In Vitro Comparative Study on Oppositely Charged Donepezil-Loaded Intranasal Liposomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Liposomes
2.3. Determination of Average Hydrodynamic Diameter, Polydispersity Index, and Surface Charge
2.4. Encapsulation Efficiency Determination
2.5. In Vitro Drug Release Studies
2.6. Droplet Size Distribution Study
2.7. In Vitro Drug Diffusion Studies
2.8. HPLC Method
2.9. Mucoadhesion Studies
2.10. Statistical Analysis
3. Results and Discussion
3.1. Selection of Liposomal Composition
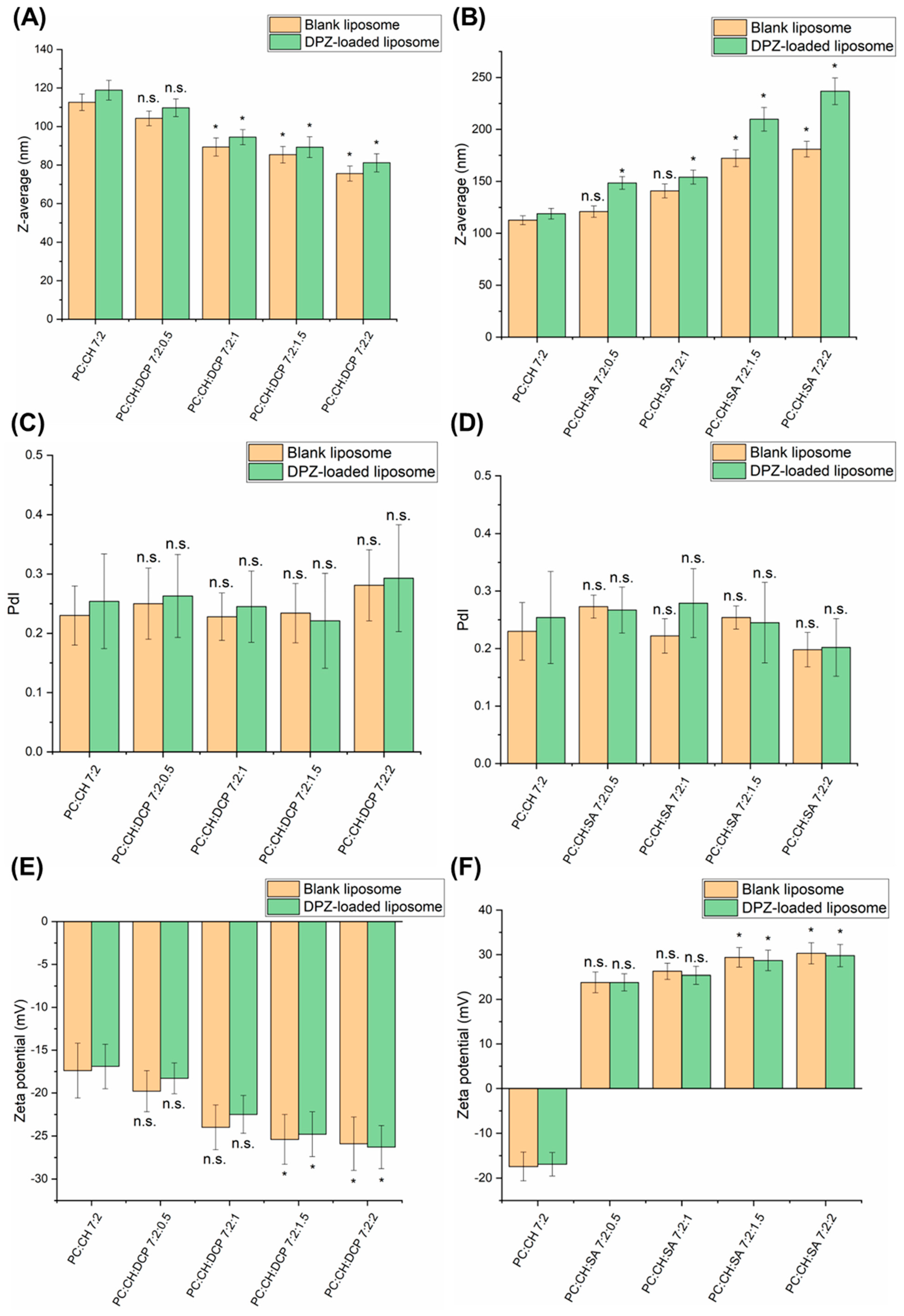
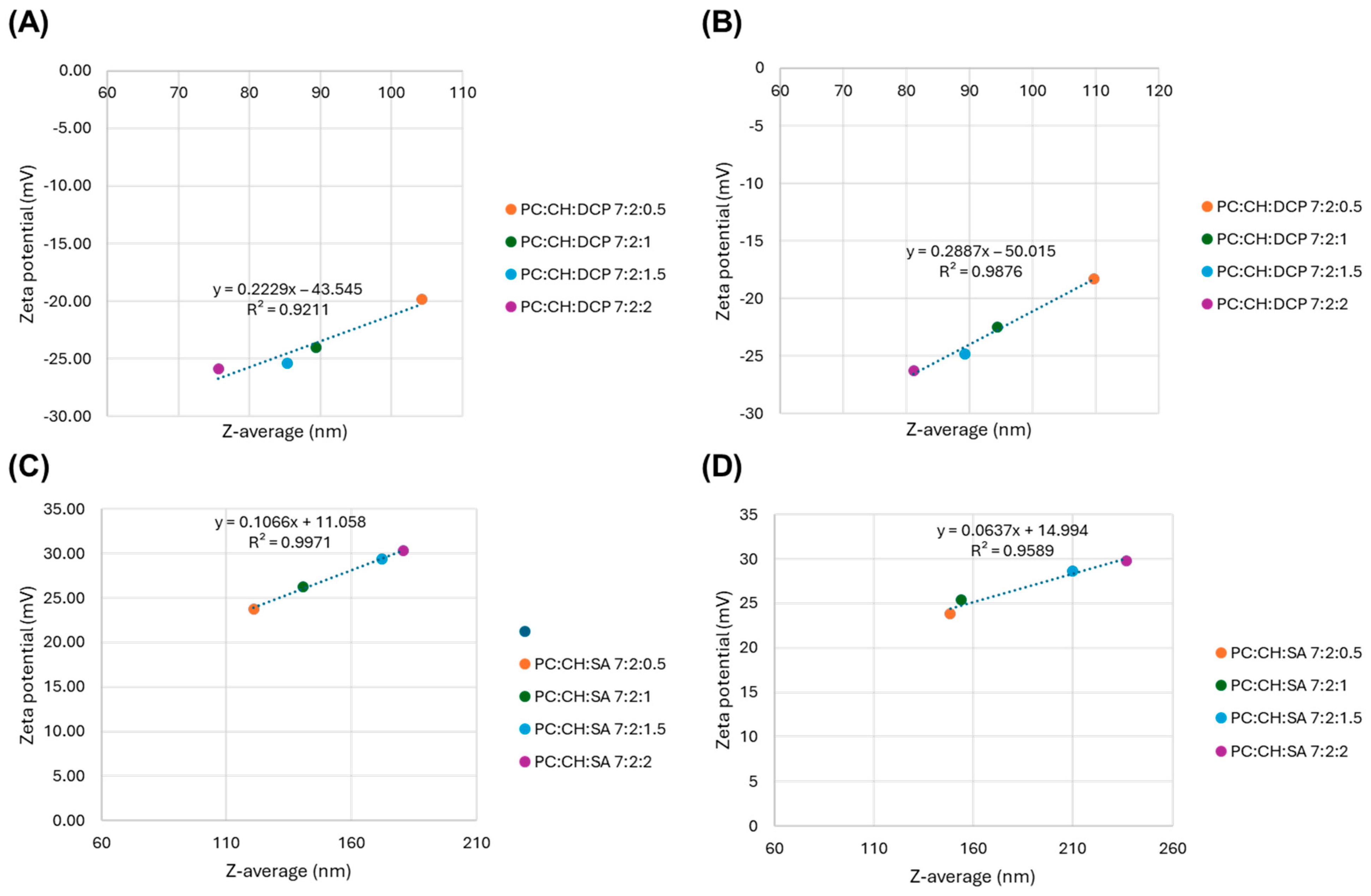
3.2. Characterization of Colloidal Parameters
3.3. Droplet Size Distribution
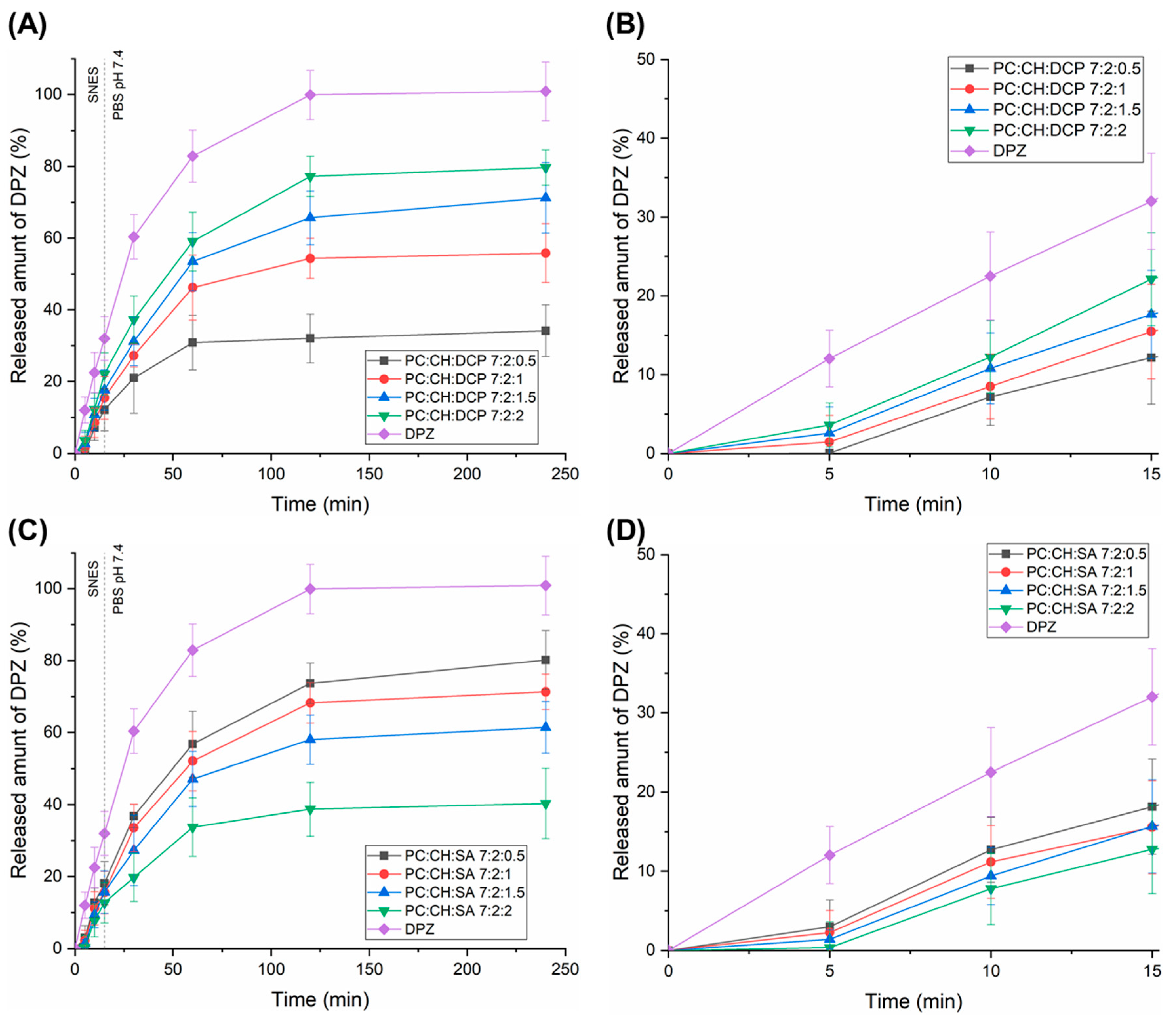
3.4. Drug Release of Liposomal Formulations
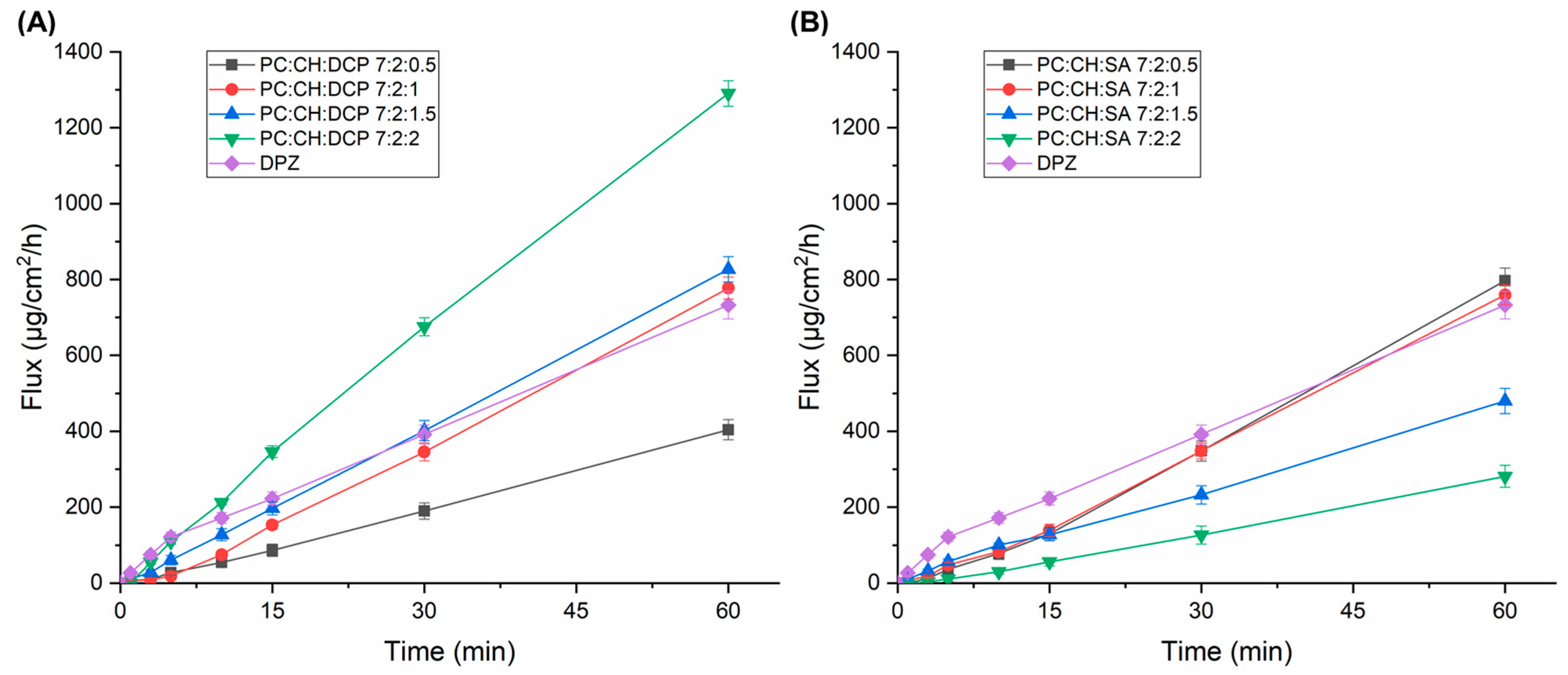
3.5. Drug Diffusion Test
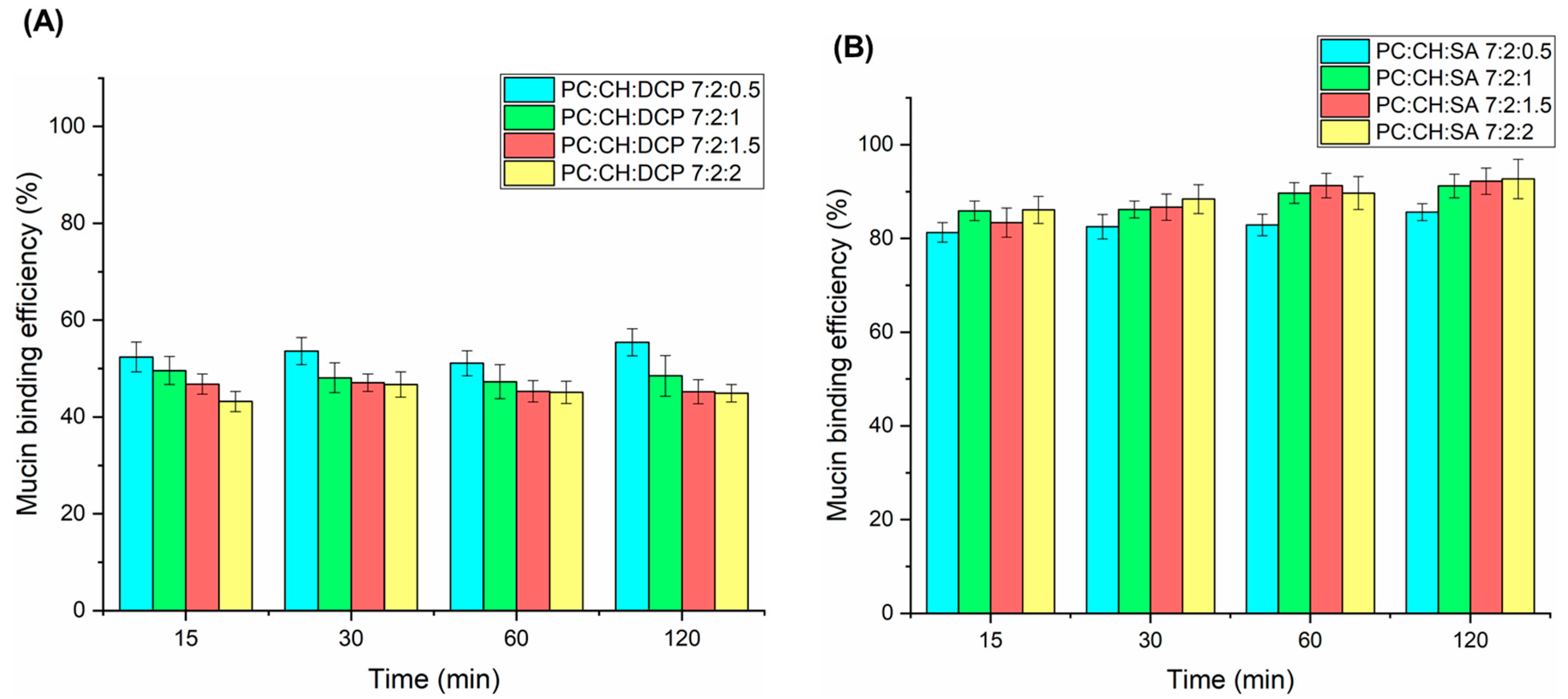
3.6. Mucoadhesive Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DPZ | Donepezil hydrochloride |
| ChEIs | Cholinesterase inhibitors |
| BBB | Blood–brain barrier |
| CNS | Central nervous system |
| DCP | Dicethyl phosphate |
| SA | Stearylamine |
| CH | Cholesterol |
| PC | Phosphatidylcholine |
