MIL-100(Fe)-Enabled Oral Delivery of Syringic Acid with Enhanced Pharmacokinetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Animals
2.3. Methods
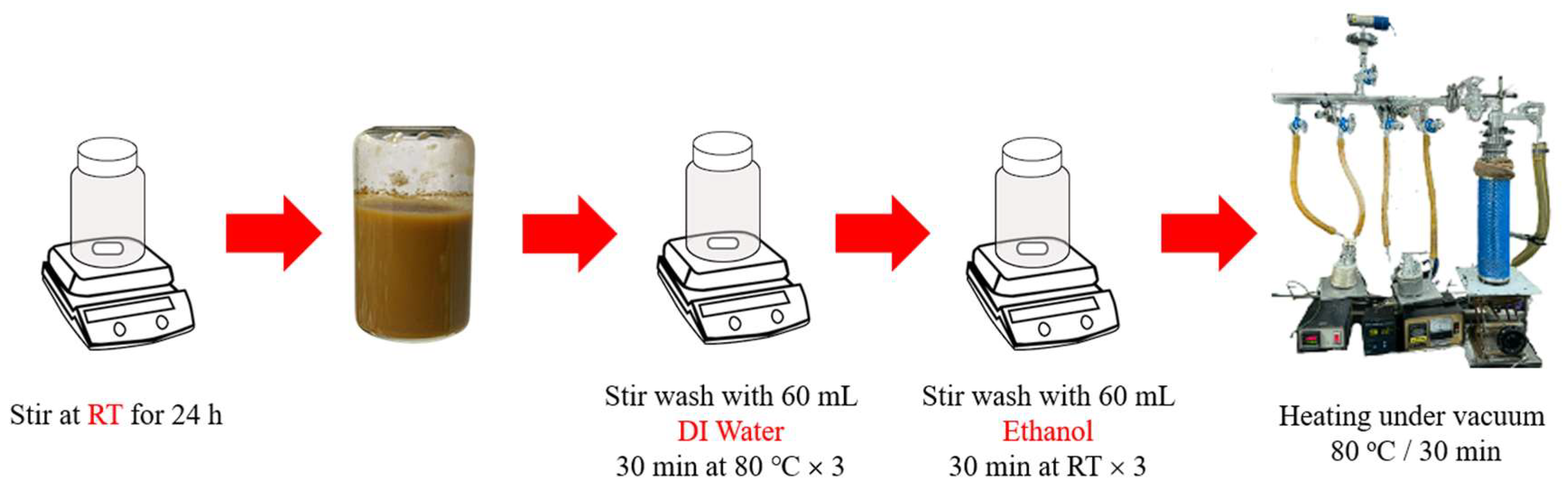
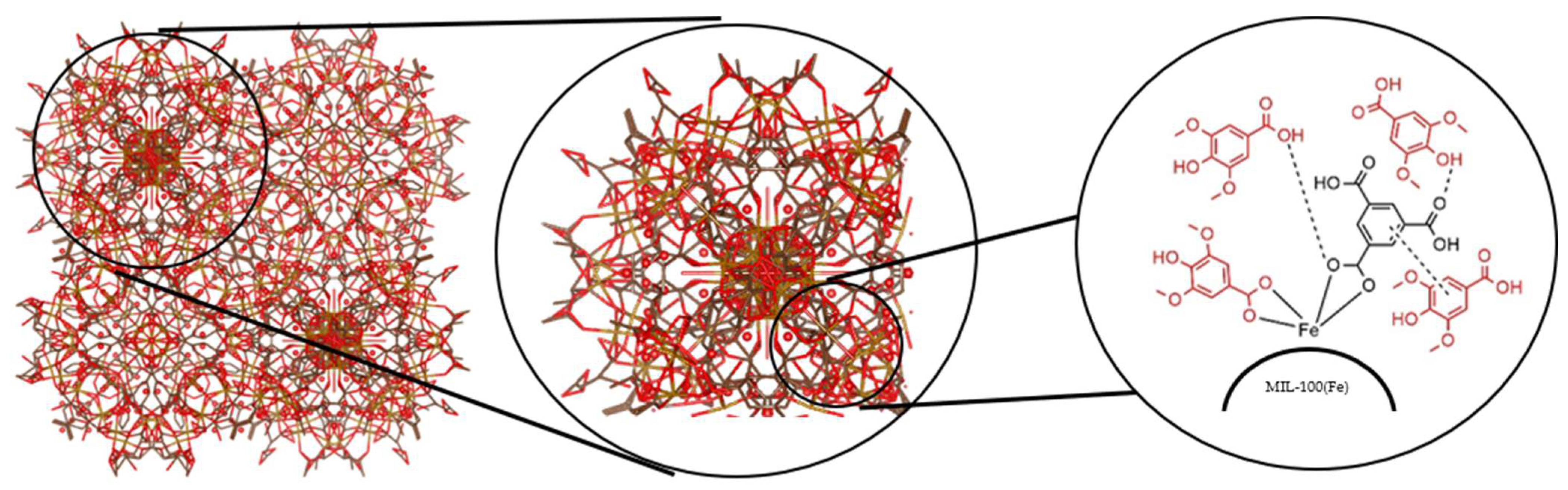
2.3.1. Synthesis of MIL-100
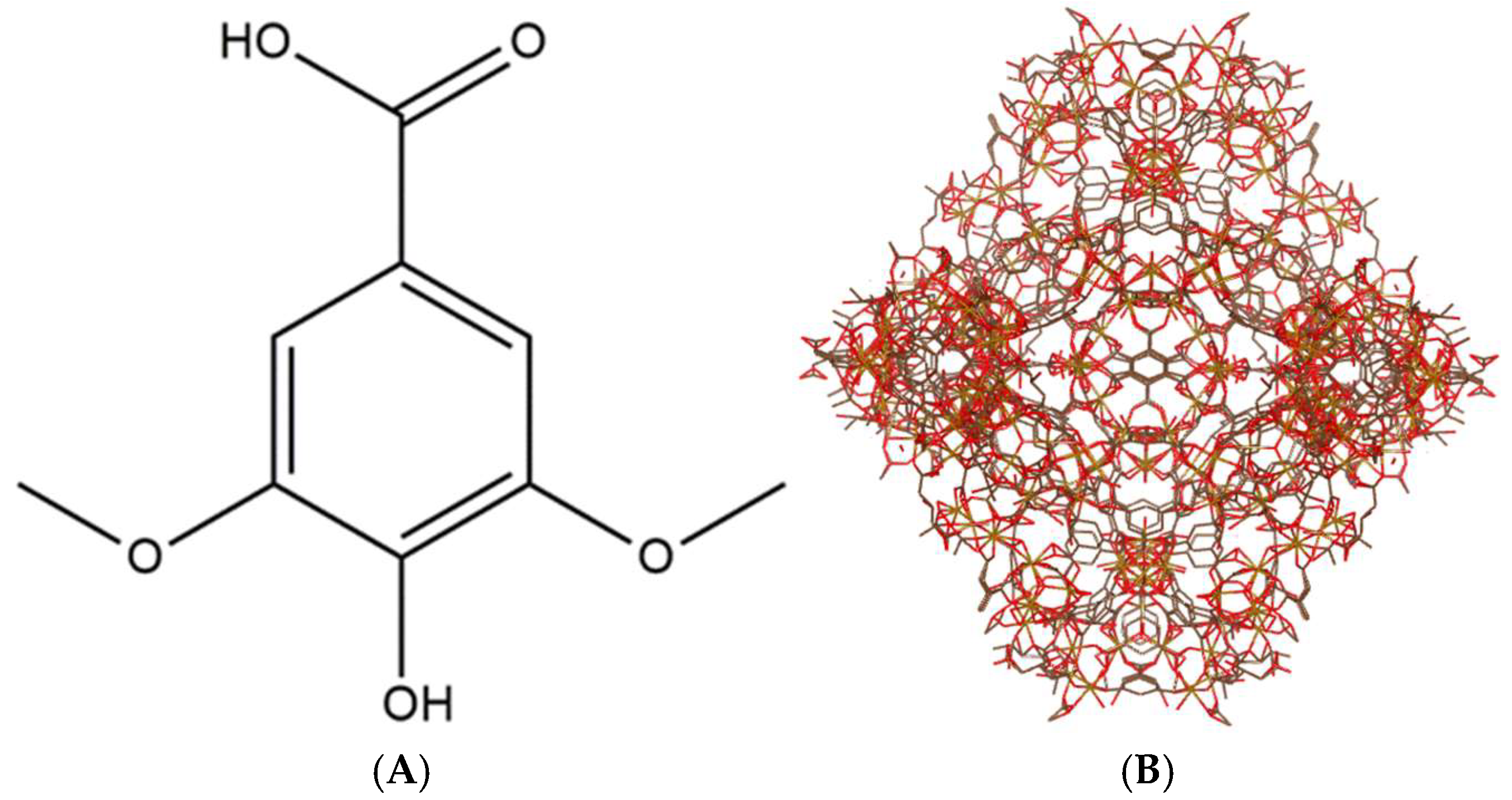
2.3.2. Syringic Acid Impregnation and Quantification
2.3.3. Characterization of SYA@MIL-100(Fe)
Nitrogen Sorption Isotherms
Thermogravimetric Analysis (TGA)
Powder X-Ray Diffraction (PXRD)
Fourier Transform Infrared Spectroscopy (FTIR)
Scanning Electron Microscopy (SEM)
Particle Size Determination
2.3.4. In Vitro Drug Release Study
2.3.5. Acute Oral Toxicity
2.3.6. Oral Bioavailability and Tissue Distribution
Collection and Processing of Blood Samples
Collection and Processing of Organ Samples
Syringic Acid Quantification
2.3.7. Statistical Analysis
3. Results
3.1. Syringic Acid Impregnation and Quantification
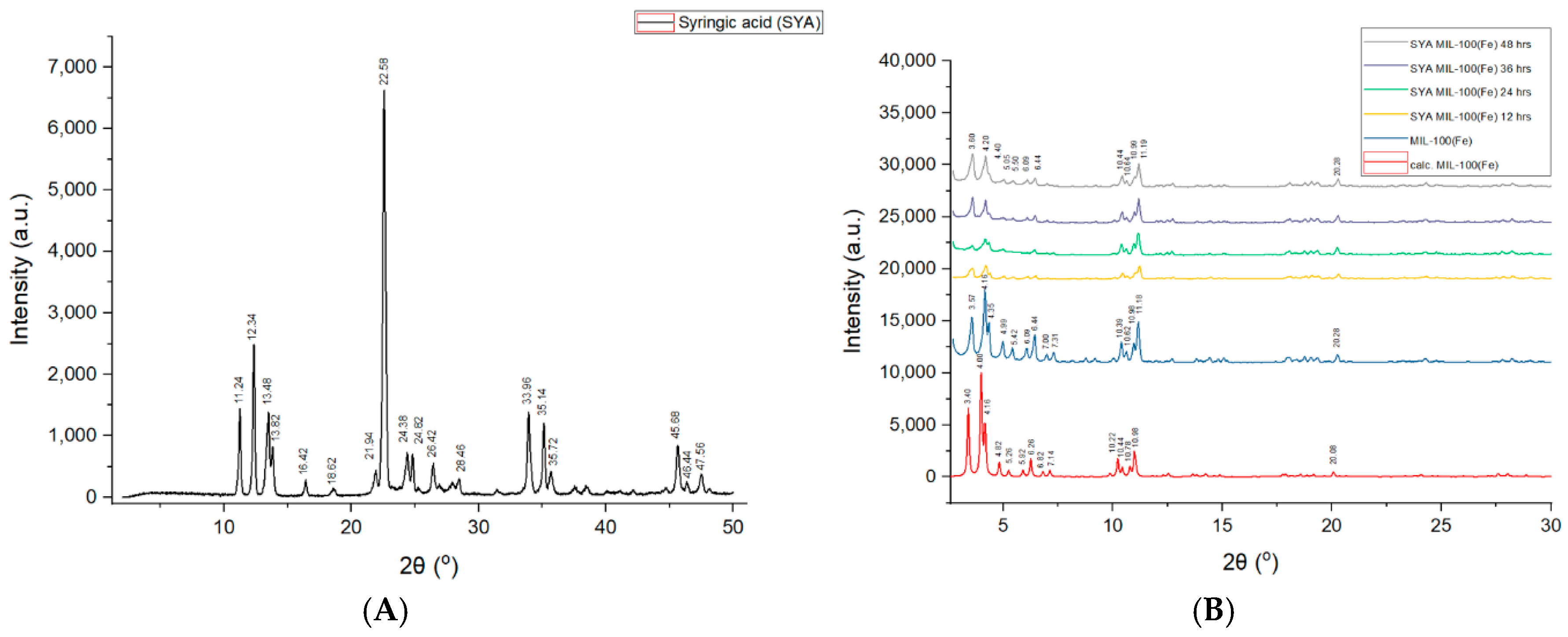
3.2. Powder X-Ray Diffraction (PXRD)
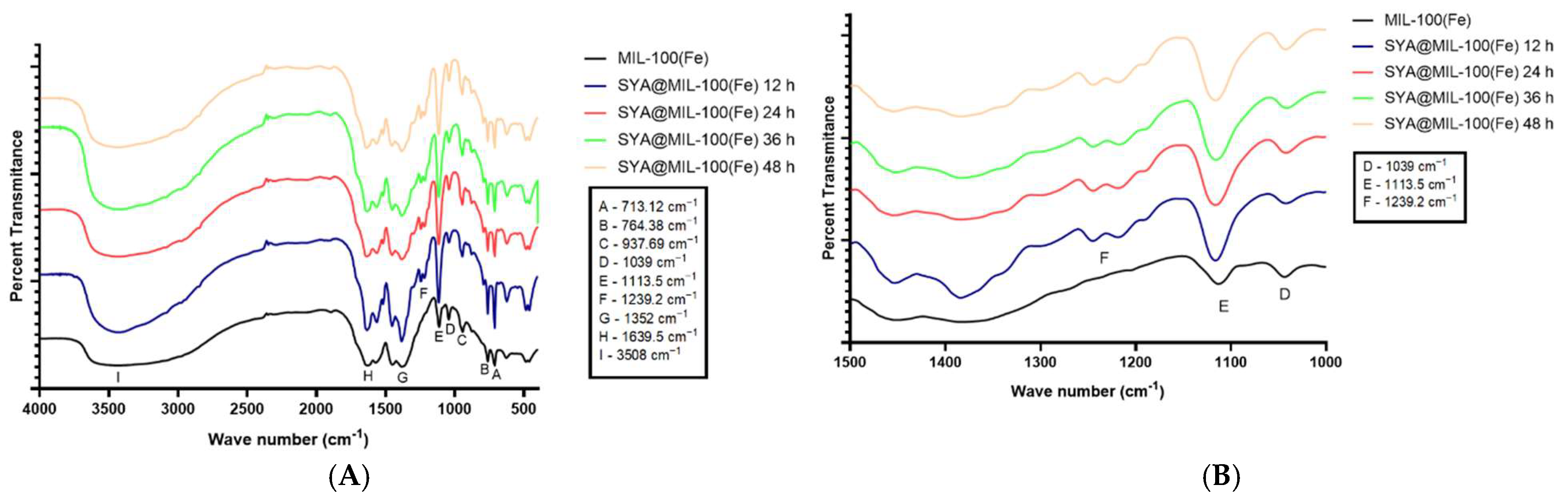
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.4. Nitrogen Adsorption–Desorption
3.5. Thermogravimetric Analysis (TGA)
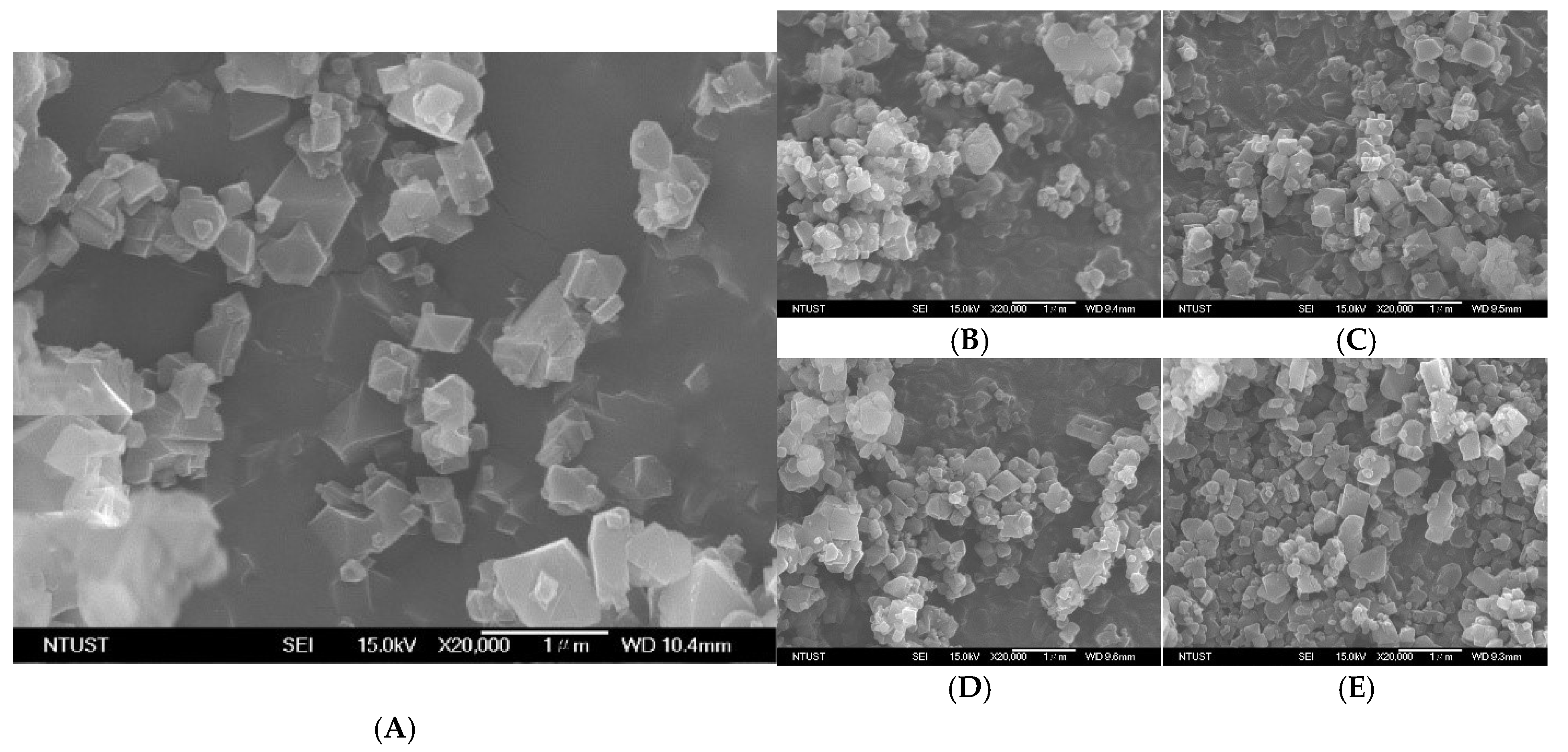
3.6. Surface Morphology and Particle Size Analysis
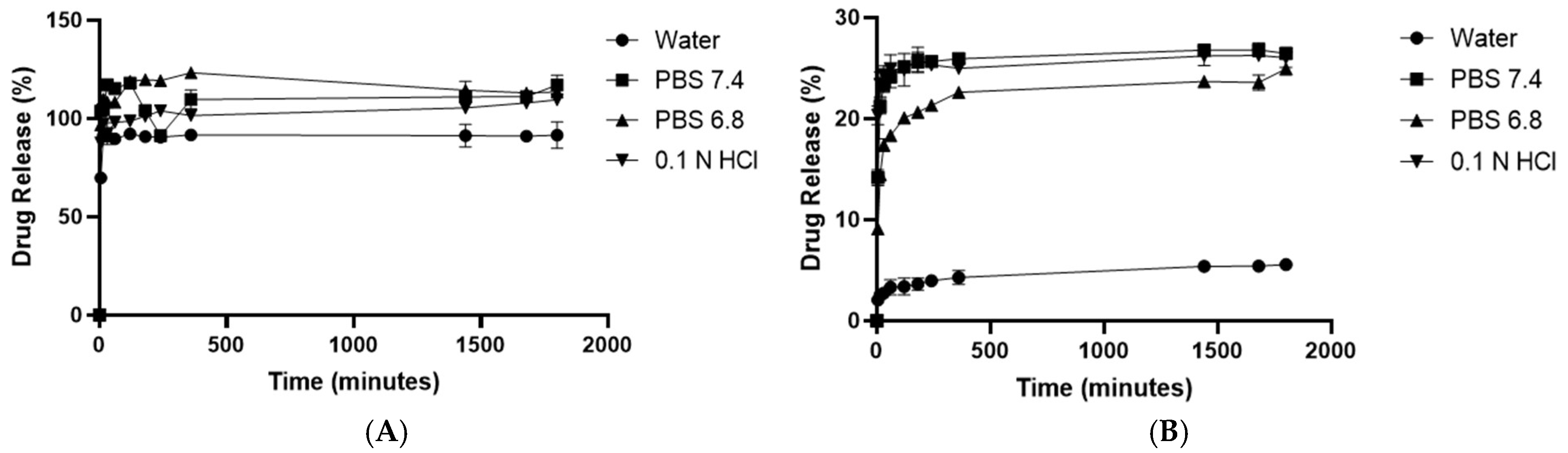
3.7. In Vitro Drug Release
3.8. Acute Oral Toxicity
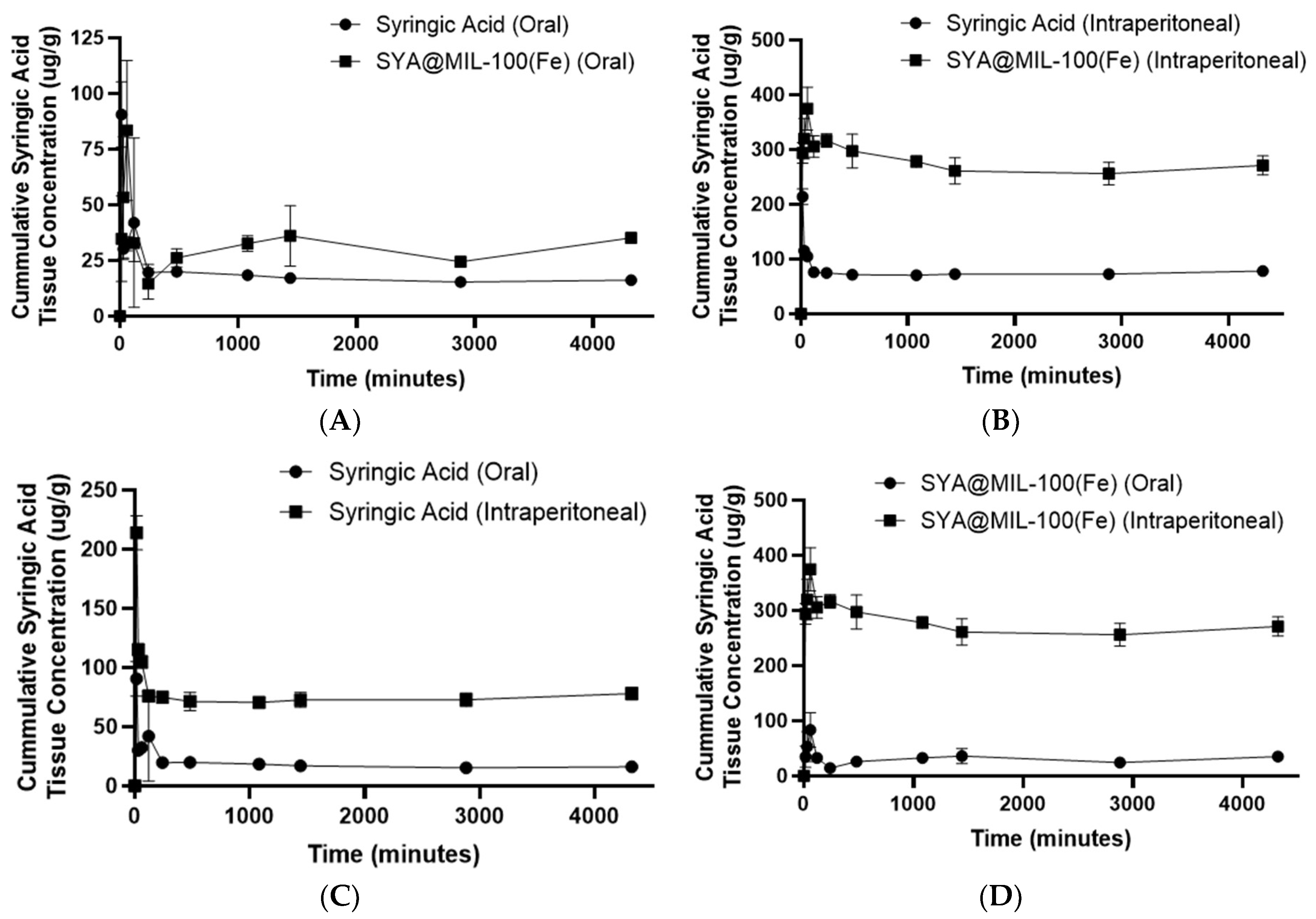
3.9. Bioavailability
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MIL | Materials of Institut Lavoisier |
| MOF | Metal–Organic Framework |
| AUC | Area under the Curve |
| Cmax | Maximum Concentration |
| Tmax | Time to Reach Maximum Concentration |
| T1/2 | Half-life |
| SD | Sprague Dawley |
| SYA@MIL-100(Fe) | Syringic Acid-Loaded MIL-100(Fe) |
| PXRD | Powder X-Ray Diffraction |
| FTIR | Fourier Transform Infrared Spectroscopy |
| h | Hour |
| min | Minute |
| s | Second |
| mL | Milliliter |
| Mcg | Microgram |
References
- Molinari, G. Natural Products in Drug Discovery: Present Status and Perspectives. In Pharmaceutical Biotechnology; Guzmán, C.A., Feuerstein, G.Z., Eds.; Springer: New York, NY, USA, 2009; Volume 655, pp. 13–27. ISBN 978-1-4419-1131-5. [Google Scholar]
- Gao, S.; Hu, M. Bioavailability challenges associated with development of anti-cancer phenolics. Mini Rev. Med. Chem. 2010, 10, 550–567. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Ono, K.; Murase, A.; Yamada, M. Phenolic Compounds Prevent Alzheimer’s Pathology through Different Effects on the Amyloid-β Aggregation Pathway. Am. J. Pathol. 2009, 175, 2557–2565. [Google Scholar] [CrossRef]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef]
- Chirinos, R.; Betalleluz-Pallardel, I.; Huamán, A.; Arbizu, C.; Pedreschi, R.; Campos, D. HPLC-DAD characterisation of phenolic compounds from Andean oca (Oxalis tuberosa Mol.) tubers and their contribution to the antioxidant capacity. Food Chem. 2009, 113, 1243–1251. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Valentão, P.; Pereira, J.; Andrade, P. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical Composition, Antioxidant Properties, and Thermal Stability of a Phytochemical Enriched Oil from Açai ( Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef]
- Pezzuto, J.M. Grapes and Human Health: A Perspective. J. Agric. Food Chem. 2008, 56, 6777–6784. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, C.; Li, W.; Adu-Frimpong, M.; Wang, Q.; Yu, J.; Xu, X. Preparation and Characterization of Syringic Acid–Loaded TPGS Liposome with Enhanced Oral Bioavailability and In Vivo Antioxidant Efficiency. AAPS PharmSciTech 2019, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, X.; Lü, Z.; Xie, W. Study on the pharmacokinetics and bioavailability of syringic acid in rabbits. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2003, 26, 798–801. [Google Scholar]
- Kumar, S.; Dilbaghi, N.; Rani, R.; Bhanjana, G.; Umar, A. Novel Approaches for Enhancement of Drug Bioavailability. Rev. Adv. Sci. Eng. 2013, 2, 133–154. [Google Scholar] [CrossRef]
