Development of 3D-Printed Gel-Based Supplement-Containing Tablets with Tailored Release Profiles for Neurological Pain Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Powders Physical Properties Evaluation
2.2.1. Powder Flow
2.2.2. Moisture of the Powder
2.3. Three-Dimensional Printing
2.3.1. Three-Dimensional Gel Tablets’ Base Preparation
2.3.2. Three-Dimensional Tablet Printing Parameters
2.4. Texture Analysis and Stability of the Chewable Gel Tablets
2.5. Antioxidant Activity and Quantification of Active Compounds in the Tablet
2.5.1. Antioxidant Activity of the Active Compounds and Tablets
2.5.2. Active Compounds’ Release In Vitro
2.5.3. Quantification of Active Compounds via HPLC
2.6. Statistical Analysis
3. Results and Discussion
3.1. The Quality of Active Ingredients
3.2. Antioxidant Activity of Active Ingredients
3.3. SEE 3D Printing of the Tablets and the Analysis of the Physical Parameters
3.3.1. Three-Dimensional Printing Parameters
3.3.2. The Analysis of the Tablets’ Physical Parameters
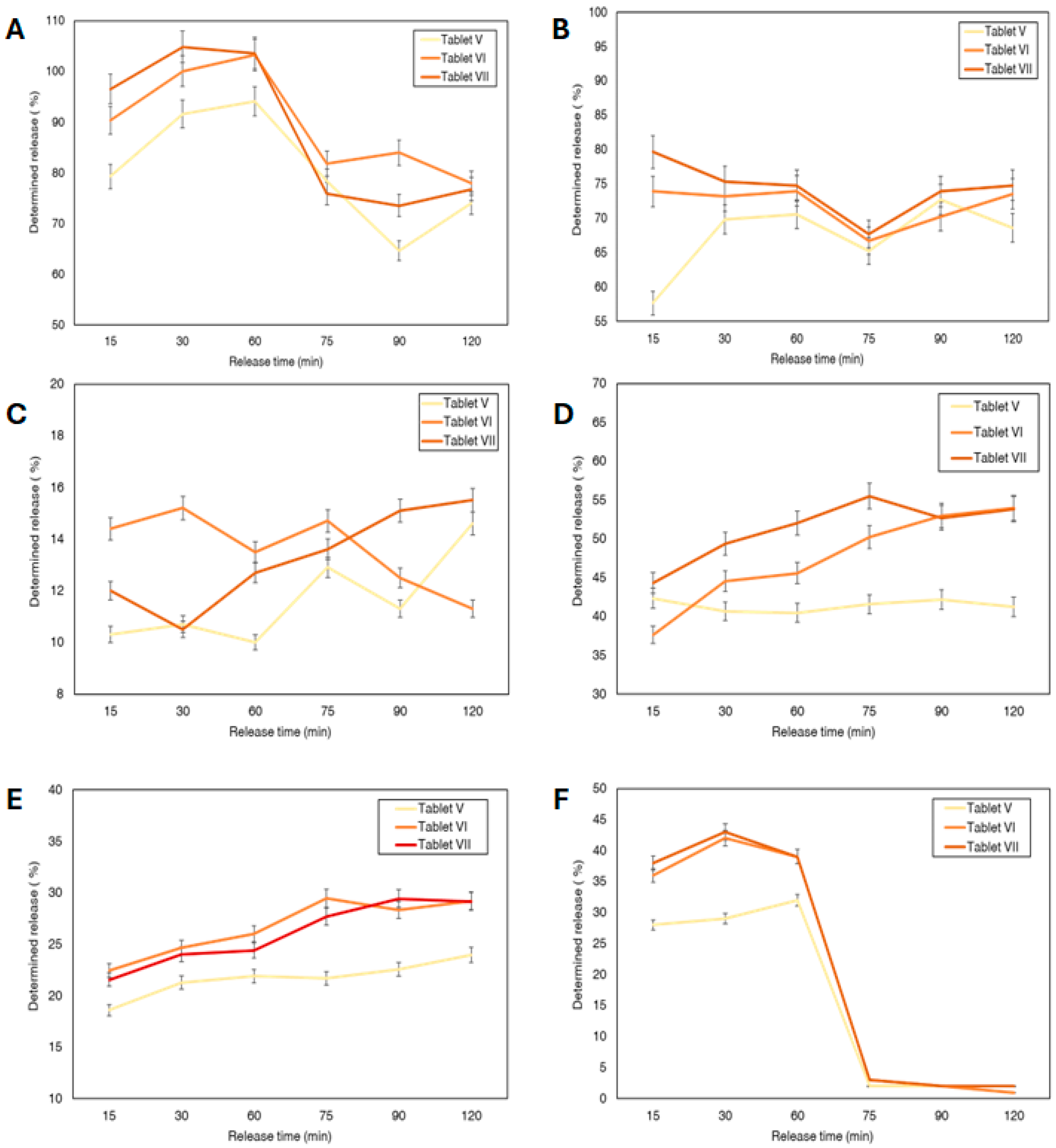
3.4. In Vitro Release of the Selected 3D Tablets
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| 3DP | Three-dimensional printing |
| HPLC | High-performance liquid chromatography |
| SEE | Semi-Solid Extrusion |
| FDM | Fused Deposition Modelling |
| API | Active pharmaceutical ingredient |
References
- Bouhassira, D. Neuropathic Pain: Definition, Assessment and Epidemiology. Rev. Neurol. 2019, 175, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Song, K.-S.; Cho, J.H.; Hong, J.-Y.; Lee, J.H.; Kang, H.; Ham, D.-W.; Ryu, H.-J. Neuropathic Pain Related with Spinal Disorders: A Systematic Review. Asian Spine J. 2017, 11, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.N.; Meyer, R.A. Mechanisms of Neuropathic Pain. Neuron 2006, 52, 77–92. [Google Scholar] [CrossRef]
- Wright, M.E.; Rizzolo, D. An Update on the Pharmacologic Management and Treatment of Neuropathic Pain. JAAPA 2017, 30, 13–17. [Google Scholar] [CrossRef]
- Mishra, G.; Singh, P.; Molla, M.; Shumet Yimer, Y.; Ewunetie, A.; Yimer Tadesse, T.; Mengie Ayele, T.; Kefale, B. Nutraceuticals: A Source of Benefaction for Neuropathic Pain and Fibromyalgia. J. Funct. Foods 2022, 97, 105260. [Google Scholar] [CrossRef]
- Abdelrahman, K.M.; Hackshaw, K.V. Nutritional Supplements for the Treatment of Neuropathic Pain. Biomedicines 2021, 9, 674. [Google Scholar] [CrossRef]
