Nanoformulation of Polymyxin E Through Complex Coacervation: A Pharmacokinetic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Formulation of the PME–Polyion Coacervation Complex (PME Nanoformulation)
2.3. The Minimum Inhibitory Concentration (MIC) Test
2.4. Determination of Maximum Tolerated Dose (MTD)
2.5. In Vivo Efficacy of the Nanoformulation
- -
- Group 1: Blank control. No treatment administered.
- -
- Group 2: Negative control. Dosage: 10 mL/kg; administration: twice daily (BID), every 12 h; no drug substance was added.
- -
- Group 3: Excipient control. Dosage: equivalent to 32 mpk; administration: twice daily (BID), every 12 h; an excipient formulation was used.
- -
- Group 4: Positive Control. Dosage: 8 mpk; administration: twice daily (BID), every 12 h; PME was administered at a dose of 8 mpk.
- -
- Group 5–8: SOP-3. Dosages: 8 mpk, 16 mpk, 24 mpk, and 32 mpk; administration: twice daily (BID), every 12 h; different dosages of the SOP-3 formulation were administered.
- -
- Groups 9–10: SOP-2. Dosages: 8 mpk and 24 mpk; administration: twice daily (BID), every 12 h; SOP-2 formulation at varying dosages was administered.
- -
- Groups 11–12: HA2-1.5. Dosages: 8 mpk and 24 mpk; administration: twice daily (BID), every 12 h; HA2-1.5 formulation was administered at differing dosages.
2.6. Pharmacokinetic Studies
2.7. Toxicity of PME Nanoformulation
3. Results
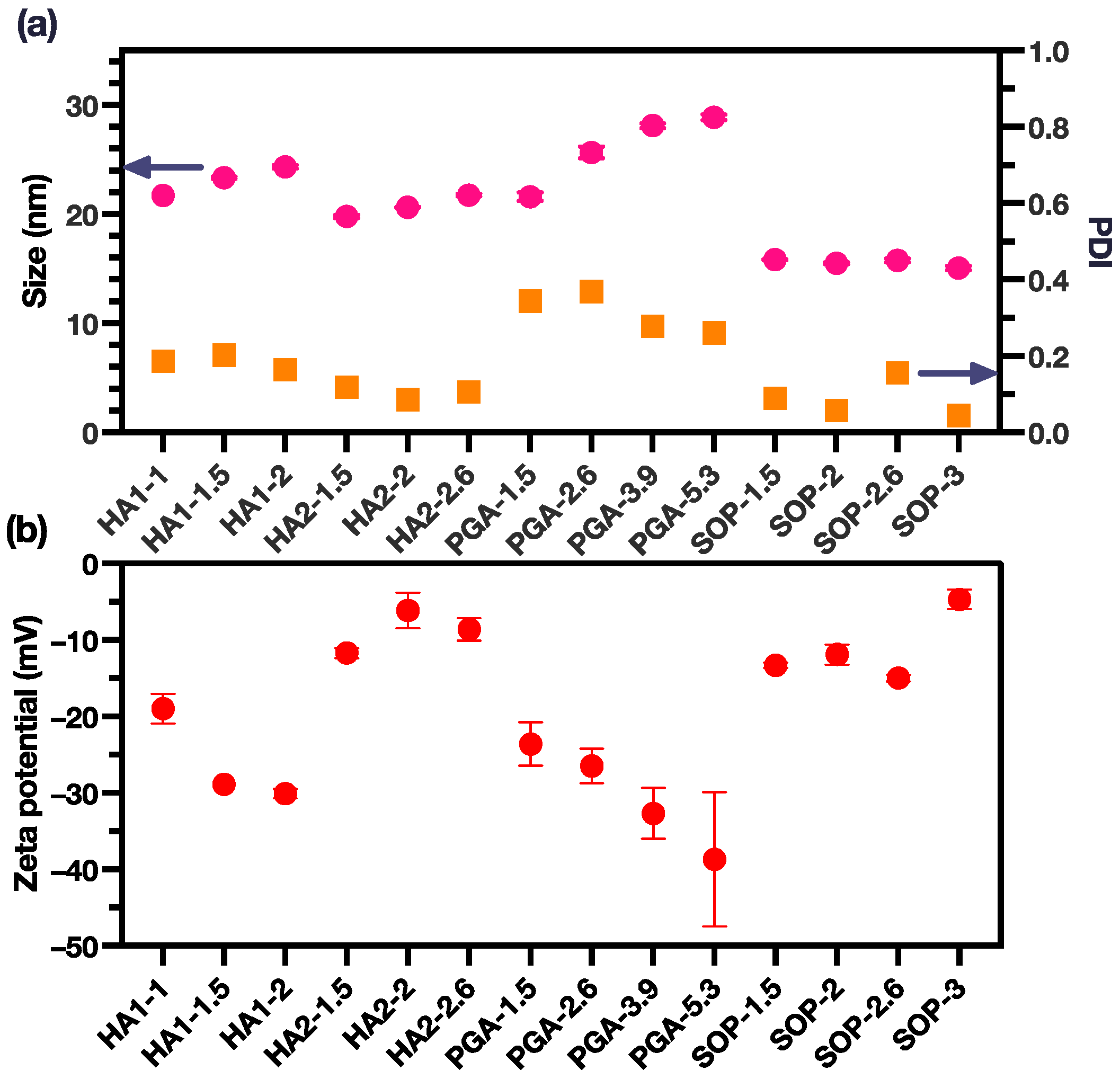
3.1. PME-Polyion Coacervation Complex
3.2. Antimicrobial Activity of PME Nanoformulations
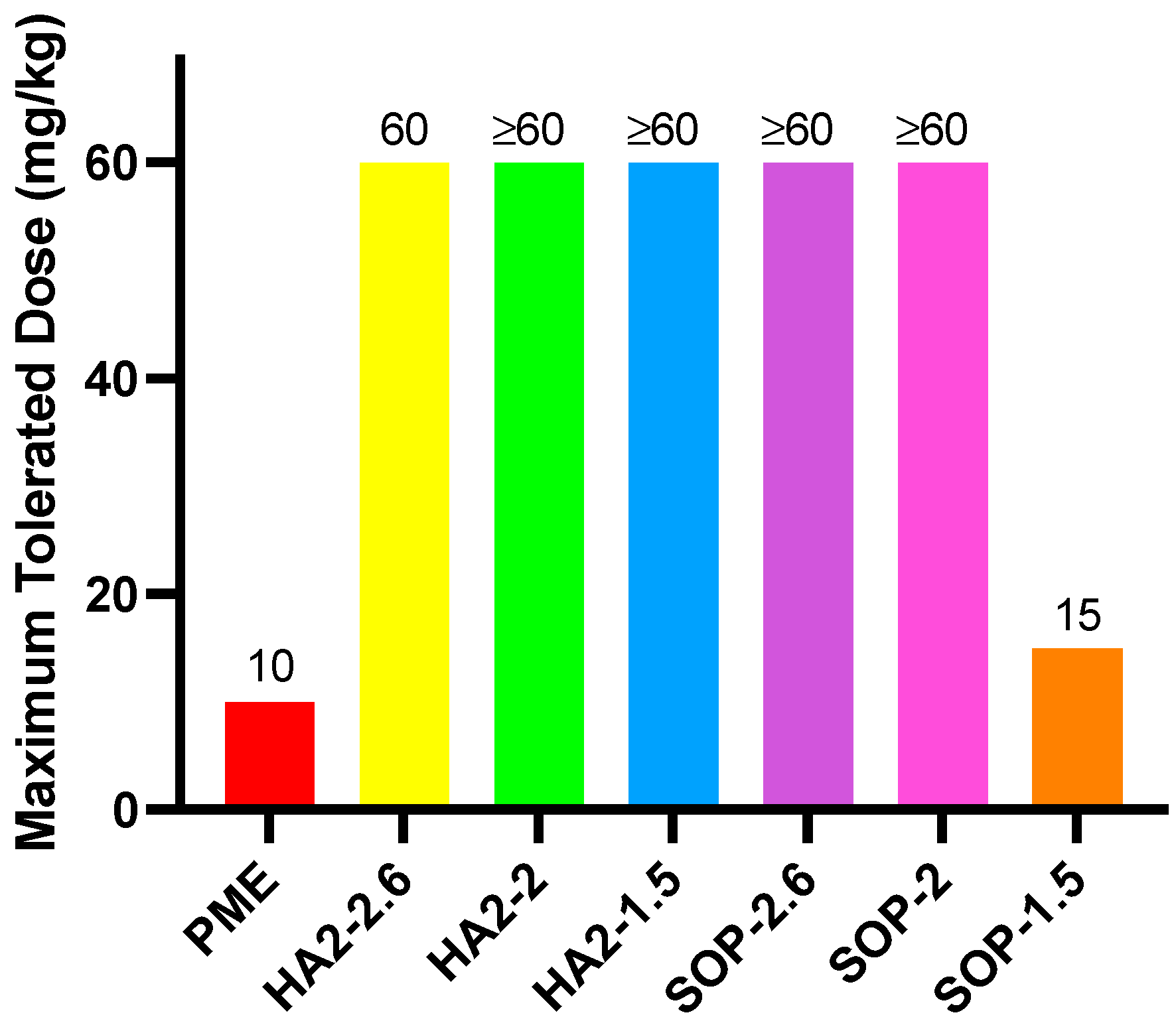
3.3. MTD
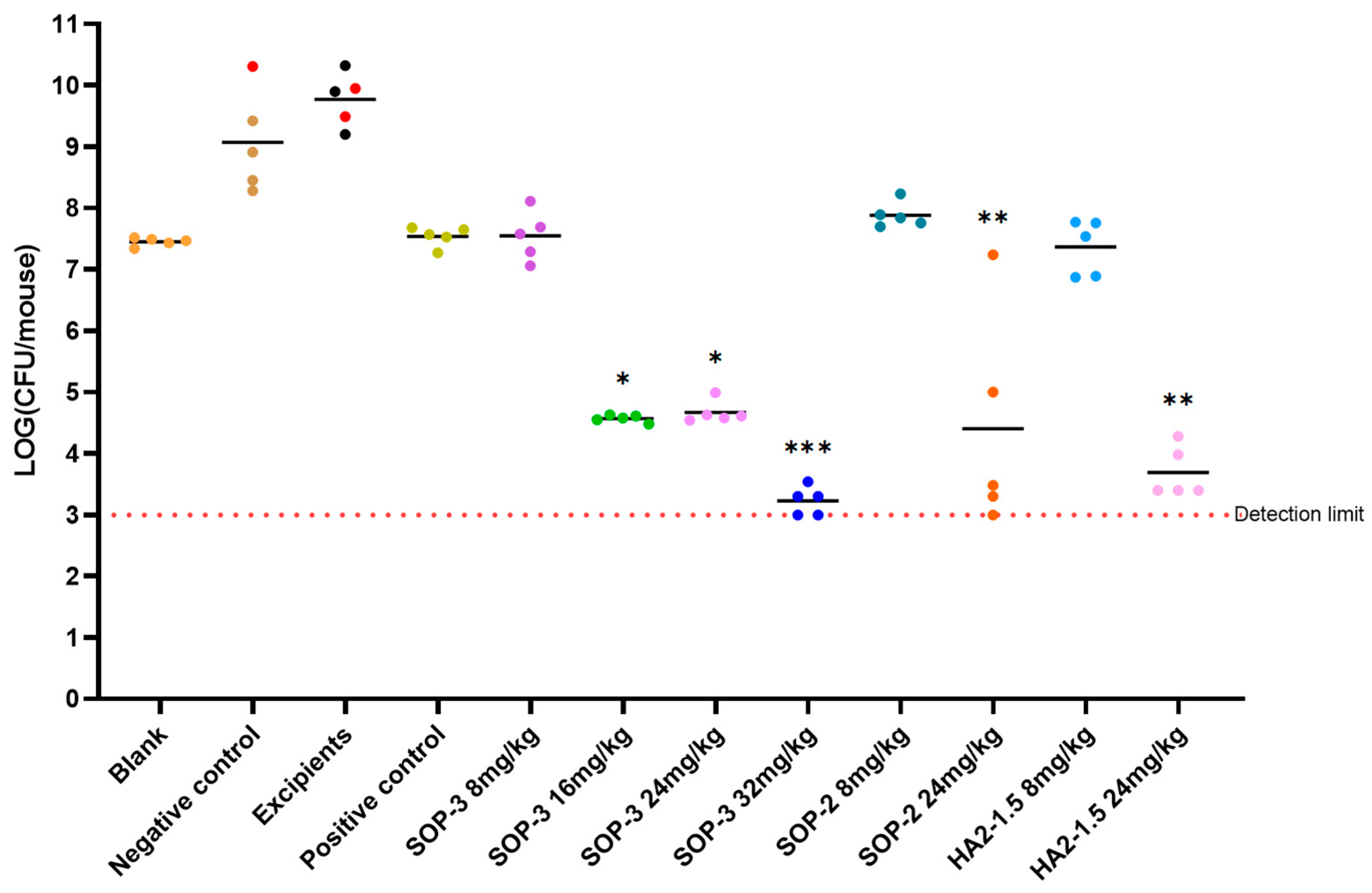
