Boronic Derivatives of Thiosemicarbazones as Tyrosinase Inhibitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
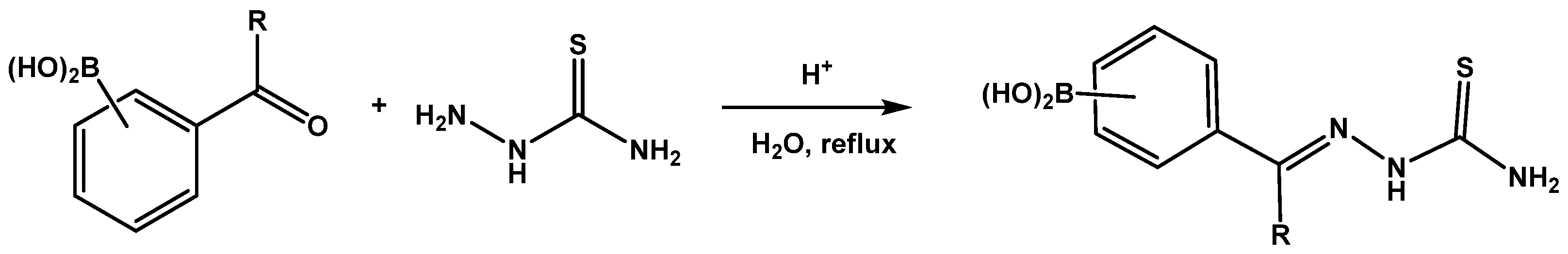
General Procedure for the Synthesis of 1–6
2.2. Tyrosinase Enzymatic Assay
2.3. Molecular Modeling
2.4. Antiproliferative Studies
3. Results and Discussion
3.1. Chemistry
3.2. Effect of Compound 1–6 on the Diphenolase Activity of Mushroom Tyrosinase
3.3. Structure-Activity Relationship
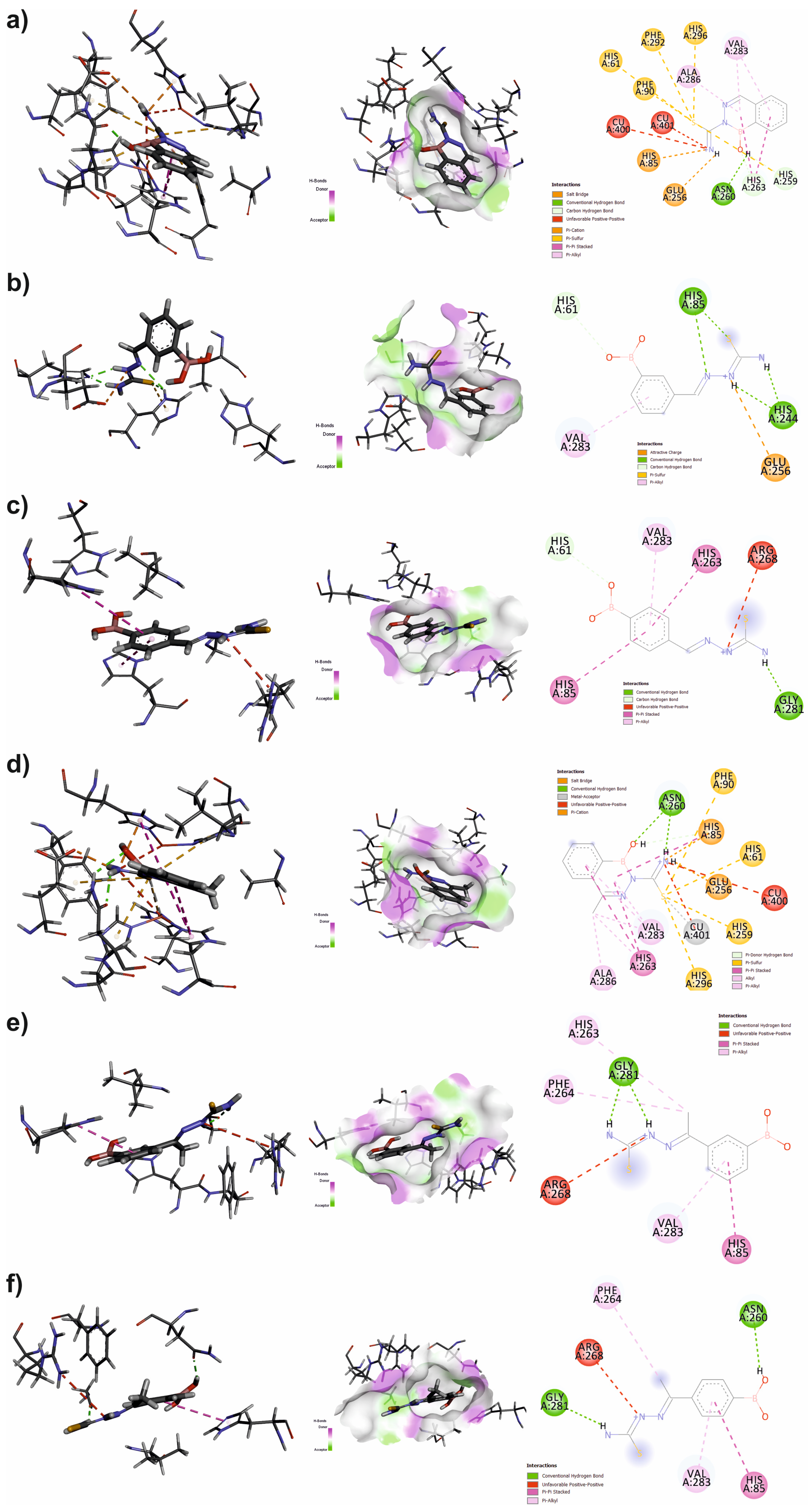
3.4. Molecular Docking
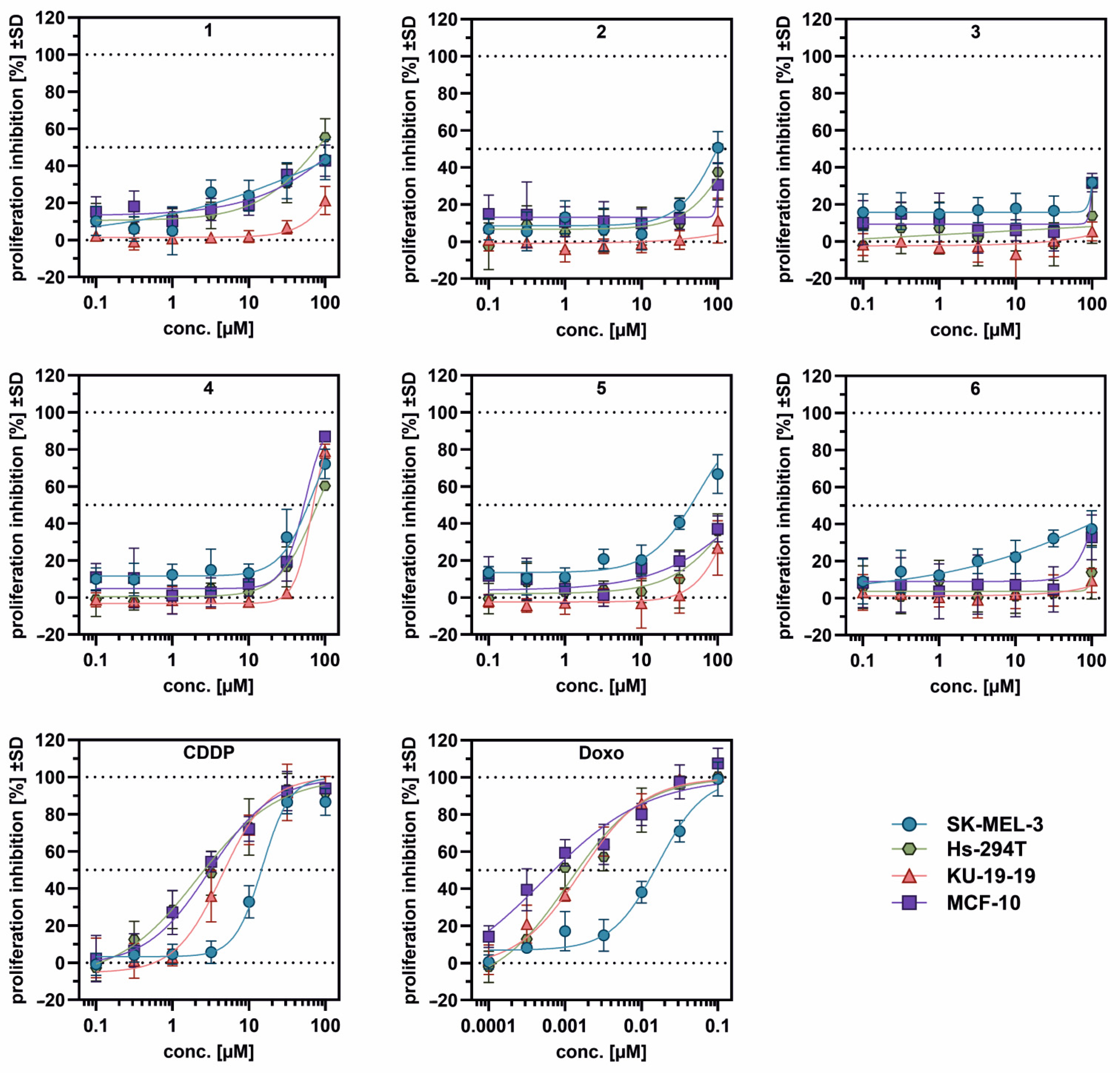
3.5. Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| KA | Kojic Acid |
References
- Claus, H.; Decker, H. Bacterial tyrosinases. Syst. Appl. Microbiol. 2006, 29, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal Structure of Agaricus bisporus Mushroom Tyrosinase: Identity of the Tetramer Subunits and Interaction with Tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure and Function of Human Tyrosinase and Tyrosinase-Related Proteins. Chem.—A Eur. J. 2018, 24, 47–55. [Google Scholar] [CrossRef]
- Pongkai, P.; Saisavoey, T.; Sangtanoo, P.; Sangvanich, P.; Karnchanatat, A. Effects of protein hydrolysate from chicken feather meal on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. Food Sci. Biotechnol. 2017, 26, 1199–1208. [Google Scholar] [CrossRef]
- Hridya, H.; Amrita, A.; Mohan, S.; Gopalakrishnan, M.; Dakshinamurthy, T.K.; Doss, G.P.; Siva, R. Functionality study of santalin as tyrosinase inhibitor: A potential depigmentation agent. Int. J. Biol. Macromol. 2016, 86, 383–389. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Jung, S.-H. Recent development of signaling pathways inhibitors of melanogenesis. Cell. Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, X.; Karangwa, E.; Xia, S. Correlating enzymatic browning inhibition and antioxidant ability of Maillard reaction products derived from different amino acids. J. Sci. Food Agric. 2017, 97, 4210–4218. [Google Scholar] [CrossRef] [PubMed]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Final Report of the Safety Assessment of Kojic Acid as Used in Cosmetics. Int. J. Toxicol. 2010, 29, 244S–273S. [Google Scholar] [CrossRef]
- Andersen, F.A.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W. Final Amended Safety Assessment of Hydroquinone as Used in Cosmetics. Int. J. Toxicol. 2010, 29, 274S–287S. [Google Scholar] [CrossRef]
- Yusuf, M.; Jain, P. Synthesis and biological significances of 1,3,4-thiadiazolines and related heterocyclic compounds. Arab. J. Chem. 2014, 7, 525–552. [Google Scholar] [CrossRef]
- Xue, C.-B.; Zhang, L.; Luo, W.-C.; Xie, X.-Y.; Jiang, L.; Xiao, T. 3D-QSAR and molecular docking studies of benzaldehyde thiosemicarbazone, benzaldehyde, benzoic acid, and their derivatives as phenoloxidase inhibitors. Bioorg. Med. Chem. 2007, 15, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Cao, R.-H.; Chen, Z.-Y.; Yu, L.; Ma, L.; Song, H.-C. Design, Synthesis and Biological Evaluation of Hydroxy- or Methoxy-Substituted Phenylmethylenethiosemicarbazones as Tyrosinase Inhibitors. Chem. Pharm. Bull. 2009, 57, 1273–1277. [Google Scholar] [CrossRef]
- Chen, L.-H.; Hu, Y.-H.; Song, W.; Song, K.-K.; Liu, X.; Jia, Y.-L.; Zhuang, J.-X.; Chen, Q.-X. Synthesis and Antityrosinase Mechanism of Benzaldehyde Thiosemicarbazones: Novel Tyrosinase Inhibitors. J. Agric. Food Chem. 2012, 60, 1542–1547. [Google Scholar] [CrossRef]