| SNES | Simulated nasal electrolyte solution |
| Z-average | Average hydrodynamic diameter |
| PdI | Polydispersity index |
| EE | Encapsulation efficiency |
| JSS | Steady-state flux |
| MBE | Mucin binding efficiency |
References
- Espinoza, L.C.; Vacacela, M.; Clares, B.; Garcia, M.L.; Fabrega, M.J.; Calpena, A.C. Development of a Nasal Donepezil-loaded Microemulsion for the Treatment of Alzheimer’s Disease: In vitro and ex vivo Characterization. CNS Neurol. Disord. Drug Targets 2018, 17, 43–53. [Google Scholar] [CrossRef]
- Ruela, A.L.M.; de Figueiredo, E.C.; de Araújo, M.B.; Carvalho, F.C.; Pereira, G.R. Molecularly imprinted microparticles in lipid-based formulations for sustained release of donepezil. Eur. J. Pharm. Sci. 2016, 93, 114–122. [Google Scholar] [CrossRef]
- Al Harthi, S.; Alavi, S.E.; Radwan, M.A.; El Khatib, M.M.; AlSarra, I.A. Nasal delivery of donepezil HCl-loaded hydrogels for the treatment of Alzheimer’s disease. Sci. Rep. 2019, 9, 9563. [Google Scholar] [CrossRef]
- Musumeci, T.; Pellitteri, R.; Spatuzza, M.; Puglisi, G. Nose-to-brain delivery: Evaluation of polymeric nanoparticles on olfactory ensheathing cells uptake. J. Pharm. Sci. 2014, 103, 628–635. [Google Scholar] [CrossRef]