3.4. In Vivo Efficacy of PME Nanoformulations
3.5. Pharmacokinetic Study
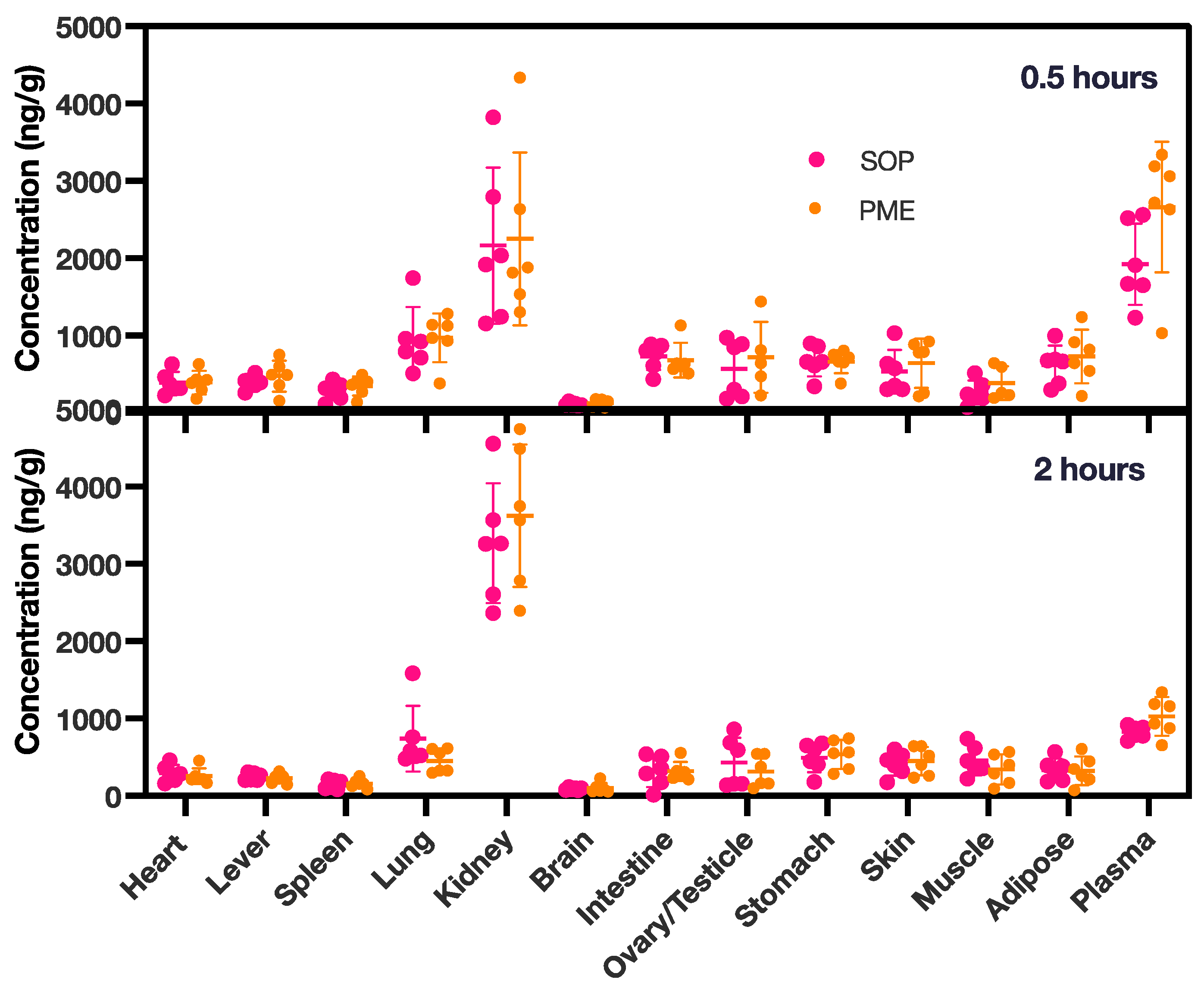
3.6. Tissue Distribution in PME Group and SOP Nanoformulation
3.7. Toxicity of the SOP Nanoformulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Årdal, C.; Balasegaram, M.; Laxminarayan, R.; McAdams, D.; Outterson, K.; Rex, J.H.; Sumpradit, N. Antibiotic Development—Economic, Regulatory and Societal Challenges. Nat. Rev. Microbiol. 2020, 18, 267–274. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed Ahmed, M.A.E.-G.; Zhong, L.-L.; Shen, C.; Yang, Y.; Doi, Y.; Tian, G.-B. Colistin and Its Role in the Era of Antibiotic Resistance: An Extended Review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nation, R.L.; Kaye, K.S. (Eds.) Polymyxin Antibiotics: From Laboratory Bench to Bedside; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2019; Volume 1145, ISBN 978-3-030-16371-6. [Google Scholar]
- Yu, Z.; Qin, W.; Lin, J.; Fang, S.; Qiu, J. Antibacterial Mechanisms of Polymyxin and Bacterial Resistance. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Doymaz, M.Z.; Karaaslan, E. Comparison of Antibacterial Activities of Polymyxin B and Colistin against Multidrug Resistant Gram Negative Bacteria. Infect. Dis. 2019, 51, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.-P.S.; Hancock, R.E.W. The Relationship between Peptide Structure and Antibacterial Activity. Peptides 2003, 24, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Velkov, T.; Thompson, P.E.; Nation, R.L.; Li, J. Structure−Activity Relationships of Polymyxin Antibiotics. J. Med. Chem. 2010, 53, 1898–1916. [Google Scholar] [CrossRef] [PubMed]
