Research on Enhancing the Solubility and Bioavailability of Canagliflozin Using Spray Drying Techniques with a Quality-by-Design Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HPLC Condition
2.3. Drug Solubility Test
2.4. Polymer Screening
2.5. Identification of CQAs, CMAs, and CPPs
2.6. Production of CFZ Solid Dispersion via Spray Drying
2.7. Yield
2.8. Solubility
2.9. Particle Size
2.10. Morphological and Physicochemical Characterization
2.10.1. Scanning Electron Microscopy (SEM)
2.10.2. Differential Scanning Calorimetry (DSC)
2.10.3. Powder X-Ray Diffractometer (PXRD)
2.11. Dissolution Test
2.12. In Vivo Pharmacokinetic Study
3. Results and Discussion
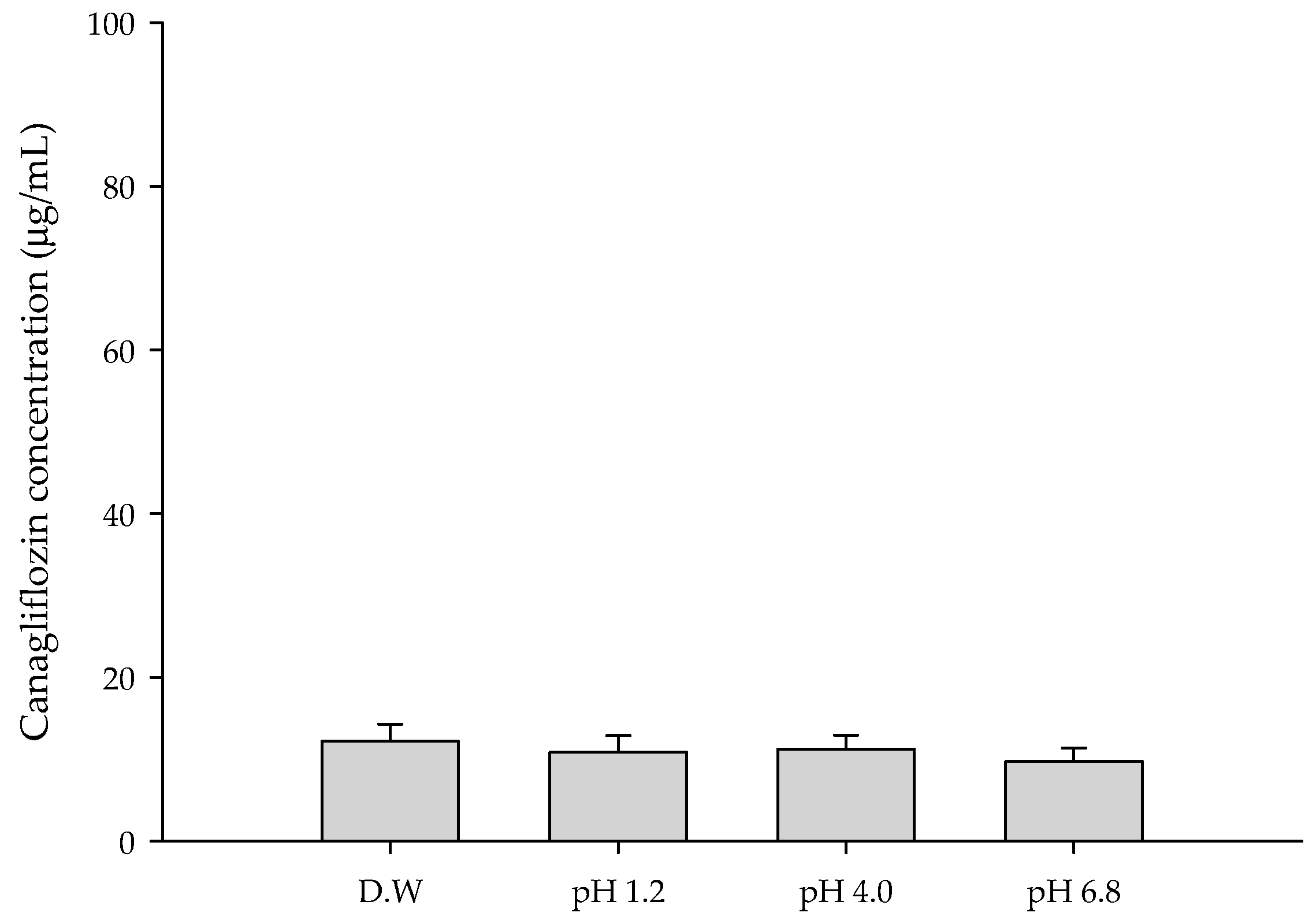
3.1. Solubility of CFZ
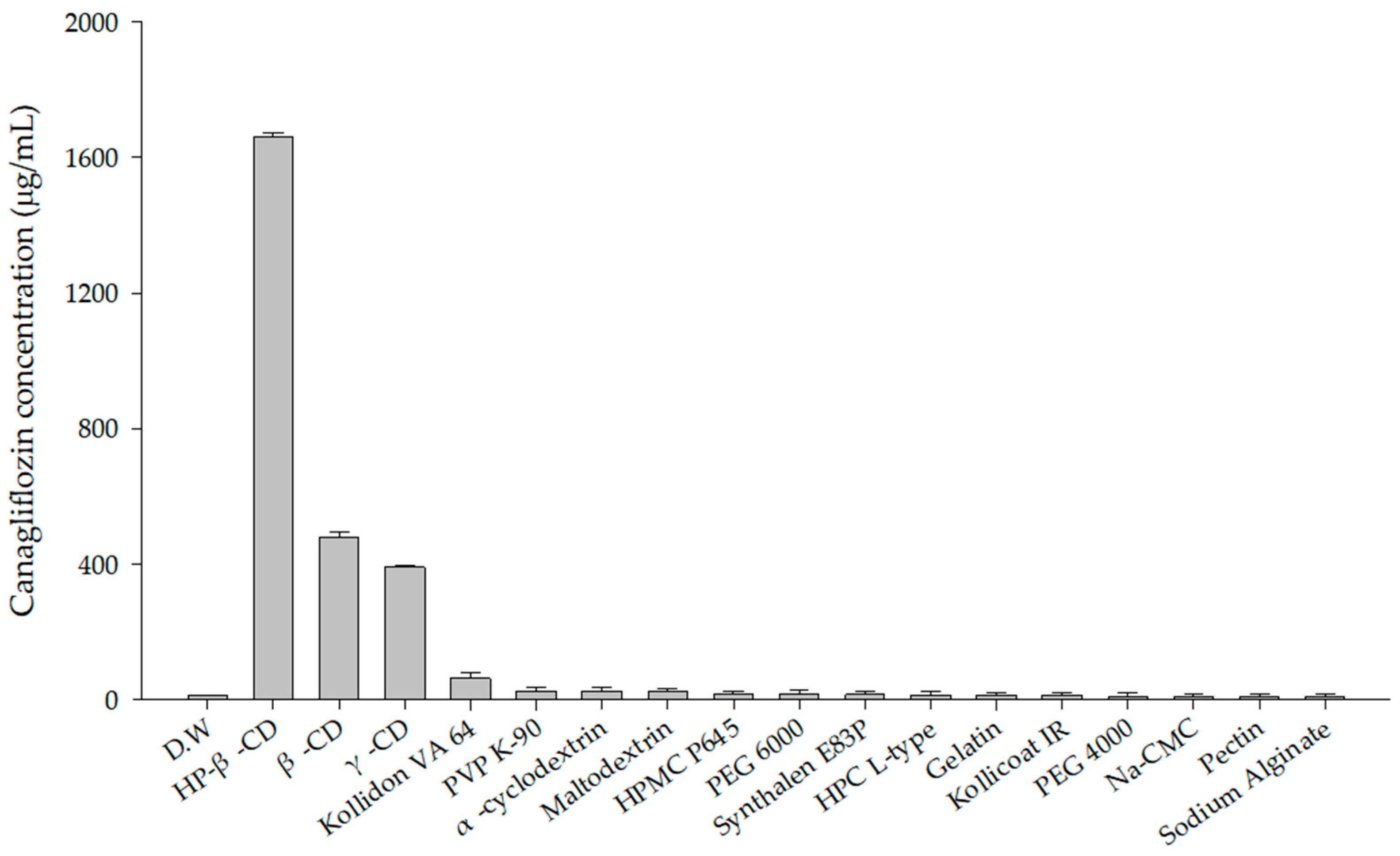
3.2. Polymer Screening
3.3. Selection of Factors and Levels in Box–Behnken Design
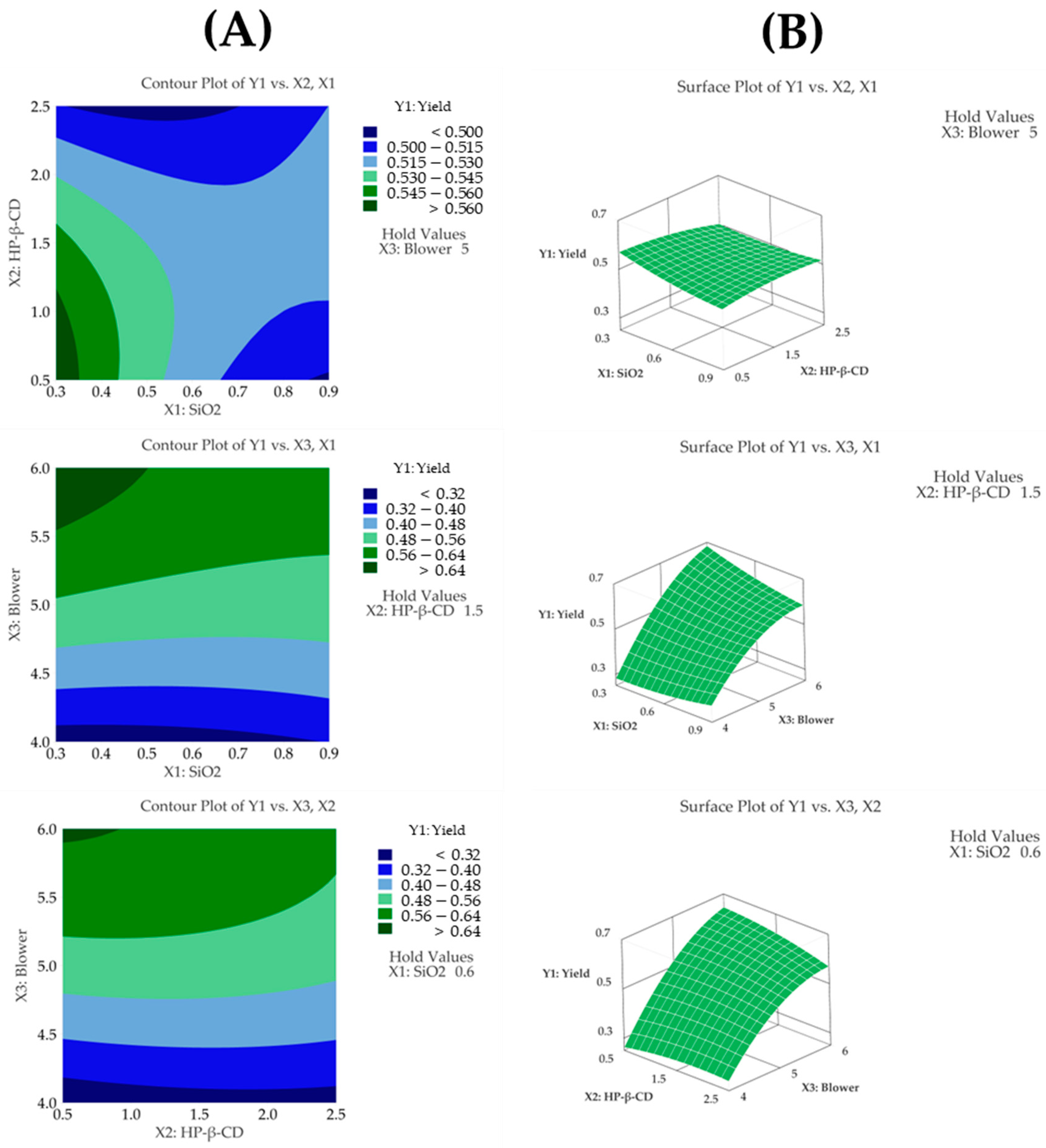
3.4. Spray Drying Process Applied with Box–Behnken Design
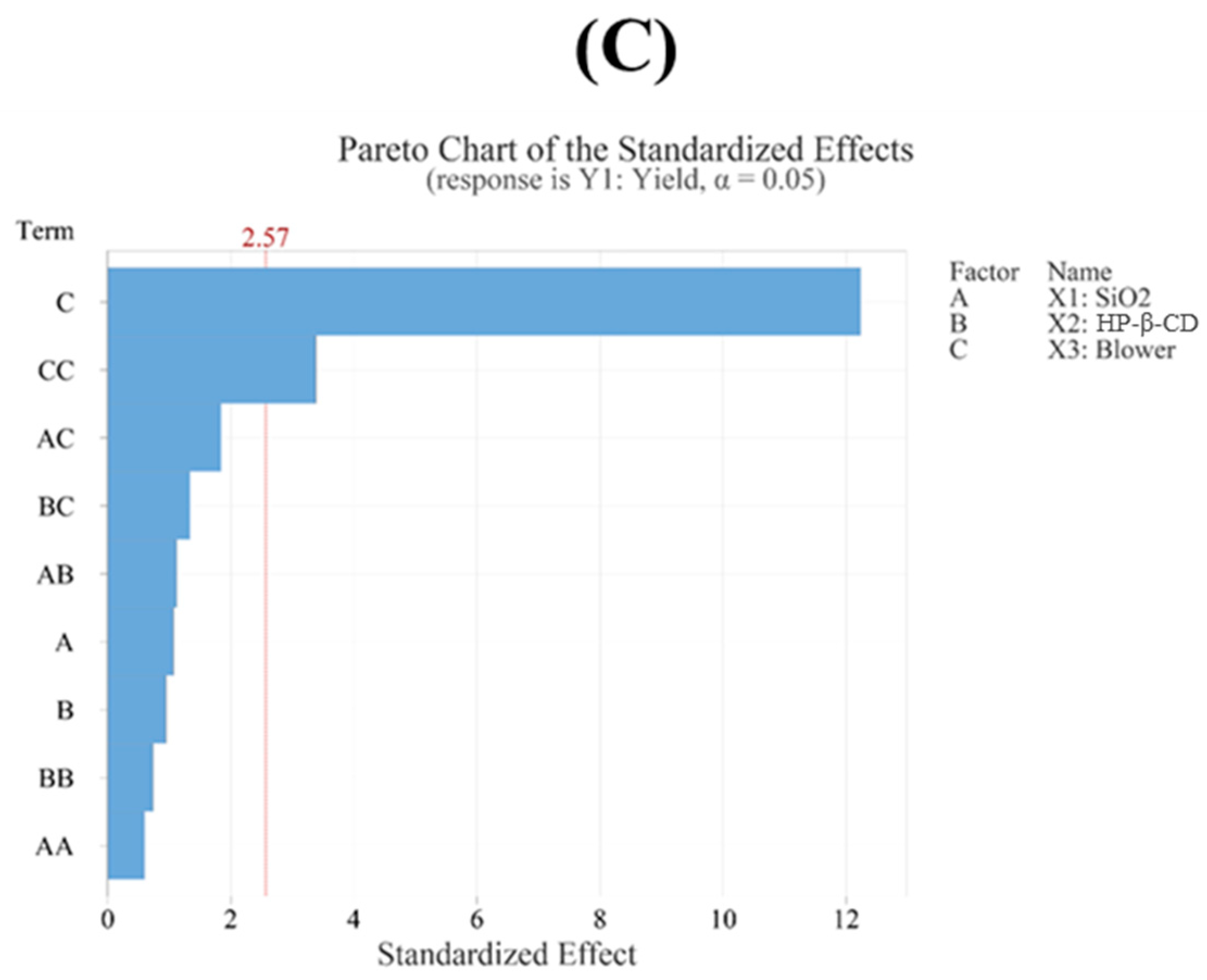
3.4.1. Yield
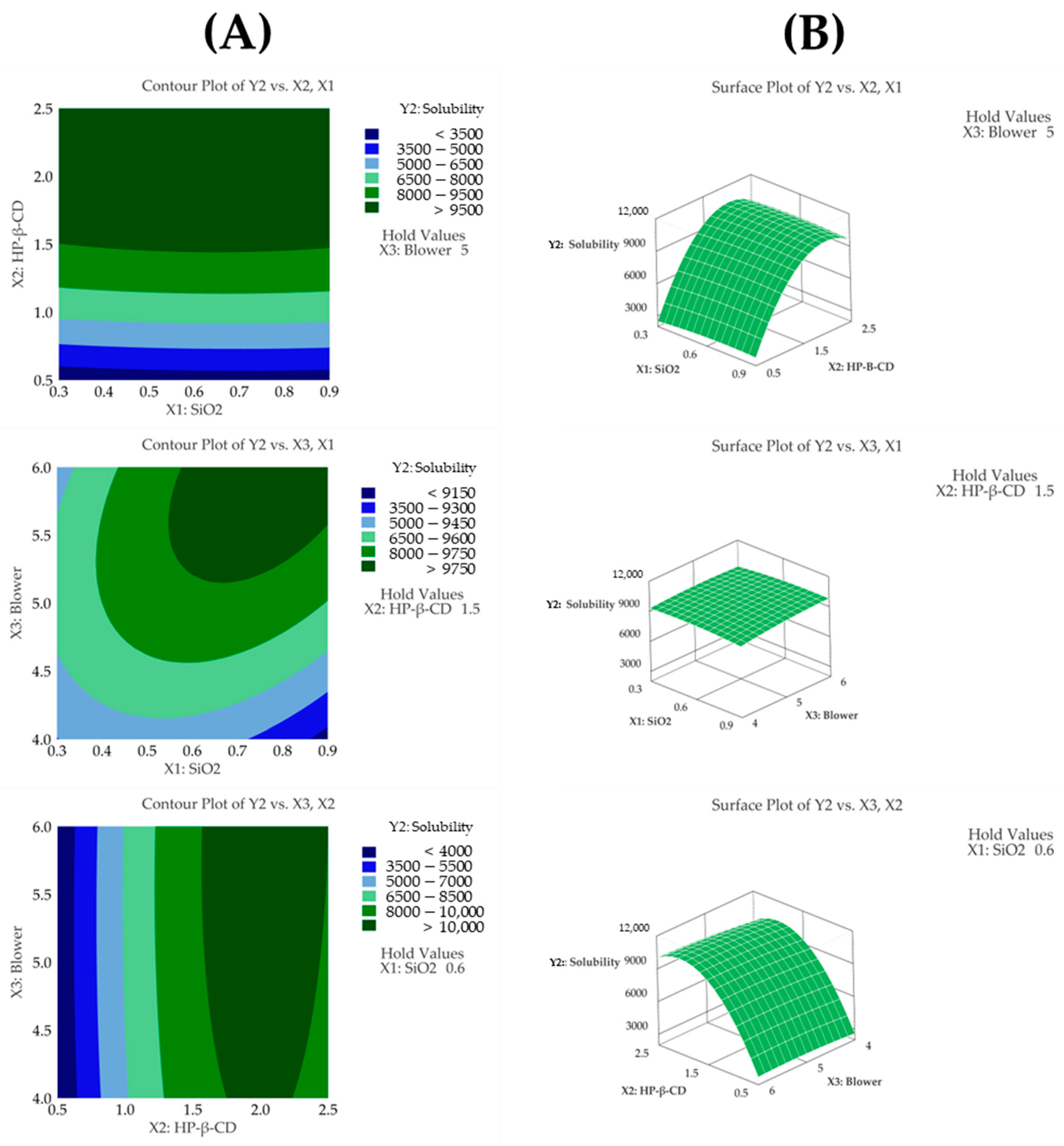
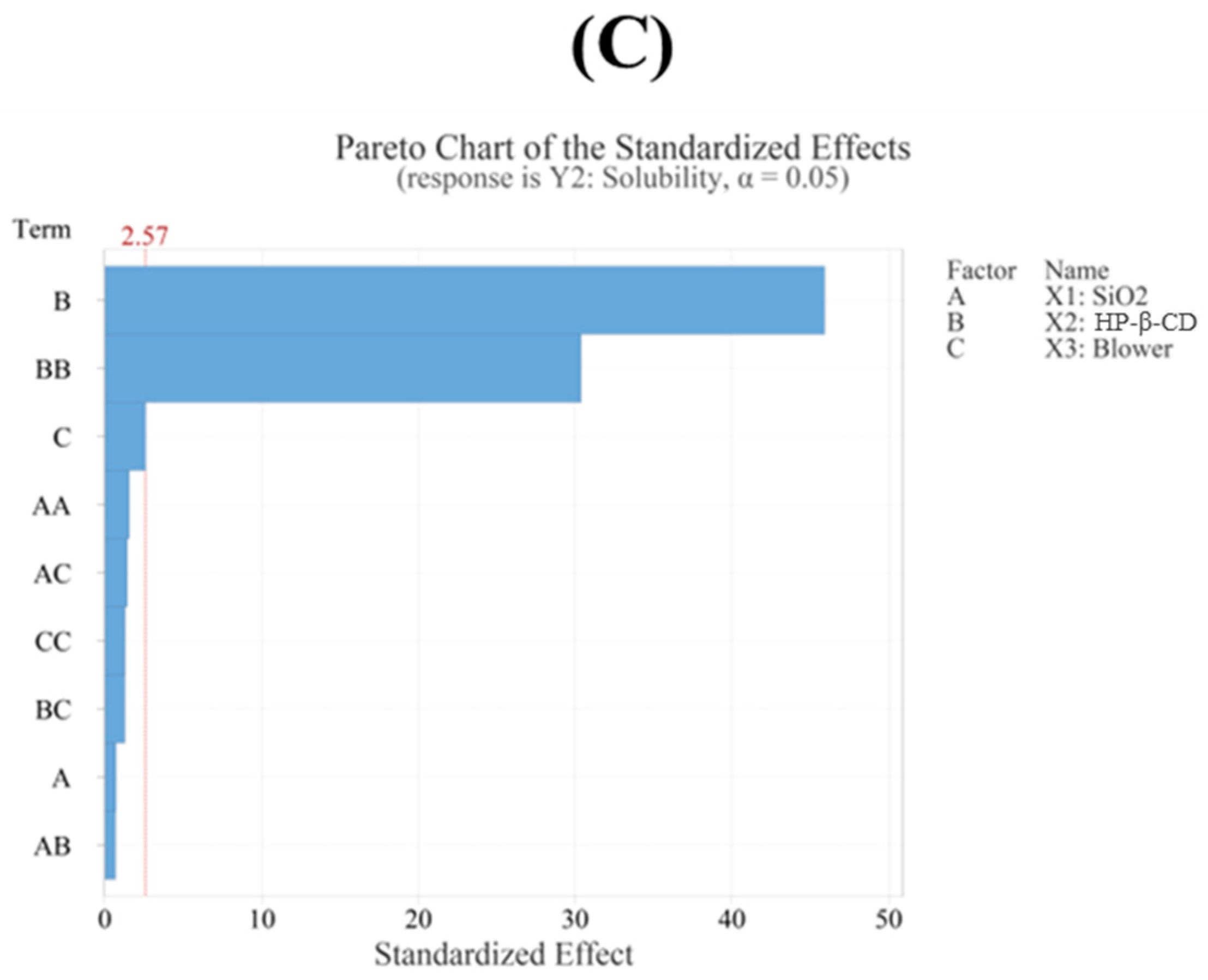
3.4.2. Solubility
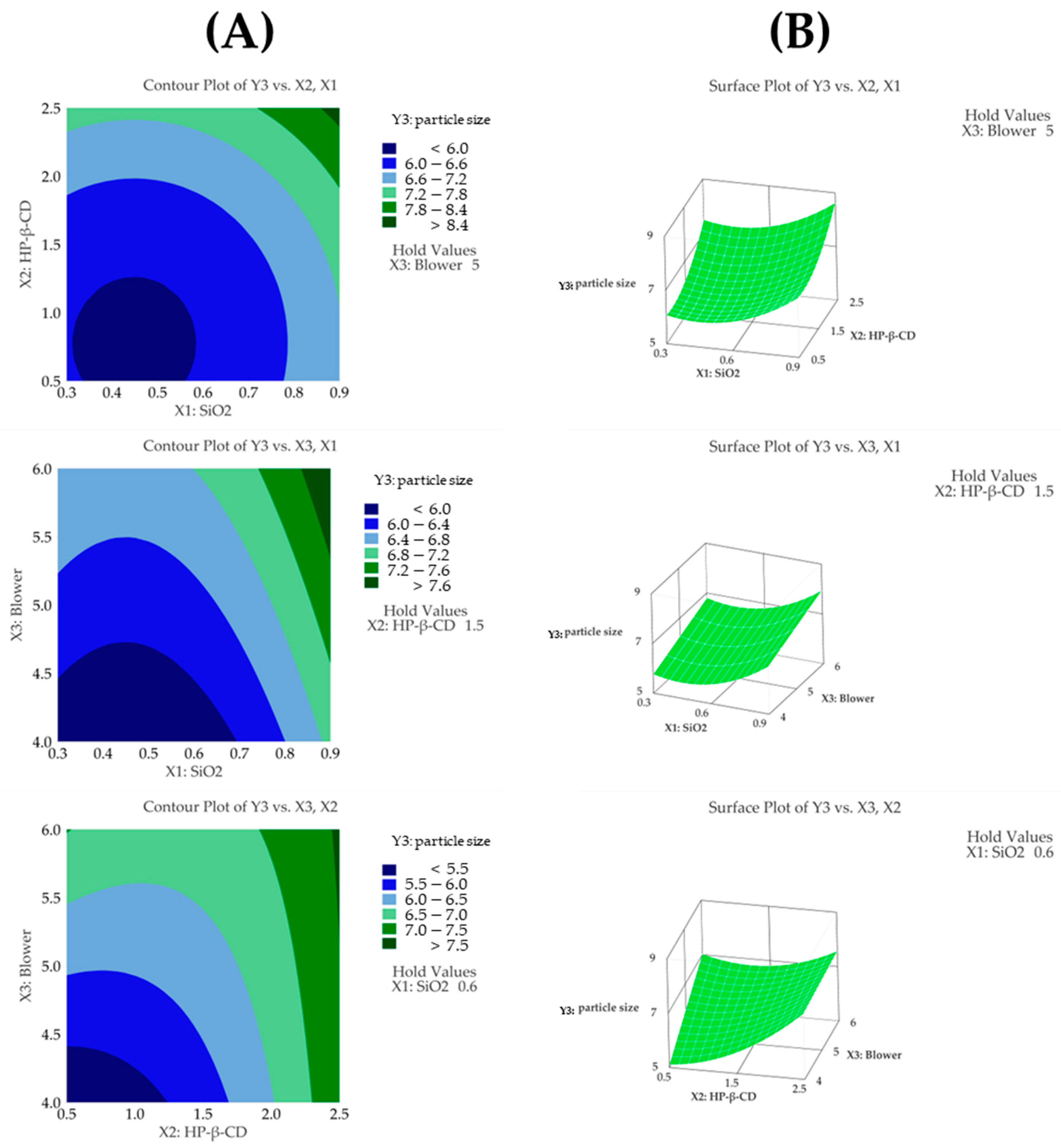
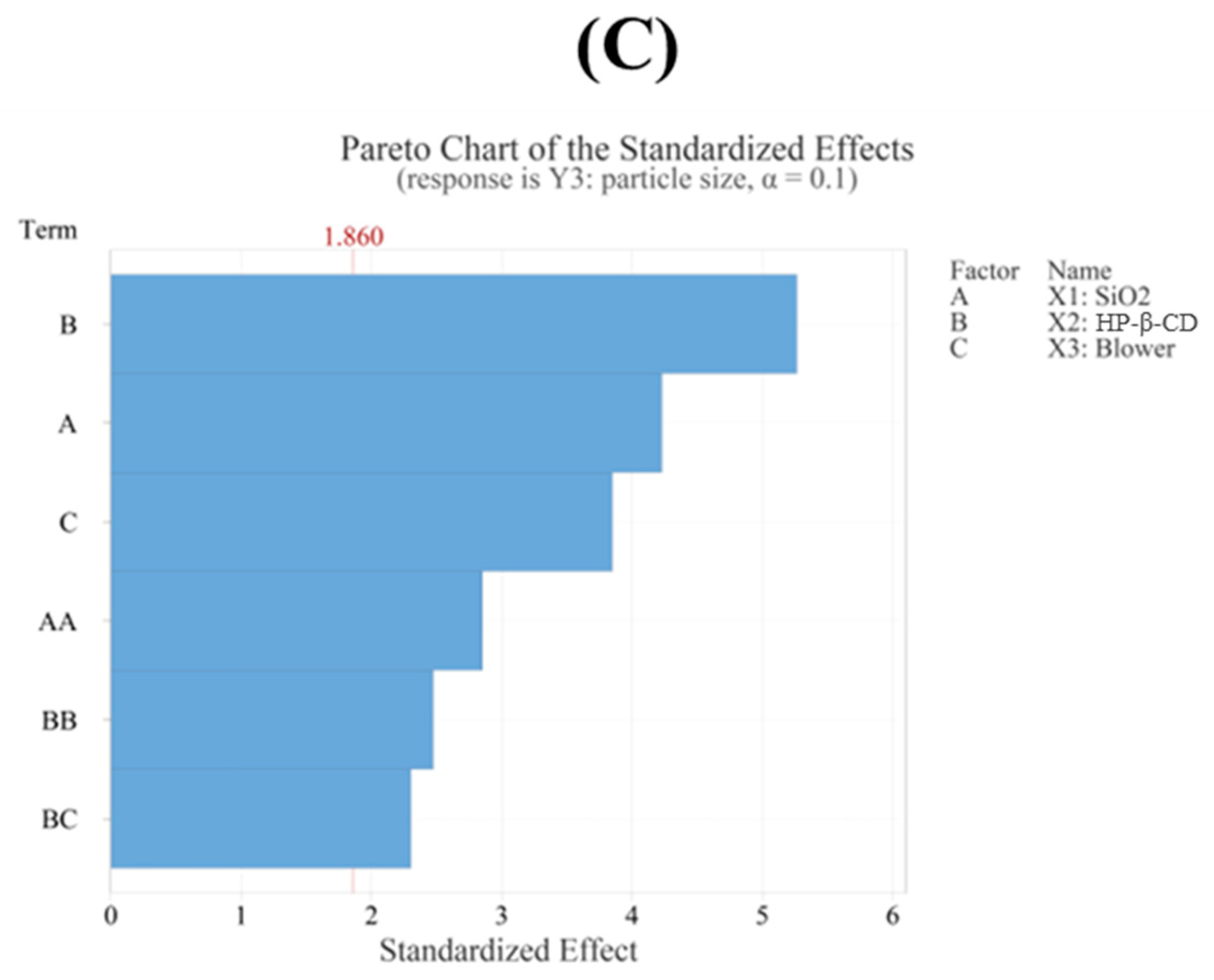
3.4.3. Particle Size
3.5. Optimization of CFZ-SD Using BBD
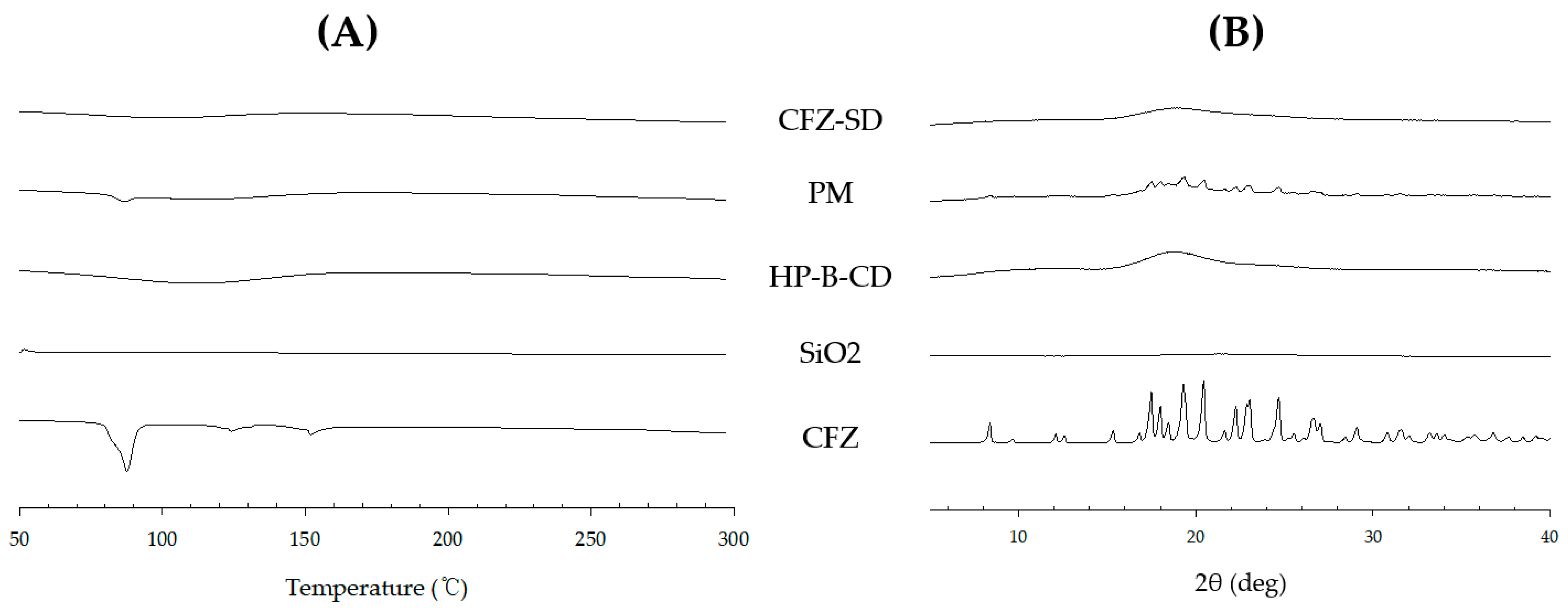
3.6. Morphological and Physiochemical Characterization of CFZ-SD
3.7. In Vitro Dissolution Test of CFZ-SD
3.8. In Vivo Pharmacokinetic Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Danaei, G.; Finucane, M.M.; Lu, Y.; Singh, G.M.; Cowan, M.J.; Paciorek, C.J.; Lin, J.K.; Farzadfar, F.; Khang, Y.-H.; Stevens, G.A. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 2011, 378, 31–40. [Google Scholar] [CrossRef]
- Lamos, E.M.; Younk, L.M.; Davis, S.N. Canagliflozin, an inhibitor of sodium–glucose cotransporter 2, for the treatment of type 2 diabetes mellitus. Expert Opin. Drug Metab. Toxicol. 2013, 9, 763–775. [Google Scholar] [CrossRef]
- Haneef, J.; Khan, M.D. Crystal Engineering of Canagliflozin: Comprehensive Overview of Diverse Solid Forms and Future Perspectives. J. Mol. Struct. 2025, 1322, 140318. [Google Scholar] [CrossRef]
- Deeks, E.D.; Scheen, A.J. Canagliflozin: A review in type 2 diabetes. Drugs 2017, 77, 1577–1592. [Google Scholar] [CrossRef]
