Microneedle-Mediated Transdermal Drug Delivery for the Treatment of Multiple Skin Diseases
Abstract
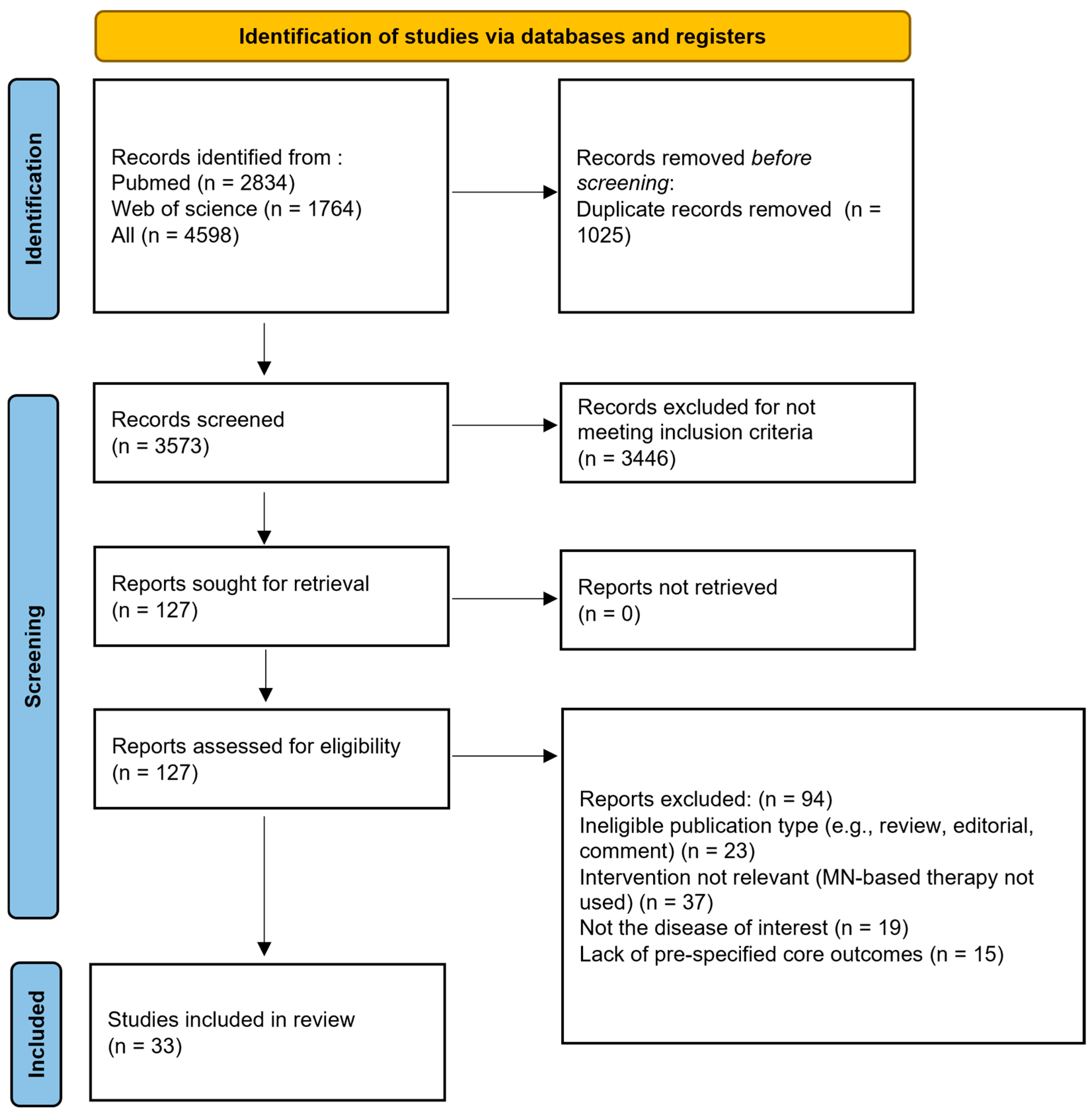
1. Introduction
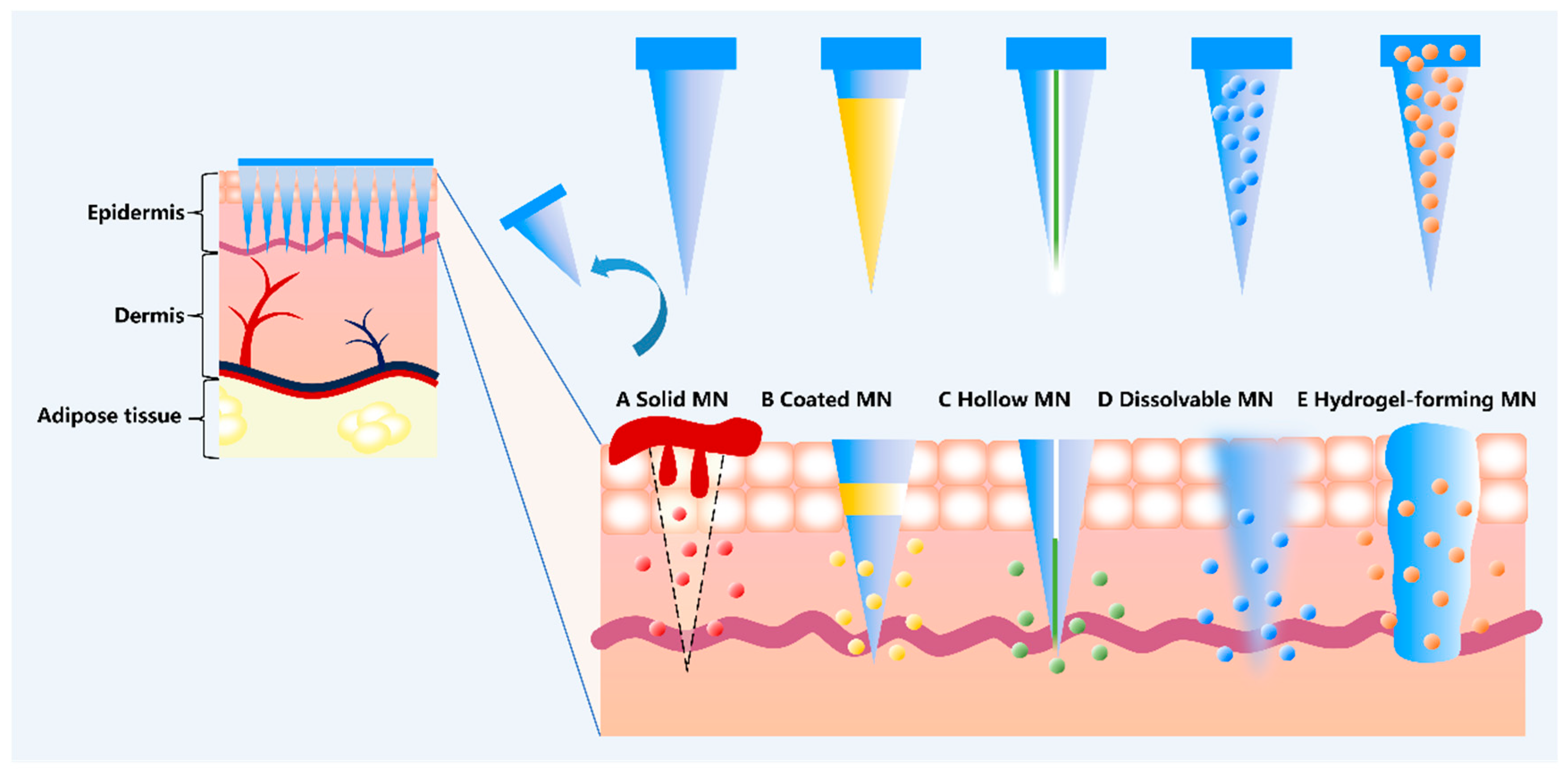
| Classification | Characteristics | Advantages | Limitations | References |
|---|---|---|---|---|
| Solid MNs | Made from materials such as metal, silicon, and titanium, these devices are prepared using technologies such as laser microprocessing and form micron-level channels after piercing the SC. | High mechanical strength, reusable; Suitable for transdermal delivery of drugs applied to the surface. | Poor biocompatibility, risk of fracture, may cause infection or inflammation. | [30,31,32] |
| Coated MNs | The drug is coated onto the surface of the MNs in the form of a solid film using micron-level dip coating or inkjet printing technology. After insertion into the skin, the coating dissolves and releases the drug. | Easy to use, rapid drug release; Suitable for local delivery of small-molecule drugs. | Drug loading capacity is limited and depends on coating thickness and needle tip geometry; Precautions must be taken to prevent premature drug release prior to use. | [33,34,35] |
| Hollow MNs | The needle body has a cavity, and the needle tip has micro-holes, which can inject small volumes of solution into the dermis at a controlled rate, and can also deliver particles or nanoparticles. | Higher drug load than coated MNs; Supports liquid and particulate drug delivery, suitable for precise dosing. | After insertion, the needle tip may be blocked by dermal tissue, resulting in obstructed drug flow or inaccurate dosage. | [36,37] |
| Dissolving MNs | Made from biodegradable polymers (such as hyaluronic acid, chitosan, and PLGA), it degrades and releases the encapsulated drug after being inserted into the skin. | High drug loading capacity (drugs can be encapsulated in the entire needle tip matrix); Good biocompatibility and degradability, with no risk of residue. | Insufficient physical stability and mechanical strength require careful consideration during storage and use. | [38,39] |
| Hydrogel-forming MNs | Made from water-expandable polymers (such as gelatin and cellulose derivatives), it is inserted into the skin, where it absorbs tissue fluid and dissolves, forming a porous water microchannel for drug delivery. | Removable to avoid polymer residue; Suitable for water-soluble drugs, with a gentle drug release process. | Low mechanical strength may affect the puncture effect; similar to dissolvable MNs, stability is the main challenge. | [40,41,42] |
2. Application of MNs for Skin Disease Therapy
2.1. MNs to Treat and Rogenetic Alopecia
2.2. MNs to Treat Acne
2.3. MNs to Treat Scar
2.4. MNs to Treat Melanoma
2.5. MNs to Treat Psoriasis
2.6. MNs to Treat Atopic Dermatitis
2.7. MNs to Treat Vitiligo
3. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MNs | Microneedles | SC | Stratum Corneum |
| TDDS | Transdermal Drug Delivery System | AGA | Androgenetic Alopecia |
| FIN-NP | Finasteride nanocrystals | CS | Cationic chitosan |
| CeNZ | Cerium dioxide nano-enzyme | CAT | Catalase |
| SOD | Superoxide Dismutase | SeNPs | Selenium nanozymes |
| HEVs | Hypoxia-pretreated extracellular vesicles | APS | Astragalus polysaccharide |
| HIF1α | Hypoxia-inducible factor 1α | VEGF | Vascular Endothelial Growth Factor |
| PQFN | Puerarin/quercetin iron-chelating nanoparticles | aPDT | Antimicrobial Photodynamic Therapy |
| ICG | Indocyanine Green | ZIF-8 | Zeolitic Imidazolate Framework-8 |
| 2-MeIM | 2-Methylimidazole | PDA NP | Polydopamine Nanoparticles |
| EO | Eugenol | BSP | Bletilla striata polysaccharide |
| EGCG | Epigallocatechin gallate | PVA | Polyvinyl Alcohol |
| DE | Diatomaceous Earth | ZnTCPP@ZnO | Zinc tetra(4-carboxyphenyl)porphyrin@Zinc Oxide |
| CS | Chitosan | CUR | Curcumin |
| TSIIA | Tanshinone IIA | HSF | Hypertrophic Scar Fibroblasts |
| CDF | Cyclodextrin metal–organic Framework | QUE | Quercetin |
| AuNCs | Gold Nanoclusters | DHA | Dihydroartemisinin |
| CB[n]s | Cucurbiturils | CAD NPs | Cucurbituril-Assembled Drug Nanoparticles |
| OUSMNs | Orthogonally Upconverting Supramolecular MNs | UCNPs | Upconversion Nanoparticles |
| RB | Rose Bengal | KFs | Keloid Fibroblasts |
| PDT | Photodynamic Therapy | PLGA | Poly(lactic-co-glycolic acid) |
| ZnO NPs | Zinc Oxide Nanoparticles | SDA | Dopamine-functionalized Sericin |
| HA-FPBA | Hyaluronic Acid-Fluorophenylboronic Acid | ACT | Adoptive T Cell Therapy |
| TCR | T Cell Receptor | CAR | Chimeric Antigen Receptor |
| TME | Tumor Microenvironment | Treg | Regulatory T cells |
| CCL22 | Chemokine (C-C motif) ligand 22 | CLG | Collagenase |
| Pc | Photosensitizer | TRA | Trametinib |
| HA-Tyr | Hyaluronic Acid-Tyramine | PpIX | Protoporphyrin IX |
| PA-Fe3+ | Phenanthroline-Fe3+ complex | PATC | Thermosensitive polymer with AIE property |
| AIE | Aggregation-Induced Emission | SERS | Surface-Enhanced Raman Scattering |
| TYR | Tyrosinase | Deu | Deucravacitinib |
| Cal | Calcipotriol | cfDNA | Cell-free DNA |
| BGC | Biguanide-modified Chitosan | Ber | Berberine |
| LPs | Liposomes | PEGDA | Poly(ethylene glycol) diacrylate |
| IL-17 mAbs | Interleukin-17 monoclonal antibodies | MXene | Two-dimensional niobium carbide |
| MTX | Methotrexate | Ph | Phellin |
| PLA | Polylactic Acid | AD | Atopic Dermatitis |
| CET | Cetirizine | PB NP | Prussian Blue Nanoparticles |
| Bs | Bacillus subtilis | TA | Triamcinolone Acetonide |
| BPQDs | Black Phosphorus Quantum Dots | PDA | Polydopamine |
| JAKi | Janus Kinase inhibitor | HMGB1 | High Mobility Group Box 1 |
| DexMA | Dextran Methacrylate | HGSM | Host–Guest Supramolecular |
| α-MSH | Alpha-Melanocyte Stimulating Hormone | MC1R | Melanocortin 1 Receptor |
| SFMA | Silk Fibroin Methacrylate | AC | Adenylate Cyclase |
| cAMP | Cyclic Adenosine Monophosphate | PKA | Protein Kinase A |
| NF-κB | Nuclear Factor kappa B |
References
- Bajza, Á.; Kocsis, D.; Berezvai, O.; Laki, A.J.; Lukács, B.; Imre, T.; Iván, K.; Szabó, P.; Erdő, F. Verification of P-Glycoprotein Function at the Dermal Barrier in Diffusion Cells and Dynamic “Skin-On-A-Chip” Microfluidic Device. Pharmaceutics 2020, 12, 804. [Google Scholar] [CrossRef]
- Czekalla, C.; Schönborn, K.H.; Lademann, J.; Meinke, M.C. Noninvasive Determination of Epidermal and Stratum Corneum Thickness in vivo Using Two-Photon Microscopy and Optical Coherence Tomography: Impact of Body Area, Age, and Gender. Ski. Pharmacol. Physiol. 2019, 32, 142–150. [Google Scholar] [CrossRef]
- Chen, Z.; Lv, Y.; Qi, J.; Zhu, Q.; Lu, Y.; Wu, W. Overcoming or circumventing the stratum corneum barrier for efficient transcutaneous immunization. Drug Discov. Today 2018, 23, 181–186. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Danso, M.O.; Berkers, T.; Mieremet, A.; Hausil, F.; Bouwstra, J.A. An ex vivo human skin model for studying skin barrier repair. Exp. Dermatol. 2014, 24, 48–54. [Google Scholar] [CrossRef]
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209. [Google Scholar] [CrossRef]