- Vink, R.; Nechifor, M. (Eds.) Magnesium in the Central Nervous System; University of Adelaide Press: Adelaide, Australia, 2011; ISBN 978-0-9870730-5-1. [Google Scholar]
- Stein, J.; Geisel, J.; Obeid, R. Association between Neuropathy and B-vitamins: A Systematic Review and Meta-analysis. Euro J. Neurol. 2021, 28, 2054–2064. [Google Scholar] [CrossRef]
- Jolivalt, C.G.; Mizisin, L.M.; Nelson, A.; Cunha, J.M.; Ramos, K.M.; Bonke, D.; Calcutt, N.A. B Vitamins Alleviate Indices of Neuropathic Pain in Diabetic Rats. Eur. J. Pharmacol. 2009, 612, 41–47. [Google Scholar] [CrossRef]
- Yousefi-Manesh, H.; Shirooie, S.; Noori, T.; Sheibani, M.; Tavangar, S.M.; Hemmati, S.; Sadeghi, M.A.; Akbarniakhaky, H.; Mohammadi, Z.; Foroutani, L.; et al. Spermidine Reduced Neuropathic Pain in Chronic Constriction Injury-induced Peripheral Neuropathy in Rats. Fundamemntal Clin. Pharma 2023, 37, 779–785. [Google Scholar] [CrossRef]
- Hou, H.; Wang, L.; Fu, T.; Papasergi, M.; Yule, D.I.; Xia, H. Magnesium Acts as a Second Messenger in the Regulation of NMDA Receptor-Mediated CREB Signaling in Neurons. Mol. Neurobiol. 2020, 57, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Negrão, L.; Almeida, P.; Alcino, S.; Duro, H.; Libório, T.; Melo Silva, U.; Figueira, R.; Gonçalves, S.; Neto Parra, L. Effect of the Combination of Uridine Nucleotides, Folic Acid and Vitamin B12 on the Clinical Expression of Peripheral Neuropathies. Pain Manag. 2014, 4, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Bauer, M.A.; Carmona-Gutierrez, D.; Kroemer, G. Spermidine: A Physiological Autophagy Inducer Acting as an Anti-Aging Vitamin in Humans? Autophagy 2019, 15, 165–168. [Google Scholar] [CrossRef]
- Noah, L.; Dye, L.; Bois De Fer, B.; Mazur, A.; Pickering, G.; Pouteau, E. Effect of Magnesium and Vitamin B6 Supplementation on Mental Health and Quality of Life in Stressed Healthy Adults: Post-hoc Analysis of a Randomised Controlled Trial. Stress Health 2021, 37, 1000–1009. [Google Scholar] [CrossRef]
- Tracy, T.; Wu, L.; Liu, X.; Cheng, S.; Li, X. 3D Printing: Innovative Solutions for Patients and Pharmaceutical Industry. Int. J. Pharm. 2023, 631, 122480. [Google Scholar] [CrossRef]
- Yasin, H.; Al-Tabakha, M.M.A.; Chan, S.Y. Fabrication of Polypill Pharmaceutical Dosage Forms Using Fused Deposition Modeling 3D Printing: A Systematic Review. Pharmaceutics 2024, 16, 1285. [Google Scholar] [CrossRef]