- Yang, X.-P.; Lai, D.; Zhong, X.-Y.; Shen, H.-P.; Huang, Y.-L. Efficacy and safety of canagliflozin in subjects with type 2 diabetes: Systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2014, 70, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Fathy Elhabal, S.; El-Nabarawi, M.A.; Abdelaal, N.; Elrefai, M.F.M.; Ghaffar, S.A.; Khalifa, M.M.; Mohie, P.M.; Waggas, D.S.; Hamdan, A.M.E.; Alshawwa, S.Z. Development of canagliflozin nanocrystals sublingual tablets in the presence of sodium caprate permeability enhancer: Formulation optimization, characterization, in-vitro, in silico, and in-vivo study. Drug Deliv. 2023, 30, 2241665. [Google Scholar] [CrossRef]
- Devineni, D.; Murphy, J.; Wang, S.S.; Stieltjes, H.; Rothenberg, P.; Scheers, E.; Mamidi, R.N. Absolute oral bioavailability and pharmacokinetics of canagliflozin: A microdose study in healthy participants. Clin. Pharmacol. Drug Dev. 2015, 4, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.C.; Patel, H.A. A recent solidification approach for nanosuspension: Formulation, optimisation and evaluation of canagliflozin immediate release pellets. Folia Medica 2022, 64, 488–500. [Google Scholar] [CrossRef]
- ElSayed, N.A.; McCoy, R.G.; Aleppo, G.; Bajaj, M.; Balapattabi, K.; Beverly, E.A.; Briggs Early, K.; Bruemmer, D.; Echouffo-Tcheugui, J.B. 9. Pharmacologic approaches to glycemic treatment: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S181–S206. [Google Scholar]
- Cal, K.; Sollohub, K. Spray drying technique. I: Hardware and process parameters. J. Pharm. Sci. 2010, 99, 575–586. [Google Scholar] [CrossRef]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray drying of pharmaceuticals and biopharmaceuticals: Critical parameters and experimental process optimization approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.; Knipp, G.; Zografi, G. Assessing the performance of amorphous solid dispersions. J. Pharm. Sci. 2012, 101, 1355–1377. [Google Scholar] [CrossRef]
- Okada, K.; Ono, T.; Hayashi, Y.; Kumada, S.; Onuki, Y. Use of time-domain NMR for 1H T1 relaxation measurement and fitting analysis in homogeneity evaluation of amorphous solid dispersion. J. Pharm. Sci. 2024, 113, 680–687. [Google Scholar] [CrossRef]
- Lin, X.; Hu, Y.; Liu, L.; Su, L.; Li, N.; Yu, J.; Tang, B.; Yang, Z. Physical stability of amorphous solid dispersions: A physicochemical perspective with thermodynamic, kinetic and environmental aspects. Pharm. Res. 2018, 35, 125. [Google Scholar] [CrossRef]
- Rusdin, A.; Mohd Gazzali, A.; Ain Thomas, N.; Megantara, S.; Aulifa, D.L.; Budiman, A.; Muchtaridi, M. Advancing drug delivery paradigms: Polyvinyl pyrolidone (PVP)-based amorphous solid dispersion for enhanced physicochemical properties and therapeutic efficacy. Polymers 2024, 16, 286. [Google Scholar] [CrossRef]