- You, A.; Zhou, J.; Song, S.; Zhu, G.; Song, H.; Yi, W. Rational design, synthesis and structure–activity relationships of 4-alkoxy- and 4-acyloxy-phenylethylenethiosemicarbazone analogues as novel tyrosinase inhibitors. Bioorg. Med. Chem. 2015, 23, 924–931. [Google Scholar] [CrossRef]
- Yang, M.-H.; Chen, C.-M.; Hu, Y.-H.; Zheng, C.-Y.; Li, Z.-C.; Ni, L.-L.; Sun, L.; Chen, Q.-X. Inhibitory kinetics of DABT and DABPT as novel tyrosinase inhibitors. J. Biosci. Bioeng. 2013, 115, 514–517. [Google Scholar] [CrossRef]
- You, A.; Zhou, J.; Song, S.; Zhu, G.; Song, H.; Yi, W. Structure-based modification of 3-/4-aminoacetophenones giving a profound change of activity on tyrosinase: From potent activators to highly efficient inhibitors. Eur. J. Med. Chem. 2015, 93, 255–262. [Google Scholar] [CrossRef]
- Li, Z.-C.; Chen, L.-H.; Yu, X.-J.; Hu, Y.-H.; Song, K.-K.; Zhou, X.-W.; Chen, Q.-X. Inhibition Kinetics of Chlorobenzaldehyde Thiosemicarbazones on Mushroom Tyrosinase. J. Agric. Food Chem. 2010, 58, 12537–12540. [Google Scholar] [CrossRef]
- Liu, J.; Yi, W.; Wan, Y.; Ma, L.; Song, H. 1-(1-Arylethylidene)thiosemicarbazide derivatives: A new class of tyrosinase inhibitors. Bioorg. Med. Chem. 2008, 16, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Hałdys, K.; Goldeman, W.; Jewgiński, M.; Wolińska, E.; Anger-Góra, N.; Rossowska, J.; Latajka, R. Halogenated aromatic thiosemicarbazones as potent inhibitors of tyrosinase and melanogenesis. Bioorg. Chem. 2020, 94, 103419. [Google Scholar] [CrossRef]
- Dong, H.; Liu, J.; Liu, X.; Yu, Y.; Cao, S. Molecular docking and QSAR analyses of aromatic heterocycle thiosemicarbazone analogues for finding novel tyrosinase inhibitors. Bioorg. Chem. 2017, 75, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, J.; Zhu, X.; Yu, Y.; Cao, S. Novel inhibitors of tyrosinase produced