- Białek, A.; Krysztofiak, J.; Hozakowska, A.; Wojszel, Z.; Osmałek, T.; Wojtyłko, M.; Froelich, A. Novel Soft Dosage Forms for Paediatric Applications: Can We 3D-Print Them or Not? Gels 2025, 11, 187. [Google Scholar] [CrossRef]
- Funk, N.L.; Leão, J.; De Oliveira, T.V.; Beck, R.C.R. Semi-Solid Extrusion (SSE) in Pharmaceuticals. In Additive Manufacturing in Pharmaceuticals; Banerjee, S., Ed.; Springer Nature: Singapore, 2023; pp. 171–200. ISBN 978-981-99-2403-5. [Google Scholar]
- Bg, P.K.; Mehrotra, S.; Marques, S.M.; Kumar, L.; Verma, R. 3D Printing in Personalized Medicines: A Focus on Applications of the Technology. Mater. Today Commun. 2023, 35, 105875. [Google Scholar] [CrossRef]
- Aina, M.; Baillon, F.; Sescousse, R.; Sanchez-Ballester, N.M.; Begu, S.; Soulairol, I.; Sauceau, M. From Conception to Consumption: Applications of Semi-Solid Extrusion 3D Printing in Oral Drug Delivery. Int. J. Pharm. 2025, 674, 125436. [Google Scholar] [CrossRef]
- Adeleke, O.A.; Abedin, S. Characterization of Prototype Gummy Formulations Provides Insight into Setting Quality Standards. AAPS PharmSciTech 2024, 25, 155. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pombo, L.; Awad, A.; Basit, A.W.; Alvarez-Lorenzo, C.; Goyanes, A. Innovations in Chewable Formulations: The Novelty and Applications of 3D Printing in Drug Product Design. Pharmaceutics 2022, 14, 1732. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Takahashi, A.; Itoh, K.; Ishitani, M.; Dairaku, M.; Togashi, M.; Mikami, R.; Attwood, D. Preparation and Evaluation of Gel Formulations for Oral Sustained Delivery to Dysphagic Patients. Drug Dev. Ind. Pharm. 2009, 35, 780–787. [Google Scholar] [CrossRef]
- Powder Flow (2.9.36). In European Pharmacopoeia; European Directorate for the Quality of Medicines & HealthCare (EDQM): Strasbourg, France, 2024.
- Kazlauskaite, J.A.; Ivanauskas, L.; Marksa, M.; Bernatoniene, J. The Effect of Traditional and Cyclodextrin-Assisted Extraction Methods on Trifolium pratense L. (Red Clover) Extracts Antioxidant Potential. Antioxidants 2022, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Dissolution Test for Solid Dosage Forms (2.9.3.). In European Pharmacopoeia; European Directorate for the Quality of Medicines & HealthCare (EDQM): Strasbourg, France, 2024.