- Velkov, T.; Roberts, K.D.; Nation, R.L.; Thompson, P.E.; Li, J. Pharmacology of Polymyxins: New Insights into an ‘Old’ Class of Antibiotics. Future Microbiol. 2013, 8, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Guo, J.; Xie, J.; Xu, M.; Hao, X.; Ma, K.; Rao, Y. Population Pharmacokinetics of Polymyxin B: A Systematic Review. Ann. Transl. Med. 2022, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Karaiskos, I.; Bassetti, M. How Do We Optimize the Prescribing of Intravenous Polymyxins to Increase Their Longevity and Efficacy in Critically Ill Patients? Expert Opin. Pharmacother. 2022, 23, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Ardebili, A.; Izanloo, A.; Rastegar, M. Polymyxin Combination Therapy for Multidrug-Resistant, Extensively-Drug Resistant, and Difficult-to-Treat Drug-Resistant Gram-Negative Infections: Is It Superior to Polymyxin Monotherapy? Expert Rev. Anti-Infect. Ther. 2023, 21, 387–429. [Google Scholar] [CrossRef] [PubMed]
- Dubashynskaya, N.V.; Skorik, Y.A. Polymyxin Delivery Systems: Recent Advances and Challenges. Pharmaceuticals 2020, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Abbott, E.; Abdulle, O.; Boakes, S.; Coleman, S.; Divall, N.; Duperchy, E.; Moss, S.; Rivers, D.; Simonovic, M.; et al. Design of Next Generation Polymyxins with Lower Toxicity: The Discovery of SPR206. ACS Infect. Dis. 2019, 5, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Aslan, A.T.; Akova, M.; Paterson, D.L. Next-Generation Polymyxin Class of Antibiotics: A Ray of Hope Illuminating a Dark Road. Antibiotics 2022, 11, 1711. [Google Scholar] [CrossRef] [PubMed]