- Marasini, N.; Yan, Y.D.; Poudel, B.K.; Choi, H.G.; Yong, C.S.; Kim, J.O. Development and optimization of self-nanoemulsifying drug delivery system with enhanced bioavailability by Box–Behnken design and desirability function. J. Pharm. Sci. 2012, 101, 4584–4596. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, B.S.; McConville, C. Box–Behnken design of experiments of polycaprolactone nanoparticles loaded with irinotecan hydrochloride. Pharmaceutics 2023, 15, 1271. [Google Scholar] [CrossRef]
- Guterres, S.S.; Weiss, V.; Freitas, L.D.L.; Pohlmann, A.R. Influence of benzyl benzoate as oil core on the physicochemical properties of spray-dried powders from polymeric nanocapsules containing indomethacin. Drug Deliv. 2000, 7, 195–199. [Google Scholar] [CrossRef]
- Cho, D.Y.; Kim, M.J.; Kim, K.S. Quaternary Solid Dispersion System containing Rebamipide with Enhanced Solubility. Yakhak Hoeji 2024, 68, 215–222. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Yun, T.; Lee, S.; Yun, S.; Cho, D.; Bang, K.; Kim, K. Investigation of stabilized amorphous solid dispersions to improve oral olaparib absorption. Pharmaceutics 2024, 16, 958. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H.; Kifuji, T.; Maruyama, N.; Inagaki, N. Pharmacokinetics, pharmacodynamics, and safety of canagliflozin in Japanese patients with type 2 diabetes mellitus. Adv. Ther. 2015, 32, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Bonakdar, T.; Ali, M.; Ghadiri, M. Particle breakability assessment using an Aero S disperser. Int. J. Pharm. 2021, 597, 120365. [Google Scholar] [CrossRef] [PubMed]
- Anwer, M.K.; Ahmed, M.M.; Alshetaili, A.; Almutairy, B.K.; Alalaiwe, A.; Fatima, F.; Ansari, M.N.; Iqbal, M. Preparation of spray dried amorphous solid dispersion of diosmin in soluplus with improved hepato-renoprotective activity: In vitro anti-oxidant and in-vivo safety studies. J. Drug Deliv. Sci. Technol. 2020, 60, 102101. [Google Scholar] [CrossRef]
- Lee, H.I.; Kim, J.S.; Woo, M.R.; Cheon, S.; Park, S.; Woo, S.; Jin, S.G.; Choi, H.-G. Physicochemical characterization and in vivo assessment of novel apixaban-loaded polymeric nano-aggregates. J. Pharm. Investig. 2024, 55, 707–719. [Google Scholar]
- Choi, H.J.; Kim, K.S. Development of a novel ticagrelor solid dispersion-loaded tablet with enhanced solubility. Yakhak Hoeji 2020, 64, 21–27. [Google Scholar] [CrossRef]