- Boyaci, A.; Tutoglu, A.; Boyaci, N.; Aridici, R.; Koca, I. Comparison of the efficacy of ketoprofen phonophoresis, ultrasound, and short-wave diathermy in knee osteoarthritis. Rheumatol. Int. 2013, 33, 2811–2818. [Google Scholar] [CrossRef] [PubMed]
- Bozorg, B.D.; Bhattaccharjee, S.A.; Somayaji, M.R.; Banga, A.K. Topical and transdermal delivery with diseased human skin: Passive and iontophoretic delivery of hydrocortisone into psoriatic and eczematous skin. Drug Deliv. Transl. Res. 2022, 12, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Sriram, D.; Yogeeswari, P.; Srichakravarthy, N.; Bal, T.R. Synthesis of stavudine amino acid ester prodrugs with broad-spectrum chemotherapeutic properties for the effective treatment of HIV/AIDS. Bioorg. Med. Chem. Lett. 2004, 14, 1085–1087. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Olatunji, O.; Das, D.B.; Garland, M.J.; Belaid, L.; Donnelly, R.F. Influence of Array Interspacing on the Force Required for Successful Microneedle Skin Penetration: Theoretical and Practical Approaches. J. Pharm. Sci. 2013, 102, 1209–1221. [Google Scholar] [CrossRef]
- Cheung, K.; Han, T.; Das, D.B. Effect of Force of Microneedle Insertion on the Permeability of Insulin in Skin. J. Diabetes Sci. Technol. 2014, 8, 444–452. [Google Scholar] [CrossRef]
- Kaur, M.; Ita, K.B.; Popova, I.E.; Parikh, S.J.; Bair, D.A. Microneedle-assisted delivery of verapamil hydrochloride and amlodipine besylate. Eur. J. Pharm. Biopharm. 2014, 86, 284–291. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Yang, G.; Zhang, S.; Zhao, X.; Gao, Y. Dissolving Microneedles Loaded with Etonogestrel Microcrystal Particles for Intradermal Sustained Delivery. J. Pharm. Sci. 2018, 107, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Hathout, R.M.; Nasr, M.; Nejadnik, M.R.; Tu, J.; Koning, R.I.; Koster, A.J.; Slütter, B.; Kros, A.; Jiskoot, W.; et al. Intradermal vaccination with hollow microneedles: A comparative study of various protein antigen and adjuvant encapsulated nanoparticles. J. Control. Release 2017, 266, 109–118. [Google Scholar] [CrossRef]
- Singh, V.; Kesharwani, P. Recent advances in microneedles-based drug delivery device in the diagnosis and treatment of cancer. J. Control. Release 2021, 338, 394–409. [Google Scholar] [CrossRef]
- Reyna, D.; Bejster, I.; Chadderdon, A.; Harteg, C.; Anjani, Q.K.; Bin Sabri, A.H.; Brown, A.N.; Drusano, G.L.; Westover, J.; Tarbet, E.B.; et al. A five-day treatment course of zanamivir for the flu with a single, self-administered, painless microneedle array patch: Revolutionizing delivery of poorly membrane-permeable therapeutics. Int. J. Pharm. 2023, 641, 123081. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Vora, L.K.; Kelly, S.A.; Li, L.; Larrañeta, E.; McCarthy, H.O.; Donnelly, R.F. Hydrogel-forming microarray patch mediated transdermal delivery of tetracycline hydrochloride. J. Control. Release 2023, 356, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Flohr, C.; Hay, R. Putting the burden of skin diseases on the global map. Br. J. Dermatol. 2021, 184, 189–190. [Google Scholar] [CrossRef]
- Goren, A.; Naccarato, T. Minoxidil in the treatment of androgenetic alopecia. Dermatol. Ther. 2018, 31, e12686. [Google Scholar] [CrossRef]
- Liu, R.H.; Xu, L.J.; McCarty, J.C.; Xiao, R.; Chen, J.X.; Lee, L.N. A Scoping Review on Complications in Modern Hair Transplantation: More than Just Splitting Hairs. Aesthetic Plast. Surg. 2025, 49, 585–595. [Google Scholar] [CrossRef]
- Iordanidis, T.N.; Spyrou, A.; Roudi, S.; Swartling, F.J.; Stemme, G.; El Andaloussi, S.; Roxhed, N. Rolling Ultrasharp Microneedle Spheres Enable Topical Delivery of Biologics Through the Skin. Adv. Healthc. Mater. 2025, e00990. [Google Scholar] [CrossRef]
- Sahu, N.; Jain, P.; Sahu, D.; Kaur, K.; Nagori, K.; Ajazuddin. Recent trends in the treatment of vitiligo using novel drug delivery system. Int. J. Pharm. 2025, 670, 125106. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, M.; Wang, Y.; Ling, G.; Zhang, P. Advances in MOFs-based microneedles in the treatment of skin diseases. Appl. Mater. Today 2025, 42, 102616. [Google Scholar] [CrossRef]
- Wu, D.; Wu, X.; Luan, Q.; Tang, Q.; Fan, L.; Shou, X.; Gao, X.; Qian, X.; Zhao, Y. Dynamic hydrogel-integrated microneedle patch with extracellular vesicles encapsulation for wound healing. Chem. Eng. J. 2024, 493, 152252. [Google Scholar] [CrossRef]
- Abdi, P.; Awad, C.; Anthony, M.R.; Farkouh, C.; Kenny, B.; Maibach, H.I.; Ogunyemi, B. Efficacy and safety of combinational therapy using topical minoxidil and microneedling for the treatment of androgenetic alopecia: A systematic review and meta-analysis. Arch. Dermatol. Res. 2023, 315, 2775–2785. [Google Scholar] [CrossRef]
- Yuan, A.; Xia, F.; Bian, Q.; Wu, H.; Gu, Y.; Wang, T.; Wang, R.; Huang, L.; Huang, Q.; Rao, Y.; et al. Ceria Nanozyme-Integrated Microneedles Reshape the Perifollicular Microenvironment for Androgenetic Alopecia Treatment. ACS Nano 2021, 15, 13759–13769. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Yang, J.; Liu, Z.; Guo, T.; Feng, N. Recent progress of microneedles in transdermal immunotherapy: A review. Int. J. Pharm. 2024, 662, 124481. [Google Scholar] [CrossRef] [PubMed]
- Yeo, D.C.; Balmayor, E.R.; Schantz, J.-T.; Xu, C. Microneedle physical contact as a therapeutic for abnormal scars. Eur. J. Med. Res. 2017, 22, 28. [Google Scholar] [CrossRef]
- Rajabi, M.; Roxhed, N.; Shafagh, R.Z.; Haraldson, T.; Fischer, A.C.; van der Wijngaart, W.; Stemme, G.; Niklaus, F. Flexible and Stretchable Microneedle Patches with Integrated Rigid Stainless Steel Microneedles for Transdermal Biointerfacing. PLoS ONE 2016, 11, e0166330. [Google Scholar] [CrossRef]
- Narayan, R.J. Transdermal Delivery of Insulin via Microneedles. J. Biomed. Nanotechnol. 2014, 10, 2244–2260. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Morrow, D.I.J.; McCrudden, M.T.C.; Alkilani, A.Z.; Vicente-Pérez, E.M.; O’Mahony, C.; González-Vázquez, P.; McCarron, P.A.; Woolfson, A.D. Hydrogel-Forming and Dissolving Microneedles for Enhanced Delivery of Photosensitizers and Precursors. Photochem. Photobiol. 2013, 90, 641–647. [Google Scholar] [CrossRef]
- Tuan-Mahmood, T.-M.; McCrudden, M.T.C.; Torrisi, B.M.; McAlister, E.; Garland, M.J.; Singh, T.R.R.; Donnelly, R.F. Microneedles for intradermal and transdermal drug delivery. Eur. J. Pharm. Sci. 2013, 50, 623–637. [Google Scholar] [CrossRef]
- Sharma, S.; Hatware, K.; Bhadane, P.; Sindhikar, S.; Mishra, D.K. Recent advances in microneedle composites for biomedical applications: Advanced drug delivery technologies. Mater. Sci. Eng. C 2019, 103, 109717. [Google Scholar] [CrossRef]
- Gill, H.S.; Prausnitz, M.R. Coated microneedles for transdermal delivery. J. Control. Release 2007, 117, 227–237. [Google Scholar] [CrossRef]
- Gupta, J.; Gill, H.S.; Andrews, S.N.; Prausnitz, M.R. Kinetics of skin resealing after insertion of microneedles in human subjects. J. Control. Release 2011, 154, 148–155. [Google Scholar] [CrossRef]