- Theuretzbacher, U.; Bush, K.; Harbarth, S.; Paul, M.; Rex, J.H.; Tacconelli, E.; Thwaites, G.E. Critical Analysis of Antibacterial Agents in Clinical Development. Nat. Rev. Microbiol. 2020, 18, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Sing, C.E. Recent Progress in the Science of Complex Coacervation. Soft Matter 2020, 16, 2885–2914. [Google Scholar] [CrossRef] [PubMed]
- Blocher, W.C.; Perry, S.L. Complex Coacervate-based Materials for Biomedicine. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1442. [Google Scholar] [CrossRef] [PubMed]
- Satlin, M.J.; Lewis, J.S.; Weinstein, M.P.; Patel, J.; Humphries, R.M.; Kahlmeter, G.; Giske, C.G.; Turnidge, J. Clinical and Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing Position Statements on Polymyxin B and Colistin Clinical Breakpoints. Clin. Infect. Dis. 2020, 71, e523–e529. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Marchand, S.; Grégoire, N.; Couet, W. Pharmacokinetics of Polymyxins in Animals. In Polymyxin Antibiotics: From Laboratory Bench to Bedside; Li, J., Nation, R.L., Kaye, K.S., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2019; Volume 1145, pp. 89–103. ISBN 978-3-030-16371-6. [Google Scholar]
- Avedissian, S.N.; Liu, J.; Rhodes, N.J.; Lee, A.; Pais, G.M.; Hauser, A.R.; Scheetz, M.H. A Review of the Clinical Pharmacokinetics of Polymyxin B. Antibiotics 2019, 8, 31. [Google Scholar] [CrossRef] [PubMed]

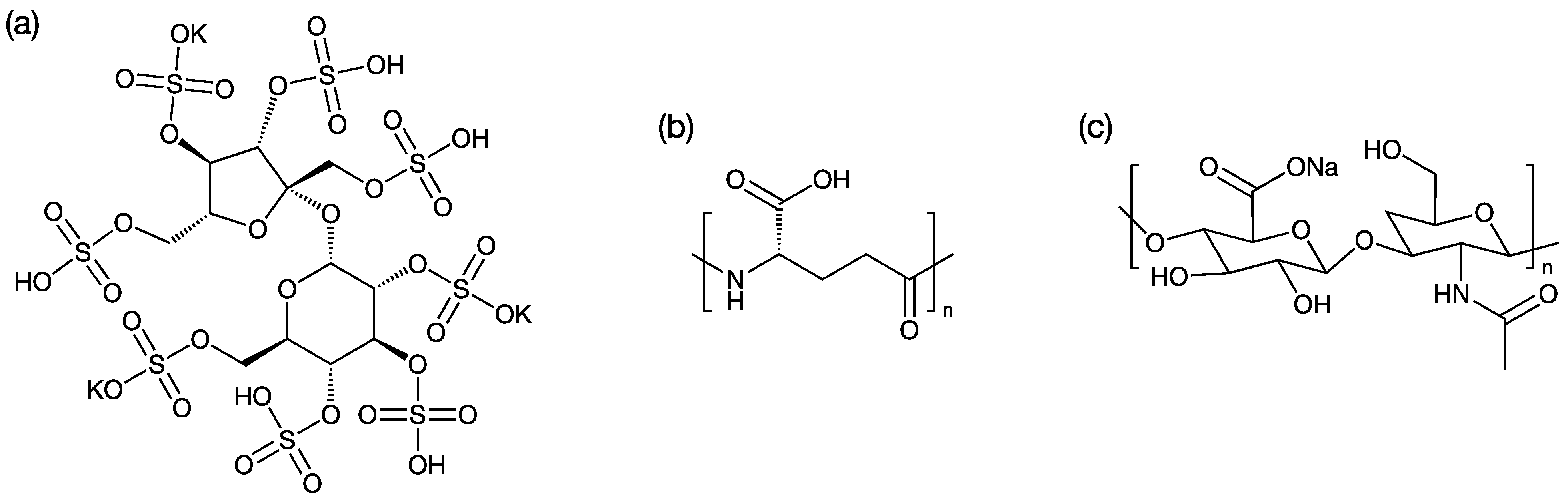



| Charge Ratio of Polyion/PME | Molar Ratio of PME/Polyion/MPEG200-DSPE | |
|---|---|---|
| HA1-1 | 1:1 | 1:0.002:5 |
| HA1-1.5 | 1.5:1 | 1:0.003:5 |