- Brubaker, J.; Moghtadernejad, S. A Comprehensive Review of the Rheological Properties of Powders in Pharmaceuticals. Powders 2024, 3, 233–254. [Google Scholar] [CrossRef]
- Crouter, A.; Briens, L. The Effect of Moisture on the Flowability of Pharmaceutical Excipients. AAPS PharmSciTech 2014, 15, 65–74. [Google Scholar] [CrossRef]
- Emery, E.; Oliver, J.; Pugsley, T.; Sharma, J.; Zhou, J. Flowability of Moist Pharmaceutical Powders. Powder Technol. 2009, 189, 409–415. [Google Scholar] [CrossRef]
- Cuyubamba, O.; Braga, C.P.; Swift, D.; Stickney, J.T.; Viel, C. The Combination of Neurotropic Vitamins B1, B6, and B12 Enhances Neural Cell Maturation and Connectivity Superior to Single B Vitamins. Cells 2025, 14, 477. [Google Scholar] [CrossRef]
- Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The Role of Magnesium in Neurological Disorders. Nutrients 2018, 10, 730. [Google Scholar] [CrossRef] [PubMed]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative Stress: The Role of Antioxidant Phytochemicals in the Prevention and Treatment of Diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef]
- Dominguez, L.; Veronese, N.; Sabico, S.; Al-Daghri, N.; Barbagallo, M. Magnesium and Migraine. Nutrients 2025, 17, 725. [Google Scholar] [CrossRef]
- Reno, A.M.; Green, M.; Killen, L.G.; O’Neal, E.K.; Pritchett, K.; Hanson, Z. Effects of Magnesium Supplementation on Muscle Soreness and Performance. J. Strength Cond. Res. 2022, 36, 2198–2203. [Google Scholar] [CrossRef] [PubMed]
- Maglione, M.; Kochlamazashvili, G.; Eisenberg, T.; Rácz, B.; Michael, E.; Toppe, D.; Stumpf, A.; Wirth, A.; Zeug, A.; Müller, F.E.; et al. Spermidine Protects from Age-Related Synaptic Alterations at Hippocampal Mossy Fiber-CA3 Synapses. Sci. Rep. 2019, 9, 19616. [Google Scholar] [CrossRef] [PubMed]
