Targeted Delivery of VEGF-siRNA to Glioblastoma Using Orientation-Controlled Anti-PD-L1 Antibody-Modified Lipid Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of VEGF-siRNA LNPs
2.3. Post-Insertion of the FcBP-HFQ Lipid and Antibody Decoration
2.4. Physicochemical Characterization
2.5. Cell Culture and In Vitro Uptake Assay
2.6. In Vitro Gene Silencing Assay
2.7. In Vivo Tumor Model and Therapeutic Study
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Statistical Analysis
3. Results
3.1. In Vitro and In Vivo Evaluation of PD-L1-Targeted VEGF-siRNA LNPs
3.1.1. Physicochemical Properties of Antibody-Modified LNPs
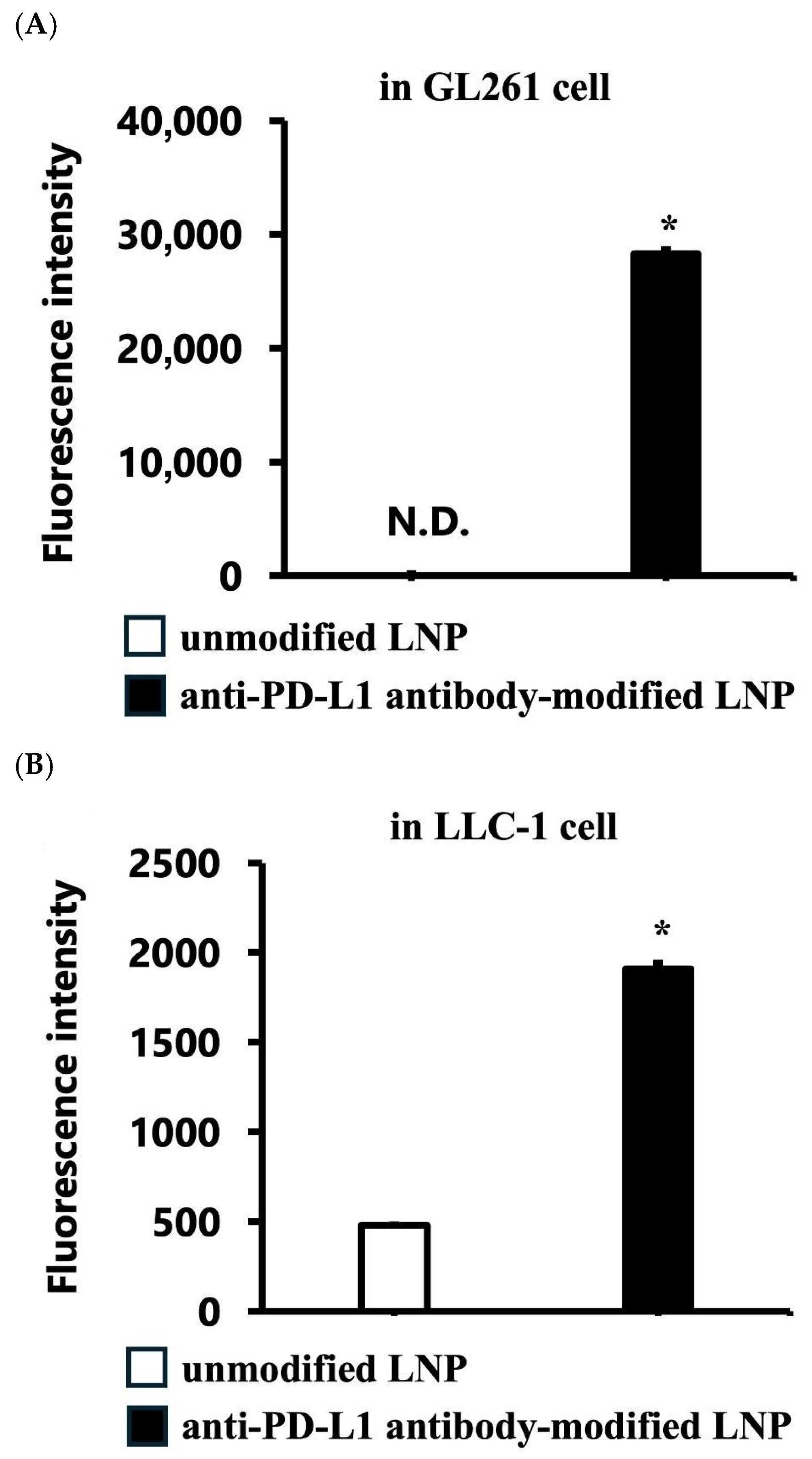
3.1.2. Targeted Cellular Uptake of LNPs
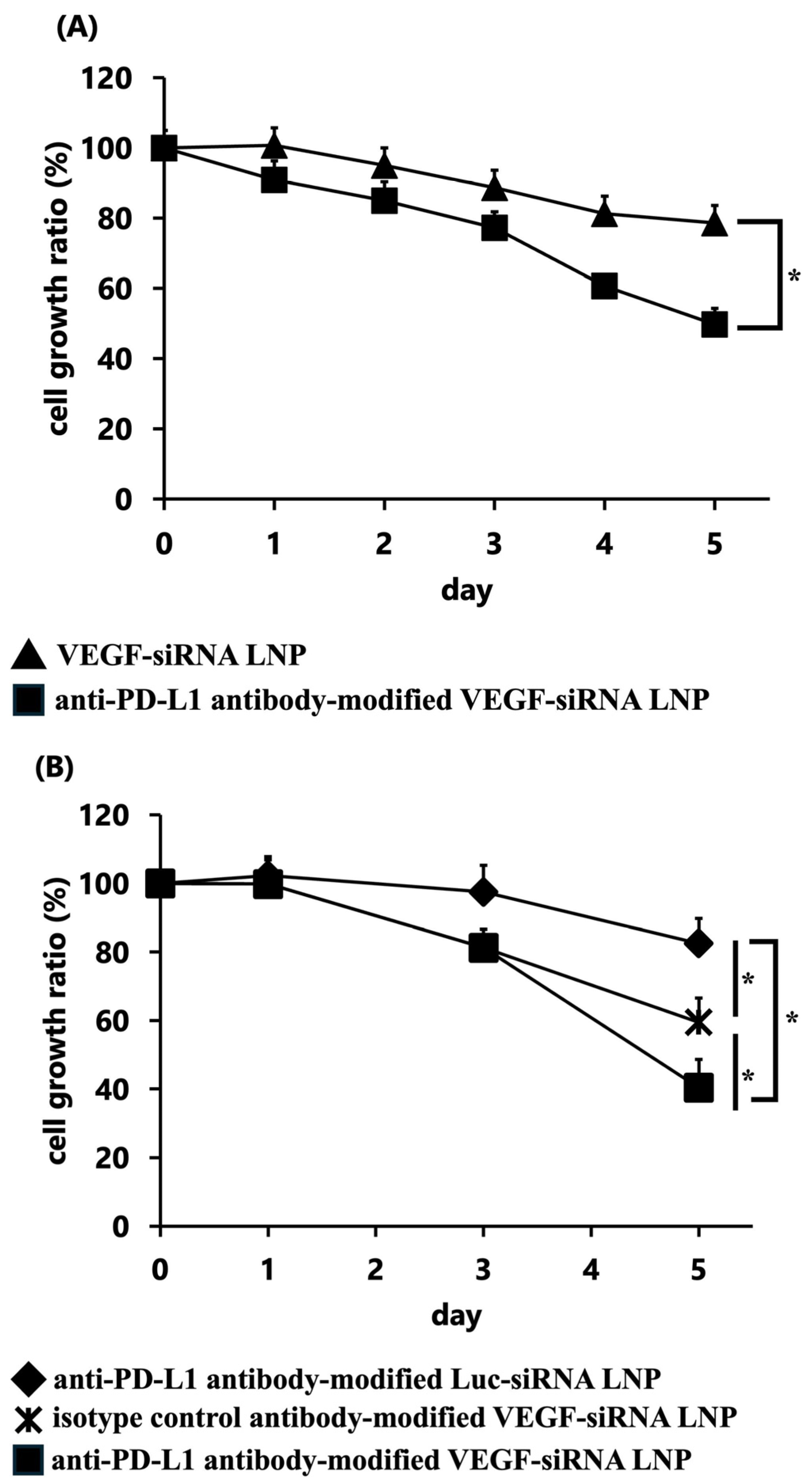
3.1.3. In Vitro Gene Silencing of VEGF
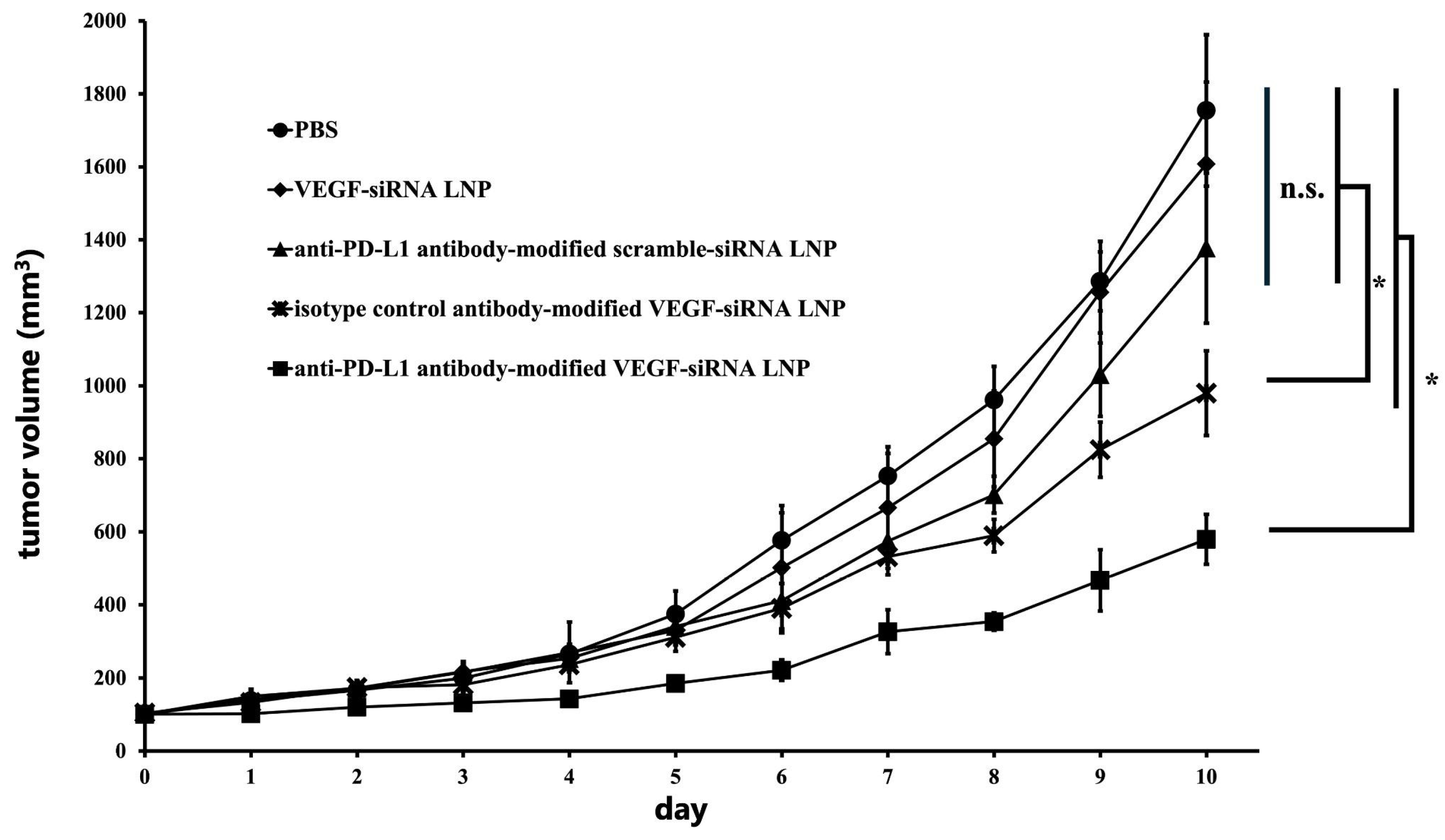
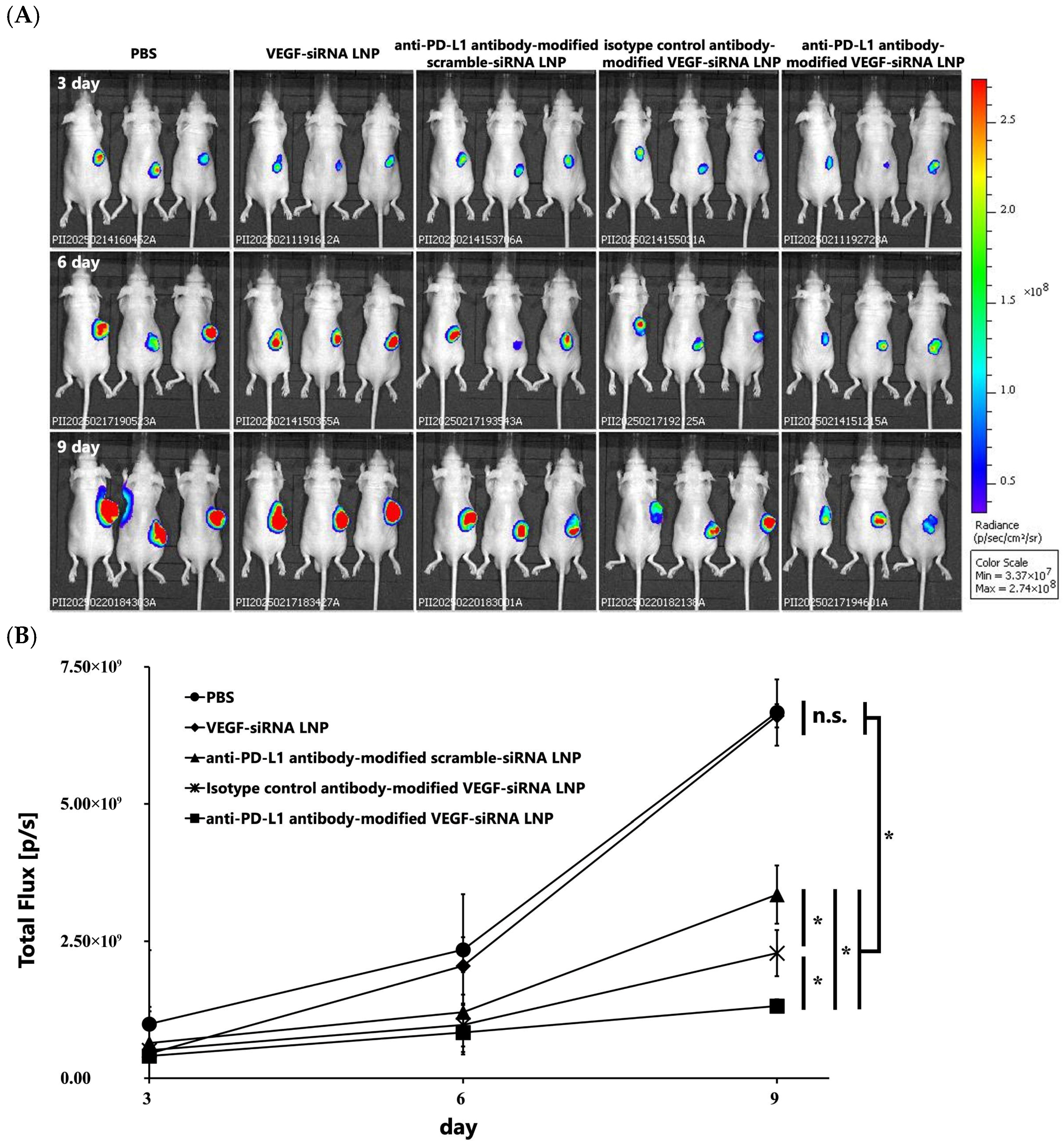
3.1.4. Inhibition of Tumor Growth Through VEGF Gene Silencing In Vivo
3.1.5. Tumor Weight and Size
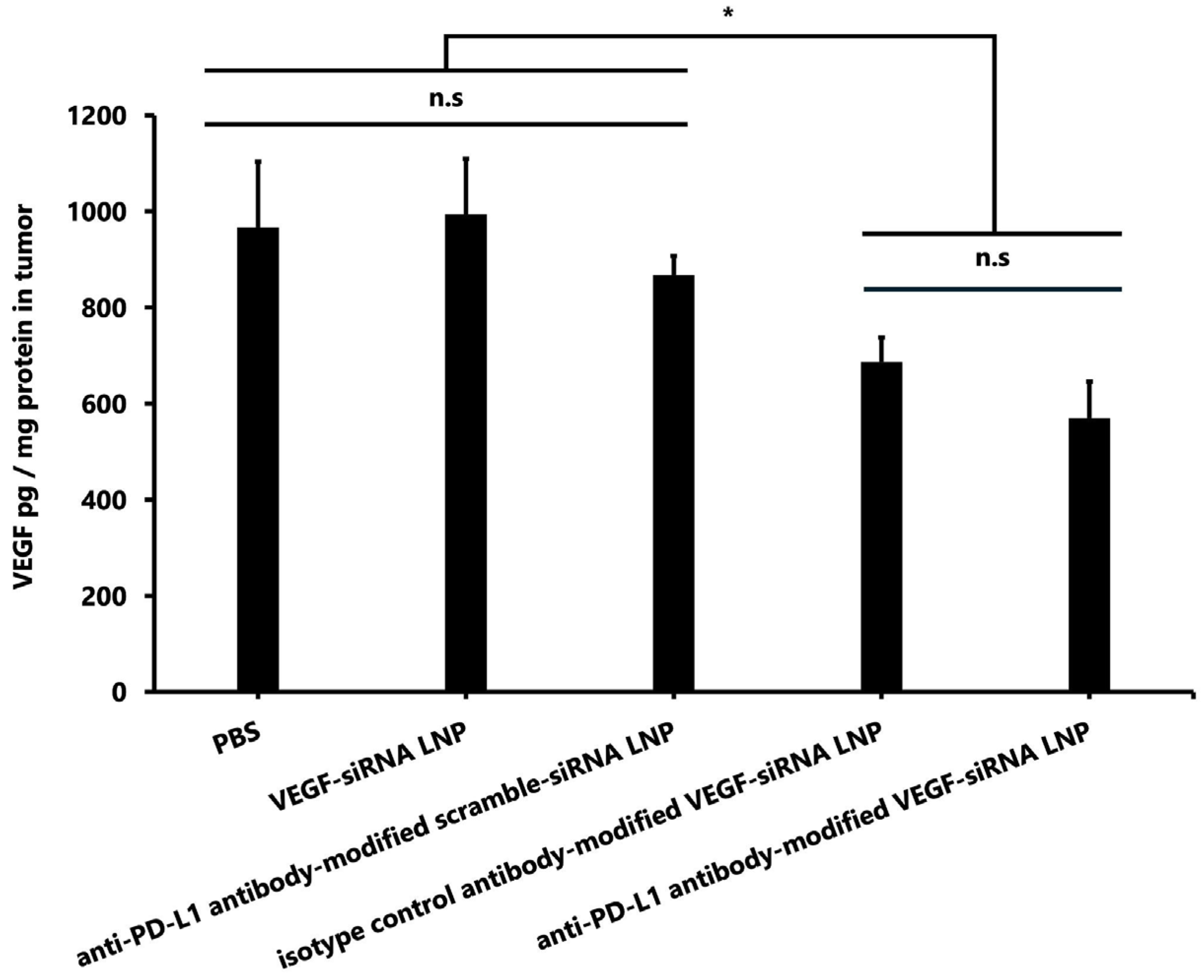
3.1.6. VEGF Concentration in Tumors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GBM | Glioblastoma |
| siRNA | Small interfering RNA |
| LNPs | Lipid nanoparticles |