| HA1-2 | 2:1 | 1:0.004:5 |
| HA2-1.5 | 1.5:1 | 1:0.12:6.73 |
| HA2-2 | 2:1 | 1:0.17:6.98 |
| HA2-2.6 | 2.6:1 | 1:0.22:7.28 |
| PGA-1.5 | 1.5:1 | 1:0.12:5.23 |
| PGA-2.6 | 2.6:1 | 1:0.22:7.28 |
| PGA-3.9 | 3.9:1 | 1:0.33:7.93 |
| PGA-5.3 | 5.3:1 | 1:0.43:8.58 |
| SOP-1.5 | 1.5:1 | 1:0.93:5.35 |
| SOP-2 | 2:1 | 1:1.25:6.24 |
| SOP-2.6 | 2.6:1 | 1:1.63:7.28 |
| SOP-3 | 3:1 | 1:1.88:6.00 |
| Organism (No. of Isolates) | Formulation | MIC Range | MIC50 | Resistant (%) | Susceptible (%) |
|---|---|---|---|---|---|
| Carbapenem-sensitive E. coli (12) | PGA-2.6 | ≤0.015–0.06 | 0.03 | 0 | 100 |
| HA2-2.6 | 0.03–0.06 | 0.03 | 0 | 100 | |
| HA2-2 | ≤0.015–0.06 | 0.03 | 0 | 100 | |
| HA2-1.5 | 0.03–0.06 | 0.03 | 0 | 100 | |
| SOP-2.6 | 0.03–0.06 | 0.06 | 0 | 100 | |
| SOP-2 | 0.03–0.06 | 0.06 | 0 | 100 | |
| SOP-1.5 | 0.03–0.125 | 0.06 | 0 | 100 | |
| PME | 0.125–0.5 | 0.125 | 0 | 100 | |
| Carbapenem-resistant E. coli (11) | PGA-2.6 | 0.03–2 | 0.06 | 0 | 100 |
| HA2-2.6 | 0.03–1 | 0.06 | 0 | 100 | |
| HA2-2 | 0.03–1 | 0.06 | 0 | 100 | |
| HA2-1.5 | 0.03–2 | 0.06 | 0 | 100 | |
| SOP-2.6 | 0.06–1 | 0.06 | 0 | 100 | |
| SOP-2 | 0.06–1 | 0.125 | 0 | 100 | |
| SOP-1.5 | 0.06–2 | 0.06 | 0 | 100 | |
| PME | 0.125–4 | 0.25 | 9.1 | 90.9 | |
| Total (23) | PGA-2.6 | ≤0.015–2 | 0.06 | 0 | 100 |
| HA2-2.6 | 0.03–1 | 0.03 | 0 | 100 | |
| HA2-2 | ≤0.015–1 | 0.06 | 0 | 100 | |
| HA2-1.5 | 0.03–2 | 0.06 | 0 | 100 | |
| SOP-2.6 | 0.03–1 | 0.06 | 0 | 100 | |
| SOP-2 | 0.03–1 | 0.06 | 0 | 100 | |
| SOP-1.5 | 0.03–2 | 0.06 | 0 | 100 | |
| PME | 0.125–4 | 0.25 | 4.3 | 95.7 |