- Said, N.S.; Olawuyi, I.F.; Lee, W.Y. Pectin Hydrogels: Gel-Forming Behaviors, Mechanisms, and Food Applications. Gels 2023, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A Review of Gelatin: Properties, Sources, Process, Applications, and Commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Wang, R.; Hartel, R.W. Citric Acid and Heating on Gelatin Hydrolysis and Gelation in Confectionery Gels. Food Hydrocoll. 2022, 129, 107642. [Google Scholar] [CrossRef]
- Maruli Tua, S.; Apul, S.; Dewi Restuana, S.; Rosa, T.; Maruba, P.; Posman, S.; Delima, P.; Sisilia, Y.; Devi Oktavia, T. Effect of Citric Acid and Sucrose Concentration on the Quality of Passion Fruit Jelly with Dutch Eggplant. IOP Conf. Ser. Earth Environ. Sci. 2018, 205, 012050. [Google Scholar] [CrossRef]
- Sunarharum, W.B.; Renika, F.; Ali, D.Y.; Asih, N.E. Optimization of Pectin and Citric Acid Concentration on the Physical and Organoleptic Characteristics of Barhi Date Jam Using Response Surface Methodology. IOP Conf. Ser. Earth Environ. Sci. 2021, 782, 032092. [Google Scholar] [CrossRef]
- Sanprasert, S.; Kumnerdsiri, P.; Seubsai, A.; Lueangjaroenkit, P.; Pongsetkul, J.; Indriani, S.; Petcharat, T.; Sai-ut, S.; Hunsakul, K.; Issara, U.; et al. Techno-Functional, Rheological, and Physico-Chemical Properties of Gelatin Capsule By-Product for Future Functional Food Ingredients. Foods 2025, 14, 1279. [Google Scholar] [CrossRef]
- Wan, Z.; Zheng, J.; Zhu, Z.; Sang, L.; Zhu, J.; Luo, S.; Zhao, Y.; Wang, R.; Zhang, Y.; Hao, K.; et al. Intermediate Role of Gut Microbiota in Vitamin B Nutrition and Its Influences on Human Health. Front. Nutr. 2022, 9, 1031502. [Google Scholar] [CrossRef]
