Application of Biomimetic SPIONs in Targeted Lung Cancer Therapy: Cell-Membrane Camouflage Technology and Lung Retention Enhancement Strategies
Abstract
1. Introduction
1.1. Current Status and Challenges of Lung Cancer Treatment
1.2. Therapeutic Potential of SPIONs
1.3. Innovative Value of Biomimetic Strategies
2. Literature Search Strategy
- Studies investigating biomimetic SPIONs (e.g., cell-membrane-camouflaged or other biomimetic strategies) for lung cancer diagnosis or therapy;
- Reports including in vitro cellular or in vivo animal experiments;
- Detailed description of fabrication protocols, mechanisms of action, or therapeutic efficacy;
- Original research articles, reviews, or meta-analyses.
- Conference abstracts, patents, editorials, commentaries, or news reports;
- Studies unrelated to lung cancer or SPIONs;
- Full text unavailable or duplicate publications.
3. Cell-Membrane Camouflage Technology
3.1. Technical Principles
3.2. Preparation and Characterization
3.3. Major Membrane Types and Their Applications
3.3.1. Macrophage Membrane
3.3.2. Neutrophil Membrane
3.3.3. Cancer-Cell Membrane (CCM)
3.3.4. Formulation–Process–Performance Nexus: Toward Reproparable, Scale-Ready Manufacturing
3.4. Tumor Heterogeneity: A Touchstone for Cell-Membrane Camouflage
3.4.1. The Challenge of Heterogeneity—Why “One-Size-Fits-All” No Longer Works
3.4.2. Multi-Receptor Synergy—How Membrane Camouflage Can Fight Back
3.5. Cell-Membrane Camouflage in the Metastatic Cascade: Mechanisms Beyond Homotypic Targeting
4. Strategies for Enhanced Pulmonary Retention
4.1. Pulmonary Vascular Architecture and the Air–Blood Barrier: Biological Constraints on SPIONs Targeting
4.2. Optimization of Magnetic Targeting Systems
4.3. Respiratory-Compensated Magnetic Targeting: Overcoming Lung Motion for Precision SPIONs Accumulation
4.4. Multifunctional Surface Engineering Strategies
5. Therapeutic Applications and Mechanisms
5.1. Strategies and Mechanisms for Overcoming Physiological Barriers
5.2. Synergistic Immunotherapy
6. Translational Medicine Challenges
7. Narrative Comparison: Biomimetic SPIONs vs. Pegylated Liposomes vs. PLGA-PEG Nanoparticles
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDT | hemodynamic therapy |
| SPIONs | Superparamagnetic iron-oxide nanoparticles |
| MHT | magnetic hyperthermia therapy |
| MRI | magnetic resonance imaging |
| MPI | magnetic particle imaging |
| EPR | enhanced permeability and retention |
| MDR | multidrug resistance |
| NETs | neutrophil extracellular traps |
| GMP | Good Manufacturing Practice |
| PLA-PEG | poly(lactic acid)-poly(ethylene glycol) |
| Tf | transferrin |
| ICD | immunogenic cell death |
| TAMs | tumor-associated macrophages |
| iPSC | induced pluripotent stem cell |
References
- Smolarz, B.; Łukasiewicz, H.; Samulak, D.; Piekarska, E.; Kołaciński, R.; Romanowicz, H. Lung Cancer-Epidemiology, Pathogenesis, Treatment and Molecular Aspect (Review of Literature). Int. J. Mol. Sci. 2025, 26, 5. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Sosa Iglesias, V.; Giuranno, L.; Dubois, L.J.; Theys, J.; Vooijs, M. Drug Resistance in Non-Small Cell Lung Cancer: A Potential for NOTCH Targeting? Front. Oncol. 2018, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R. Targeted therapy vs. chemotherapy: Which has had more impact on survival in lung cancer? Does targeted therapy make patients live longer? Hard to prove, but impossible to ignore. Clin. Adv. Hematol. Oncol. 2014, 12, 763–766. [Google Scholar]
- Liu, Y.; Wen, S.; Wang, W.; Liu, Q.; Su, L.; Zhang, Y.; Bai, C.; Pu, S.; Zhang, Q.; Wang, J. PSMD14 promotes breast cancer progression by reducing K63-linked ubiquitination on FOXM1 and activating the PI3K/AKT/mTOR pathway. Int. J. Biol. Macromol. 2025, 284 Pt 3, 131921. [Google Scholar] [CrossRef]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Lecomte, V.; Ternad, I.; Van Leuven, L.; Muller, R.N.; Stanicki, D.; Laurent, S. Superparamagnetic Iron Oxide Nanoparticles (SPION): From Fundamentals to State-of-the-Art Innovative Applications for Cancer Therapy. Pharmaceutics 2023, 15, 1. [Google Scholar] [CrossRef]
- Bahig, A.E.; Walaa MAbd, E.-R.; Shehab, E. Green synthesis of biocompatible Fe3O4 magnetic nanoparticles using Citrus Sinensis peels extract for their biological activities and magnetic-hyperthermia applications. Sci. Rep. 2023, 13, 20846. [Google Scholar]