- Westin, U.E.; Boström, E.; Gråsjö, J.; Hammarlund-Udenaes, M.; Björk, E. Direct nose-to-brain transfer of morphine after nasal administration to rats. Pharm. Res. 2006, 23, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Nabar, S.; Dandekar, P.; Patravale, V. Micellar nanocarriers: Potential nose-to-brain delivery of zolmitriptan as novel migraine therapy. Pharm. Res. 2010, 27, 655–664. [Google Scholar] [CrossRef]
- Türker, S.; Onur, E.; Ózer, Y. Nasal route and drug delivery systems. Pharm. World Sci. 2004, 26, 137–142. [Google Scholar] [CrossRef]
- Szabó-Révész, P. Modifying the physicochemical properties of NSAIDs for nasal and pulmonary administration. Drug Discov. Today Technol. 2018, 27, 87–93. [Google Scholar] [CrossRef]
- Papakyriakopoulou, P.; Rekkas, D.M.; Colombo, G.; Valsami, G. Development and in vitro-ex vivo evaluation of novel polymeric nasal donepezil films for potential use in Alzheimer’s disease using experimental design. Pharmaceutics 2022, 14, 1742. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, L.C.; Silva-Abreu, M.; Clares, B.; Rodríguez-Lagunas, M.J.; Halbaut, L.; Cañas, M.A.; Calpena, A.C. Formulation strategies to improve nose-to-brain delivery