- Wang, P.M.; Cornwell, M.; Hill, J.; Prausnitz, M.R. Precise Microinjection into Skin Using Hollow Microneedles. J. Investig. Dermatol. 2006, 126, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, P.; Zhu, J.; Lan, J.; Li, Y.; Zhang, L.; Zhu, J.; Tao, J. Hyaluronic Acid-Based Dissolving Microneedle Patch Loaded with Methotrexate for Improved Treatment of Psoriasis. ACS Appl. Mater. Interfaces 2019, 11, 43588–43598. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Gao, N.; Zhou, Q.; Wang, Y.; Ling, G.; Zhang, P. Collagen-Based Dissolving Microneedles with Flexible Pedestals: A Transdermal Delivery System for Both Anti-Aging and Skin Diseases. Adv. Health Mater. 2023, 12, e2203295. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, Y.; Gui, S.; Wu, X.; Chen, L.; Cao, Y.; Yin, D.; Ma, P. Sinomenine hydrochloride-loaded dissolving microneedles enhanced its absorption in rabbits. Pharm. Dev. Technol. 2015, 21, 787–793. [Google Scholar] [CrossRef]
- Courtenay, A.J.; McAlister, E.; McCrudden, M.T.C.; Vora, L.; Steiner, L.; Levin, G.; Levy-Nissenbaum, E.; Shterman, N.; Kearney, M.-C.; McCarthy, H.O.; et al. Hydrogel-forming microneedle arrays as a therapeutic option for transdermal esketamine delivery. J. Control. Release 2020, 322, 177–186. [Google Scholar] [CrossRef]
- Paredes, A.J.; McKenna, P.E.; Ramöller, I.K.; Naser, Y.A.; Volpe-Zanutto, F.; Li, M.; Abbate, M.T.A.; Zhao, L.; Zhang, C.; Abu-Ershaid, J.M.; et al. Microarray Patches: Poking a Hole in the Challenges Faced When Delivering Poorly Soluble Drugs. Adv. Funct. Mater. 2020, 31, 2005792. [Google Scholar] [CrossRef]
- Zhao, J.; Xiang, H.; Liu, Y.; Miao, X. Positively charged chitosan-dissolving microneedles promote follicular accumulation of finasteride nanocrystals to treat androgenetic alopecia. Int. J. Biol. Macromol. 2025, 321, 146223. [Google Scholar] [CrossRef]
- Jing, S.; Liu, Y.; Wang, B.; Zhou, H.; Zhang, H.; Siwakoti, P.; Qu, X.; Ye, P.; He, Y.; Kumeria, T.; et al. Microneedle-mediated hypoxic extracellular vesicle-encapsulated selenium nanoparticles delivery to treat androgenetic alopecia. J. Control. Release 2025, 381, 113597. [Google Scholar] [CrossRef]
- Ding, Y.-W.; Li, Y.; Zhang, Z.-W.; Dao, J.-W.; Wei, D.-X. Hydrogel forming microneedles loaded with VEGF and Ritlecitinib/polyhydroxyalkanoates nanoparticles for mini-invasive androgenetic alopecia treatment. Bioact. Mater. 2024, 38, 95–108. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, T.; Wu, W.; Yang, B.; Lu, A.; Pan, K.; Xu, J.; Lu, C.; Quan, G.; Wu, C.; et al. Gas-propelled anti-hair follicle aging microneedle patch for the treatment of androgenetic alopecia. J. Control. Release 2025, 379, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Lin, Z.; Zhao, Y.; Zhou, Y.; Niu, B.; Shi, C.; Lu, C.; Wen, X.; Zhang, M.; Quan, G.; et al. Bioresponsive Nanoarchitectonics-Integrated Microneedles for Amplified Chemo-Photodynamic Therapy against Acne Vulgaris. ACS Appl. Mater. Interfaces 2021, 13, 48433–48448. [Google Scholar] [CrossRef]
- Wang, Q.; Gan, Z.; Wang, X.; Li, X.; Zhao, L.; Li, D.; Xu, Z.; Mu, C.; Ge, L.; Li, D. Dissolving Hyaluronic Acid-Based Microneedles to Transdermally Deliver Eugenol Combined with Photothermal Therapy for Acne Vulgaris Treatment. ACS Appl. Mater. Interfaces 2024, 16, 21595–21609. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, P.; Qiu, M.; Zhong, G.; Yang, Q.; Lei, P.; Gou, K.; Zeng, R.; Zhang, C.; Qu, Y. A novel natural polysaccharide dissolving microneedle capable of adsorbing pus to load EGCG for the treatment of acne vulgaris. Mater. Des. 2024, 238, 112639. [Google Scholar] [CrossRef]
- Xiang, Y.; Lu, J.; Mao, C.; Zhu, Y.; Wang, C.; Wu, J.; Liu, X.; Wu, S.; Kwan, K.Y.H.; Cheung, K.M.C.; et al. Ultrasound-triggered interfacial engineering-based microneedle for bacterial infection acne treatment. Sci. Adv. 2023, 9, eadf0854. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, H.; Kou, Y.; Chen, W.; He, K.; Wen, D.; Wen, L.; He, J.; Chen, G. Multifunctional nanocomposite-driven microneedles enabling bacteria capturing, detecting, and killing for precision treatment of acne with varying severities. Chem. Eng. J. 2025, 514, 163339. [Google Scholar] [CrossRef]
- Wu, T.; Hou, X.; Li, J.; Ruan, H.; Pei, L.; Guo, T.; Wang, Z.; Ci, T.; Ruan, S.; He, Y.; et al. Microneedle-Mediated Biomimetic Cyclodextrin Metal Organic Frameworks for Active Targeting and Treatment of Hypertrophic Scars. ACS Nano 2021, 15, 20087–20104. [Google Scholar] [CrossRef]
- Zhao, B.; Wei, M.; Zhou, X.; Liu, W.; Li, Q.; Xue, Y.; Tan, L.; Shang, L. Supramolecular Assembly-Enabled Transdermal Therapy of Hypertrophic Scarring Through Concurrent Ferroptosis-Apoptosis. Adv. Funct. Mater. 2025, 35, 2416011. [Google Scholar] [CrossRef]
- Lv, W.; Zhang, Y.; Wu, Y.; Chen, H. Orthogonal upconversion supramolecular microneedles promote endogenous ferroptosis in keloids. Theranostics 2025, 15, 6184–6202. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.; Liu, N.; Wang, J.; Guo, Q.; You, Y.; Mao, K.; Wang, Y.; Zhang, W.; Wu, T. Curcumin-loaded PLGA microparticles integrated with ZnO/GelMA hydrogel microneedles for infectious wound healing and reduction of hypertrophic scars. J. Nanobiotechnol. 2025, 23, 455. [Google Scholar] [CrossRef]
- Liu, H.; Qin, S.; Zhang, H.; Chen, Z.; Zhao, Y.; Liu, J.; Deng, Y.; Liu, M.; Chen, W.; Wang, Z.; et al. Silk Sericin-based ROS-Responsive Oxygen Generating Microneedle Platform Promotes Angiogenesis and Decreases Inflammation for Scarless Diabetic Wound Healing. Adv. Funct. Mater. 2024, 35, 2404461. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, H.; Sheng, T.; Wu, Y.; Chen, Y.; You, J.; Yang, Y.; Luo, B.; Zhao, S.; Zheng, Y.; et al. Grooved Microneedle Patch Augments Adoptive T Cell Therapy Against Solid Tumors via Diverting Regulatory T Cells. Adv. Mater. 2024, 36, e2401667. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Kang, Y.; Zhou, S.; Zhang, T.; Zheng, Y.; Jin, Q.; Zhong, W.; Xu, K. A Multifunctional Rocket-Like Microneedle System with Thrusters for Self-Promoted Deep Drug Penetration and Combination Treatment in Melanoma. Adv. Funct. Mater. 2024, 34, 2405696. [Google Scholar] [CrossRef]
- Huang, Y.; Lai, H.; Jiang, J.; Xu, X.; Zeng, Z.; Ren, L.; Liu, Q.; Chen, M.; Zhang, T.; Ding, X.; et al. pH-activatable oxidative stress amplifying dissolving microneedles for combined chemo-photodynamic therapy of melanoma. Asian J. Pharm. Sci. 2022, 17, 679–696. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, L.; Sha, T.; Lin, Y.; Zeng, R.; Xu, J.; Chen, S.; Cai, H.-H.; Zhang, J.; Zhou, H.; et al. In Situ Tyrosinase Monitoring by Wearable Microneedle Patch toward Clinical Melanoma Screening. ACS Nano 2023, 17, 20073–20086. [Google Scholar] [CrossRef]