- Park, J.H.; Cho, J.H.; Kim, D.S.; Kim, J.S.; Din, F.U.; Kim, J.O.; Yong, C.S.; Youn, Y.S.; Oh, K.T.; Kim, D.W. Revaprazan-loaded surface-modified solid dispersion: Physicochemical characterization and in vivo evaluation. Pharm. Dev. Technol. 2019, 24, 788–793. [Google Scholar] [CrossRef]
- Cho, D.-Y.; Lee, J.-G.; Kim, M.-J.; Cho, H.-J.; Cho, J.-H.; Kim, K.-S. Approaches for Inclusion Complexes of Ezetimibe with Cyclodextrins: Strategies for Solubility Enhancement and Interaction Analysis via Molecular Docking. Int. J. Mol. Sci. 2025, 26, 1686. [Google Scholar] [CrossRef]
- Deshmukh, R.; Deshmane, S.; Sawant, A.; Deshmane, S.; Jain, S. Transforming canagliflozin solubility using lipidic carrier and its pharmacokinetic study. Pharm. Dev. Technol. 2024, 29, 1175–1184. [Google Scholar] [CrossRef]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef]
- Araujo-Fernandez, A.S.; Uribe-Villarreal, J.C.; Perez-Chauca, E.; Alva-Plasencia, P.M.; Caballero-Aquiño, O.E.; Ganoza-Yupanqui, M.L. Validation of a UV spectrophotometric method to quantify losartan potassium in tablets from the dissolution test at pH 1.2, 4.5 and 6.8. J. Pharm. Pharmacogn. Res 2022, 10, 310–317. [Google Scholar] [CrossRef]
- Loftsson, T.; Saokham, P.; Couto, A.R.S. Self-association of cyclodextrins and cyclodextrin complexes in aqueous solutions. Int. J. Pharm. 2019, 560, 228–234. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X. Preparation and characterization of the inclusion complex of Ofloxacin with β-CD and HP-β-CD. J. Incl. Phenom. Macrocycl. Chem. 2011, 69, 173–179. [Google Scholar] [CrossRef]
- Kim, S.; Gupta, B.; Moon, C.; Oh, E.; Jeong, J.-H.; Yong, C.S.; Kim, J.O. Employing an optimized spray-drying process to produce ezetimibe tablets with an improved dissolution profile. J. Pharm. Investig. 2016, 46, 583–592. [Google Scholar] [CrossRef]
- Box, G.E.; Behnken, D.W. Some new three level designs for the study of quantitative variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Horváth, M.; Sinkó, K. Hierarchical porous SiO2 cryogel via sol-gel process. Gels 2022, 8, 808. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Alonso, R.; Morales-Guillermo, M.; Salgado-Cervantes, M.; Robles-Olvera, V.; García-Alvarado, M.; Rodríguez-Jimenes, G. Effect of process variables of spray drying employing heat pump and nitrogen on aromatic compound yield in powders obtained from vanilla (Vanilla planifolia Andrews) ethanolic extract. Dry. Technol. 2019, 37, 1806–1820. [Google Scholar] [CrossRef]
- Pohlmann, A.R.; Weiss, V.; Mertins, O.; da Silveira, N.P.; Guterres, S.L.S. Spray-dried indomethacin-loaded polyester nanocapsules and nanospheres: Development, stability evaluation and nanostructure models. Eur. J. Pharm. Sci. 2002, 16, 305–312. [Google Scholar] [CrossRef]