- Kozyraki, R.; Cases, O. Vitamin B12 Absorption: Mammalian Physiology and Acquired and Inherited Disorders. Biochimie 2013, 95, 1002–1007. [Google Scholar] [CrossRef]
- Stach, K.; Stach, W.; Augoff, K. Vitamin B6 in Health and Disease. Nutrients 2021, 13, 3229. [Google Scholar] [CrossRef]
- Hossain, K.S.; Amarasena, S.; Mayengbam, S. B Vitamins and Their Roles in Gut Health. Microorganisms 2022, 10, 1168. [Google Scholar] [CrossRef] [PubMed]
- Suter, P.M. The B-Vitamins. In Essential and Toxic Trace Elements and Vitamins in Human Health; Elsevier: Amsterdam, The Netherlands, 2020; pp. 217–239. ISBN 978-0-12-805378-2. [Google Scholar]
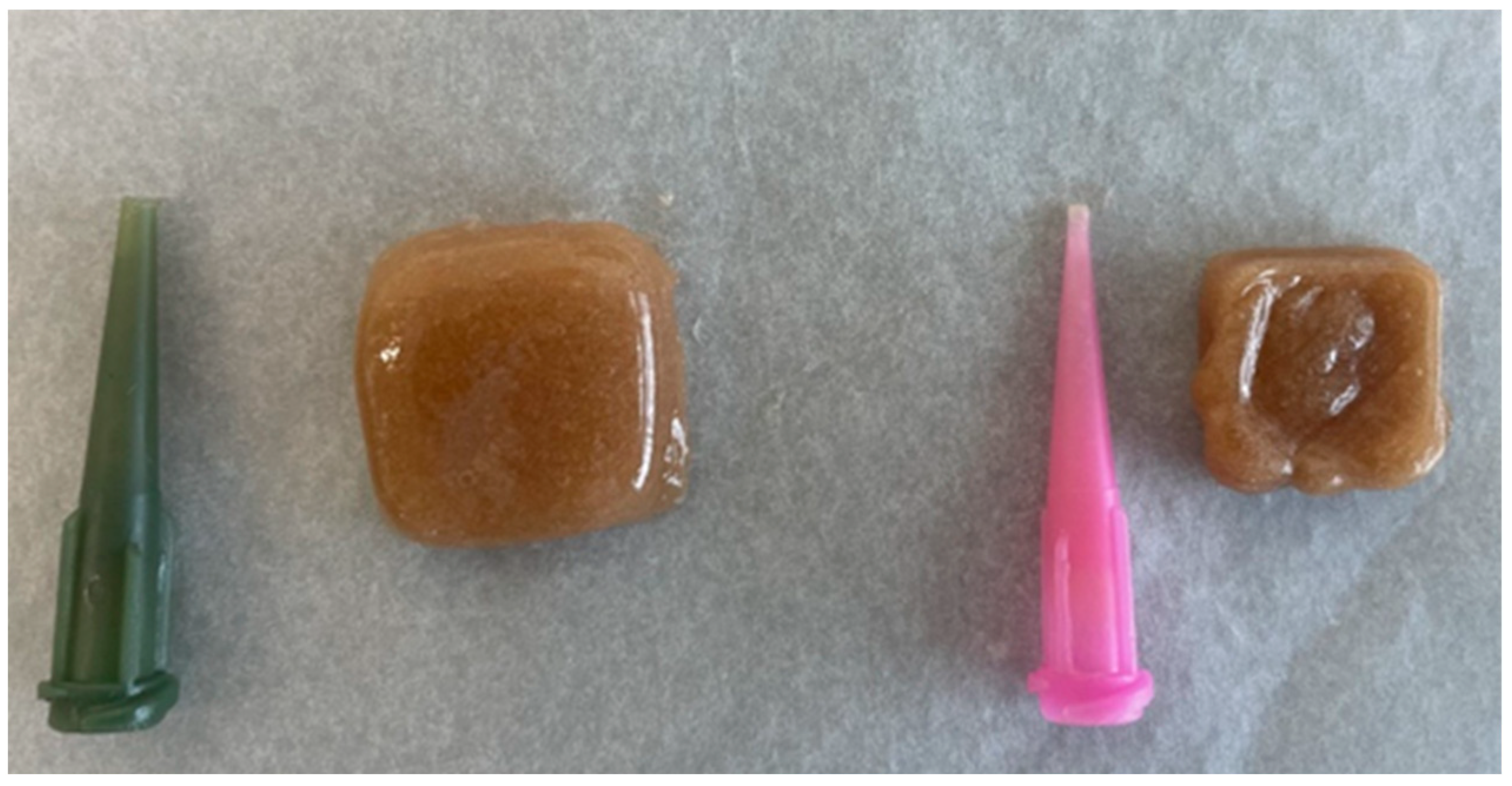
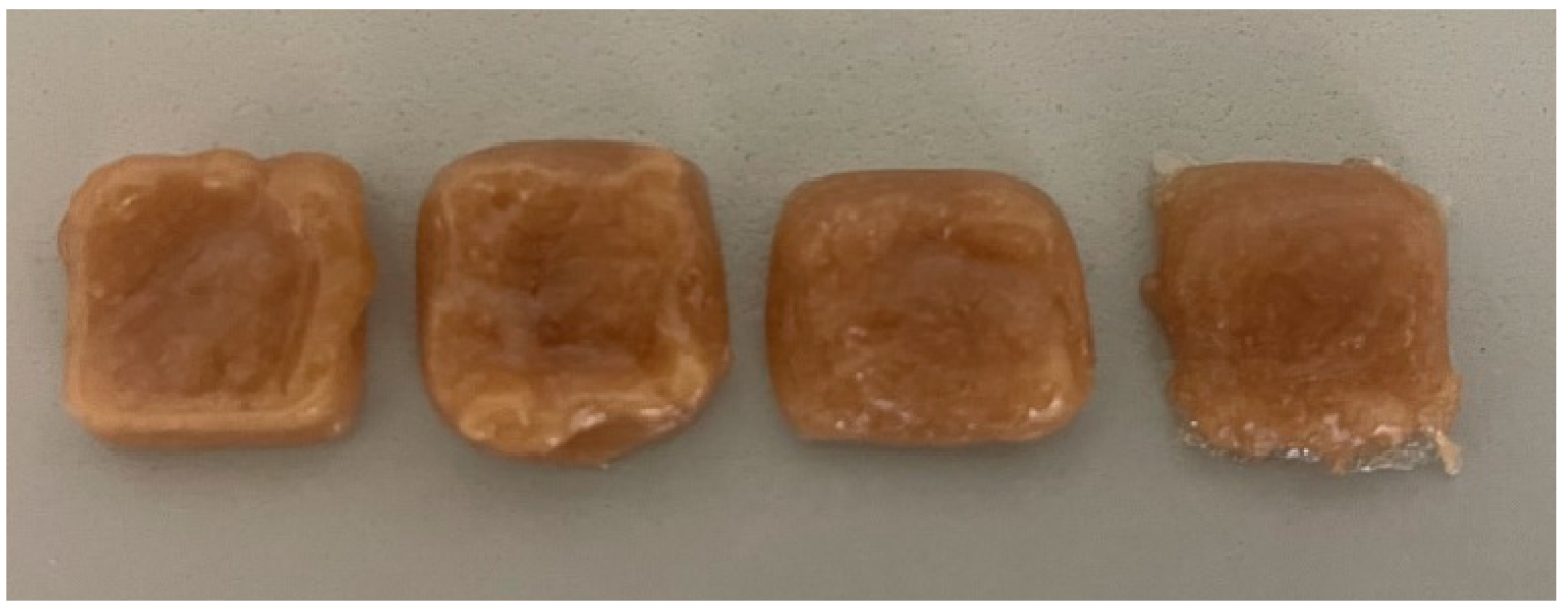
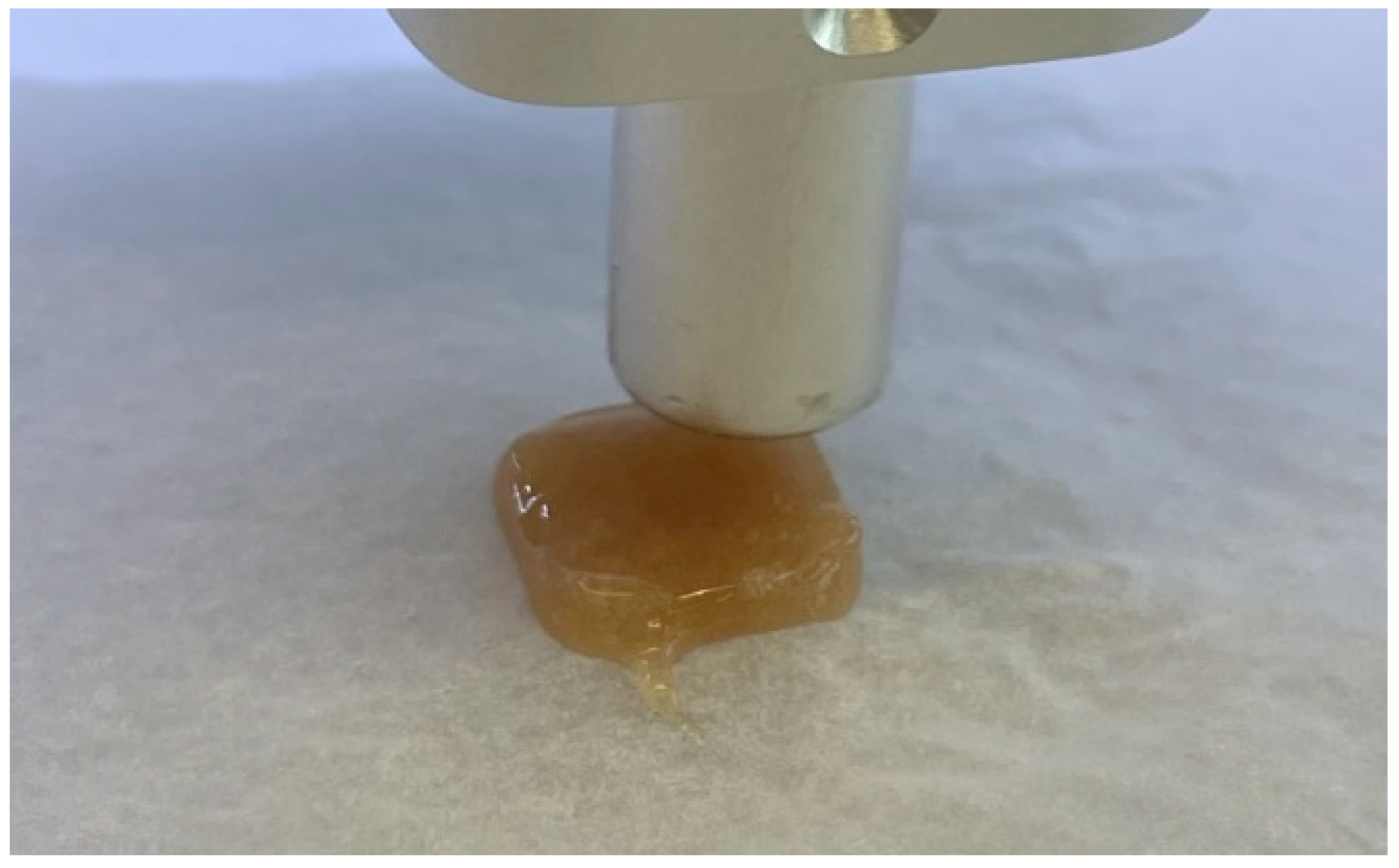

| I | II | III | IV | V | VI | VII | VIII | ||
|---|---|---|---|---|---|---|---|---|---|
| Gelatin | 9 | 9 | 10 | 10 | 9 | 9 | 10 | 10 | |
| Pectin | 7 | 7 | 8 | 8 | 7 | 7 | 8 | 8 | |
| Sugar | 28 | 30 | 28 | 30 | 28 | 30 | 28 | 30 | |
| Citric acid | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 0.5 | 0.5 | |
| Water | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | |
| Hausner Ratio | Compressibility Index | Moisture, % | ||
|---|---|---|---|---|
| Magnesium citrate | 1.182 ± 0.05 | 15.38 ± 0.54 | 2.12 ± 0.24 | |
| Uridine monophosphate | 1.300 ± 0.15 | 23.08 ± 0.70 | 16.11 ± 1.84 | |
| Niacin | 1.353 ± 0.21 | 26.09 ± 0.32 | 3.54 ± 1.01 | |
| Pyridoxine | 1.300 ± 0.04 | 23.08 ± 0.43 | 2.15 ± 0.68 | |
| Cobalamin | 1.333 ± 0.07 | 25.00 ± 0.29 | 4.18 ± 0.63 | |
| Folic acid | 1.667 ± 0.04 | 40.00 ± 0.63 | 7.75 ± 0.41 | |