- Wang, C.; Zeng, Y.; Chen, K.-F.; Lin, J.; Yuan, Q.; Jiang, X.; Wu, G.; Wang, F.; Jia, Y.-G.; Li, W. A self-monitoring microneedle patch for light-controlled synergistic treatment of melanoma. Bioact. Mater. 2023, 27, 58–71. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhao, Z.Q.; Sheng, Y.J.; Chen, K.J.; Chen, B.Z.; Guo, X.D.; Cui, Y. Dual-Action Psoriasis Therapy: Antiproliferative and Immunomodulatory Effects via Self-Locking Microneedles. Adv. Sci. 2024, 11, e2409359. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Zhang, Y.; Hu, L.; Chen, B.; Li, Y.; Guo, X.; Yu, B.; Xu, F.-J. Biguanide chitosan microneedles with cell-free DNA scavenging ability for psoriasis therapy. Bioact. Mater. 2024, 33, 497–505. [Google Scholar] [CrossRef]
- Shen, S.; Shen, W.; Wang, L.; Sun, B.; Zhang, Y.; Jia, R.; Wu, Y.; Chen, X.; Cao, K.; Fang, Y.; et al. Berberine hydrochloride-loaded liposomes-in-hydrogel microneedles achieve the efficient treatment for psoriasis. Mater. Today Bio 2025, 32, 101795. [Google Scholar] [CrossRef]
- Wu, D.; Shou, X.; Yu, Y.; Wang, X.; Chen, G.; Zhao, Y.; Sun, L. Biologics-Loaded Photothermally Dissolvable Hyaluronic Acid Microneedle Patch for Psoriasis Treatment. Adv. Funct. Mater. 2022, 32, 2205847. [Google Scholar] [CrossRef]
- Moawad, F.; Ruel, Y.; Rezaei, N.; Alsarraf, J.; Pichette, A.; Legault, J.; Pouliot, R.; Brambilla, D. Microneedles with Implantable Tip-Accumulated Therapeutics for the Long-Term Management of Psoriasis. Small 2024, 20, 2405927. [Google Scholar] [CrossRef]
- Zhang, W.; Lei, J.; Jiang, P.; Hao, T.; Yuan, Y.; Hu, H.; Li, W. Double-Layered Microneedle Patch Integrated with Multifunctional Nanoparticles and Live Bacteria for Long-Term Treatment of Atopic Dermatitis. Small 2025, 21, e2409121. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, Z.; Lu, S.; Zhao, P.; Wang, X.; Jia, F.; Chang, H. Microsphere-integrated hydrogel microneedle patch for sustained methotrexate delivery in the long-term management of atopic dermatitis. Eur. Polym. J. 2025, 229, 113877. [Google Scholar] [CrossRef]
- Song, L.; Fan, L.; Zhang, Q.; Huang, S.; Kong, B.; Xiao, J.; Xu, Y. Multifunctional Triamcinolone Acetonide Microneedle Patches for Atopic Dermatitis Treatment. Small Struct. 2024, 5, 2400302. [Google Scholar] [CrossRef]
- Jang, M.; Kang, B.M.; Yang, H.; Ohn, J.; Kwon, O.; Jung, H. High-Dose Steroid Dissolving Microneedle for Relieving Atopic Dermatitis. Adv. Health Mater. 2021, 10, 2001691. [Google Scholar] [CrossRef]
- Liang, S.; Liu, S.; Long, Z.; Li, X.; Han, Q.; Wei, X.; Xing, L.; Xue, X.; Chen, M. A self-powered hydration-monitoring and drug-delivery skin patch for closed-loop treatment of atopic dermatitis. Microsyst. Nanoeng. 2025, 11, 156. [Google Scholar] [CrossRef]
- Li, C.; Wang, W.; Shao, J.; Zhou, S.; Ji, X.; Xi, Y.; Xu, Q.; Huang, Y.; Wang, J.; Wan, Y.; et al. Biomimetic polydopamine loaded with janus kinase inhibitor for synergistic vitiligo therapy via hydrogel microneedles. J. Nanobiotechnol. 2025, 23, 63. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yu, Y.; Li, C.; Li, Q.; Chen, P.; Li, W.; Liu, W.; Li, Z.; Liu, Y.; Zhang, S.; et al. Tofacitinib combined with melanocyte protector α-MSH to treat vitiligo through dextran based hydrogel microneedles. Carbohydr. Polym. 2023, 305, 120549. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.; Wei, C.; Feng, L.; Guo, R. Silk Fibroin-Based Hydrogel Microneedles Deliver α-MSH to Promote Melanosome Delivery for Vitiligo Treatment. ACS Biomater. Sci. Eng. 2023, 9, 3368–3378. [Google Scholar] [CrossRef] [PubMed]
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic alopecia: A review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef]
- Chen, W.; Thiboutot, D.; Zouboulis, C.C. Cutaneous Androgen Metabolism: Basic Research and Clinical Perspectives. J. Investig. Dermatol. 2002, 119, 992–1007. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Degitz, K. Androgen action on human skin—From basic research to clinical significance. Exp. Dermatol. 2004, 13, 5–10. [Google Scholar] [CrossRef]
- Inui, S.; Itami, S. Androgen actions on the human hair follicle: Perspectives. Exp. Dermatol. 2012, 22, 168–171. [Google Scholar] [CrossRef]
- Gordon, K.; Gordon, K.; Tosti, A. Alopecia: Evaluation; treatment, Clinical. Cosmet. Investig. Dermatol. 2011, 101–106. [Google Scholar] [CrossRef]
- Lee, S.-I.; Nagayya-Sriraman, S.-K.; Shanmugam, S.; Baskaran, R.; Yong, C.-S.; Yoon, S.-K.; Choi, H.-G.; Yoo, B.-K. Effect of Charge Carrier Lipid on Skin Penetration, Retention, and Hair Growth of Topically Applied Finasteride-Containing Liposomes. Biomol. Ther. 2011, 19, 231–236. [Google Scholar] [CrossRef]
- Upton, J.H.; Hannen, R.F.; Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Oxidative Stress–Associated Senescence in Dermal Papilla Cells of Men with Androgenetic Alopecia. J. Investig. Dermatol. 2015, 135, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Huang, Y.-C.; Huang, K.-S.; Chan, C.-C.; Chiu, H.-Y.; Tsai, R.-Y.; Chan, J.-Y.; Lin, S.-J. Stress-induced premature senescence of dermal papilla cells compromises hair follicle epithelial-mesenchymal interaction. J. Dermatol. Sci. 2017, 86, 114–122. [Google Scholar] [CrossRef]
- Chu, S.-Y.; Chou, C.-H.; Huang, H.-D.; Yen, M.-H.; Hong, H.-C.; Chao, P.-H.; Wang, Y.-H.; Chen, P.-Y.; Nian, S.-X.; Chen, Y.-R.; et al. Mechanical stretch induces hair regeneration through the alternative activation of macrophages. Nat. Commun. 2019, 10, 1524. [Google Scholar] [CrossRef]
- Kim, Y.S.; Jeong, K.H.; Kim, J.E.; Woo, Y.J.; Kim, B.J.; Kang, H. Repeated Microneedle Stimulation Induces Enhanced Hair Growth in a Murine Model. Ann. Dermatol. 2016, 28, 586–592. [Google Scholar] [CrossRef]
- Alsalhi, W.A.; Alalola, A.S.; Randolph, M.J.; Gwillim, E.C.; Tosti, A. Novel drug delivery approaches for the management of hair loss. Expert Opin. Drug Deliv. 2020, 17, 287–295. [Google Scholar] [CrossRef]
- Heng, A.H.S.; Chew, F.T. Systematic review of the epidemiology of acne vulgaris. Sci. Rep. 2020, 10, 5754. [Google Scholar] [CrossRef]
- Ju, Q.; Tao, T.; Hu, T.; Karadağ, A.S.; Al-Khuzaei, S.; Chen, W. Sex hormones and acne. Clin. Dermatol. 2017, 35, 130–137. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Solomon, C.G.; Vulgaris, A. New England. J. Med. 2018, 379, 1343–1352. [Google Scholar]
- Das, S.; Reynolds, R.V. Recent Advances in Acne Pathogenesis: Implications for Therapy. Am. J. Clin. Dermatol. 2014, 15, 479–488. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, J.; Im, B.N.; Park, H.; Na, K. Combined photodynamic and antibiotic therapy for skin disorder via lipase-sensitive liposomes with enhanced antimicrobial performance. Biomaterials 2017, 141, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, J.; Jeong, S.; Im, B.N.; Kim, M.; Yang, S.; Na, K. Lipase-Sensitive Transfersomes Based on Photosensitizer/Polymerizable Lipid Conjugate for Selective Antimicrobial Photodynamic Therapy of Acne. Adv. Health Mater. 2016, 5, 3139–3147. [Google Scholar] [CrossRef]
- Wen, T.; Quan, G.; Niu, B.; Zhou, Y.; Zhao, Y.; Lu, C.; Pan, X.; Wu, C. Versatile Nanoscale Metal–Organic Frameworks (nMOFs): An Emerging 3D Nanoplatform for Drug Delivery and Therapeutic Applications. Small 2021, 17, 2005064. [Google Scholar] [CrossRef]