- Hummler, H.; Stillhart, C.; Meilicke, L.; Grimm, M.; Krause, E.; Mannaa, M.; Gollasch, M.; Weitschies, W.; Page, S. Impact of tablet size and shape on the swallowability in older adults. Pharmaceutics 2023, 15, 1042. [Google Scholar] [CrossRef]
- Gupta, B.; Poudel, B.K.; Tran, T.H.; Pradhan, R.; Cho, H.-J.; Jeong, J.-H.; Shin, B.S.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Modulation of pharmacokinetic and cytotoxicity profile of imatinib base by employing optimized nanostructured lipid carriers. Pharm. Res. 2015, 32, 2912–2927. [Google Scholar] [CrossRef]
- Nyamba, I.; Sombie, C.B.; Yabre, M.; Zime-Diawara, H.; Yameogo, J.; Ouedraogo, S.; Lechanteur, A.; Semde, R.; Evrard, B. Pharmaceutical approaches for enhancing solubility and oral bioavailability of poorly soluble drugs. Eur. J. Pharm. Biopharm. 2024, 204, 114513. [Google Scholar] [CrossRef]
- Gupta, V.; Davis, M.; Hope-Weeks, L.J.; Ahsan, F. PLGA microparticles encapsulating prostaglandin E 1-hydroxypropyl-β-cyclodextrin (PGE 1-HPβCD) complex for the treatment of pulmonary arterial hypertension (PAH). Pharm. Res. 2011, 28, 1733–1749. [Google Scholar] [CrossRef]
- ICH. ICH Harmonised Tripartite Guideline. In Pharmaceutical Development, Q8 (2R); As revised in August; ICH: Geneva, Switzerland, 2009; p. 23. [Google Scholar]
- Jeong, J.-H.; Yoon, T.-H.; Ryu, S.-W.; Kim, M.-G.; Kim, G.-H.; Oh, Y.-J.; Lee, S.-J.; Kwak, N.-W.; Bang, K.-H.; Kim, K.-S. Quality by Design (QbD)-Based Development of a Self-Nanoemulsifying Drug Delivery System for the Ocular Delivery of Flurbiprofen. Pharmaceutics 2025, 17, 629. [Google Scholar] [CrossRef]
- Wang, C.; Yan, T.; Yan, T.; Wang, Z. Fabrication of hesperetin/hydroxypropyl-β-cyclodextrin complex nanoparticles for enhancement of bioactivity using supercritical antisolvent technology. J. Mol. Struct. 2023, 1279, 134947. [Google Scholar] [CrossRef]
- Salt, A. Commentary: Concerns Regarding FDA Guidance on Dissolution Testing. Dissolution Technol. 2018, 25, 6–7. [Google Scholar] [CrossRef]
- Singh, A.; Van den Mooter, G. Spray drying formulation of amorphous solid dispersions. Adv. Drug Deliv. Rev. 2016, 100, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.T.; Tran, P.H.; Tran, T.T. pH-independent dissolution enhancement for multiple poorly water-soluble drugs by nano-sized solid dispersions based on hydrophobic–hydrophilic conjugates. Drug Dev. Ind. Pharm. 2019, 45, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, Y.; Tin, Y.-Y.; Soe, M.-T.-P.; Ko, B.; Park, S.; Lee, J. Recent technologies for amorphization of poorly water-soluble drugs. Pharmaceutics 2021, 13, 1318. [Google Scholar] [CrossRef] [PubMed]