of donepezil. Pharmaceutics 2019, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; Aboud, H.M.; Abdellatif, M.M.; Abou-Taleb, H.A. Nose-to-Brain Targeted Delivery of Donepezil Hydrochloride via Novel Hyaluronic Acid-Doped Nanotransfersomes for Alzheimer’s Disease Mitigation. J. Pharm. Sci. 2024, 113, 1934–1945. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.A.; Nguyen, T.T.L.; Maeng, H.J. Recent advances in intranasal liposomes for drug, gene, and vaccine delivery. Pharmaceutics 2023, 15, 207. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Lim, C.; Oh, K.T. Recent advances in intranasal administration for brain-targeting delivery: A comprehensive review of lipid-based nanoparticles and stimuli-responsive gel formulations. Int. J. Nanomed. 2024, 23, 1767–1807. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.S.; Oh, K.T.; Choi, H.G.; Lim, S.J. Liposomal formulations for nose-to-brain delivery: Recent advances and future perspectives. Pharmaceutics 2019, 11, 540. [Google Scholar] [CrossRef]
- Kaur, A.; Nigam, K.; Bhatnagar, I.; Sukhpal, H.; Awasthy, S.; Shankar, S.; Tyagi, A.; Dang, S. Treatment of Alzheimer’s diseases using donepezil nanoemulsion: An intranasal approach. Drug Deliv. Transl. Res. 2020, 10, 1862–1875. [Google Scholar] [CrossRef]