- Gonçalves, C.; Pereira, P.; Gama, M. Self-Assembled Hydrogel Nanoparticles for Drug Delivery Applications. Materials 2010, 3, 1420–1460. [Google Scholar] [CrossRef]
- Sayed, E.; Haj-Ahmad, R.; Ruparelia, K.; Arshad, M.S.; Chang, M.-W.; Ahmad, Z. Porous Inorganic Drug Delivery Systems—A Review. AAPS PharmSciTech 2017, 18, 1507–1525. [Google Scholar] [CrossRef]
- Liu, S.; Pan, J.; Ma, Y.; Qiu, F.; Niu, X.; Zhang, T.; Yang, L. Three-in-one strategy for selective adsorption and effective separation of cis -diol containing luteolin from peanut shell coarse extract using PU/GO/BA-MOF composite. Chem. Eng. J. 2016, 306, 655–666. [Google Scholar] [CrossRef]
- He, S.; Wu, L.; Li, X.; Sun, H.; Xiong, T.; Liu, J.; Huang, C.; Xu, H.; Sun, H.; Chen, W.; et al. Metal-organic frameworks for advanced drug delivery. Acta Pharm. Sin. B 2021, 11, 2362–2395. [Google Scholar] [CrossRef] [PubMed]
- Abánades Lázaro, I.; Abánades Lázaro, S.; Forgan, R.S. Enhancing anticancer cytotoxicity through bimodal drug delivery from ultrasmall Zr MOF nanoparticles. Chem. Commun. 2018, 54, 2792–2795. [Google Scholar] [CrossRef]
- Bag, P.P.; Wang, D.; Chen, Z.; Cao, R. Outstanding drug loading capacity by water stable microporous MOF: A potential drug carrier. Chem. Commun. 2016, 52, 3669–3672. [Google Scholar] [CrossRef]
- Cunha, D.; Ben Yahia, M.; Hall, S.; Miller, S.R.; Chevreau, H.; Elkaïm, E.; Maurin, G.; Horcajada, P.; Serre, C. Rationale of Drug Encapsulation and Release from Biocompatible Porous Metal–Organic Frameworks. Chem. Mater. 2013, 25, 2767–2776. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Y.; Chi, B.; Lin, C.; Tian, F.; Xu, M.; Wang, Y.; Xu, Z.; Li, L.; Wang, J. Synthesis of ‘dual-key-and-lock’ drug carriers for imaging and improved drug release. Nanotechnology 2020, 31, 445102. [Google Scholar] [CrossRef]
- He, C.; Lu, K.; Liu, D.; Lin, W. Nanoscale Metal–Organic Frameworks for the Co-Delivery of Cisplatin and Pooled siRNAs to Enhance Therapeutic Efficacy in Drug-Resistant Ovarian Cancer Cells. J. Am. Chem. Soc. 2014, 136, 5181–5184. [Google Scholar] [CrossRef]
- He, Y.; Zhang, W.; Guo, T.; Zhang, G.; Qin, W.; Zhang, L.; Wang, C.; Zhu, W.; Yang, M.; Hu, X.; et al. Drug nanoclusters formed in confined nano-cages of CD-MOF: Dramatic enhancement of solubility and bioavailability of azilsartan. Acta Pharm. Sin. B 2019, 9, 97–106. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Maurin, G.; Ramsahye, N.A.; Balas, F.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Flexible Porous Metal-Organic Frameworks for a Controlled Drug Delivery. J. Am. Chem. Soc. 2008, 130, 6774–6780. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- Hu, X.; Wang, C.; Wang, L.; Liu, Z.; Wu, L.; Zhang, G.; Yu, L.; Ren, X.; York, P.; Sun, L.; et al. Nanoporous CD-MOF particles with uniform and inhalable size for pulmonary delivery of budesonide. Int. J. Pharm. 2019, 564, 153–161. [Google Scholar] [CrossRef]
- Leng, X.; Dong, X.; Wang, W.; Sai, N.; Yang, C.; You, L.; Huang, H.; Yin, X.; Ni, J. Biocompatible Fe-Based Micropore Metal-Organic Frameworks as Sustained-Release Anticancer Drug Carriers. Molecules 2018, 23, 2490. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, T.; Lachmanski, L.; Manoli, F.; Menendez-Miranda, M.; Manet, I.; Guo, Z.; Wu, L.; Zhang, J.; Gref, R. Cyclodextrin-based metal-organic frameworks particles as efficient carriers for lansoprazole: Study of morphology and chemical composition of individual particles. Int. J. Pharm. 2017, 531, 424–432. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, S.; Wang, H.; Peng, Y.; Tan, Z.; Tang, B. Functional groups influence and mechanism research of UiO-66-type metal-organic frameworks for ketoprofen delivery. Colloids Surf. B Biointerfaces 2019, 178, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Almaraz, M.T.; Gref, R.; Agostoni, V.; Kreuz, C.; Clayette, P.; Serre, C.; Couvreur, P.; Horcajada, P. Towards improved HIV-microbicide activity through the co-encapsulation of NRTI drugs in biocompatible metal organic framework nanocarriers. J. Mater. Chem. B 2017, 5, 8563–8569. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Li, T.; An, J. Drug Release Properties of a Series of Adenine-Based Metal-Organic Frameworks. Chem. A Eur. J. 2015, 21, 17010–17015. [Google Scholar] [CrossRef]