- Schleich, N.; Sibret, P.; Danhier, P.; Ucakar, B.; Laurent, S.; Muller, R.N.; Jérôme, C.; Gallez, B.; Préat, V.; Danhier, F. Dual anticancer drug/superparamagnetic iron oxide-loaded PLGA-based nanoparticles for cancer therapy and magnetic resonance imaging. Int. J. Pharm. 2013, 447, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Quan, Q.; Xie, J.; Gao, H.; Yang, M.; Zhang, F.; Liu, G.; Lin, X.; Wang, A.; Eden, H.S.; Lee, S.; et al. HSA coated iron oxide nanoparticles as drug delivery vehicles for cancer therapy. Mol. Pharm. 2011, 8, 1669–1676. [Google Scholar] [CrossRef]
- da Costa Araujo, J.F.; Nogueira da Silva Avelino Oliveira Rocha, G.; Rodrigues Silva, J.Y.; Ribeiro Rocha, J.V.; Figueiroa Bakuzis, A.; Alves Junior, S. Fe3O4/ZIF-8-90 Nanocomposite as a Strategy for Oncological Treatment. ACS Omega 2025, 10, 30236–30249. [Google Scholar] [CrossRef]
- Yue, L.; Sun, C.; Kwong, C.H.T.; Wang, R. Cucurbit[7]uril-functionalized magnetic nanoparticles for imaging-guided cancer therapy. J. Mater. Chem. B 2020, 8, 2749–2753. [Google Scholar] [CrossRef] [PubMed]
- Stueber, D.D.; Villanova, J.; Aponte, I.; Xiao, Z.; Colvin, V.L. Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends. Pharmaceutics 2021, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Bulte, J.W.M. Superparamagnetic iron oxides as MPI tracers: A primer and review of early applications. Adv. Drug Deliv. Rev. 2019, 138, 293–301. [Google Scholar] [CrossRef]
- Jung, K.O.; Jo, H.; Yu, J.H.; Gambhir, S.S.; Pratx, G. Development and MPI tracking of novel hypoxia-targeted theranostic exosomes. Biomaterials 2018, 177, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, K.; Du, J.; Tian, J.; Zhang, H. The first visualization of chemotherapy-induced tumor apoptosis via magnetic particle imaging in a mouse model. Phys. Med. Biol. 2020, 65, 195004. [Google Scholar] [CrossRef]
- Zhu, X.; Li, J.; Peng, P.; Hosseini Nassab, N.; Smith, B.R. Quantitative Drug Release Monitoring in Tumors of Living Subjects by Magnetic Particle Imaging Nanocomposite. Nano Lett. 2019, 19, 6725–6733. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Chao, M.P.; Weissman, I.L.; Majeti, R. The CD47-SIRPα pathway in cancer immune evasion and potential therapeutic implications. Curr. Opin. Immunol. 2012, 24, 225–230. [Google Scholar] [CrossRef]
- Mateo, V.; Lagneaux, L.; Bron, D.; Biron, G.; Armant, M.; Delespesse, G.; Sarfati, M. CD47 ligation induces caspase-independent cell death in chronic lymphocytic leukemia. Nat. Med. 1999, 5, 1277–1284. [Google Scholar] [CrossRef]
- Marure-Rojano, A.E.; Cano-García, J.R.; Luna-Agulo, A.B.; Sánchez-Chapul, L.; Santos-Cuevas, C.L.; Aguilar-Gaytán, M.D.R.; Flores-Berrios, E.P.; Couder-García, B.D.C.; Lara-Hernández, G.; Bahena-Ocampo, I.U.; et al. The cytotoxic effect of quercetin-induced apoptosis on lung metastatic cells from giant cell tumor of bone. Cell. Mol. Biol. 2025, 71, 214–222. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef]
- Davis, P.J.; Lin, H.Y.; Hercbergs, A.; Keating, K.A.; Mousa, S.A. Coronaviruses and Integrin αvβ3: Does Thyroid Hormone Modify the Relationship? Endocr. Res. 2020, 45, 210–215. [Google Scholar] [CrossRef]
- Wettersten, H.I.; Weis, S.M.; Pathria, P.; Von Schalscha, T.; Minami, T.; Varner, J.A.; Cheresh, D.A. Arming Tumor-Associated Macrophages to Reverse Epithelial Cancer Progression. Cancer Res. 2019, 79, 5048–5059. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Zheng, Y.; Zhu, Y.; Zhou, Z.; Liu, Z.; Lin, C.; Wan, Y.; Wen, Y.; Liu, C.; et al. Autocrine pro-legumain promotes breast cancer metastasis via binding to integrin αvβ3. Oncogene 2022, 41, 4066–4077. [Google Scholar] [CrossRef]
- Zhou, W.; Ma, J.; Meng, L.; Liu, D.; Chen, J. Deletion of TRIB3 disrupts the tumor progression induced by integrin αvβ3 in lung cancer. BMC Cancer 2022, 22, 459. [Google Scholar] [CrossRef]
- Hu, C.M.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef] [PubMed]
- Asrorov, A.M.; Gu, Z.; Li, F.; Liu, L.; Huang, Y. Biomimetic camouflage delivery strategies for cancer therapy. Nanoscale 2021, 13, 8693–8706. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, H.; Cao, D.; Guo, Y.; Wu, D.; Yang, M.; Wang, H.; Shao, X.; Li, Y.; Liang, Y. Overcoming Biological Barriers in Cancer Therapy: Cell Membrane-Based Nanocarrier Strategies for Precision Delivery. Int. J. Nanomed. 2025, 20, 3113–3145. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Su, J.; Ran, W.; Zhang, P.; Yin, Q.; Zhang, Z.; Yu, H.; Li, Y. Preparation and Application of Cell Membrane-Camouflaged Nanoparticles for Cancer Therapy. Theranostics 2017, 7, 2575–2592. [Google Scholar] [CrossRef]
- Tao, X.; Zhao, C.; Mackinnon, R. Membrane protein isolation and structure determination in cell-derived membrane vesicles. Proc. Natl. Acad. Sci. USA 2023, 120, e2302325120. [Google Scholar] [CrossRef]