| Organism (No. of Isolates) | Formulation | MIC Range | MIC50 | Resistant (%) | Susceptible (%) |
|---|---|---|---|---|---|
| carbapenem-sensitive K. pneumoniae (12) | PGA-2.6 | 0.06–0.25 | 0.06 | 0 | 100 |
| HA2-2.6 | 0.06–0.125 | 0.06 | 0 | 100 | |
| HA2-2 | 0.06–0.125 | 0.06 | 0 | 100 | |
| HA2-1.5 | 0.06–0.125 | 0.06 | 0 | 100 | |
| SOP-2.6 | 0.06–0.125 | 0.125 | 0 | 100 | |
| SOP-2 | 0.06–0.125 | 0.125 | 0 | 100 | |
| SOP-1.5 | >0.06–0.125 | 0.125 | 0 | 100 | |
| PME | 0.25–0.5 | 0.5 | 0 | 100 | |
| carbapenem-resistant K. pneumoniae (10) | PGA-2.6 | 0.03–0.125 | 0.06 | 0 | 100 |
| HA2-2.6 | 0.03–0.06 | 0.06 | 0 | 100 | |
| HA2-2 | 0.03–0.125 | 0.06 | 0 | 100 | |
| HA2-1.5 | 0.06–0.125 | 0.06 | 0 | 100 | |
| SOP-2.6 | 0.06–0.125 | 0.06 | 0 | 100 | |
| SOP-2 | 0.06–0.125 | 0.125 | 0 | 100 | |
| SOP-1.5 | 0.06–0.5 | 0.125 | 0 | 100 | |
| PME | 0.125–0.5 | 0.5 | 0 | 100 | |
| Total (22) | PGA-2.6 | 0.03–0.25 | 0.06 | 0 | 100 |
| HA2-2.6 | 0.03–0.125 | 0.06 | 0 | 100 | |
| HA2-2 | 0.03–0.125 | 0.06 | 0 | 100 | |
| HA2-1.5 | 0.06–0.125 | 0.06 | 0 | 100 | |
| SOP-2.6 | 0.06–0.125 | 0.06 | 0 | 100 | |
| SOP-2 | 0.06–0.125 | 0.125 | 0 | 100 | |
| SOP-1.5 | 0.06–0.5 | 0.125 | 0 | 100 | |
| PME | 0.125–0.5 | 0.5 | 0 | 100 |
| Organism (No. of Isolates) | Formulation | MIC range | MIC50 | Resistant (%) | Susceptible (%) |
|---|---|---|---|---|---|
| Carbapenem-sensitive A. baumannii (10) | PGA-2.6 | 0.06–0.125 | 0.125 | 0 | 100 |
| HA2-2.6 | 0.06–0.125 | 0.125 | 0 | 100 | |
| HA2-2 | 0.06–0.125 | 0.125 | 0 | 100 | |
| HA2-1.5 | 0.06–0.25 | 0.125 | 0 | 100 | |
| SOP-2.6 | 0.06–0.25 | 0.125 | 0 | 100 | |
| SOP-2 | 0.125–0.5 | 0.25 | 0 | 100 | |