- Rezaei, M.; Abbasi, A.; Varshochian, R.; Dinarvand, R.; Jeddi-Tehrani, M. NanoMIL-100(Fe) containing docetaxel for breast cancer therapy. Artif. Cells Nanomedicine Biotechnol. 2018, 46, 1390–1401. [Google Scholar] [CrossRef]
- Rojas, S.; Carmona, F.J.; Maldonado, C.R.; Horcajada, P.; Hidalgo, T.; Serre, C.; Navarro, J.A.R.; Barea, E. Nanoscaled Zinc Pyrazolate Metal–Organic Frameworks as Drug-Delivery Systems. Inorg. Chem. 2016, 55, 2650–2663. [Google Scholar] [CrossRef]
- Simon, M.A.; Anggraeni, E.; Soetaredjo, F.E.; Santoso, S.P.; Irawaty, W.; Thanh, T.C.; Hartono, S.B.; Yuliana, M.; Ismadji, S. Hydrothermal Synthesize of HF-Free MIL-100(Fe) for Isoniazid-Drug Delivery. Sci. Rep. 2019, 9, 16907. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Qin, C.; Wang, C.-G.; Su, Z.-M.; Wang, S.; Wang, X.-L.; Yang, G.-S.; Shao, K.-Z.; Lan, Y.-Q.; Wang, E.-B. Chiral Nanoporous Metal-Organic Frameworks with High Porosity as Materials for Drug Delivery. Adv. Mater. 2011, 23, 5629–5632. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Y.; Qin, C.; Wang, X.-L.; Yang, G.-S.; Shao, K.-Z.; Lan, Y.-Q.; Su, Z.-M.; Huang, P.; Wang, C.-G.; Wang, E.-B. Zeolitic imidazolate framework-8 as efficient pH-sensitive drug delivery vehicle. Dalton Trans. 2012, 41, 6906. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Li, L.; Yu, X.; Liu, L.; Meng, Q.; Wang, F.; Zhang, R. Functionalization of mixed ligand metal-organic frameworks as the transport vehicles for drugs. J. Colloid Interface Sci. 2017, 486, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Taherzade, S.; Soleimannejad, J.; Tarlani, A. Application of Metal-Organic Framework Nano-MIL-100(Fe) for Sustainable Release of Doxycycline and Tetracycline. Nanomaterials 2017, 7, 215. [Google Scholar] [CrossRef]
- Taylor-Pashow, K.M.L.; Della Rocca, J.; Xie, Z.; Tran, S.; Lin, W. Postsynthetic Modifications of Iron-Carboxylate Nanoscale Metal−Organic Frameworks for Imaging and Drug Delivery. J. Am. Chem. Soc. 2009, 131, 14261–14263. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, T.; Wang, C.; He, Y.; Zhang, X.; Li, G.; Chen, Y.; Li, J.; Lin, Y.; Xu, X.; et al. MOF Capacitates Cyclodextrin to Mega-Load Mode for High-Efficient Delivery of Valsartan. Pharm. Res. 2019, 36, 117. [Google Scholar] [CrossRef]
- Luo, Y.; Tan, B.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Low-Temperature Rapid Synthesis and Performance of the MIL-100(Fe) Monolithic Adsorbent for Dehumidification. Ind. Eng. Chem. Res. 2020, 59, 7291–7298. [Google Scholar] [CrossRef]
- Horcajada, P.; Surblé, S.; Serre, C.; Hong, D.-Y.; Seo, Y.-K.; Chang, J.-S.; Grenèche, J.-M.; Margiolaki, I.; Férey, G. Synthesis and catalytic properties of MIL-100(Fe), an iron(III) carboxylate with large pores. Chem. Commun. 2007, 27, 2820–2822. [Google Scholar] [CrossRef]
- Singco, B.; Liu, L.-H.; Chen, Y.-T.; Shih, Y.-H.; Huang, H.-Y.; Lin, C.-H. Approaches to drug delivery: Confinement of aspirin in MIL-100(Fe) and aspirin in the de novo synthesis of metal–organic frameworks. Microporous Mesoporous Mater. 2016, 223, 254–260. [Google Scholar] [CrossRef]
- Santos, J.H.; Quimque, M.T.J.; Macabeo, A.P.G.; Corpuz, M.J.-A.T.; Wang, Y.-M.; Lu, T.-T.; Lin, C.-H.; Villaflores, O.B. Enhanced Oral Bioavailability of the Pharmacologically Active Lignin Magnolol via Zr-Based Metal Organic Framework Impregnation. Pharmaceutics 2020, 12, 437. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Shahbazi, S.; Stratz, S.A.; Auxier, J.D.; Hanson, D.E.; Marsh, M.L.; Hall, H.L. Characterization and thermogravimetric analysis of lanthanide hexafluoroacetylacetone chelates. J. Radioanal. Nucl. Chem. 2017, 311, 617–626. [Google Scholar] [CrossRef]