- Huang, X.; Guo, H.; Wang, L.; Zhang, Z.; Zhang, W. Biomimetic cell membrane-coated nanocarriers for targeted siRNA delivery in cancer therapy. Drug Discov. Today 2023, 28, 103514. [Google Scholar] [CrossRef]
- Shen, X.; Lu, Q.; Peng, T.; Zhang, Y.; Tan, W.; Yang, Y.; Tan, J.; Yuan, Q. Bionic Potassium Ion Channel in Live Cells Repairs Cardiomyocyte Function. J. Am. Chem. Soc. 2024, 146, 20388–20398. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, J.; Chen, X.; Liu, W.; Chen, T. Cell Membrane Coating Technology: A Promising Strategy for Biomedical Applications. Nano-Micro Lett. 2019, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Mu, W.; Yuan, S.; Fu, S.; Liu, Y.; Zhang, N. Cell Membrane Biomimetic Nano-Delivery Systems for Cancer Therapy. Pharmaceutics 2023, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Harisa, G.I.; Ibrahim, M.F.; Alanazi, F.K. Erythrocyte-mediated delivery of pravastatin: In vitro study of effect of hypotonic lysis on biochemical parameters and loading efficiency. Arch. Pharmacal Res. 2012, 35, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Flatmark, T.; Saraste, J. The p58-positive pre-golgi intermediates consist of distinct subpopulations of particles that show differential binding of COPI and COPII coats and contain vacuolar H+-ATPase. J. Cell Sci. 2000, 113 Pt 11, 2023–2031. [Google Scholar] [CrossRef]
- Fang, R.H.; Hu, C.M.; Luk, B.T.; Gao, W.; Copp, J.A.; Tai, Y.; O’Connor, D.E.; Zhang, L. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014, 14, 2181–2188. [Google Scholar] [CrossRef]
- Wu, Y.; Wan, S.; Yang, S.; Hu, H.; Zhang, C.; Lai, J.; Zhou, J.; Chen, W.; Tang, X.; Luo, J.; et al. Macrophage cell membrane-based nanoparticles: A new promising biomimetic platform for targeted delivery and treatment. J. Nanobiotechnol. 2022, 20, 542. [Google Scholar] [CrossRef]
- Horvat, N.K.; Chocarro, S.; Marques, O.; Bauer, T.A.; Qiu, R.; Diaz-Jimenez, A.; Helm, B.; Chen, Y.; Sawall, S.; Sparla, R.; et al. Superparamagnetic Iron Oxide Nanoparticles Reprogram the Tumor Microenvironment and Reduce Lung Cancer Regrowth after Crizotinib Treatment. ACS Nano 2024, 18, 11025–11041. [Google Scholar] [CrossRef]
- Zheng, Y.; Cai, J.; Ji, Q.; Liu, L.; Liao, K.; Dong, L.; Gao, J.; Huang, Y. Tumor-Activated Neutrophils Promote Lung Cancer Progression through the IL-8/PD-L1 Pathway. Curr. Cancer Drug Targets 2025, 25, 294–305. [Google Scholar] [CrossRef]
- Han, L.; Chen, Y.; Huang, N.; Zhou, X.; Lv, Y.; Li, H.; Chai, D.; Zheng, J.; Wang, G. Cancer-educated neutrophils promote lung cancer progression via PARP-1-ALOX5-mediated MMP-9 expression. Cancer Biol. Med. 2024, 21, 175–192. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Xu, L.; Tu, F.; Rui, X.; Zhang, L.; Yan, Z.; Liu, Y.; Hu, R. Neutrophil membrane-coated nanoparticles exhibit increased antimicrobial activities in an anti-microbial resistant, K. pneumonia infection model. Nanomedicine 2023, 48, 102640. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Hu, L.; Zheng, H.; Liu, M.; Liu, X.; Li, C.; Qiu, Q.; Zhao, Z.; Cheng, X.; Lai, C.; et al. Neutrophil-mediated delivery of pixantrone-loaded liposomes decorated with poly(sialic acid)-octadecylamine conjugate for lung cancer treatment. Drug Deliv. 2018, 25, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cui, H.; Zhang, J.; Bei, Y.; Huang, Y.; Li, M.; Liu, J.; Wu, Y.; Gao, J. Application of Cell Membrane-Coated Nanomaterials for Tumor Treatment. Mini Rev. Med. Chem. 2023, 23, 1535–1559. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Gao, H.; Zhang, X.; Sun, L.; Huang, Y.; Zhang, J.; Ding, B. Cell Membrane-Coated Biomimetic Nanoparticles in Cancer Treatment. Pharmaceutics 2024, 16, 531. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, S.; Li, S.; Song, G.; Meng, T.; Yuan, H.; Hu, F. Normalizing Tumor Blood Vessels to Improve Chemotherapy and Inhibit Breast Cancer Metastasis by Multifunctional Nanoparticles. Mol. Pharm. 2023, 20, 5078–5089. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Cai, H.; Yang, Y.; Peng, F.; Song, M.; Sun, K.; Yan, F.; Liu, Y. Hybrid membrane camouflaged copper sulfide nanoparticles for photothermal-chemotherapy of hepatocellular carcinoma. Acta Biomater. 2020, 111, 363–372. [Google Scholar] [CrossRef]
