | - Acceptability study on children aged 4 to 12 years old, 77% found them acceptable for a daily intake - Tablet size, followed by taste, texture, and finally smell, were the factors mentioned as important for the acceptability of a medicine | [66] |
| Hydrocortisone, 10–15%, 2.5 mg to 7.5 mg/tablet | 65.45% Eudragit EPO | Triethylcitrate 4.55% | Sodium stearyl fumarate 4%, TiO2 1%, Talc | Caplet | Diameter < 10 mm | - High drug-loading filaments were incompatible with 3D printing | [40] |
| Caffeine citrate 5%, 10%, 15%, 20% | HPC LF 60% –95% | HPMC K4M 20%—sustained release agent, Eudragit EPO 5–20%—taste masking agent | Doughnut | 10 mm diameter | - API or Eudragit EPO concentration increase led to poor filament printability - The association of polymers demonstrated efficient taste masking | [47] | |
| Tinidazole, 10–15% | Kollidon 25 | Hexagonal, heart, pentagon, mickey-mouse, star, fish | 12 mm/9 mm/6 mm/4 mm diameter and 3 mm height | - By incorporating the API into the polymer matrix, HME is considered to cover up bitter flavours by avoiding direct contact with the taste buds - Complex shapes and geometries were successfully prepared - No additional excipients, hence reducing the excipient toxicity risks | [52] | ||
| Sodium valproate, 10% and 30% | PEO 49-67-70-100% | PEG 6000 21–23–30%/PEG 35000 21 –23–30% | Scaffolds | 13 × 19 × 2.5 mm (width × length × heights), decreased | - To improve water contact, the printed form was made using a grid pattern without a shell - Dosage forms designed to be administered in a liquid form after 15 min of dispersing in water | [54] | |
| Sprinklets | |||||||
| Celecoxib, 10% | 80–90% Aquazol P500 | TPGS 10%, SPL5%, surfactants | Doughnut, heart, star | <5 mm diameter, 2 mm height | - Surfactants also had a plasticizing effect and inhibited precipitation of the API during filament production - Supposed to result in improved swallowability - Celecoxib’s solubility and dissolution profile were greatly enhanced by the amorphous solid dispersion produced by HME | [44] | |
| Mini-tablets | |||||||
| Caffeine or Propranolol hydrochloride, 10% | HPMC (AFFINISOL™ HME 15LV)/ HPC (Klucel ELF) | PEG 6000 10% | Fumed silica 0.5%—flow regulator | Cylindrical | 1.5 to 4 mm diameter | - Printed shape was found to be more irregular the smaller the diameter - Release was similar for both APIs when using HPC | [42] |
| Griseofulvin, 1–20% | 84%/75%/65% HPC SL and 15% Kollicoat Protect | - | - | Cylindrical | 1.5 mm diameter | - Ability to titrate doses in increments of 0.19 mg using single unit mini-tablets | [41] |
| Hydrocortisone, 20%, 2–8 mg/unit | PVP (Kollidon VA64), HPMC (AFFINISOL HME 15LV) | PEG 6000 10%, sorbitol 10% and triethylcitrate 3% | Red iron oxide—colouring agent | Waffle | 31.79 to 132.4 mm3 volume | - Immediate release - Content and mass consistency were proven | [49] |
| Baclofen, 10% | PVA (Parteck MXP) | Sorbitol 10% | - | Caplet | 5 mm/7.5 mm/10 mm diameter | -Printing at higher temperatures leads to high weight uniformity - From the tested patterns (shark fill, linear, hexagonal, diamond), the diamond infill led to the fastest disintegration | [50] |
| Pastilles/Candy-like dosage form | |||||||
| Indomethacin, 20%, 25 mg/unit | HPMCAS 60% (AQOAT AS-MF) | PEG6000 20% | Bottle, heart, ring, bear | 10 to 20 mm diameter | - The drug–polymer interaction through HME process allowed effective taste masking | [51] | |
| Chewable tablets | |||||||
| Diphenhydramine hydrochloride, 2.5%, 12.5 mg/unit | Klucel® ELF 80%, | Gelucire 48/16 14.5%—surfactant, food colours 1%, strawberry flavour 1.1%, sucralose 0.9%, | Smurf, banana, cherry, palm tree | 10 to 20 mm diameter | - Smurf design demonstrated the quickest API release | [43] | |
| Orodispersible films | |||||||
| Aripiprazole, 3.5% | PVA (Poval 4-88) 96.5% | Rectangle-shaped film | 6 cm2 | - The method of 3D printing is compared to electrospinning and solvent casting - Cast films have demonstrated the best stability; after storage, the 3D printed films showed improvements in tensile strength and Young’s modulus - Packaging must ensure stability for a long time | [53] | ||
| Suppositories | |||||||