- Chao, M.-Y.; Zhang, W.-H.; Lang, J.-P. Co2 and Co3 Mixed Cluster Secondary Building Unit Approach toward a Three-Dimensional Metal-Organic Framework with Permanent Porosity. Molecules 2018, 23, 755. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Udriştioiu, E.G.; Aboul-Enein, H.Y. X-Ray Diffraction: Instrumentation and Applications. Crit. Rev. Anal. Chem. 2015, 45, 289–299. [Google Scholar] [CrossRef]
- So, P.B.; Chen, H.-T.; Lin, C.-H. De novo synthesis and particle size control of iron metal organic framework for diclofenac drug delivery. Microporous Mesoporous Mater. 2020, 309, 110495. [Google Scholar] [CrossRef]
- Danhier, F.; Magotteaux, N.; Ucakar, B.; Lecouturier, N.; Brewster, M.; Préat, V. Novel self-assembling PEG-p-(CL-co-TMC) polymeric micelles as safe and effective delivery system for Paclitaxel. Eur. J. Pharm. Biopharm. 2009, 73, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Baishya, H. Application of Mathematical Models in Drug Release Kinetics of Carbidopa and Levodopa ER Tablets. J. Dev. Drugs 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Ding, P.; Shen, H.; Wang, J.; Ju, J. Improved oral bioavailability of magnolol by using a binary mixed micelle system. Artif. Cells Nanomedicine Biotechnol. 2018, 46, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, W.; Zhang, H.; Adu-Frimpong, M.; Ma, P.; Zhu, Y.; Deng, W.; Yu, J.; Xu, X. Improved Oral Bioavailability and Hypolipidemic Effect of Syringic Acid via a Self-microemulsifying Drug Delivery System. AAPS PharmSciTech 2021, 22, 45. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal–Organic Frameworks as Efficient Materials for Drug Delivery. Angew. Chem. Int. Ed. 2006, 45, 5974–5978. [Google Scholar] [CrossRef] [PubMed]
- Isaeva, V.I.; Kustov, L.M. The application of metal-organic frameworks in catalysis (Review). Pet. Chem. 2010, 50, 167–180. [Google Scholar] [CrossRef]
- Li, X.; Lachmanski, L.; Safi, S.; Sene, S.; Serre, C.; Grenèche, J.M.; Zhang, J.; Gref, R. New insights into the degradation mechanism of metal-organic frameworks drug carriers. Sci. Rep. 2017, 7, 13142. [Google Scholar] [CrossRef]
- Shano, L.B.; Karthikeyan, S.; Kennedy, L.J.; Chinnathambi, S.; Pandian, G.N. MOFs for next-generation cancer therapeutics through a biophysical approach—A review. Front. Bioeng. Biotechnol. 2024, 12, 1397804. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhang, X.; Zhang, Z.; She, J.; Wu, D.; Gao, W. High Drug-Loading Nanomedicines for Tumor Chemo–Photo Combination Therapy: Advances and Perspectives. Pharmaceutics 2022, 14, 1735. [Google Scholar] [CrossRef]
- Bavnhøj, C.G.; Knopp, M.M.; Madsen, C.M.; Löbmann, K. The role interplay between mesoporous silica pore volume and surface area and their effect on drug loading capacity. Int. J. Pharm. X 2019, 1, 100008. [Google Scholar] [CrossRef]
- Raza, A.; Wu, W. Metal-organic frameworks in oral drug delivery. Asian J. Pharm. Sci. 2024, 19, 100951. [Google Scholar] [CrossRef]
- Cui, R.; Zhao, P.; Yan, Y.; Bao, G.; Damirin, A.; Liu, Z. Outstanding Drug-Loading/Release Capacity of Hollow Fe-Metal–Organic Framework-Based Microcapsules: A Potential Multifunctional Drug-Delivery Platform. Inorg. Chem. 2021, 60, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Ayyoubzadeh, S.M.; Ghorbani-Bidkorbeh, F.; Shahhosseini, S.; Dadashzadeh, S.; Asadian, E.; Mosayebnia, M.; Siavashy, S. An investigation of affecting factors on MOF characteristics for biomedical applications: A systematic review. Heliyon 2021, 7, e06914. [Google Scholar] [CrossRef]