| FcBP | Fc-binding peptide |
| BBB | Blood–brain barrier |
| VEGF | Vascular endothelial growth factor |
| PD-L1 | Programmed death-ligand 1 |
| MC3 | DLin-MC3-DMA |
| DSPC | 1,2-distearoyl-sn-glycero-3-phosphocholine |
| DSG-PEG2000 | 1,2-distearoyl-rac-glycero-3-methoxypolyethylene glycol-2000 |
| FcBP-HFQ lipid | Fc-binding high-functionality and quality lipid |
| PDI | Polydispersity index |
| SD | Standard deviation |
References
- Grochans, S.; Cybulska, A.M.; Siminska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme-Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neurooncology 2022, 24 (Suppl. S5), v1–v95. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Loureiro, L.V.M.; Neder, L.; Callegaro-Filho, D.; de Oliveira Koch, L.; Stavale, J.N.; Malheiros, S.M.F. The immunohistochemical landscape of the Vegf family and its receptors in glioblastomas. Surg. Exp. Pathol. 2020, 3, 9. [Google Scholar] [CrossRef]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Chen, X.; Mangala, L.S.; Rodriguez-Aguayo, C.; Kong, X.; Lopez-Berestein, G.; Sood, A.K. RNA interference-based therapy and its delivery systems. Cancer Metastasis Rev. 2018, 37, 107–124. [Google Scholar] [CrossRef]