- Rajput, A.; Butani, S. Donepezil HCl liposomes: Development, characterization, cytotoxicity, and pharmacokinetic study. AAPS PharmSciTech 2022, 23, 74. [Google Scholar] [CrossRef]
- Yasir, M.; Zafar, A.; Noorulla, K.M.; Tura, A.J.; Sara, U.V.S.; Panjwani, D.; Khalid, M.; Haji, M.J.; Gobena, W.G.; Gebissa, T.; et al. Nose to brain delivery of donepezil through surface modified NLCs: Formulation development, optimization, and brain targeting study. J. Drug Deliv. Sci. Technol. 2022, 75, 103631. [Google Scholar] [CrossRef]
- Bhandari, M.; Rasool, N.; Singh, Y. Polymeric lipid nanoparticles for donepezil delivery. In Polymeric Biomaterials and Bioengineering: Select Proceedings of APA Bioforum 2021; Springer: New York, NY, USA, 2022; pp. 51–63. [Google Scholar]
- Garg, Y.; Sharma, G.; Katare, O.P.; Chopra, S.; Bhatia, A. Design and Optimization of Donepezil Hydrochloride Loaded Nanoparticles Using Doe Approach: A Novel Approach for Treating Alzheimer’s Diseases Through Nose-To-Brain Delivery. Available at SSRN 4255393. 2022. Available online: https://ssrn.com/abstract=4255393 (accessed on 11 September 2025).