- Fang, C.; Cen, D.; Wang, Y.; Wu, Y.; Cai, X.; Li, X.; Han, G. ZnS@ZIF-8 core-shell nanoparticles incorporated with ICG and TPZ to enable H2S-amplified synergistic therapy. Theranostics 2020, 10, 7671–7682. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Wamsley, M.; Zhang, D.; Wang, H. Colloidal Polydopamine Beads: A Photothermally Active Support for Noble Metal Nanocatalysts. ACS Appl. Mater. Interfaces 2022, 14, 17560–17569. [Google Scholar] [CrossRef]
- An, J.; Hu, Y.-G.; Cheng, K.; Li, C.; Hou, X.-L.; Wang, G.-L.; Zhang, X.-S.; Liu, B.; Zhao, Y.-D.; Zhang, M.-Z. ROS-augmented and tumor-microenvironment responsive biodegradable nanoplatform for enhancing chemo-sonodynamic therapy. Biomaterials 2020, 234, 119761. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J.; Bruggeman, J.P.; Misra, A.; Borenstein, J.T.; Langer, R. Biocompatibility of biodegradable semiconducting melanin films for nerve tissue engineering. Biomaterials 2009, 30, 3050–3057. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Hoing, J.L.; Newman, M.; Simman, R. Role of Hyaluronic Acid Treatment in the Prevention of Keloid Scarring. J. Am. Coll. Clin. Wound Spec. 2012, 4, 23–31. [Google Scholar] [CrossRef]
- Ogawa, R. The Most Current Algorithms for the Treatment and Prevention of Hypertrophic Scars and Keloids: A 2020 Update of the Algorithms Published 10 Years Ago. Plast. Reconstr. Surg. 2021, 149, 79e–94e. [Google Scholar] [CrossRef]
- Liu, J.; Luo, T.; Xue, Y.; Mao, L.; Stang, P.J.; Wang, M. Hierarchical Self-assembly of Discrete Metal–Organic Cages into Supramolecular Nanoparticles for Intracellular Protein Delivery. Angew. Chem. Int. Ed. Engl. 2021, 60, 5429–5435. [Google Scholar] [CrossRef]
- Zhou, Y.; Niu, B.; Wu, B.; Luo, S.; Fu, J.; Zhao, Y.; Quan, G.; Pan, X.; Wu, C. A homogenous nanoporous pulmonary drug delivery system based on metal-organic frameworks with fine aerosolization performance and good compatibility. Acta Pharm. Sin. B 2020, 10, 2404–2416. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, D.; Yang, F.; Ye, C.; Chen, Z.; Chen, Y.; Yu, X.; Xie, J.; Dou, Y.; Chang, J. In Situ Self-Assembled Phytopolyphenol-Coordinated Intelligent Nanotherapeutics for Multipronged Management of Ferroptosis-Driven Alzheimer’s Disease. ACS Nano 2024, 18, 7890–7906. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Kesharwani, P.; Almalki, W.H.; Almujri, S.S.; Dai, L.; Chen, Z.-S.; Sahebkar, A.; Gao, F. Understanding the Novel Approach of Nanoferroptosis for Cancer Therapy. Nano-Micro Lett. 2024, 16, 188. [Google Scholar] [CrossRef]
- Li, X.; Shen, M.; Yang, J.; Liu, L.; Yang, Y. Pillararene-Based Stimuli-Responsive Supramolecular Delivery Systems for Cancer Therapy. Adv. Mater. 2024, 36, e2313317. [Google Scholar] [CrossRef]
- Zhao, J.-Y.; Cui, R.; Zhang, Z.-L.; Zhang, M.; Xie, Z.-X.; Pang, D.-W. Cytotoxicity of nucleus-targeting fluorescent gold nanoclusters. Nanoscale 2014, 6, 13126–13134. [Google Scholar] [CrossRef]
- Bhattacharya, S.R.; Bhattacharya, K.; Xavier, V.J.; Ziarati, A.; Picard, D.; Bürgi, T. The Atomically Precise Gold/Captopril Nanocluster Au25(Capt)18 Gains Anticancer Activity by Inhibiting Mitochondrial Oxidative Phosphorylation. ACS Appl. Mater. Interfaces 2022, 14, 29521–29536. [Google Scholar] [CrossRef]
- Song, T.; Yang, G.; Zhang, H.; Li, M.; Zhou, W.; Zheng, C.; You, F.; Wu, C.; Liu, Y.; Yang, H. Enhanced ferroptosis therapy with a “nano-destructor” by disrupting intracellular redox and iron homeostasis. Nano Today 2023, 51, 101896. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Kluger, H.M. Driver Mutations in Melanoma: Lessons Learned from Bench-to-Bedside Studies. Curr. Oncol. Rep. 2012, 14, 449–457. [Google Scholar] [CrossRef]
- Ullah, R.; Yin, Q.; Snell, A.H.; Wan, L. RAF-MEK-ERK pathway in cancer evolution and treatment. Semin. Cancer Biol. 2021, 85, 123–154. [Google Scholar] [CrossRef]
- Tangella, L.P.; Clark, M.E.; Gray, E.S. Resistance mechanisms to targeted therapy in BRAF-mutant melanoma—A mini review. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2021, 1865, 129736. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Hou, A.J.; Chen, L.C.; Chen, Y.Y. Navigating CAR-T cells through the solid-tumour microenvironment. Nat. Rev. Drug Discov. 2021, 20, 531–550. [Google Scholar] [CrossRef]
- Fu, R.; Li, H.; Li, R.; McGrath, K.; Dotti, G.; Gu, Z. Delivery Techniques for Enhancing CAR T Cell Therapy against Solid Tumors. Adv. Funct. Mater. 2021, 31, 2009489. [Google Scholar] [CrossRef]
- Eby, J.M.; Kang, H.-K.; Tully, S.T.; Bindeman, W.E.; Peiffer, D.S.; Chatterjee, S.; Mehrotra, S.; Le Poole, I.C. CCL22 to Activate Treg Migration and Suppress Depigmentation in Vitiligo. J. Investig. Dermatol. 2015, 135, 1574–1580. [Google Scholar] [CrossRef]
- Rapp, M.; Wintergerst, M.W.; Kunz, W.G.; Vetter, V.K.; Knott, M.M.; Lisowski, D.; Haubner, S.; Moder, S.; Thaler, R.; Eiber, S.; et al. CCL22 controls immunity by promoting regulatory T cell communication with dendritic cells in lymph nodes. J. Exp. Med. 2019, 216, 1170–1181. [Google Scholar] [CrossRef]
- Kohli, K.; Pillarisetty, V.G.; Kim, T.S. Key chemokines direct migration of immune cells in solid tumors. Cancer Gene Ther. 2021, 29, 10–21. [Google Scholar] [CrossRef]
- Abyaneh, H.S.; Regenold, M.; McKee, T.D.; Allen, C.; Gauthier, M.A. Towards extracellular matrix normalization for im-proved treatment of solid tumors. Theranostics 2020, 10, 1960–1980. [Google Scholar] [CrossRef]
- Xu, F.; Huang, X.; Wang, Y.; Zhou, S. A Size-Changeable Collagenase-Modified Nanoscavenger for Increasing Penetration and Retention of Nanomedicine in Deep Tumor Tissue. Adv. Mater. 2020, 32, e1906745. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.-H.; Schön, M.P. Psoriasis. Lancet 2015, 983–994. [Google Scholar] [CrossRef]
- Di Meglio, P.; Villanova, F.; Navarini, A.A.; Mylonas, A.; Tosi, I.; Nestle, F.O.; Conrad, C. Targeting CD8+ T cells prevents psoriasis development. J. Allergy Clin. Immunol. 2016, 138, 274–276.e6. [Google Scholar] [CrossRef] [PubMed]
- Res, P.C.M.; Piskin, G.; De Boer, O.J.; Van Der Loos, C.M.; Teeling, P.; Bos, J.D.; Teunissen, M.B.M. Overrepresentation of IL-17A and IL-22 Producing CD8 T Cells in Lesional Skin Suggests Their Involvement in the Pathogenesis of Psoriasis. PLoS ONE 2010, 5, e14108. [Google Scholar] [CrossRef]
- Casciano, F.; Diani, M.; Altomare, A.; Granucci, F.; Secchiero, P.; Banfi, G.; Reali, E. CCR4+ Skin-Tropic Phenotype as a Feature of Central Memory CD8+ T Cells in Healthy Subjects and Psoriasis Patients. Front. Immunol. 2020, 11, 529. [Google Scholar] [CrossRef]
- Strober, B.; Thaçi, D.; Sofen, H.; Kircik, L.; Gordon, K.B.; Foley, P.; Rich, P.; Paul, C.; Bagel, J.; Colston, E.; et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J. Am. Acad. Dermatol. 2023, 88, 40–51. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Warren, R.B.; Zhong, Y.; Zhuo, J.; Cichewicz, A.; Kadambi, A.; Junqueira, D.; Westley, T.; Kisa, R.; Daamen, C.; et al. Short-, Mid-, and Long-Term Efficacy of Deucravacitinib Versus Biologics and Nonbiologics for Plaque Psoriasis: A Network Meta-Analysis. Dermatol. Ther. 2023, 13, 2839–2857. [Google Scholar] [CrossRef]