- Teng, X.Q.; Qu, J.; Li, G.H.; Zhuang, H.H.; Qu, Q. Small Interfering RNA for Gliomas Treatment: Overcoming Hurdles in Delivery. Front. Cell Dev. Biol. 2022, 10, 824299. [Google Scholar] [CrossRef]
- Lv, Z.; Dai, Y. mRNA vaccines and SiRNAs targeting cancer immunotherapy: Challenges and opportunities. Discov. Oncol. 2025, 16, 1265. [Google Scholar] [CrossRef]
- Samaridou, E.; Heyes, J.; Lutwyche, P. Lipid nanoparticles for nucleic acid delivery: Current perspectives. Adv. Drug Deliv. Rev. 2020, 154–155, 37–63. [Google Scholar] [CrossRef]
- Wu, L.; Li, X.; Qian, X.; Wang, S.; Liu, J.; Yan, J. Lipid Nanoparticle (LNP) Delivery Carrier-Assisted Targeted Controlled Release mRNA Vaccines in Tumor Immunity. Vaccines 2024, 12, 186. [Google Scholar] [CrossRef]
- Mukai, H.; Ogawa, K.; Kato, N.; Kawakami, S. Recent advances in lipid nanoparticles for delivery of nucleic acid, mRNA, and gene editing-based therapeutics. Drug Metab. Pharmacokinet. 2022, 44, 100450. [Google Scholar] [CrossRef]
- Bruckner, M.; Simon, J.; Landfester, K.; Mailander, V. The conjugation strategy affects antibody orientation and targeting properties of nanocarriers. Nanoscale 2021, 13, 9816–9824. [Google Scholar] [CrossRef]