- Holm, R.; Olesen, N.E.; Hartvig, R.A.; Jørgensen, E.B.; Larsen, D.B.; Westh, P. Effect of cyclodextrin concentration on the oral bioavailability of danazol and cinnarizine in rats. Eur. J. Pharm. Biopharm. 2016, 101, 9–14. [Google Scholar] [CrossRef]
- Beig, A.; Agbaria, R.; Dahan, A. The use of captisol (SBE7-β-CD) in oral solubility-enabling formulations: Comparison to HPβCD and the solubility–permeability interplay. Eur. J. Pharm. Sci. 2015, 77, 73–78. [Google Scholar] [CrossRef]
- Kaur, I.; Wakode, S.; Pal Singh, H.; Manachanda, S. Development and Validation of a Stability-Indicating Reverse Phase HPLC-PDA Method for Determination of Canagliflozin in Bulk and Pharmaceutical Dosage Form. Pharm. Methods 2016, 7, 54–62. [Google Scholar] [CrossRef]
- Beig, A.; Agbaria, R.; Dahan, A. Oral delivery of lipophilic drugs: The tradeoff between solubility increase and permeability decrease when using cyclodextrin-based formulations. PLoS ONE 2013, 8, e68237. [Google Scholar] [CrossRef]
- Másson, M.; Loftsson, T.; Másson, G.S.; Stefánsson, E. Cyclodextrins as permeation enhancers: Some theoretical evaluations and in vitro testing. J. Control. Release 1999, 59, 107–118. [Google Scholar] [CrossRef] [PubMed]




| X Factors | Variation Intervals | |
|---|---|---|
| Low | High | |
| X1: SiO2 ratio (w/w) | 1:0.3 | 1:0.9 |
| X2: polymer ratio (mol/mol) | 1:0.5 | 1:2.5 |
| X3: blower | 4.0 | 6.0 |
| Y factors | Goal | |
| Y1: yield (%) | Maximize | |
| Y2: solubility (μg/mL) | Maximize | |
| Y3: particle size (μm) | Minimize | |
| Run | X Factors | Y Factors | ||||
|---|---|---|---|---|---|---|
| Unit | X1: SiO2 Ratio (w/w) | X2: HP-β-CD Ratio (mol/mol) | X3: Blower | Y1: Yield (%) | Y2: Solubility (μg/mL) | Y3: Particle Size (μm) |
| F1 | 0.3 | 0.5 | 5 | 58.5 | 2547 | 5.67 |
| F2 | 0.9 | 0.5 | 5 | 46.3 | 2577 | 7.61 |
| F3 | 0.3 | 2.5 | 5 | 53.6 | 9806 | 7.39 |
| F4 | 0.9 | 2.5 | 5 | 50.0 | 9541 | 8.86 |
| F5 | 0.3 | 1.5 | 4 | 26.5 | 9135 | 6.06 |
| F6 | 0.9 | 1.5 | 4 | 35.7 | 9160 | 6.87 |
| F7 | 0.3 | 1.5 | 6 | 65.0 | 9314 | 6.98 |
| F8 | 0.9 | 1.5 | 6 | 60.0 | 9963 | 7.33 |
| F9 | 0.6 | 0.5 | 4 | 26.2 | 2667 | 4.92 |
| F10 | 0.6 | 2.5 | 4 | 26.8 | 9325 | 7.16 |
| F11 | 0.6 | 0.5 | 6 | 66.6 | 2694 | 7.19 |
| F12 | 0.6 | 2.5 | 6 | 56.9 | 9908 | 7.67 |