- Cretu, C.; Nicola, R.; Marinescu, S.-A.; Picioruș, E.-M.; Suba, M.; Duda-Seiman, C.; Len, A.; Illés, L.; Horváth, Z.E.; Putz, A.-M. Performance of Zr-Based Metal–Organic Framework Materials as In Vitro Systems for the Oral Delivery of Captopril and Ibuprofen. Int. J. Mol. Sci. 2023, 24, 13887. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Wang, L.; Hou, J. Emerging microporous materials as novel templates for quantum dots. Microstructures 2023, 3. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, L.; Yang, Y.; Qian, X.; Fu, T.; Li, X.; Yang, Z.; Yan, H.; Cui, C.; Tan, W. Metal–Organic Framework Nanocarriers for Drug Delivery in Biomedical Applications. Nano-Micro Lett. 2020, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Marqués, M.; Hidalgo, T.; Serre, C.; Horcajada, P. Nanostructured metal–organic frameworks and their bio-related applications. Coord. Chem. Rev. 2016, 307, 342–360. [Google Scholar] [CrossRef]
- Wittmann, T.; Tschense, C.B.L.; Zappe, L.; Koschnick, C.; Siegel, R.; Stäglich, R.; Lotsch, B.V.; Senker, J. Selective host–guest interactions in metal–organic frameworks via multiple hydrogen bond donor–acceptor recognition sites. J. Mater. Chem. A 2019, 7, 10379–10388. [Google Scholar] [CrossRef]
- Chobot, V.; Hadacek, F. Iron and its complexation by phenolic cellular metabolites: From oxidative stress to chemical weapons. Plant Signal. Behav. 2010, 5, 4–8. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, A. Kinetics of complex formation of Fe(III) with syringic acid: Experimental and theoretical study. Food Chem. 2018, 265, 96–100. [Google Scholar] [CrossRef]
- Quintero-Álvarez, F.G.; Rojas-Mayorga, C.K.; Mendoza-Castillo, D.I.; Aguayo-Villarreal, I.A.; Bonilla-Petriciolet, A. Physicochemical Modeling of the Adsorption of Pharmaceuticals on MIL-100-Fe and MIL-101-Fe MOFs. Adsorpt. Sci. Technol. 2022, 2022, 4482263. [Google Scholar] [CrossRef]
- Sun, B.; Zheng, X.; Zhang, X.; Zhang, H.; Jiang, Y. Oxaliplatin-Loaded Mil-100(Fe) for Chemotherapy–Ferroptosis Combined Therapy for Gastric Cancer. ACS Omega 2024, 9, 16676–16686. [Google Scholar] [CrossRef] [PubMed]
- Parsa, F.; Setoodehkhah, M.; Atyabi, S.M. Design, fabrication and characterization of a magnetite-chitosan coated iron-based metal–organic framework (Fe3O4@chitosan/MIL-100(Fe)) for efficient curcumin delivery as a magnetic nanocarrier. RSC Adv. 2025, 15, 18518–18534. [Google Scholar] [CrossRef]
- Tohidi, S.; Aghaie-Khafri, M. Cyclophosphamide Loading and Controlled Release in MIL-100(Fe) as anAnti-breast Cancer Carrier: In vivo In vitro Study. Curr. Drug Deliv. 2024, 21, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Le, B.T.; La, D.D.; Nguyen, P.T.H. Ultrasonic-Assisted Fabrication of MIL-100(Fe) Metal–Organic Frameworks as a Carrier for the Controlled Delivery of the Chloroquine Drug. ACS Omega 2023, 8, 1262–1270. [Google Scholar] [CrossRef]
- Lajevardi, A.; Hossaini Sadr, M.; Tavakkoli Yaraki, M.; Badiei, A.; Armaghan, M. A pH-responsive and magnetic Fe3O4@silica@MIL-100(Fe)/β-CD nanocomposite as a drug nanocarrier: Loading and release study of cephalexin. New J. Chem. 2018, 42, 9690–9701. [Google Scholar] [CrossRef]
- Sucharitha, P.; Reddy, R.; Jahnavi, I.; Prakruthi, H.; Sruthi, T.; Gowd, M.R.G.B.; Haseena, B.; Babu, N. Design of Lamivudine Loaded Metal Organic Frameworks MIL 100 (Fe) by Microwave Assisted Chemistry. Indian J. Pharm. Educ. Res. 2024, 58, s1083–s1092. [Google Scholar] [CrossRef]
- Al Haydar, M.; Abid, H.R.; Sunderland, B.; Wang, S. Multimetal organic frameworks as drug carriers: Aceclofenac as a drug candidate. Drug Des. Devel. Ther. 2018, 13, 23–35. [Google Scholar] [CrossRef]
- Lin, Y.; Yu, R.; Yin, G.; Chen, Z.; Lin, H. Syringic acid delivered via mPEG-PLGA-PLL nanoparticles enhances peripheral nerve regeneration effect. Nanomedicine 2020, 15, 1487–1499. [Google Scholar] [CrossRef]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High drug-loading nanomedicines: Progress, current status, and prospects. Int. J. Nanomed. 2017, 12, 4085–4109. [Google Scholar] [CrossRef]
- Souza, B.; Moslein, A.; Titov, K.; Taylor, J.D.; Rudic, S.; Tan, J.-C. Drug Guest Molecules as Modulators During One-Step Mechanochemical Encapsulation in MIL-100 (Fe) Framework. ChemRxiv 2020, preprint. [Google Scholar] [CrossRef]