- Tsai, C.W.; Jheng, S.L.; Chen, W.Y.; Ruaan, R.C. Strategy of Fc-recognizable Peptide ligand design for oriented immobilization of antibody. Anal. Chem. 2014, 86, 2931–2938. [Google Scholar] [CrossRef]
- Kato, N.; Moriya, N.; Matsumoto, M.; Matsuo, A.; Yoshida, M.; Hiu, T.; Matsuo, T.; Takashima, Y.; Kamiya, M.; Mukai, H.; et al. Synthesis of a novel adapter lipid using Fc-region mediated antibody modification for post-insert preparation of transferrin receptor targeted messenger RNA-loaded lipid nanoparticles. Eur. J. Pharm. Biopharm. 2024, 203, 114468. [Google Scholar] [CrossRef] [PubMed]
- Nduom, E.K.; Wei, J.; Yaghi, N.K.; Huang, N.; Kong, L.Y.; Gabrusiewicz, K.; Ling, X.; Zhou, S.; Ivan, C.; Chen, J.Q.; et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2016, 18, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Song, G.; Yu, J. The prognostic significance of PD-L1 expression in patients with glioma: A meta-analysis. Sci. Rep. 2017, 7, 4231. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Kato, N.; Kodama, Y.; Mukai, H.; Kawakami, S. Influence of lipid composition of messenger RNA-loaded lipid nanoparticles on the protein expression via intratracheal administration in mice. Int. J. Pharm. 2023, 637, 122896. [Google Scholar] [CrossRef]