| ABTS, mg TE/g | ±SD | DPPH, mg TE/g | ±SD | ||
|---|---|---|---|---|---|
| Magnesium citrate | 70.29 | 0.47 | 4.26 | 0.03 | |
| Uridine monophosphate | 62.08 | 3.54 | 4.09 | 0.03 | |
| Niacin | 65.94 | 0.68 | 4.26 | 0.08 | |
| Pyridoxine | 345.64 | 2.06 | 7.21 | 0.08 | |
| Cobalamin | 59.86 | 2.53 | 4.15 | 0.04 | |
| Folic acid | 76.18 | 0.80 | 4.41 | 0.04 | |
| Spermidine | 68.16 | 1.45 | 4.16 | 0.09 | |
| Samples | Firmness * | Springiness * | Hardness * | Stickiness * | ||||
|---|---|---|---|---|---|---|---|---|
| Prepared | After 2 Weeks | Prepared | After 2 Weeks | Prepared | After 2 Weeks | Prepared | After 2 Weeks | |
| I | 933.94 ± 49.74 | - | 33.31 ± 6.17 | - | 538.06 ± 10.62 | - | −45.57 ± 4.26 | - |
| AI | 1094.34 ± 45.55 | - | 25.1 ± 1.65 | - | 1281.02 ± 22.66 | - | −434.63 ± 1.7 | - |
| II | 465.94 ± 99.37 | 5829.82 ± 161.96 | 35.35 ± 6.6 | 61.39 ± 3.68 | 701.29 ± 14.34 | - | −46.77 ± 2.56 | −1.15 ± 0.04 |
| AII | 900.45 ± 5.97 | - | 21.27 ± 1.12 | - | 1228.78 ± 38.48 | - | −406.63 ± 23.89 | - |
| III | 902.42 ± 44.34 | - | 46.22 ± 2.73 | - | 1041.68 ± 33.57 | - | −68.35 ± 0.68 | - |
| AIII | 1021.29 ± 185.01 | - | 23.43 ± 2.22 | - | 1218.39 ± 35.76 | - | −309.4 ± 9.18 | - |
| IV | 1012.16 ± 119.62 | - | 40.81 ± 6.17 | - | 976.92 ± 36.25 | - | −76.51 ± 3.99 | - |
| AIV | 1092.17 ± 144.78 | - | 26.87 ± 0.73 | - | 1249.62 ± 39.56 | - | −249.19 ± 2.6 | - |
| V | 1275.69 ± 71.75 | - | 37.91 ± 0.42 | - | 1617.94 ± 39.93 | - | −129.02 ± 12.81 | - |
| AV | 289.58 ± 116.11 | 3280.68 ± 774.6 | 8.84 ± 0.6 | 35.54 ± 2.12 | 413.35 ± 72.03 | 5354.99 ± 350.98 | −30.11 ± 5.71 | −0.12 ± 0.07 |
| VI | 1036.4 ± 200.46 | - | 36.7 ± 2.82 | - | 1359.33 ± 35.1 | - | −80.43 ± 6.11 | - |