- Chen, H.Y.; Deng, J.; Wang, Y.; Wu, C.Q.; Li, X.; Dai, H.W. Hybrid cell membrane-coated nanoparticles: A multifunctional biomimetic platform for cancer diagnosis and therapy. Acta Biomater. 2020, 112, 1–13. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Y.; Xu, X.; Wang, F.; Shen, W.; Leng, X.; Zhao, J.; Liu, B.; Wang, Y.; Liu, P. Surface PEGylated Cancer Cell Membrane-Coated Nanoparticles for Codelivery of Curcumin and Doxorubicin for the Treatment of Multidrug Resistant Esophageal Carcinoma. Front. Cell Dev. Biol. 2021, 9, 688070. [Google Scholar] [CrossRef]
- Kimball, A.S.; Obi, A.T.; Diaz, J.A.; Henke, P.K. The Emerging Role of NETs in Venous Thrombosis and Immunothrombosis. Front. Immunol. 2016, 7, 236. [Google Scholar] [CrossRef]
- Kandasamy, G.; Maity, D. Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int. J. Pharm. 2015, 496, 191–218. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Y.; Zhang, C.; Zhao, Y.; Lu, C.; Yin, B.; Yang, Y.; Gong, X.; Teng, L.; Liu, Y.; et al. An Acidity-Unlocked Magnetic Nanoplatform Enables Self-Boosting ROS Generation through Upregulation of Lactate for Imaging-Guided Highly Specific Chemodynamic Therapy. Angew. Chem. Int. Ed. 2021, 60, 9562–9572. [Google Scholar] [CrossRef]
- Xie, P.; Du, P.; Li, J.; Liu, P. Stimuli-responsive hybrid cluster bombs of PEGylated chitosan encapsulated DOX-loaded superparamagnetic nanoparticles enabling tumor-specific disassembly for on-demand drug delivery and enhanced MR imaging. Carbohydr. Polym. 2019, 205, 377–384. [Google Scholar] [CrossRef]
- Lv, Y.; Kan, J.; Luo, M.; Yang, C.; Luo, X.; Lin, X.; Li, H.; Li, X.; Li, Y.; Yang, C.; et al. Multifunctional Nanosnowflakes for T1-T2 Double-Contrast Enhanced MRI and PAI Guided Oxygen Self-Supplementing Effective Anti-Tumor Therapy. Int. J. Nanomed. 2022, 17, 4619–4638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ji, C.; Zhang, H.; Shi, H.; Mao, F.; Qian, H.; Xu, W.; Wang, D.; Pan, J.; Fang, X.; et al. Engineered neutrophil-derived exosome-like vesicles for targeted cancer therapy. Sci. Adv. 2022, 8, eabj8207. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.Q.; Ji, A.P.; Zhang, J.; Bo, L.; Jiao, Q.; Qi, N.; Zhang, D.S.; Du, M.H. Preparation and biodistribution study of 131I-labeled bevacizumab-conjugated paclitaxel-loaded superparamagnetic iron oxide nanoparticles. Nucl. Tech. 2023, 46, 36–43. [Google Scholar]
- Carter, T.J.; Agliardi, G.; Lin, F.Y.; Ellis, M.; Jones, C.; Robson, M.; Richard-Londt, A.; Southern, P.; Lythgoe, M.; Zaw Thin, M.; et al. Potential of Magnetic Hyperthermia to Stimulate Localized Immune Activation. Small 2021, 17, e2005241. [Google Scholar] [CrossRef]
- Vural, V.; Yilmaz, O.C. The Turkish SentiMAG feasibility trial: Preliminary results. Breast Cancer 2020, 27, 261–265. [Google Scholar] [CrossRef]
- Alvarado, M.D.; Mittendorf, E.A.; Teshome, M.; Thompson, A.M.; Bold, R.J.; Gittleman, M.A.; Beitsch, P.D.; Blair, S.L.; Kivilaid, K.; Harmer, Q.J.; et al. SentimagIC: A Non-inferiority Trial Comparing Superparamagnetic Iron Oxide Versus Technetium-99m and Blue Dye in the Detection of Axillary Sentinel Nodes in Patients with Early-Stage Breast Cancer. Ann. Surg. Oncol. 2019, 26, 3510–3516. [Google Scholar] [CrossRef]
- Khan, S.; Amin, F.M.; Fliedner, F.P.; Christensen, C.E.; Tolnai, D.; Younis, S.; Olinger, A.C.R.; Birgens, H.; Daldrup-Link, H.; Kjær, A.; et al. Investigating macrophage-mediated inflammation in migraine using ultrasmall superparamagnetic iron oxide-enhanced 3T magnetic resonance imaging. Cephalalgia 2019, 39, 1407–1420. [Google Scholar] [CrossRef]
- Akhtar, N.; Mohammed, H.A.; Yusuf, M.; Al-Subaiyel, A.; Sulaiman, G.M.; Khan, R.A. SPIONs Conjugate Supported Anticancer Drug Doxorubicin’s Delivery: Current Status, Challenges, and Prospects. Nanomaterials 2022, 12, 3686. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, W.; Wang, M.; Liao, Z. Magnetic nanoparticles for cancer theranostics: Advances and prospects. J. Control. Release 2021, 335, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jia, Y.; Zhu, X.; Zhi, W.; Li, D.; Huang, S. Research Progress of Metal-Phenolic Networks in Tumor Diagnosis and Treatment. China Oncol. 2022, 31, 463–472. [Google Scholar]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Sun, D.; Meng, Z.; Ying, W.; Gao, W.; Hou, R.; Zheng, Y.; Cai, X.; Hu, B.; et al. Hollow magnetic nanosystem-boosting synergistic effect between magnetic hyperthermia and sonodynamic therapy via modulating reactive oxygen species and heat shock proteins. Chem. Eng. J. 2020, 390, 124521. [Google Scholar] [CrossRef]
- Pfister, F.; Dörrie, J.; Schaft, N.; Buchele, V.; Unterweger, H.; Carnell, L.R.; Schreier, P.; Stein, R.; Kubánková, M.; Guck, J.; et al. Human T cells loaded with superparamagnetic iron oxide nanoparticles retain antigen-specific TCR functionality. Front. Immunol. 2023, 14, 1223695. [Google Scholar] [CrossRef]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef]