- Ogawa, K.; Kato, N.; Yoshida, M.; Hiu, T.; Matsuo, T.; Mizukami, S.; Omata, D.; Suzuki, R.; Maruyama, K.; Mukai, H.; et al. Focused ultrasound/microbubbles-assisted BBB opening enhances LNP-mediated mRNA delivery to brain. J. Control Release 2022, 348, 34–41. [Google Scholar] [CrossRef]
- Strand, M.S.; Krasnick, B.A.; Pan, H.; Zhang, X.; Bi, Y.; Brooks, C.; Wetzel, C.; Sankpal, N.; Fleming, T.; Goedegebuure, S.P.; et al. Precision delivery of RAS-inhibiting siRNA to KRAS driven cancer via peptide-based nanoparticles. Oncotarget 2019, 10, 4761–4775. [Google Scholar] [CrossRef]
- Ali Zaidi, S.S.; Fatima, F.; Ali Zaidi, S.A.; Zhou, D.; Deng, W.; Liu, S. Engineering siRNA therapeutics: Challenges and strategies. J. Nanobiotechnol. 2023, 21, 381. [Google Scholar] [CrossRef]
- Tatiparti, K.; Sau, S.; Kashaw, S.K.; Iyer, A.K. siRNA Delivery Strategies: A Comprehensive Review of Recent Developments. Nanomaterials 2017, 7, 77. [Google Scholar] [CrossRef]