- Feng, D.; Feng, Y.; Zang, Y.; Li, P.; Zhang, X. Phase change in modified metal organic frameworks MIL-101(Cr): Mechanism on highly improved energy storage performance. Microporous Mesoporous Mater. 2019, 280, 124–132. [Google Scholar] [CrossRef]
- Miri, B.; Motakef-Kazemi, N.; Shojaosadati, S.A.; Morsali, A. Application of a Nanoporous Metal Organic Framework Based on Iron Carboxylate as Drug Delivery System. Iran. J. Pharm. Res. IJPR 2018, 17, 1164–1171. [Google Scholar]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Kohane, D.S. Microparticles and nanoparticles for drug delivery. Biotechnol. Bioeng. 2007, 96, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Pan, H.; He, F.; Wang, X.; Li, J.; Yang, X.; Pan, W. Effect of particle size on oral absorption of carvedilol nanosuspensions: In vitro and in vivo evaluation. Int. J. Nanomed. 2015, 10, 6425–6434. [Google Scholar] [CrossRef]
- Zhu, J.; Liao, L.; Zhu, L.; Zhang, P.; Guo, K.; Kong, J.; Ji, C.; Liu, B. Size-dependent cellular uptake efficiency, mechanism, and cytotoxicity of silica nanoparticles toward HeLa cells. Talanta 2013, 107, 408–415. [Google Scholar] [CrossRef]
- Linnane, E.; Haddad, S.; Melle, F.; Mei, Z.; Fairen-Jimenez, D. The uptake of metal–organic frameworks: A journey into the cell. Chem. Soc. Rev. 2022, 51, 6065–6086. [Google Scholar] [CrossRef]
- Quijia, C.R.; Lima, C.; Silva, C.; Alves, R.C.; Frem, R.; Chorilli, M. Application of MIL-100(Fe) in drug delivery and biomedicine. J. Drug Deliv. Sci. Technol. 2021, 61, 102217. [Google Scholar] [CrossRef]
- Neuer, A.L.; Herrmann, I.K.; Gogos, A. Biochemical transformations of inorganic nanomedicines in buffers, cell cultures and organisms. Nanoscale 2023, 15, 18139–18155. [Google Scholar] [CrossRef] [PubMed]
- Pirzadeh, K.; Ghoreyshi, A.A.; Rohani, S.; Rahimnejad, M. Strong Influence of Amine Grafting on MIL-101 (Cr) Metal–Organic Framework with Exceptional CO2/N2 Selectivity. Ind. Eng. Chem. Res. 2020, 59, 366–378. [Google Scholar] [CrossRef]
- Marson Armando, R.A.; Abuçafy, M.P.; Graminha, A.E.; Silva, R.S.D.; Frem, R.C.G. Ru-90@bio-MOF-1: A ruthenium(II) metallodrug occluded in porous Zn-based MOF as a strategy to develop anticancer agents. J. Solid State Chem. 2021, 297, 122081. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y.; Xia, X.; Cao, Z.; Deng, Y.; Tang, B. Sr/PTA Metal Organic Framework as A Drug Delivery System for Osteoarthritis Treatment. Sci. Rep. 2019, 9, 17570. [Google Scholar] [CrossRef]
- Lu, L.; Ma, M.; Gao, C.; Li, H.; Li, L.; Dong, F.; Xiong, Y. Metal Organic Framework@Polysilsesequioxane Core/Shell-Structured Nanoplatform for Drug Delivery. Pharmaceutics 2020, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Gharehdaghi, Z.; Naghib, S.M.; Rahimi, R.; Bakhshi, A.; Kefayat, A.; Shamaeizadeh, A.; Molaabasi, F. Highly improved pH-Responsive anticancer drug delivery and T2-Weighted MRI imaging by magnetic MOF CuBTC-based nano/microcomposite. Front. Mol. Biosci. 2023, 10, 1071376. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xiong, Y.; Dong, F. Cyclodextrin metal–organic framework@SiO2 nanocomposites for poorly soluble drug loading and release. RSC Adv. 2024, 14, 31868–31876. [Google Scholar] [CrossRef]
- Mirza, A.C.; Panchal, S.S. Safety evaluation of syringic acid: Subacute oral toxicity studies in Wistar rats. Heliyon 2019, 5, e02129. [Google Scholar] [CrossRef]
- Chen, G.; Leng, X.; Luo, J.; You, L.; Qu, C.; Dong, X.; Huang, H.; Yin, X.; Ni, J. In Vitro Toxicity Study of a Porous Iron(III) Metal—Organic Framework. Molecules 2019, 24, 1211. [Google Scholar] [CrossRef]
- Tariq, T.; Bibi, S.; Ahmad Shah, S.S.; Wattoo, M.A.; Salem, M.A.; El-Haroun, H.; El-Bahy, Z.M.; Rehman, A.U.; Bao, S. MIL materials: Synthesis strategies, morphology control, and biomedical application: A critical review. J. Drug Deliv. Sci. Technol. 2025, 104, 106532. [Google Scholar] [CrossRef]