| F13 | 0.6 | 1.5 | 5 | 54.8 | 9743 | 6.65 |
| F14 | 0.6 | 1.5 | 5 | 51.3 | 9481 | 6.16 |
| F15 | 0.6 | 1.5 | 5 | 51.0 | 9921 | 6.21 |
| Source | DF * | Adj SS * | Adj MS * | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 9 | 0.2531 | 0.0281 | 18.97 | 0.002 |
| Linear Model | 3 | 0.2251 | 0.0750 | 50.61 | 0.000 |
| X1: SiO2 ratio (w/w) | 1 | 0.0016 | 0.0016 | 1.13 | 0.336 |
| X2: HP-β-CD ratio (mol/mol) | 1 | 0.0013 | 0.0013 | 0.89 | 0.388 |
| X3: blower | 1 | 0.2221 | 0.2221 | 149.79 | 0.000 |
| Quadratic Model | 3 | 0.0184 | 0.0061 | 4.15 | 0.080 |
| X1: SiO2 ratio | 1 | 0.0005 | 0.0005 | 0.36 | 0.574 |
| X2: HP-β-CD ratio | 1 | 0.0007 | 0.0007 | 0.54 | 0.496 |
| X3: blower | 1 | 0.0169 | 0.0169 | 11.42 | 0.020 |
| Two-Way Interaction | 3 | 0.0095 | 0.0031 | 2.15 | 0.213 |
| X1: SiO2 ratio X2: HP-β-CD ratio | 1 | 0.0018 | 0.0018 | 1.25 | 0.315 |
| X1: SiO2 ratio X3: blower | 1 | 0.0050 | 0.0050 | 3.40 | 0.125 |
| X2: HP-β-CD ratio X3: blower | 1 | 0.0026 | 0.0026 | 1.79 | 0.239 |
| Error | 5 | 0.0074 | 0.0015 | ||
| Lack-of-fit | 3 | 0.0065 | 0.0022 | 4.87 | 0.175 |
| Pure error | 2 | 0.0009 | 0.0004 | ||
| S | R | R2 (Retouch) | |||
| 0.0385069 | 97.15% | 92.03% | |||
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 9 | 142,486,449 | 15,831,828 | 338.35 | 0.000 |
| Linear Model | 3 | 99,007,026 | 33,002,342 | 705.30 | 0.000 |
| X1: SiO2 ratio (w/w) | 1 | 24,090 | 24,090 | 0.51 | 0.505 |
| X2: HP-β-CD ratio (mol/mol) | 1 | 98,666,128 | 98,666,128 | 2108.62 | 0.000 |
| X3: blower | 1 | 316,808 | 316,808 | 6.77 | 0.048 |
| Quadratic Model | 3 | 43,283,039 | 14,427,680 | 308.34 | 0.000 |
| X1: SiO2 ratio | 1 | 114,861 | 114,861 | 2.45 | 0.178 |
| X2: HP-β-CD ratio | 1 | 43,208,809 | 43,208,809 | 923.43 | 0.000 |
| X3: blower | 1 | 78,301 | 78,301 | 1.67 | 0.252 |
| Two-Way Interaction | 3 | 196,384 | 65,461 | 1.40 | 0.346 |
| X1: SiO2 X2: HP-β-CD ratio | 1 | 21,756 | 21,756 | 0.46 | 0.526 |
| X1: SiO2 ratio X3: blower | 1 | 97,344 | 97,344 | 2.08 | 0.209 |
| X2: HP-β-CD ratio X3: blower | 1 | 77,284 | 77,284 | 1.65 | 0.255 |
| Error | 5 | 233,959 | 46,792 | ||
| Lack-of-fit | 3 | 135,983 | 45,328 | 0.93 | 0.557 |
| Pure error | 2 | 97,976 | 48,988 | ||
| S | R | R2 (Retouch) | |||
| 216.314 | 99.84% | 99.54% | |||
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 6 | 11.5399 | 1.9233 | 13.17 | 0.001 |
| Linear Model | 3 | 8.8208 | 2.9403 | 20.13 | 0.000 |
| X1: SiO2 ratio (w/w) | 1 | 2.6106 | 2.6106 | 17.88 | 0.003 |
| X2: HP-β-CD ratio (mol/mol) | 1 | 4.0470 | 4.0470 | 27.71 | 0.001 |