| SOP-1.5 | 0.125–0.25 | 0.125 | 0 | 100 | |
| PME | >0.25–0.5 | 0.5 | 0 | 100 | |
| Carbapenem-resistant A. baumannii (10) | PGA-2.6 | 0.125–0.5 | 0.25 | 0 | 100 |
| HA2-2.6 | 0.125–0.5 | 0.25 | 0 | 100 | |
| HA2-2 | 0.125–0.5 | 0.25 | 0 | 100 | |
| HA2-1.5 | 0.125–0.5 | 0.25 | 0 | 100 | |
| SOP-2.6 | 0.25–0.5 | 0.25 | 0 | 100 | |
| SOP-2 | 0.25–0.5 | 0.5 | 0 | 100 | |
| SOP-1.5 | 0.25–0.5 | 0.25 | 0 | 100 | |
| PME | 0.5–1 | 0.5 | 0 | 100 | |
| Total (20) | PGA-2.6 | 0.06–0.5 | 0.125 | 0 | 100 |
| HA2-2.6 | 0.06–0.5 | 0.125 | 0 | 100 | |
| HA2-2 | 0.06–0.5 | 0.125 | 0 | 100 | |
| HA2-1.5 | 0.06–0.5 | 0.125 | 0 | 100 | |
| SOP-2.6 | 0.06–0.5 | 0.25 | 0 | 100 | |
| SOP-2 | 0.125–0.5 | 0.25 | 0 | 100 | |
| SOP-1.5 | 0.125–0.5 | 0.25 | 0 | 100 | |
| PME | 0.5–1 | 0.5 | 0 | 100 |
| Organism (No. of Isolates) | Formulation | MIC Range | MIC50 | Resistant (%) | Susceptible (%) |
|---|---|---|---|---|---|
| Carbapenem-sensitive P. aeruginosa (10) | PGA-2.6 | 0.03–1 | 0.25 | 0 | 100 |
| HA2-2.6 | 0.03–4 | 0.25 | 10 | 90 | |
| HA2-2 | 0.06–1 | 0.25 | 0 | 100 | |
| HA2-1.5 | 0.06–1 | 0.25 | 0 | 100 | |
| SOP-2.6 | 0.06–2 | 0.5 | 0 | 100 | |
| SOP-2 | 0.125–2 | 0.5 | 0 | 100 | |
| SOP-1.5 | 0.06–2 | 0.25 | 0 | 100 | |
| PME | 0.125–2 | 1 | 0 | 100 | |
| Carbapenem-resistant P. aeruginosa (11) | PGA-2.6 | 0.125–2 | 1 | 0 | 100 |
| HA2-2.6 | 0.25–2 | 0.5 | 0 | 100 | |
| HA2-2 | 0.125–2 | 1 | 0 | 100 | |
| HA2-1.5 | 0.125–4 | 1 | 9.1 | 90.9 | |
| SOP-2.6 | 0.25–2 | 1 | 0 | 100 | |
| SOP-2 | 0.25–2 | 1 | 0 | 100 | |
| SOP-1.5 | 0.25–4 | 1 | 9.1 | 90.9 | |
| PME | 1–2 | 2 | 0 | 100 | |
| Total (21) | PGA-2.6 | 0.03–2 | 0.5 | 0 | 100 |