- Itoh, A.; Isoda, K.; Kondoh, M.; Kawase, M.; Watari, A.; Kobayashi, M.; Tamesada, M.; Yagi, K. Hepatoprotective Effect of Syringic Acid and Vanillic Acid on CCl4-Induced Liver Injury. Biol. Pharm. Bull. 2010, 33, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.; Raja, B. Protective Effects of Syringic Acid against Acetaminophen-Induced Hepatic Damage in Albino Rats. J. Basic Clin. Physiol. Pharmacol. 2010, 21, 369–386. [Google Scholar] [CrossRef]
- Ferah Okkay, I.; Okkay, U.; Gundogdu, O.L.; Bayram, C.; Mendil, A.S.; Ertugrul, M.S.; Hacimuftuoglu, A. Syringic acid protects against thioacetamide-induced hepatic encephalopathy: Behavioral, biochemical, and molecular evidence. Neurosci. Lett. 2022, 769, 136385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, S.; Li, Y.; Yang, J.; Mao, X.; Liu, T.; Zhang, Q.; Fu, Z.; Zhu, X.; Xu, L. Protective effects of syringic acid in nonalcoholic fatty liver in rats through regulation of Nrf2/HO-1 signaling pathway. J. Biochem. Mol. Toxicol. 2024, 38, e23809. [Google Scholar] [CrossRef]
- Sherkhane, B.; Yerra, V.G.; Sharma, A.; Kumar, K.A.; Chayanika, G.; Kumar, A.V.; Kumar, A. Nephroprotective potential of syringic acid in experimental diabetic nephropathy: Focus on oxidative stress and autophagy. Indian J. Pharmacol. 2023, 55, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Zabad, O.M.; Samra, Y.A.; Eissa, L.A. Syringic acid ameliorates experimental diabetic nephropathy in rats through its antiinflammatory, anti-oxidant and anti-fibrotic effects by suppressing Toll like receptor-4 pathway. Metabolism 2022, 128, 154966. [Google Scholar] [CrossRef]
- Mirza, A.C.; Panchal, S.S.; Allam, A.A.; Othman, S.I.; Satia, M.; Mandhane, S.N. Syringic Acid Ameliorates Cardiac, Hepatic, Renal and Neuronal Damage Induced by Chronic Hyperglycaemia in Wistar Rats: A Behavioural, Biochemical and Histological Analysis. Molecules 2022, 27, 6722. [Google Scholar] [CrossRef]
- Rashedinia, M.; Khoshnoud, M.J.; Fahlyan, B.K.; Hashemi, S.-S.; Alimohammadi, M.; Sabahi, Z. Syringic Acid: A Potential Natural Compound for the Management of Renal Oxidative Stress and Mitochondrial Biogenesis in Diabetic Rats. Curr. Drug Discov. Technol. 2021, 18, 405–413. [Google Scholar] [CrossRef]
- Akram, M.U.; Nesrullah, M.; Afaq, S.; Malik, W.M.A.; Ghafoor, A.; Ismail, M.; Nawaz, H.; Ibrahim, M.; Verpoort, F.; Chughtai, A.H. An easy approach towards once a day sustained release dosage form using microporous Cu-MOFs as drug delivery vehicles. New J. Chem. 2024, 48, 11542–11554. [Google Scholar] [CrossRef]
- Li, L.; Han, S.; Zhao, S.; Li, X.; Liu, B.; Liu, Y. Chitosan modified metal-organic frameworks as a promising carrier for oral drug delivery. RSC Adv. 2020, 10, 45130–45138. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Kant, A.; Kumar, M.; Masram, D.T. Synthesis, characterizations and kinetic study of metal organic framework nanocomposite excipient used as extended release delivery vehicle for an antibiotic drug. Inorganica Chim. Acta 2019, 496, 119036. [Google Scholar] [CrossRef]
- Sun, C.; Li, W.; Ma, P.; Li, Y.; Zhu, Y.; Zhang, H.; Adu-Frimpong, M.; Deng, W.; Yu, J.; Xu, X. Development of TPGS/F127/F68 mixed polymeric micelles: Enhanced oral bioavailability and hepatoprotection of syringic acid against carbon tetrachloride-induced hepatotoxicity. Food Chem. Toxicol. 2020, 137, 111126. [Google Scholar] [CrossRef]
- Coughlin, J.E.; Pandey, R.K.; Padmanabhan, S.; O’Loughlin, K.G.; Marquis, J.; Green, C.E.; Mirsalis, J.C.; Iyer, R.P. Metabolism, Pharmacokinetics, Tissue Distribution, and Stability Studies of the Prodrug Analog of an Anti-Hepatitis B Virus Dinucleoside Phosphorothioate. Drug Metab. Dispos. 2012, 40, 970–981. [Google Scholar] [CrossRef] [PubMed]