- Vonarbourg, A.; Passirani, C.; Saulnier, P.; Benoit, J.P. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials 2006, 27, 4356–4373. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Lee, B.J. Protein corona: A new approach for nanomedicine design. Int. J. Nanomed. 2017, 12, 3137–3151. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, J.S.; Malissek, M.; Simon, S.; Knauer, S.K.; Maskos, M.; Stauber, R.H.; Peukert, W.; Treuel, L. Impact of the nanoparticle-protein corona on colloidal stability and protein structure. Langmuir 2012, 28, 9673–9679. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, M.P.; Pitek, A.S.; Lynch, I.; Dawson, K.A. Formation and characterization of the nanoparticle-protein corona. Methods Mol. Biol. 2013, 1025, 137–155. [Google Scholar]
- Owens, D.E., III.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Martinod, K.; Wagner, D.D. Thrombosis: Tangled up in NETs. Blood 2014, 123, 2768–2776. [Google Scholar] [CrossRef]
- Unterweger, H.; Dézsi, L.; Matuszak, J.; Janko, C.; Poettler, M.; Jordan, J.; Bäuerle, T.; Szebeni, J.; Fey, T.; Boccaccini, A.R.; et al. Dextran-coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging: Evaluation of size-dependent imaging properties, storage stability and safety. Int. J. Nanomed. 2018, 13, 1899–1915. [Google Scholar] [CrossRef]
- Zaloga, J.; Janko, C.; Nowak, J.; Matuszak, J.; Knaup, S.; Eberbeck, D.; Tietze, R.; Unterweger, H.; Friedrich, R.P.; Duerr, S.; et al. Development of a lauric acid/albumin hybrid iron oxide nanoparticle system with improved biocompatibility. Int. J. Nanomed. 2014, 9, 4847–4866. [Google Scholar] [CrossRef]
- Unterweger, H.; Janko, C.; Schwarz, M.; Dézsi, L.; Urbanics, R.; Matuszak, J.; Őrfi, E.; Fülöp, T.; Bäuerle, T.; Szebeni, J.; et al. Non-immunogenic dextran-coated superparamagnetic iron oxide nanoparticles: A biocompatible, size-tunable contrast agent for magnetic resonance imaging. Int. J. Nanomed. 2017, 12, 5223–5238. [Google Scholar] [CrossRef] [PubMed]
- Sadauskas, E.; Wallin, H.; Stoltenberg, M.; Vogel, U.; Doering, P.; Larsen, A.; Danscher, G. Kupffer cells are central in the removal of nanoparticles from the organism. Part. Fibre Toxicol. 2007, 4, 10. [Google Scholar] [CrossRef]
- Alexiou, C.; Arnold, W.; Klein, R.J.; Parak, F.G.; Hulin, P.; Bergemann, C.; Erhardt, W.; Wagenpfeil, S.; Lübbe, A.S. Locoregional cancer treatment with magnetic drug targeting. Cancer Res. 2000, 60, 6641–6648. [Google Scholar]
- Tietze, R.; Lyer, S.; Dürr, S.; Struffert, T.; Engelhorn, T.; Schwarz, M.; Eckert, E.; Göen, T.; Vasylyev, S.; Peukert, W.; et al. Efficient drug-delivery using magnetic nanoparticles–biodistribution and therapeutic effects in tumour bearing rabbits. Nanomedicine 2013, 9, 961–971. [Google Scholar] [CrossRef] [PubMed]
- de Las Heras, B.M.; Rubio-Aparicio, P.M.; Rubio-San-Simón, A.; Moreno, L.; Mazorra, P.; Almaraz, R.L.; López, M.L.; Guill, J.B.; Segura, V.; Bermúdez, M.; et al. Management and outcome of children with high-risk neuroblastoma: Insights from the Spanish Society of Pediatric Hematology and Oncology (SEHOP) neuroblastoma group on refractory and relapse/progressive disease. Clin. Transl. Oncol. 2025, 27, 2345–2356. [Google Scholar] [CrossRef]
- Wu, C.; Chen, W.; Yan, S.; Zhong, J.; Du, L.; Yang, C.; Pu, Y.; Li, Y.; Lin, J.; Zeng, M.; et al. MRI-guided photothermal/photodynamic immune activation combined with PD-1 inhibitor for the multimodal combination therapy of melanoma and metastases. Regen. Biomater. 2024, 11, rbae019. [Google Scholar] [CrossRef]
- Mosafer, J.; Abnous, K.; Tafaghodi, M.; Mokhtarzadeh, A.; Ramezani, M. In vitro and in vivo evaluation of anti-nucleolin-targeted magnetic PLGA nanoparticles loaded with doxorubicin as a theranostic agent for enhanced targeted cancer imaging and therapy. Eur. J. Pharm. Biopharm. 2017, 113, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Karati, D.; Mukherjee, S.; Prajapati, B.G. Unveiling Spanlastics as a Novel Carrier for Drug Delivery: A Review. Pharm. Nanotechnol. 2025, 13, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Karakatsanis, A.; Olofsson, H.; Stålberg, P.; Bergkvist, L.; Abdsaleh, S.; Wärnberg, F. Simplifying Logistics and Avoiding the Unnecessary in Patients with Breast Cancer Undergoing Sentinel Node Biopsy. A Prospective Feasibility Trial of the Preoperative Injection of Super Paramagnetic Iron Oxide Nanoparticles. Scand. J. Surg. 2018, 107, 130–137. [Google Scholar] [CrossRef]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef]