| HA2-2.6 | 0.03–4 | 0.5 | 4.8 | 95.2 | |
| HA2-2 | 0.06–2 | 0.5 | 0 | 100 | |
| HA2-1.5 | 0.06–4 | 0.5 | 4.8 | 95.2 | |
| SOP-2.6 | 0.06–2 | 0.5 | 0 | 100 | |
| SOP-2 | 0.125–2 | 1 | 0 | 100 | |
| SOP-1.5 | 0.06–4 | 0.5 | 4.8 | 95.2 | |
| PME | 0.125–2 | 2 | 0 | 100 |
| T1/2 (h) | Tmax (h) | Cmax (μg/mL) | AUC (h•g/mL) | AUCINF (h•μg/mL) | Cl (mL/min/kg) | MRTINF (h) | Vss (mL/kg) | |
|---|---|---|---|---|---|---|---|---|
| PME | 1.33 ± 0.05 | 0.08 | 10.26 ± 1.78 | 12.12 ± 2.08 | 12.29 ± 2.12 | 4.16 ± 0.78 | 1.63 ± 0.15 | 403.50 ± 54.46 |
| SOP | 1.48 ± 0.19 | 0.08 | 10.50 ± 0.81 | 11.13 ± 0.92 | 11.35 ± 0.90 | 4.42 ± 0.34 | 1.69 ± 0.20 | 448.24 ± 68.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Liu, L.; Wang, W.; Yuan, Y.; Wang, W. Nanoformulation of Polymyxin E Through Complex Coacervation: A Pharmacokinetic Analysis. Pharmaceutics 2025, 17, 76. https://doi.org/10.3390/pharmaceutics17010076
Chen X, Liu L, Wang W, Yuan Y, Wang W. Nanoformulation of Polymyxin E Through Complex Coacervation: A Pharmacokinetic Analysis. Pharmaceutics. 2025; 17(1):76. https://doi.org/10.3390/pharmaceutics17010076
Chicago/Turabian StyleChen, Xiaobao, Li Liu, Weidan Wang, Yuan Yuan, and Wei Wang. 2025. "Nanoformulation of Polymyxin E Through Complex Coacervation: A Pharmacokinetic Analysis" Pharmaceutics 17, no. 1: 76. https://doi.org/10.3390/pharmaceutics17010076
APA StyleChen, X., Liu, L., Wang, W., Yuan, Y., & Wang, W. (2025). Nanoformulation of Polymyxin E Through Complex Coacervation: A Pharmacokinetic Analysis. Pharmaceutics, 17(1), 76. https://doi.org/10.3390/pharmaceutics17010076