by the 4-substitution of TCT. Food Chem. 2017, 221, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Dong, H.; Yu, Y.; Cao, S. Inhibitory effect of synthetic aromatic heterocycle thiosemicarbazone derivatives on mushroom tyrosinase: Insights from fluorescence, 1H NMR titration and molecular docking studies. Food Chem. 2016, 190, 709–716. [Google Scholar] [CrossRef]
- Dong, H.; Liu, J.; Liu, X.; Yu, Y.; Cao, S. Combining molecular docking and QSAR studies for modeling the anti-tyrosinase activity of aromatic heterocycle thiosemicarbazone analogues. J. Mol. Struct. 2018, 1151, 353–365. [Google Scholar] [CrossRef]
- Zhu, T.-H.; Cao, S.-W.; Yu, Y.-Y. Synthesis, characterization and biological evaluation of paeonol thiosemicarbazone analogues as mushroom tyrosinase inhibitors. Int. J. Biol. Macromol. 2013, 62, 589–595. [Google Scholar] [CrossRef]
- Arslan, H.; Duran, N.; Borekci, G.; Koray Ozer, C.; Akbay, C. Antimicrobial Activity of Some Thiourea Derivatives and Their Nickel and Copper Complexes. Molecules 2009, 14, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Hałdys, K.; Goldeman, W.; Jewgiński, M.; Wolińska, E.; Anger, N.; Rossowska, J.; Latajka, R. Inhibitory properties of aromatic thiosemicarbazones on mushroom tyrosinase: Synthesis, kinetic studies, molecular docking and effectiveness in melanogenesis inhibition. Bioorg. Chem. 2018, 81, 577–586. [Google Scholar] [CrossRef]
- Belitsky, J.M. Aryl boronic acid inhibition of synthetic melanin polymerization. Bioorg. Med. Chem. Lett. 2010, 20, 4475–4478. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.W.; Kyle, C.B.; Vogels, C.M.; Wheaton, S.L.; Baerlocher, F.J.; Decken, A.; Westcott, S.A. Synthesis, Characterization, and Antifungal Activity of Boron-Containing Thiosemicarbazones. Chem. Biodivers. 2008, 5, 2415–2422. [Google Scholar] [CrossRef]