- Ramli, N.A.; Ali, N.; Hamzah, S.; Yatim, N.I. Physicochemical characteristics of liposome encapsulation of stingless bees’ propolis. Heliyon 2021, 7, e06649. [Google Scholar] [CrossRef]
- Salamah, M.; Volk, B.; Lekli, I.; Bak, I.; Gyöngyösi, A.; Kozma, G.; Kónya, Z.; Szalenkó-Tőkés, Á.; Kiricsi, Á.; Rovó, L.; et al. Preparation, and ex vivo and in vivo Characterization of Favipiravir-Loaded Aspasomes and Niosomes for Nose-to-Brain Administration. Int. J. Nanomed. 2025, 20, 6489–6514. [Google Scholar] [CrossRef]
- Mardikasari, S.A.; Katona, G.; Budai-Szűcs, M.; Kiricsi, Á.; Rovó, L.; Csóka, I. Mucoadhesive in Situ Nasal Gel of Amoxicillin Trihydrate for Improved Local Delivery: Ex Vivo Mucosal Permeation and Retention Studies. Eur. J. Pharm. Sci. 2024, 202, 106897. [Google Scholar] [CrossRef]
- Gieszinger, P.; Kiss, T.; Szabó-Révész, P.; Ambrus, R. The development of an in vitro horizontal diffusion cell to monitor nasal powder penetration inline. Pharmaceutics 2021, 13, 809. [Google Scholar] [CrossRef] [PubMed]
- Liew, K.B.; Peh, K.K.; Tze Fung Tan, Y. RP-HPLC analytical method development and optimization for quantification of donepezil hydrochloride in orally disintegrating. Pak. J. Pharm. Sci. 2013, 26, 961–966. [Google Scholar]
- Akel, H.; Ismail, R.; Katona, G.; Sabir, F.; Ambrus, R.; Csóka, I. A Comparison Study of Lipid and Polymeric Nanoparticles in the Nasal Delivery of Meloxicam: Formulation, Characterization, and in Vitro Evaluation. Int. J. Pharm. 2021, 604, 120724. [Google Scholar] [CrossRef] [PubMed]
- Rençber, S.; Karavana, S.Y.; Yılmaz, F.F.; Eraç, B.; Nenni, M.; Özbal, S.; Pekçetin, Ç.; Gurer-Orhan, H.; Hoşgör-Limoncu, M.; Güneri, P.; et al. Development, Characterization, and in Vivo Assessment of Mucoadhesive Nanoparticles Containing Fluconazole for the Local Treatment of Oral Candidiasis. Int. J. Nanomed. 2016, 11, 2641–2653. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Garrido, A.; Molina-Bolívar, J.A.; Gálvez-Ruiz, M.J.; Galisteo-González, F. Mucoadhesive Properties of Liquid Lipid Nanocapsules Enhanced by Hyaluronic Acid. J. Mol. Liq. 2019, 296, 111965. [Google Scholar] [CrossRef]
- Senior, J.H.; Trimble, K.R.; Maskiewicz, R. Interaction of positively-charged liposomes with blood: Implications for their application in vivo. Biochim. Biophys. Acta (BBA)-Biomembr. 1991, 1070, 173–179. [Google Scholar] [CrossRef]
- Lee, D.; Minko, T. Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier. Pharmaceutics 2021, 13, 2049. [Google Scholar] [CrossRef]
- Németh, Z.; Csóka, I.; Semnani Jazani, R.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by design-driven zeta potential optimisation study of liposomes with charge imparting membrane additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef]
- Dymek, M.; Olechowska, K.; Hąc-Wydro, K.; Sikora, E. Liposomes as carriers of GHK-Cu tripeptide for cosmetic application. Pharmaceutics 2023, 15, 2485. [Google Scholar] [CrossRef]
- Amer, S.S.; Nasr, M.; Abdel-Aziz, R.T.A.; Moftah, N.H.; El Shaer, A.; Polycarpou, E.; Mamdouh, W.; Sammour, O. Cosm-Nutraceutical Nanovesicles for Acne Treatment: Physicochemical Characterization and Exploratory Clinical Experimentation. Int. J. Pharm. 2020, 577, 119092. [Google Scholar] [CrossRef]
- Zaid Alkilani, A.; Abu-Zour, H.; Alshishani, A.; Abu-Huwaij, R.; Basheer, H.A.; Abo-Zour, H. Formulation and Evaluation of Niosomal Alendronate Sodium Encapsulated in Polymeric Microneedles: In Vitro Studies, Stability Study and Cytotoxicity Study. Nanomaterials 2022, 12, 3570. [Google Scholar] [CrossRef]
- Kotyńska, J.; Figaszewski, Z.A. Adsorption equilibria at interface separating electrolyte solution and phosphatidylcholine-stearylamine liposome membrane. Biophys. Chem. 2007, 127, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Jagwani, S.; Jalalpure, S.; Dhamecha, D.; Jadhav, K.; Bohara, R. Pharmacokinetic and Pharmacodynamic Evaluation of Resveratrol Loaded Cationic Liposomes for Targeting Hepatocellular Carcinoma. ACS Biomater. Sci. Eng. 2020, 6, 4969–4984. [Google Scholar] [CrossRef] [PubMed]