| Prednisolone sodium phosphate, 4%, 6–30 mg/unit | HPC (Klucel EF) 48%/73%/96% | Mannitol 23–48% | Torpedo-shaped | 16 mm/21 mm/26 mm height | - Size was chosen to match the size of a 1.15 mL infant suppository mold - The mannitol-containing filament had high brittleness - Slow release with small mannitol ratios and immediate release with high mannitol ratios | [45] | |
| Artesunate, 500 mg/unit | PVA | PEG3350, PEG1000 | - Comparison of the 3D-printed products with fused suppositories with free API and API-loaded micelles - The suppository shell was 3D printed and filled with API-PEG mixtures | [56] | |||
| Cannabidiol | Placebo spring with thermoplastic urethane Shell with PVA | Shell with PEG | PEG3350, PEG1000 | - PVA shell absorbs water, dissolves, and slowly releases the API - The spring was made by 3D printing thermoplastic urethane filaments, while the API-loaded shell was made using a 3D-printed metal mold - The hollow structure was designed to increase patient compliance | [57] | ||
| API Name, %, Dose/Unit | Excipients | Design | Size/Volume | Observations | Ref. | ||
|---|---|---|---|---|---|---|---|
| Matrix-Forming Polymers | Plasticizers | Others | |||||
| Tablets | |||||||
| Praziquantel, 35 or 50%, 100 mg API/unit | Kollidon VA 64 50%, 60%, 65%/ PEO 100000 60%/HPC ELF 95% | - | Span 20, Kolliphor SLS Fine 5%—surfactants | Cylindrical | 10 mm diameter | - Efficient taste-masking - Printlets demonstrated stability after a three-month evaluation | [81] |
| Ibuprofen, 40% | Kollidon VA 64, Soluplus, or Eudragit EPO | Cylindrical | 10 mm diameter | - Micro-extrusion technique enables a reduction in the total processing time of 3D printing as well as the waste of produced materials | [83] | ||
| Biotin, 5% | PEO 100000 60%/HPC ELF 95% | Mannitol 35% in the PEO pharma-ink | Capsule-shaped | 2.7 mm diameter, 8.6 mm length | - Examined the implementation of a software-controlled, in-line analytical balance in a pharmaceutical multi-printhead DPE printer - Developed immediate release capsule-shaped tablets with PEO and extended-release ones with HPC | [84] | |
| Mini-tablets | |||||||
| Ritonavir or lopinavir, 25%, 65 mg API/unit | AQOAT® LG (HPMCAS) 51.75% | 22% PEG4000 | 0.75% magnesium stearate | Spherical | 6 mm diameter | - Tablets were first prepared through HME and FDM, but this resulted in drug degradation - DPE was selected due to its decreased process temperature, 80 °C - Printed tablets were compared to commercially produced Kaletra; the mini-tablets had a zero-order release profile | [86] |
| Budesonide 0.59% | AFFINISOL HPMC HME 15 LV 41.84–75.44% | PEG6000 3.97–2.99% | Adjuvant blend 8.17–20% hydroxypropyl-β-cyclodextrin (HP-β-CD) 46.61%—solubility enhancer Eudragit FS 30D—coating agent | Cylindrical | 5 mm diameter | - Targeted delivery of API to the colon was accomplished - Three-month stability evaluation demonstrated an absence of API degradation | [80] |
| Captopril | PVA 4-88 and AFFINISOL HPMC HME 15 LV | Cylindrical | - As a co-polymer, HPMC was added to improve drug encapsulation and guarantee structural integrity - By varying the infill, immediate release and sustained release tablets were created | [85] | |||
| Mucoadhesive films | |||||||
| Clobetasol propionate, 0.20%, 125 µg/film | AFFINISOL HPMC HME 15 LV 0.35% | Polyox™ WSR N10 (PEO) 66.45–86.45% | HP-β-CD 3%—solubility enhancer Chitosan 10–30%—mucoadhesive | Cylindrical | 20 mm diameter | - Retention of API inside the epithelium prevented systemic absorption - A progressive release of the drug over time was demonstrated - Three-month stability was established | [82] |
| FDM | DPE | SSE | |
|---|---|---|---|
| Advantages | - Most researched method - High design versatility - Good resolution - Printed products with good mechanical properties [149] | - Powder mixtures as input materials, no pre-processing - Lower thermal stress on API, compared to FDM [150] - Allows higher drug loads than FDM | - Low thermal stress on API, fit for thermosensitive APIs - Faster overall compared to other technologies - Products obtained via SSE accommodate wide dose ranges |