- Jung, H.N.; Lee, S.Y.; Lee, S.; Youn, H.; Im, H.J. Lipid nanoparticles for delivery of RNA therapeutics: Current status and the role of in vivo imaging. Theranostics 2022, 12, 7509–7531. [Google Scholar] [CrossRef]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhang, Y.; Li, W.; Jeanty, C.; Xiang, G.; Dong, Y. Recent advances of antibody drug conjugates for clinical applications. Acta Pharm. Sin. B 2020, 10, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Hu, M.; Iyer, V.; Yu, J. Blocking the PD-1/PD-L1 pathway in glioma: A potential new treatment strategy. J. Hematol. Oncol. 2017, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Caccese, M.; Indraccolo, S.; Zagonel, V.; Lombardi, G. PD-1/PD-L1 immune-checkpoint inhibitors in glioblastoma: A concise review. Crit. Rev. Oncol. Hematol. 2019, 135, 128–134. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, R.; Wang, Y.; Liu, M.; Hu, D.; Wang, Y.; Yang, L. A blood-brain barrier- and blood-brain tumor barrier-penetrating siRNA delivery system targeting gliomas for brain tumor immunotherapy. J. Control Release 2024, 369, 642–657. [Google Scholar] [CrossRef]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: A phase 2 trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef]