- Hałdys, K.; Goldeman, W.; Anger-Góra, N.; Rossowska, J.; Latajka, R. Monosubstituted acetophenone thiosemicarbazones as potent inhibitors of tyrosinase: Synthesis, inhibitory studies, and molecular docking. Pharmaceuticals 2021, 14, 74. [Google Scholar] [CrossRef]
- Ledwoń, P.; Goldeman, W.; Hałdys, K.; Jewgiński, M.; Calamai, G.; Rossowska, J.; Papini, A.M.; Rovero, P.; Latajka, R. Tripeptides conjugated with thiosemicarbazones: New inhibitors of tyrosinase for cosmeceutical use. J. Enzyme Inhib. Med. Chem. 2023, 38, 2193676. [Google Scholar] [CrossRef]
- Kaminski, G.A.; Friesner, R.A.; Tirado-Rives, J.; Jorgensen, W.L. Evaluation and Reparametrization of the OPLS-AA Force Field for Proteins via Comparison with Accurate Quantum Chemical Calculations on Peptides. J. Phys. Chem. B 2001, 105, 6474–6487. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. JNCI J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Briganti, S.; Camera, E.; Picardo, M. Chemical and Instrumental Approaches to Treat Hyperpigmentation. Pigment Cell Res. 2003, 16, 101–110. [Google Scholar] [CrossRef]
- Bagherzadeh, K.; Shirgahi Talari, F.; Sharifi, A.; Ganjali, M.R.; Saboury, A.A.; Amanlou, M. A new insight into mushroom tyrosinase inhibitors: Docking, pharmacophore-based virtual screening, and molecular modeling studies. J. Biomol. Struct. Dyn. 2015, 33, 487–501. [Google Scholar] [CrossRef]
- Parvez, S.; Kang, M.; Chung, H.S.; Bae, H. Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytother. Res. 2007, 21, 805–816. [Google Scholar] [CrossRef] [PubMed]
- GraphPad GraphPad Prism for Windows, 10.5.0; GraphPad Software: San Diego, CA, USA, 2025.