| Disadvantages | - Filament formulation must grant printability - Thermal treatment applied during extrusion and printing can lead to API degradation - API doses limited by filament printability [149] | - Materials are exposed to heat for a longer time than in FDM [149] - Powder properties (flowability) influence printability | - Lower resolution when compared to FDM and DPE [150] - Material viscosity must be controlled to have a printable feedstock [150] - Variable solidification time [150] -Solvent use |
| Method complexity | - Medium complexity - Three stages: powder mixing, hot-melt extrusion and printing | - Low complexity - Two stages: powder mixing and printing | - Medium complexity - Three stages: semisolid ink Preparation, printing and solidification (cooling/drying) |
| Costs | - Relatively low | - Medium | - medium to high |
| Required know-how | - Basic 3DP principles and design software knowledge - Hot-melt extrusion process and formulation principles - Mechanical characterisation and optimisation of filaments - Pharmaceutical characterization of 3DP dosage forms | - Basic 3DP principles and design software knowledge - Powder flow characterisation and optimisation - Pharmaceutical characterization of 3DP dosage forms | - Basic 3DP principles and design software knowledge - Understanding of the rheology of semisolid products - Cooling/drying process knowledge - Pharmaceutical characterisation of 3DP dosage forms |
| Suitability for printing paediatric dosage forms | - Suitable for customised sizes, shapes, and doses - Dose flexibility and accurate dose titration confirmed - Various paediatric-friendly dosage forms: tablets, mini-tablets, pastilles, chewable tablets, orodispersible dosage forms, chewable tablets, and suppositories - High stability | - Suitable for tablets, minitablets, and mucoadhesive films - Good stability for several months | - Highly suitable for chewable formulations, orodispersible dosage forms, tablets and mini-tablets |
| Translational challenges | - Need for industrially manufactured filaments and GMP scale-up [90] - Non-destructive and inline quality control demand - Few or no clinical implementations | - GMP-scale reproducibility needs a powder feed and flowability control for a consistent API distribution in printlets [79] - Establishing quality control methods | - Rheology and solidification have to be controlled for large scale production [151] - Establishing automated quality control methods |
| Clinical translation and Acceptability | - Used in early acceptability studies with placebo tablets with the highest acceptability score for torus geometry [152], and another study with an 83% overall acceptability, based on the evaluation of 30 participants [66] | - Limited clinical translation for children so far | - Hospital clinical trials for children have been conducted with success [122,103] and stability, human sensory evaluations were assessed in a hospital setting [121] - Established feasibility in a community pharmacy setting [153] |
| Development maturity level | Mid-stage | Emerging | Advanced (for community and hospital pharmacies) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couți, N.; Iurian, S.; Porfire, A.; Casian, T.; Iovanov, R.; Tomuță, I. Three-Dimensional Printing in Paediatrics: Innovative Technology for Manufacturing Patient-Centred Drug Delivery Systems. Pharmaceutics 2025, 17, 1364. https://doi.org/10.3390/pharmaceutics17111364
Couți N, Iurian S, Porfire A, Casian T, Iovanov R, Tomuță I. Three-Dimensional Printing in Paediatrics: Innovative Technology for Manufacturing Patient-Centred Drug Delivery Systems. Pharmaceutics. 2025; 17(11):1364. https://doi.org/10.3390/pharmaceutics17111364
Chicago/Turabian StyleCouți, Nadine, Sonia Iurian, Alina Porfire, Tibor Casian, Rareș Iovanov, and Ioan Tomuță. 2025. "Three-Dimensional Printing in Paediatrics: Innovative Technology for Manufacturing Patient-Centred Drug Delivery Systems" Pharmaceutics 17, no. 11: 1364. https://doi.org/10.3390/pharmaceutics17111364
APA StyleCouți, N., Iurian, S., Porfire, A., Casian, T., Iovanov, R., & Tomuță, I. (2025). Three-Dimensional Printing in Paediatrics: Innovative Technology for Manufacturing Patient-Centred Drug Delivery Systems. Pharmaceutics, 17(11), 1364. https://doi.org/10.3390/pharmaceutics17111364