- Jeengar, M.K.; Kurakula, M.; Patil, P.; More, A.; Sistla, R.; Parashar, D. Effect of Cationic Lipid Nanoparticle Loaded siRNA with Stearylamine against Chikungunya Virus. Molecules 2022, 27, 1170. [Google Scholar] [CrossRef]
- Mattheolabakis, G.; Nie, T.; Constantinides, P.P.; Rigas, B. Sterically stabilized liposomes incorporating the novel anticancer agent phospho-ibuprofen (MDC-917): Preparation, characterization, and in vitro/in vivo evaluation. Pharm. Res. 2011, 29, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Liu, J.; Gao, Y.; Hao, Y.; Yang, X.; Huang, G. Preparation and evaluation of stearylamine-bearing pemetrexed disodium-loaded cationic liposomes in vitro and in vivo. AAPS PharmSciTech 2020, 21, 193. [Google Scholar] [CrossRef]
- Doub, W.H.; Suman, J.M.; Copley, M.; Goodey, A.P.; Hosseini, S.; Mitchell, J.P. Laboratory Performance Testing of Aqueous Nasal Inhalation Products for Droplet/Particle Size Distribution: An Assessment from the International Pharmaceutical Aerosol Consortium on Regulation and Science (IPAC-RS). AAPS PharmSciTech 2023, 24, 208. [Google Scholar] [CrossRef]
- Casula, E.; Manconi, M.; Vázquez, J.; Lopez-Mendez, T.; Pedraz, J.; Calvo, E.; Lozano, A.; Zaru, M.; Ascenso, A.; Manca, M. Design of a Nasal Spray Based on Cardiospermum Halicacabum Extract Loaded in Phospholipid Vesicles Enriched with Gelatin or Chondroitin Sulfate. Molecules 2021, 26, 6670. [Google Scholar] [CrossRef]
- Pina Costa, C.; Nižić Nodilo, L.; Silva, R.; Martins, E.; Zadravec, D.; Kalogjera, L.; Nuno Moreira, J.; Manuel Sousa Lobo, J.; Hafner, A.; Catarina Silva, A. In Situ Hydrogel Containing Diazepam-Loaded Nanostructured Lipid Carriers (DZP-NLC) for Nose-to-Brain Delivery: Development, Characterization and Deposition Studies in a 3D-Printed Human Nasal Cavity Model. Int. J. Pharm. 2023, 644, 123345. [Google Scholar] [CrossRef]
- Fang, J.Y.; Hung, C.F.; Hwang, T.L.; Huang, Y.L. Physicochemical characteristics and in vivo deposition of liposome-encapsulated tea catechins by topical and intratumor administrations. J. Drug Target. 2005, 13, 19–27. [Google Scholar] [CrossRef]
- Webb, M.S.; Wheeler, J.J.; Bally, M.B.; Mayer, L.D. The cationic lipid stearylamine reduces the permeability of the cationic drugs verapamil and prochlorperazine to lipid bilayers: Implications for drug delivery. BBA-Biomembr. 1995, 1238, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Srinath, P.; Vyas, S.P.; Diwan, P.V. Preparation and pharmacodynamic evaluation of liposomes of indomethacin. Drug Dev. Ind. Pharm. 2000, 26, 313–321. [Google Scholar] [CrossRef]
- Vasileva, L.; Gaynanova, G.; Valeeva, F.; Belyaev, G.; Zueva, I.; Bushmeleva, K.; Sibgatullina, G.; Samigullin, D.; Vyshtakalyuk, A.; Petrov, K.; et al. Mitochondria-targeted delivery strategy of dual-loaded liposomes for Alzheimer’s disease therapy. Int. J. Mol. Sci. 2023, 24, 10494. [Google Scholar] [CrossRef]
- Sutthapitaksakul, L.; Dass, C.R.; Sriamornsak, P. Donepezil—An updated review of challenges in dosage form design. J. Drug Deliv. Sci. Technol. 2021, 63, 102549. [Google Scholar] [CrossRef]
- Pham, Q.D.; Nöjd, S.; Edman, M.; Lindell, K.; Topgaard, D.; Wahlgren, M. Mucoadhesion: Mucin-Polymer Molecular Interactions. Int. J. Pharm. 2021, 610, 121245. [Google Scholar] [CrossRef] [PubMed]
- Guerini, M.; Condrò, G.; Perugini, P. Evaluation of the Mucoadhesive Properties of Chitosan-Based Microstructured Lipid Carrier (CH-MLC). Pharmaceutics 2022, 14, 170. [Google Scholar] [CrossRef]
- Kumari, K.; Kumar, A.; Manjur, A.T.; Rakshit, S. Bioactives Promiscuity of Mucin: Insight from Multi-Spectroscopic, Thermodynamic, and Molecular Dynamic Simulation Analyses. Langmuir 2023, 39, 4589–4600. [Google Scholar] [CrossRef] [PubMed]

| Drug Delivery System | Formulation | Charge Inducer | Result/Outcome | Reference |
|---|---|---|---|---|
| Nanoemulsion | Labrasol Cetyl pyridinium chloride Glycerol | Cetyl pyridinium chloride | Z-average: 65 nm -PdI: 0.084 Zeta potential: −10.7 mV Release property: Fast release in PBS (pH 7.4), ACSF, simulated nasal fluid Cell viability: 76.3% (Neuro 2a) | [15] |