- Oyama, T.; Yoshimori, A.; Ogawa, H.; Shirai, Y.; Abe, H.; Kamiya, T.; Tanuma, S. The structural differences between mushroom and human tyrosinase cleared by investigating the inhibitory activities of stilbenes. J. Mol. Struct. 2023, 1272, 134180. [Google Scholar] [CrossRef]
- Ewelina, W.; Julita, K.; Urszula, B. Superior Drug Delivery Performance of Multifunctional Bilosomes: Innovative Strategy to Kill Skin Cancer Cells for Nanomedicine Application. Int. J. Nanomed. 2024, 19, 4701–4717. [Google Scholar] [CrossRef] [PubMed]
- Kulbacka, J.; Wilk, K.A.; Bazylińska, U.; Dubińska-Magiera, M.; Potoczek, S.; Saczko, J. Curcumin Loaded Nanocarriers with Varying Charges Augmented with Electroporation Designed for Colon Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 1377. [Google Scholar] [CrossRef]
- Lewińska, A.; Domżał-Kędzia, M.; Wójtowicz, K.; Bazylińska, U. Surfactin-stabilized poly(D,L-lactide) nanoparticles for potential skin application. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 648, 129216. [Google Scholar] [CrossRef]

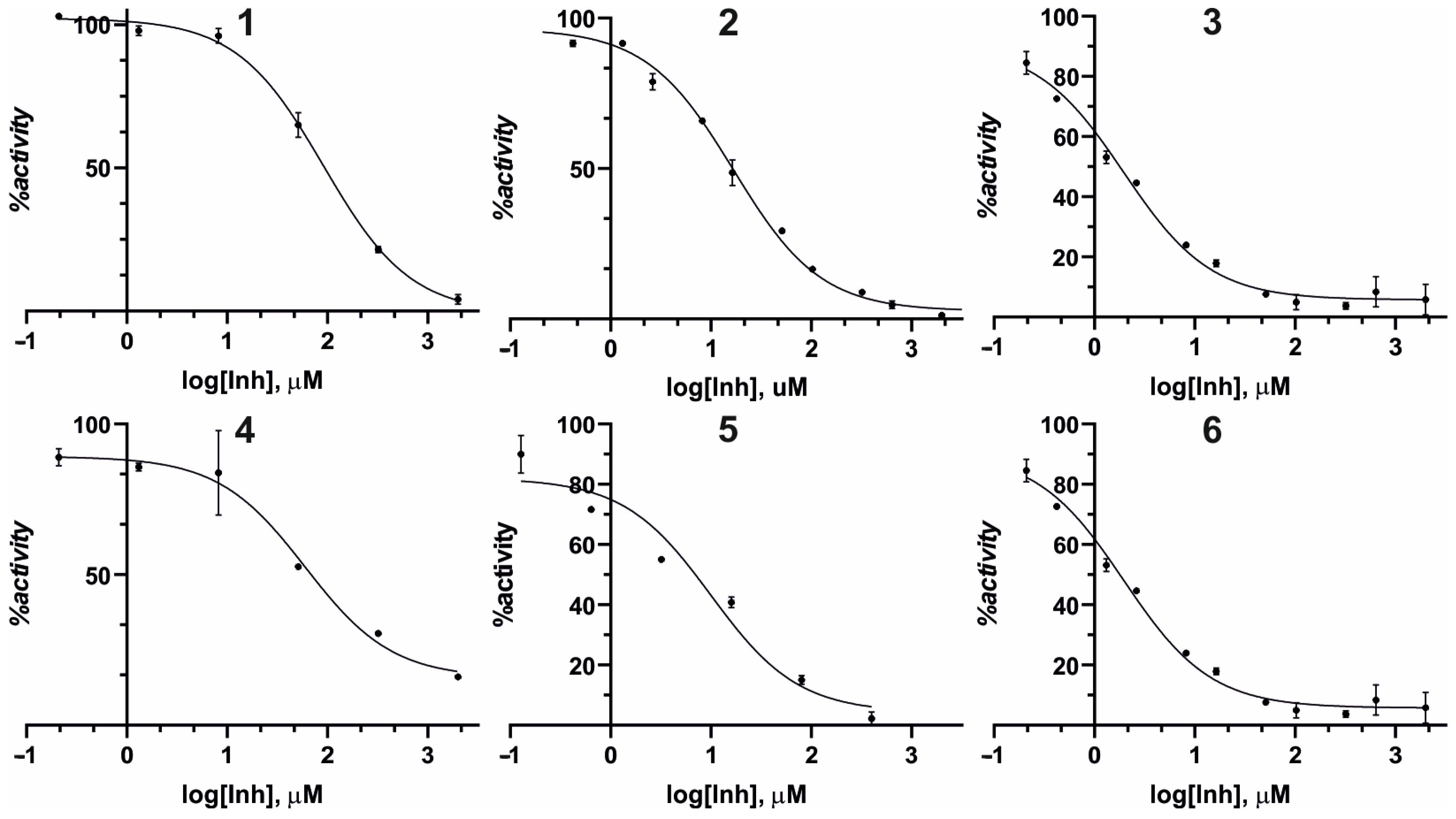
| Inhibitor | IC50 a [μM] | 95% CI b IC50 [µM] |
|---|---|---|
| 1 | 87.9 | 70.6–112.5 |
| 2 | 16.3 | 13.6–19.4 |
| 3 | 11.7 | 9.8–13.9 |
| 4 | 66.1 | 51.1–91.3 |
| 5 | 9.9 | 3.2–28.5 |
| 6 | 1.4 | 0.6–2.3 |
| KA c | 14.3 | 10.9–18.4 |
| Inhibitor | IFD Score | MMGBSA dG Binding Energy [kcal/mol] | |
|---|---|---|---|
| 1 | pose 1 | −807.44 | −22.91 |
| pose 2 | −807.33 | −22.69 | |
| pose 3 | −807.28 | −23.15 | |
| 2 | pose 1 | −809.80 | −35.32 |
| pose 2 | −809.11 | −28.33 | |
| pose 3 | −809.03 | −25.21 | |
| 3 | pose 1 | −808.78 | −28.20 |
| pose 2 | −808,47 | −28.32 | |
| pose 3 | −808.08 | −34.63 | |
| 4 | pose 1 | −810.81 | −27.99 |
| pose 2 | −810.50 | −24.57 | |
| pose 3 | −810.06 | −25.00 | |
| 5 | pose 1 | −809.74 | −35.36 |
| pose 2 | −808.00 | −26.44 | |
| pose 3 | −806.93 | −35.58 | |