- Hoy, S.M. Deucravacitinib: First Approval. Drugs 2022, 82, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, L.; Wang, J.; Luo, X.; Wang, M.; Wang, C.; Chen, J.; Zhou, Y.; Yin, H.; Song, Y.; et al. Targeting STING in dendritic cells alleviates psoriatic inflammation by suppressing IL-17A production. Cell. Mol. Immunol. 2024, 21, 738–751. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Kuo, I.-H.; Carpenter-Mendini, A.; Yoshida, T.; McGirt, L.Y.; Ivanov, A.I.; Barnes, K.C.; Gallo, R.L.; Borkowski, A.W.; Yamasaki, K.; Leung, D.Y.; et al. Activation of Epidermal Toll-Like Receptor 2 Enhances Tight Junction Function: Implications for Atopic Dermatitis and Skin Barrier Repair. J. Investig. Dermatol. 2013, 133, 988–998. [Google Scholar] [CrossRef]
- Ito, Y.; Sasaki, T.; Li, Y.; Tanoue, T.; Sugiura, Y.; Skelly, A.N.; Suda, W.; Kawashima, Y.; Okahashi, N.; Watanabe, E.; et al. Staphylococcus cohnii is a potentially biotherapeutic skin commensal alleviating skin inflammation. Cell Rep. 2021, 35, 109052. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, S.; Li, C. Mechanisms of melanocyte death in vitiligo. Med. Res. Rev. 2020, 41, 1138–1166. [Google Scholar] [CrossRef]
- Cui, T.; Zhang, W.; Li, S.; Chen, X.; Chang, Y.; Yi, X.; Kang, P.; Yang, Y.; Chen, J.; Liu, L.; et al. Oxidative Stress–Induced HMGB1 Release from Melanocytes: A Paracrine Mechanism Underlying the Cutaneous Inflammation in Vitiligo. J. Investig. Dermatol. 2019, 139, 2174–2184.e4. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, W.; Niu, L.; Zhou, Y.; Wang, Z.; Chen, J.; Chen, J.; Ma, J.; Zhang, J.; Jiang, Z.; et al. 3D-hUMSCs Exosomes Ameliorate Vitiligo by Simultaneously Potentiating Treg Cells-Mediated Immunosuppression and Suppressing Oxidative Stress-Induced Melanocyte Damage. Adv. Sci. 2024, 11, e2404064. [Google Scholar] [CrossRef]
- Akl, J.; Lee, S.; Ju, H.J.; Parisi, R.; Kim, J.Y.; Jeon, J.J.; Heo, Y.-W.; Eleftheriadou, V.; Hamzavi, I.; Griffiths, C.E.M.; et al. Estimating the burden of vitiligo: A systematic review and modelling study. Lancet Public Health 2024, 9, e386–e396. [Google Scholar] [CrossRef]
- Bastonini, E.; Kovacs, D.; Briganti, S.; Ottaviani, M.; D’ARino, A.; Migliano, E.; Pacifico, A.; Iacovelli, P.; Picardo, M. Effects of pioglitazone on the differentiation and inflammation in vitiligo keratinocytes. J. Eur. Acad. Dermatol. Venereol. 2024, 38, E573–E575. [Google Scholar] [CrossRef]
- Mukhatayev, Z.; Le Poole, I.C. Vitiligo: Advances in pathophysiology research and treatment development. Trends Mol. Med. 2024, 30, 844–862. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Hu, Z.; Yue, X.; Proetto, M.T.; Jones, Y.; Gianneschi, N.C. Mimicking Melanosomes: Polydopamine Nanoparticles as Artificial Microparasols. ACS Cent. Sci. 2017, 3, 564–569. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Jung, S.-H. Recent development of signaling pathways inhibitors of melanogenesis. Cell. Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zhou, F.; Liu, L.; Zhu, G.; Li, Q.; Li, C.; Gao, T. Vitiligo: How do oxidative stress-induced autoantigens trigger autoimmunity? J. Dermatol. Sci. 2016, 81, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Dinparastisaleh, R.; Mirsaeidi, M. Antifibrotic and Anti-Inflammatory Actions of α-Melanocytic Hormone: New Roles for an Old Player. Pharmaceuticals 2021, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Menon, I.; Bagwe, P.; Gomes, K.B.; Bajaj, L.; Gala, R.; Uddin, M.N.; D’souza, M.J.; Zughaier, S.M. Microneedles: A New Generation Vaccine Delivery System. Micromachines 2021, 12, 435. [Google Scholar] [CrossRef]
- Priya, S.; Tomar, Y.; Desai, V.M.; Singhvi, G. Enhanced skin drug delivery using dissolving microneedles: A potential approach for the management of skin disorders. Expert Opin. Drug Deliv. 2023, 20, 721–738. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, H.; Li, W. Microneedle-Mediated Transdermal Delivery of Drug-Carrying Nanoparticles. Front. Bioeng. Biotechnol. 2022, 10, 840395. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, H.; Sun, X.; Wang, Y.; Xu, F.; Xia, W.; Chen, L.; Li, M.; Yang, T.; Qiao, Y.; et al. From mechanism to ap-plications: Advanced microneedles for clinical medicine. Bioact. Mater. 2025, 51, 1–45. [Google Scholar]
- Zhang, X.; Wang, Y.; Chi, J.; Zhao, Y. Smart Microneedles for Therapy and Diagnosis. Research 2020, 2020, 7462915. [Google Scholar] [CrossRef]
- Ashraf, G.; Ahmed, K.; Aziz, A.; Asif, M.; Kong, J.; Fang, X. Microneedle wearables in advanced microsystems: Unlocking next-generation biosensing with AI. TrAC Trends Anal. Chem. 2025, 187, 118208. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, Q.; Wang, Q.; Qiu, H.; Li, H.; Xu, T. Fully integrated microneedle biosensor array for wearable multiplexed fitness biomarkers monitoring. Biosens. Bioelectron. 2024, 265, 116697. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Wang, H.; Ullah, I.; Xie, W.; Lin, T.; Tan, Q.; Pan, X.; Yuan, Y. A Wireless Operated Flexible Bioelectronic Microneedle Patch for Actively Controlled Transdermal Drug Delivery. Adv. Mater. 2025, 37, e2417136. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Lyu, L.; Xie, Y.; Yan, S.; Zhao, Y.; Chen, W.; Liu, Y.-N.; Zhao, Y. Active Chemical Messenger-Driven Immune Activation via Electrochemical Patch for In-Situ Tumor Vaccination. ACS Nano 2025, 19, 22424–22441. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Flynn, C.D.; Geraili, A.; Mirzaie, S.; Esmaeili, F.; Zargartalebi, H.; Ahmed, S.; Juska, V.B.; Das, J.; Abdrabou, A.; et al. Continuous insulin monitoring using an antibody-protecting zwitterionic microneedle patch. Nat. Biomed. Eng. 2025, 1–13. [Google Scholar] [CrossRef]
- Khairnar, P.; Phatale, V.; Shukla, S.; Tijani, A.O.; Hedaoo, A.; Strauss, J.; Verana, G.; Vambhurkar, G.; Puri, A.; Srivastava, S. Nanocarrier-Integrated Microneedles: Divulging the Potential of Novel Frontiers for Fostering the Management of Skin Ailments. Mol. Pharm. 2024, 21, 2118–2147. [Google Scholar] [CrossRef] [PubMed]
- Olowe, M.; Parupelli, S.K.; Desai, S. A Review of 3D-Printing of Microneedles. Pharmaceutics 2022, 14, 2693. [Google Scholar] [CrossRef]
- Avcil, M.; Çelik, A. Microneedles in Drug Delivery: Progress and Challenges. Micromachines 2021, 12, 1321. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, H.; Hu, T.; Xu, C.; Jiang, L.; Zhang, Y.S.; Xie, M. Recent advances of microneedles used towards stimuli-responsive drug delivery, disease theranostics, and bioinspired applications. Chem. Eng. J. 2021, 426, 130561. [Google Scholar] [CrossRef]
- Economidou, S.N.; Douroumis, D. 3D printing as a transformative tool for microneedle systems: Recent advances, manufacturing considerations and market potential. Adv. Drug Deliv. Rev. 2021, 173, 60–69. [Google Scholar] [CrossRef]