| Liposomes | Hydrogenated soy phosphatidyl cholin Cholesterol -Ammonium sulfate | Ammonium sulfate | Z-average: 103 nm PdI: 0.108 -EE: 93% Nasal mucosa permeation: 80.11% Mucoadhesive strength: 2320 dyne/cm2 | [16] |
| Solid lipid nanoparticles (SLNs) | Compritol Capryol 90 Poloxamer 188 Chitosan | Chitosan | Z-average: 193 nm PdI: 0.298 Zeta potential: 38.9 mV EE: 89.85% Release property: sustained release (84.82%) up to 24 h Highly Mucoadhesive | [17] |
| Polymeric Lipid Nanoparticles | Soy lecithin Glutaraldehyde Chitosan Glacial acetic acid Gelatin | Chitosan | Z-average: 237 nm Zeta potential: positive Drug loading: 10.24% Release property: burst release of up to 99.99% for 5 days Cell viability: safe toward mouse fibroblast cells (L929) Highly Mucoadhesive | [18] |
| Nanosuspension | Chitosan Sodium tripolyphosphate Glacial acetic acid | Chitosan | Z-average: 177.8 nm PdI: 0.593 Zeta potential: 16.6 mV Release property: sustained, 90.82% (24 h) Mucodhesive force: 9.26 g | [19] |
| Formulation Code | Amount of Lipids | Amount of Charge Inducers | ||
|---|---|---|---|---|
| Molar Ratio of PC | Molar Ratio of CH | Molar Ratio of DCP | Molar Ratio of SA | |
| PC:CH:DCP 7:2:0.5 | 7 | 2 | 0.5 | 0 |
| PC:CH:DCP 7:2:1 | 7 | 2 | 1 | 0 |
| PC:CH:DCP 7:2:1.5 | 7 | 2 | 1.5 | 0 |
| PC:CH:DCP 7:2:2 | 7 | 2 | 2 | 0 |
| PC:CH:SA 7:2:0.5 | 7 | 2 | 0 | 0.5 |
| PC:CH:SA 7:2:1 | 7 | 2 | 0 | 1 |
| PC:CH:SA 7:2:1.5 | 7 | 2 | 0 | 1.5 |
| PC:CH:SA 7:2:2 | 7 | 2 | 0 | 2 |
| Liposomal Formulation | EE (%) |
|---|---|
| PC:CH:DCP 7:2:0.5 | 58.4 ± 2.1 |
| PC:CH:DCP 7:2:1 | 62.1 ± 2.7 |
| PC:CH:DCP 7:2:1.5 | 69.6 ± 3.4 |
| PC:CH:DCP 7:2:2 | 73.8 ± 1.8 |
| PC:CH:SA 7:2:0.5 | 67.6 ± 3.9 |
| PC:CH:SA 7:2:1 | 72.8 ± 4.1 |
| PC:CH:SA 7:2:1.5 | 75.5 ± 2.2 |
| PC:CH:SA 7:2:2 | 81.3 ± 2.7 |
| Formulation | Dv10 (µm) | Dv50 (µm) | Dv90 (µm) | Span |
|---|---|---|---|---|
| PC:CH 7:2 | 131.2 ± 6.3 | 173.9 ± 11.4 | 228.2 ± 10.3 | 0.56 ± 0.21 |
| PC:CH:DCP 7:2:0.5 | 125.4 ± 4.7 | 173.2 ± 8.8 | 203.1 ± 12.6 | 0.48 ± 0.13 |
| PC:CH:DCP 7:2:1 | 113.2 ± 5.9 | 160.0 ± 6.3 | 258.9 ± 13.8 | 0.84 ± 0.17 |
| PC:CH:DCP 7:2:1.5 | 110.4 ± 1.8 | 160.1 ± 4.1 | 232.7 ± 9.7 | 0.76 ± 0.09 |
| PC:CH:DCP 7:2:2 | 101.4 ± 3.6 | 151.3 ± 8.7 | 198.4 ± 12.5 | 0.55 ± 0.23 |
| PC:CH:SA 7:2:0.5 | 121.4 ± 7.4 | 184.3 ± 7.5 | 376.9 ± 24.6 | 0.72 ± 0.14 |
| PC:CH:SA 7:2:1 | 118.4 ± 2.2 | 168.2 ± 5.3 | 260.2 ± 13.3 | 0.68 ± 0.18 |
| PC:CH:SA 7:2:1.5 | 111.5 ± 6.8 | 171.8 ± 6.7 | 236.6 ± 15.1 | 0.86 ± 0.06 |
| PC:CH:SA 7:2:2 | 81.1 ± 9.7 | 125.4 ± 4.1 | 186 ± 9.5 | 0.83 ± 0.02 |
| Formulation | Flux (µg/cm2/h) at t = 1 h |
|---|---|
| DPZ solution | 732.81 ± 36.5 |
| PC:CH:DCP 7:2:0.5 | 404.24 ± 26.7 |
| PC:CH:DCP 7:2:1 | 777.33 ± 28.9 |
| PC:CH:DCP 7:2:1.5 | 827.19 ± 33.4 |
| PC:CH:DCP 7:2:2 | 1290.21 ± 33.6 |
| PC:CH:SA 7:2:0.5 | 796.92 ± 33.1 |
| PC:CH:SA 7:2:1 | 759.52 ± 34.2 |
| PC:CH:SA 7:2:1.5 | 479.93 ± 27.2 |
| PC:CH:SA 7:2:2 | 281.37 ± 26.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valehi, E.; Katona, G.; Dobó, D.G.; Csóka, I. In Vitro Comparative Study on Oppositely Charged Donepezil-Loaded Intranasal Liposomes. Pharmaceutics 2025, 17, 1250. https://doi.org/10.3390/pharmaceutics17101250
Valehi E, Katona G, Dobó DG, Csóka I. In Vitro Comparative Study on Oppositely Charged Donepezil-Loaded Intranasal Liposomes. Pharmaceutics. 2025; 17(10):1250. https://doi.org/10.3390/pharmaceutics17101250
Chicago/Turabian StyleValehi, Elika, Gábor Katona, Dorina Gabriella Dobó, and Ildikó Csóka. 2025. "In Vitro Comparative Study on Oppositely Charged Donepezil-Loaded Intranasal Liposomes" Pharmaceutics 17, no. 10: 1250. https://doi.org/10.3390/pharmaceutics17101250
APA StyleValehi, E., Katona, G., Dobó, D. G., & Csóka, I. (2025). In Vitro Comparative Study on Oppositely Charged Donepezil-Loaded Intranasal Liposomes. Pharmaceutics, 17(10), 1250. https://doi.org/10.3390/pharmaceutics17101250