| 6 | pose 1 | −809.98 | −35.13 |
| pose 2 | −809.25 | −33.48 | |
| pose 3 | −808.88 | −31.93 |
| IC50 [µM] ± 95%CI * | ||||||||
|---|---|---|---|---|---|---|---|---|
| SK-MEL-3 | Hs-294T | KU-19-19 | MCF-10A | |||||
| 1 | [43.4] * | 29.9–56.8 | [55.4] * | 43–67.8 | [21.4] * | 11.9–30.8 | [42.7] * | 34.9–50.6 |
| 2 | [50.7] * | 39.9–61.6 | [37.6] * | 18.8–56.4 | [11.3] * | −1.4–24 | [30.7] * | 15.9–45.5 |
| 3 | [31.8] * | 29.1–34.6 | [13.9] * | −4.5–32.2 | [5.4] * | −1–11.7 | [31.7] * | 25.4–38 |
| 4 | 68.5 | 57.8–80.5 | 79.4 | 65.3–97.5 | 69.0 | 64.5–74.4 | 55.0 | 43.2–70.8 |
| 5 | 56.1 | 48.9–64.3 | [35.9] * | 26.1–45.7 | [26.8] * | 13.2–40.5 | [37.1] * | 29.7–44.5 |
| 6 | [37.3] * | 28.2–46.4 | [13.9] * | −4–31.9 | [9.5] * | −0.8–19.8 | [32.8] * | 13.6–52 |
| CDDP | 15 | 13.7–16.4 | 2.17 | 1.15–3.30 | 4.46 | 3.86–5.14 | 3.03 | 2.40–3.71 |
| Doxo | 0.016 | 0.013–0.021 | 0.0011 | 0.00058–0.0017 | 0.0016 | 0.0011–0.0022 | 0.00051 | 0.0010–0.0023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jewgiński, M.; Msanif, M.; Zachary, H.; Psurski, M.; Latajka, R. Boronic Derivatives of Thiosemicarbazones as Tyrosinase Inhibitors. Pharmaceutics 2025, 17, 1300. https://doi.org/10.3390/pharmaceutics17101300
Jewgiński M, Msanif M, Zachary H, Psurski M, Latajka R. Boronic Derivatives of Thiosemicarbazones as Tyrosinase Inhibitors. Pharmaceutics. 2025; 17(10):1300. https://doi.org/10.3390/pharmaceutics17101300
Chicago/Turabian StyleJewgiński, Michał, Msanif Msanif, Honorata Zachary, Mateusz Psurski, and Rafał Latajka. 2025. "Boronic Derivatives of Thiosemicarbazones as Tyrosinase Inhibitors" Pharmaceutics 17, no. 10: 1300. https://doi.org/10.3390/pharmaceutics17101300
APA StyleJewgiński, M., Msanif, M., Zachary, H., Psurski, M., & Latajka, R. (2025). Boronic Derivatives of Thiosemicarbazones as Tyrosinase Inhibitors. Pharmaceutics, 17(10), 1300. https://doi.org/10.3390/pharmaceutics17101300