- Sica, A.; Larghi, P.; Mancino, A.; Rubino, L.; Porta, C.; Totaro, M.G.; Rimoldi, M.; Biswas, S.K.; Allavena, P.; Mantovani, A. Macrophage polarization in tumour progression. Semin. Cancer Biol. 2008, 18, 349–355. [Google Scholar] [CrossRef]
- Wang, Y.L.; Bian, K.; Zhang, D.D. Regulation Mechanism of Macrophage Phenotypic Polarization in Tumors and Research Progress of Traditional Chinese Medicine Intervention. China J. Chin. Mater. Medica 2015, 40, 180–184. [Google Scholar]
- Kaneda, M.M.; Messer, K.S.; Ralainirina, N.; Li, H.; Leem, C.J.; Gorjestani, S.; Woo, G.; Nguyen, A.V.; Figueiredo, C.C.; Foubert, P.; et al. PI3Kγ is a molecular switch that controls immune suppression. Nature 2016, 539, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Uchino, K.; Ochiya, T.; Takeshita, F. RNAi therapeutics and applications of microRNAs in cancer treatment. Jpn. J. Clin. Oncol. 2013, 43, 596–607. [Google Scholar] [CrossRef]
- Deng, Y.C.; Zhang, Z.P.; Cheng, Q.S.; Wang, X.P.; Zhou, Y.A.; Lu, Y.; Li, X.F. Preparation of Functionalized Fe3O4 and Its Application in Gene Transfection. Biomed. Eng. Clin. Med. 2009, 13, 62–68. [Google Scholar]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef]
- Bardoel, B.W.; Kenny, E.F.; Sollberger, G.; Zychlinsky, A. The balancing act of neutrophils. Cell Host Microbe 2014, 15, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zafar, H.; Zia, M.; Ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Alcázar, M.; Rangaswamy, C.; Panda, R.; Bitterling, J.; Simsek, Y.J.; Long, A.T.; Bilyy, R.; Krenn, V.; Renné, C.; Renné, T.; et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science 2017, 358, 1202–1206. [Google Scholar] [CrossRef]
- Janko, C.; Zaloga, J.; Pöttler, M.; Dürr, S.; Eberbeck, D.; Tietze, R.; Lyer, S.; Alexiou, C. Strategies to optimize the biocompatibility of iron oxide nanoparticles—“SPIONs safe by design”. J. Magn. Magn. Mater. 2017, 431, 281–284. [Google Scholar] [CrossRef]
- Friedrich, R.P.; Janko, C.; Poettler, M.; Tripal, P.; Zaloga, J.; Cicha, I.; Dürr, S.; Nowak, J.; Odenbach, S.; Slabu, I.; et al. Flow cytometry for intracellular SPION quantification: Specificity and sensitivity in comparison with spectroscopic methods. Int. J. Nanomed. 2015, 10, 4185–4201. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 28–51. [Google Scholar] [CrossRef]
- Warnatsch, A.; Tsourouktsoglou, T.D.; Branzk, N.; Wang, Q.; Reincke, S.; Herbst, S.; Gutierrez, M.; Papayannopoulos, V. Reactive Oxygen Species Localization Programs Inflammation to Clear Microbes of Different Size. Immunity 2017, 46, 421–432. [Google Scholar] [CrossRef]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef]
- Jhunjhunwala, S.; Aresta-DaSilva, S.; Tang, K.; Alvarez, D.; Webber, M.J.; Tang, B.C.; Lavin, D.M.; Veiseh, O.; Doloff, J.C.; Bose, S.; et al. Neutrophil Responses to Sterile Implant Materials. PLoS ONE 2015, 10, e0137550. [Google Scholar] [CrossRef]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef]
- Massberg, S.; Grahl, L.; von Bruehl, M.L.; Manukyan, D.; Pfeiler, S.; Goosmann, C.; Brinkmann, V.; Lorenz, M.; Bidzhekov, K.; Khandagale, A.B.; et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010, 16, 887–896. [Google Scholar] [CrossRef]
- von Brühl, M.L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Köllnberger, M.; et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef]
- De Bont, C.M.; Boelens, W.C.; Pruijn, G.J.M. NETosis, complement, and coagulation: A triangular relationship. Cell. Mol. Immunol. 2019, 16, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Banda, N.K.; Mehta, G.; Chao, Y.; Wang, G.; Inturi, S.; Fossati-Jimack, L.; Botto, M.; Wu, L.; Moghimi, S.M.; Simberg, D. Mechanisms of complement activation by dextran-coated superparamagnetic iron oxide (SPIO) nanoworms in mouse versus human serum. Part. Fibre Toxicol. 2014, 11, 64. [Google Scholar] [CrossRef]
- Szebeni, J.; Fishbane, S.; Hedenus, M.; Howaldt, S.; Locatelli, F.; Patni, S.; Rampton, D.; Weiss, G.; Folkersen, J. Hypersensitivity to intravenous iron: Classification, terminology, mechanisms and management. Br. J. Pharmacol. 2015, 172, 5025–5036. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Li, X.; Gruosso, T.; Zuo, D.; Omeroglu, A.; Meterissian, S.; Guiot, M.C.; Salazar, A.; Park, M.; Levine, H. Infiltration of CD8+ T cells into tumor cell clusters in triple-negative breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 3678–3687. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, G.; Lorkowski, M.E.; Sims, H.M.; Loutrianakis, G.; Rahmy, A.; Cha, A.; Abenojar, E.; Wickramasinghe, S.; Moon, T.J.; Samia, A.C.S.; et al. Hyperthermia-mediated changes in the tumor immune microenvironment using iron oxide nanoparticles. Nanoscale Adv. 2021, 3, 5890–5899. [Google Scholar] [CrossRef]