- Detamornrat, U.; McAlister, E.; Hutton, A.R.J.; Larrañeta, E.; Donnelly, R.F. The Role of 3D Printing Technology in Microengineering of Microneedles. Small 2022, 18, 2106392. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Huang, Y.; Wu, W.; Deng, X.; Liu, H.; Li, R.; Tao, J.; Li, X.; Liu, X.; et al. A 3D-Printed Self-Adhesive Bandage with Drug Release for Peripheral Nerve Repair. Adv. Sci. 2020, 7, 2002601. [Google Scholar] [CrossRef]
- Wang, C.; Rubakhin, S.S.; Enright, M.J.; Sweedler, J.V.; Nuzzo, R.G. 3D Particle-Free Printing of Biocompatible Conductive Hydrogel Platforms for Neuron Growth and Electrophysiological Recording. Adv. Funct. Mater. 2021, 31, 2010246. [Google Scholar] [CrossRef] [PubMed]
- Caudill, C.L.; Perry, J.L.; Tian, S.; Luft, J.C.; DeSimone, J.M. Spatially controlled coating of continuous liquid interface production microneedles for transdermal protein delivery. J. Control. Release 2018, 284, 122–132. [Google Scholar] [CrossRef]
- Krieger, K.J.; Bertollo, N.; Dangol, M.; Sheridan, J.T.; Lowery, M.M.; O’cEarbhaill, E.D. Simple and customizable method for fabrication of high-aspect ratio microneedle molds using low-cost 3D printing. Microsyst. Nanoeng. 2019, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, L.; Jiang, X.; Li, L.; Wu, S.; Yuan, X.; Cheng, H.; Jiang, X.; Gou, M. 3D-printed microneedle arrays for drug delivery. J. Control. Release 2022, 350, 933–948. [Google Scholar] [CrossRef] [PubMed]

| Diseases | MNs’ Names | Drug-Loading Agents and Their Corresponding Functions | References |
|---|---|---|---|
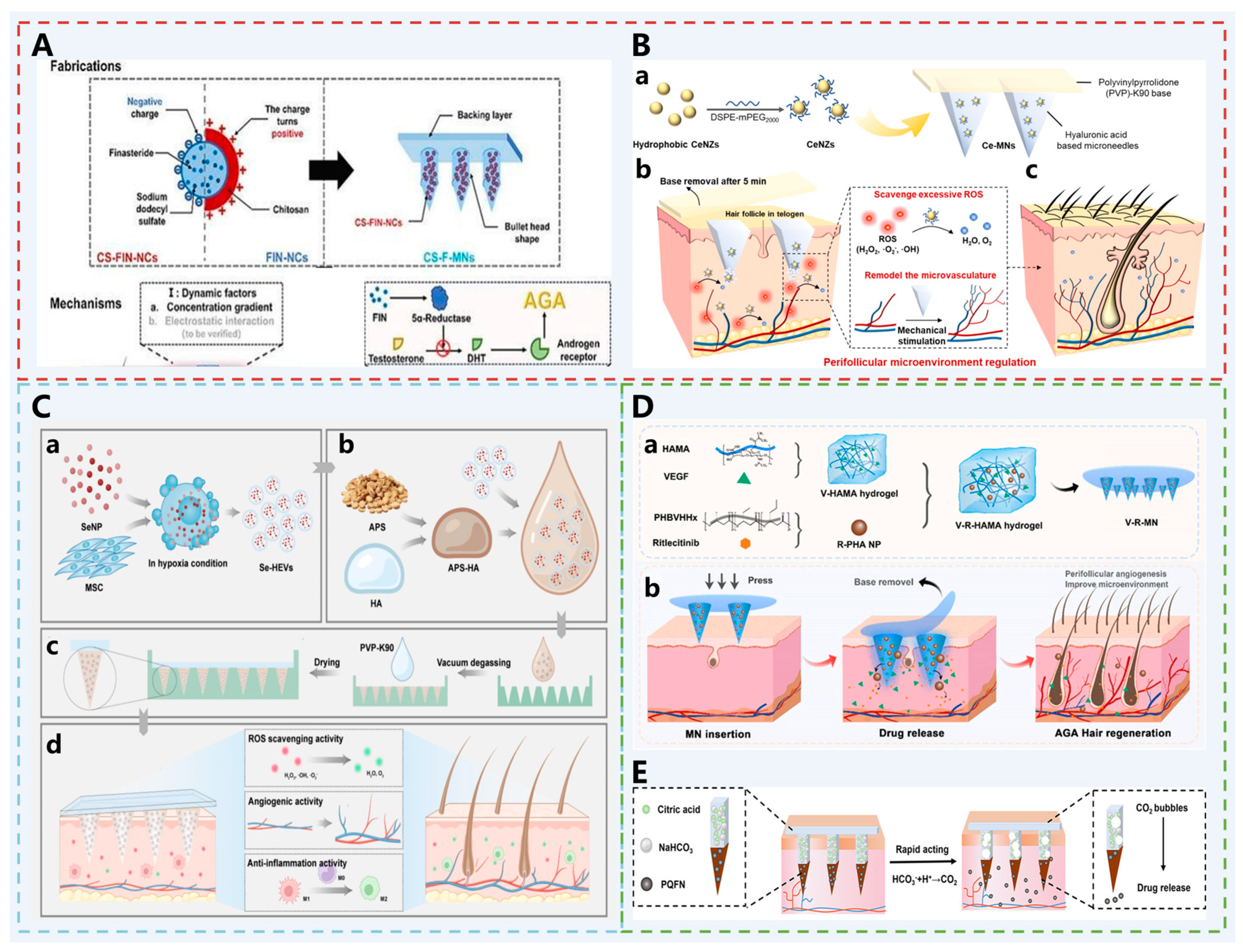
| AGA | CS-F-MN | FIN-NCs: Serves as the main drug to inhibit 5α-reductase and reduce follicle miniaturization. CS: Coats the nanocrystals to enhance positive charge for improved follicular targeting and accumulation. | [43] |
| CeMN | Possesses CAT and SOD activities, alleviates oxidative stress in the pathological microenvironment of AGA, protects dermal papilla cells. | [27] | |
| Se-HEVs-AMN | SeNPs: Antioxidant and anti-androgenic; HEVs: Hypoxia pretreatment upregulates HIF1α, promotes VEGF transcription and angiogenesis; APS: Activates the Nrf2/HO-1 pathway, inhibits inflammation and induces M2 macrophage polarization, synergistically promoting hair growth. | [44] | |
| V-R-MN | Ritlecitinib: inhibits the attack of hair follicle cells by CD8+ T cells and natural killer cells, and regulates the perifollicular immune microenvironment; VEGF: Improves the perifollicular vascular microenvironment, and provides nutrients for hair follicles. | [45] | |
| PQFN MN | PQFN: Scavenges ROS, promotes angiogenesis, and reverses DHT-induced senescence of dermal papilla cells; NaHCO3 and Citric Acid: React with skin interstitial fluid to generate CO2, driving PQFN into the deeper dermis. | [46] | |
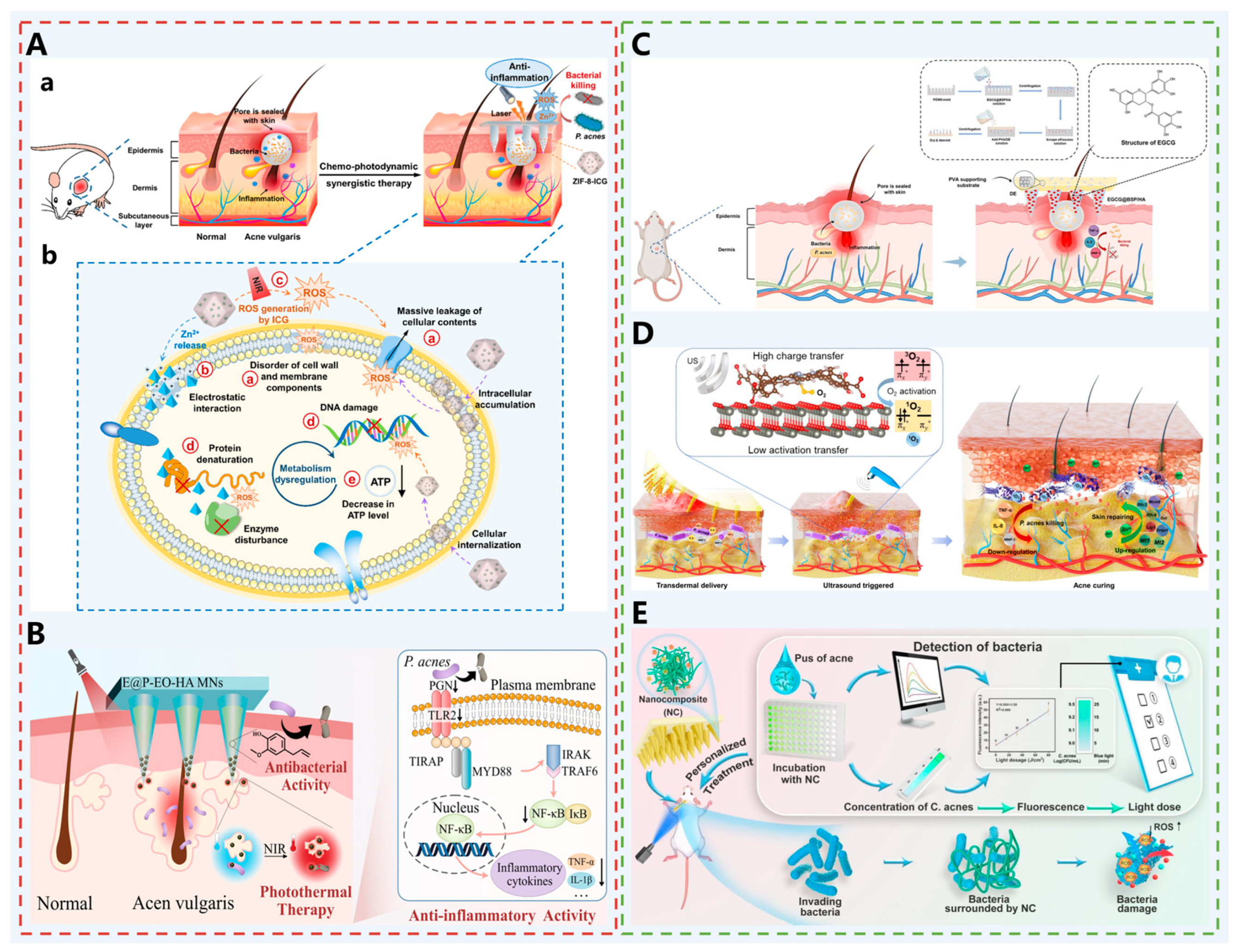
| Acne | ZIF-8-ICG @ MNs | ICG: As a photosensitizer, generates ROS under NIR laser activation, inhibits P. acnes proliferation and reduces inflammatory factor expression; ZIF-8: A pH-responsive carrier that degrades in the acidic microenvironment of bacterial infection, releases Zn2+ to destroy bacterial cell membranes, while avoiding aggregation-induced quenching of ICG and improving its photostability. | [47] |
| E @ P-EO-HA MNs | EO: Antibacterial and anti-inflammatory effects, promoting the repair of P. acnes-infected skin; PDA NP: As a photothermal agent, rapidly generates heat under 808 nm NIR laser irradiation, destroys sebaceous glands and inhibits P. acnes proliferation. | [48] | |
| EGCG @ BSP/HA MNs | EGCG: Exerts broad-spectrum antibacterial, anti-inflammatory, and antioxidant effects; BSP and HA: Constitute the needle body, with anti-inflammatory, antioxidant, and wound-healing promoting functions; PVA and DE: Constitute the base, and the porous structure of DE absorbs acne pus, prevents bacterial reinfection, and provides a clean environment for drug action. | [49] | |
| ZnTCPP@ZnO MN | ZnTCPP@ZnO: Ultrasound-responsive nanocomposites that promote oxygen activation and generate singlet oxygen (1O2) under ultrasound, achieving a 99.73% clearance rate of P. acnes; Zn2+: Released from the composites, upregulates metallothionein and DNA replication-related genes, and promotes cell proliferation and skin repair. | [50] | |
| Multifunctional nanocomposites MNs | CS: Positively charged, efficiently captures P. acnes; CUR: Has fluorescent properties, enabling rapid visual detection of bacterial concentration; TSIIA: Exerts anti-inflammatory effects, and combined with PDT, matches personalized light doses according to fluorescence intensity to accurately eliminate bacteria and alleviate inflammation. | [51] | |
| Scar | BSP-MNs-QUE @ HSF/CDF | QUE: Regulates the Wnt/β-catenin and JAK2/STAT3 signaling pathways, and reduces the expression of type I and type III collagen in hypertrophic scars; CDF: A drug carrier with high drug loading capacity and good biocompatibility; BSP: Constitutes the MNs matrix, with anti-inflammatory, antioxidant, and wound-healing promoting functions. HSF Membrane: Enables QUE@HSF/CDF to specifically target HSF. | [52] |
| CAD NPs MNs | AuNCs: Promote ROS production, consume GSH, and induce cell apoptosis; DHA: Generates free radicals, triggers oxidative stress and induces iron deficiency; CB[n]s: Self-assembles with AuNCs and DHA into pH-responsive CAD NPs, mediating ferroptosis–apoptosis combined effects to inhibit excessive proliferation of scar fibroblasts. | [53] | |
| OUSMN | UCNPs: Surface-modified with HKN15 and RB, emit red light for real-time imaging to monitor the targeting process under 990 nm laser excitation, generate 1O2 under 808 nm laser excitation, degrade ferritin highly expressed in KFs, release iron ions to induce endogenous ferroptosis, and simultaneously synergize with PDT to inhibit the PI3K-AKT and mTOR pathways. | [54] | |
| MN-C/P-Z | CUR: Continuously inhibits the expression of α-SMA and type I collagen, prevents fibroblasts from differentiating into myofibroblasts, and reduces excessive collagen deposition; ZnO NPs: Constitute the MNs base, form a physical barrier, inhibit Staphylococcus aureus and Escherichia coli, prevent infection and create a moist healing environment; | [55] | |
| HFSVM | Verteporfin: A YAP inhibitor that inhibits the YAP signaling pathway, reduces the transformation of En-1-positive fibroblasts, and inhibits scar formation; SDA: Consumes ROS and decomposes to produce oxygen under high ROS conditions, alleviates tissue hypoxia, and activates the ERK1/2 and HO-1 pathways to promote angiogenesis; HA-FPBA: Crosslinks with SDA via borate ester bonds to form MNs, and the borate ester bonds dissociate under high glucose conditions to control the release of Verteporfin. | [56] | |
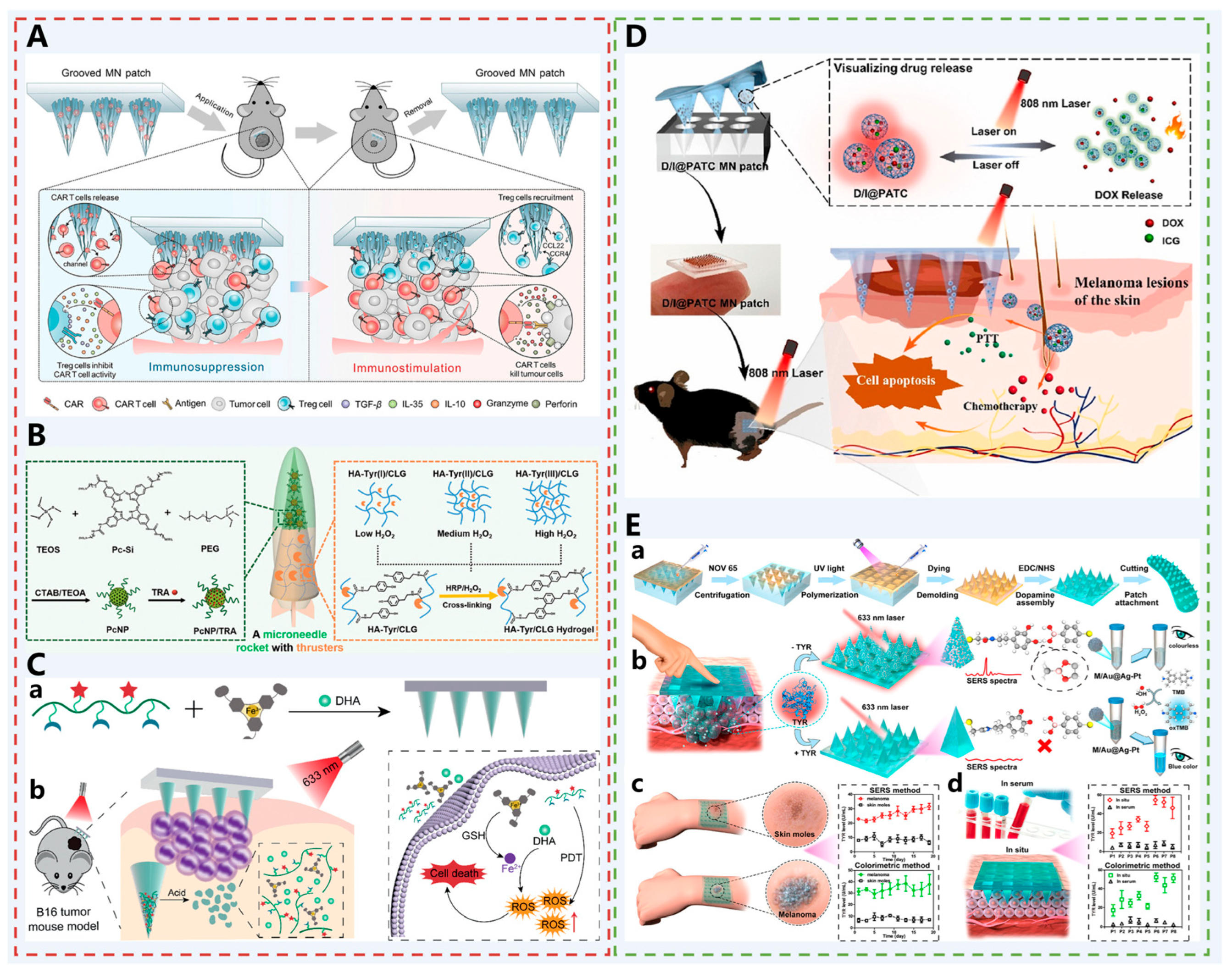
| Melanoma | Groove MN patch | CCL22: Modified on the surface of MNs, acts as a chemoattractant to divert Treg from the TME, increases the ratio of CD8+ T cells/Treg cells, and enhances the inhibitory effect of TCR T cells or CAR T cells on melanoma in ACT. | [57] |