- Spizzirri, U.G.; Aiello, F.; Carullo, G.; Facente, A.; Restuccia, D. Nanotechnologies: An Innovative Tool to Release Natural Extracts with Antimicrobial Properties. Pharmaceutics 2021, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-L.; Zeng, X.-S.; Zhou, X.; Yang, J.-L.; Ren, L.-L.; Long, X.-Y.; Wang, F.-Q.; Olaleye, O.E.; Tian, N.-N.; Zhu, Y.-X.; et al. Molecular Basis Underlying Hepatobiliary and Renal Excretion of Phenolic Acids of Salvia miltiorrhiza Roots (Danshen). Front. Pharmacol. 2022, 13, 911982. [Google Scholar] [CrossRef]
- Wu, Y.; Li, L.; Ming, G.; Ma, X.; Liang, C.; Li, Y.; He, X. Measurement of Pharmacokinetics and Tissue Distribution of Four Compounds from Nauclea officinalis in Rat Plasma and Tissues through HPLC-MS/MS. J. Anal. Methods Chem. 2022, 2022, 5297603. [Google Scholar] [CrossRef] [PubMed]

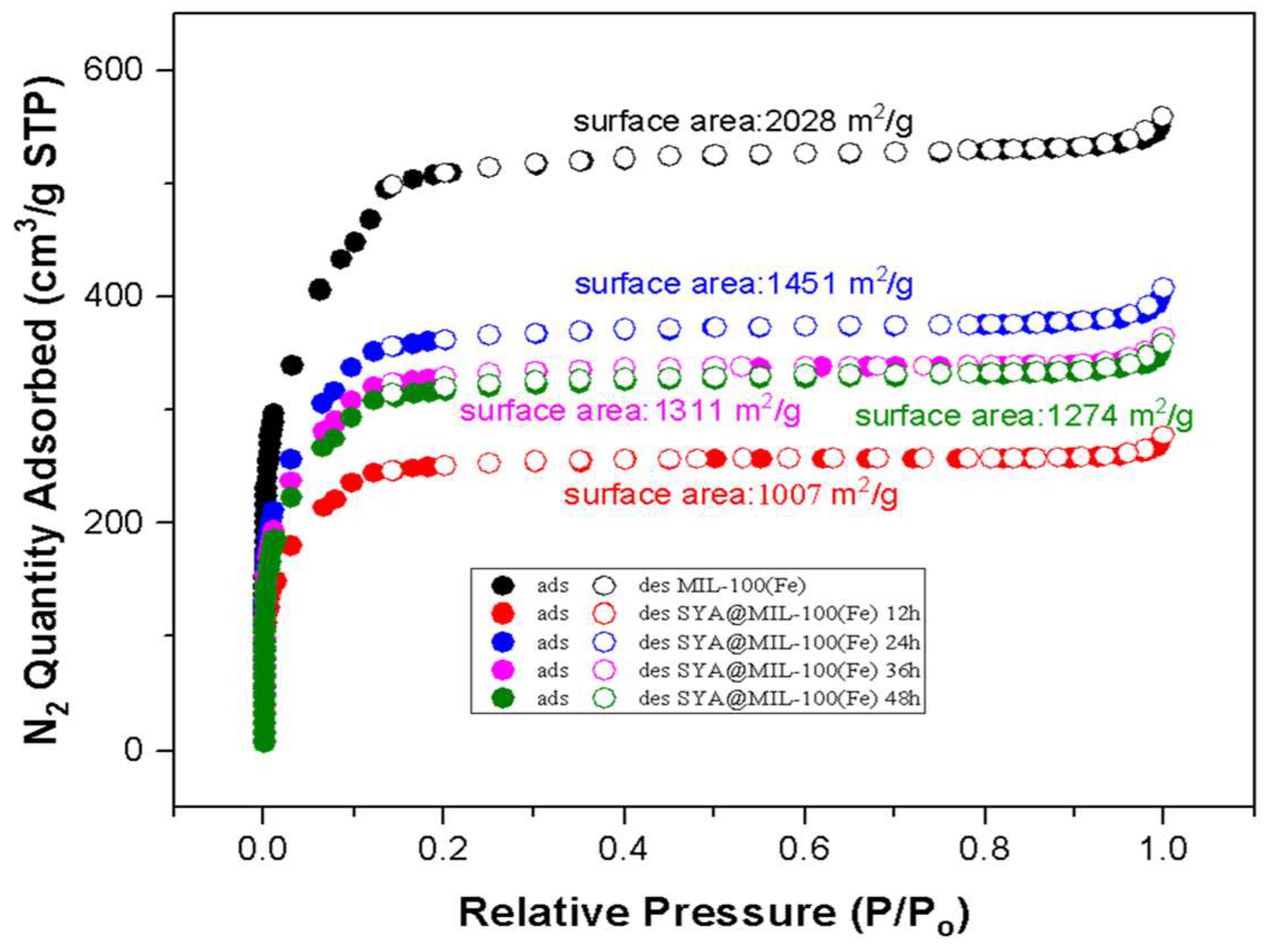



| Total Pore Volume (cm3 g−1) * | Micropore Volume (cm3 g−1) ** | Mesopore Volume (cm3 g−1) *** | Pore Width (Å) **** | |
|---|---|---|---|---|
| MIL-100(Fe) | 0.855916 | 0.297397 | 0.558519 | 29.717 |
| SYA@MIL-100(Fe)—12 h | 0.422034 | 0.231148 | 0.190886 | 35.259 |
| SYA@MIL-100(Fe)—24 h | 0.619344 | 0.400067 | 0.219277 | 37.399 |
| SYA@MIL-100(Fe)—36 h | 0.555394 | 0.373316 | 0.182078 | 35.937 |
| SYA@MIL-100(Fe)—48 h | 0.546359 | 0.339412 | 0.206947 | 37.102 |
| Test Compound | Biological Sample | Route | AUC0–72 * | AUC0–∞ * | Cmax ** | Tmax *** | T1/2 *** |
|---|---|---|---|---|---|---|---|
| Syringic Acid | Blood | Oral | 1419 ± 142.15 | 1460 ± 143.84 | 2.33 ± 0.19 | 66.78 ± 7.56 ‡ | 118.77 ± 30.76 |
| Intraperitoneal | 4368.33 ± 489.25 ‡ | 5400.84 ± 964.81 ‡ | 2.55 ± 0.04 ‡ | 55.97 ± 2.11 | 999.64 ± 410 ‡ | ||
| Liver | Oral | 32,000.33 ± 3544.16 ‡ | 47,401.91 ± 8515.77 ‡ | 16.28 ± 3.54 | 515.96 ± 4.44 ‡ | 2288.81 ± 812.66 | |
| Intraperitoneal | 102,784.67 ± 1510.75 | 166,522.15 ± 34,487.20 | 40.13 ± 0.56 ‡ | 24.70 ±0.21 | 1852.40 ± 1105.09 | ||
| Kidney | Oral | 77,103.33 ± 2531.03 ‡ | 78,035.27 ± 2458.81 ‡ | 105.36 ± 9.79 | 28.21 ± 0.33 ‡ | 37.56 ± 4.69 ‡ | |
| Intraperitoneal | 321,104.67 ± 11,949.32 | 808,296.73 ± 429,472.17 | 218.21 ± 8.73 ‡ | 24.15 ± 0.96 | 4805.81 ± 3271.08 | ||
| SYA@MIL-100(Fe) | Blood | Oral | 15,606 ± 1936.03 | 131,269.97 ± 61,666.27 | 3.79 ± 0.43 | 549.02 ± 159.41 | 24,392.75 ± 10,593.37 |
| Intraperitoneal | 56,022.33 ± 2240.13 ‡ | 206,758.55 ± 31,210.34 ‡ | 26.54 ± 0.55 ‡ | 1236.64 ± 91.50 ‡ | 11,504.68 ± 2306 ‡ | ||
| Liver | Oral | 17,309.33 ± 1351.33 ‡ | 22,623.03 ± 2085.19 ‡ | 11.42 ±2.30 | 385.57 ± 42.44 ‡ | 913.33 ± 223.58 | |
| Intraperitoneal | 363,982.67 ± 5429.14 | 522,988.13 ± 44,624.56 | 150.25 ± 6.65 ‡ | 111.01 ± 4.91 | 1309.25 ± 359.93 | ||
| Kidney | Oral | 130,698 ± 7713.74 ‡ | 144,112.76 ± 8494.44 ‡ | 89.27 ± 17.80 | 97.30 ± 9.65 ‡ | 265.21 ± 22.01 ‡ | |
| Intraperitoneal | 1,169,999.33 ± 36,457.71 | 2,971,634.10 ± 1,163,138.16 | 393.47 ± 14.44 ‡ | 118.08 ± 0.86 | 2799.01 ± 1717.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.H.; Victoriano, H.J.; Sepulveda, M.; Liu, H.-E.; Valencia, S.M.N.; Walde, R.Z.M.L.; Ongo, E.A.; Lin, C.-H. MIL-100(Fe)-Enabled Oral Delivery of Syringic Acid with Enhanced Pharmacokinetics. Pharmaceutics 2025, 17, 1282. https://doi.org/10.3390/pharmaceutics17101282
Santos JH, Victoriano HJ, Sepulveda M, Liu H-E, Valencia SMN, Walde RZML, Ongo EA, Lin C-H. MIL-100(Fe)-Enabled Oral Delivery of Syringic Acid with Enhanced Pharmacokinetics. Pharmaceutics. 2025; 17(10):1282. https://doi.org/10.3390/pharmaceutics17101282
Chicago/Turabian StyleSantos, Joshua H., Hannah Jean Victoriano, Mary Sepulveda, Hung-En Liu, Shierrie Mae N. Valencia, Rikkamae Zinca Marie L. Walde, Emelda A. Ongo, and Chia-Her Lin. 2025. "MIL-100(Fe)-Enabled Oral Delivery of Syringic Acid with Enhanced Pharmacokinetics" Pharmaceutics 17, no. 10: 1282. https://doi.org/10.3390/pharmaceutics17101282
APA StyleSantos, J. H., Victoriano, H. J., Sepulveda, M., Liu, H.-E., Valencia, S. M. N., Walde, R. Z. M. L., Ongo, E. A., & Lin, C.-H. (2025). MIL-100(Fe)-Enabled Oral Delivery of Syringic Acid with Enhanced Pharmacokinetics. Pharmaceutics, 17(10), 1282. https://doi.org/10.3390/pharmaceutics17101282