- Dias, A.M.M.; Courteau, A.; Bellaye, P.S.; Kohli, E.; Oudot, A.; Doulain, P.E.; Petitot, C.; Walker, P.M.; Decréau, R.; Collin, B. Superparamagnetic Iron Oxide Nanoparticles for Immunotherapy of Cancers through Macrophages and Magnetic Hyperthermia. Pharmaceutics 2022, 14, 2388. [Google Scholar] [CrossRef]
- Janko, C.; Ratschker, T.; Nguyen, K.; Zschiesche, L.; Tietze, R.; Lyer, S.; Alexiou, C. Functionalized Superparamagnetic Iron Oxide Nanoparticles (SPIONs) as Platform for the Targeted Multimodal Tumor Therapy. Front. Oncol. 2019, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Fessas, P.; Lee, H.; Ikemizu, S.; Janowitz, T. A molecular and preclinical comparison of the PD-1-targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin. Oncol. 2017, 44, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S. Cancer Nanomedicine: Lessons for Immuno-Oncology. Trends Cancer 2017, 3, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Park, C.G.; Hartl, C.A.; Subedi, N.; Cartwright, A.N.; Puerto, R.B.; Zheng, Y.; Maiarana, J.; Freeman, G.J.; Wucherpfennig, K.W.; et al. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat. Commun. 2017, 8, 1747. [Google Scholar] [CrossRef]
- Mühlberger, M.; Unterweger, H.; Band, J.; Lehmann, C.; Heger, L.; Dudziak, D.; Alexiou, C.; Lee, G.; Janko, C. Loading of Primary Human T Lymphocytes with Citrate-Coated Superparamagnetic Iron Oxide Nanoparticles Does Not Impair Their Activation after Polyclonal Stimulation. Cells 2020, 9, 342. [Google Scholar] [CrossRef]
- Ngema, L.M.; Adeyemi, S.A.; Marimuthu, T.; Ubanako, P.N.; Ngwa, W.; Choonara, Y.E. Surface Immobilization of Anti-VEGF Peptide on SPIONs for Antiangiogenic and Targeted Delivery of Paclitaxel in Non-Small-Cell Lung Carcinoma. ACS Appl. Bio Mater. 2023, 6, 2747–2759. [Google Scholar] [CrossRef]
- Shahbazi-Gahrouei, D.; Abdi, N.; Shahbazi-Gahrouei, S.; Hejazi, S.H.; Salehnia, Z. In vivo study of anti-epidermal growth factor receptor antibody-based iron oxide nanoparticles (anti-EGFR-SPIONs) as a novel MR imaging contrast agent for lung cancer (LLC1) cells detection. IET Nanobiotechnol. 2020, 14, 369–374. [Google Scholar] [CrossRef]
- Persano, S.; Vicini, F.; Poggi, A.; Fernandez, J.L.C.; Rizzo, G.M.R.; Gavilán, H.; Silvestri, N.; Pellegrino, T. Elucidating the Innate Immunological Effects of Mild Magnetic Hyperthermia on U87 Human Glioblastoma Cells: An In Vitro Study. Pharmaceutics 2021, 13, 10. [Google Scholar] [CrossRef]
- Birkhäuser, F.D.; Studer, U.E.; Froehlich, J.M.; Triantafyllou, M.; Bains, L.J.; Petralia, G.; Vermathen, P.; Fleischmann, A.; Thoeny, H.C. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging facilitates detection of metastases in normal-sized pelvic lymph nodes of patients with bladder and prostate cancer. Eur. Urol. 2013, 64, 953–960. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Ma, M.; Chen, Z.; Tang, X.L.; Wang, Z. Self-Assembly Iron Oxide Nanoclusters for Photothermal-Mediated Synergistic Chemo/Chemodynamic Therapy. J. Immunol. Res. 2021, 2021, 9958239. [Google Scholar] [CrossRef] [PubMed]
- Aghighi, M.; Pisani, L.; Theruvath, A.J.; Muehe, A.M.; Donig, J.; Khan, R.; Holdsworth, S.J.; Kambham, N.; Concepcion, W.; Grimm, P.C.; et al. Ferumoxytol Is Not Retained in Kidney Allografts in Patients Undergoing Acute Rejection. Mol. Imaging Biol. 2018, 20, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Dengler, M.A.; van der Kuip, H.; Yildiz, H.; Rösch, S.; Klumpp, S.; Klingel, K.; Kandolf, R.; Helluy, X.; Hiller, K.H.; et al. Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: A human study using a multi-parametric cardiovascular magnetic resonance imaging approach. Eur. Heart J. 2013, 34, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Florian, A.; Ludwig, A.; Rösch, S.; Yildiz, H.; Klumpp, S.; Sechtem, U.; Yilmaz, A. Positive effect of intravenous iron-oxide administration on left ventricular remodelling in patients with acute ST-elevation myocardial infarction—A cardiovascular magnetic resonance (CMR) study. Int. J. Cardiol. 2014, 173, 184–189. [Google Scholar] [CrossRef]
- Aoki, T.; Saito, M.; Koseki, H.; Tsuji, K.; Tsuji, A.; Murata, K.; Kasuya, H.; Morita, A.; Narumiya, S.; Nozaki, K. Macrophage Imaging of Cerebral Aneurysms with Ferumoxytol: An Exploratory Study in an Animal Model and in Patients. J. Stroke Cerebrovasc. Dis. 2017, 26, 2055–2064. [Google Scholar] [CrossRef]