| PcNP/TRA-HA-Tyr/CLG-MN | Pc: As a photosensitizer, exerts PDT effects under NIR irradiation to kill tumor cells; TRA: Targets the MEK pathway and inhibits melanoma growth and proliferation; CLG: degrades collagen in the tumor ECM, and improves drug diffusion and distribution in tumors. | [58] | |
| DHA @ HPFe-MN | DHA: Catalyzed by Fe2+ generated from the reduction of PA-Fe3+ in the acidic tumor microenvironment, produces a large amount of ROS to achieve oxygen-independent chemotherapy; HA-ADH-PpIX: A pH-responsive conjugate that degrades to release PpIX in acidic environments, exerting PDT effects under NIR irradiation to further amplify oxidative stress; PA-Fe3+: Serves as an “iron reservoir” to provide Fe3+ for participating in the catalytic reaction of DHA. | [59] | |
| D/I @ PATC MN | PATC: Has AIE property to realize visual monitoring of drug release process; also has light-controlled pulsed drug release capability, and a single administration can trigger multiple drug releases through external light irradiation, combining chemotherapy and photothermal therapy to enhance the therapeutic effect on melanoma. | [60] | |
| Au @ Ag-Pt NPs MN | Au@Ag-Pt NPs: Surface-modified with dopamine, combined with MNs via reversible borate bonds; in the presence of TYR, dopamine is oxidized to benzoquinone, inhibiting the combination of nanoparticles and MNs, resulting in a dual-mode response of SERS signal attenuation and colorimetric signal enhancement; used for early detection and monitoring of melanoma. | [61] | |
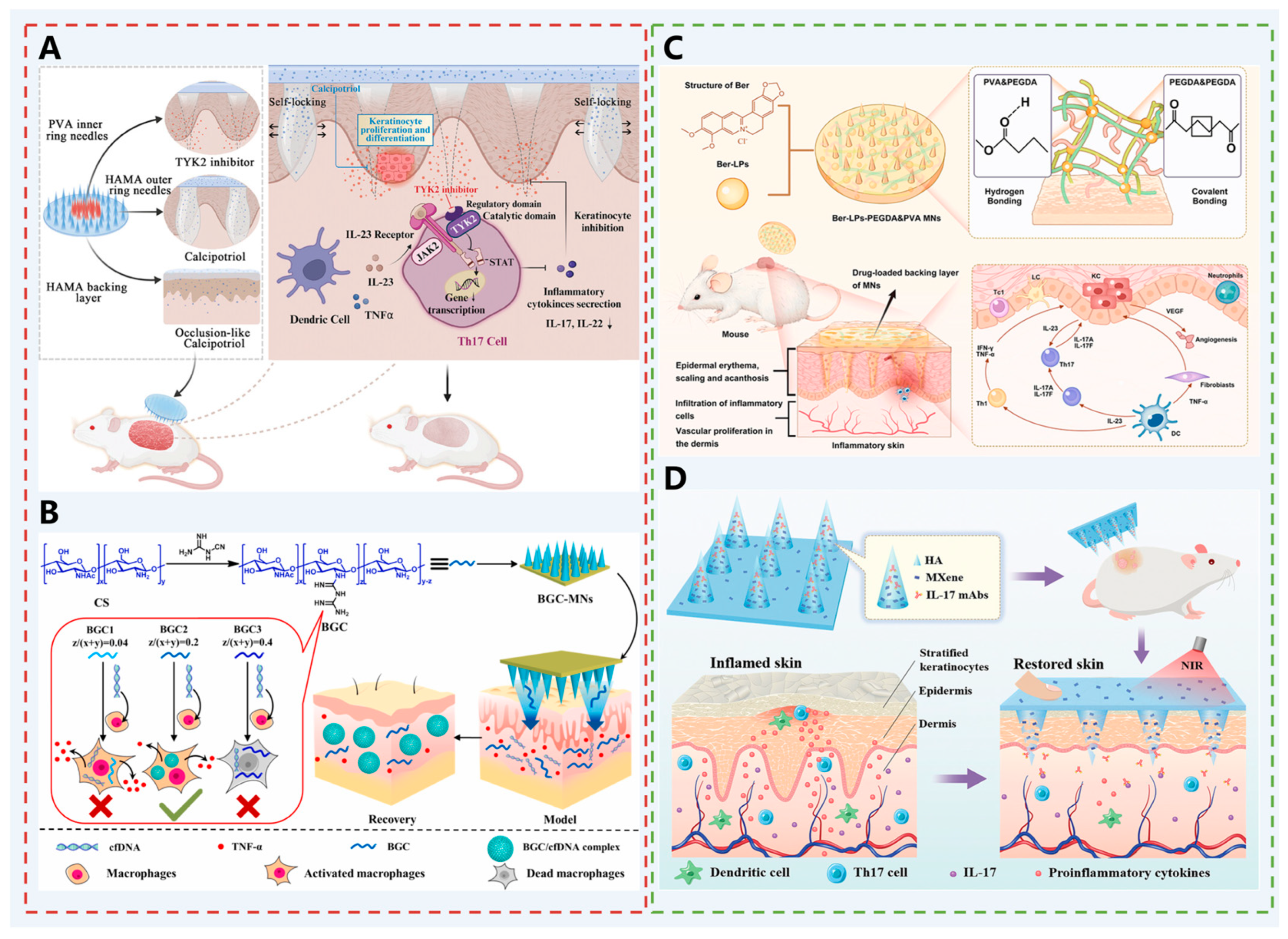
| Psoriasis | Deu @ Cal MN | Deu: A TYK2 mutation inhibitor that is rapidly released to regulate the local immune microenvironment, inhibit the spread of skin-derived systemic inflammation, and downregulate the psoriasis-related IL-23/IL-17 pathway; Cal: A vitamin D derivative that is slowly released to inhibit keratinocyte proliferation and regulate keratinocyte differentiation. | [62] |
| BGC-MN | BGC: A cationic polymer that binds cfDNA in the dermis through electrostatic interaction, preventing cfDNA from activating the cGAS-STING pathway, and inhibiting inflammatory response and abnormal proliferation of keratinocytes. | [63] | |
| Ber-LPs-PEGDA and PVA MN | Ber: Has anti-inflammatory and antioxidant effects, inhibits the expression of inflammatory factors, and downregulates angiogenesis-related proteins; LPs: Improve the encapsulation efficiency and stability of Ber, achieving efficient transdermal drug delivery and controlled release. | [64] | |
| Mxene-MN | IL-17 mAbs: Inhibits the IL-17 signaling pathway, inhibits epidermal thickening, and reduces inflammatory cell infiltration and inflammatory factors; Mxene: Has excellent photothermal conversion properties, generates heat under NIR irradiation to dissolve the HA matrix, triggering on-demand release of IL-17 mAbs. | [65] | |
| MTX and Ph-loaded PLGA-tipped MNs | MTX: Inhibits cell proliferation and immune response, a “gold standard” drug for psoriasis treatment; Ph: A natural flavonoid compound with anti-inflammatory, antioxidant, and antiproliferative effects and high safety. | [66] | |
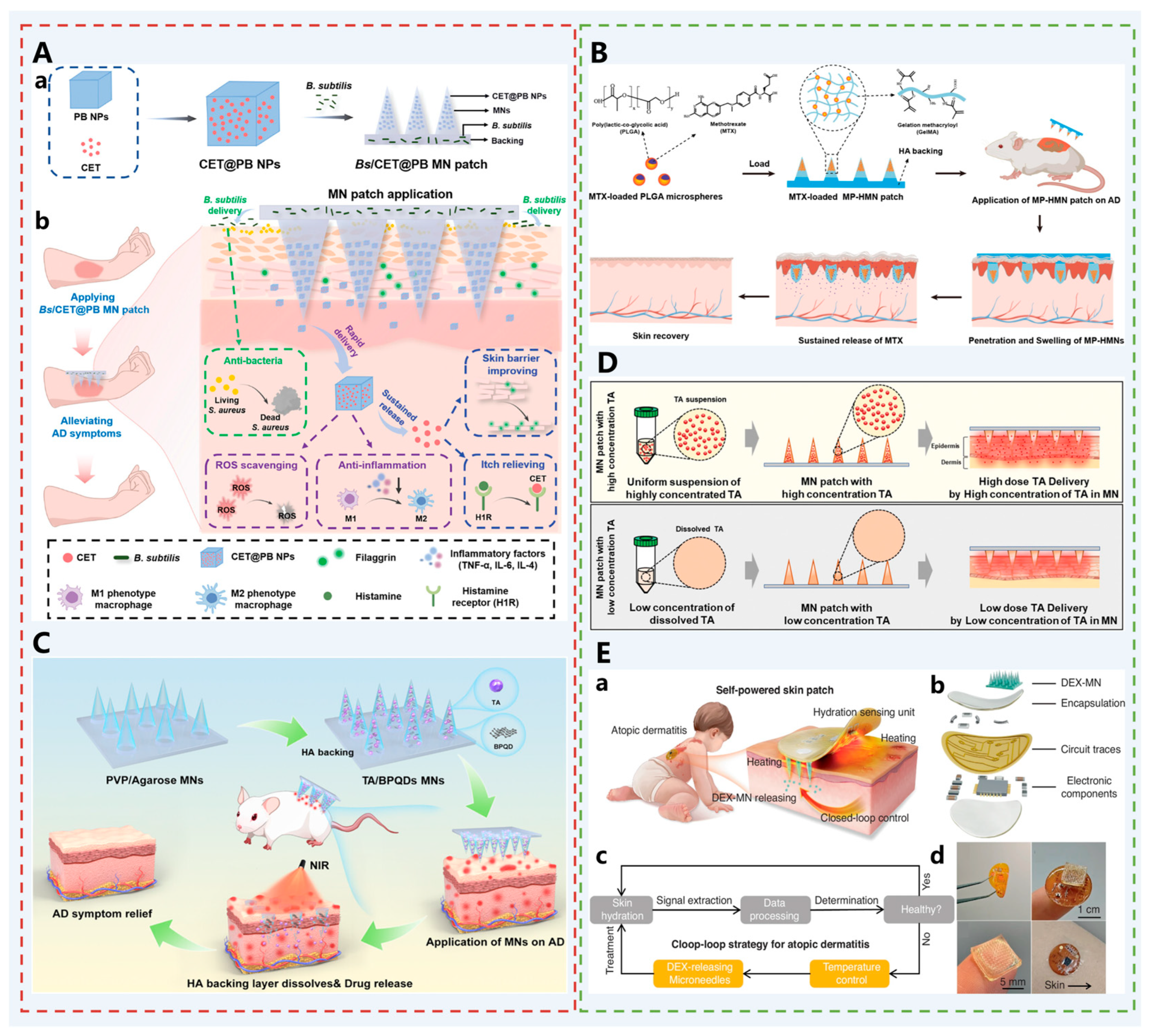
| Atopic dermatitis | Bs/CET @ BP MN patch | CET: An H1 receptor antagonist that blocks the binding of histamine to receptors, relieves itching, and reduces skin damage and inflammation caused by scratching; PB NP: Scavenges ROS, reduces the level of inflammatory factors, and improves the pathological microenvironment of oxidative stress and immune disorders in AD; Bs: Survives on the skin surface and competitively inhibits S. aureus, improving skin microbial imbalance. | [67] |
| TA/BPQDS MNs | MTX: Inhibits DNA synthesis and immune cell proliferation, exerting anti-inflammatory effects; PLGA: Encapsulates MTX into microspheres to achieve sustained drug release for up to 12 days, reducing administration frequency. | [68] | |
| MP-HMN | TA: A glucocorticoid that exerts anti-inflammatory effects for AD treatment; BPQDs: Generates heat under NIR irradiation, inducing agarose phase transition to control TA release; | [69] | |
| TA-DMN | TA: Anti-inflammatory effect for AD treatment; PVP and HA: Optimize polymer composition, and combine with ultrasonic treatment to reduce the TA particle size from 25.1 μm to 5.2 μm, enhance suspension stability, avoid particle sedimentation, and achieve encapsulation of 2 mg TA per MN patch (containing 108 needles) to reach the clinical therapeutic dose. | [70] | |
| Self-powered closed-loop skin patch | Dexamethasone Sodium Phosphate: An anti-inflammatory drug that, when the skin hydration signal is abnormal (lasting 65 s), the piezoelectric generator generates heat to melt the phase-change material, achieving on-demand release; Piezoelectric Generator: Collects mechanical energy from human daily activities and converts it into electrical energy to power the system; Hydration Sensing Unit: Monitors skin hydration status in real-time based on changes in skin thermal conductivity to trigger drug release; | [71] | |
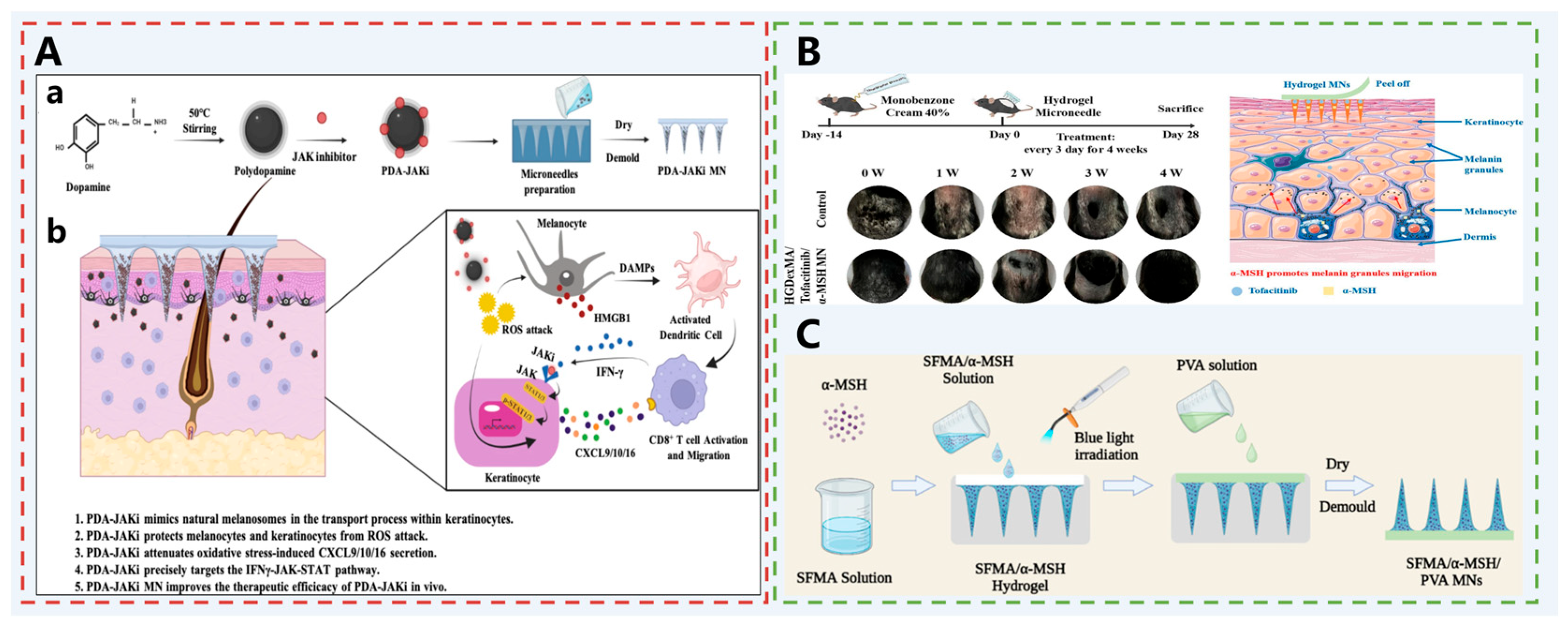
| Vitiligo | PDA-JAKi MN | PDA: Melanin-like nanoparticles that scavenge ROS, protect the mitochondrial integrity of melanocytes, inhibit the release of HMGB1, and prevent melanocyte apoptosis; JAKi: Inhibits the IFN-γ-JAK-STAT signaling pathway, reduces CD8+ T cell activation and infiltration, and decreases the expression of inflammatory factors and chemokines; | [72] |
| HGDexMA, hydrogel MNs | JAKi: Inhibits the JAK pathway, and reduces CD8+ T cell-mediated inflammation; α-MSH: Activates the MC1R to promote melanin synthesis and migration; DexMA: Provides biocompatibility and mechanical strength; HGSM: Enhances the hydrogel network density and mechanical properties. | [73] | |
| SFMA/α-MSH/PVA MNs | α-MSH: Activates the MC1R on melanocytes, upregulates intracellular cAMP, activates Tyr, promotes melanin synthesis and transport, and simultaneously inhibits NF-κB activation induced by inflammatory factors; SFMA: Constitutes the MN matrix with good biocompatibility and mechanical strength; PVA: Assists in forming MNs and enhances stability. | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Xu, S.; Li, S. Microneedle-Mediated Transdermal Drug Delivery for the Treatment of Multiple Skin Diseases. Pharmaceutics 2025, 17, 1281. https://doi.org/10.3390/pharmaceutics17101281
Zhou L, Xu S, Li S. Microneedle-Mediated Transdermal Drug Delivery for the Treatment of Multiple Skin Diseases. Pharmaceutics. 2025; 17(10):1281. https://doi.org/10.3390/pharmaceutics17101281
Chicago/Turabian StyleZhou, Lian, Shilong Xu, and Siwen Li. 2025. "Microneedle-Mediated Transdermal Drug Delivery for the Treatment of Multiple Skin Diseases" Pharmaceutics 17, no. 10: 1281. https://doi.org/10.3390/pharmaceutics17101281
APA StyleZhou, L., Xu, S., & Li, S. (2025). Microneedle-Mediated Transdermal Drug Delivery for the Treatment of Multiple Skin Diseases. Pharmaceutics, 17(10), 1281. https://doi.org/10.3390/pharmaceutics17101281