| AVI | 388.48 ± 127.61 | 4962.15 ± 252.92 | 7.06 ± 0.63 | 37.91 ± 0.42 | 618.19 ± 245.13 | 5845.23 ± 209.88 | −1.66 ± 0.03 | −0.13 ± 0.07 |
| VII | 989.49 ± 61.49 | - | 38.47 ± 2.23 | - | 1352.21 ± 29.08 | - | −88.56 ± 1.99 | - |
| AVII | 394.5 ±110.27 | 5087.64 ± 809.44 | 11.17 ± 0.46 | 36.27 ± 4.47 | 704.63 ± 66.82 | 6045.34 ± 4.56 | −15.08 ± 6.26 | −0.09 ± 0.05 |
| VIII | 2754.46 ± 152.66 | - | 48.53 ± 0.25 | - | 2283.46 ± 21.96 | - | −31.14 ± 6.89 | - |
| AVIII | 290.22 | - | 10.19 ± 2.37 | - | 506.37 ± 136.65 | - | −1.53 ±0.05 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazlauskaite, J.A.; Matulyte, I.; Bernatoniene, J. Development of 3D-Printed Gel-Based Supplement-Containing Tablets with Tailored Release Profiles for Neurological Pain Management. Pharmaceutics 2025, 17, 1168. https://doi.org/10.3390/pharmaceutics17091168
Kazlauskaite JA, Matulyte I, Bernatoniene J. Development of 3D-Printed Gel-Based Supplement-Containing Tablets with Tailored Release Profiles for Neurological Pain Management. Pharmaceutics. 2025; 17(9):1168. https://doi.org/10.3390/pharmaceutics17091168
Chicago/Turabian StyleKazlauskaite, Jurga Andreja, Inga Matulyte, and Jurga Bernatoniene. 2025. "Development of 3D-Printed Gel-Based Supplement-Containing Tablets with Tailored Release Profiles for Neurological Pain Management" Pharmaceutics 17, no. 9: 1168. https://doi.org/10.3390/pharmaceutics17091168
APA StyleKazlauskaite, J. A., Matulyte, I., & Bernatoniene, J. (2025). Development of 3D-Printed Gel-Based Supplement-Containing Tablets with Tailored Release Profiles for Neurological Pain Management. Pharmaceutics, 17(9), 1168. https://doi.org/10.3390/pharmaceutics17091168