- Ruan, W.; Jiao, M.; Xu, S.; Ismail, M.; Xie, X.; An, Y.; Guo, H.; Qian, R.; Shi, B.; Zheng, M. Brain-targeted CRISPR/Cas9 nanomedicine for effective glioblastoma therapy. J. Control Release 2022, 351, 739–751. [Google Scholar] [CrossRef]
- Zhao, P.; Tian, Y.; Lu, Y.; Zhang, J.; Tao, A.; Xiang, G.; Liu, Y. Biomimetic calcium carbonate nanoparticles delivered IL-12 mRNA for targeted glioblastoma sono-immunotherapy by ultrasound-induced necroptosis. J. Nanobiotechnol. 2022, 20, 525. [Google Scholar] [CrossRef]

| Size (nm) | PDI | Zeta Potential (mV) | EE (%) | |
|---|---|---|---|---|
| VEGF-siRNA LNP | 109.93 ± 1.82 | 0.05 ± 0.01 | –5.09 ± 3.55 | 94.23 ± 0.93 |
| Anti-PD-L1 antibody-modified scramble-siRNA LNP | 118.97 ± 1.27 | 0.18 ± 0.04 | –3.79 ± 1.47 | 93.77 ± 1.35 |
| Isotype control antibody- modified VEGF-siRNA LNP | 119.2 ± 1.82 | 0.13 ± 0.01 | –2.97 ± 0.85 | 93.53 ± 0.93 |
| Anti-PD-L1 antibody-modified VEGF-siRNA LNP | 125.23 ± 1.27 | 0.18 ± 0.02 | –0.74 ± 2.47 | 93.37 ± 1.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuo-Tani, A.; Matsumoto, M.; Hiu, T.; Kamiya, M.; Geng, L.; Takayama, R.; Ushiroda, Y.; Kato, N.; Nakamura, H.; Yoshida, M.; et al. Targeted Delivery of VEGF-siRNA to Glioblastoma Using Orientation-Controlled Anti-PD-L1 Antibody-Modified Lipid Nanoparticles. Pharmaceutics 2025, 17, 1298. https://doi.org/10.3390/pharmaceutics17101298