- MA3RS Study Investigators. Aortic Wall Inflammation Predicts Abdominal Aortic Aneurysm Expansion, Rupture, and Need for Surgical Repair. Circulation 2017, 136, 787–797. [Google Scholar] [CrossRef]
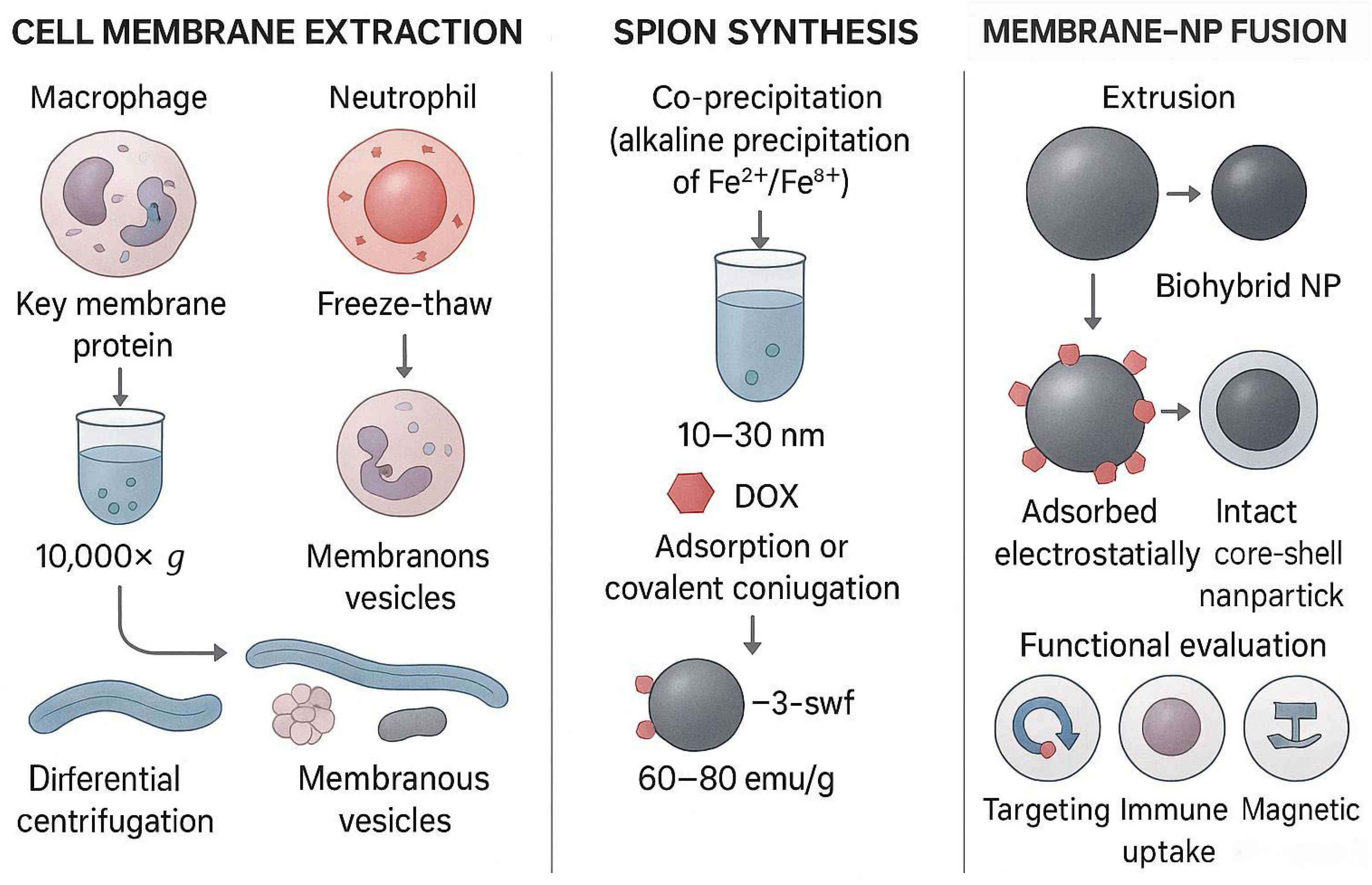

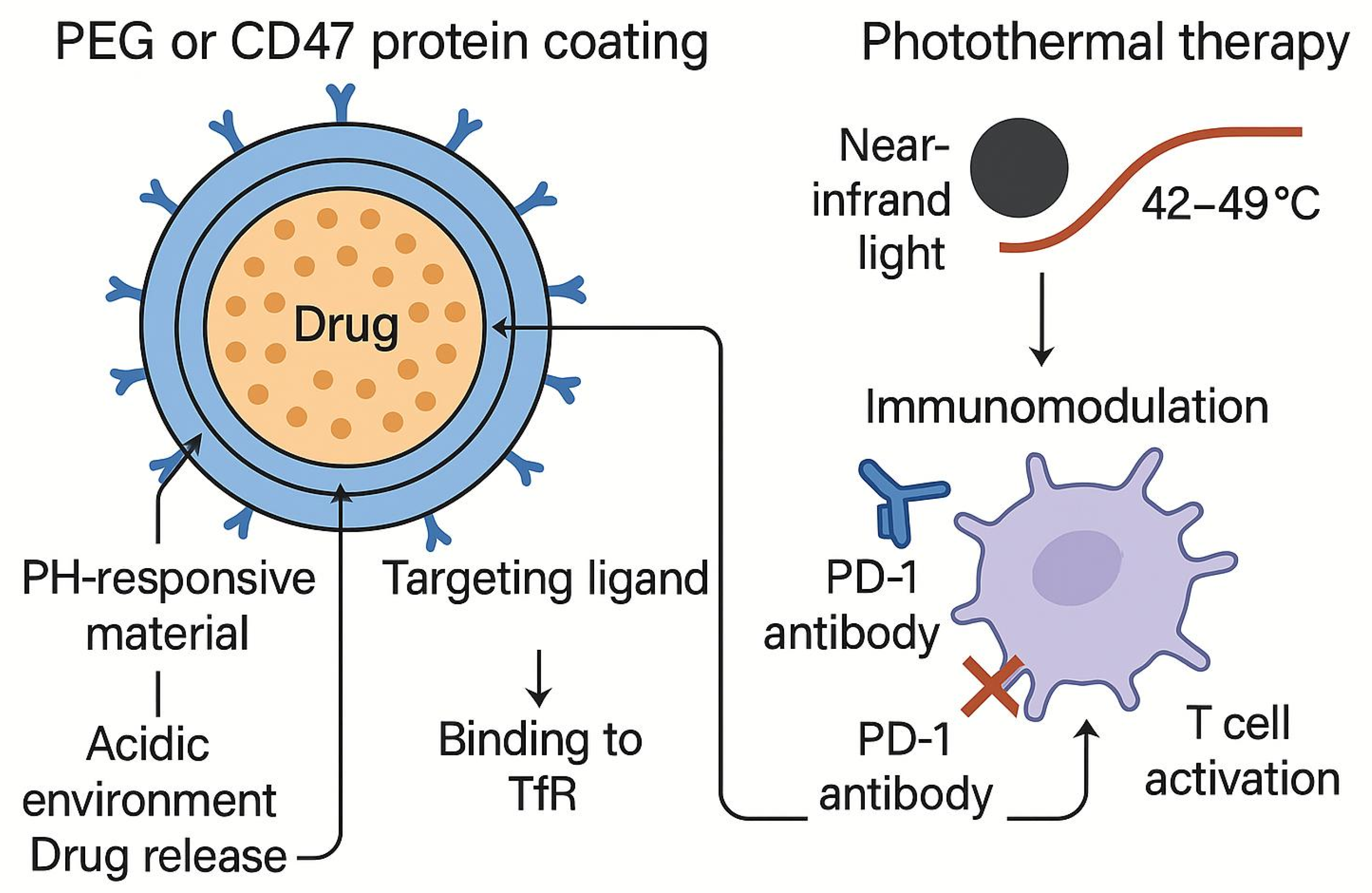
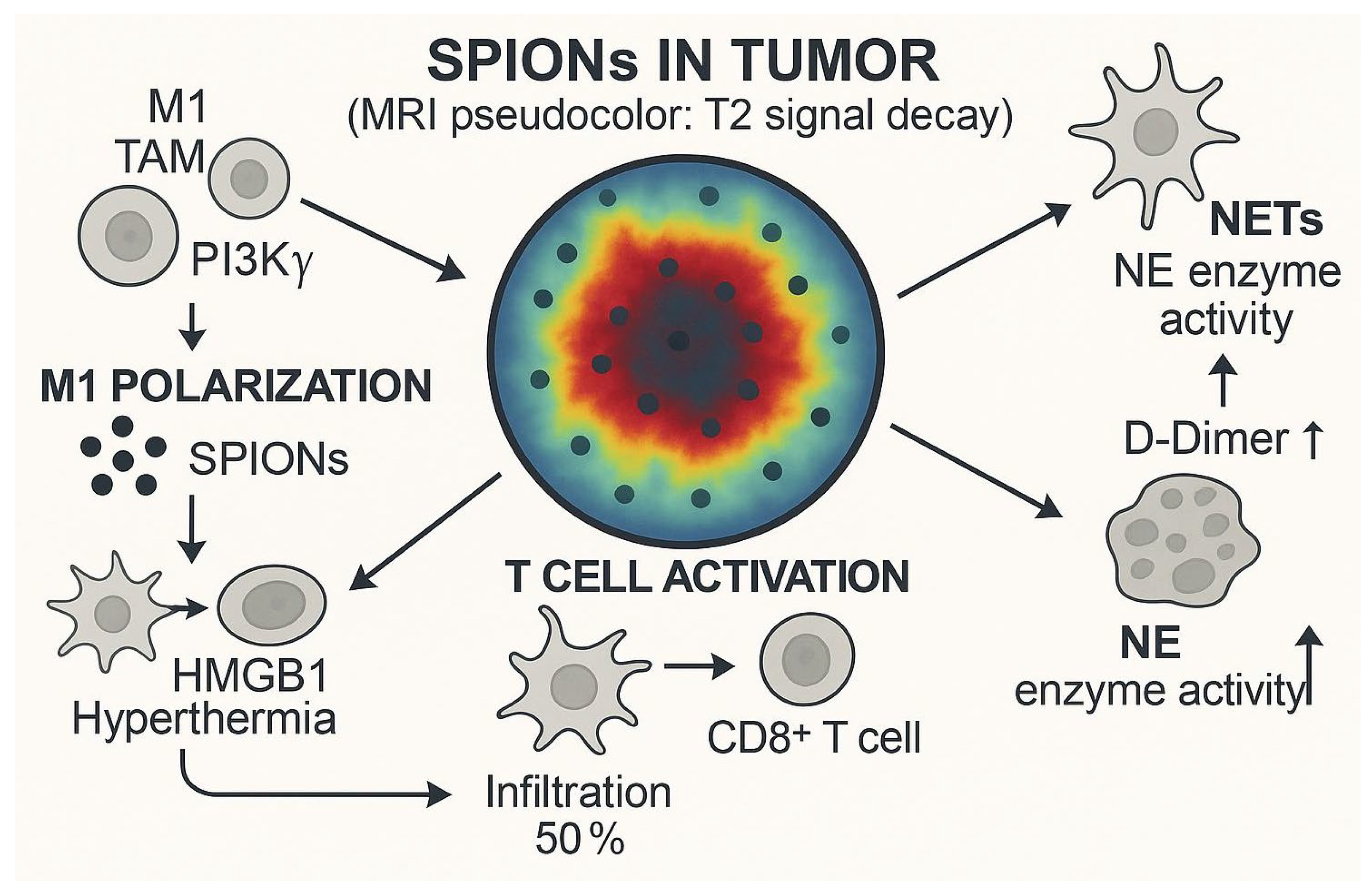
| Membrane Type | Major Membrane-Protein Components | Targeting Characteristics | Immune-Evasion Capability | Circulation Time | Preparation Difficulty | Application Scenarios | References |
|---|---|---|---|---|---|---|---|
| Macrophage membrane | PSGL-1, LFA-1, VLA-4 | VCAM-1-mediated inflammation/tumor-targeting | Moderate (retains partial self-recognition signals) | Moderate (days) | Moderate | Inflammatory sites, tumor microenvironment targeting | [40,41] |
| Neutrophil membrane | CD11b/CD18, CD62L | Integrin-ICAM-1-mediated blood-air barrier penetration | Strong (natural immune-evasion properties) | Short (hours) | Difficult | Lung targeting, acute inflammation therapy | [42,43,44,45] |
| Cancer-cell membrane | Tumor-specific antigens (e.g., EGFR), integrin αvβ3 | Homologous targeting (same tumor type) | Strong (expresses “don’t-eat-me” signals) | Moderate (days) | Moderate | Primary tumor and metastasis therapy | [49,51] |
| Cell-Membrane Type | Functional Modification | Therapeutic Mechanism | Model | Key Findings | Conclusion | References |
|---|---|---|---|---|---|---|
| Neutrophil membrane | poly(sialic acid)-octadecylamine | Neutrophil-mediated delivery | Mouse lung cancer model | Enhanced drug delivery to lung tumor site | Neutrophil membrane improves lung targeting | [45] |
| Cancer-cell membrane | Curcumin + DOX co-loading | Homologous targeting + MDR reversal | Esophageal cancer model (extensible to lung) | Effective inhibition of drug-resistant tumor growth | CCM enhances tumor-specific accumulation | [51] |
| No membrane (MnO2 shell) | Ce6 photosensitizer + O2 generation | Self-oxygenated PDT + MRI/PA imaging | Mouse lung cancer model | Alleviated hypoxia, enhanced ROS production | Overcomes hypoxia-induced PDT resistance | [56] |
| Neutrophil exosome hybrid | Transferrin (Tf) conjugation | Magnetic targeting + exosome homing | Mouse lung-metastasis model | Enhanced lung accumulation via Tf and neutrophil tropism | Dual targeting improves lung retention | [57] |
| Lung cancer cell membrane | Tumor-associated antigens preserved | Homologous targeting + immune evasion | Mouse lung cancer model | Enhanced tumor enrichment, evaded immune clearance | Cancer membrane improves tumor-specific targeting | [65] |
| Mesoporous silica shell | Fe3O4@mSiO2 core–shell | High paclitaxel loading via mesopores | In vitro/vivo tumor models | Improved hydrophobic drug-loading and release | Mesoporous shell enhances drug compatibility | [66] |
| Hollow SPIONs | Hematoporphyrin + US-triggered H2O2 decomposition | SDT + MHT synergy | Mouse tumor model | Overcame light penetration limit, enhanced deep tumor therapy | Combined SDT-MHT effective for deep tumors | [67] |
| T-cell membrane | SPIONs loaded into human T-cells | T-cell function preservation + magnetic navigation | Human T-cells in vitro | T-cell activation and cytotoxicity unaffected | SPIONs can serve as T-cell carriers for immunotherapy | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Jiang, L.; Wang, K.; Dai, J.; Liu, X. Application of Biomimetic SPIONs in Targeted Lung Cancer Therapy: Cell-Membrane Camouflage Technology and Lung Retention Enhancement Strategies. Pharmaceutics 2025, 17, 1301. https://doi.org/10.3390/pharmaceutics17101301
Liu Q, Jiang L, Wang K, Dai J, Liu X. Application of Biomimetic SPIONs in Targeted Lung Cancer Therapy: Cell-Membrane Camouflage Technology and Lung Retention Enhancement Strategies. Pharmaceutics. 2025; 17(10):1301. https://doi.org/10.3390/pharmaceutics17101301
Chicago/Turabian StyleLiu, Quanxing, Li Jiang, Kai Wang, Jigang Dai, and Xiaobing Liu. 2025. "Application of Biomimetic SPIONs in Targeted Lung Cancer Therapy: Cell-Membrane Camouflage Technology and Lung Retention Enhancement Strategies" Pharmaceutics 17, no. 10: 1301. https://doi.org/10.3390/pharmaceutics17101301
APA StyleLiu, Q., Jiang, L., Wang, K., Dai, J., & Liu, X. (2025). Application of Biomimetic SPIONs in Targeted Lung Cancer Therapy: Cell-Membrane Camouflage Technology and Lung Retention Enhancement Strategies. Pharmaceutics, 17(10), 1301. https://doi.org/10.3390/pharmaceutics17101301