| X3: blower | 1 | 2.1632 | 2.1632 | 14.81 | 0.005 |
| Quadratic Model | 2 | 1.9447 | 0.9724 | 6.66 | 0.020 |
| X1: SiO2 ratio | 1 | 1.1881 | 1.1881 | 8.14 | 0.021 |
| X2: HP-β-CD ratio | 1 | 0.8939 | 0.8939 | 6.12 | 0.038 |
| Two-Way Interaction | 1 | 0.7744 | 0.7744 | 5.30 | 0.050 |
| X2: HP-β-CD ratio X3: blower | 1 | 0.7744 | 0.7744 | 5.30 | 0.050 |
| Error | 8 | 1.1682 | 0.1460 | ||
| Lack-of-fit | 6 | 1.0228 | 0.1705 | 2.34 | 0.329 |
| Pure error | 2 | 0.1454 | 0.0727 | ||
| S | R | R2 (Retouch) | |||
| 0.382138 | 90.81% | 83.91% | |||
| Factor | Setting | ||||
|---|---|---|---|---|---|
| X1: SiO2 ratio (w/w) | 1:0.4 | ||||
| X2: HP-β-CD ratio (mol/mol) | 1:1.6 | ||||
| X3: blower | 6 | ||||
| Response | Suitable Value SE | Suitable Value | 95% CI | 95% PI | |
| Y1: yield | 0.6470 | 0.0219 | (0.5983, 0.6958) | (0.5487, 0.7453) | |
| Y2: solubility | 9971 | 137 | (9619, 10,324) | (9313, 10,630) | |
| Y3: particle size | 6.661 | 0.209 | (6.179, 7.143) | (5.657, 7.665) | |
| PK Parameter | CFZ-SD | CFZ |
|---|---|---|
| AUC0–48 (ng·h/mL) | 14,650.43 ± 2383.81 | 7675.32 ± 595.33 |
| Cmax (ng/mL) | 1089.59 ± 199.22 | 870.05 ± 33.95 |
| Tmax (h) | 5.97 ± 0.88 | 3.11 ± 0.64 |
| T1/2 | 4.69 ± 1.01 | 4.48 ± 1.10 |
| Kel | 0.16 ± 0.03 | 0.17 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Choi, S.U.; Kim, T.J.; Jeong, N.Y.; Paeng, H.S.; Kim, K.S. Research on Enhancing the Solubility and Bioavailability of Canagliflozin Using Spray Drying Techniques with a Quality-by-Design Approach. Pharmaceutics 2025, 17, 1319. https://doi.org/10.3390/pharmaceutics17101319
Lee JH, Choi SU, Kim TJ, Jeong NY, Paeng HS, Kim KS. Research on Enhancing the Solubility and Bioavailability of Canagliflozin Using Spray Drying Techniques with a Quality-by-Design Approach. Pharmaceutics. 2025; 17(10):1319. https://doi.org/10.3390/pharmaceutics17101319
Chicago/Turabian StyleLee, Ji Ho, Seong Uk Choi, Tae Jong Kim, Na Yoon Jeong, Hyun Seo Paeng, and Kyeong Soo Kim. 2025. "Research on Enhancing the Solubility and Bioavailability of Canagliflozin Using Spray Drying Techniques with a Quality-by-Design Approach" Pharmaceutics 17, no. 10: 1319. https://doi.org/10.3390/pharmaceutics17101319
APA StyleLee, J. H., Choi, S. U., Kim, T. J., Jeong, N. Y., Paeng, H. S., & Kim, K. S. (2025). Research on Enhancing the Solubility and Bioavailability of Canagliflozin Using Spray Drying Techniques with a Quality-by-Design Approach. Pharmaceutics, 17(10), 1319. https://doi.org/10.3390/pharmaceutics17101319