Matsuo-Tani A, Matsumoto M, Hiu T, Kamiya M, Geng L, Takayama R, Ushiroda Y, Kato N, Nakamura H, Yoshida M, et al. Targeted Delivery of VEGF-siRNA to Glioblastoma Using Orientation-Controlled Anti-PD-L1 Antibody-Modified Lipid Nanoparticles. Pharmaceutics. 2025; 17(10):1298. https://doi.org/10.3390/pharmaceutics17101298
Chicago/Turabian StyleMatsuo-Tani, Ayaka, Makoto Matsumoto, Takeshi Hiu, Mariko Kamiya, Longjian Geng, Riku Takayama, Yusuke Ushiroda, Naoya Kato, Hikaru Nakamura, Michiharu Yoshida, and et al. 2025. "Targeted Delivery of VEGF-siRNA to Glioblastoma Using Orientation-Controlled Anti-PD-L1 Antibody-Modified Lipid Nanoparticles" Pharmaceutics 17, no. 10: 1298. https://doi.org/10.3390/pharmaceutics17101298
APA StyleMatsuo-Tani, A., Matsumoto, M., Hiu, T., Kamiya, M., Geng, L., Takayama, R., Ushiroda, Y., Kato, N., Nakamura, H., Yoshida, M., Mukai, H., Matsuo, T., & Kawakami, S. (2025). Targeted Delivery of VEGF-siRNA to Glioblastoma Using Orientation-Controlled Anti-PD-L1 Antibody-Modified Lipid Nanoparticles. Pharmaceutics, 17(10), 1298. https://doi.org/10.3390/pharmaceutics17101298








